Abstract
The current research aims to develop a simple, sensitive, and green analytical method for the group extraction/monitoring of 19 organochlorine and organophosphorus pesticides from fruit juices using microwave radiation to assist a cloud point extraction (MW-CPE) in combination with re-extraction in hexane and GC-MS/MS detection. The main experimental factors affecting the CPE and re-extraction have been optimized. The matrix-matched calibration was performed, and the limit of quantification (LOQ) for all studied pesticides at optimized conditions ranged between 5 and 47 ng L−1. When applying only 0.25 mL of hexane for re-extraction, the proposed method shows good accuracy and precision. The “greenness” of the developed MW-CPE-GC-MS/MS method was assessed using the AGREE prep software. The method has been successfully implemented in pesticide analysis in commercially available fruit juices (lemon concentrate and red apple juice). The recovery values obtained for most analytes were within the range of 71% and 114% and RSD below 20% (exept Heptahlor, Aldrin, o,p-DDD, p,p-DDD and o,p-DDT, p,p-DDT). The developed method combines a preconcentration with a sample clean-up step due to the extraction of the pigments into the non-polar micelles during the extraction step, and deposition in the intermediate layer of MgSO4 during the re-extraction step.
1. Introduction
Pesticides include a vast range of natural (plant-based) or synthetic compounds used to prevent or eliminate insects, weeds, and pests. Some inorganic compounds are also employed as pesticides, but most modern pesticides are organic chemicals [1,2,3]. Overall, pesticides benefit farmers and people worldwide by increasing agricultural productivity, reducing losses from pests, diseases, and weeds, and indirectly benefiting society in numerous ways. In public health, pesticides are used to control disease vectors, thereby helping prevent illnesses such as malaria, etc. Despite its advantages, uncontrolled pesticide use has resulted in various forms of pollution and food contamination. Pesticide residues can spread beyond application sites through evaporation and airborne movement, as well as soil leaching, erosion, and water runoff. Pesticide drift poses risks to human health, affects nearby crops, contaminates conservation areas, soil, water, grass, and other vegetation, and harms non-target organisms [2,4]. It often leads to indiscriminate damage to aquatic and terrestrial food webs, ecosystems, and the biodiversity of non-target plants and animals, including humans [5]. Numerous health problems, including various cancers, diabetes mellitus, respiratory illnesses, neurological disorders, neurotoxicity, reproductive syndromes, leukemia, and oxidative stress, can arise from direct exposure to pesticides and pesticide residues in food products [1].
There are many classifications (chemical nature, toxicity, target pests, and various combined classifications) [3], but the most common and practical method of categorizing pesticides is by their chemical composition and the nature of active ingredients. Among the various pesticide classes, organochlorine pesticides (OCPs) and organophosphorus pesticides (OPPs) are particularly notable due to their toxicity, tendency to bioaccumulate, and potential endocrine-disrupting effects [6,7,8,9]. Although some OCPs have been banned or restricted, due to their persistent nature, residues still appear [10,11,12]. Meanwhile, some OPPs are still frequently used in current farming practices and pose risks of both acute and chronic health effects, particularly on the nervous system [8,9]. The hazards to the environment and human health connected with the widespread use of pesticides in modern agriculture have raised questions regarding the safety of these chemicals and serious concerns about their persistence in food products.
Fruit juices are staples in the human diet due to their high nutritional value and beneficial health properties. They are considered a healthy food for both adults and children because their production involves fruits containing many vitamins, minerals, organic acids, sugars, and other nutrients. However, they can also be a source of pesticide contamination in juice, as pesticides can penetrate through apple skins and remain in fresh fruit or juice [13]. Since juices are widely consumed, effective methods for detecting pesticide residues are crucial. To ensure food safety, many countries enforce strict residue monitoring standards. The maximum residue limits (MRL) for pesticide residues and/or their degradation products in products of plant or animal origin are set in Regulation (EC) No 396/2005 [14] and the Codex Alimentarius (Food and Agriculture Organisation of the United Nations and World Health Organisation) [15]. Apple and lemon juices are complex food matrices rich in organic acids, sugars, and aromatic compounds, all of which can interfere with analytical detection [16]. Accurate and sensitive quantification of trace-level pesticide residues in such matrices requires an efficient sample preparation method combined with a robust analytical technique. Traditional extraction techniques—such as liquid–liquid extraction or solid-phase extraction—often require large volumes of organic solvents, lengthy procedures, or have limited selectivity [17,18]. In contrast, cloud point extraction (CPE) has emerged as an environmentally friendly and efficient alternative [19,20,21,22,23,24,25,26]. Based on the phase separation of non-ionic surfactants at specific temperatures (the cloud point), CPE provides high enrichment factors while reducing solvent consumption. This makes it particularly suitable for food safety applications where minimizing environmental impact and maximizing analyte recovery are both crucial. Due to its high sensitivity, selectivity, and ability to handle volatile and semi-volatile compounds, gas chromatography–mass spectrometry (GC-MS) stands out as a preferred technique for pesticide residue detection and quantification [16]. However, only a limited number of studies have explored the synergy between CPE and GC-MS for the simultaneous determination of multiple pesticide classes in complex juice matrices [27,28,29,30,31,32,33,34,35,36,37,38]. This study is based on a previous investigation into the compatibility of cloud point extraction combined with re-extraction in organic solvents and GC-MS/MS detection without a clean-up step prior to injection [38]. The aim is to develop and optimize a CPE-based method for the extraction and preconcentration of selected organochlorine and organophosphorus pesticides from apple and lemon juices, followed by GC-MS analysis. The results are expected to provide a sensitive, reproducible, and environmentally conscious approach suitable for routine food safety monitoring.
2. Materials and Methods
2.1. Chemicals and Reagents
The studied pesticides, o,p-DDE, p,p-DDE, o,p-DDT, p,p-DDT, o,p-DDD, p,p-DDD, alpha-HCH, beta-HCH, gamma-HCH, alpha-Endosulfan, Aldrin, Dieldrin, Endrin, Heptachlor, Heptachlor-endo-epoxide-A, Hexachlorobenzene, Pentachlorobenzene, Chlorpyrifos, Chlorpyrifos-methyl (purity ≥ 95%), were supplied from Dr. Ehrenrshtorfer GmbH (Augsburg, Germany). Ultrapure water (0.056 µS cm−1) was obtained from a Chorus 1 Complete Ultrapure Water System, ELGA, PURELAB® (High Wycombe, UK). Stock solutions were prepared in acetonitrile at a concentration of 1000 mg L−1, and dilutions were carried out in hexane. All stock solutions were stored at 4 °C. Acetonitrile LC-MS grade was obtained from Honeywell (Charlotte, NC, USA), and hexane and Triton X-100 were purchased from Sigma–Aldrich (Taufkirchen, Germany).
2.2. Instrumentation, Apparatus and Software
The study was performed by Trace 1300 gas chromatograph (GC) equipped with a PTV injector using a glass liner (PTV Liner with Three Baffles, 1 mm ID, 2.75 mm OD, 120 mm Length, Thermo Fisher Scientific, Waltham, MA, USA), combined with TSQ 9000 mass spectrometer (MS/MS) (Thermo Scientific, Waltham, MA, USA). TG SQC MS column (15 m × 0.25 mm × 0.25 µm, Thermo Fisher Scientific, Waltham, MA, USA) was used for chromatographic separations. Helium (99.999%) was used as a carrier gas, and Argon (99.996%) as a collision gas.
The pesticide analysis was performed under the following conditions: flow rate 1.2 mL min−1, injection volume 1 µL, split ratio 5:1, PTV gradient from 65 °C at 14.5 °C s−1 to 260 °C. The temperature of the column oven was held at 120 °C for 1 min, rated with 40 °C min−1 to 155 °C, 4 °C min−1 to 187 °C, 1 °C min−1 to 194 °C, and 12 °C min−1 to 260 °C, held for 5 min. The solvent cut time was 5 min, and the analysis was 28 min. The transfer line and the EI temperatures were 250 °C and 230 °C, respectively. The quantitative analysis was performed in selected reaction monitoring (SRM) mode [38,39]. It should be noted that beta-HCH and gamma-HCH, p,p-DDD and o,p-DDT were analyzed as a sum of their signals because of coelution and the identical SRM transitions.
The limits of detection (LOD) and limits of quantification (LOQ) were calculated using standard deviations of the weighted regression line intercepts (weighting factor 1 c−2), based on the 3 s and 10 s criteria [38].
2.3. Other Equipment
As a source of energy input, a microwave system MDS-81D (CEM Corp., Matthews, NC, USA), with a maximum power of 600 W, was used for conducting CPE. The heating program applies a maximum power for 4 min, followed by six heating cycles of 5 min each, with 1 min breaks between (Table 1).

Table 1.
Working conditions for microwave heating in MW-CPE-GC-MS/MS.
