Abstract
Polypeptides exhibit significant health-promoting effects through diverse biological activities, including antihypertensive, antidiabetic, anti-cancer, antimicrobial, and antioxidant properties. Membrane technology offers an efficient separation approach for polypeptides due to its high efficiency, low energy consumption, operational simplicity, and environmental sustainability. This review briefly described the advancements in membrane separation of polypeptides and highlighted the major implementation challenges, such as membrane fouling, peptide adsorption losses, and compromised separation efficiency caused by peptide aggregation. Contributing factors for each issue based on the progress and reports of relevant research were analyzed. And solutions and strategies were also summarized as feed pretreatment, operational parameter optimization, aggregate elimination, and membrane surface modification. These approaches could reduce product loss and enhance peptide yield during purification. This review can provide reference for the research on efficient membrane separation of polypeptide products.
1. Introduction
Peptides are composed of different amino acids, which constitute the structure and function of proteins, and are one of the important components of organisms. They have a variety of biological activities, including anti-hypertension, anti-diabetes, anti-cancer, antibacterial, and antioxidant [1,2,3,4], which have many positive effects on health. While the health-promoting properties of bioactive peptides are well known, the lack of economical scale-up purification technology would limit commercial production of bioactive peptides [4]. In recent years, a number of feasible methods have been proposed for the isolation and purification of peptides, such as salt chromatography, ion exchange chromatography, reversed-phase high-performance liquid chromatography, affinity chromatography, etc. However, the complexity of separation and the excessive product loss have prevented the application of these techniques in large-scale production of bioactive peptides in the pharmaceutical industry.
Membrane separation technology is a set of separation, purification, and concentration, and is high-tech and highly efficient, as well as having low energy consumption, simple operation, and environmental friendliness [5,6]. Since the introduction of membrane separation technology in the last century, microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), and other membrane separation technologies have been developed successively, and have now been applied in the fields of medicine, food, the chemical industry, environmental protection, and so on. Due to its mild operating conditions and environment, membrane separation can be carried out at normal temperature and does not require any chemical additives [7], which is suitable for the separation of bioactive substances, and has little impact on the activity of peptide substances, providing a further feasible means for the separation and purification of peptides.
Membrane separation process is driven by pressure, difference, concentration difference, potential difference, etc., and, according to the selective permeability of the membrane, small molecules can freely pass through the semi-permeable membrane, while large molecules are selectively intercepted by the membrane, so as to achieve the separation of the multi-component mixture, and to achieve the extraction, concentration, and purification of products. However, during the membrane separation process, solutes, suspended solids, colloids, biological particles, and other impurities gradually accumulate and pile up on the surface of the membrane or in the pore. The membrane flux is reduced, and then blockage is formed, which affects the separation process as well as the purification effect, and membrane contamination is formed. In addition, the adsorption of peptide has always been an important topic of concern in the membrane separation process. The concentration of peptides in a solution is extremely low compared with common bio-based products; the adsorption of peptides on the membrane surface could not be neglected. Mainly due to the interaction between the peptide molecules and the membrane surface, peptides might be adsorbed or rejected by the membrane with electrostatic force or hydrophobic interaction and so on, which will affect selectivity of membrane separation, which has become a major obstacle to membrane separation [8].
This paper first describes the progress of the application of membrane technology in peptide separation and purification, and then focuses on the existing problems. The resolution strategies were also discussed, which could help to improve the separation selectivity and recovery of peptides, further promoting the application of membrane separation in bioactive peptide production and purification.
For this review, a search of the literature was conducted using the ScienceDirect and Wiley core collection databases, covering publications since 2005. The search strategy primarily employed combinations of keywords such as “protein/peptide”, “fouling”, “adsorption”, “aggregation”, “membrane separation”, and related synonyms. Additionally, the reference lists of retrieved studies were manually screened to identify further relevant publications. The flowchart is shown in Figure 1.
Figure 1.
Study flowchart.
2. Application of Membranes in the Separation and Purification of Peptides
According to the principle of membrane action, pore size. and different applications, types of membrane technology can be divided into MF, UF, NF, RO, electrodialysis (EDFM), affinity membrane separation, etc. Compared with other separation processes, the general membrane separation process has mild reaction conditions and simple operation, which maintains the bioactivity of peptides. In recent years, membrane technology has been used for peptide separation and purification.
2.1. Microfiltration
MF is a process that utilizes membranes with pore sizes roughly between 0.05 and 10 um to retain macromolecules, suspending particles using pressure difference as the driving force, and is widely used for the separation, purification, and clarification of protein solutions [9]. Van der Schaaf et al. extracted and purified β-casein from micellar casein concentrate by MF; the results showed that the content of αs –casein in the permeate of MF was reduced to below 0.1% and successfully separated β-casein from casein micelles at 5 °C [10]. Kongsinkaew et al. successfully separated a 4.1 kD candidate cell-penetrating antimicrobial peptide (CAP) from most of the contaminating proteins and the pigments by using MF with a pore size of 0.2 μm [11]. Swaminathan et al. removed the peptides from the hydrolyzed protein solution by MF composed of polyvinylidene fluoride (PVDF) with a pore size of 0.1 µm to concentrate the phospholipids (PL) and fats, thus producing a partially enriched PL concentrate in the final product [12]. However, the MF membrane has difficulty effectively separating soluble organic substances with different molecular weights at the molecular level. Therefore, the application range of MF is relatively narrow, and it can only be used for the preliminary crude separation of peptides to remove insoluble particulate matters and colloids.
2.2. Ultrafiltration
Compared to MF, UF membranes have a different molecular weight cut off (MWCO), thus improving the selectivity of separation of organics with different molecular weights. The process of UF involves the passage of solvent and small molecule compounds through the membrane under a pressure difference, while colloids and biomacromolecules are retained to achieve the separation effect. Proteins, peptides, amino acids, salts, and other compounds (e.g., organic acids, sugars, vitamins, etc.) can be separated by UF membrane with different MWCO [9]. In many biological downstream processes, UF is a practical and cost-effective method for the purification and concentration of products in large-scale and continuous production. Numerous studies have shown that the extraction and concentration of peptides using UF was a routine and successful operation. Tam et al. performed UF of colostrum whey using 10 kD hollow fiber membranes and found that the best peptide permeation of 95% was achieved at a trans-membrane pressure of 2 bar with a feed flow rate of 3 L/min [13]. It showed the permeation decreased if the pressure was further increased. Marcet et al. investigated the effect of pH on the selective permeation of peptides in tryptic hydrolysates using polyethersulfone membrane with a MWCO of 1 kD and cellulose-based membranes with a MWCO of 2 kD, respectively, under the pH 4.0, 7.0, and 10.0 [14]. The results showed that the permeate contained the most peptides with molecular weights lower than 1240 Da, and the peptide concentration in the permeate filtered by the two membranes was the highest at pH 4.0. Ghalamara et al. chose polysulfone membranes with a MWCO of 3 kD to separate the higher molecular weight (≥10 kD) protein fraction from the lower molecular weight (≤1.2 kD) peptide fraction of the sardine cooking wastewater [15]. The results showed that the fraction with MWCO less than 1.2 kD was preferentially permeated, while those with MWCO more than 10 kD were completely rejected by the UF membrane.
2.3. Nanofiltration and Reverse Osmosis
NF membrane is a membrane between RO and UF, and the pore size is usually 1 nm. Compared with UF, NF membrane can retain smaller sizes of substances and multivalent salts, and it also has lower operation pressure, low energy consumption, and higher flux compared with RO [16,17]. In the NF process, the sieve effect is utilized to separate neutral components and the Donnan effect to separate charged components [18]. The two principles are synergistic with each other to retain small molecular organics and can be dialyzed and desalted at the same time, which combines concentration and dialysis. Fjerbæk et al. utilized NF to sequester proteins and small peptides from herring brine and selectively separate small molecules such as salt and acetic acid, showing that proteins were concentrated 30-fold and small peptides 11-fold, which facilitated later recycling and processing [19]. Saidi et al. separated bioactive peptides from tuna protein hydrolysate using 1 KD NF membranes and showed that the permeate recovered by UF in the previous step was finally concentrated by the NF step and that 22.5% of the peptides had a MWCO in the range of 0.3–1 kD, and approximately 72.5% of peptides were less than 0.3 kD [20].
In the presence of a concentration difference, the process of spontaneous movement of water molecules in solution to a region of higher concentration is called osmosis, and the driving force that prompts osmosis of water molecules is called the osmotic pressure. If the pressure exerted on the region of higher concentration is greater than the osmotic pressure, the water molecules flow in the direction opposite to the process of osmosis, which is called RO. RO can be used for the simultaneous removal of ions, particles, and organic components. By combining RO with UF and NF, Ghalamara et al. demonstrated the extraction of small peptides exhibiting antioxidant capacity and antibacterial activity from sardine cooking wastewater fractions [15].
2.4. Electrodialysis
EDFM technology is under the action of DC electric field, due to the barrier effect of ion exchange membrane, with electrostatic gravity as the driving force, to realize the purification and concentration of the solution, low energy consumption, and high efficiency. EDFM can also be coupled with other techniques such as UF, which further improves the separation efficiency by replacing a portion of the ion-exchange membrane with an UF membrane, and it also overcomes the membrane contamination problem of UF membranes while separating charged substances. Firdaous et al. selectively isolated anionic/cationic peptides from antihypertensive peptides from alfalfa white protein hydrolysate by EDFM [21]. The results demonstrated that, during continuous EDFM, a 12-fold membrane area expansion combined with controlled KCl solution pH increased yields by 11.6-fold for the target cationic peptide (pH 3.0) and 9.8-fold for the target anionic peptide (pH 9.0). Doyen et al. used EDFM with UF membrane to separate antimicrobial peptide fractions from snow crab by-product hydrolysate, with peptide recoveries of up to 94% of the peptides with molecular weights in the range of 300–600 Da [22].
3. Challenges in Membrane Separation of Polypeptides
Although membrane technology is widely used in the separation and purification of polypeptide, there are some unavoidable challenges which limit the application of membrane technology in this field. These include membrane fouling, membrane adsorption, and poor membrane selectivity.
