Exploring the Influence of Extraction Methods, Solvents, and Temperature on Total Phenolic Recovery and Antioxidant Capacity in Olive Leaf Extracts: A Systematic Review with Quantitative Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. In- and Exclusion Criteria
2.3. Outcomes
2.4. Data Extraction and Statistical Analysis
3. Results and Discussion
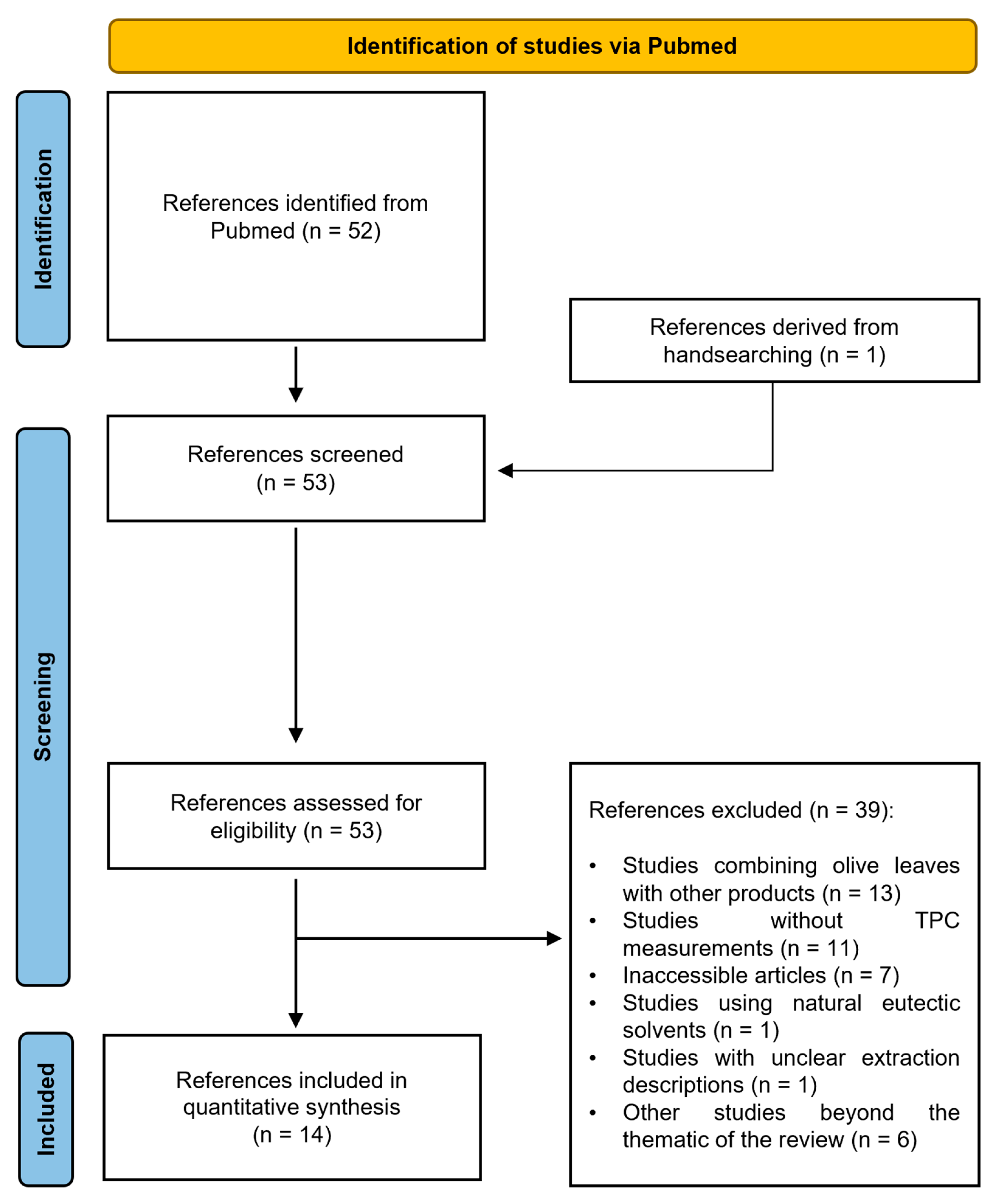
3.1. Study Selection and Characteristics
3.2. Influence of Extraction Method in TPC, TFC and Antioxidant Capacity
3.3. Influence of Solvent Used in TPC, TFC and Antioxidant Capacity
3.4. Influence of Extraction Time and Temperature in TPC, TFC and Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.d.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 660582. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Navarro-Hortal, M.D.; Quirantes-Piné, R.; Grosso, G.; Giampieri, F.; Lipari, V.; Sánchez-González, C.; Battino, M.; Quiles, J.L. Molecular Mechanisms of the Protective Effects of Olive Leaf Polyphenols against Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Sánchez-González, C.; Sumalla-Cano, S.; Battino, M.; García-Ruiz, R.; Sánchez, S.; et al. Effect of olive leaf phytochemicals on the anti-acetylcholinesterase, anti-cyclooxygenase-2 and ferric reducing antioxidant capacity. Food Chem. 2024, 444, 138516. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef]
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Delgado Noya, I.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Vera-Ramírez, L.; Forbes-Hernández, T.J.; Esteban-Muñoz, A.; Giampieri, F.; Bullón, P.; Battino, M.; Sánchez-González, C.; et al. An oleuropein rich-olive (Olea europaea L.) leaf extract reduces β-amyloid and tau proteotoxicity through regulation of oxidative- and heat shock-stress responses in Caenorhabditis elegans. Food Chem. Toxicol. 2022, 162, 112914. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xin, X.; Zhu, S.; Niu, E.; Wu, Q.; Li, T.; Liu, D. Changes in Phytochemical Profiles and Biological Activity of Olive Leaves Treated by Two Drying Methods. Front. Nutr. 2022, 9, 854680. [Google Scholar] [CrossRef]
- Muhammad, N.; Hussain, I.; Fu, X.-A.; Ali, A.; Guo, D.; Noureen, L.; Subhani, Q.; Ahmad, N.; Zhu, Q.-F.; Cui, H.; et al. A Comprehensive Review of Instrumentation and Applications in Post-Column and In-Source Derivatization for LC-MS. Mass Spectrom. Rev. 2025. [Google Scholar] [CrossRef]
- Márquez, K.; Márquez, N.; Ávila, F.; Cruz, N.; Burgos-Edwards, A.; Pardo, X.; Carrasco, B. Oleuropein-Enriched Extract From Olive Mill Leaves by Homogenizer-Assisted Extraction and Its Antioxidant and Antiglycating Activities. Front. Nutr. 2022, 9, 895070. [Google Scholar] [CrossRef]