The following additional equipment was used: Lab Dancer vortex (IKA, Staufen im Breisgau, Germany), KA-1000 centrifuge (Jiangsu Zhengji Instruments Co., Ltd., Jintan, China), Kerry US ultrasonic bath, and a hot plate. Glass conical tubes with a volume of 12 mL and polypropylene caps were used for sample preparation. The software Statistica® version 14.1 (TIBCO, Santa Clara, CA, USA) was used for the optimization study and the visualization of the results. The evaluation of the greenness metric of the developed method was applied using AGREEprep software: https://git.pg.edu.pl/p174235/agreeprep, accessed on 15 July 2025 [40].
2.4. Microwave-Assisted Cloud Point Extraction in Combination with Re-Extraction in an Organic Solvent for the Pesticide Analysis in Fruit Juices
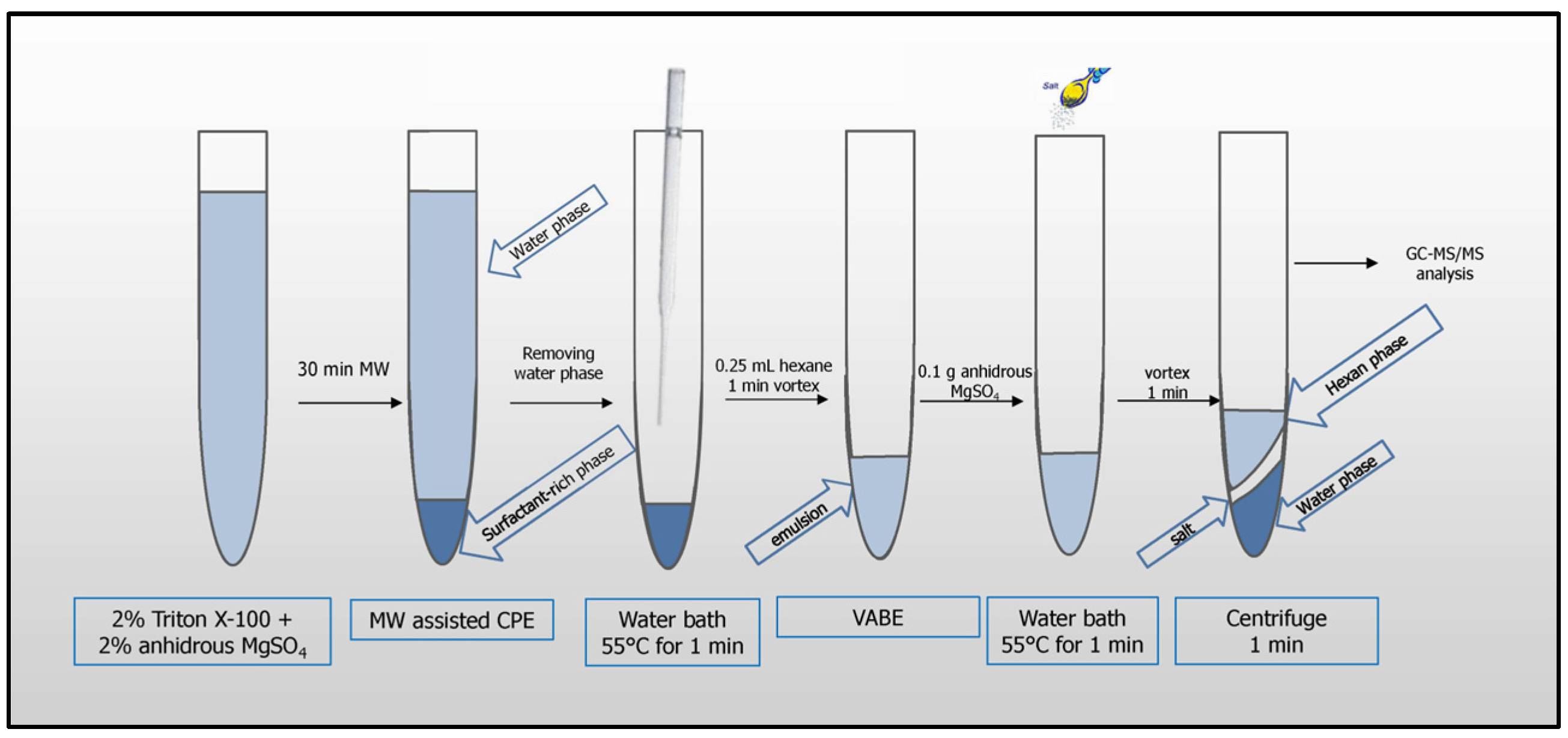
The following procedure was optimized and applied for pesticide analysis: 8 mL of apple juice or 2 mL of lemon juice diluted with 6 mL of water were pipetted into a 12 mL glass tube. 2 mL of 10% (m m−1) aqueous solution of Triton X-100 was added to the samples (0.2 g of MgSO4 was also added to the diluted lemon juice). The extraction systems were placed in a water bath and heated in a microwave system (Section 2.3) above the cloud point temperature for 30 min until two phases were formed: a surfactant-rich phase (lower layer) and an aqueous phase (upper layer). The tubes were left at room temperature, 24 °C ± 2 °C, to cool down (~10 min) and placed in a refrigerator (~30 min) to increase the viscosity of the surfactant-rich phase. The supernatant was removed with a Pasteur pipette. To reduce viscosity, the surfactant-rich phase was placed in a water bath at 55 °C for 1 min, then 0.25 mL of hexane was added, and re-extraction was performed by vortex agitation for 1 min. Then, 0.1 g of MgSO4 was added to the resulting emulsion, followed by subsequent heating in a water bath at 55 °C for 1 min, and vortex agitation for 1 min. The samples were centrifuged for 1 min at 900× g, and then the aliquot volume of 1 µL from the supernatant (hexane phase) was injected for analysis (Figure 1).
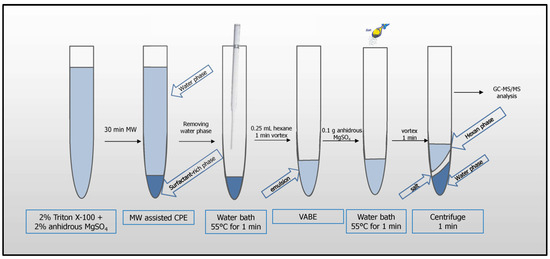
Figure 1.
MW-CPE-GC-MS/MS procedure for pesticide analysis.
3. Results and Discussion
The application of cloud point extraction (CPE) as an approach for preliminary separation and concentration of organochlorine and organophosphorus pesticides was investigated in our previous study [38]. As a result, Triton X-100 was selected as a surfactant to implement the CPE at a concentration of 2% (m m−1), and hexane was chosen as an organic solvent for re-extraction. It was found that the procedure is highly robust to the pH of the initial water sample (from 3 to 11), since it does not affect the extraction efficiency of most target pesticides. Considering the positive matrix effects evaluated, it was established that a matrix-matched calibration is the appropriate calibration approach. However, the initial procedure applied has significant disadvantages, including the use of a low-efficiency source of energy (hot plate) for the CPE, long re-extraction time (over 12 h), and relatively large volume (2 mL) of hexane for re-extraction.
In light of these conclusions, this study has focused on endeavors aimed at substituting or minimizing the volume of organic solvents used, as well as reducing the time required for sample preparation. The objective is to develop an innovative “green” analytical methodology for the preconcentration of organochlorine and organophosphorus pesticides extracted from apple and lemon juices.
3.1. Optimization of Cloud Point Extraction in Combination with Re-Extraction in Organic Solvent
A series of experiments was conducted in the following optimization study to enhance the characteristics of CPE and re-extraction, using both a one-factor-at-a-time approach and a factorial design (central composite design—CCD) [41,42,43]. Following the principles of green chemistry, the second approach allows a reduction in the number of experiments and the consumption of samples, reagents, and energy [44,45,46].
3.1.1. Optimization of the Extraction Step
- Microwave-assisted cloud point extraction (MW-CPE).
The feasibility of replacing the conventional hot plate with microwave irradiation was investigated to enhance the energy transfer efficiency to the extraction systems. The effect of microwave incubation time was examined through a one-factor experiment at 10, 20 and 30 min of microwave treatment. For this purpose, a microwave system equipped with a carousel, with six external vessels filled with ballast water (~80 mL), was positioned so that the glass tubes containing the model aqueous solutions (2% (m m−1) Triton X-100, pesticide concentration 50 µg L−1) were immersed in the water bath. A previously optimized microwave temperature program, described in Section 2.3, was applied for heating of the extraction systems. After the prescribed time, 2 mL of water was added to the surfactant-rich phase and re-extraction with 2 mL hexane was performed, followed by GC-MS/MS analysis. From the results in Table 2, it can be seen that for most analytes, the analytical yields obtained were above 80% at 30 min MW treatment. The exceptions were only found for alpha-HCH, beta and gamma-HCH, whose analytical yields ranged from 60 to 80%.

Table 2.
Analytical yields at 30 min MW incubation time with corresponding SD (%). Conditions: CPE—2% (m m−1) Triton X-100, initial pesticide concentration 50 µg L−1; re-extraction with 2 mL added water and 2 mL hexane, 10 min vortex agitation, 15 min centrifugation.