3.1. Membrane Fouling
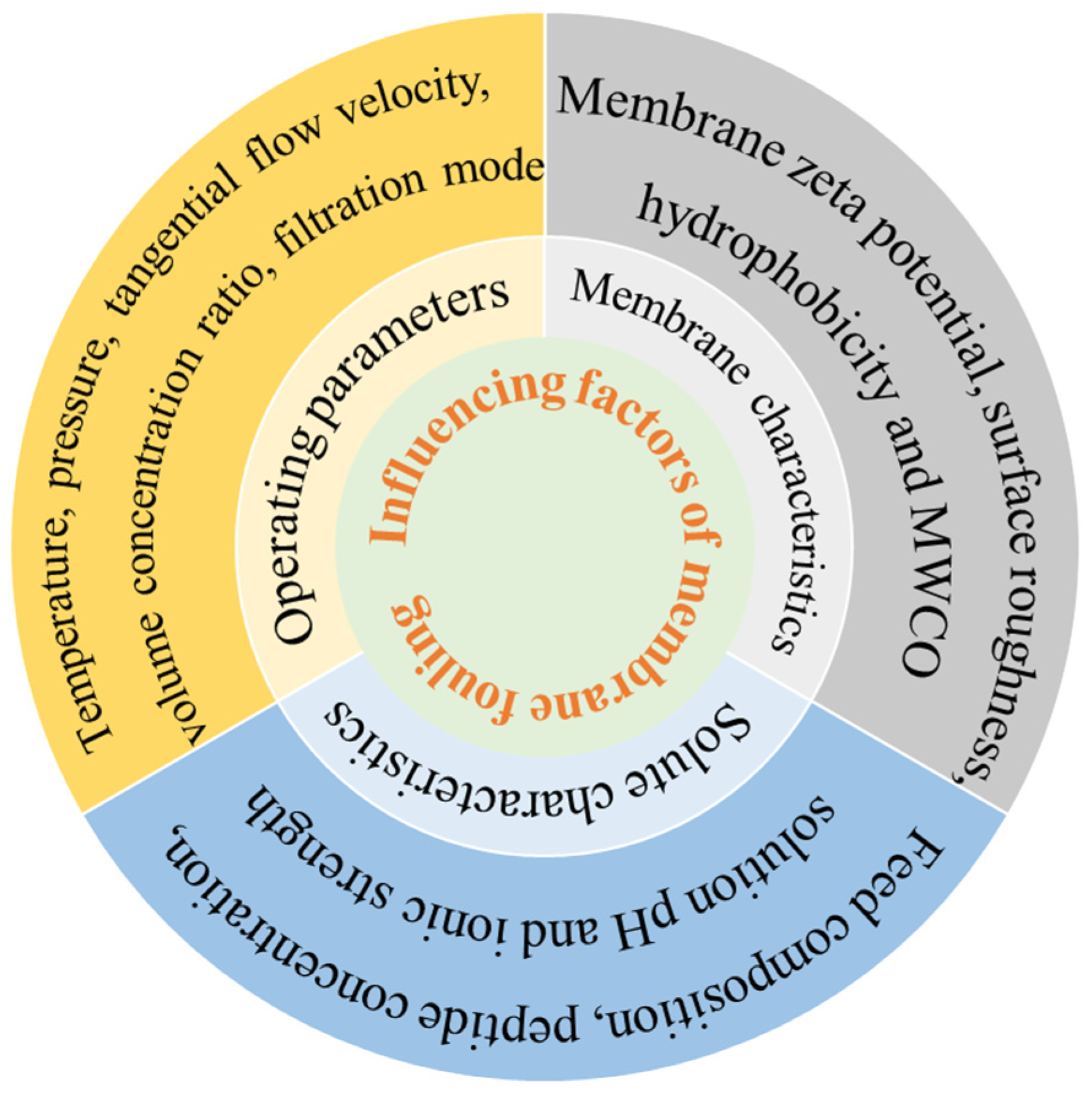
Membrane fouling is a major barrier to membrane separation technology. It consists of the gradual deposition and accumulation of suspended/dissolved substances on the membrane surface or within the membrane pore. Membrane fouling has a direct impact on membrane flux and membrane life [23]. The reductions in pore size and filtration capacity are mainly manifested by a gradual decrease in osmotic fluid flux over time during constant pressure operation [24]. Membrane fouling is mainly divided into two cases: one is the concentration polarization of the membrane surface that eventually forms a filter cake layer (external fouling); the second is caused by the adsorption of the membrane surface and the membrane hole (internal fouling) [25]. Proteins are typical contaminants of UF [26], which are easily absorbed and accumulated on the membrane surface, resulting in high loss and severe membrane fouling. The factors affecting membrane contamination can be divided into three areas: physical and chemical properties of the solute and solvent, membrane characteristics, and operating parameters during separation [27], as outlined in Figure 2.
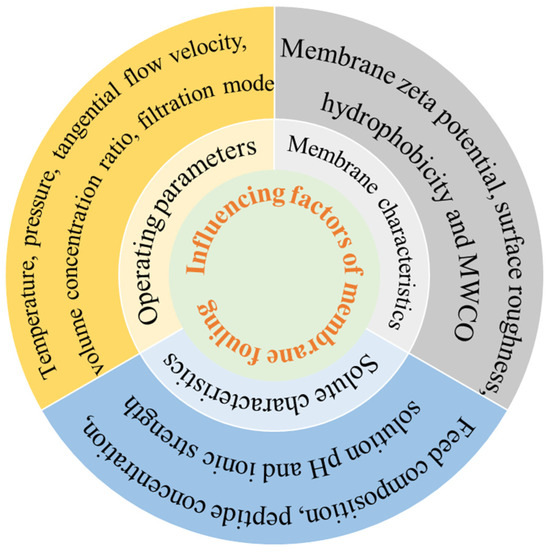
Figure 2.
Influence factors of membrane fouling.
Physical and chemical properties of the solute and solvent are directly related to membrane contamination. Feed composition, peptide concentration, solution pH, and ionic strength played important roles in membrane contamination [28]. Beaubier et al. [29] found that the permeation of the antimicrobial peptide of bovine hemoglobin hydrolysate (NKT, 653Da) by UF (1 kD MWCO regenerated cellulose membrane) was the highest when the degree of hydrolysis was 3%. He found that the content of peptides with small molecular weight increased as the degree of hydrolysis rose; therefore, more peptides could pass through the membrane, and then the blockage of the membrane hole was more serious. Similarly, when tertiary UF membranes were used to separate polypeptides from yeast wastewater hydrolysates, Marson et al. [30] compared the UF sequence of method 1 (50 kD-8 kD-1 kD) and method 2 (15 kD-8 kD-1 kD); the flux of method 2 (16.5 L m−2 h−1) was much higher than that of method 1 (9.2 L m−2 h−1) at 1 kD membrane separation. In method 1, the permeable components of 50 kD UF still existed in the permeate of 8 kD UF, which was more conducive to the plugging of the internal pores of the 1 kD membrane. In method 2, a more important cake layer was formed in UF by 15 kD membrane, and some smaller molecular weight substances were intercepted, and no pollution was formed in subsequent UF. Saidi et al. [31] separated protein hydrolysate from Prolastin® (a commercial fish hydrolysate) by polyethersulfone (PES) UF membrane and found that the membrane separation flux decreased with the increase in Prolastin® concentration. And the retention rate for peptides of all molecular weights remained almost unchanged; as the initial concentration rose from 30 to 120 g/L, it confirmed the key effect of concentration polarization phenomena and fouling which are enhanced at high concentration. At the same time, Saidi found that pH decreased from 8.0 to 3.0 and membrane flux decreased threefold. This is attributed to the decrease in pH in the solution causing an increase in the negative charge of the peptide and a decrease in the repulsion between the peptide and the surface of the negatively charged PES membrane to aggravate contamination. Miao et al. [32] found that the negative property of bovine serum albumin (BSA) and PVDF membrane decreased with the increase in ionic strength (0~1 mM), which reduced the electrostatic repulsion between membrane and protein and between proteins, resulting in more serious membrane pollution. Roslan et al. [33] also reported that during UF fractionation of tilapia by-product protein hydrolysate, the permeation flux decreased at salt concentrations of 0.2~0.6 M compared with the absence of salt. High salt concentrations disrupted the hydrated layer, enhanced peptide interactions, and exacerbated membrane fouling.
As a separation medium, the physicochemical properties of the membrane, such as membrane zeta potential, surface roughness, hydrophobicity, and MWCO, play an important role in the trend of membrane fouling. Persico et al. [34] separated whey protein hydrolysates by MF membranes and found that zeta potential and surface roughness were highly correlated with total membrane fouling. Specifically, the peptide adsorption capacity of the S11+ membrane (functionalized PVC-silica, 0.076 μm) was up to four times higher than those of the S11 (PVC-silica, 0.079 μm) and S11− (differently functionalized PVC-silica, 0.071 μm) membranes. The zeta potential of S11+ was +0.3 mV at pH 7.0, while those of S11 and S11− were −7.4 mV and −5.8 mV, respectively. Furthermore, the CF55 membrane (PVC-silica, 0.26 μm), which had a zeta potential similar to S11, exhibited a lower polypeptide adsorption capacity than S11 (236 μg/cm2 compared with 296 μg/cm2). This difference is attributed to CF55’s larger pore size, lower surface roughness, and fewer adsorption sites. Hashino et al. [35] studied the scaling of membranes in UF of BSA, which indicated that PVDF membranes with high BSA retention rate had a more serious decrease in permeability. This is due to the stronger hydrophobicity of the PVDF membrane surface, and the PVDF-BSA interaction is much stronger than the BSA-BSA force. When separating active peptides from whey protein hydrolysates, Arrutia et al. [36] found that both the flux (10.6 L·m−2·h−1) and the average peptide permeability (4.37%~90.84%) of the 5 kD membrane were higher than those of the 1 kD membrane (2.0 L·m−2·h−1 and 5.97%~43.15%, respectively). However, the peptide composition in the permeate solutions was similar for both membranes, a result attributable to the larger pore size of the 5 kD membrane. However, Persico et al. [37] observed that PES UF membranes with MWCOs ranging from 5 to 300 kD showed no significant difference in their separation of whey protein hydrolysates or in membrane fouling, which may be attributed to the molecular weight of its hydrolysate peptides below 2.3 kD, much lower than the MWCO of the membrane.
Operation parameters during filtration, such as temperature, pressure, tangential flow velocity, volume concentration ratio (VCR), and filtration modes, directly affected the adsorption and blockage of proteins and peptides in the membrane separation process. Barukčić et al. [38] used ceramic membranes for MF of sweet whey at temperatures of 20, 40, and 50 °C and the highest flux was achieved at 50 °C, which was explained by the decrease in viscosity as the temperature increased. Steinhauer et al. [39] investigated the effect of temperature on the MF flux and scaling layer properties of suspension prepared from the isolated main whey protein β-Lg by dead-end filtration. The results showed that the flux increased gradually at 10~30 °C, but the scaling resistance did not change; the flux was only affected by the viscosity. However, at 30~40 °C, the flux decreased significantly, and the scaling resistance increased rapidly for the cross-linking between proteins at high temperature although they were not denaturated. Arias et al. [40] optimized the conditions for obtaining antioxidant peptides from red tilapia viscera hydrolysates via ceramic membrane UF. They found that the concentration polarization layer was intensified at lower flow rates. Higher pressure can obtain stronger driving force, which is conducive to the increase in flux, but at the same time, more serious plugging of gel layer and membrane hole occurs, increasing irreversible pollution. Wang et al. [41] investigated the effect of VCR on osmotic flux during NF of glutathione and found that higher VCR corresponded to lower flux due to the increased concentration of linearized glutathione produced by higher VCR. Cirillo et al. [42] investigated fouling evolution during MF of BSA with dead-end (DE) and cross-flow (CF) mode. They found that no BSA was detected in the permeate during DE mode. SEM characterization confirmed that a lot of BSA was adsorbed onto the membrane pores and surface. During DE mode filtration, individual proteins accumulated on the membrane surface and within the pores due to hydrophobic interactions, significantly reducing the pore size and hindering the flow of the permeate. The slowed permeation flux led to a longer residence time within the membrane, which promoted the adsorption of albumin within the membrane.