- Ezz El-Din Ibrahim, M.; Alqurashi, R.M.; Alfaraj, F.Y. Antioxidant Activity of Moringa oleifera and Olive Olea europaea L. Leaf Powders and Extracts on Quality and Oxidation Stability of Chicken Burgers. Antioxidants 2022, 11, 496. [Google Scholar] [CrossRef]
- Žuntar, I.; Putnik, P.; Bursać Kovačević, D.; Nutrizio, M.; Šupljika, F.; Poljanec, A.; Dubrović, I.; Barba, F.; Režek Jambrak, A. Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED). Foods 2019, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef] [PubMed]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Penkacik, K. Genotype-Related Differences in the Phenolic Compound Profile and Antioxidant Activity of Extracts from Olive (Olea europaea L.) Leaves. Molecules 2019, 24, 1130. [Google Scholar] [CrossRef] [PubMed]
- Alhakim, F.; Laham, A.; Hasian, J. Comparative Study of Alcoholic Extracts of Different Syrian Grapevine and Olive Leaf Cultivars for Their Antioxidant Activity and Photoprotective Effects. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 7027281. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Prevete, G.; Carvalho, L.G.; Del Carmen Razola-Diaz, M.; Verardo, V.; Mancini, G.; Fiore, A.; Mazzonna, M. Ultrasound assisted extraction and liposome encapsulation of olive leaves and orange peels: How to transform biomass waste into valuable resources with antimicrobial activity. Ultrason. Sonochem. 2024, 102, 106765. [Google Scholar] [CrossRef]
- Benčić, Đ.; Barbarić, M.; Mornar, A.; Klarić, D.A.; Brozovic, A.; Dabelić, S.; Fadljević, M.; Marković, A.K. Oleuropein in olive leaf, branch, and stem extracts: Stability and biological activity in human cervical carcinoma and melanoma cells. Acta Pharm. 2023, 73, 601–616. [Google Scholar] [CrossRef]
- Pyrka, I.; Koutra, C.; Siderakis, V.; Stathopoulos, P.; Skaltsounis, A.-L.; Nenadis, N. Exploring the Bioactive Content of Liquid Waste and Byproducts Produced by Two-Phase Olive Mills in Laconia (Greece): Is There a Prospect for Added-Value Applications? Foods 2023, 12, 4421. [Google Scholar] [CrossRef]
- Li, T.; Wu, W.; Zhang, J.; Wu, Q.; Zhu, S.; Niu, E.; Wang, S.; Jiang, C.; Liu, D.; Zhang, C. Antioxidant Capacity of Free and Bound Phenolics from Olive Leaves: In Vitro and In Vivo Responses. Antioxidants 2023, 12, 2033. [Google Scholar] [CrossRef]
