Abstract
The oxalic acid leachate of vanadium-bearing shale (OALS) is a complex system in which the ion states and coordination mechanisms of the primary metallic elements—vanadium, iron, and aluminum—are not fully understood. This study investigated the ionic speciation and coordination mechanisms of vanadium, iron, and aluminum in OALS. The results indicate that vanadium predominantly existed as VO(C2O4)22− anions, iron as Fe(C2O4)2− and Fe(C2O4)33− anions, and aluminum as Al(C2O4)2− and Al(C2O4)33− anions. The coordination reaction processes and equations of various oxalate complexes were examined. Regardless of whether the molar ratio was 1:1 or 1:2, the iron–oxalate complex exhibited the lowest reaction Gibbs free energy (ΔG), with values of −5343.69 and −1470.72 kJ/mol, respectively. The aluminum–oxalate complex followed, with ΔG values of −5169.23 and −1318.87 kJ/mol, respectively. The vanadium–oxalate complex displayed the highest reaction ΔG, at −2760.65 and −714.12 kJ/mol, respectively. Therefore, the coordination mechanism of vanadium, iron, and aluminum with oxalate ions in OALS is such that iron coordinated with oxalate first, followed by aluminum, and finally vanadium. The research results have important guiding significance for the purification, enrichment, and coordination mechanisms of complex solutions.
1. Introduction
Vanadium-bearing shale is a significant source of vanadium in China [1]. With the growing market demand for vanadium in recent years, extracting vanadium from vanadium-bearing shale has emerged as an important research direction. Traditional vanadium extraction methods from vanadium-bearing shale primarily involved roasting, sulfuric acid leaching, and ammonium salt precipitation [2,3]. However, these methods were associated with issues such as strong sulfuric acid corrosion and the generation of ammonia nitrogen wastewater [4]. To address these challenges, Hu et al. [5,6] proposed using oxalic acid, a clean and environmentally friendly organic acid, to extract vanadium from vanadium-bearing shale. Compared to traditional inorganic acids (e.g., sulfuric acid and hydrochloric acid), oxalic acid leaching offers advantages such as high selectivity, ease of operation, safety, and environmental protection [7]. However, vanadium in vanadium-bearing shale typically exists as aluminum isomorphous substitution in octahedral aluminum silicate minerals [8]. Consequently, regardless of whether inorganic or organic acids were used, aluminum was co-leached with vanadium, leading to a high concentration of impurity aluminum in the oxalic acid leachate of vanadium-bearing shale (OALS), often 10 to 20 times that of vanadium [9,10]. Additionally, vanadium-bearing shale contained minor iron-containing minerals, which are also leached during the process [11]. Therefore, the main ions presented in the OALS include vanadium, aluminum, iron, and oxalate ions. However, the OALS is a highly complex system, and the ionic speciation as well as the coordination reaction processes and mechanisms between metal ions and oxalate ions remain poorly understood. Therefore, conducting in-depth research on the coordination mechanisms within the OALS is of significant importance.
Oxalic acid has good reducing and complexing properties and can form anionic complexes with various high-valent metal cations, such as Fe3+, Al3+, VO2+, Cr3+, Cu2+, and Ti2+ [12,13]. The oxalate leaching-solvent extraction system demonstrates significant potential for practical industrial application in the green recovery of vanadium from resources like shale, red mud, and spent catalysts. Its core advantages lie in high selective leaching, efficient separation, oxalate regeneration, and environmental sustainability. Based on these characteristics, the use of oxalic acid as a leaching agent to extract valuable metals from minerals and solid secondary resources or to remove impurity iron from non-metallic minerals has been reported in recent years [14,15,16]. Red mud contains abundant iron resources. Due to the reducing and complexing properties of oxalic acid, it is often used to extract iron from red mud. In the oxalic acid leaching solution for red mud, iron mainly exists in the form of Fe(C2O4)33−, and iron can be recovered in the form of FeC2O4 by using the iron powder reduction precipitation method [16,17]. Yakabe and Minami [18] used tri-n-octylamine (TOA) solvent extraction to extract hafnium from oxalic acid solutions containing hafnium. With 0.08 mol/L TOA as the extractant, hafnium and oxalate ions were co-extracted. The extraction mechanism was that the complex Hf(C2O4)44− of hafnium and oxalic acid formed an ionic association with TOA, and the structure of the hafnium complex was [(R3NH)4·Hf(C2O4)4]. Djordjević et al. [19] used di-n-octylamino ethanol (DOAE) and di-n-octylamino propanol (DOAP) to study the extraction of tantalum (Ta) and niobium (Nb) from oxalic acid–metal solutions. Firstly, the complex of M (M = Ta, Nb) and oxalate ions was speculated to be MO(C2O4)33− based on solution chemistry. The mechanism was that the complex MO(C2O4)33− of M and oxalate ions formed an ionic association with the extractant DOAP, and the structure of the complex was speculated to be [(R2R’NH)3·MO(C2O4)3] [20]. Overall, in recent years, oxalic acid has increasingly been utilized in the field of hydrometallurgy. However, the existing literature predominantly focuses on the extraction of valuable metals or the removal of impurity ions, with limited attention given to the ionic speciation and coordination processes involving metal and oxalate ions. In particular, detailed studies on the coordination mechanisms are scarce. Although our previous work has reported on the ionic speciation of some oxalate complexes [21,22], a systematic investigation of the coordination reaction processes and mechanisms for vanadium, iron, and aluminum with oxalate ions has yet to be conducted.