3.2. Loss of Polypeptide Caused by Membrane Adsorption
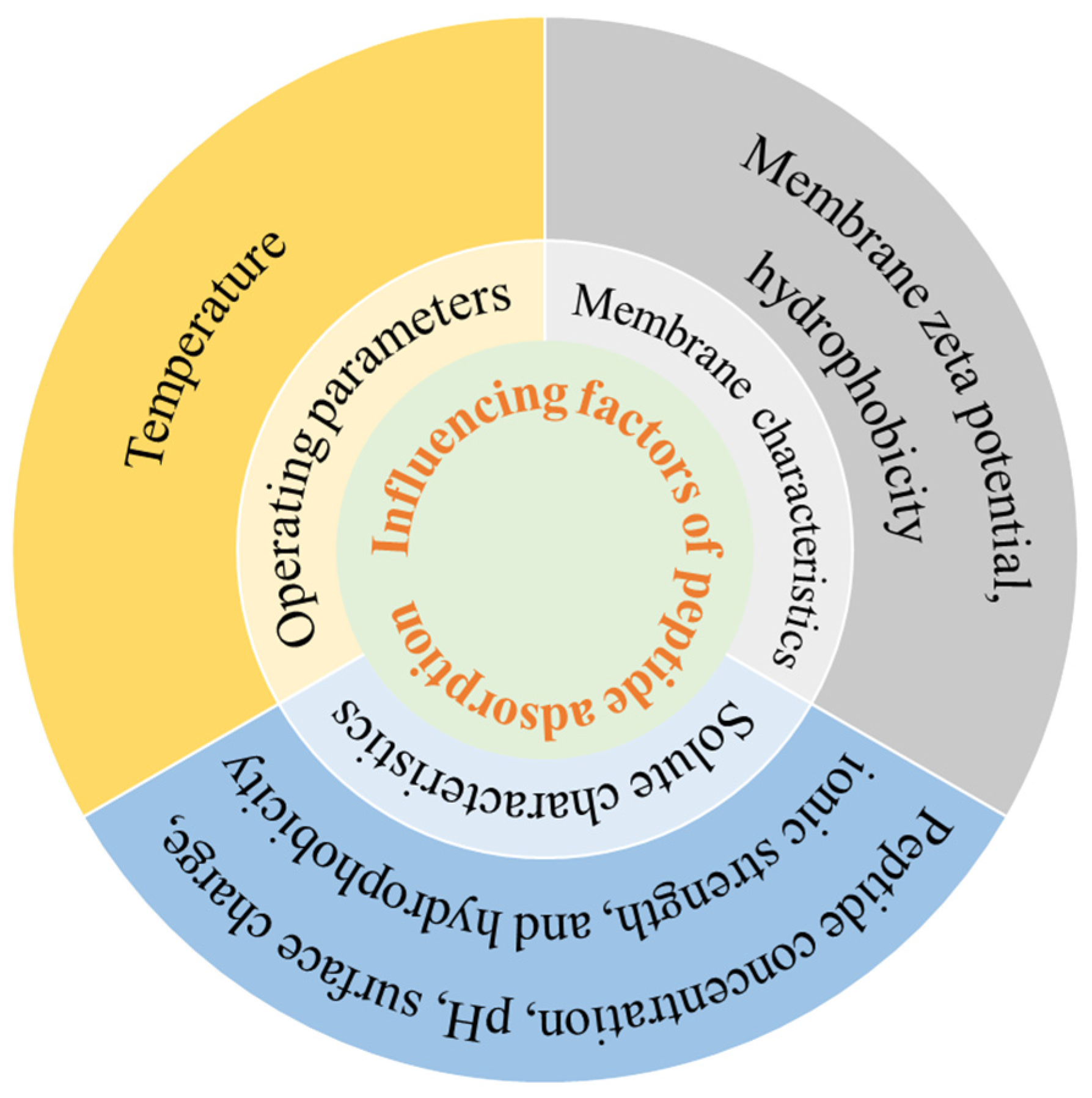
Due to the presence of membrane contaminants, polypeptides are inevitably adsorbed to the pore or surface of the membrane. Protein–membrane interactions are key factors determining protein adsorption in membrane filtration systems, which are mainly manifested as electrostatic interactions and hydrophobic interactions between peptides and membranes [43,44]. Membrane adsorption is a component of membrane fouling and is also affected by polypeptide properties, membrane properties, and operation conditions, as outlined in Figure 3.
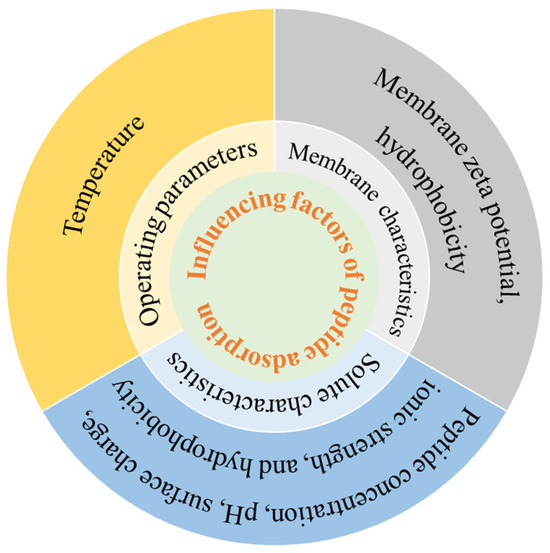
Figure 3.
Influence factors of peptide adsorption.
Factors affecting peptide adsorption include the peptide concentration, pH, surface charge, ionic strength, and hydrophobicity. Chabeaud et al. [45] reported higher initial peptide concentrations of fish protein hydrolysates reduced permeate flux of UF. When the UF with low peptide concentrations (5 g/L and 30 g/L) was completed, 95% of the permeation flux was restored after a single five-step cleaning cycle. In contrast, after UF with high concentrations (90 g/L and 150 g/L), a second regeneration step was required, which suggested that severe adsorption occurred at high concentrations. When brewer’s yeast protein hydrolysate was separated by 30 kD PES membrane, the water flux and average pore size decreased more significantly at pH 5.0 than pH 8.0 [46], which was attributed to the fact that pH 5.0 was closer to the isoelectric point of yeast protein and the adsorption was more severe. Marcet et al. [14] reported that during the UF of trypsin hydrolysate under alkaline conditions (pH 10.0), compared to acidic conditions (pH 4.0), the permeate exhibited a lower peptide concentration and a higher adsorption capacity. This difference was attributed to the significantly higher negative surface charges of the PES and RC membranes at pH 10.0 (−35 mV and −20 mV, respectively) relative to pH 4.0 (both −5 mV). The significant increase in negative surface charges on the membrane significantly enhances the electrostatic adsorption of peptides. Kilmer et al. [47] observed more rapid scaling at ionic strengths below 100 mM, attributing to reduced electrostatic repulsion between the protein and the membrane at lower ionic strength, which resulted in faster adsorption of protein. Fernandez et al. [48] studied the effect of peptide hydrophobicity on the separation of β-lactoglobulin trypsin digestives using 5 kD PES membranes. They found hydrophobic adsorption interactions between peptides and membranes were enhanced with increasing peptide hydrophobicity. Loh et al. [49] found that 2.0 g/L hemoglobin (Hb) was adsorbed at both pH 4.7 and 7.4 in the UF by PVDF membranes, whereas 4.0 g/L BSA adsorption occurred only at pH 4.7 (BSA pI). This difference was attributed to Hb’s stronger inherent hydrophobicity, which promoted hydrophobic interactions with the PVDF membrane.
The effect of membrane properties on the adsorption of polypeptides is directly related to the zeta potential and hydrophobicity of the membrane surface. Different graft modifications on cellulose membranes were performed to study their adsorption of human serum immunoglobulin (IgG) at pH 9.0 [50], which showed that the IgG adsorption capacity of the sulfonic acid-modified membrane (approximately 100 μg/cm2) was lower than the basic membrane (930 μg/cm2). This significant difference was due to the contrast in zeta potential between the sulfonic acid-modified membrane (−9.4 mV) and the basic membrane (+5.0 mV). Specifically, the strong electrostatic attraction between the negatively charged IgG and the positively charged amino groups on the aminated membrane promoted its higher adsorption. Compared with hydrophilic membranes, hydrophobic membranes were more prone to protein adsorption [51]. Hadidi et al. also reported that the hydrophobic membrane exhibited nearly double the IgG adsorption capacity of the hydrophilic membrane (490 μg/cm2 vs. 250 μg/cm2) [50].
Temperature is another factor affecting the adsorption of protein and polypeptide. In general, the diffusion rate of proteins to the adsorbent surface is accelerated at high temperatures, and the adsorption rate would increase. When the RO membranes were used to study the influence of temperature on BSA adsorption [52], the flux decline rate was enhanced when the temperature increased (10~40 °C), and the adsorption of BSA by the membranes was more serious. It could be explained in two ways. Firstly, the increase in temperature decreased the negative zeta potential of RO membrane and reduced the electrostatic repulsion between BSA molecules and the surface of the membrane. On the other hand, BSA exposed more hydrophobic residues at higher temperatures, and the hydrophobic aggregation between BSA molecules was enhanced.
3.3. Poor Selectivity Caused by Polypeptide Aggregation
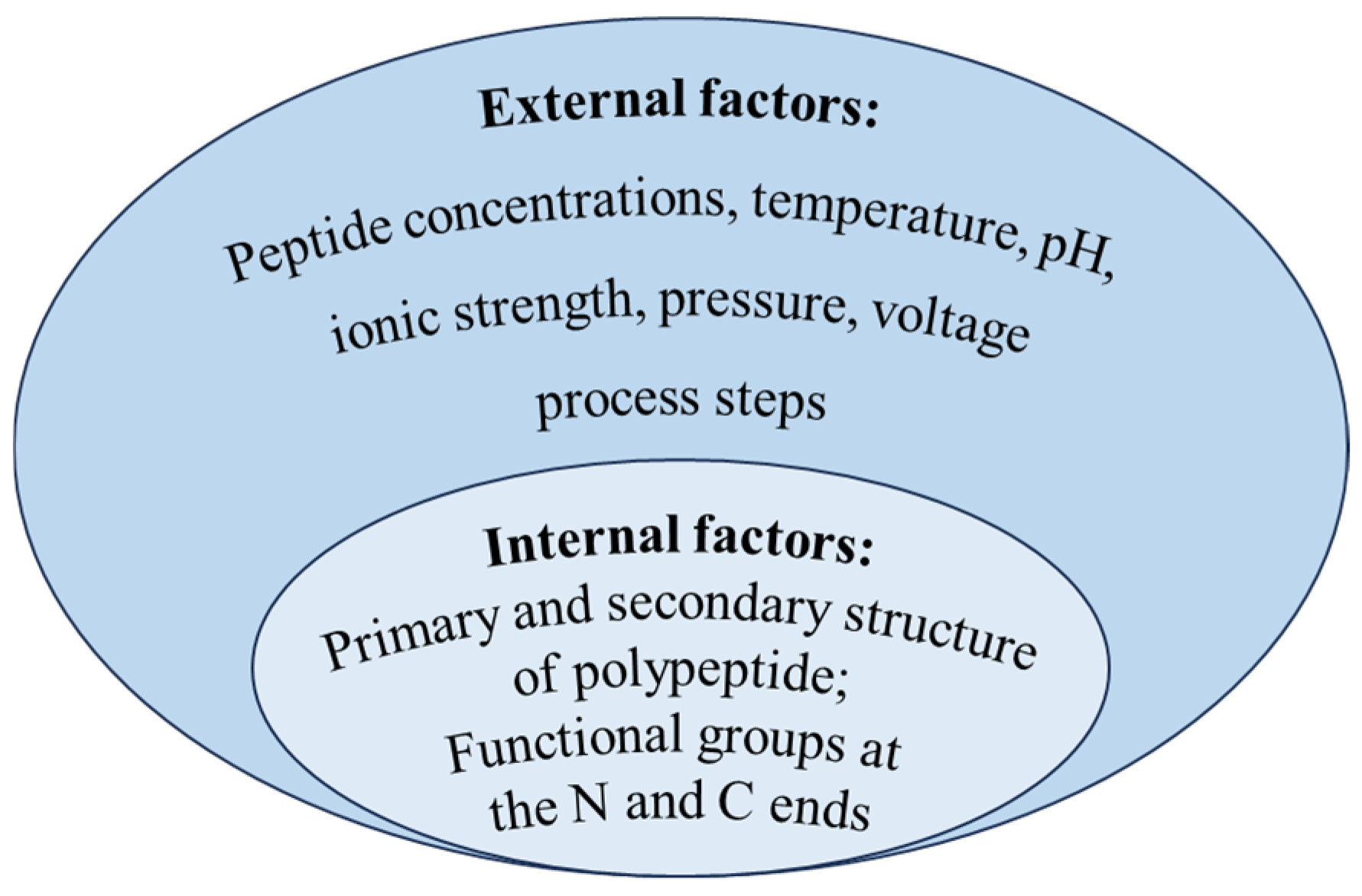
The aggregation of polypeptides multiplies the size of the polymer, which will prevent polypeptides from penetrating the membrane, and results in poor selectivity for membrane separation based on the screening effect. Although studies on the aggregation behavior of peptides remains limited, analogous phenomena of protein self-assembly has been extensively documented. Starting with internal conformational changes, some protein–protein interactions led to the formation of dimers and oligomers [53], then dimers or oligomers continued to aggregate and grow under hydrophobic, electrostatic, and covalent interactions, finally forming polymers and even visible insoluble particles [54,55]. Protein aggregation can occur through different mechanisms or pathways, and different pathways may occur simultaneously. Majid et al. [56] summarized five main polymerization mechanisms as follows: reversible self-assembly of the native monomer, aggregation of conformationally altered monomeric proteins, aggregation of chemically modified protein, nucleation-controlled aggregation of protein, and surface-induced aggregation of protein. However, regardless of the mechanism, peptide aggregation may be affected by both internal and external factors, as outlined in Figure 4.
