Synergistic Extraction of Samarium(III) from Water via Emulsion Liquid Membrane Using a Low-Concentration D2EHPA–TOPO System: Operational Parameters and Salt Effects
Abstract
1. Introduction
2. Material and Methods
2.1. Material
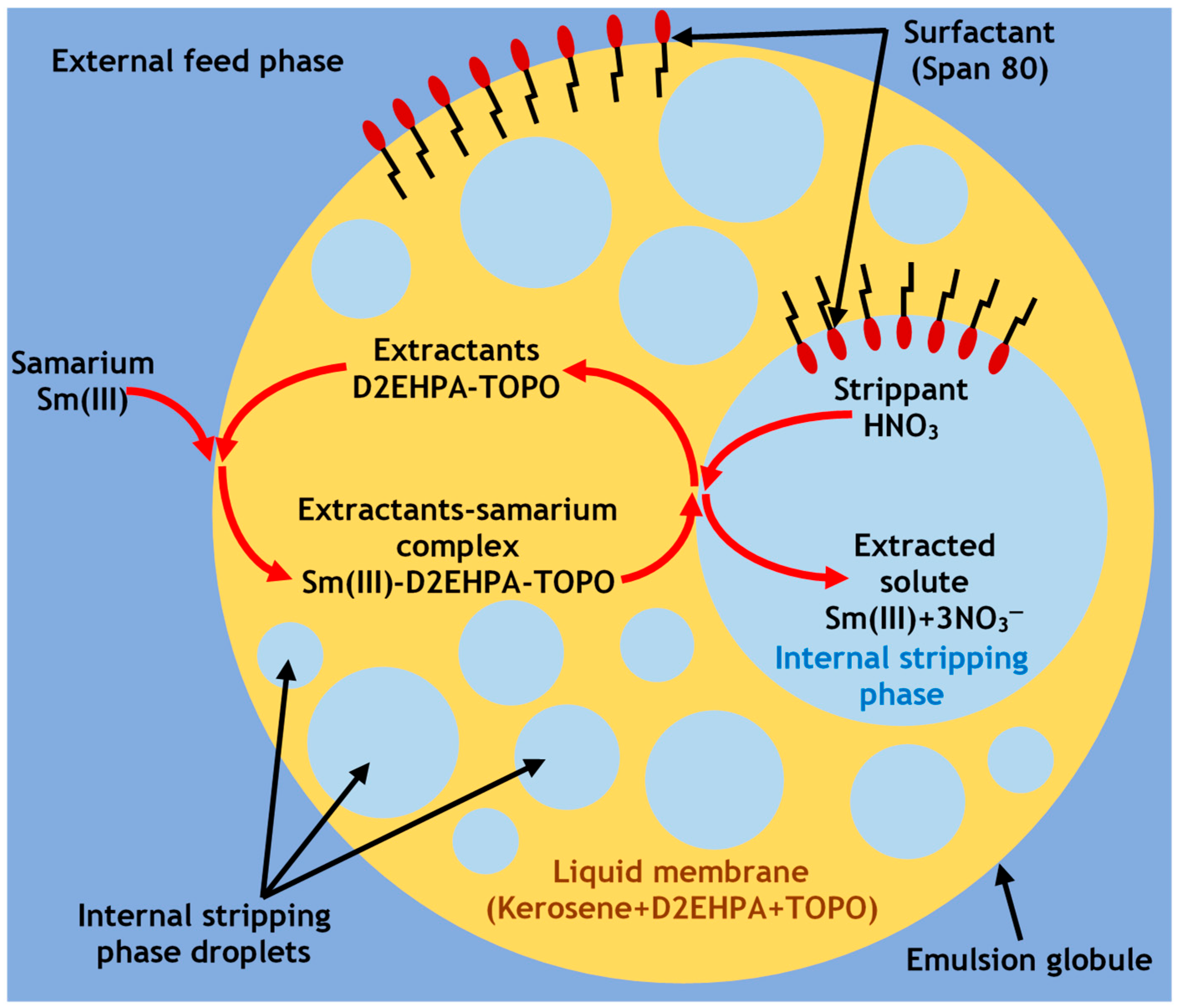