In this study, the ionic speciation and coordination mechanisms of vanadium, iron, and aluminum in the OALS were investigated. Thermodynamic software was employed to analyze the ionic speciation. Additionally, the coordination reaction processes of various coordinating ions were examined. Finally, quantum chemical calculations were performed on the complexes to elucidate the coordination mechanisms of vanadium, iron, and aluminum with oxalate ions. These results provide valuable insights for the coordination and separation of metal ions.
2. Materials and Methods
2.1. Experimental Materials
The OALS was derived from vanadium-bearing shale collected in Tongshan County, Hubei Province, using a roasting-followed-by-leaching process. Figure 1 presents the phase composition of the vanadium shale, while Table 1 lists its primary chemical constituents.
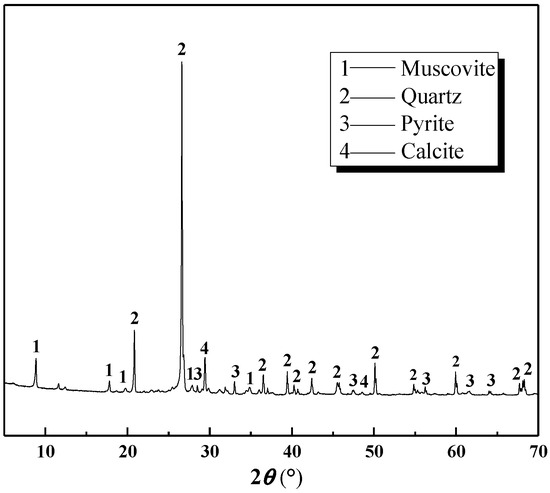
Figure 1.
XRD of vanadium-bearing shale.

Table 1.
Primary chemical constituents of vanadium-bearing shale (wt.%).
The OALS was prepared according to previous studies [23,24]. The vanadium-bearing shale, with a particle size finer than −0.074 mm (100% passing), was subjected to roasting at 850 °C for 1 h. Subsequently, the roasted material was leached under the optimal conditions: a solution containing 60% oxalic acid (H2C2O4) and 5% calcium fluoride (CaF2), with a liquid-to-solid ratio of 1:1, at a temperature of 95 °C for 4 h [23]. Upon completion of the reactions, the OALS was obtained through filtration. The primary chemical composition of the OALS is presented in Table 2. It is indicated that the OALS is a very complex solution with low vanadium concentration and high aluminum and iron concentrations; in addition, the OALS contains both organic ligands oxalate and inorganic ligands fluoride and phosphate ions.

Table 2.
The main chemical composition of the OALS (mol/L).
2.2. Experimental Operations
The free thermodynamic software Medusa [25] was primarily employed to study ionic speciation. Given the highly acidic nature of the OALS, the pH range for the ionic speciation analysis in this study was set from −1.0 to 6.0. In the simulation of the actual OALS, all constituent ions were included, and their concentrations were set to match those measured in the original OALS. In contrast, for the simulation of the pure oxalic acid system, only oxalate ions and a single metal ion (V, Fe, or Al) were introduced, with their concentrations also matching those present in the actual OALS.
Quantum chemical calculations were performed using Gaussian 16W software [26]. Structural models were constructed with GaussView 6.0 software. Subsequently, density functional theory (DFT) calculations at the B3LYP/6-31G(d,p) level were employed to optimize the structures of vanadium, iron, and aluminum complexes with oxalate. Frequency calculations were then conducted on the optimized structures using the same basis set to determine their thermodynamic parameters. The Gibbs free energy (ΔG) of the oxalate complexes was calculated to elucidate the preferential coordination mechanism of vanadium, iron, and aluminum with oxalate.
2.3. Determination Method
The concentration of vanadium was determined using the ammonium ferrous sulfate titration method [27]. The iron concentration was measured by 1,10-phenanthroline spectrophotometry with a UV-5500 spectrophotometer (Metash, Shanghai, China) [28]. The oxalate concentration was determined via potassium permanganate titration [29]. Concentrations of other elements were analyzed using an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 4300DV, PerkinElmer, Boston, MA, USA). The phase compositions were identified by X-ray diffraction (XRD, D8 Advance, Bruker, Berlin, Germany).
3. Results and Discussion
3.1. Ionic Speciation of Vanadium, Iron, and Aluminum in the OLAS
3.1.1. Ionic Speciation of Vanadium
Generally, both tetravalent (V(IV)) and pentavalent vanadium(V(V)) exist in the vanadium-bearing shale. In the sulfuric acid leachate, vanadium usually coexists in the form of VO2+ or VO2+ [30], while in the oxalic acid system, vanadium can form various complexes with oxalate ions. Therefore, the ionic speciation of V(IV) and V(V) in the pure oxalic acid solution were studied, and the results are shown in Figure 2.
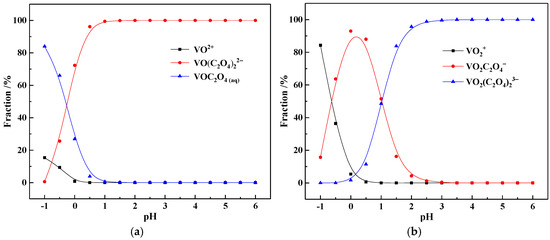
Figure 2.
The ionic speciation of V(IV) (a) and V(V) (b) in the pure oxalic acid solution.
As shown in Figure 2a, when the pH is in the range of −1.0 to 0, V(IV) mainly exists in the form of free VOC2O4 (aq) molecules; when the pH rises to 0–1.0, V(IV) mainly exists in the form of VO(C2O4)22−; and when the pH is greater than 1, over 99% of V(IV) exists in the form of VO(C2O4)22−. In contrast, as shown in Figure 2b, when the pH is in the range of −1.0 to −0.5, V(V) mainly exists in the form of VO2+; when the pH rises to −0.5 to 1.2, V(V) mainly exists in the form of VO2C2O4−; and when the pH is greater than 1.2, V(V) mainly exists in the form of VO2(C2O4)33− anions. Due to the strong reducing property of oxalate ions, the coordinated anions VO2C2O4− and VO2(C2O4)33− are further reduced to VO(C2O4)22− in high-concentration oxalate solutions. Therefore, in the pure oxalic acid solutions, vanadium mainly exists in the form of coordinated anions of tetravalent vanadium.