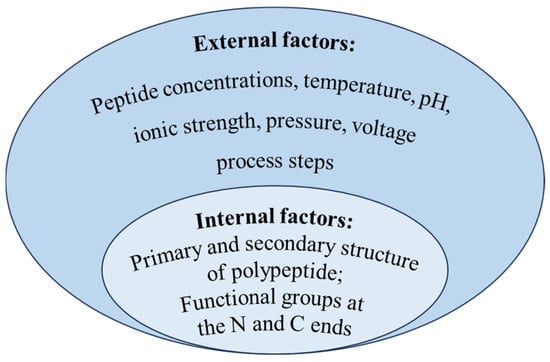
Figure 4.
Influence factors of polypeptide aggregation.
Relevant studies have shown that the intrinsic factor affecting peptide aggregation was related to the structure of peptides [57]. There are many regions or sequences of polypeptide amino acids which are easy to aggregate to form aggregates; they are called aggregation prone regions (APRs) [58]. The hydrophobic interaction, hydrogen bonding force, and van der Waals force among these APRs are the main forces for aggregate stability [59]; the higher the relative number of hydrophobic amino acids in the primary structure, the more APRs are formed. Tartaglia et al. [60] compared the aggregation tendency of six unstructured polypeptides. Among them, α-Synuclein could self-assemble to form an inclusion body in neuron cells. The other five peptides (Aβ1-42, calcitonin, glucagon, the second WW domain of CA150, and islet amyloid polypeptide) were aggregated as amyloid fibrils. It was mainly explained by the longer aggregation core of α-Synuclein (residues 30–95), which included four APRs. The alpha helix structure in the secondary structure of proteins had been shown to be a precursor to the formation of beta sheet aggregates [61]. Pawar et al. [62] evaluated the intrinsic tendency of aggregates of unstructured polypeptide chains and found that beta sheets favored aggregation. The aggregation region of Aβ42 peptide was identified as residues 15–21 and 30–42, and it was verified that the residue sequences of Aβ42 peptide formed β sheet structure in the aggregation. Relevant studies had indicated that various functional groups at the N-terminal and C-terminal of the polypeptide could affect the formation of fibril, and it was verified that the hydrogen bond between amino acids promotes the formation of fibril [8]. The (105–115) peptide fragments of transthyroxin protein (TTR) with different N-terminal and C-terminal groups were synthesized by solid-phase synthesis. When the C-end is carboxyl or amide group and the N-end is α-amino, the fragment of N-acetyl-α-amino will form fibril, while the fragment of peptide with the group N, N-dimethyl-α-amino will not form fibril. The N, N-dimethyl-alpha-amino group inhibits hydrogen bond formation.
High concentrations of proteins increase intermolecular interactions, while the presence of an upper solubility limit for proteins can promote aggregation [63]. Adachi et al. [64] employed differential interference contrast (DIC) microscopy to investigate the formation of deoxyhemoglobin (deoxy-HbS) fibers under temperature jump conditions (0~22 °C). They observed that numerous HbS fibers formed when the concentration of deoxygenated HbS exceeded its solubility limit (~17 g/dL). Furthermore, increasing HbS concentration progressively shorted the fiber nucleation time. At concentrations above 25 g/dL, fiber nucleation occurred almost instantaneously.
Temperature is an important factor in protein conformation and stability, and both high and low temperatures can induce protein aggregation. Oliva et al. [65] investigated the stability of Bevacizumab at 5–50 °C. It was found to be stable at 5 °C (18 months); a total of 3.2% of the monomers formed dimers at 25 °C after 5 days, and dimers, trimers and high molecular weight aggregates appeared at 50 °C after 3 days. Meliga et al. [66] found that the cold-induced precipitation of monoclonal IgM had negative activation enthalpy, and the cold-induced precipitation of monoclonal IgM cryoglobulin occurred when the temperature decreased to 14~15 °C.
Protein stability is also affected by the pH of the solution, which can alter protein conformation [67] and led to protein aggregation. Li et al. [68] studied the effect of pH on BSA (pI 4.7) aggregation and found that aggregation increased with decreasing pH in the pH range 3.0~7.0. When the pH dropped from 7.0 to 4.7, the net charge of the BSA molecules was reduced, and the electrostatic repulsion between the proteins was weakened, increasing the likelihood of aggregation. When pH decreased from 4.7 to 3.0, the structure of BSA expanded, the hydrophobic region increased, the content of secondary structure β sheet increased, and the aggregation increased. Filipe et al. [69] found that IgG appeared significantly clustered (from dimer to 10 μm particles) at pH 1.0 versus pH 6.0, attributing more APRs to changes in tertiary structure at low pH.
Ionic strength is a critical solution parameter affecting protein aggregation. Ions of both charges present in solution can combine with, or electrostatically interact with, proteins. These interactions may alter inter-protein charge–charge interactions and induce conformational changes, thereby promoting varied aggregation behavior [70]. When studying the aggregation of insulin, Giger et al. [71] observed that at pH 5.2 and 10 mM ionic strength, the turbidity of a 0.1 g/L insulin solution increased significantly, indicating maximum aggregation. This phenomenon occurred because added salt neutralized protein surface charges, thereby reducing electrostatic repulsion between molecules. Varga et al. [67] observed that at pH 7.0, BSA existed predominantly as monomers (2.2 nm particle size) in salt-free conditions but formed significant dimers (5.3 nm) with 150 mM NaCl. This shift was attributed to salt-induced reduction in zeta potential, which decreased from −69 mV (no salt) to −2.4 mV.
Roche et al. [72] found that high pressure caused sulfhydryl (SH)-disulfide exchange reaction led to the formation and stabilization of protein aggregates. Cordeiro et al. [73] found that high pressure treatment caused transthyroxin protein (TTR) to become a pre-aggregated tetramer state, due to the severe deformation of tryptophan in TTR under high pressure. In this state, aggregates would form within 30 min after decompression, while the aggregation of TTR was formed after 72 h under normal pressure.
Voltage is a critical operational parameter in electrodialysis that directly influences the extent of protein aggregation. Wang et al. [74] demonstrated that the particle size of soy protein isolate (SPI) progressively increased under electric field intensities ranging from 4 to 10 V/cm. They attributed this phenomenon to electric field-induced exposure of hydrophobic groups, which facilitated the formation of disulfide bonds and ultimately promoted protein aggregation. Hu et al. [75] reported that whey protein experienced more pronounced aggregation under low electric field intensities (5–10 V/cm), whereas, at higher voltages (15–20 V/cm), initial aggregates formed and subsequently dissociated, resulting in increased solubility. This behavior was attributed to the enhanced formation of disulfide bonds and greater surface hydrophobicity at lower voltages. In contrast, higher voltages promoted the formation of α-helices and increased net surface charge, thereby weakening hydrophobic interactions and leading to the dissociation of aggregates.
Process steps, such as agitation, pumping, sonication, and exposure to light, could also affect peptide aggregation [76]. The first three processes all increase the rate of collision motion between polypeptide molecules while creating an air–water interface, where shear forces expose hydrophobic regions and induce protein aggregation [77,78]. Bovine insulin subjected to shear rates of 20~150 s−1 for 3000 s exhibited concentration reduction from 1 mg/mL to undetectable levels due to severe aggregation. By contrast, no significant concentration change occurred under static conditions [79]. The explanation is not that the shear force destroys the tertiary structure of the protein, such as hydrogen bonds, but that the hydrophobic residues adsorbed at the hydrophobic interface at high shear are transformed into denaturated states, inducing the enhancement of aggregation [80]. Exposure to light might cause protein degradation. Sreedhara et al. [81] observed significant degradation in five IgG1 monoclonal antibodies (mAbs 1–5) following exposure to both visible light (≥1.2 × 106 lux·h) and UV radiation (≥200 W·h/m2). This light-induced damage promoted extensive oxidation of Trp residues (including conserved Trp motifs) and Met, while also triggering antibody aggregation.
4. Methods and Strategies for Solving Problems
As mentioned previously, membrane fouling, adsorption, and polypeptide aggregation affect membrane separation performance and separation yield of polypeptide. Many studies are underway to reduce membrane fouling and peptide adsorption.
4.1. Pretreatment Methods
Pretreatment is a key step to prevent membrane fouling, which mainly includes chlorination, ultrasound, adsorption, MF, and their combination in the process of protein separation [82], which can be used as a reference for polypeptide separation. The purpose of pretreatment is to reduce the impurity composition in the feed liquid or change the form of the product (peptide) by simple treatment, so as to reduce the concentration polarization, filter cake formation, and membrane surface adsorption caused by membrane filtration. The summary is shown in Table 1.

Table 1.
Summary of preprocessing solution strategies.
Miao et al. explored the influence mechanism of pre-chlorination on protein fouling of UF membranes [83]. They found pre-chlorination could weaken the membrane–BSA and BSA–BSA hydrophobic and hydrogen bonds; it also broke the disulfide bonds between BSA molecules and the electrostatic repulsive forces between proteins were reinforced.
Ultrasonic pretreatment (UP) produces tiny bubbles by generating vibrations. The collapse of these bubbles generates powerful shock waves and shear forces, which disrupts hydrophobic interactions and reduces aggregate formation in solution. When Koh et al. [84] separated whey protein using 10 kD flat UF membranes, they found that UP significantly decreased the particle size (from 24.7 µm to 0.19 µm) and viscosity (from 3.2 cP to 3.0 cP) before UF, thereby effectively mitigating membrane fouling.
Poletto et al. [85] pretreated pectinase using sequential activated carbon adsorption and MF, followed by 10 kD UF concentration. This pretreatment enhanced permeate flux from 11 to 24 L·m−2·h−1 and reduced fouling resistance by 70%. Specifically, activated carbon pretreatment removed 11% of impurities and 72% of colorants, whereas MF eliminated 10% of impurities and 96.8% of turbidity.
4.2. Optimization of Separation Processes
The optimization of membrane separation conditions, including temperature, pressure, feed flow rate, filtering mode, and the application of external forces during the separation process, including electric fields, pulsation, ultrasound, and the addition of particles could be used to reduce membrane fouling and adsorption. The summary of the optimized operating conditions is shown in Table 2.

Table 2.
Summary of optimization strategies for operating conditions.
The influence of temperature on the separation of polypeptide cannot be ignored in membrane separation. While elevated temperatures reduce feed viscosity and enhance permeate flux, they simultaneously disrupt peptide secondary structures, inducing conformational changes that accelerate fouling and flux decline [91]. Kenneth et al. [86] identified the fouling layer as primarily composed of α-lactalbumin (α-LA) in skimmed milk UF. The adsorption capacity of this protein reached 273.8 mg/m2 at 10 °C and increased to 369.0 mg/m2 at 50 °C. Although higher UF temperatures promote pore dilation, this enlargement exacerbates α-LA pore blockage. Consequently, the operating temperature was maintained at 10 °C to minimize peptide adsorption.
Feed flow rate and pressure are critical parameters in membrane separation. Generally, increasing the feed flow rate can mitigate solute deposition on the membrane surface by reducing concentration polarization and gel layer resistance, which could control membrane fouling [92]. Elevating pressure enhances the driving force, suppresses gel layer formation on the membrane surface, and improves permeation flux. However, excessive pressure may force particles into membrane pores, causing adsorption or pore blockage and intensifying irreversible fouling [93]. Tam et al. [13] used a 10 kD hollow fiber membrane to isolate whey protein and small molecule peptides (≤10 kD) from bovine colostrum whey, and a higher permeation flux was achieved at higher feed flow rates and moderate transmembrane pressure (TMP). This enhancement was attributed to the turbulence generated by the high flow rate, which mitigated concentration polarization and reduced fouling. However, at moderate TMP (2 bar), the flux was higher than other pressures. It was explained by the fact that higher pressures could promote the thickening of the membrane fouling layer and pore constriction. Arias et al. observed a significant linear correlation between peptide permeability and feed flow rate in visceral antioxidant peptide separation by cross-flow ceramic membrane filtration [40]. Additionally, selectivity for small peptides (<3 kD) remained stable at pressures ≤10 bar, enabling determination of the apparent molecular weight cut-off.