- Musolino, V.; Macrì, R.; Cardamone, A.; Serra, M.; Coppoletta, A.R.; Tucci, L.; Maiuolo, J.; Lupia, C.; Scarano, F.; Carresi, C.; et al. Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity. Plants 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Smole Možina, S.; Ozogul, Y.; et al. Antioxidant and Antimicrobial Activity of Hydroethanolic Leaf Extracts from Six Mediterranean Olive Cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; De Montijo-Prieto, S.; Jiménez-Valera, M.; Carrasco-Pancorbo, A.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Comparative Extraction of Phenolic Compounds from Olive Leaves Using a Sonotrode and an Ultrasonic Bath and the Evaluation of Both Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients (Recast) (Text with EEA Relevance). Off. J. Eur. Union 2009, 141, 3–11. Available online: http://data.europa.eu/eli/dir/2009/32/oj/eng (accessed on 26 August 2025).
- Orak, H.H.; Isbilir, S.S.; Yagar, H. Determination of antioxidant properties of lyophilized olive leaf water extracts obtained from 21 different cultivars. Food Sci. Biotechnol. 2012, 21, 1065–1074. [Google Scholar] [CrossRef]
- El Mannoubi, I. Impact of different solvents on extraction yield, phenolic composition, in vitro antioxidant and antibacterial activities of deseeded Opuntia stricta fruit. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 176–184. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the Aqueous Extraction of Phenolic Compounds from Olive Leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the usage of olive leaves, bioactive compounds, biological activities, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A. Effect of storage, temperature, and pH on the preservation of the oleuropein content of olive leaf extracts. Sustain. Food Technol. 2024, 2, 750–759. [Google Scholar] [CrossRef]
| Olive Variety | Solvent | Extraction Time | Extraction Temp. | Additional Methods | TPC (mg GAE/g) | Refs. |
|---|---|---|---|---|---|---|
| Arbequina and not specified OL varieties | Pure water, ethanol/water (80/20, v/v), ethanol/water (50/50, v/v), and ethanol/water (25/75 v/v) | 30 s, 45 s, 3 min, 9 min, 24 h, and 48 h | 35–40 °C and 22 °C | HAE, HVED, and shaking | 15.88–53.00 | [11] |
| Koroneiki | Ethanol/water (80/20, v/v) | 5 h | 30 °C | Shaking | 1.97 | [12] |
| Not specified OL varieties | Water, ethanol/water (25/75 v/v) and ethanol/water (50/50, v/v) | 3 and 9 min | 22 °C | Shaking | 5.32–20.61 | [13] |
| Apollo, Ascolana tenera, Carolea, Cellina, Cipressino, Itrana, Maurino, Minerva, Moraio, Nociara, Ogliarola, Pendolino, Sant Agostino, Ravece, and Taggiasca | Ethanol/water (60/40, v/v) | 2 h | 25 °C | Shaking | 12.00–47.00 | [14] |
| Ascolana, Ayvalik, Cekiste, Esek Zeytini, Gemlik, Kilis Yaglik, Memecik, Saurani, and Uslu | Methanol/water (80/20, v/v) | 45 min | 65 °C | Shaking | 110.00–268.00 | [15] |