Based on the above results, the ionic speciation of vanadium in the actual OALS was studied (Figure 3). The results show that the ionic speciation of vanadium in the actual OALS was basically consistent with that of V(IV) in the pure oxalic acid system. When pH is less than 0, it exists in the form of VOC2O4(aq) molecules, and when pH is greater than 0, it exists in the form of VO(C2O4)22− complex anions. When pH is 0.65, the proportion of VO(C2O4)22− complex anions is 92.65%, and the proportion of VOC2O4(aq) molecules is 7.35%. This indicates that in the high-concentration oxalic acid system, V in the vanadium shale acid leaching solution mainly coordinates with the organic ligand C2O42− to form VO(C2O4)22− anions and does not coordinate with the inorganic ligands P and F.
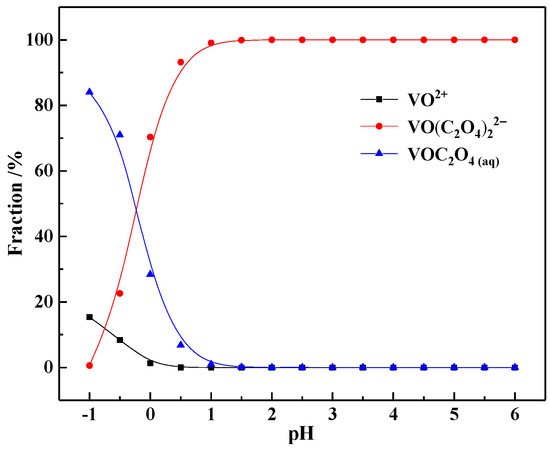
Figure 3.
The ionic speciation of V in the OALS.
3.1.2. Ionic Speciation of Iron
In sulfuric acid system, iron usually exists in two valence states: divalent (Fe2+) and trivalent (Fe3+) [31]. Due to the reducing property of oxalic acid, Fe3+ is usually reduced to Fe2+ by oxalate ions. In the OALS, Fe3+ accounts for 60% of the total iron, while Fe2+ makes up 40%. Therefore, the ionic speciation of Fe(III) and Fe(II) in pure oxalic acid systems and actual OALS was studied, and the results are shown in Figure 4 and Figure 5.
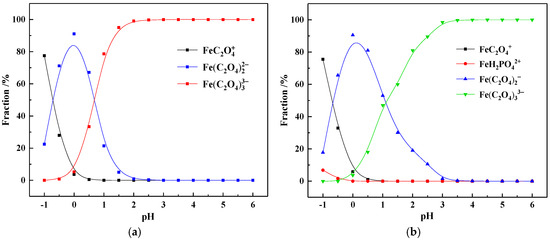
Figure 4.
The ionic speciation of Fe(III) in the pure oxalic acid system (a) and OALS (b).
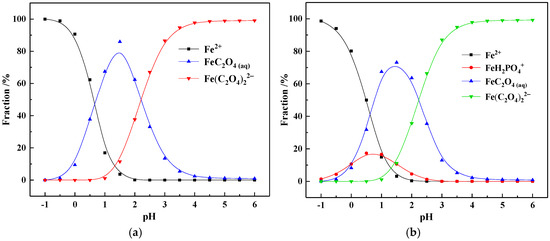
Figure 5.
The ionic speciation of Fe(II) in the pure oxalic acid system (a) and OALS (b).
As can be seen from Figure 4a, when the pH is between −1.0 and −0.5, Fe(III) mainly exists as FeC2O4+ cation; when the pH rises to −0.5 to 1.0, Fe(III) is mainly in the form of Fe(C2O4)2− anion; and when the pH is greater than 1, Fe(III) mainly exists as Fe(C2O4)33− anions. Compared with Figure 4b, in the actual OALS, the ionic speciation of Fe(III) is relatively similar to that in the pure oxalic acid system. However, when the pH is between −1.0 and 0, the FeH2PO42+ species is formed, indicating that Fe(III) can form complexes with P. When the pH is between −0.5 and 1.0 and greater than 1.0, the species of Fe(III) are mainly Fe(C2O4)2− and Fe(C2O4)33− coordinated anions, respectively. When the solution pH is 0.65, the proportion of Fe(C2O4)2− ions is 73.66%, that of Fe(C2O4)33− ions is 25.64%, and that of FeC2O4+ is 0.70%. Therefore, in the oxalic acid leaching solution of vanadium shale, Fe(III) mainly exists in the form of Fe(C2O4)2− and Fe(C2O4)33− coordinated anions.
As can be seen from Figure 5a, when the pH is between −1.0 and 0.5, Fe(II) mainly exists in the form of Fe2+; when the pH rises to 0.5 to 2.5, Fe(II) is mainly in the form of free FeC2O4 molecules; and when the pH is greater than 2.5, Fe(II) mainly exists as the Fe(C2O4)22− anions. Compared with Figure 5b, in the actual OALS, similar to Fe(III), the existence state of Fe(II) is also quite similar to that in the pure oxalic acid system. In the solution with pH ranging from −1.0 to 3.0, the FeH2PO4+ ion exists; while when the pH is 0.5 to 2.5 and greater than 2.5, Fe(II) mainly exists as FeC2O4 and Fe(C2O4)22− species, respectively. When the solution pH is 0.65, the proportions of Fe2+, FeC2O4(aq), FeH2PO4+, and Fe(C2O4)22− are 41.28%, 41.37%, 17.03%, and 0.27%. The results show that in the OALS, Fe(II) mainly exists in the form of FeC2O4 and Fe(C2O4)22− complex anions.