Cirillo et al. [42] compared the effects of different pumping systems in MF of BSA and found that the CF configuration using a peristaltic pump yielded higher BSA permeability. It was attributed to the pulsating TMP characteristic of peristaltic pumps in CF systems. The pulsating TMP reduced BSA residence time near the membrane surface and mitigated membrane pore fouling, and then higher fluxes were sustained.
Application of external forces for membrane fouling was shown in previous research [94], such as ultrasonic treatment [95], electric field [96], and pulsating flow [97]. Ultrasonic wave was used in the UF process to improve the separation efficiency of whey protein [87]. They found that the cavitations caused by ultrasound led to turbulence on the membrane surface, reducing concentration polarization and helping to prevent the formation of a dense filter cake layer, which effectively increased the separation flux. Holder et al. [88] demonstrated that electric field application during long-term cross-flow filtration of micellar casein hydrolysates increased protein mass flow by 267%, as the field slowed convective transport of counter-charged peptides, reducing concentration polarization. Wilharm et al. [89] found that long duration low-amplitude pulses during UF of 1% BSA and 0.3% IgG solutions tripled BSA mass flux compared to pulse-free operation, resulting from pulse-induced pore clearance of retained solutes that enabled solute transport in subsequent cycles.
Furthermore, Suwal et al. [90] found that during EDFM fractionation of protein hydrolysates, the membrane resistance of the anion exchange membrane (AEM) under a 40 V pulsed electric field (PEF) was lower than under a direct current electric field (DC). When PEF was combined with DC, the AEM’s conductivity decreased more significantly, while its nitrogen content increased by 22%. These results demonstrated that PEF effectively mitigated peptide/amino acid fouling on the AEM. This effect was attributed to the ability of unstable electric fields like PEF to disrupt or delay the formation of concentration polarization layers and reduce water molecule dissociation.
4.3. Elimination of Polypeptide Aggregates
The elimination of aggregates can be divided into molecular structure modification, adjustment of solution composition, and separation technology, as outlined in Table 3.

Table 3.
Summary of strategies for eliminating polypeptide aggregates.
Molecular structure modification mainly involves modification of aggregation “hot spots”, removing aggregation sites from molecular structure, and reducing protein aggregation [109]. Singh et al. [98] revealed that m-cresol (CR) induced cytochrome c aggregation by populating an “invisible” partially unfolded intermediate with a locally unfolded Met80 region, identifying this structural hotspot as critical for aggregation. Stabilizing the Met80 region significantly suppressed aggregation.
At the isoelectric point, the net charge on the polypeptide surface is zero, minimizing intermolecular repulsive forces and promoting aggregation. Hence, the solution pH should be far away from the isoelectric point to reduce membrane fouling. Butylina [110] demonstrated that NF of whey UF permeate was significantly more effective in alkaline conditions (pH = 9.5) than under acidic environments. It was owing to the increase in negatively charged peptides under alkaline pH, the increase in electrostatic repulsion with the membrane, and the reduction in irreversible scaling. Schermeyer et al. [99] demonstrated that at pH 5.0 (vs. pH 9.0), humanized mAbs showed 3.6% higher monodispersity due to increased zeta potential (5.7 mV vs. near 0 mV) when distanced from their pI (8.7), where enhanced electrostatic repulsion reduced aggregation by strengthening intermolecular forces. Zhang et al. [100] found that a 1 h weakly acidic treatment (pH 4.0) converted half of the monospecific antibody aggregate fraction into monomers, which was attributed to the induced electrostatic repulsion at weakly acidic pH that overcomes intermolecular attraction.
The addition of a lot of salt can disrupt the electrostatic repulsion between protein molecules, and the existence of salt solubility is beneficial for increasing proteins’ solubility under a certain ionic strength. Miao et al. [32] showed that increased ionic strength (10~100 mM) reduced membrane fouling during UF of BSA with PVDF membranes due to heightened hydration repulsion between the membrane and BSA molecules, and between BSA molecules themselves, which masked electrostatic forces. Ding et al. [101] found that a high NaCl concentration (300–500 mM) promoted the dissociation and solubilization of egg white protein (EWP) aggregates because the hydrated sodium ion completely encapsulated the surface of EWPs, resulting in a strong intermolecular hydration force.
In general, higher temperature conditions promoted the formation of larger aggregates via monomer-independent cluster–cluster aggregation steps [111]. Zhang et al. reported ultra-high temperature treatment and further observed hydrogen bond disruption and β-barrel opening, which enhanced the electrostatic interactions and hydrophobicity of β-lactoglobulin surfaces, leading to non-covalent aggregation [112]. However, higher temperatures within a certain range also provide enough energy to break the interaction of proteins. Bryant et al. [102] found that, when the whey protein solution was heated at 90 °C for 30 min, a large part of the aggregates with a diameter exceeding 10 μm would be dispersed into 1 μm particles.
In addition, ultrasonic treatment can alleviate protein aggregation to a certain extent. Cao et al. [103] found that quinoa protein treated with 400 W ultrasound for 30 min exhibited the best dispersion stability in water, corresponding to the highest zeta potential, smallest particle size, and most uniform distribution, which mainly attributed to the fact that ultrasound destroyed the secondary structure of the protein [113,114]. However, sometimes ultrasonic treatment only provides temporary relief. When it stops, the aggregation may reoccur [115].
A variety of stabilizers have been described to reduce protein aggregation, including surfactants, sugars, amino acids, amines, cyclodextrins (CDs), etc., which were attributed to improving conformational stability, colloidal stability, or solubility [116]. Surfactants typically stabilize proteins in solutions through the following mechanisms: competing with proteins for sites where proteins can denature or aggregate, stabilizing protein structures, and weakening protein–protein interactions. Hanson et al. [104] demonstrated the role of hydrophobic tail length in surfactant-mediated stabilization of model therapeutic protein IgG, observing that 14 carbon-long tail surfactants were most effective in preventing IgG aggregation. Kim et al. [105] found that sucrose and trehalose increased exothermic enthalpy when mixed with protein, proving to be effective stabilizers of etanercept, the effect depended on the concentration of sugar. Yuen et al. used 50 mM L-arginine to effectively inhibit the heat-induced amorphous aggregation of insulin and preserve the natural structure of insulin by binding arginine to some easily aggregated residues [106]. Allantoin, as a small molecule amine compound, could strongly inhibit the aggregation of lysozyme, which resulted in reversibility of thermal unfolding of lysozyme [107]. Fukuda et al. [108] found that 2-hydroxypropyl-β-cyclodextrin and L(+)-arginine hydrochloride combination synergistically increased the onset temperature of insulin aggregation. The mechanism could potentially involve enhanced interactions between hydrophobic residues on the protein surface and CDs, thereby effectively inhibiting protein–protein interactions in the bulk solution.
4.4. Selection and Modification of Membrane
Different membrane materials and preparation methods result in different physical and chemical properties such as hydrophobicity, chargeability, porosity, pore size, and surface roughness [117]. It is very important to select appropriate membranes when using membranes to separate specific polypeptides. Similarly, a lot of research has focused on hydrophilic modification and charge modification of membranes for protein separation. The summary of membrane modification used to improve separation performance is shown in Table 4.

Table 4.
Summary of membrane modification strategies.
Intrinsic hydrophobicity of membrane materials was one of the main reasons for membrane fouling [128]. Various approaches including surface coating, immerse precipitation, chemical grafting, and blending method had been investigated for membrane modification [129]. Bubela et al. immobilized iron (III) oxide nanoparticles on PVDF membranes via polyethyleneimine (PEI) grafting, significantly enhancing membrane hydrophilicity (contact angle decreased from 68.1° to 50.8°) [118], which also reduced irreversible fouling resistance during BSA/lysozyme separation by 48%, attributed to the nanoparticles slowing down concentration polarization. Yun et al. fabricated hydrophilic PES MF membranes by blending polyethylene glycol methacrylate (PEGMA) via combined non-solvent-induced (NIPS) and vapor-induced phase separation (VIPS) [119]; it exhibited enhanced hydrophilicity (contact angle decreased from 100° to 71.2°) and significantly reduced BSA adsorption capacity by 50%. Kanagaraj et al. [120] prepared hydrophilic cellulose acetate (CA) membrane for egg albumin separation by mixing diethylene glycol (DEG) and hydroxy benzene sulfonate (HBS) into CA castable, which had the lowest irreversible fouling (15.4%), confirming their improved antifouling performance. Wang et al. [121] grafted 2-hydroxyethyl acrylate-terminated poly (styrene-alt-maleic anhydride) (SMA-HEA) onto PVDF membranes via novel amphiphilic splices; the contact angle reduced from 85° to 55° and protein absorption decreased from 54.31 μg/cm2 to 5.65 μg/cm2. Jin et al. [122] synthesized a novel amphoteric PES membrane (amphoteric PES-zPES); the water contact angle decreased from 80.5° to 58°, and its BSA adsorption was reduced by 65%. Incorporating PEG into PES membranes reduced pore size and pure water flux by 75%, but maintained similar initial soy protein flux (57 vs. 46 mm·s−1) while increasing the flux decay rate from 28% to 42% [123].
Charge modification is to directly change the zeta potential of the membrane and reduce the adsorption of the opposite charge protein on the surface of the membrane through electrostatic repulsion effect [130]. A negatively charged tight ceramic UF membrane was effectively prepared via a two-step grafting polyacrylic acid (PAA), which improved the antifouling and easy-cleaning properties of ceramic membrane during the BSA filtration process [124]. The modified ceramic membrane had a lower permeance decline (38%) and a higher flux recovery ratio (93%) than that of the original membrane (55% and 69%). In addition, zwitterion polymer modification changed the surface charge of the membrane while enhancing the hydrophilicity, which was conducive to reducing the adsorption of proteins [131]. Novel negatively charged PVDF membranes were synthesized with PVDF-g-PAA copolymer additives [125], and the surface zeta potential confirmed strong negative charge (pH 3.0~10.0), achieving 100% BSA flux recovery ratio, which resulted from synergistic hydrogen bonding and electrostatic repulsion by grafted sulfonic/carboxylic groups against protein foulants. Whey protein was isolated by modifying polyvinylidene fluoride (PVDF) membranes through the grafting of polyamidoamine (PAMAM) dendritic macromolecules followed by carboxyl group functionalization [126]. The modified membrane exhibited an increased whey protein retention rate (from 58.9% to 79%) and a doubling of protein flux (from 15.3 L·m−2·h−1 to 30.3 L·m−2·h−1) compared to the original membrane. This improvement was attributed to the more negative zeta potential of the modified membrane (–55 mV vs. –42 mV), which enhanced electrostatic repulsion between the membrane and the protein. In a subsequent study, Wang et al. [127] optimized the modification conditions. The further-modified membrane achieved a significantly higher whey protein retention rate (98.8%), but the protein flux decreased dramatically to 2.3 L·m−2·h−1. This decline in flux was ascribed to the even lower zeta potential (–64 mV) of the modified membrane.