| Picual, Hojiblanca, Arbequina, and OL mixtures | Ethanol/water (80/20, v/v) | 2 h | 25 °C | Shaking | 27.19–52.78 | [3] |
| Mousaabi, Khouderi, Zaity, and Nipali | Ethanol/water (80/20, v/v) | 4 h | 60 °C | Soxhlet extraction | 38.39–72.78 | [16] |
| Hojiblanca | Ethanol/water (50/50, v/v) and ethanol/water (75/25 v/v) | 5 h | 80 °C | Soxhlet extraction | 71.90–76.10 | [17] |
| Not specified OL varieties | Water | 25 min | <75 °C | UAE | 162.00 | [18] |
| Rosinjola and Istarska bjelica | Ethanol 100% | 30 min | <30 °C | UAE | 10.80–14.15 | [19] |
| Koroneiki | Methanol 100% | 30 min | 50 °C | UAE | 15.40–41.80 | [20] |
| Not specified OL varieties | Ethanol 70% (1/10 w/v) | 30 min | 50 °C | UAE | 79.43 | [21] |
| Nocellara del Belice and Carolea | Ethanol/water (50/50 v/v) | 3 h | 25 °C | UAE | 10.83–92.93 | [22] |
| Lastovka, Levantinka, Oblica, Moraiolo, Frantoio, Nostrana di Brisighella, and Arbequina | Ethanol/water (80/20, v/v) | 45 s, 30 min and 1 h | 25 °C and 35–40 °C | UAE | 16.50–44.00 | [23] |
| Measurement | Studies | Samples | Mean | SEM | Refs. |
|---|---|---|---|---|---|
| Total phenolic content (mg GAE/g DW) | 14 | 149 | 45 | 5 | [3,11,12,13,14,15,16,17,18,19,20,21,22,23] |
| Total flavonoid content (mg CAE/g DW) | 5 | 67 | 17 | 2 | [3,19,20,21,22] |
| DPPH (μmol TE/g) | 5 | 105 | 121 | 11 | [3,11,13,14,15] |
| FRAP (μmol TE/g) | 5 | 91 | 458 | 44 | [3,11,13,15,18,21] |
| ABTS (μmol TE/g) | 4 | 61 | 438 | 19 | [3,11,15,21] |
| Extraction Method | Samples | TPC | TFC | DPPH | FRAP | ABTS | Refs. |
|---|---|---|---|---|---|---|---|
| Heat-Assisted Extraction | 3 | 46 (5) a | - | - | - | - | [11] |
| High Voltage Electrical Discharge-assisted extraction | 24 | 31 (5) b | - | 30 (1) a | 273 (25) a | - | [11] |
| Shaking | 85 | 52 (6) ac | 21 (8) a | 150 (63) b | 527 (277) b | 449 (81) a | [3,11,12,13,14,15] |
| Soxhlet extraction | 6 | 63 (8) c | - | - | - | - | [16,17] |
| Ultrasound-assisted extraction | 30 | 34 (6) b | 6 (3) b | - | 421 (357) ab | 119 (47) b | [18,19,20,21,22,23] |
| Solvent | Samples | TPC | TFC | DPPH | FRAP | ABTS | Refs. |
|---|---|---|---|---|---|---|---|
| Water 100% | 12 | 35 (12) a | - | 29 (1) a | 270 (53) a | - | [11,13,18] |
| Methanol ≥ 75% in water | 22 | 103 (20) b | 4 (1) a | 48 (4) b | 1606 (117) b | 223 (10) a | [15,20] |
| Ethanol ≤ 25% in water | 11 | 33 (5) a | - | 29 (1) a | 263 (19) a | - | [11,13] |
| Ethanol 25–75% in water | 28 | 34 (4) a | - | 23 (2) a | 246 (26) a | - | [11,13,14,17,21] |
| Ethanol > 75% in water | 75 | 36 (1) a | 20 (2) b | 222 (10) c | 378 (23) c | 490 (16) b | [3,11,12,19,23] |