2.2. Experimental Protocols
3. Results and Discussion
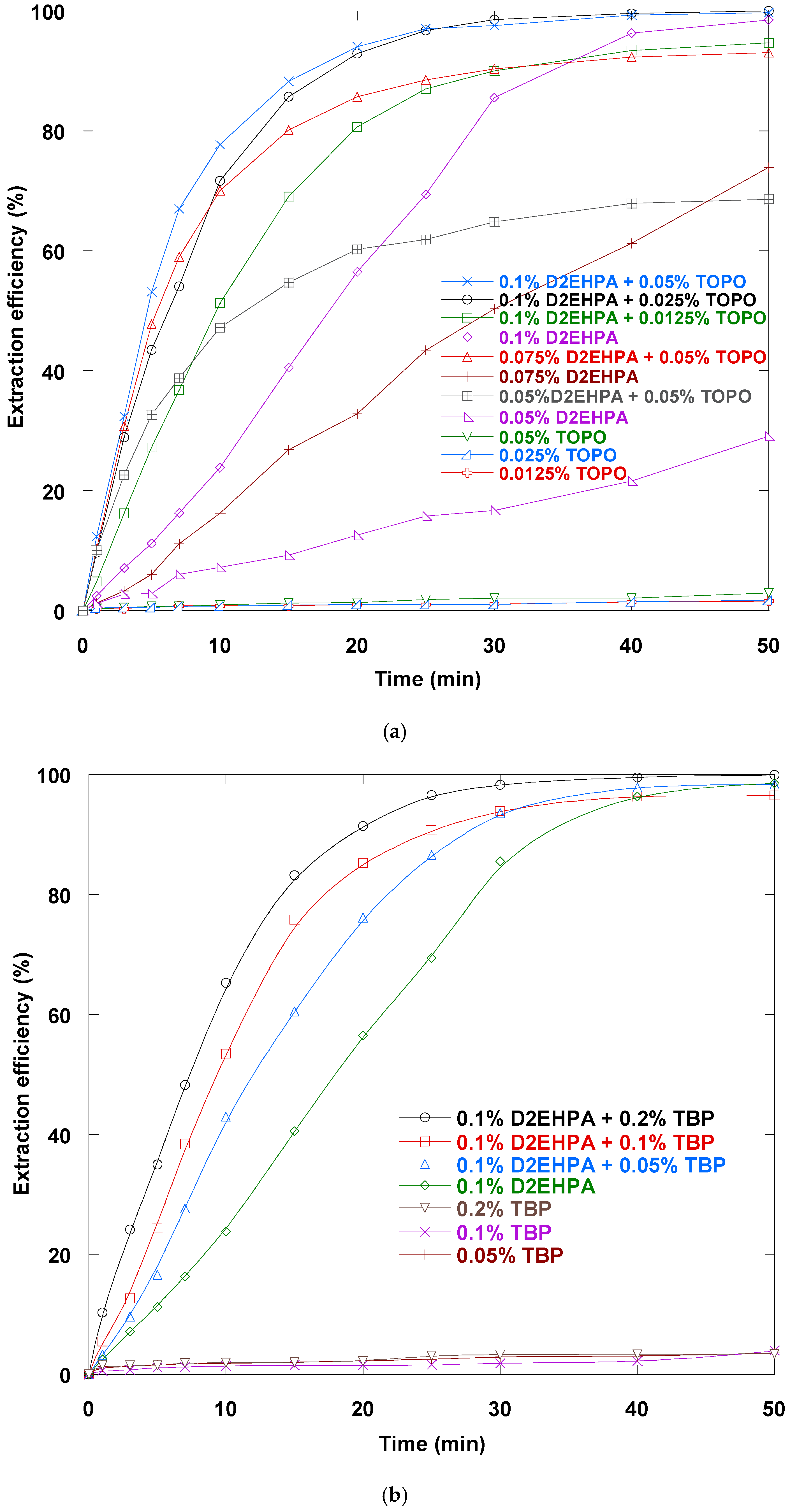
3.1. Synergistic Extractant Selection
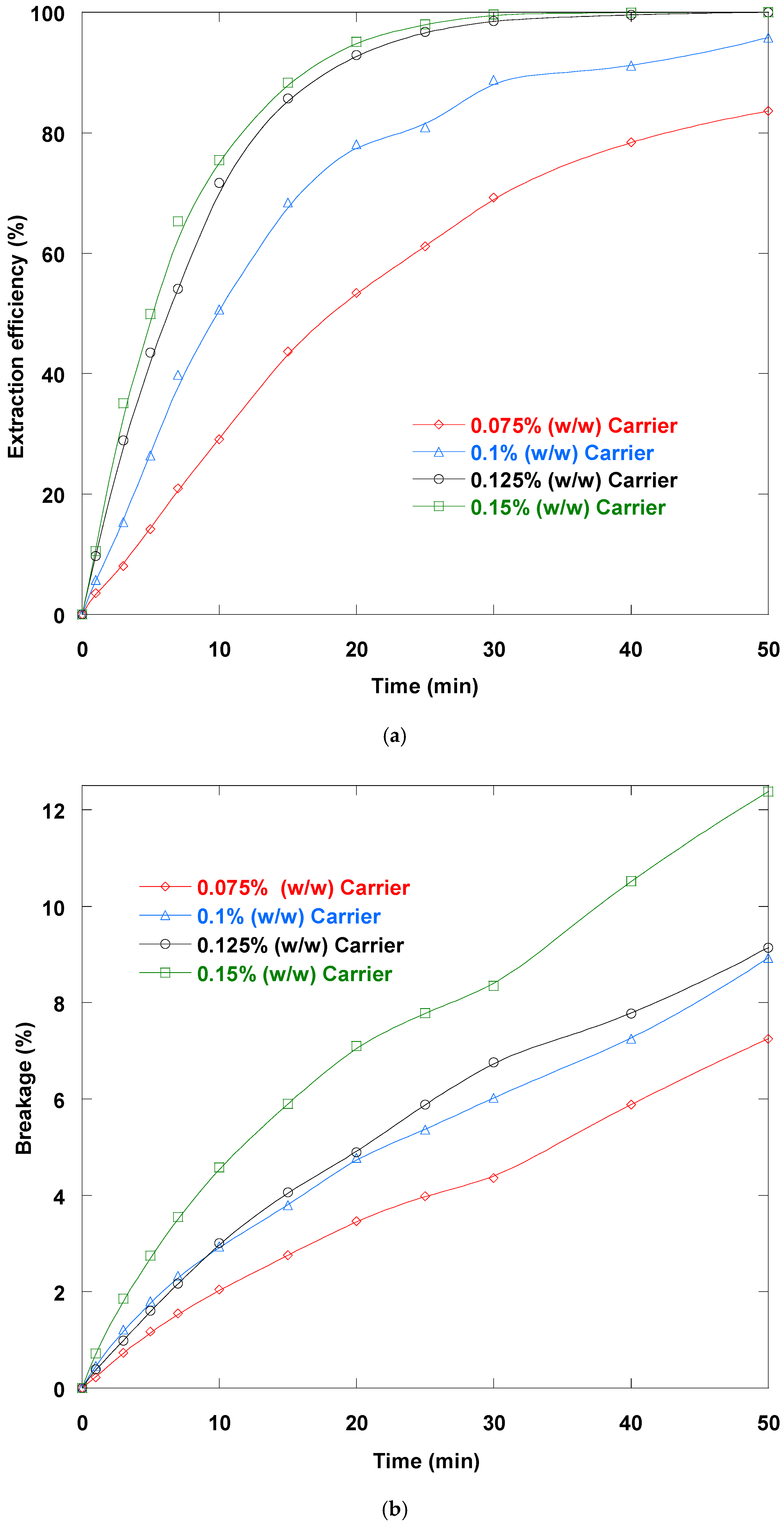
3.2. D2EHPA–TOPO Loading Impact
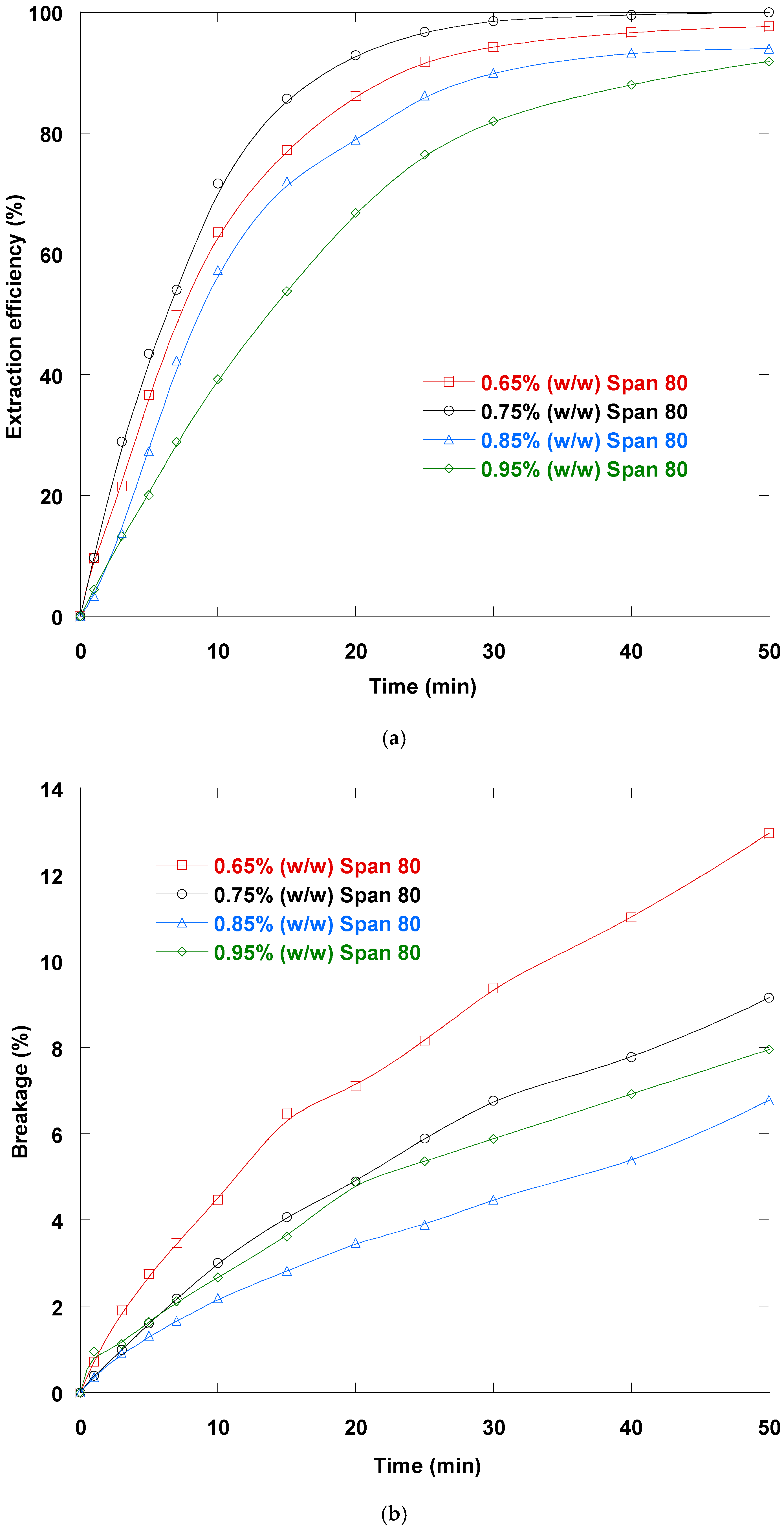