It is well-established that the stability of a complex increases with the number of coordinated ligands. As evidenced by the coordination stability constants (Table 3) [32], the formation constants of Fe(III)-C2O4 complexes consistently exceed those of Fe(II)-C2O4 complexes. This indicates that when Fe3+ and Fe2+ coexist in an oxalate solution, Fe3+ preferentially coordinates with C2O42−. Consequently, Fe(III)-C2O4 complexes exhibit greater stability in the OLAS. Furthermore, the coordination constant of Fe(II)-C2O4 surpasses that of Fe(II)-F complexes, rendering Fe2+ more likely to coordinate with C2O42− and precluding the formation of Fe(II)-F complexes in the OLAS. Similarly, regardless of coordination number, the formation constants of Fe(III)-C2O4 complexes consistently dominate over those of Fe(III)-F complexes, thereby eliminating Fe(III)-F complexes in the OLAS. These findings were in full agreement with the simulation results presented in Figure 3 and Figure 4.

Table 3.
Stability constants of Fe-C2O4 and Fe-F complexes.
Based on the aforementioned findings, since Fe also exists in anionic forms within the OALS, this may significantly impede vanadium extraction from the system. Whether employing solvent extraction or ion exchange methodologies, the selection of extractants or ion-exchange resins with high vanadium selectivity was of paramount importance.
3.1.3. Ionic Speciation of Aluminum
Aluminum mainly exists in the form of Al3+ in sulfuric acid solutions [31], while in oxalic acid solutions, aluminum readily coordinates with oxalate ions to form various complexes. Thus, the ionic speciation of aluminum in the pure oxalic acid systems and actual OALS was investigated, and the results are shown in Figure 6.
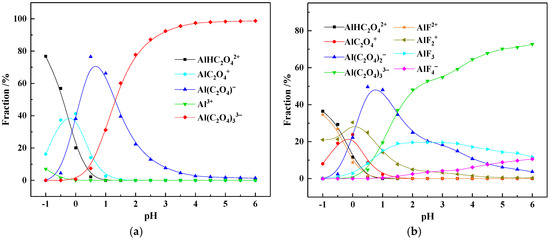
Figure 6.
Ionic speciation of Al in the pure oxalic acid system (a) and actual OALS (b).
The results in Figure 6a indicate that aluminum can form four complexes, namely, AlHC2O42+, AlC2O4+, Al(C2O4)2−, and Al(C2O4)33−, in the pure oxalic acid solutions of different pH values. When the pH is between 0 and 1.5, the Al(C2O4)2− anion is dominant, and when the pH is greater than 1.5, the Al(C2O4)33− complex anion is stably present.
Compared with the ionic speciation of aluminum in the pure oxalic acid solution, the ionic speciation of aluminum in the actual OALS is more complex. The results in Figure 6b show that, in addition to the above four aluminum–oxalate complexes, aluminum forms various complexes with fluorine, including AlF2+, AlF2+, AlF3, and AlF4−. However, when the pH is between 0 and 1.5, the Al(C2O4)2− anion is still dominant, and when the pH is greater than 1.5, the Al(C2O4)33− complex anion is still stably present. Moreover, it can be seen from the results in Figure 5b that as the pH increases, the acidity of the solution continuously decreases, and the proportions of C2O42− and F− increase, which leads to the increasing coordination numbers of Al3+ with C2O42− and F−, thereby forming various aluminum–oxalate and aluminum-fluoride complexes. In the actual OALS, when the pH is 0.65, the proportions of Al(C2O4)2−, Al(C2O4)33−, AlF2+, AlF3, and AlC2O4+ ions are 48.95%, 8.06%, 20.76%, 10.85%, and 5.05%.
Based on the stability constants of Al-F and Al-C2O4 complexes (Table 4) [32], when the coordination number ranges from 1 to 3, the stability constants of Al-C2O4 complexes consistently exceed those of Al-F complexes. However, when the coordination number increases to 4–6, the stability constants of Al-F complexes surpass 18, indicating that Al-F complexes with coordination numbers exceeding 4 exhibit exceptional stability. It should be noted that higher coordination numbers require elevated ligand ion concentrations. Given that the C2O42− concentration (1.40 mol/L) in OALS solution significantly exceeds the F− concentration (0.32 mol/L), under pH 0.65 conditions, aluminum primarily forms complexes with C2O42− ions, with minimal complexation occurring with fluoride ions.

Table 4.
Stability constants of Al-F and Al-C2O4 complexes [32].
Similar to the research results on iron, since Al also exists in anionic forms within the OALS, this also may significantly impede vanadium extraction from the system. Whether employing solvent extraction or ion exchange methodologies, the selection of extractants or ion-exchange resins with high vanadium selectivity was of paramount importance.
3.2. Coordination Reaction Process of Vanadium, Iron, and Aluminum with Oxalate Ions
Section 3.1. investigated the ionic forms of vanadium, iron, and aluminum in the OALS. The results showed that vanadium, iron, and aluminum mainly formed complexes with oxalate ions, but the coordination process was not clear. Therefore, a systematic study was further conducted on the coordination process of vanadium, iron, and aluminum with oxalate ions.
3.2.1. Coordination Process of Vanadium and Oxalate Ions
In the inorganic acid sulfuric acid system, vanadium in the leachate of vanadium-bearing shale usually exists in the form of VO2+ [30]. However, according to the research results of Section 3.1., vanadium in the OALS mainly exists in the form of VOC2O4 molecules and VO(C2O4)22− complex anions, and their coordination reactions are shown in Equations (1) and (2) [33].