It was evident that the analytical yields obtained were comparable to or higher than those obtained with heating on a hot plate [38], but the energy consumption was reduced by more than quadruple (17 W per sample with MW-CPE versus 70 W per sample with conventional CPE). Additionally, the time reduction was also significant, as only 4 min were required to heat a 12 × 80 mL water bath compared to heating the water on a hot plate (30 min). For this reason, the subsequent CPE experiments were performed using microwave heating.
- Salting out effect
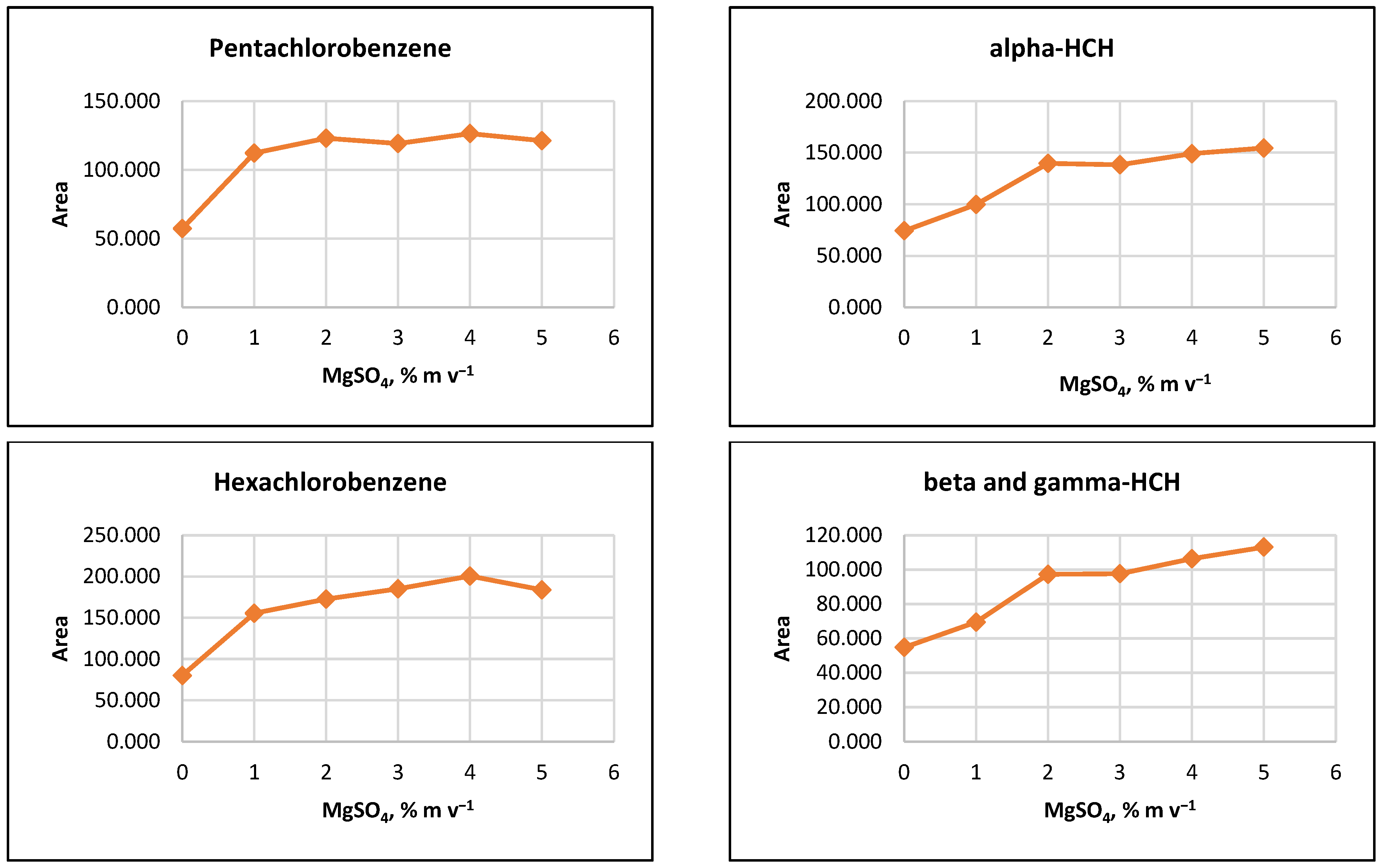
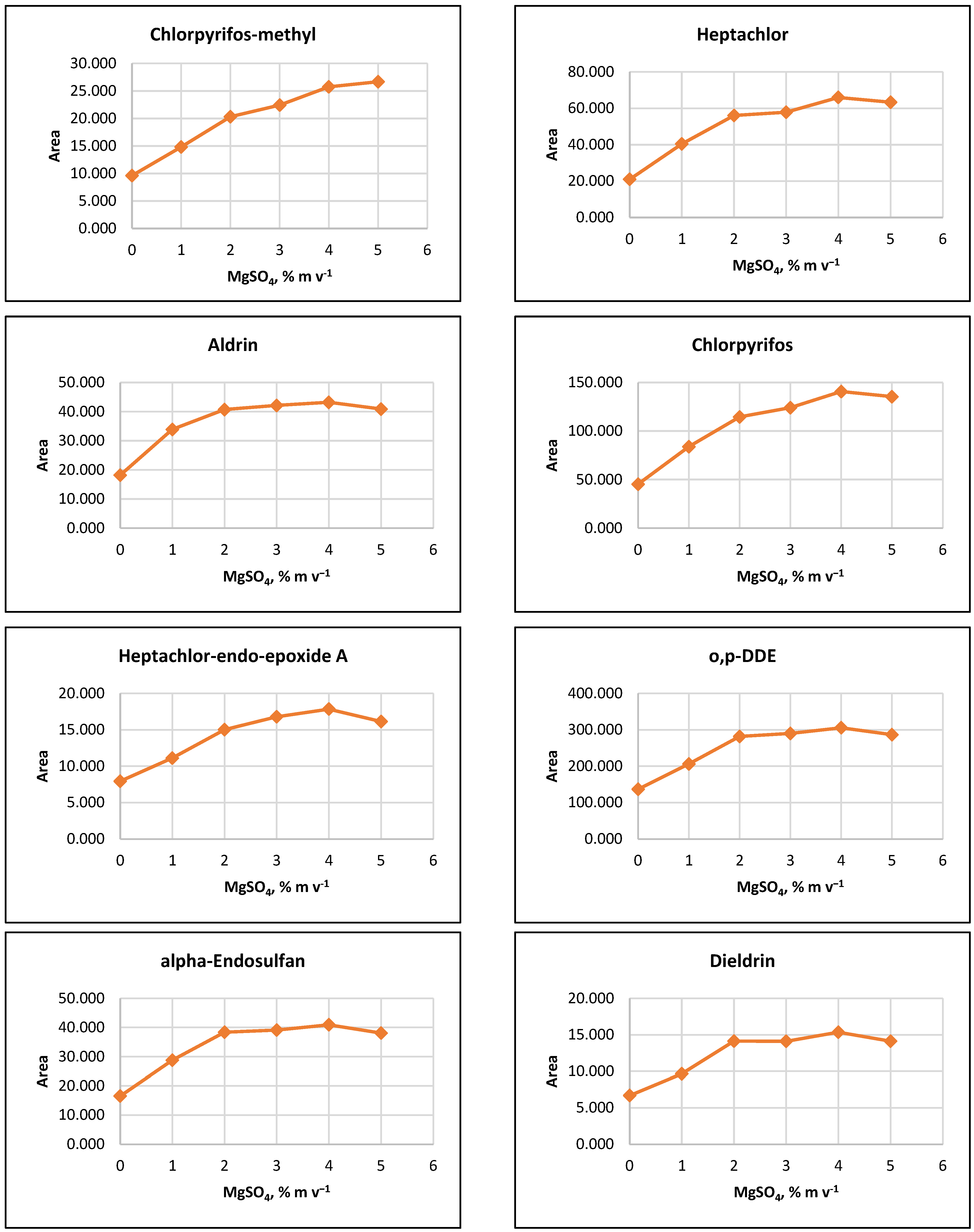
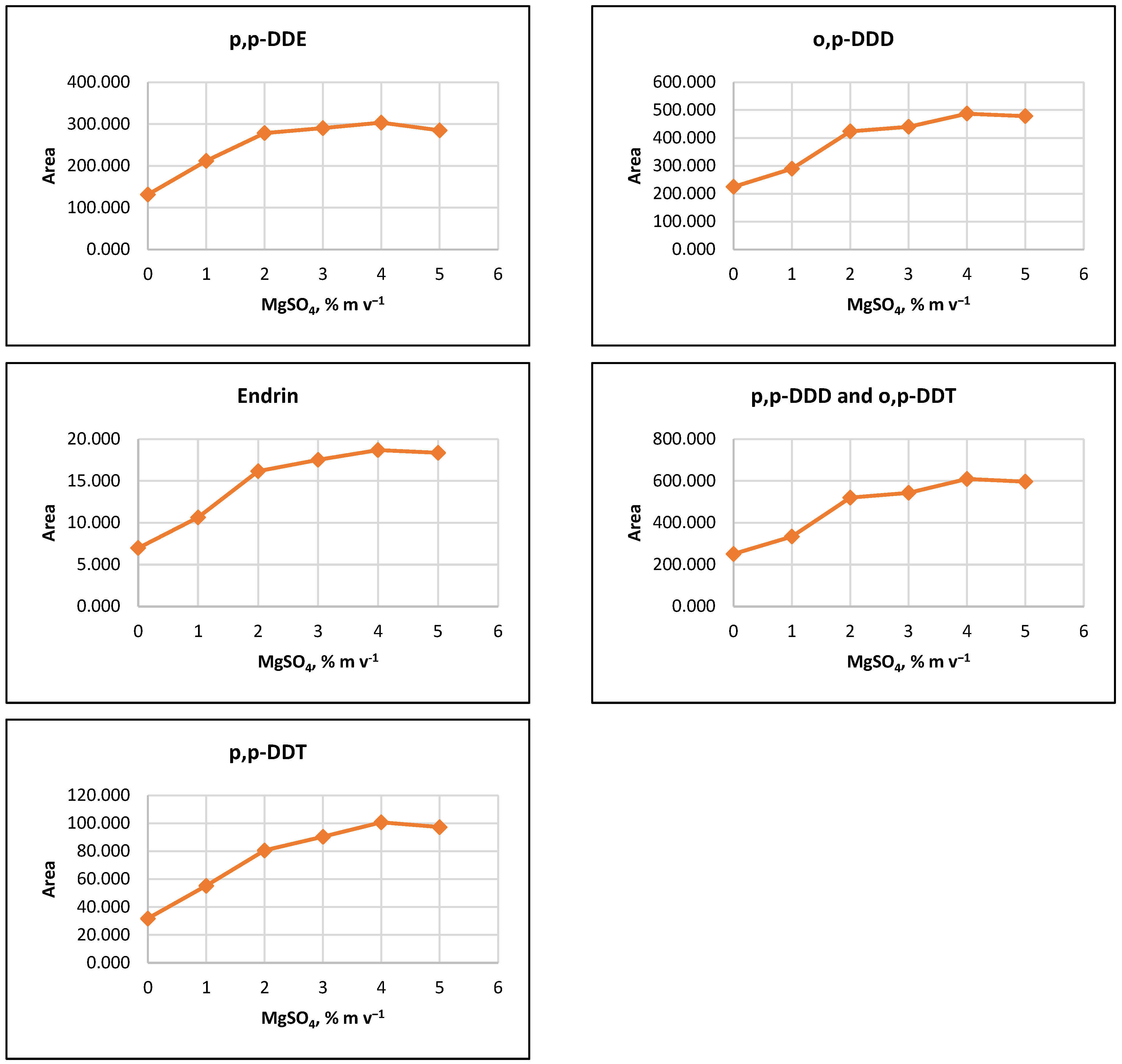
The influence of ion strength on CPE efficiency was examined by performing the CPE procedure with added MgSO4 salt at concentrations of 1%, 2%, 3%, 4%, 5%, or 10% (m v−1). The results indicated that the signals of the target analytes increased with increasing salt concentration up to 2% (m v−1). For some pesticides, a slight increase in signals was observed as MgSO4 content rose to 5% (m v−1) (Figure 2).
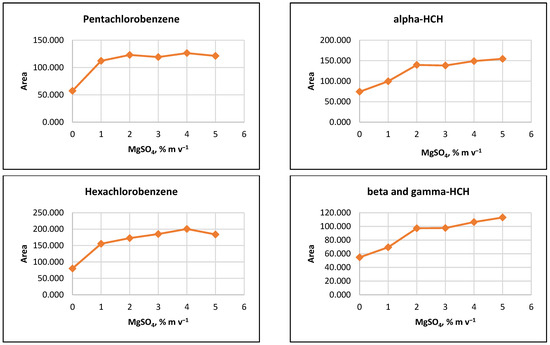
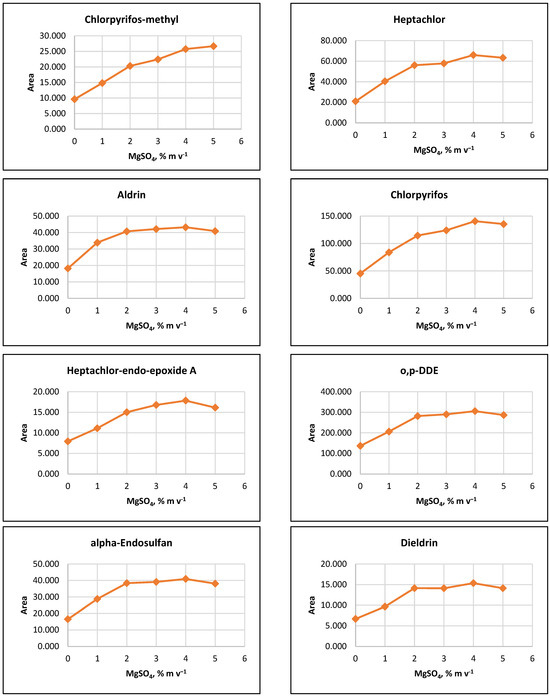
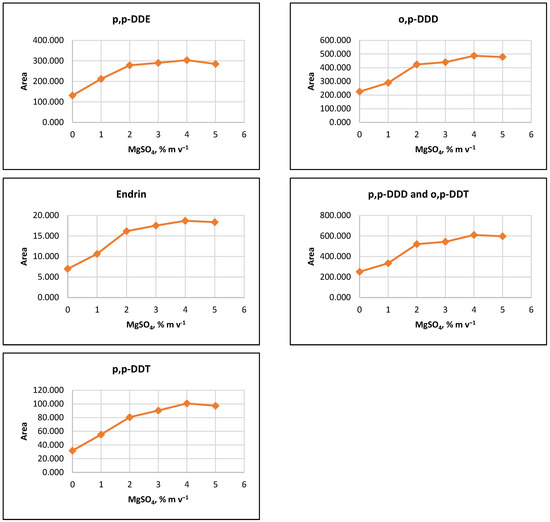
Figure 2.
Salting out effect at 1%, 2%, 3%, 4% and 5% (m v−1) MgSO4 and without salt content. Conditions: CPE—2% (m m−1) Triton X-100, pesticide concentration 10 µg L−1, 30 min MW incubation time; re-extraction—2 mL hexane, without added water.