5. Conclusions
Polypeptides have attracted wide concern because of their high efficacy, high specificity, and low toxicity. However, membrane separation of polypeptides still has three major drawbacks: poor selectivity, large loss, and membrane fouling, which limits its wide application. Focusing on these challenges, this review summarized previous studies, analyzed the underlying causes of the issues, and provided a detailed description of feasible methods and potential strategies within the relevant field. Firstly, the peptide’s dispersibility and solubility can be enhanced by adjusting the solution pH away from its isoelectric point and maintaining adequate ionic strength. Aggregation also can be prevented by employing pretreatment to remove impurities or by adding protein stabilizers. Secondly, the development of new membrane materials with enhanced hydrophilicity and a tailored surface charge are recommended, so that a repulsive interaction can be established with the peptide’s surface potential, thus resulting in enhanced separation efficiency. Finally, the operational conditions for membrane separation should be optimized to reduce fouling and gel polarization. Membrane separations should be performed at low temperature, appropriate pressure, and high flow rate. On the other hand, application of pulsatile flow, electric field, or ultrasound also could be employed to promote peptide dispersion or prevent precipitation. These summaries could provide valuable guidance for research on membrane separation of polypeptide products.
Author Contributions
Conceptualization, H.W.; investigation, Y.Y.; resources, H.W.; data curation, Y.Y. and L.D.; writing—original draft preparation, Y.Y. and L.D.; writing—review and editing, H.W.; project administration, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (Grant number 2021YFC2103902).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Quintero-Soto, M.F.; Chávez-Ontiveros, J.; Garzón-Tiznado, J.A.; Salazar-Salas, N.Y.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; López-Valenzuela, J.A. Characterization of peptides with antioxidant activity and antidiabetic potential obtained from chickpea (Cicer arietinum L.) protein hydrolyzates. J. Food Sci. 2021, 86, 2962–2977. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Proteins and bioactive peptides from algae: Insights into antioxidant, anti-hypertensive, anti-diabetic and anti-cancer activities. Trends Food Sci. Technol. 2024, 145, 104352. [Google Scholar] [CrossRef]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.; Ciftci, O.N. Purification and fractionation of bioactive peptides through membrane filtration: A critical and application review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar] [CrossRef]
- Li, L.; Ye, M.; Gan, X.; Xiao, T.; Zhu, Z. Development of membrane separation technology and membrane-based biore-actor in wastewater treatment: Conventional membrane and dynamic membrane. Desalin. Water Treat. 2023, 304, 36–46. [Google Scholar] [CrossRef]
- Zhu, Q.; Cai, Z.; Zhou, P.; Sun, X.; Xu, J. Recent progress of membrane technology for chiral separation: A comprehensive review. Sep. Purif. Technol. 2023, 309, 123077. [Google Scholar] [CrossRef]
- Wen, J.; Han, Q.; Qiu, M.; Jiang, L.; Chen, X.; Fan, Y. Membrane technologies for the separation and purification of functional oligosaccharides: A review. Sep. Purif. Technol. 2024, 346, 127463. [Google Scholar] [CrossRef]
- Grelich-Mucha, M.; Bachelart, T.; Torbeev, V.; Ożga, K.; Berlicki, Ł.; Olesiak-Bańska, J. Amyloid engineering–how terminal capping modifies morphology and secondary structure of supramolecular peptide aggregates. Biomater. Sci. 2024, 12, 1590–1602. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef]
- Van der Schaaf, J.M.; Goulding, D.A.; Fuerer, C.; O’Regan, J.; O’Mahony, J.A.; Kelly, A.L. A novel approach to isolation of β-casein from micellar casein concentrate by cold microfiltration combined with chymosin treatment. Int. Dairy J. 2024, 148, 105796. [Google Scholar] [CrossRef]
- Kongsinkaew, C.; Ajariyakhajorn, K.; Boonyaratanakornkit, V.; Sooksai, S.; Pornpukdeewattana, S.; Krusong, W.; Sitanggang, A.B.; Charoenrat, T. Membrane-based approach for the removal of pigment impurities secreted by Pichia pastoris. Food Bioprod. Process. 2023, 139, 178–189. [Google Scholar] [CrossRef]
- Swaminathan, A.V.; Molitor, M.S.; Burrington, K.J.; Otter, D.; Lucey, J.A. Partial enrichment of phospholipids by enzymatic hydrolysis and membrane filtration of whey protein phospholipid concentrate. JDS Commun. 2023, 4, 175–180. [Google Scholar] [CrossRef]
- Tam, A.J.C.; Durham, S.D.; Barile, D.; de Moura Bell, J.M.L.N. Application of hollow fiber membranes for producing an ultrafiltration permeate from colostrum whey enriched in bioactive compounds. Food Bioprod. Process. 2024, 146, 185–194. [Google Scholar] [CrossRef]
- Marcet, I.; Delgado, J.; Díaz, N.; Rendueles, M.; Díaz, M. Peptides recovery from egg yolk lipovitellins by ultrafiltration and their in silico bioactivity analysis. Food Chem. 2022, 379, 132145. [Google Scholar] [CrossRef] [PubMed]
- Ghalamara, S.; Coscueta, E.R.; Silva, S.; Brazinha, C.; Pereira, C.D.; Pintado, M.E. Integrated ultrafiltration, nanofiltration, and reverse osmosis pilot process to produce bioactive protein/peptide fractions from sardine cooking effluent. J. Environ. Manag. 2022, 317, 115344. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, Z.; Graham, N.J.D.; Liu, H.; Sun, K.; Yu, W. Double positively charged polyamide nanofiltration membrane with PEI/Zr4+ for Cr3+ and trimethoprim removal. Chem. Eng. J. 2023, 469, 144074. [Google Scholar] [CrossRef]
- Zhao, J.; Ouyang, F.; Yang, Y.; Tang, W. Degradation of recalcitrant organics in nanofiltration concentrate from biologically pretreated landfill leachate by ultraviolet-Fenton method. Sep. Purif. Technol. 2020, 235, 116076. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Tang, S.; Xu, Y.; Zhi, X. Recent advances of nanofiltration separation in pharmaceutical field from water to organic solution. J. Mol. Liq. 2024, 409, 125482. [Google Scholar] [CrossRef]
- Fjerbæk Søtoft, L.; Lizarazu, J.M.; Razi Parjikolaei, B.; Karring, H.; Christensen, K.V. Membrane fractionation of herring marinade for separation and recovery of fats, proteins, amino acids, salt, acetic acid and water. J. Food Eng. 2015, 158, 39–47. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Belleville, M.-P.; Amar, R.B. Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: Impact on interesting peptides fractions and nutritional properties. Food Res. Int. 2014, 65, 453–461. [Google Scholar] [CrossRef]
- Firdaous, L.; Dhulster, P.; Amiot, J.; Doyen, A.; Lutin, F.; Vezina, L.-P.; Bazinet, L. Investigation of the large-scale bioseparation of an antihypertensive peptide from alfalfa white protein hydrolysate by an electromembrane process. J. Membr. Sci. 2010, 355, 175–181. [Google Scholar] [CrossRef]
- Doyen, A.; Saucier, L.; Beaulieu, L.; Pouliot, Y.; Bazinet, L. Electroseparation of an antibacterial peptide fraction from snow crab by-products hydrolysate by electrodialysis with ultrafiltration membranes. Food Chem. 2012, 132, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Castro-Munoz, R.; Garcia-Depraect, O. Membrane-based harvesting processes for microalgae and their valuable-related molecules: A review. Membranes 2021, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, A.; Tomaiuolo, G.; Guido, S. Membrane fouling phenomena in microfluidic systems: From technical challenges to scientific opportunities. Micromachines 2021, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Corbaton-Baguena, M.-J.; Alvarez-Blanco, S.; Vincent-Vela, M.-C. Fouling mechanisms of ultrafiltration membranes fouled with whey model solutions. Desalination 2015, 360, 87–96. [Google Scholar] [CrossRef]
- Celik, E.; Liu, L.; Choi, H. Protein fouling behavior of carbon nanotube/polyethersulfone composite membranes during water filtration. Water Res. 2011, 45, 5287–5294. [Google Scholar] [CrossRef]
- Boukil, A.; Suwal, S.; Chamberland, J.; Pouliot, Y.; Doyen, A. Ultrafiltration performance and recovery of bioactive peptides after fractionation of tryptic hydrolysate generated from pressure-treated β-lactoglobulin. J. Membr. Sci. 2018, 556, 42–53. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhu, L. Fouling mechanism of hydrophobic polytetrafluoroethylene (PTFE) membrane by differently charged organics during direct contact membrane distillation (DCMD) process: An especial interest in the feed properties. J. Membr. Sci. 2018, 548, 125–135. [Google Scholar] [CrossRef]
- Beaubier, S.; Przybylski, R.; Bodin, A.; Nedjar, N.; Dhulster, P.; Kapel, R. Ultrafiltration fractionation of bovine hemoglobin hydrolysates: Prediction of separation performances for optimal enrichment in antimicrobial peptide. Membranes 2021, 11, 73. [Google Scholar] [CrossRef]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.-P. Serial fractionation of spent brewer’s yeast protein hydrolysate by ultrafiltration: A peptide-rich product with low RNA content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Ben Amar, R.; Belleville, M.-P. Fractionation of a tuna dark muscle hydrolysate by a two-step membrane process. Sep. Purif. Technol. 2013, 108, 28–36. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Mi, N.; Gao, Z.; Liu, T.; Lv, Y.; Wang, X.; Meng, X.; Yang, Y. Enhancement and mitigation mechanisms of protein fouling of ultrafiltration membranes under different ionic strengths. Environ. Sci. Technol. 2015, 49, 6574–6580. [Google Scholar] [CrossRef] [PubMed]
- Roslan, J.; Mustapa Kamal, S.M.; Md. Yunos, K.F.; Abdullah, N. Fractionation of Tilapia by-product protein hydrolysate using multilayer configuration of ultrafiltration membrane. Processes 2021, 9, 446. [Google Scholar] [CrossRef]
- Persico, M.; Dhulster, P.; Bazinet, L. Redundancy analysis for determination of the main physicochemical characteristics of filtration membranes explaining their fouling by peptides. J. Membr. Sci. 2018, 563, 708–717. [Google Scholar] [CrossRef]
- Hashino, M.; Hirami, K.; Ishigami, T.; Ohmukai, Y.; Maruyama, T.; Kubota, N.; Matsuyama, H. Effect of kinds of membrane materials on membrane fouling with BSA. J. Membr. Sci. 2011, 384, 157–165. [Google Scholar] [CrossRef]
- Arrutia, F.; Rubio, R.; Riera, F.A. Production and membrane fractionation of bioactive peptides from a whey protein concentrate. J. Food Eng. 2016, 184, 1–9. [Google Scholar] [CrossRef]
- Persico, M.; Daigle, G.; Kadel, S.; Perreault, V.; Pellerin, G.; Thibodeau, J.; Bazinet, L. Predictive models for determination of peptide fouling based on the physicochemical characteristics of filtration membranes. Sep. Purif. Technol. 2020, 240, 116602. [Google Scholar] [CrossRef]
- Barukčić, I.; Božanić, R.; Kulozik, U. Effect of pore size and process temperature on flux, microbial reduction and fouling mechanisms during sweet whey cross-flow microfiltration by ceramic membranes. Int. Dairy J. 2014, 39, 8–15. [Google Scholar] [CrossRef]