According to reference [34], pentavalent vanadium can form coordination anions VO2C2O4− and VO2(C2O4)23− with oxalate ions. The reaction equations are as follows:
However, in the OALS, there is a large number of oxalate ions. Under the conditions of heating and an excess of oxalate ions, the V(V) complex with oxalate is further reduced to a V(IV) complex. Therefore, in the OALS, vanadium mainly exists in the form of VOC2O4 molecules and VO(C2O4)22− complex anions. The reaction equation was as follows [35]:
Therefore, when vanadium-bearing shale is leached with oxalic acid, the coordination process of vanadium is as follows: V(IV) directly coordinates with C2O42− to form VOC2O4. Under conditions of excess C2O42−, VOC2O4 is further coordinated to form the VO(C2O4)22− complex; V(V) is first coordinated by C2O42− to form the VO2C2O4− complex. Under conditions of excess C2O42−, VO2C2O4− is further coordinated to form the VO2(C2O4)23− complex. However, under heating conditions, the VO2C2O4− and VO2(C2O4)23− complexes are further reduced by C2O42−, forming the VOC2O4 and VO2(C2O4)23− complexes.
3.2.2. Coordination Process of Iron and Oxalate Ions
In the sulfate leachate of vanadium-bearing shale, iron usually exists simultaneously as Fe2+ and Fe3+ [31]. However, in the oxalic acid system, iron exists in multiple complex forms. According to the research results in Section 3.1, the main complexes of Fe2+ with C2O42− are FeC2O4 and Fe(C2O4)22−, and the reaction equations are shown in Equations (11) and (12) [36,37]; the main complexes of Fe3+ with C2O42− are FeC2O4+, Fe(C2O4)2−, and Fe(C2O4)33−, and the reaction equations are shown in Equations (13)–(15) [38].
The initial pH of the OALS is 0.65. According to Figure 3 and Figure 4, Fe2+ exists simultaneously as FeC2O4 and Fe(C2O4)22−, and Fe3+ exists simultaneously as Fe(C2O4)2− and Fe(C2O4)33−. Therefore, the coordination process of Fe2+ with C2O42− is that Fe2+ first coordinates with C2O42− to form C2O42− and then is further coordinated by C2O42− to form the Fe(C2O4)22− complex. The coordination process of Fe3+ with oxalate ions is as follows: Fe3+ first coordinates with C2O42− to form FeC2O4+, and then under the condition of excess oxalate ions, FeC2O4+ is further coordinated to form the Fe(C2O4)2− and Fe(C2O4)33− complexes.
3.2.3. Coordination Process of Aluminum and Oxalate Ions
In the sulfate leachate, aluminum usually exists in the form of Al3+, Al(OH)2+, and Al(OH)2+ [39]. However, in the oxalic acid system, aluminum exists in various complex forms, such as AlC2O4+, Al(C2O4)2−, and Al(C2O4)33−. The coordination reaction of aluminum with oxalate ions are shown in Equations (16)–(19) [40].
According to the results in Figure 5, Al exists in the form of both Al(C2O4)2− and Al(C2O4)33− in the OALS. Therefore, during the oxalic acid leaching of vanadium-bearing shale, the coordination process of Al with C2O42− is as follows: Al3+ first coordinates with HC2O4− and C2O42− to form AlHC2O42+ and AlC2O4+, respectively. Under the condition of excess oxalate ions, AlC2O4+ is further coordinated to form Al(C2O4)2− and Al(C2O4)33− complexes.
3.3. Coordination Mechanism of Vanadium, Iron, and Aluminum with Oxalate Ions
The previous section determined the ionic speciation and coordination process of vanadium, iron, and aluminum in OALS. The results indicated that vanadium, iron, and aluminum mainly exist in the form of oxalate complexes in OALS, and the complexes with inorganic ligands P and F were relatively few. However, the coordination mechanism between metal ions and oxalate ions was not clear. Therefore, the quantum chemistry software Gaussian 16W was used to conduct an in-depth study on the coordination mechanism of vanadium, iron, and aluminum with oxalate ions.
3.3.1. Structural Optimization of Vanadium–Oxalate Complexes
According to Equations (1) and (2), the vanadium–oxalate complexes were formed by the coordination reaction between VO2+ and C2O42−, resulting in VOC2O4 and VO(C2O4)22−. Therefore, the molecular structures of VO2+ and C2O42− were first optimized, and the results are shown in Figure 7.
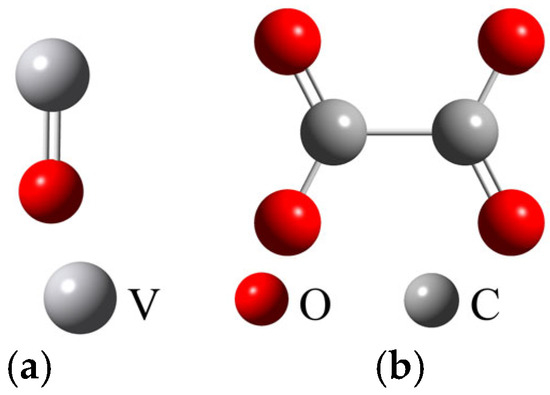
Figure 7.
Electrostatic potential of VO2+ (a) and C2O42−(b).
As shown in Figure 7a, the electrostatic potential of VO2+ was deep blue at the V atom, indicating that the V atom had a higher electrostatic potential and exhibited electron acceptor characteristics. As shown in Figure 6b, the C2O42− molecule has good structural symmetry, with the electrostatic potential around the O atoms being red and evenly distributed among the four O atoms, demonstrating electron donor characteristics.