It was observed that the gravitational sedimentation of the surfactant-rich phase was significantly hindered at a concentration of 10% MgSO4 (m v−1), as a phase with a flocculent structure and diffuse boundaries settled at the bottom of the reaction tube, most likely due to the increase in the density of the aqueous medium. Due to the relatively small increase in analyte signals at concentrations above 2% (m v−1) and to minimize reagents used, it was preferred to add 2% (m v−1) MgSO4 to the extraction systems at the CPE step.
3.1.2. Re-Extraction Optimization
- Decreasing the surfactant-rich phase viscosity
After the gravitational sedimentation, the obtained surfactant-rich phase was isolated by pipetting the aqueous phase. The separated surfactant-rich phase still had considerable viscosity and was not free-flowing, which led to problems in mass transfer during the re-extraction step. Two approaches were applied to reduce the viscosity of the surfactant-rich phase: (i) dilution with water in combination with different agitation methods for dispersing the organic solvent in the surfactant-rich phase—ultrasonic treatment (ultrasound-assisted back-extraction—USABE) or vortex agitation (vortex-assisted back-extraction—VABE), or (ii) heating of the surfactant-rich phase before re-extraction in hexane.
To investigate the effect of adding water in combination with ultrasonic or vortex agitation, two central composite designs were created for each agitation method using the statistical software Statistica 14.1.0.8 (TIBCO Statistica, USA). The two factors (water volume and re-extraction time) have been varied as shown in Supplementary Materials Tables S1 and S2. A hot plate was used to perform the CPE, and the volume of hexane for re-extraction was fixed at 2 mL. The remaining experimental parameters were set as specified in point 2.4.