3.3. Surfactant Concentration Impact
3.4. Emulsification Duration Impact
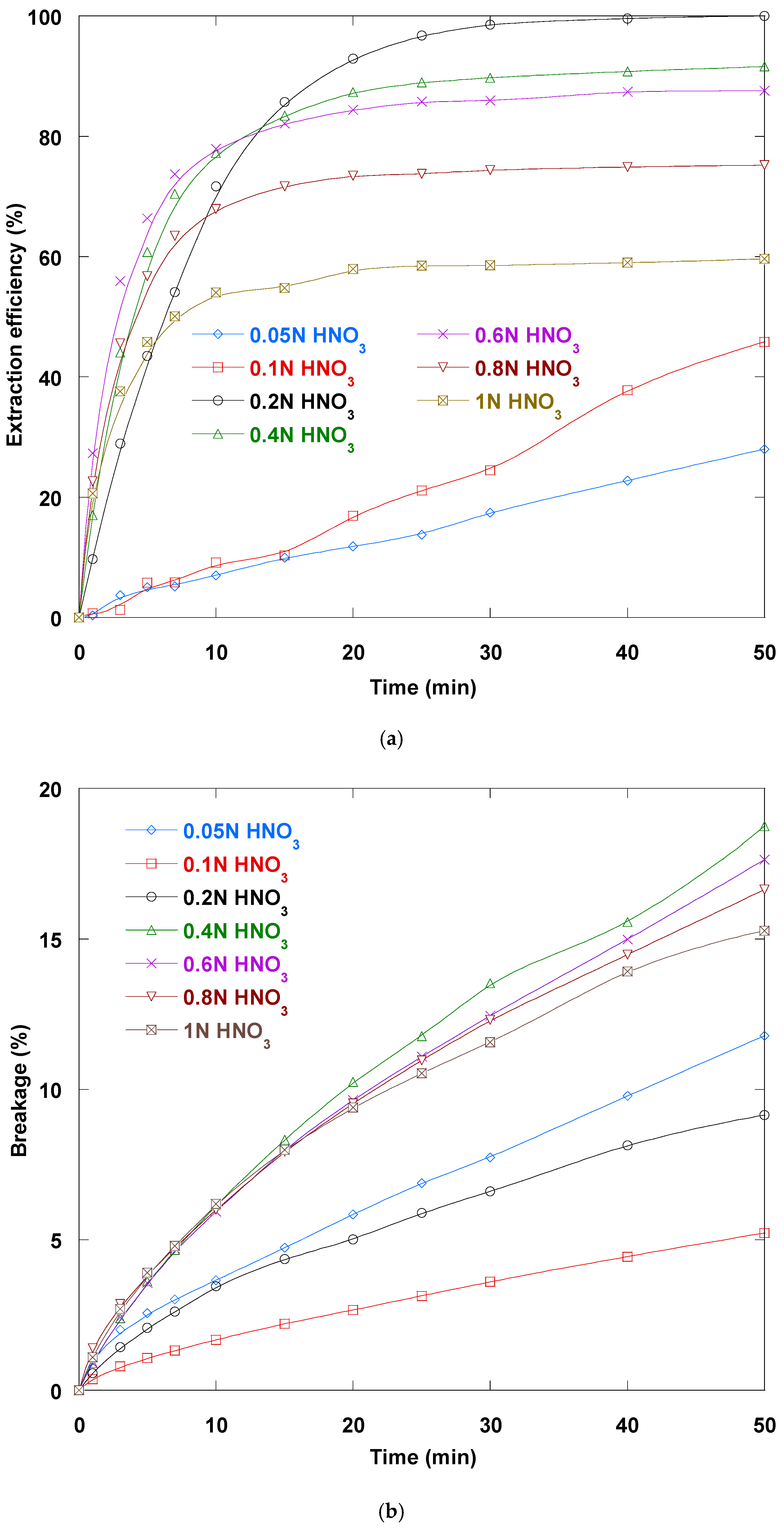
3.5. Strippant (HNO3) Concentration Impact
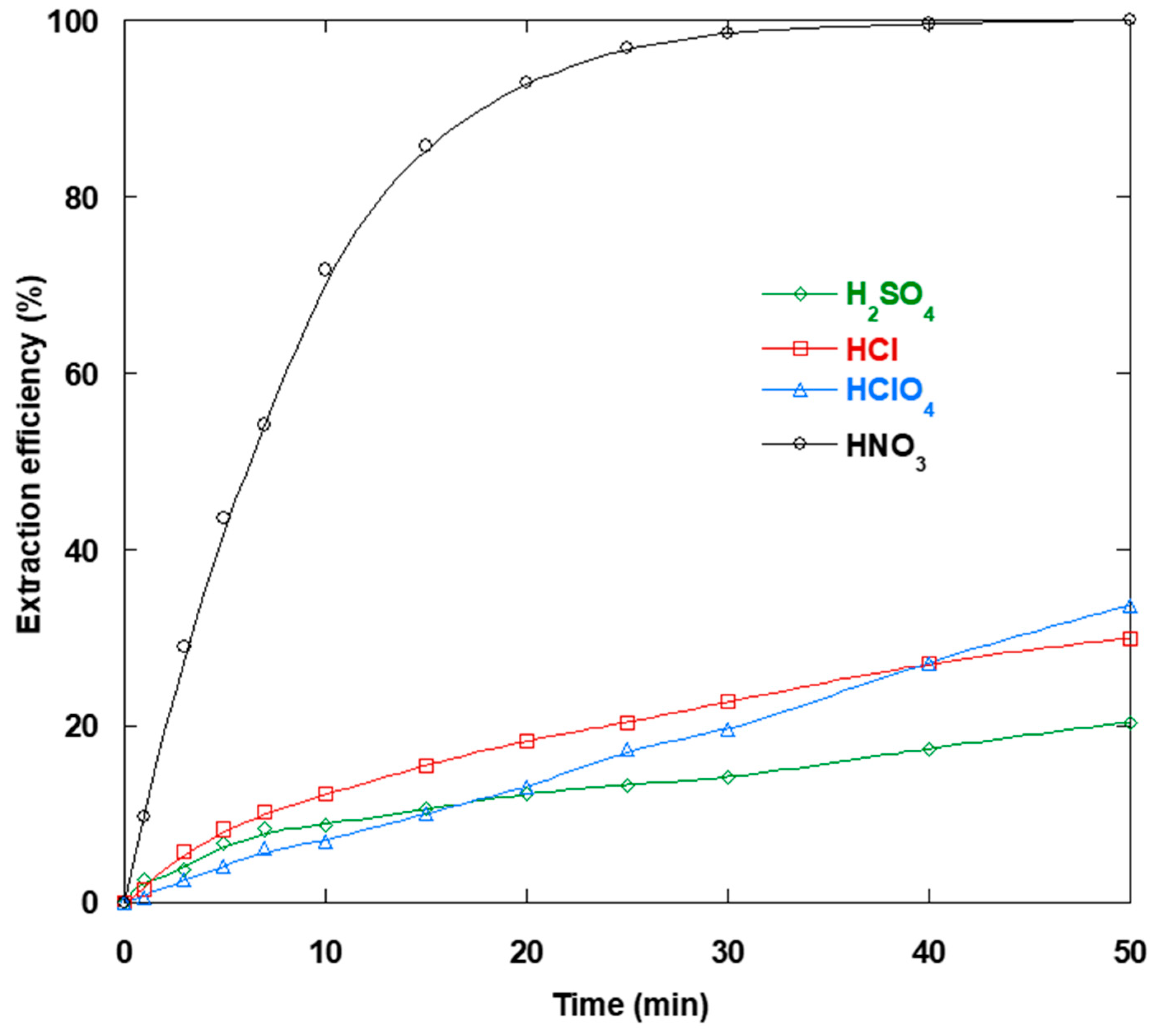
3.6. Strippant Type Impact