Based on the above analysis, the structure of the complex VOC2O4 was optimized, and the results are shown in Figure 8a. On this basis, the structure of the complex VO(C2O4)22− was further optimized, and the results are shown in Figure 8b and Table 5. The results indicate that the molecular structure of VO(C2O4)22− is a symmetrical structure centered on the V=O double bond, with two oxalate ions arranged at certain angles on both sides.
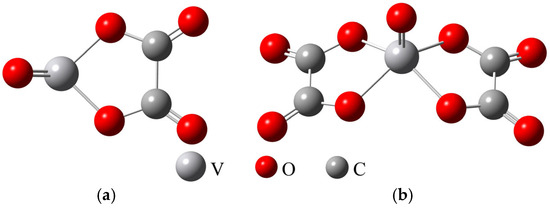
Figure 8.
Optimized molecular structures of VOC2O4 (a) and VO(C2O4)22− (b).

Table 5.
Bond length and angles of VO(C2O4)22−.
3.3.2. Structural Optimization of Iron–Oxalate Complexes
In the OALS, Fe3+ had a larger proportion and was more likely to coordinate with oxalate ions. Therefore, this paper only examines the coordination process between Fe3+ and oxalate ions. According to Equations (13) and (14), Fe3+ first coordinated with C2O42− to form FeC2O4+, and its optimized result is shown in Figure 9a. Then, under the condition of excess oxalate ions, it further coordinated to form Fe(C2O4)2−, and its optimized result is shown in Figure 9b. Then Bond length and angles of Fe(C2O4)2− is listed in Table 6. The results show that Fe(C2O4)2− had a planar symmetrical structure with iron atoms at the center and two oxalate ions on both sides.
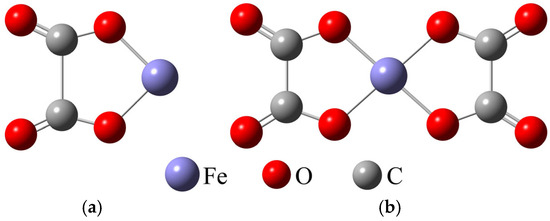
Figure 9.
Optimized molecular structures of FeC2O4+ (a) and Fe(C2O4)2− (b).

Table 6.
Bond length and angles of Fe(C2O4)2−.
3.3.3. Structural Optimization of Aluminum–Oxalate Complexes
According to Equations (16)–(18), Al3+ coordinates with HC2O4− to form AlHC2O42+, and then further forms AlC2O4+ and Al(C2O4)2−. To compare with vanadium–oxalate and iron–oxalate complexes in the same system, the aluminum–oxalate complexes with molar ratios of 1:1 and 1:2 were optimized, that is, the structures of AlC2O4+ and Al(C2O4)2− were optimized. The results are shown in Figure 10. The results indicate that the molecular structure of Al(C2O4)2− is a structure centered on the aluminum atom, with two oxalate ions arranged in a cross-symmetric manner. Bond length and angles of Al(C2O4)2− are listed in Table 7.
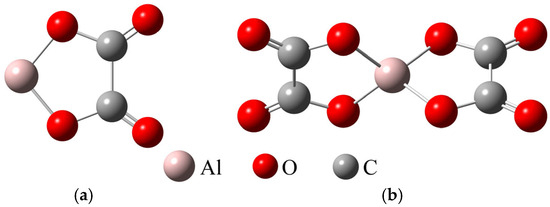
Figure 10.
Optimized molecular structures of AlC2O4+ (a) and Al(C2O4)2− (b).

Table 7.
Bond length and angles of Al(C2O4)2−.
3.3.4. Coordination Mechanism of Vanadium, Iron, and Aluminum with Oxalate Anions
The frequencies of the optimized VO2+, Fe3+, Al3+, C2O42−, VOC2O4, VO(C2O4)22−, FeC2O4+, Fe(C2O4)2−, AlC2O4+, and Al(C2O4)2− were calculated to determine the ΔG of these structures. At this stage, the ΔG of these compounds was precisely determined. The ΔG of the coordination reactions of vanadium, iron, and aluminum with oxalate ions (Equations (1), (2), (13), (14), (17) and (18)) were calculated. The results are shown in Table 8.

Table 8.
ΔG of coordination reactions between V, Fe, Al, and C2O42− ions.
As shown in Table 8, regardless of whether the molar ratio was 1:1 or 1:2, the reaction ΔG of the iron–oxalate complex is the smallest, being −5343.69 and −1470.72 kJ/mol, respectively. Next is the aluminum–oxalate complex, with reaction ΔG values of −5169.23 and −1318.87 kJ/mol, respectively. The vanadium–oxalate complex has the largest reaction ΔG, being −2760.65 and −714.12 kJ/mol, respectively. Therefore, the order of coordination ability of vanadium, iron, and aluminum with oxalate is iron > aluminum > vanadium. From this, it can be known that the coordination mechanism of vanadium, iron, and aluminum with oxalate ions is as follows: iron coordinates with oxalate ions first, followed by aluminum, and finally vanadium.
4. Conclusions
- (1)
- Vanadium, iron, and aluminum can form various complexes with oxalate ions. In the OALS (pH range of 0.5 to 1.0), vanadium predominantly exists as VO(C2O4)22− anions, iron primarily exists as the Fe(C2O4)2− and Fe(C2O4)33− anions, and aluminum mainly exists as the Al(C2O4)2− and Al(C2O4)33− anions.
- (2)
- In the OALS, vanadium, iron, and aluminum initially coordinate with oxalate ions to form 1:1 molar ratio complexes. Subsequently, these complexes further coordinate to generate anionic complexes with molar ratios of 1:2 and 1:3 between the metal ions and oxalate ions.