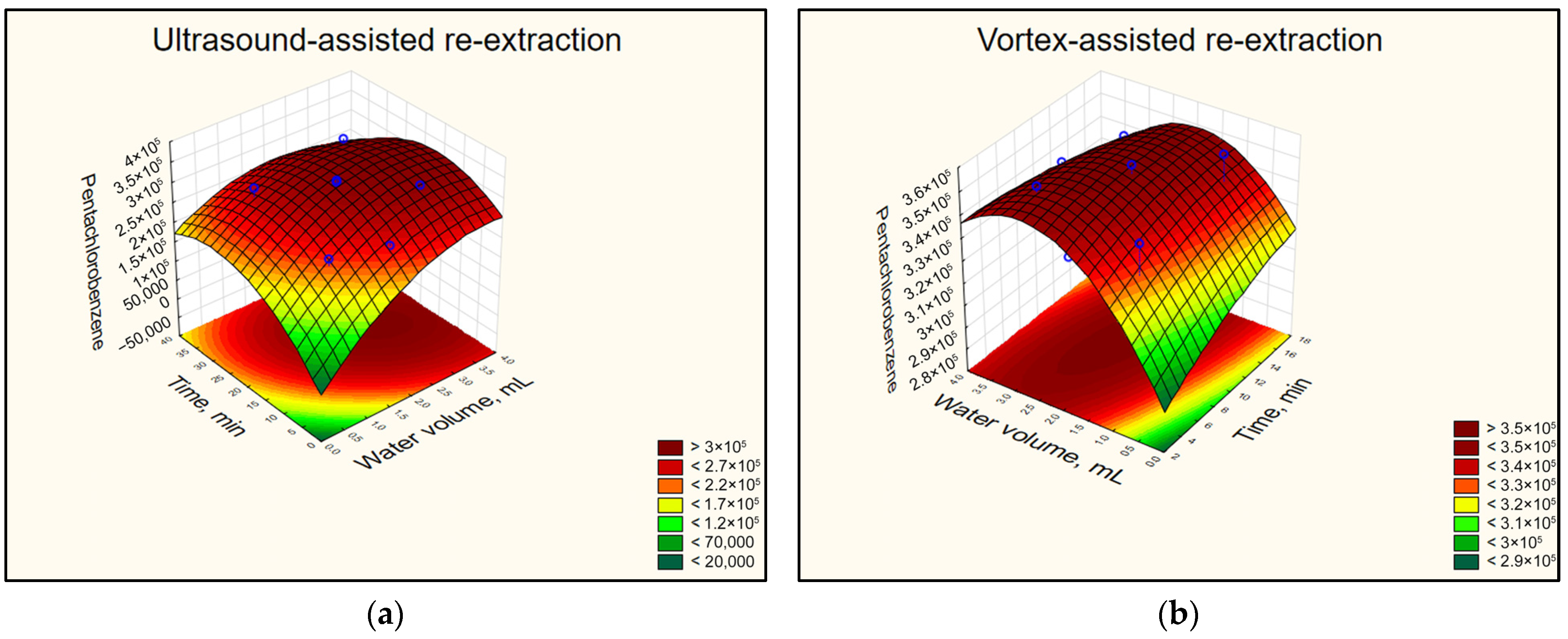
The results from the two CCDs indicated that both ultrasonic treatment and vortex agitation lead to comparable responses for all pesticides studied. The dilution of the surfactant-rich phase leads to an increase in re-extraction efficiency, and the effect becomes significant when at least 1 mL of water is introduced. The results obtained for Pentachlorobenzene applying USABE (a) or VABE (b) are presented in Figure 3. Regarding the time required for dispersing the organic solvent in the surfactant-rich phase, the results showed that using ultrasound requires at least 20 min. However, when the vortex is applied, this time is halved to 10 min. From the perspective of reducing extraction time and enhancing the “green” character of CPE, a vortex can be considered as a preferable agitation method in further experiments at the re-extraction step.
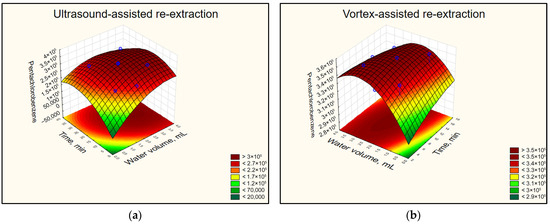
Figure 3.
The response surface diagrams for Pentachlorobenzene from CCDs representing the signal dependence on the amount of added water to the surfactant-rich phase and the re-extraction time by (a) USABE and (b) VABE. Conditions: CPE—2% (m m−1) Triton X-100, initial pesticide concentration 25 µg L−1, MW incubation time 30 min; re-extraction with 2 mL hexane, 15 min centrifugation.
Despite the beneficial effect of the added water on mass transfer efficiency, a lengthy cooling period (~12 h) in a freezer was still required for the physical separation of hexane from the surfactant-rich phase. Consequently, the plan to reduce the volume of the organic solvent would complicate phase separation after re-extraction. Therefore, the possibility of lowering the viscosity of the surfactant-rich phase through a different method, specifically heating, was explored. The maximum heating temperature was set as high as possible without exceeding the coagulation temperature of Triton X-100. It was found that heating at 55 °C for 1 min, immediately before adding hexane, decreased the viscosity of the surfactant-rich phase. The analytical yields achieved were comparable to those obtained when 2 mL of water was added to the surfactant-rich phase. Additionally, the reduced viscosity resulted in a shorter re-extraction time of 1 min by using vortex agitation.
- Minimization of the organic solvent volume for re-extraction.
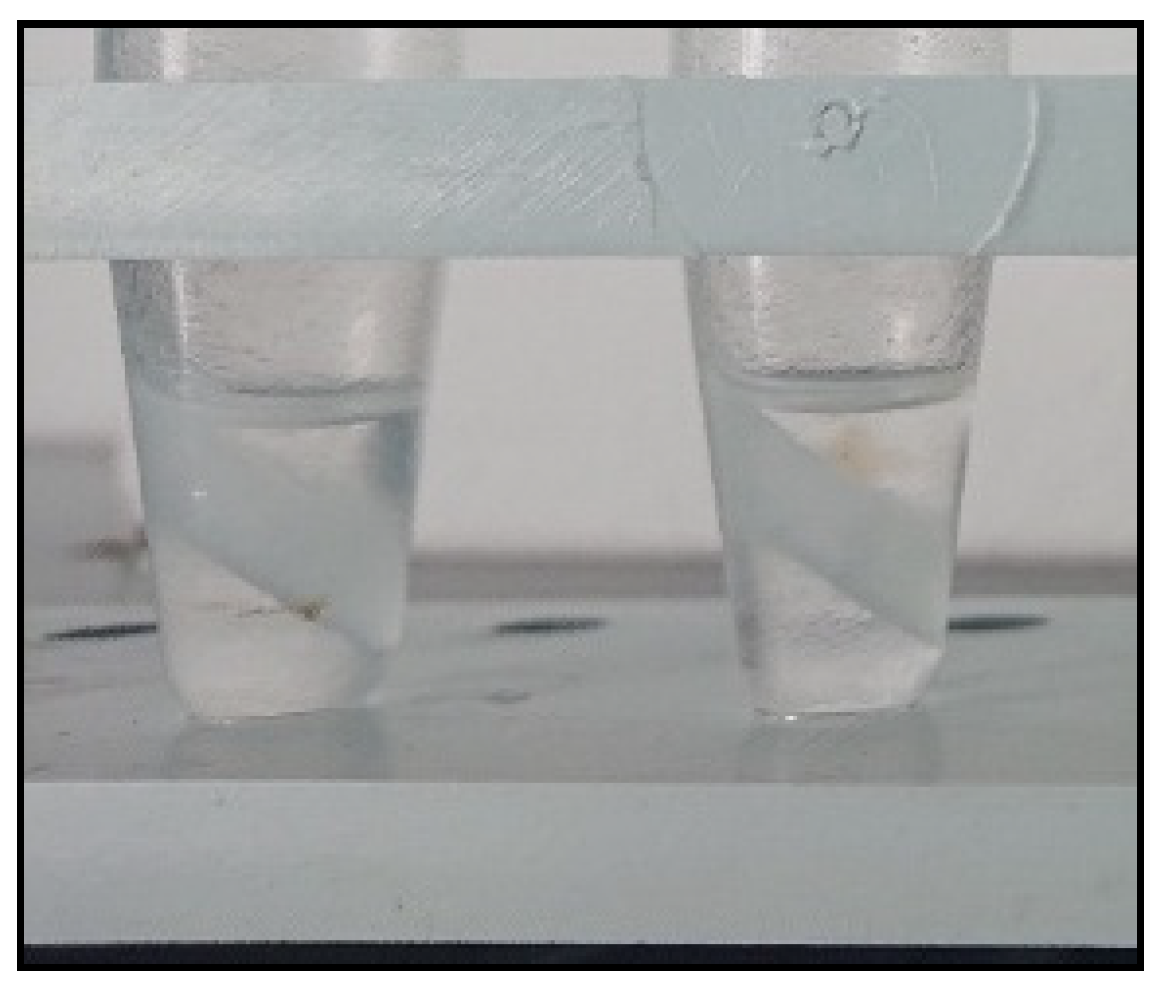
In alignment with the trends of “green” analytical chemistry, the feasibility of reducing the amount of organic solvent used during the re-extraction phase was systematically examined. A one-variable-at-a-time methodology was employed to achieve this objective, with the volume of hexane varied at 0.25 mL, 0.5 mL, 1.0 mL, and 2.0 mL. It was noted that, despite the lower viscosity of the surfactant-rich phase, volumes less than 1.0 mL resulted in the formation of a stable emulsion, which could only be broken down through extended cooling in a freezer for a duration exceeding 12 h. This prompted an investigation into an alternative strategy to break down the emulsion. For this purpose, the salting-out effect was examined by the addition of 0.05 g, 0.1 g, and 0.2 g of anhydrous MgSO4. The other experimental parameters for the CPE step were set as specified in Section 2.4. To facilitate the migration of MgSO4 into the emulsified extraction system, a 1-min tempering at 55 °C was employed, followed by 1 min of vortex agitation and centrifugation at 900× g for 1 min. It was found that the addition of 0.1 g of salt resulted in the formation of a distinct upper layer of hexane, even with only 0.25 mL of organic solvent (Figure 4).