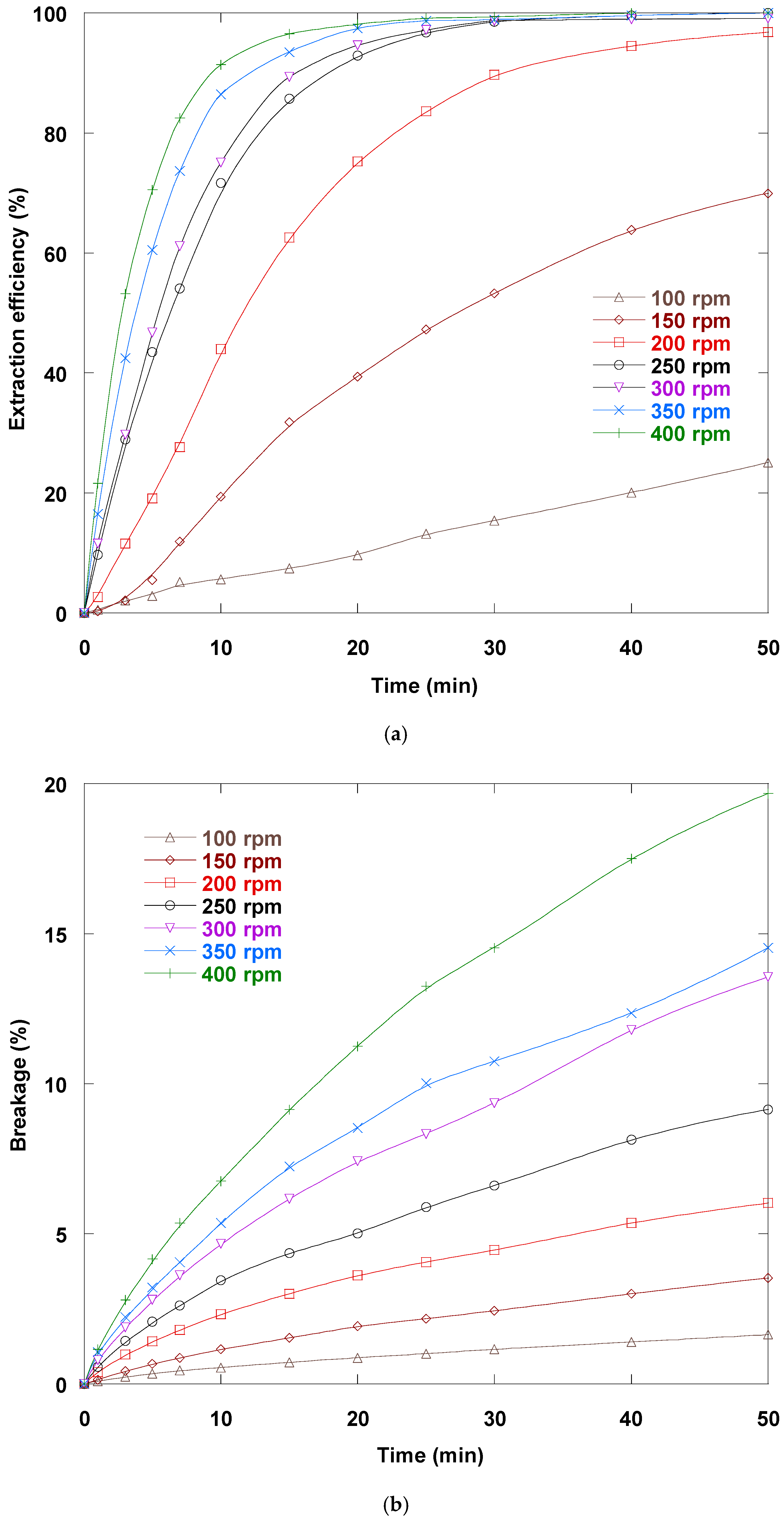
3.7. Mixing Rate Impact
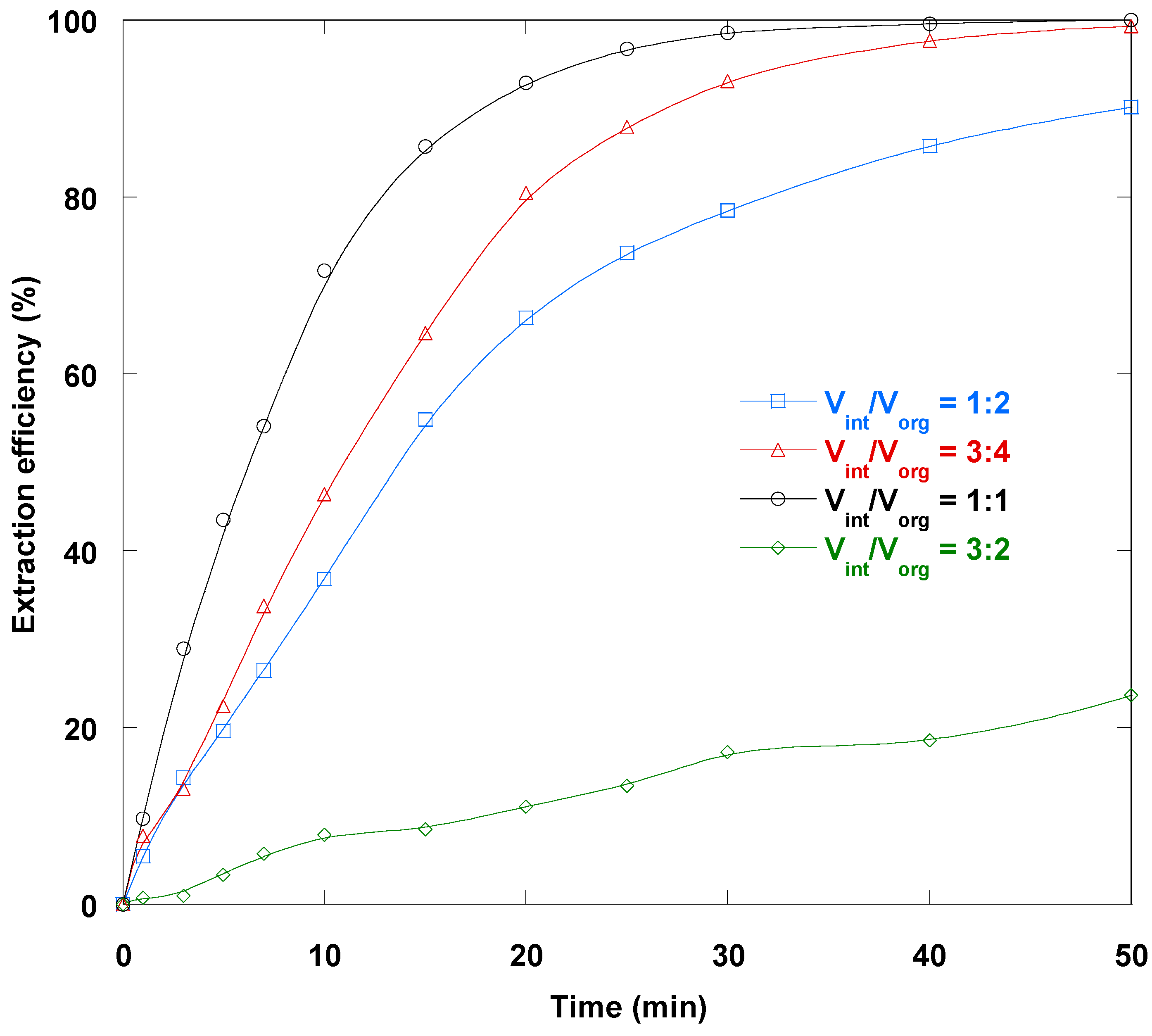
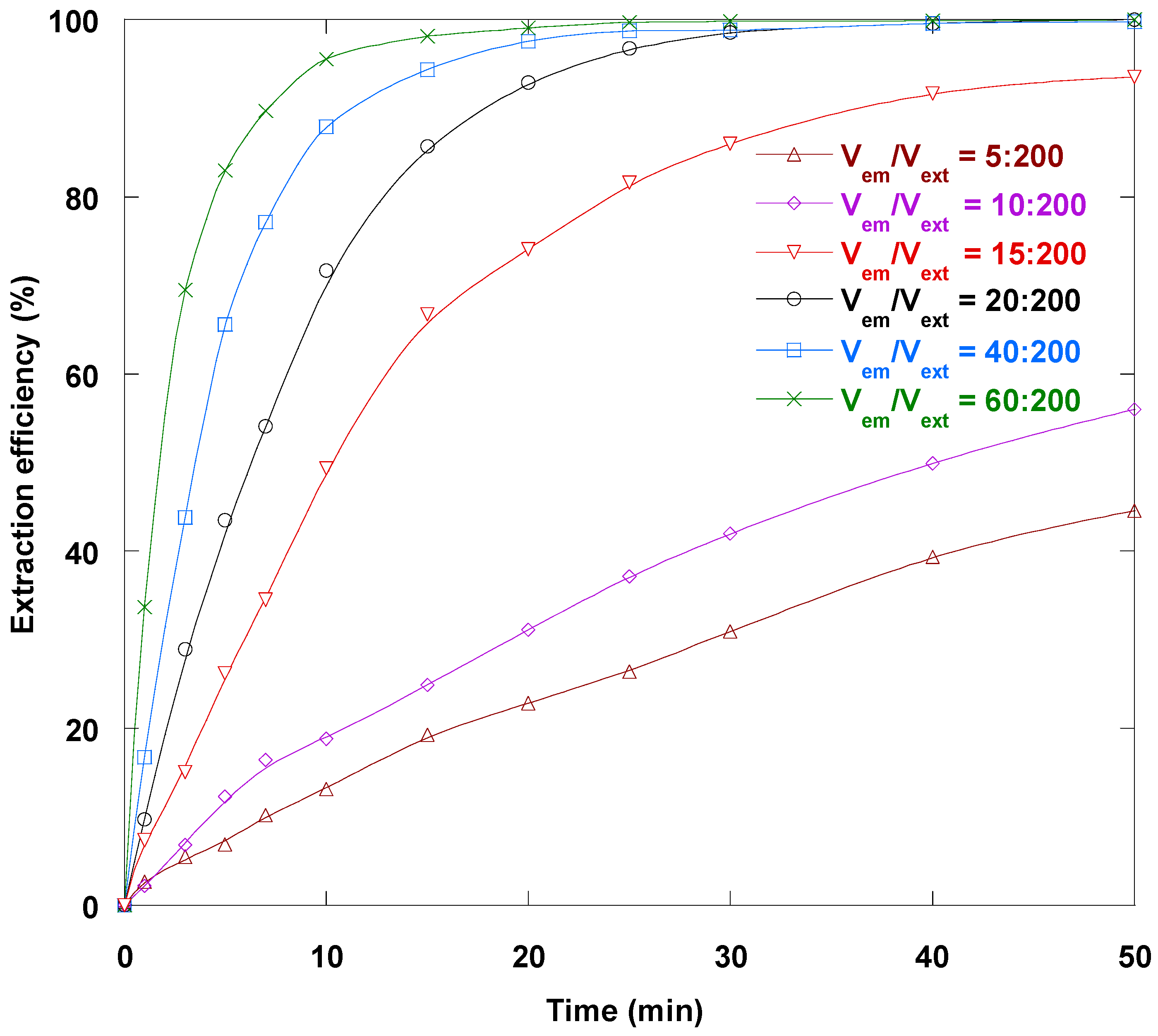
3.8. Internal/Membrane Phase Volume Ratio Impact
3.9. Treatment Ratio Impact
3.10. Diluent Type Impact