- Steinhauer, T.; Hanély, S.; Bogendörfer, K.; Kulozik, U. Temperature dependent membrane fouling during filtration of whey and whey proteins. J. Membr. Sci. 2015, 492, 364–370. [Google Scholar] [CrossRef]
- Arias, L.; Sánchez-Henao, C.P.; Zapata, J.E. Optimization of the separation conditions of antioxidant peptides from red tilapia (Oreochromis spp.) viscera on cross-flow filtration ceramic membranes. S. Afr. J. Chem. Eng. 2023, 45, 100–110. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, T.; Zhang, Z.; Feng, X. Extraction and concentration of glutathione from yeast by membranes. Can. J. Chem. Eng. 2022, 100, S195–S204. [Google Scholar] [CrossRef]
- Cirillo, A.I.; Tomaiuolo, G.; Guido, S.J.C.E.J.A. Microfiltration of concentrated albumin solutions and the role of processing conditions on membrane fouling. Chem. Eng. J. Adv. 2023, 16, 100561. [Google Scholar] [CrossRef]
- Marshall, A.D.; Munro, P.A.; Tragardh, G. The effect of protein fouling in microfiltration and ultrafiltration on permeate flux, protein retention and selectivity: A literature review. Desalination 1993, 91, 65–108. [Google Scholar] [CrossRef]
- Chew, J.W.; Kilduff, J.; Belfort, G. The behavior of suspensions and macromolecular solutions in crossflow microfiltration: An update. J. Membr. Sci. 2020, 601, 117865. [Google Scholar] [CrossRef]
- Chabeaud, A.; Vandanjon, L.; Bourseau, P.; Jaouen, P.; Chaplain-Derouiniot, M.; Guerard, F. Performances of ultrafiltration membranes for fractionating a fish protein hydrolysate: Application to the refining of bioactive peptidic fractions. Sep. Purif. Technol. 2009, 66, 463–471. [Google Scholar] [CrossRef]
- Marson, G.V.; Pereira, D.T.V.; da Costa Machado, M.T.; Di Luccio, M.; Martínez, J.; Belleville, M.-P.; Hubinger, M.D. Ultrafiltration performance of spent brewer’s yeast protein hydrolysate: Impact of pH and membrane material on fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Kilmer, N.T.; Huss, R.L.; George, C.C.; Stennett, E.M.S. The influence of ion identity and ionic strength on membrane biofouling of a binary protein solution. Sep. Purif. Technol. 2021, 255, 117769. [Google Scholar] [CrossRef]
- Fernández, A.; Suárez, A.; Zhu, Y.; FitzGerald, R.J.; Riera, F.A. Membrane fractionation of a β-lactoglobulin tryptic digest: Effect of the pH. J. Food Eng. 2013, 114, 83–89. [Google Scholar] [CrossRef]
- Loh, S.; Beuscher, U.; Poddar, T.K.; Porter, A.G.; Wingard, J.M.; Husson, S.M.; Wickramasinghe, S.R. Interplay among membrane properties, protein properties and operating conditions on protein fouling during normal-flow microfiltration. J. Membr. Sci. 2009, 332, 93–103. [Google Scholar] [CrossRef]
- Hadidi, M.; Zydney, A.L. Fouling behavior of zwitterionic membranes: Impact of electrostatic and hydrophobic interactions. J. Membr. Sci. 2014, 452, 97–103. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Tay, K.G.; Ng, H.Y. Fouling of reverse osmosis membrane by protein (BSA): Effects of pH, calcium, magnesium, ionic strength and temperature. J. Membr. Sci. 2008, 315, 28–35. [Google Scholar] [CrossRef]
- Barnett, G.V.; Drenski, M.; Razinkov, V.; Reed, W.F.; Roberts, C.J. Identifying protein aggregation mechanisms and quantifying aggregation rates from combined monomer depletion and continuous scattering. Anal. Biochem. 2016, 511, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Creusot, N.; Gruppen, H. Protein-peptide interactions in mixtures of whey peptides and whey proteins. J. Agric. Food Chem. 2007, 55, 2474–2481. [Google Scholar] [CrossRef]
- Creusot, N.; Gruppen, H.; van Koningsveld, G.A.; de Kruif, C.G.; Voragen, A.G.J. Peptide-peptide and protein-peptide interactions in mixtures of whey protein isolate and whey protein isolate hydrolysates. Int. Dairy J. 2006, 16, 840–849. [Google Scholar] [CrossRef]
- Majid, N.; Khan, R.H. Protein aggregation: Consequences, mechanism, characterization and inhibitory strategies. Int. J. Biol. Macromol. 2023, 242, 125123. [Google Scholar] [CrossRef]
- Wang, W.; Roberts, C.J. Protein aggregation-Mechanisms, detection, and control. Int. J. Pharm. 2018, 550, 251–268. [Google Scholar] [CrossRef]
- Buck, P.M.; Kumar, S.; Singh, S.K. On the role of aggregation prone regions in protein evolution, stability, and enzymatic catalysis: Insights from diverse analyses. PLoS. Comput. Biol. 2013, 9, e1003291. [Google Scholar] [CrossRef]
- Meric, G.; Robinson, A.S.; Roberts, C.J. Driving forces for nonnative protein aggregation and approaches to predict aggregation-prone regions. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 139–159. [Google Scholar] [CrossRef]
- Tartaglia, G.G.; Pawar, A.P.; Campioni, S.; Dobson, C.M.; Chiti, F.; Vendruscolo, M. Prediction of aggregation-prone regions in structured proteins. J. Mol. Biol. 2008, 380, 425–436. [Google Scholar] [CrossRef]
- Steckmann, T.; Awan, Z.; Gerstman, B.S.; Chapagain, P.P. Kinetics of peptide secondary structure conversion during amyloid β-protein fibrillogenesis. J. Theor. Biol. 2012, 301, 95–102. [Google Scholar] [CrossRef]
- Pawar, A.P.; DuBay, K.F.; Zurdo, J.; Chiti, F.; Vendruscolo, M.; Dobson, C.M. Prediction of “Aggregation-prone” and “Aggregation-susceptible” regions in proteins associated with neurodegenerative diseases. J. Mol. Biol. 2005, 350, 379–392. [Google Scholar] [CrossRef]
- Adachi, K.; Ding, M.; Asakura, T.; Surrey, S. Relationship between β4 hydrogen bond and β6 hydrophobic interactions during aggregate, fiber or crystal formation in oversaturated solutions of hemoglobin A and S. Arch. Biochem. Biophys. 2009, 481, 137–144. [Google Scholar] [CrossRef][Green Version]
- Adachi, K.; Ding, M.; Surrey, S. Role of the β4Thr−β73Asp hydrogen bond in HbS polymer and domain formation from multinucleate-containing clusters. Biochemistry 2008, 47, 5441–5449. [Google Scholar] [CrossRef]
- Oliva, A.; Fariña, J.B.; Llabrés, M. Pre-study and in-study validation of a size-exclusion chromatography method with different detection modes for the analysis of monoclonal antibody aggregates. J. Chromatogr. B. 2016, 1022, 206–212. [Google Scholar] [CrossRef]
- Meliga, S.C.; Farrugia, W.; Ramsland, P.A.; Falconer, R.J. Cold-induced precipitation of a monoclonal IgM: A negative activation enthalpy reaction. J. Phys. Chem. B. 2013, 117, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.; Hornok, V.; Sebok, D.; Dekany, I. Comprehensive study on the structure of the BSA from extended-to aged form in wide (2–12) pH range. Int. J. Biol. Macromol. 2016, 88, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, Z.; Wangb, Y.; Ding, L.; Wang, Y. Role of pH-induced structural change in protein aggregation in foam fractionation of bovine serum albumin. Biotechnol. Rep. 2016, 9, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; KüKrer, B.; Hawe, A.; Jiskoot, W. Transient molten globules and metastable aggregates induced by brief exposure of a monoclonal IgG to low pH. J. Pharm. Sci. 2012, 101, 2327–2339. [Google Scholar] [CrossRef]
- Wang, W.; Nema, S.; Teagarden, D. Protein aggregation—Pathways and influencing factors. Int. J. Pharm. 2010, 390, 89–99. [Google Scholar] [CrossRef]
- Giger, K.; Vanam, R.P.; Seyrek, E.; Dubin, P.L. Suppression of Insulin Aggregation by Heparin. Biomacromolecules 2008, 9, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Royer, C.A. Lessons from pressure denaturation of proteins. J. R. Soc. Interface 2018, 15, 20180244. [Google Scholar] [CrossRef]
- Cordeiro, Y.; Foguel, D.; Silva, J.L. Pressure–temperature folding landscape in proteins involved in neurodegenerative diseases and cancer. Biophys. Chem. 2013, 183, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, N.; Chen, X.; Wu, Z.; Zhong, W.; Yu, D.; Zhang, H. Effects of moderate electric field on the structural properties and aggregation characteristics of soybean protein isolate. Food Hydrocoll. 2022, 133, 107911. [Google Scholar] [CrossRef]
- Hu, X.; Wang, H.; Hu, Y.; Tu, Z. Insight into the effects of pulsed electric field on the structure, aggregation characteristics and functional properties of whey proteins. Food Hydrocoll. 2024, 154, 110111. [Google Scholar] [CrossRef]
- Devi, S.; Chaturvedi, M.; Fatima, S.; Priya, S. Environmental factors modulating protein conformations and their role in protein aggregation diseases. Toxicology 2022, 465, 153049. [Google Scholar] [CrossRef]
- Ghazvini, S.; Kalonia, C.; Volkin, D.B.; Dhar, P. Evaluating the Role of the air-solution interface on the mechanism of subvisible particle formation caused by mechanical agitation for an IgG1 mAb. J. Pharm. Sci. 2016, 105, 1643–1656. [Google Scholar] [CrossRef]
- Lin, G.L.; Pathak, J.A.; Kim, D.H.; Carlson, M.; Riguero, V.; Kim, Y.J.; Buff, J.S.; Fuller, G.G. Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions. Soft Matter. 2016, 12, 3293–3302. [Google Scholar] [CrossRef]
- McBride, S.A.; Tilger, C.F.; Sanford, S.P.; Tessier, P.M.; Hirsa, A.H. Comparison of human and bovine insulin amyloidogenesis under uniform shear. J. Phys. Chem. B. 2015, 119, 10426–10433. [Google Scholar] [CrossRef]
- McBride, S.A.; Sanford, S.P.; Lopez, J.M.; Hirsa, A.H. Shear-induced amyloid fibrillization: The role of inertia. Soft Matter 2016, 12, 3461–3467. [Google Scholar] [CrossRef]
- Sreedhara, A.; Yin, J.; Joyce, M.; Lau, K.; Wecksler, A.T.; Deperalta, G.; Yi, L.; John Wang, Y.; Kabakoff, B.; Kishore, R.S.K. Effect of ambient light on IgG1 monoclonal antibodies during drug product processing and development. Eur. J. Pharm. Biopharm. 2016, 100, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, S.; Xu, L.; Graham, N.J.D.; Yu, W. Beneficial role of pre- and post-ozonation in a low rate biofiltration-ultrafiltration process treating reclaimed water. Water Res. 2022, 226, 119284. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Zhou, Y.; Wang, P.; Lu, W.; Li, P.; Li, X.; Wang, L. A comparison of effect mechanisms of chlorination and ozonation on the interfacial forces of protein at membrane surfaces and the implications for membrane fouling control. J. Membr. Sci. 2021, 628, 119266. [Google Scholar] [CrossRef]
- Koh, L.L.A.; Nguyen, H.T.H.; Chandrapala, J.; Zisu, B.; Ashokkumar, M.; Kentish, S.E. The use of ultrasonic feed pre-treatment to reduce membrane fouling in whey ultrafiltration. J. Membr. Sci. 2014, 453, 230–239. [Google Scholar] [CrossRef]
- Poletto, P.; da Rocha Renosto, D.; Baldasso, C.; Zeni, M.; da Silveira, M.M. Activated charcoal and microfiltration as pretreatment before ultrafiltration of pectinases produced by Aspergillus niger in solid-state cultivation. Sep. Purif. Technol. 2015, 151, 102–107. [Google Scholar] [CrossRef]
- Ng, K.S.Y.; Dunstan, D.E.; Martin, G.J.O. Influence of processing temperature on flux decline during skim milk ultrafiltration. Sep. Purif. Technol. 2018, 195, 322–331. [Google Scholar] [CrossRef]