- (3)
- The coordination sequence of vanadium, iron, and aluminum with oxalate ions is as follows: iron > aluminum > vanadium. Specifically, the coordination mechanism proceeds such that iron coordinates with oxalate ions first, followed by aluminum, and finally vanadium in the OALS.
Author Contributions
Data curation, Q.X., Z.L. and Q.L.; Formal analysis, Q.X., Q.L. and X.T.; Investigation, Q.X., Z.L., Q.L., and H.L.; Writing—original draft: Q.X., Q.L. and H.L.; Visualization: H.L. and X.T.; Conceptualization, Z.L. and X.L.; Funding acquisition, Z.L.; Methodology: Z.L. and X.T.; Writing—review & editing, Z.L. and X.L.; Resources, X.T.; Visualization X.T.; Project administration, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number No. 52204269 And Jiangxi Provincial Key Laboratory of Low-Carbon Processing and Utilization of Strategic Metal Mineral Resources grant number No. 2023SSY01041 And Open Project for State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming University of Science and Technology grant number CNMRCUKF20 And Key Laboratory of Environmental Protection, Mining Resources Utilization and Pollution Control of the Ministry of Ecology and Environment grant number No. HB202102.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to express our sincere gratitude to Zhang Yimin’s research team from Wuhan University of Science and Technology for generously providing the vanadium shale raw materials as well as technical support for the use of Gaussian software.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, S.; Wang, L.; Chen, J.; Ye, L.; Du, J. Research progress of vanadium extraction processes from vanadium slag: A review. Sep. Purif. Technol. 2024, 342, 127035. [Google Scholar] [CrossRef]
- Li, C.; Jiang, T.; Wen, J.; Yu, T.; Li, F. Review of leaching, separation and recovery of vanadium from roasted products of vanadium slag. Hydrometallurgy 2024, 226, 106313. [Google Scholar] [CrossRef]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, S.; Liu, T.; Chen, T.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Yang, Y. Separation and recovery of iron impurity from a vanadium-bearing stone coal via an oxalic acid leaching-reduction precipitation process. Sep. Purif. Technol. 2017, 180, 99–106. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Xue, N. Source separation of vanadium over iron from roasted vanadium-bearing shale during acid leaching via ferric fluoride surface coating. J. Clean. Prod. 2018, 181, 399–407. [Google Scholar] [CrossRef]
- Kanižaj, L.; Molčanov, K.; Dubraja, L.A.; Klaser, T.; Jurić, M. Homo- and heterometallic oxalate-based complexes obtained using [Cr(C2O4)3]3− building block—Two polymorphs of a solvate. Polyhedron 2022, 211, 115556. [Google Scholar] [CrossRef]
- Fu, Z.; He, W.; Rao, Y.; Du, G.; Wang, N.; Wang, S. A highly selective and low-discharge method for vanadium extraction from vanadium slag: Low-calcification roasting-sodium bicarbonate leaching. Sep. Purif. Technol. 2025, 361, 131584. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Liu, T.; Huang, J.; Liu, H. Two-Stage Separation of V(IV) and Al(III) by Crystallization and Solvent Extraction from Aluminum-Rich Sulfuric Acid Leaching Solution of Stone Coal. JOM 2017, 69, 1950–1957. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Dehghani, M.H.; Ghanbari, R. Investigating the residual aluminum elimination from conventional and enhanced coagulation by phosphate compounds in wastewater treatment process. J. Mol. Liq. 2016, 221, 673–684. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Y.; Liu, T.; Huang, J.; Liu, H. Chen F. Mechanism of vanadium extraction from stone coal via hydrating and hardening of anhydrous calcium sulfate. Hydrometallurgy 2016, 166, 48–56. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Zheng, Q. Separation and recovery of iron impurities from a complex oxalic acid solution containing vanadium by K3Fe(C2O4)3·3H2O crystallization. Sep. Purif. Technol. 2020, 232, 115970. [Google Scholar] [CrossRef]
- TVSPV, S.G.; Gangu, K.K.; Maddil, S.; Jonnalagadda, S.B. Excellent catalytic activity of ethylenediamine stabilised oxalate ligated aluminium coordination complex for synthesis of novel benzoquinolines. Polyhedron 2020, 189, 114734. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Lu, F.; Chai, H.; Gu, H. Selective separation of aluminum, silicon, and titanium from red mud using oxalic acid leaching, iron precipitation and pH adjustments with calcium carbonate. Hydrometallurgy 2024, 223, 106221. [Google Scholar] [CrossRef]
- Li, W.; Yan, X.; Niu, Z.; Zhu, X. Selective recovery of vanadium from red mud by leaching with using oxalic acid and sodium sulfite. J. Environ. Chem. Eng. 2021, 9, 105669. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Wang, M.; Wang, H.; Xian, P. Iron recovery from the leached solution of red mud through the application of oxalic acid. Int. J. Miner. Process. 2016, 157, 145–151. [Google Scholar] [CrossRef]