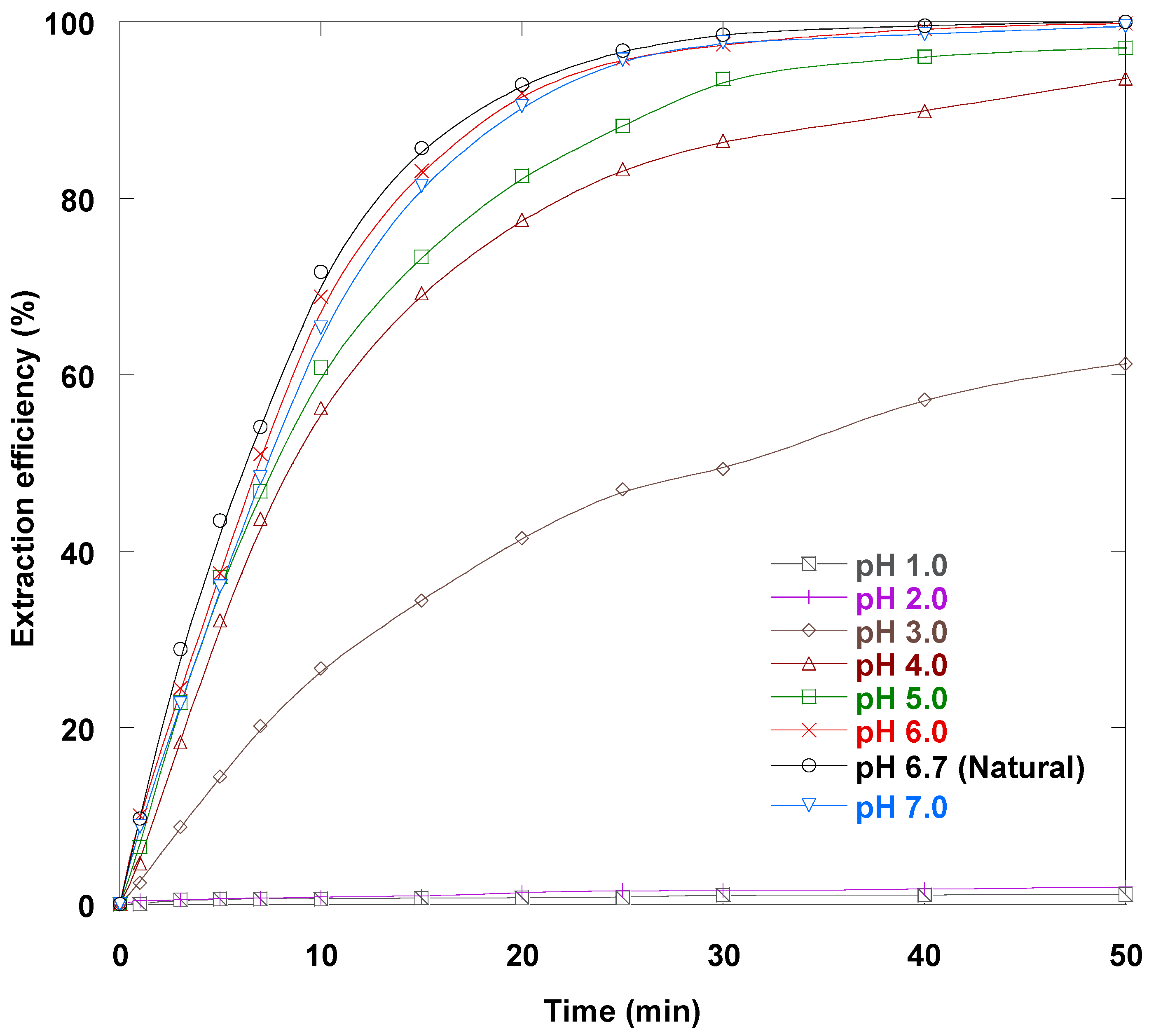
3.11. Feed Phase pH Impact
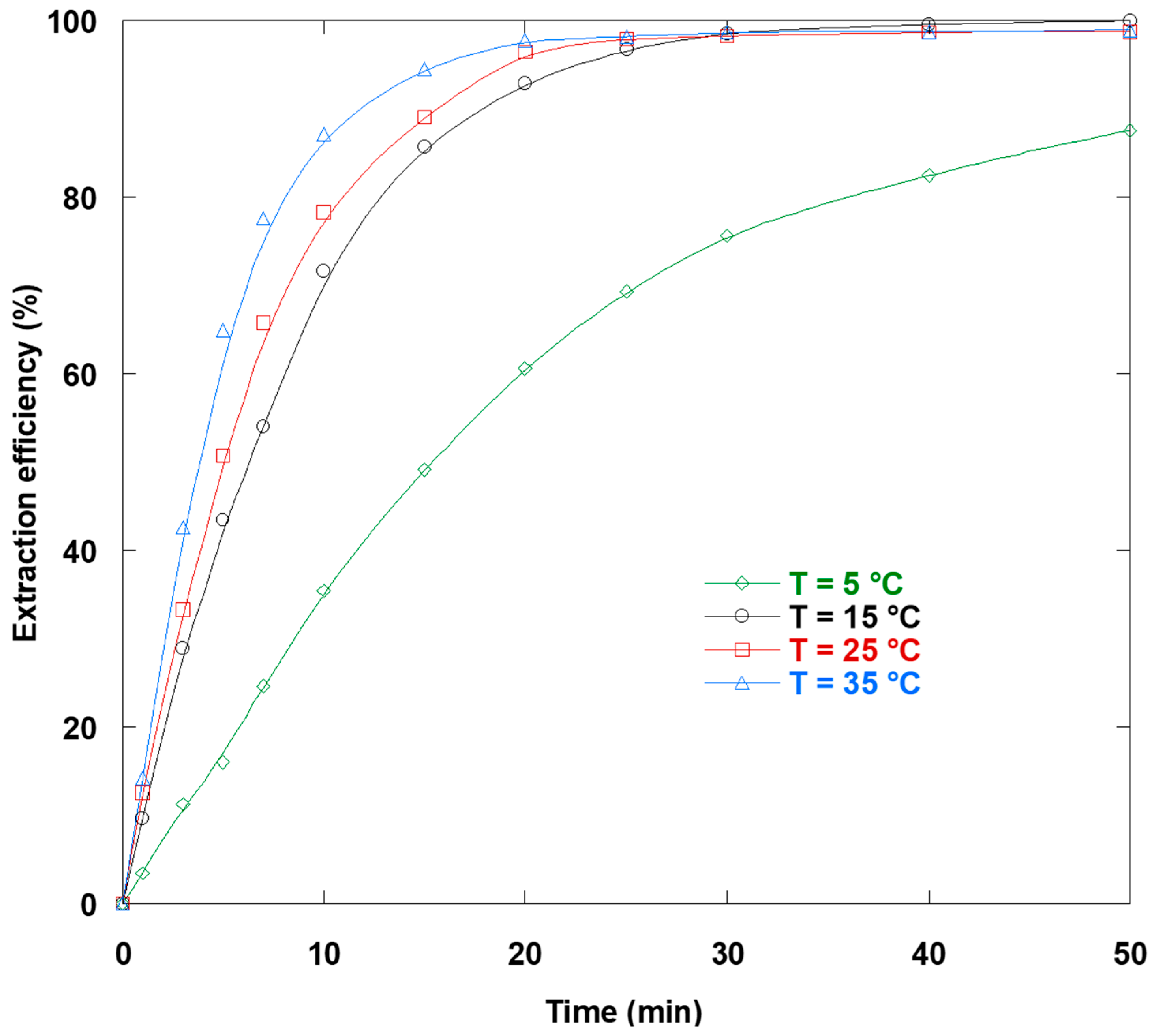
3.12. Temperature Impact
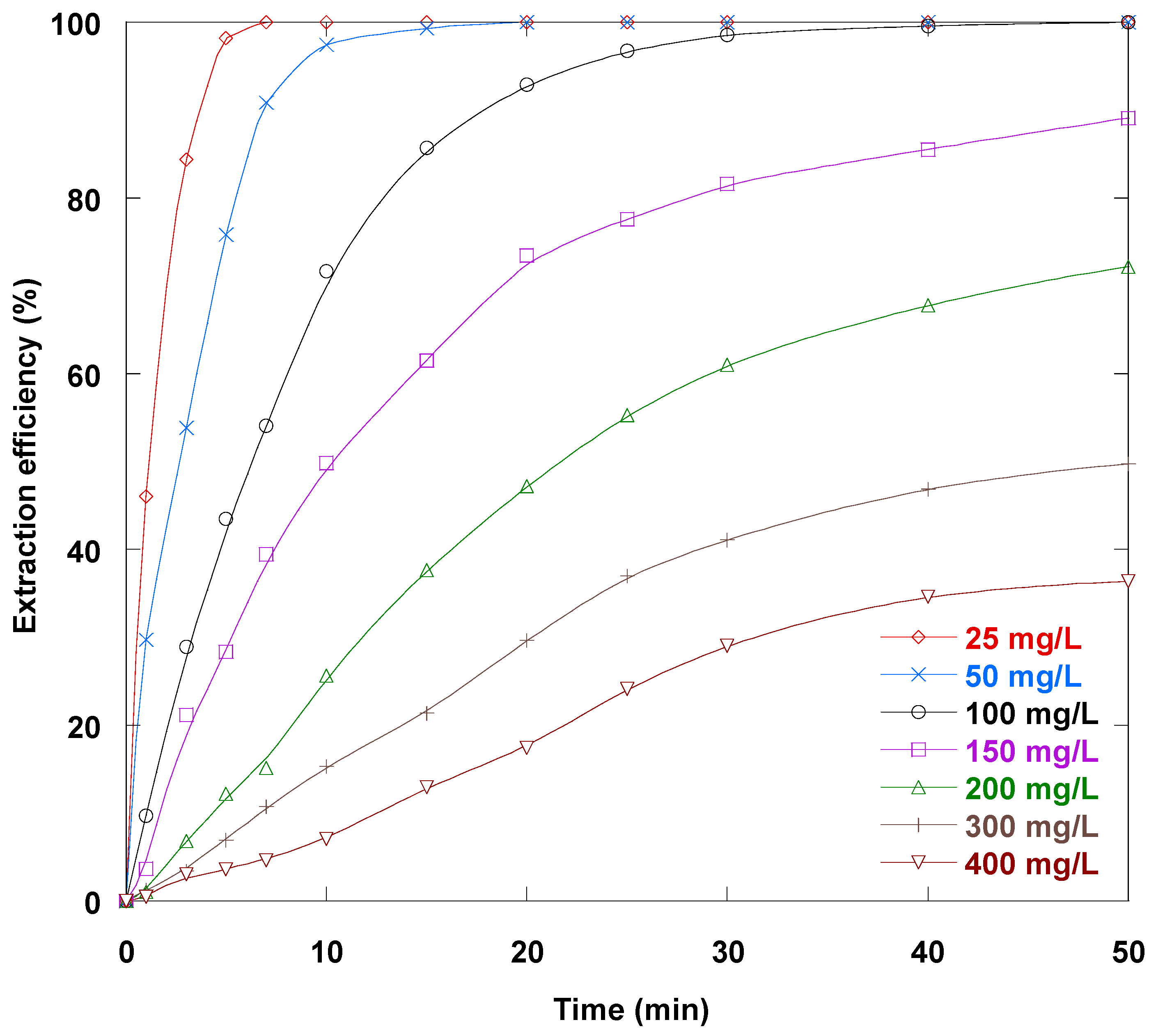
3.13. Sm(III) Initial Loading Impact
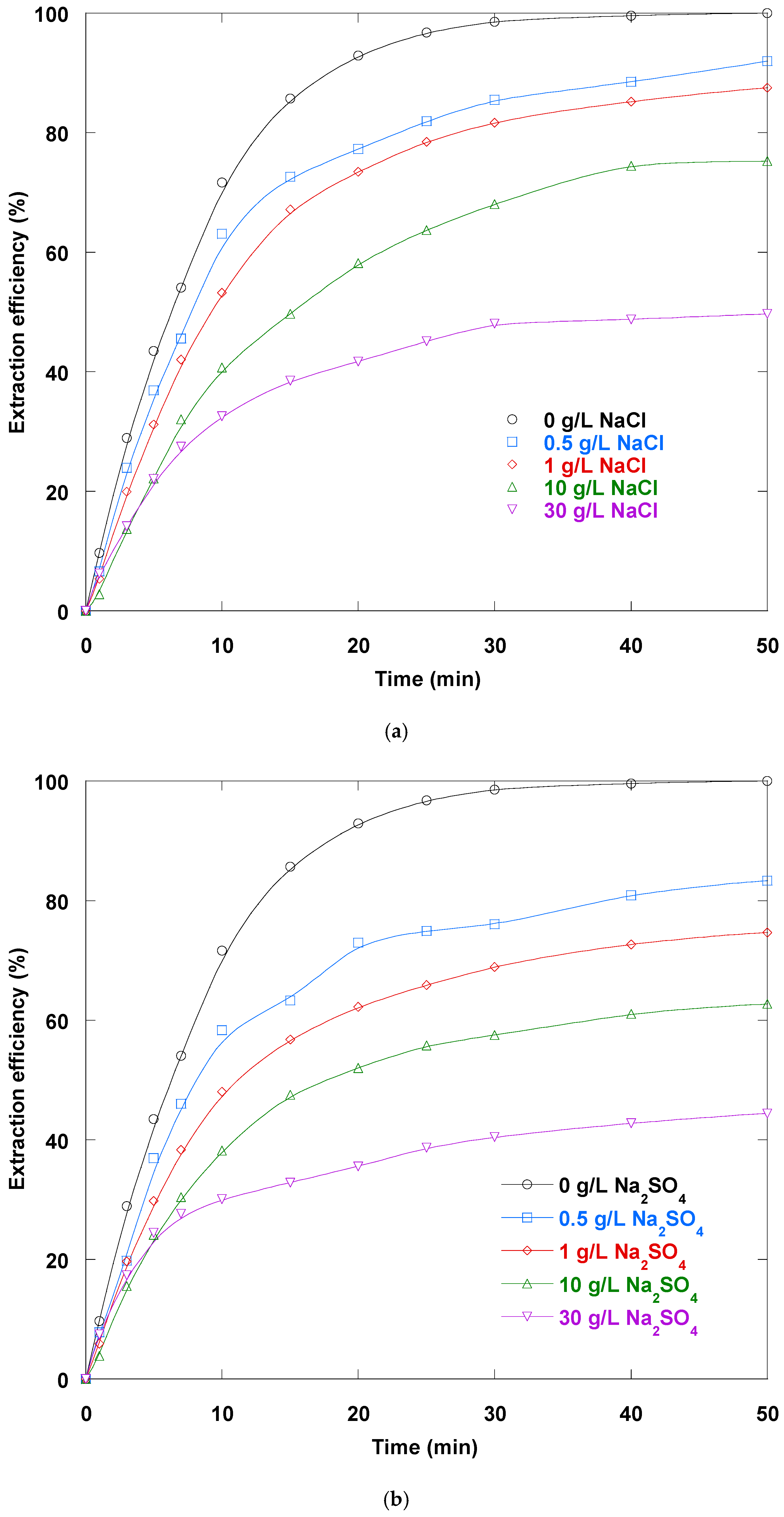