- Prabhuzantye, T.; Khaire, R.A.; Gogate, P.R. Enhancing the recovery of whey proteins based on application of ultrasound in ultrafiltration and spray drying. Ultrason. Sonochem. 2019, 55, 125–134. [Google Scholar] [CrossRef]
- Holder, A.; Weik, J.; Hinrichs, J. A study of fouling during long-term fractionation of functional peptides by means of cross-flow ultrafiltration and cross-flow electro membrane filtration. J. Membr. Sci. 2013, 446, 440–448. [Google Scholar] [CrossRef]
- Wilharm, C.; Rodgers, V.G.J. Significance of duration and amplitude in transmembrane pressure pulsed ultrafiltration of binary protein mixtures. J. Membr. Sci. 1996, 121, 217–228. [Google Scholar] [CrossRef]
- Suwal, S.; Amiot, J.; Beaulieu, L.; Bazinet, L. Effect of pulsed electric field and polarity reversal on peptide/amino acid migration, selectivity and fouling mitigation. J. Membr. Sci. 2016, 510, 405–416. [Google Scholar] [CrossRef]
- Puri, R.; Singh, U.; O’Mahony, J.A. Influence of processing temperature on membrane performance and characteristics of process streams generated during ultrafiltration of skim milk. Foods 2020, 9, 1721. [Google Scholar] [CrossRef]
- Nath, A.; Eren, B.A.; Csighy, A.; Pásztorné-Huszár, K.; Kiskó, G.; Abrankó, L.; Tóth, A.; Szerdahelyi, E.; Kovács, Z.; Koris, A.; et al. Production of liquid milk protein concentrate with antioxidant capacity, angiotensin converting enzyme inhibitory activity, antibacterial activity, and hypoallergenic property by membrane filtration and enzymatic modification of proteins. Processes 2020, 8, 871. [Google Scholar] [CrossRef]
- Gaviria, Y.S.; Zapata, J.E. Optimization of fractionation with membranes of antioxidant enzymatic hydrolysate of Californian red worm (Eisenia fetida) protein. Heliyon 2024, 10, e31169. [Google Scholar] [CrossRef] [PubMed]
- Tanudjaja, H.J.; Anantharaman, A.; Ng, A.Q.Q.; Ma, Y.; Tanis-Kanbur, M.B.; Zydney, A.L.; Chew, J.W. A review of membrane fouling by proteins in ultrafiltration and microfiltration. J. Water Process Eng. 2022, 50, 103294. [Google Scholar] [CrossRef]
- Ábel, M.; Szabó, G.; Poser, O.; László, Z.; Hodúr, C. Enzyme recovery and fouling mitigation by ultrasound-enhanced ultrafiltration. Desalin. Water Treat. 2013, 51, 4921–4926. [Google Scholar] [CrossRef]
- Chen, G.; Song, W.; Qi, B.; Ghosh, R.; Wan, Y. Separation of human serum albumin and polyethylene glycol by electro-ultrafiltration. Biochem. Eng. J. 2016, 109, 127–136. [Google Scholar] [CrossRef]
- Gironès i Nogué, M.; Akbarsyah, I.J.; Bolhuis-Versteeg, L.A.M.; Lammertink, R.G.H.; Wessling, M. Vibrating polymeric microsieves: Antifouling strategies for microfiltration. J. Membr. Sci. 2006, 285, 323–333. [Google Scholar] [CrossRef]
- Singh, S.M.; Hutchings, R.L.; Mallela, K.M.G. Mechanisms of m-cresol-induced protein aggregation studied using a model protein cytochrome c. J. Pharm. Sci. 2011, 100, 1679–1689. [Google Scholar] [CrossRef]
- Schermeyer, M.-T.; Wöll, A.K.; Kokke, B.; Eppink, M.; Hubbuch, J. Characterization of highly concentrated antibody solution-A toolbox for the description of protein long-term solution stability. mAbs 2017, 9, 1169–1185. [Google Scholar] [CrossRef]
- Zhang, T.; Li, B.; Dong, W.; Wan, Y.; Li, Y. Acidic pH or salt treatment can convert soluble antibody aggregates in culture harvest into monomers and improve Protein A chromatography step yield. Protein Exp. Purif. 2024, 215, 106391. [Google Scholar] [CrossRef]
- Ding, Y.; Xiao, N.; Guo, S.; Lin, J.; Chen, L.; Mou, X.; Ai, M. Impact of NaCl perturbation on physicochemical and structural properties of preheat–treated egg white protein modulating foaming property. Food Chem. 2024, 459, 140377. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.M.; McClements, D.J. Ultrasonic spectrometry study of the influence of temperature on whey protein aggregation. Food Hydrocoll. 1999, 13, 439–444. [Google Scholar] [CrossRef]
- Cao, H.; Sun, R.; Shi, J.; Li, M.; Guan, X.; Liu, J.; Huang, K.; Zhang, Y. Effect of ultrasonic on the structure and quality characteristics of quinoa protein oxidation aggregates. Ultrason. Sonochem. 2021, 77, 105685. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.G.; Katz, J.S.; Ma, H.; Putterman, M.; Yezer, B.A.; Petermann, O.; Reineke, T.M. Effects of hydrophobic tail length variation on surfactant-mediated protein stabilization. Mol. Pharm. 2020, 17, 4302–4311. [Google Scholar] [CrossRef]
- Kim, N.A.; Thapa, R.; Jeong, S.H. Preferential exclusion mechanism by carbohydrates on protein stabilization using thermodynamic evaluation. Int. J. Biol. Macromol. 2018, 109, 311–322. [Google Scholar] [CrossRef]
- Yuen, K.N.; Konermann, L. Mechanism of protein aggregation inhibition by arginine: Blockage of anionic side chains favors unproductive encounter complexes. J. Am. Chem. Soc. 2024, 146, 8394–8406. [Google Scholar] [CrossRef]
- Arakawa, T.; Maluf, N.K. The effects of allantoin, arginine and NaCl on thermal melting and aggregation of ribonuclease, bovine serum albumin and lysozyme. Int. J. Biol. Macromol. 2018, 107, 1692–1696. [Google Scholar] [CrossRef]
- Fukuda, M.; Takahashi, K.; Takarada, T.; Saito, S.; Tanaka, M. Synergistic effect of cyclodextrins and electrolytes at high concentrations on protein aggregation inhibition. J. Pharm. Sci. 2024, 113, 3543–3553. [Google Scholar] [CrossRef]
- Wang, X.; Singh, S.K.; Kumar, S. Potential aggregation-prone regions in complementarity-determining regions of antibodies and their contribution towards antigen recognition: A computational analysis. Pharm. Res. 2010, 27, 1512–1529. [Google Scholar] [CrossRef]
- Butylina, S.; Luque, S.; Nyström, M. Fractionation of whey-derived peptides using a combination of ultrafiltration and nanofiltration. J. Membr. Sci. 2006, 280, 418–426. [Google Scholar] [CrossRef]
- Meng, H.K.; Pang, K.T.; Wan, C.; Zheng, Z.Y.; Beiying, Q.; Yang, Y.; Zhang, W.; Ho, Y.S.; Walsh, I.; Chia, S. Thermal and pH stress dictate distinct mechanisms of monoclonal antibody aggregation. Int. J. Biol. Macromol. 2024, 282, 136601. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Cao, J.; Jiang, L.; Liu, T.; Yi, H. Experimental and molecular dynamics simulation study on the mechanism of β-lactoglobulin self-aggregation induced by ultra-high temperature. LWT 2024, 209, 116782. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, X.; Huang, J.; Wei, Y.; Wen, L.; Yang, F.; Yang, F.; Liu, W. Harnessing ultrasonic power to optimize quinoa byproduct protein for sustainable utilization. LWT 2024, 207, 116629. [Google Scholar] [CrossRef]
- Sharma, N.; Sahil; Madhumita, M.; Kumar, Y.; Prabhakar, P.K. Ultrasonic modulated rice bran protein concentrate: Induced effects on morphological, functional, rheological, and thermal characteristics. Innov. Food Sci. Emerg. 2023, 85, 103332. [Google Scholar] [CrossRef]
- Tao, C.; Ye, T.; Tang, D.; Tian, R.; Xie, Y. Isolation of Prunus persica kernel protein and its physicochemical properties and aggregating performance modulated by heating and ultrasonication. Sustain. Chem. Pharm. 2024, 38, 101508. [Google Scholar] [CrossRef]
- Kim, H.L.; McAuley, A.; Livesay, B.; Gray, W.D.; McGuire, J. Modulation of Protein Adsorption by Poloxamer 188 in Relation to Polysorbates 80 and 20 at Solid Surfaces. J. Pharm. Sci. 2014, 103, 1043–1049. [Google Scholar] [CrossRef]
- Valiño, V.; San Román, M.F.; Ibañez, R.; Ortiz, I. Improved separation of bovine serum albumin and lactoferrin mixtures using charged ultrafiltration membranes. Sep. Purif. Technol. 2014, 125, 163–169. [Google Scholar] [CrossRef]
- Bubela, H.; Konovalova, V.; Kujawa, J.; Kolesnyk, I.; Burban, A.; Kujawski, W. Enhancing of transport parameters and antifouling properties of PVDF membranes modified with Fe3O4 nanoparticles in the process of proteins fractionation. Sep. Purif. Technol. 2023, 325, 124573. [Google Scholar] [CrossRef]
- Yun, K.H.; Sharma, K.; Kim, H.U.; Bae, T.-H. Modification of a PES microfiltration membrane to enhance sterile filtration by inhibiting protein adsorption. J. Ind. Eng. Chem. 2023, 123, 311–319. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Nagendran, A.; Rana, D.; Matsuura, T. Separation of macromolecular proteins and removal of humic acid by cellulose acetate modified UF membranes. Int. J. Biol. Macromol. 2016, 89, 81–88. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, G.; Yan, Z.; Li, S.; Xu, M.; Wang, C.; Li, Y. Improving the hydrophilicity and antifouling performance of PVDF membranes via PEI amination and further poly (methyl vinyl ether-alt-maleic anhydride) modification. React. Funct. Polym. 2023, 189, 105610. [Google Scholar] [CrossRef]
- Jin, W.; Lin, Y.-F.; Xu, Z.-L.; Li, P.-P.; Dai, J.-Y.; Tong, Y.-H.; Zhang, X. Polyethersulfone membrane modified by zwitterionic groups for improving anti-fouling and antibacterial properties. J. Ind. Eng. Chem. 2023, 122, 274–284. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barredo-Damas, S.; Iborra-Clar, A.; Pascual-Garrido, J.; Iborra-Clar, M.I. Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions. Sustainability 2021, 13, 13682. [Google Scholar] [CrossRef]
- Ye, Y.; Han, Q.; Zhao, C.; Ke, W.; Qiu, M.; Chen, X.; Fan, Y. Improved negative charge of tight ceramic ultrafiltration membranes for protein-resistant and easy-cleaning performance. Sep. Purif. Technol. 2023, 309, 123082. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Seyed Dorraji, M.S. Novel negatively-charged amphiphilic copolymers of PVDF-g-PAMPS and PVDF-g-PAA to improve permeability and fouling resistance of PVDF UF membrane. React. Funct. Polym. 2022, 179, 105386. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, G.; Rana, D.; Matsuura, T.; Lan, C.Q. Engineering polyvinylidene fluoride membranes using charge tunable dendrimer polyamidoamine to enable microporous membranes for protein separation. Sep. Purif. Technol. 2025, 354, 128887. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, G.; Zhou, H.; Wang, X.; Rana, D.; Matsuura, T.; Lan, C.Q. Polyamidoamine dendrimer-modified polyvinylidene fluoride microporous membranes for protein separation. React. Funct. Polym. 2024, 204, 106021. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Kong, L.; Zhang, J.; Zuo, W. Static adsorption of protein-polysaccharide hybrids on hydrophilic modified membranes based on atomic layer deposition: Anti-fouling performance and mechanism insight. J. Membr. Sci. 2018, 548, 470–480. [Google Scholar] [CrossRef]
- Ji, M.; Li, X.; Omidvarkordshouli, M.; Sigurdardóttir, S.B.; Woodley, J.M.; Daugaard, A.E.; Luo, J.; Pinelo, M. Charge exclusion as a strategy to control retention of small proteins in polyelectrolyte-modified ultrafiltration membranes. Sep. Purif. Technol. 2020, 247, 116936. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, M.; Song, J.; Zhang, L.; Li, X.-M.; He, T. Fouling resistance of 3-[[3-(trimethoxysilane)-propyl] amino] propane-1-sulfonic acid zwitterion modified poly (vinylidene fluoride) membranes. Sep. Purif. Technol. 2020, 239, 116589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).