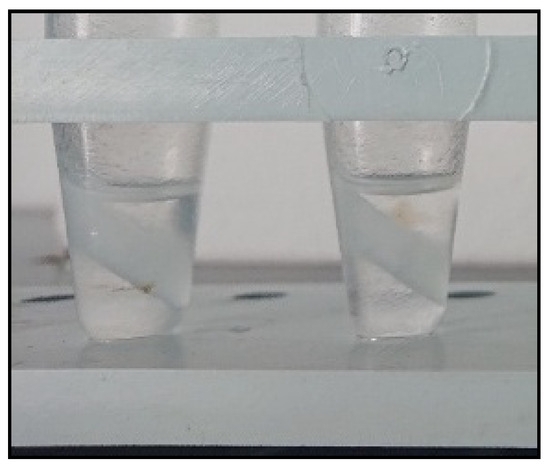
Figure 4.
Aqueous model solutions after CPE and re-extraction. Conditions: CPE—2% (m m−1) Triton X-100, 2% (m v−1) MgSO4, 30 min MW incubation time; re-extraction—tempering at 55 °C for 1 min, 0.25 mL hexane, vortex agitation for 1 min, addition of 0.1 g anhydrous MgSO4, tempering at 55 °C for 1 min, vortex agitation for 1 min and 1 min centrifugation at 900× g.
A probable explanation for this phenomenon is that the excess quantity of solid salt (MgSO4) caused the formation of a saturated salt solution within the surfactant-rich phase, increasing the polarity of the water contained therein. Thermodynamically, it can be assumed that this results in the aggregation and enlargement of the dispersed hexane microdroplets into a separate layer. The formation of an intermediate layer of undissolved MgSO4 (Figure 4) further facilitated pipetting an aliquot of the hexane for subsequent analysis. It is noteworthy that, as a consequence of the optimization carried out, the step involving cooling the samples in a freezer was rendered unnecessary.
To assess the repeatability of the procedure, model solutions were prepared according to the specified CPE and re-extraction parameters in Section 2.4, using 0.5 mL and 0.25 mL hexane for re-extraction in parallel. From the results of the analytical yields shown in Table 3, it is evident that when using 0.25 mL hexane, the analytical yields achieved decrease, and their repeatability significantly deteriorates compared to those with 0.5 mL hexane. The reduced repeatability can be explained by both the reduced contact surface between the water and the organic phase, as well as deterioration in droplet coalescence. For 0.5 mL hexane, most of the relative standard deviations ranged between 2 and 5%, whereas the corresponding values for 0.25 mL hexane were in the range from 9 to 14%. Despite the twofold higher preconcentration factor when using 0.25 mL hexane, the LOQ did not decrease by a factor of two due to the lower analytical yields. When analyte concentrations are low—near or below the LOQ—working with a higher preconcentration factor is generally preferred, even if it slightly reduces precision, as it improves the method’s detection capabilities. Considering the expected relatively low concentrations of pesticides in most samples analyzed (e.g., water, food, beverages, etc.), it was preferred to use 0.25 mL of hexane during re-extraction.

Table 3.
Analytical figures of merit of the developed MW-CPE-GC-MS/MS method. Conditions: CPE—pesticide concentration 10 μg L−1, 2% (m m−1) Triton X-100, 2% (m v−1) MgSO4, 30 min MW incubation time; re-extraction—tempering at 55 °C for 1 min, 0.25 mL hexane, vortex agitation for 1 min, addition of 0.1 g anhydrous MgSO4, tempering at 55 °C for 1 min, vortex agitation for 1 min and 1 min centrifugation at 900× g, (n = 3).
Reducing the re-extraction time (to 5 min) and the organic solvent volume (to 0.25 mL) were the final steps in developing a sample preparation method based on CPE. The final sample preparation conditions are given in Section 2.4. The analytical yields achieved under these conditions ranged from 46 to 94% for 0.5 mL hexane and from 38 to 83% for 0.25 mL hexane, respectively (Table 3).
The reduction in the hexane volume from 0.5 mL to 0.25 mL is beneficial for the greenness of the method and for increasing its detection power (lowering the methodological LODs/LOQs), but inevitably leads to an increase in the method uncertainty. So, in general, it can be recommended that when the pesticides concentrations are at very low levels (near the methodological LOQs) 0.25 mL hexane to be used. If the pesticide concentrations in the sample are relatively high (e.g., near or higher than MRL), then it could be worthy 0.5 mL of hexane to be used in order to achieve better precision (lower measurement uncertainty).
3.2. The “Greenness” Assessment of the MW-CPE-GC-MS/MS Method—AGREEprep
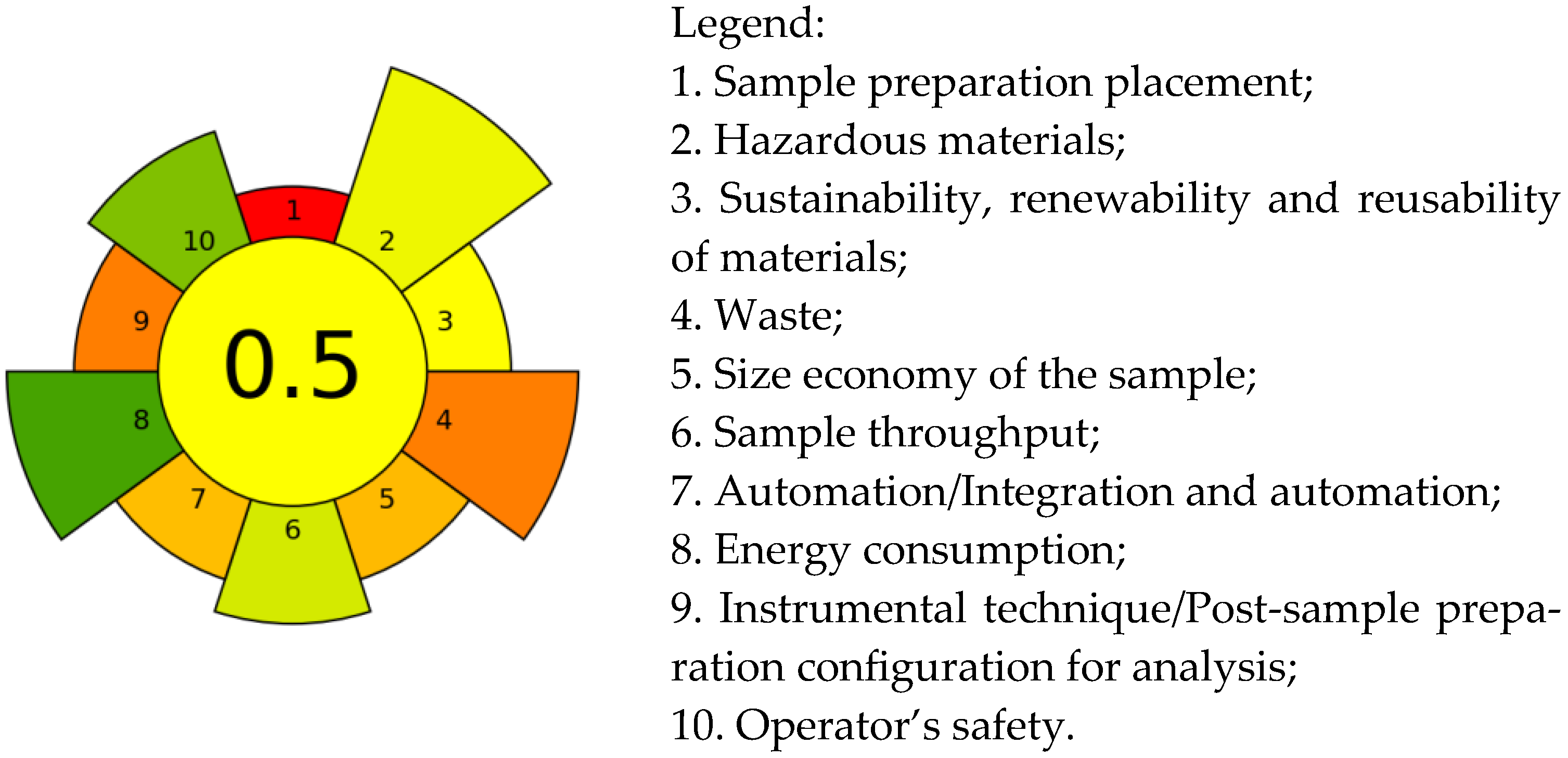
The “greenness” of the developed method was assessed using the AGREE prep software [40] with a maximum score of 1 and green color coding for the low environmental impact of the method. The pictogram of the proposed method, presented in Figure 5, displays the distribution according to relevant assessment criteria.