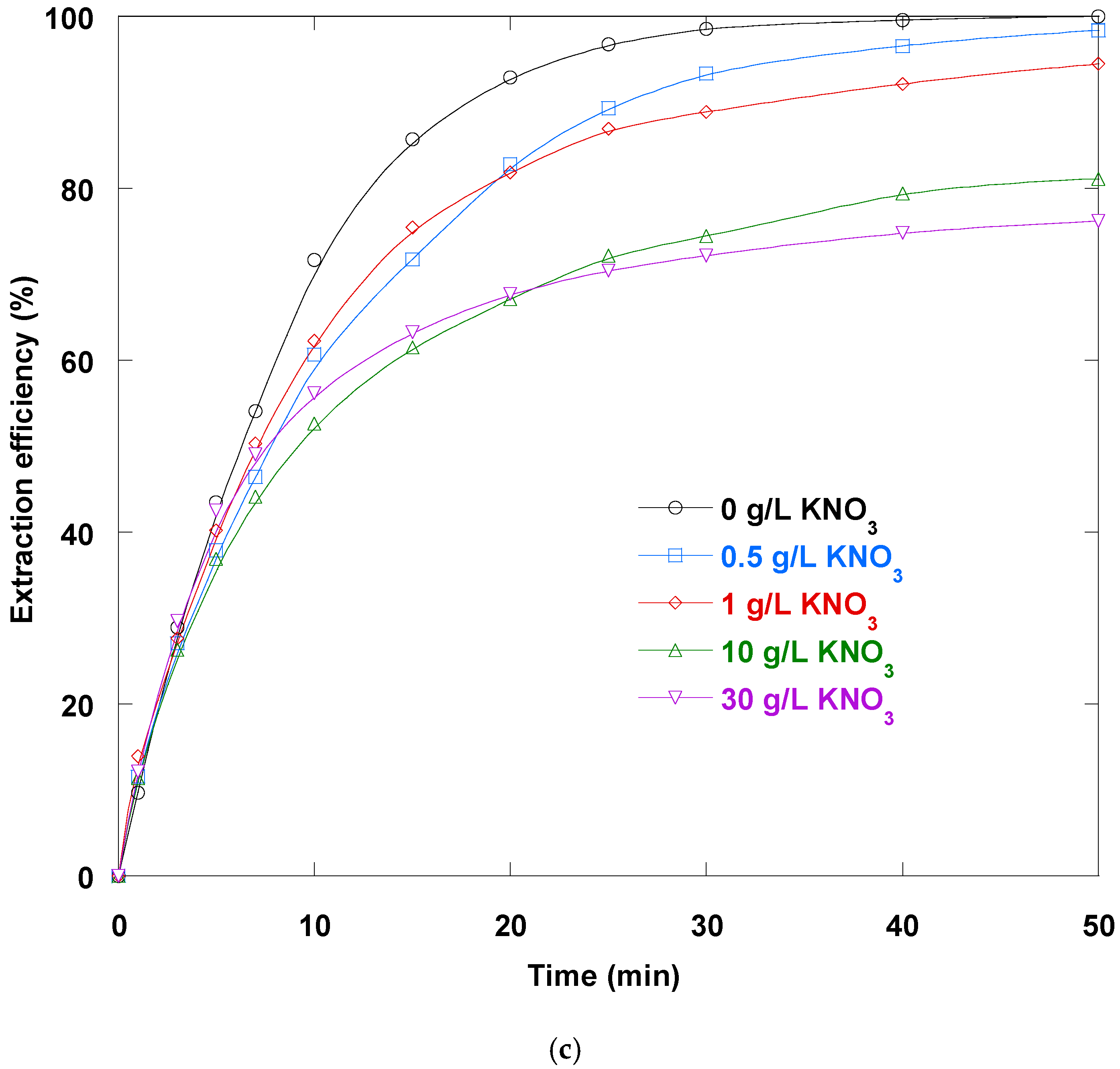
3.14. Salts Impact
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Gosen, B.S.; Verplanck, P.L.; Long, K.R.; Gambogi, J.; Seal, R.R., II. The Rare-Earth Elements: Vital to Modern Technologies and Lifestyles; USGS: Reston, VA, USA, 2014. [Google Scholar] [CrossRef]
- Rare Earth Elements–A Subset of Critical Minerals|Netl. Doe. Gov. Available online: https://www.netl.doe.gov/resource-sustainability/critical-minerals-and-materials/rare-earth-elements (accessed on 2 July 2025).