- Hayatullah; Shathi, A.S.; Mostafa, M.G.; Rahman, M.A.; Biswas, P.K.; Alam, M.S.; Rana, M.S.; Uddin, M.R.; Nuruzzaman, M.; Shahriar, M.S.; et al. Iron removal from red clay using oxalic acid leaching for enhanced ceramic industry applications. Heliyon 2024, 10, e38863. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, K.; Minami, S. Liquid-liquid extraction of hafnium complex ion from aqueous oxalic-acid solution with high molecular weight amine. J. Inorg. Nucl. Chem. 1975, 37, 1973–1976. [Google Scholar] [CrossRef]
- Djordjević, C.; Goričan, H.; Sevdić, D. Solvent extraction of niobium and tantalum-VII: Extraction with di-n-octylamino alcohols from oxalic metal solutions. J. Inorg. Nucl. Chem. 1969, 31, 1487–1494. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Mishra, D.; Yi, K.; Hong, J.; Jun, M.; Park, H. Separation of molybdenum and vanadium from oxalate leached solution of spent residue hydrodesulfurization (RHDS) catalyst by liquid-liquid extraction using amine extractant. J. Ind. Eng. Chem. 2015, 21, 1265–1269. [Google Scholar] [CrossRef]
- Liu, Z.; Sheng, M.; He, Y.; Zhou, H.; Huang, J.; Luo, X.; Zhang, Y. Coordination mechanism of aluminum with oxalate and fluoride in aluminum crystallization from vanadium extraction wastewater. J. Mol. Liq. 2022, 347, 117992. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Zheng, Q. Separation and Recovery of Vanadium and Iron from Oxalic-acid-based Shale Leachate by Coextraction and Stepwise Stripping. Sep. Purif. Technol. 2020, 244, 116532. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Luo, D. Separation and recovery of vanadium and aluminum from oxalic acid leachate of shale by solvent extraction with Aliquat 336. Sep. Purif. Technol. 2020, 249, 116867. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Huang, J.; Liu, T.; Yuan, Y.; Xue, N. Eco-Friendly Leaching and Separation of Vanadium over Iron Impurity from Vanadium-Bearing Shale Using Oxalic Acid as a Leachant. ACS Sustain. Chem. Eng. 2018, 6, 1900–1908. [Google Scholar] [CrossRef]
- Puigdomenech, L. Medusa Software; KTH University: Stockholm, Sweden, 2015. [Google Scholar]
- Liu, X.; Fang, J.; Zheng, W.; Tan, Z.; Zheng, X.; Di, J. Study on desulfurization mechanism of ionic liquid extractant based on Gaussian quantitative calculation. Comput. Theor. Chem. 2021, 1204, 113353. [Google Scholar] [CrossRef]
- GB/T 8704.5-2007; Determination of Vanadium Content in Ferrovanadium-Ammonium Ferrous Sulfate Titration Method and Potentiometric Titration Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- HJ/T-2007; Water Quality Determination of Iron-Phenanthroline Spectrophotometry. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2007.
- GB/T 601-2016; Chemical Reagent—Preparations of Reference Titration Solutions. Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- Liu, H.; Zhang, Y.; Huang, J.; Liu, T.; Xue, N.; Wang, K. Selective separation and recovery of vanadium from a multiple impurity acid leaching solution of stone coal by emulsion liquid membrane using di-(2-ethylhexyl)phosphoric acid. Chem. Eng. Res. Des. 2017, 122, 289–297. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Y.; Huang, J.; Liu, T.; Wang, L. Separation of impurities aluminum and iron during pressure acid leaching of vanadium from stone coal. J. Clean. Prod. 2017, 166, 1265–1273. [Google Scholar] [CrossRef]
- Sillén, L.G.; Martell, A.E. Stability Constants of Metal−Ion Complex; Metcalfe & Cooper Limited: London, UK, 1964. [Google Scholar]
- Romodanovskii, P.A.; Vorob’ev, P.N.; Dmitrieva, N.G.; Gridchin, S.N.; Orlova, T.D. Thermodynamic characteristics of formation reactions of the vanadium(V) malonate complex. Russ. J. Coord. Chem. 2009, 35, 479–481. [Google Scholar] [CrossRef]
- Tracey, A.S. Applications of 51V NMR spectroscopy to studies of the complexation of vanadium (V) by α−hydroxycarboxylic acids. Coord. Chem. Rev. 2003, 237, 113–121. [Google Scholar] [CrossRef]
- Bruyère, V.I.E.; Rodenas, L.A.G.; Morando, P.J.; Pedro, J.; Blesa, M.A. Reduction of vanadium(V) by oxalic−acid in aqueous acid solutions. J. Chem. Soc. Dalton Trans. 2001, 24, 3593–3597. [Google Scholar] [CrossRef]
- Panias, D.; Taxiarchou, M.; Paspaliaris, I.; Kontopoulos, A. Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy 1996, 42, 257–265. [Google Scholar] [CrossRef]
- Enrique, J.B. Natural iron oxalates and their analogous synthetic counterparts: A review. Geochemistry 2016, 76, 449–460. [Google Scholar] [CrossRef]
- Ogi, Y.; Obara, Y.; Katayama, T.; Suzuki, Y.I.; Liu, S.Y.; Bartlett, N.C.; Kurahashi, N.; Karashima, S.; Togashi, T.; Inubushi, Y.; et al. Ultraviolet photochemical reaction of [Fe(III)(C2O4)3]3− in aqueous solutions studied by femtosecond time-resolved X-ray absorption spectroscopy using an X-ray free electron laser. Struct. Dyn. 2015, 2, 034901. [Google Scholar] [CrossRef]
- Boily, J.F.; Qafoku, O.; Felmy, A.R. A Potentiometric spectrophotometric and pitzer ion−interaction study of reaction equilibria in the aqueous H+−Al3+, H+−oxalate and H+−Al3+−oxalate systems up to 5 mol·dm−3 NaCl. J. Solut. Chem. 2007, 36, 1727–1743. [Google Scholar] [CrossRef]
- Phillips, B.L.; Crawford, S.N.; Casey, W.H. Rate of water exchange between Al(C2O4)(H2O)4+(aq) complexes and aqueous solutions determined by 17O−NMR spectroscopy. Geochim. Cosmochim. Acta 1997, 61, 4965–4973. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).