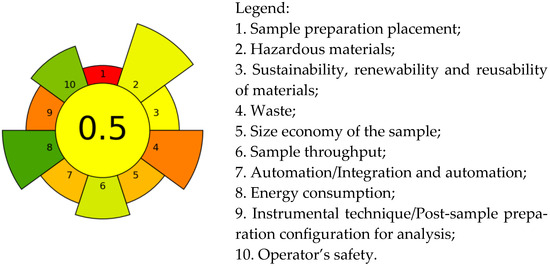
Figure 5.
Evaluation of “greenness” of the MW-CPE-GC-MS/MS method using AGREEprep.
From the diagram, it can be seen that the highest scores were obtained under criterion 8 and criterion 10. The reduction in hazardous materials (criterion 2) contributed to a positive rating under criterion 10, which relates to operator safety during sample preparation. The lower energy consumption for preparing one sample (17 W per sample), owing to the equipment employed (a 12-position MV system, an 8-position centrifuge and a vortex), contributes to a positive rating for criterion 8 compared to using a hot plate. Lower ratings were obtained for criteria 5, 4 and 3, which relate to sample volume, waste produced, and use of sustainable materials. A significant negative impact on the rating under criterion 9 is exerted by the GC-MS/MS technique, typically used for pesticide analysis. The lowest rating was observed under criterion 1 due to the requirement for laboratory sample preparation. In summary, the final score of 0.50 (yellow code) indicates that the method has a moderate environmental impact compared to the score of 0.42 calculated for the classic QuEChERS method (BDS EN 15662:2018) for foods of plant origin. The higher score obtained for MW-CPE-GC-MS/MS is mainly due to the minimization of the hazardous materials used (solvents) under criterion 2 and the reduction in the total amount of waste under criterion 4. The final score of the developed MW-CPE-GC-MS/MS method was regrettably affected by the necessity for sample preparation in the laboratory, final waste volume (including the initial sample volume) and the typical analysis technique employed.
3.3. Analysis of Food Samples Using the Developed MW-CPE-GC-MS/MS Method
Maximum residue levels (MRLs) of pesticides in or on food and feed of plant and animal origin and amendments are specified in Regulation (EC) No 396/2005. For pesticides not explicitly listed in the regulatory documents, an MRL of 0.01 mg kg−1 applies [14]. Alternative MRLs are specified in the Codex Alimentarius [15]. The low LOQs achieved for all studied pesticides (Table 3) enable us to utilize the developed MW-CPE-GC-MS/MS method for analyzing fruit juices. In this work, lemon juice (concentrate with high pulp content) and red apple juice (pasteurized with pigments) were analyzed.
3.3.1. Analysis of Lemon Juice (Concentrate)
Due to the high pulp content of the lemon juice (fruit pieces/vesicles from the fruit endocarp), different dilution factors (DF = 1.25, 1.67, 2.5 and 5) were employed during the CPE step (Section 2.4) To evaluate the accuracy of the MW-CPE-GC-MS/MS method, each sample was spiked with target analytes at a concentration of 10 µg L−1, in triplicate. It was observed that using DF = 1.25 did not result in the formation of a surfactant-rich phase, likely due to the high density of the aqueous phase. When higher dilution factors were used, the sedimentation of the surfactant-rich phase was not deteriorated. The highest analytical yields, ranging from 72 to 114% were obtained when diluting lemon juice with DF = 5 (Table 4).

Table 4.
Analytical yields of 10 µg L−1 pesticide addition to lemon juice (DF = 5) obtained by the MW-CPE-GC-MS/MS, (n = 3).
Notably, the analytical yields obtained for some of the analytes were statistically identical to those calculated for model solutions (Table 3). For certain target analytes—Pentachlorobenzene, Hexachlorobenzene, Chlorpyrifos, p,p-DDE, Endrin and p,p-DDT—the analytical yield values were close to 100%. Yields lower than 70% were observed only for beta and gamma-HCH, and Heptachlor-endo-epoxide A. The concentration of these pesticides can be easily calculated in real samples by applying a correction factor for partial extraction.
3.3.2. Analysis of Red Apple Juice
To assess the analytical potential of the developed MW-CPE-GC-MS/MS method for analyzing highly pigmented samples, red apple juice was chosen. Due to its high pigment content, the sample was diluted with DFs of 1.25, 1.67, 2.5, and 5. The accuracy was tested by spiking each sample with target analytes at a concentration of 10 µg L−1, in triplicate. The samples were subjected to the extraction procedure described in Section 2.4. It was found that for each target analyte, similar analytical yields were obtained at DF = 1.67, 2.5 and 5, unlike at DF = 1.25, where the formation of a surfactant-rich phase was not observed. The possible reason for this could be the increased density of the aqueous phase. To test this, the CPE was performed without adding 2% (m v−1) MgSO4. It was observed that without this addition, the formation of a surfactant-rich phase was not hindered (Figure 6a), and the analytical yields were comparable to the yields for diluted samples and statistically identical to those for model solutions (Table 3). Notably, the developed method combines a preconcentration step and a sample clean-up step, as shown in Figure 6. From Figure 6b), it can be seen that the pigments were extracted into the non-polar core of the micelles during CPE. In the re-extraction step, the pigments were deposited in the intermediate layer of MgSO4. Finally, the hexane phase remains colorless (Figure 6c). The last effect can be explained by the higher polarity of the pigment molecules compared to the ones of the pesticides. The analytical yields, calculated using matrix-matched calibration, are presented in Table 5.

Figure 6.
Sample preparation steps of a red apple juice at DF = 1.25 (a) surfactant-rich phase obtained without addition of MgSO4 at the CPE step, (b) at the re-extraction step with 0.25 mL hexane and (c) after centrifugation.

Table 5.
Analytical yields for red apple juice samples at 10 µg L−1 pesticide concentration (DF = 1.25) obtained by the MW-CPE-GC-MS/MS, (n = 3). Conditions: CPE—pesticide concentration 10 μg L−1, 2% (m m−1) Triton X-100, without addition of 2% (m v−1) MgSO4 in the aqueous phase, microwave incubation for 30 min; 10 min re-extraction by vortex and addition of 0.1 g MgSO4, (n = 3).
4. Conclusions
Novel microwave-assisted CPE, using Triton X-100 as a surfactant combined with re-extraction in hexane for the group separation and preconcentration of 19 studied pesticides (organochlorine and organophosphorus), has been implemented in GC-MS/MS analysis. The proposed analytical method has significantly lower methodological LOQs (for some pesticides) than the MRL values for food samples. The analytical yields achieved were in the range of 70–120% and characterized by satisfactory precision, successfully applying matrix-matched calibration. Based on the recovery studies, it can be concluded that the combined MW-CPE-GC-MS/MS method can be applied for pesticide control in fruit juices. The “green” character of the developed MW-CPE-GC-MS/MS method was evaluated with a score of 0.50, using AGREEprep software. An important emphasis and essential for the successful implementation of subsequent GC-MS/MS analysis is that the preliminary sample preparation procedure includes the preconcentration of the target analytes simultaneously with a clean-up step that separates the pigments of the samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations12090231/s1, Table S1: CCD matrix for ultrasound-assisted re-extraction optimization with two factors: water volume added and re-extraction time. Table S2: CCD matrix for vortex-assisted re-extraction optimization with two factors: water volume added and re-extraction time.
Author Contributions
Conceptualization, A.H. and K.S.; methodology, A.H.; software, A.H. and K.S.; validation, A.H. and K.S.; formal analysis, A.H.; investigation, A.H.; resources, A.H. and K.S.; data curation, A.H.; writing—original draft preparation, A.H.; writing—review and editing, K.S.; visualization, A.H.; supervision, K.S.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project Digital Sustainable Ecosystems—Technological Solutions and Social Models for Ecosystem Sustainability (DUEcoS) BG-RRP-2.004-0001-C01.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CCD | Central composite design |
| CPE | Cloud point extraction |
| DF | Dilution factor |
| EI | Electron impact |
| ER | Extraction recovery |
| GC-MS | Gas chromatography mass spectrometry |
| GC-MS/MS | Gas chromatography tandem mass spectrometry |
| ID | Internal diameter |
| LOQ | Limit of quantification |
| MW | Microwave |
| MRL | Maximum residue level |
| MW-CPE | Microwave-assisted cloud point extraction |
| MW-CPE-GC-MS/MS | Gas chromatography mass spectrometry |
| OCPs | Organochlorine pesticides |
| OD | Outer diameter |
| OPPs | Organophosphorus pesticides |
| PTV | Programmable temperature vaporizer |
| RSD | Relative standard deviation |
| SD | Standard deviation |
| USABE | Ultrasound-assisted re-extraction |
| VABE | Vortex-assisted re-extraction |
References
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides Impacts on Human Health and the Environment with Their Mechanisms of Action and Possible Countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides Classification and Its Impact on Environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Hernández, A.F. Food Safety: Pesticides. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2–4, pp. 375–388. [Google Scholar]
- Cech, R.; Zaller, J.G.; Lyssimachou, A.; Clausing, P.; Hertoge, K.; Linhart, C. Pesticide Drift Mitigation Measures Appear to Reduce Contamination of Non-Agricultural Areas, but Hazards to Humans and the Environment Remain. Sci. Total Environ. 2023, 854, 158814. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Sparling, D.W. Organochlorine Pesticides. In Ecotoxicology Essentials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–107. [Google Scholar]
- Organochlorine Pesticides—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/organochlorine-pesticides (accessed on 14 August 2025).