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Jha, A.R. Contribution of Rare Earth Materials in the Development of the Glass Industry, Crystal Technology, Glass Polishing, Electro-Optical Devices, and the Chemical Industry; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781138033870. [Google Scholar]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A Critical Review on Solvent Extraction of Rare Earths from Aqueous Solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Lobacheva, O.L.; Dzhevaga, N.V. The Experimental Study of Innovative Methods Regarding the Removal of Sm(III). Appl. Sci. 2021, 11, 7726. [Google Scholar] [CrossRef]
- Böddeker, K.W. Liquid Separations with Membranes. An Introduction to Barrier Interference; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540474517. [Google Scholar]
- Aguilar, M.; Cortina, J.L. Solvent Extraction and Liquid Membranes. Fundamentals and Applications in New Materials; CRC Press Taylor & Francis: New York, NY, USA, 2008; ISBN 978-0-8247-4015-3. [Google Scholar]
- Abdelkader, B.; Imene, B.; Lahouaria, A.; Boumediene, H.; Mostefa, K.; Ulrich, M. Emulsion Liquid Membrane Technique for Optimal Separation of Ni (II) and Sm (III) Using Response Surface Methodology and Box–Behnken Experimental Setup. Environ. Technol. 2025, 46, 1348–1368. [Google Scholar] [CrossRef]
- Larochelle, T.; Noble, A.; Strickland, K.; Ahn, A.; Ziemkiewicz, P.; Constant, J.; Hoffman, D.; Glascock, C. Recovery of Rare Earth Element from Acid Mine Drainage Using Organo-Phosphorus Extractants and Ionic Liquids. Minerals 2022, 12, 1337. [Google Scholar] [CrossRef]
- Dzulqornain, A.M.; Lee, J.C.; Yoon, H.; Kim, R.; Chung, K.W. Investigation on the Interaction Between Acidic Organophosphorous Extractants and Tri-N-Octylamine (TOA) for the Extraction of Rare Earths in HCl System. In 154th Annual Meeting & Exhibition Supplemental Proceedings (TMS 2025); Springer: Cham, Switzerland,; pp. 1130–1138. [CrossRef]
- Fujita, Y.; McCall, S.K.; Ginosar, D. Recycling Rare Earths: Perspectives and Recent Advances. MRS Bull. 2022, 47, 283–288. [Google Scholar] [CrossRef]
- Naeem, A.T.; Kashi, E.; Salehi, M.A.; Habibpour, R. Extraction and Separation of La (III), Pr (III) and Nd (III) Using Binary Mixture of D2EHPA with Cyanex 272, TOPO, and TBP Extractants. Metall. Res. Technol. 2018, 115, 612. [Google Scholar] [CrossRef]
- Essakhraoui, M.; Boukhair, A.; Bentiss, F.; Mazouz, H.; Beniazza, R.; Haneklaus, N. Advances in Heavy Metal Extraction Using Organophosphorus Compounds: A Comprehensive Review. Metals 2025, 15, 524. [Google Scholar] [CrossRef]
- Batchu, N.K.; Li, Z.; Verbelen, B.; Binnemans, K. Structural Effects of Neutral Organophosphorus Extractants on Solvent Extraction of Rare-Earth Elements from Aqueous and Non-Aqueous Nitrate Solutions. Sep. Purif. Technol. 2021, 255, 117711. [Google Scholar] [CrossRef]
- Babayan, I.I.; Kurdakova, S.V.; Kovalenko, N.A.; Uspenskaya, I.A. Bulk Properties of Di(2-Ethylhexyl)Phosphoric Acid–Samarium (Europium, Gadolinium) Di(2-Ethylhexyl)Phosphate–Organic Solvent Solutions. Russ. J. Phys. Chem. A 2022, 96, 84–92. [Google Scholar] [CrossRef]
- Kozhevnikova, A.V.; Milevskii, N.A.; Lobovich, D.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Deep Eutectic Solvent (TOPO/D2EHPA/Menthol) for Extracting Metals from Synthetic Hydrochloric Acid Leachates of NMC-LTO Batteries. Metals 2024, 14, 1441. [Google Scholar] [CrossRef]
- Medjahed, B.; Didi, M.A. Spectroscopic Study of Complexes from UO2(II), Th(IV) and Sm(III) Based on the Effects of the Characteristic TBP, TOPO and D2EHPA Bands in Various Organic Solvents. J. Radioanal. Nucl. Chem. 2018, 318, 1427–1438. [Google Scholar] [CrossRef]
- Wongsawa, T.; Koonsang, T.; Kunthakudee, N.; Prapasawat, T.; Maneeintr, K.; Pancharoen, U. The Experimental Investigations on Viscosity, Surface Tension, Interfacial Tension and Solubility of the Binary and Ternary Systems for Tributyl Phosphate (TBP) Extractant in Various Organic Solvents with Water: Thermodynamic NRTL Model and Molecular Interaction Approach. J. Mol. Liq. 2018, 251, 229–237. [Google Scholar] [CrossRef]
- Chaouchi, S.; Hamdaoui, O. Extraction of Endocrine Disrupting Compound Propylparaben from Water by Emulsion Liquid Membrane Using Trioctylphosphine Oxide as Carrier. J. Ind. Eng. Chem. 2015, 22, 296–305. [Google Scholar] [CrossRef]
- Schroën, K.; de Ruiter, J.; Berton-Carabin, C. The Importance of Interfacial Tension in Emulsification: Connecting Scaling Relations Used in Large Scale Preparation with Microfluidic Measurement Methods. ChemEngineering 2020, 4, 63. [Google Scholar] [CrossRef]
- Chen, Y.; Narayan, S.; Dutcher, C.S. Phase-Dependent Surfactant Transport on the Microscale: Interfacial Tension and Droplet Coalescence. Langmuir 2020, 36, 14904–14923. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, J.; Li, S.; Chen, Z.; Wang, Y. Mass Transfer and Droplet Behaviors in Liquid-Liquid Extraction Process Based on Multi-Scale Perspective: A Review. Separations 2023, 10, 264. [Google Scholar] [CrossRef]
- Taamallah, A.; Hamdaoui, O.; Kerboua, K.; Alghyamah, A. Extraction of Cerium(III) Ions from Dilute Aqueous Solutions by Emulsion Liquid Membrane: Effects of Operating Conditions, Salts and Natural Water Matrices. Desalination Water Treat. 2021, 238, 218–230. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Götz, V.; Peukert, W. Effect of Surfactants on the Molecular Structure of the Buried Oil/Water Interface. Angew. Chem. Int. Ed. 2021, 60, 25143–25150. [Google Scholar] [CrossRef]
- Karai, O.; Selem, N.Y.; Benabderazak, K.; Mendil, J.; Mazouz, H.; Al-Dahhan, M.H. Emulsion Liquid Membrane (ELM) Technique for Efficient Separation of Heavy Metals from Acidic Solutions Including Phosphoric Acid: A Review. Int. J. Environ. Sci. Technol. 2025, 22, 9743–9766. [Google Scholar] [CrossRef]
- Ash, T.; Han, Y.; Evans, J.W.; Windus, T.L. DFT Investigation of the Impact of Inner-Sphere Water Molecules on RE Nitrate Binding to Internal Pore and External Surface of MCM-22. Phys. Chem. Chem. Phys. 2025, 27, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Liquids-Dynamic Viscosities. Available online: https://www.engineeringtoolbox.com/absolute-viscosity-liquids-d_1259.html (accessed on 3 July 2025).
- Liquids-Dielectric Constants. Available online: https://www.engineeringtoolbox.com/liquid-dielectric-constants-d_1263.html (accessed on 3 July 2025).
- Xylene|1330-20-7. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0130912.htm (accessed on 3 July 2025).
- Trichloroethylene|79-01-6. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5406573.htm (accessed on 3 July 2025).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taamallah, A.; Hamdaoui, O. Synergistic Extraction of Samarium(III) from Water via Emulsion Liquid Membrane Using a Low-Concentration D2EHPA–TOPO System: Operational Parameters and Salt Effects. Separations 2025, 12, 233. https://doi.org/10.3390/separations12090233
Taamallah A, Hamdaoui O. Synergistic Extraction of Samarium(III) from Water via Emulsion Liquid Membrane Using a Low-Concentration D2EHPA–TOPO System: Operational Parameters and Salt Effects. Separations. 2025; 12(9):233. https://doi.org/10.3390/separations12090233
Chicago/Turabian StyleTaamallah, Ahlem, and Oualid Hamdaoui. 2025. "Synergistic Extraction of Samarium(III) from Water via Emulsion Liquid Membrane Using a Low-Concentration D2EHPA–TOPO System: Operational Parameters and Salt Effects" Separations 12, no. 9: 233. https://doi.org/10.3390/separations12090233
APA StyleTaamallah, A., & Hamdaoui, O. (2025). Synergistic Extraction of Samarium(III) from Water via Emulsion Liquid Membrane Using a Low-Concentration D2EHPA–TOPO System: Operational Parameters and Salt Effects. Separations, 12(9), 233. https://doi.org/10.3390/separations12090233