| Extraction Temperature | Samples | TPC | TFC | DPPH | FRAP | ABTS | Refs. |
|---|---|---|---|---|---|---|---|
| ≤25 °C | 103 | 32 (1) a | 20 (2) a | 129 (11) a | 330 (17) a | 490 (16) a | [3,11,13,14,22,23] |
| 25–50 °C | 33 | 33 (3) a | 7 (3) b | - | - | - | [11,12,19,20,21,23] |
| >50 °C | 16 | 152 (20) b | - | 48 (4) b | 1523 (134) b | 217 (10) b | [15,16,17,18] |
| Extraction Time | Samples | TPC | TFC | DPPH | FRAP | ABTS | Refs. |
|---|---|---|---|---|---|---|---|
| ≤1 h | 70 | 57 (8) a | 7 (3) a | 34 (2) a | 556 (93) a | 204 (16) a | [11,13,15,18,20,23] |
| 1–2 h | 65 | 34 (1) b | 21 (2) b | 175 (13) b | 378 (23) b | 490 (16) b | [3,14] |
| >2–5 h | 9 | 54 (10) a | - | - | - | - | [12,16,17,22] |
| >24 h | 4 | 43 (1) c | - | - | - | - | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Correa, M.; Montalbán-Hernández, C.; Navarro-Hortal, M.D.; Peña-Guzmán, D.; Badillo-Carrasco, A.; Varela-López, A.; Hinojosa-Nogueira, D.; Romero Márquez, J.M. Exploring the Influence of Extraction Methods, Solvents, and Temperature on Total Phenolic Recovery and Antioxidant Capacity in Olive Leaf Extracts: A Systematic Review with Quantitative Synthesis. Separations 2025, 12, 236. https://doi.org/10.3390/separations12090236
Castillo-Correa M, Montalbán-Hernández C, Navarro-Hortal MD, Peña-Guzmán D, Badillo-Carrasco A, Varela-López A, Hinojosa-Nogueira D, Romero Márquez JM. Exploring the Influence of Extraction Methods, Solvents, and Temperature on Total Phenolic Recovery and Antioxidant Capacity in Olive Leaf Extracts: A Systematic Review with Quantitative Synthesis. Separations. 2025; 12(9):236. https://doi.org/10.3390/separations12090236
Chicago/Turabian StyleCastillo-Correa, María, Cristina Montalbán-Hernández, María D. Navarro-Hortal, Diego Peña-Guzmán, Alberto Badillo-Carrasco, Alfonso Varela-López, Daniel Hinojosa-Nogueira, and Jose M. Romero Márquez. 2025. "Exploring the Influence of Extraction Methods, Solvents, and Temperature on Total Phenolic Recovery and Antioxidant Capacity in Olive Leaf Extracts: A Systematic Review with Quantitative Synthesis" Separations 12, no. 9: 236. https://doi.org/10.3390/separations12090236
APA StyleCastillo-Correa, M., Montalbán-Hernández, C., Navarro-Hortal, M. D., Peña-Guzmán, D., Badillo-Carrasco, A., Varela-López, A., Hinojosa-Nogueira, D., & Romero Márquez, J. M. (2025). Exploring the Influence of Extraction Methods, Solvents, and Temperature on Total Phenolic Recovery and Antioxidant Capacity in Olive Leaf Extracts: A Systematic Review with Quantitative Synthesis. Separations, 12(9), 236. https://doi.org/10.3390/separations12090236