- Lim, L.; Bolstad, H.M. Organophosphate Insecticides: Neurodevelopmental Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 785–791. [Google Scholar]
- Mahajan, R.; Verma, S.; Chandel, S.; Chatterjee, S. Organophosphate Pesticide: Usage, Environmental Exposure, Health Effects, and Microbial Bioremediation. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 473–490. [Google Scholar]
- The Stockholm Convention on Persistent Organic Pollutants. 2001. Available online: https://chm.pops.int/TheConvention/Overview/tabid/3351/Default.aspx (accessed on 17 July 2025).
- Stockholm Convention. 2009. Available online: https://chm.pops.int/Implementation/PublicAwareness/PressReleases/COP4Geneva,9May2009/tabid/542/Default.aspx (accessed on 7 July 2025).
- Stockholm Convention. 2011. Available online: https://chm.pops.int/Implementation/PesticidePOPs/Endosulfan/Overview/tabid/5362/Default.aspx (accessed on 7 July 2025).
- Manousi, N.; Ferracane, A.; Kalogiouri, N.P.; Kabir, A.; Furton, K.G.; Tranchida, P.Q.; Zachariadis, G.A.; Mondello, L.; Samanidou, V.F.; Rosenberg, E. Design and Development of Second-Generation Fabric Phase Sorptive Extraction Membranes: Proof-of-Concept for the Extraction of Organophosphorus Pesticides from Apple Juice Prior to GC–MS Analysis. Food Chem. 2023, 424, 136423. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Available online: https://publications.europa.eu/resource/cellar/dbb0741d-b722-11ef-91ed-01aa75ed71a1.0002.02/DOC_1 (accessed on 27 July 2025).
- Codex Alimentarius (Food and Agriculture Organization of the United Nations and World Health Organization). Available online: https://www.fao.org/fao-who-codexalimentarius/about-codex/en/ (accessed on 14 August 2025).
- Fernandes, V.C.; Domingues, V.F.; Delerue-Matos, C.; Mateus, N. Determination of Pesticides in Fruit and Fruit Juices by Chromatographic Methods. J. Chromatogr. Sci. 2011, 49, 715–730. [Google Scholar] [CrossRef]
- Kanu, A.B. Recent Developments in Sample Preparation Techniques Combined with High-Performance Liquid Chromatography: A Critical Review. J. Chromatogr. A 2021, 1654, 462444. [Google Scholar] [CrossRef]
- Notardonato, I.; Avino, P. Dispersive Liquid–Liquid Micro Extraction: An Analytical Technique Undergoing Continuous Evolution and Development—A Review of the Last 5 Years. Separations 2024, 11, 203. [Google Scholar] [CrossRef]
- Tesfaye, B.; Gure, A.; Asere, T.G.; Molole, G.J. Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction for Determination of Organochlorine Pesticides in Water and Apple Juice Samples. Microchem. J. 2023, 195, 109428. [Google Scholar] [CrossRef]
- Yamini, Y.; Ghambarian, M. Environmental Applications of Cloud-Point Extraction. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 3, pp. 657–680. [Google Scholar]
- Halko, R.; Hagarová, I.; Andruch, V. Innovative Approaches in Cloud-Point Extraction. J. Chromatogr. A 2023, 1701, 464053. [Google Scholar] [CrossRef]
- Filik, H.; Demirci, S. Cloud Point Extraction of Pesticide Residues. In Pesticides in the Modern World—Trends in Pesticides Analysis; InTech: London, UK, 2011. [Google Scholar]
- Mortada, W.I. Recent Developments and Applications of Cloud Point Extraction: A Critical Review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Kori, S. Cloud Point Extraction Coupled with Back Extraction: A Green Methodology in Analytical Chemistry. Forensic Sci. Res. 2021, 6, 19–33. [Google Scholar] [CrossRef]
- Snigur, D.; Azooz, E.A.; Zhukovetska, O.; Guzenko, O.; Mortada, W. Recent Innovations in Cloud Point Extraction Towards a More Efficient and Environmentally Friendly Procedure. TrAC Trends Anal. Chem. 2023, 164, 117113. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Faria, A.M.; Dardengo, R.P.; Lima, C.F.; Neves, A.A.; Queiroz, M.E.L.R. Determination of Disulfoton in Surface Water Samples by Cloud-Point Extraction and Gas Chromatography. Int. J. Environ. Anal. Chem. 2007, 87, 249–258. [Google Scholar] [CrossRef]
- Ohashi, A.; Ogiwara, M.; Ikeda, R.; Okada, H.; Ohashi, K. Cloud Point Extraction and Preconcentration for the Gas Chromatography of Phenothiazine Tranquilizers in Spiked Human Serum. Anal. Sci. 2004, 20, 1353–1357. [Google Scholar] [CrossRef]
- Shen, J.; Shao, X. Determination of Tobacco Alkaloids by Gas Chromatography-Mass Spectrometry Using Cloud Point Extraction as a Preconcentration Step. Anal. Chim. Acta 2006, 561, 83–87. [Google Scholar] [CrossRef]
- Sikalos, T.I.; Paleologos, E.K. Cloud Point Extraction Coupled with Microwave or Ultrasonic Assisted Back Extraction as a Preconcentration Step Prior to Gas Chromatography. Anal. Chem. 2005, 77, 2544–2549. [Google Scholar] [CrossRef]
- Takagai, Y.; Hinze, W.L. Cloud Point Extraction with Surfactant Derivatization as an Enrichment Step Prior to Gas Chromatographic or Gas Chromatography−Mass Spectrometric Analysis. Anal. Chem. 2009, 81, 7113–7122. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Cloud Point Extraction and Gas Chromatography with Direct Microvial Insert Thermal Desorption for the Determination of Haloanisoles in Alcoholic Beverages. Talanta 2016, 160, 282–288. [Google Scholar] [CrossRef]
- Zygoura, P.D.; Paleologos, E.K.; Riganakos, K.A.; Kontominas, M.G. Determination of Diethylhexyladipate and Acetyltributylcitrate in Aqueous Extracts after Cloud Point Extraction Coupled with Microwave Assisted Back Extraction and Gas Chromatographic Separation. J. Chromatogr. A 2005, 1093, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.R.; Silva, M.F.; Martínez, L.D.; Wuilloud, R.G.; Altamirano, J.C. Determination of Polybrominated Diphenyl Ethers in Water and Soil Samples by Cloud Point Extraction-Ultrasound-Assisted Back-Extraction-Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2009, 1216, 4339–4346. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, X.; Deng, X.; Huang, L.; Yang, M.; Zhou, Z. Cloud Point Extraction Coupled with Ultrasonic-Assisted Back-Extraction for the Determination of Organophosphorus Pesticides in Concentrated Fruit Juice by Gas Chromatography with Flame Photometric Detection. Food Chem. 2011, 127, 683–688. [Google Scholar] [CrossRef]
- Makarchuk, I.S.; Klovak, V.O.; Levchyk, V.M.; Doroschuk, V.O. Cloud Point Extraction Coupled with Ultrasonic-Assisted Back-Extraction for the Determination of Metalaxyl, Fludioxonil and Fenarimol in Fruits by Gas Chromatography with Flame Ionization Detection. Chem. Pap. 2022, 76, 7575–7584. [Google Scholar] [CrossRef]
- Jia, G.F.; Lv, C.G.; Zhu, W.T.; Qiu, J.; Wang, X.Q.; Zhou, Z.Q. Applicability of Cloud Point Extraction Coupled with Microwave-Assisted Back-Extraction to the Determination of Organophosphorous Pesticides in Human Urine by Gas Chromatography with Flame Photometry Detection. J. Hazard. Mater. 2008, 159, 300–305. [Google Scholar] [CrossRef]
- Hristozova, A.D.; Simitchiev, K.K.; Kmetov, V.J.; Rosenberg, E. Compatibility of Cloud Point Extraction with Gas Chromatography: Matrix Effects of Triton X-100 on GC-MS and GC-MS/MS Analysis of Organochlorine and Organophosphorus Pesticides. Talanta 2024, 269, 125445. [Google Scholar] [CrossRef]
- Hristozova, A.; Vidal, L.; Aguirre, M.Á.; Simitchiev, K.; Canals, A. Natural Deep Eutectic Solvent-Based Dispersive Liquid-Liquid Microextraction of Pesticides in Drinking Waters Combined with GC-MS/MS Detection. Talanta 2025, 282, 126967. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. Design of Experiments. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–22. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9781118146927. [Google Scholar]
- Özcan, S.; Levent, S.; Can, N.Ö. Quality by Design Approach with Design of Experiment for Sample Preparation Techniques. Adv. Sample Prep. 2023, 7, 100079. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).