Enhancing Copper Leaching from Refractory Copper Oxide Ore Using Organic Cationic Surfactant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Ore Samples
2.1.2. Leaching Reagents
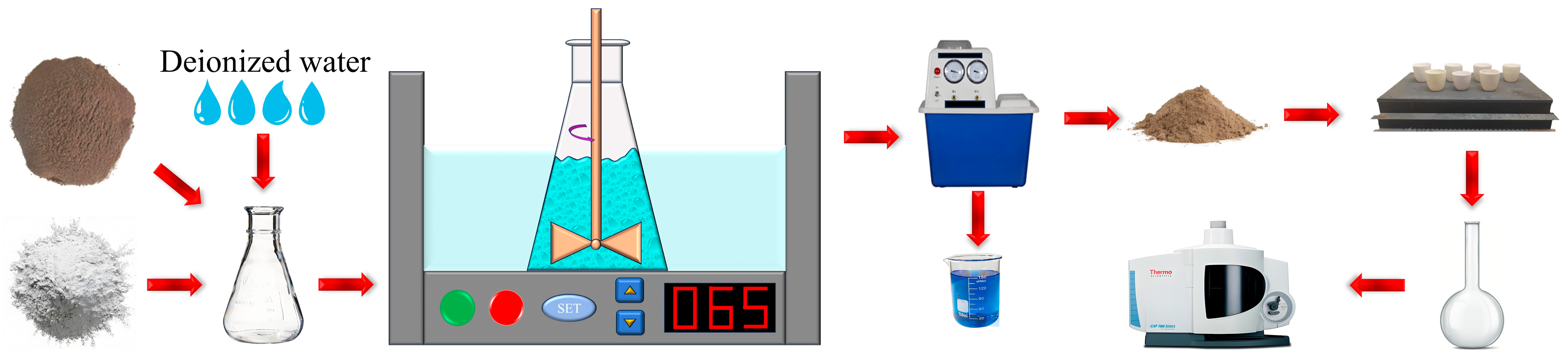
2.2. Experimental Procedure
2.3. Analysis and Characterization
3. Results and Discussion
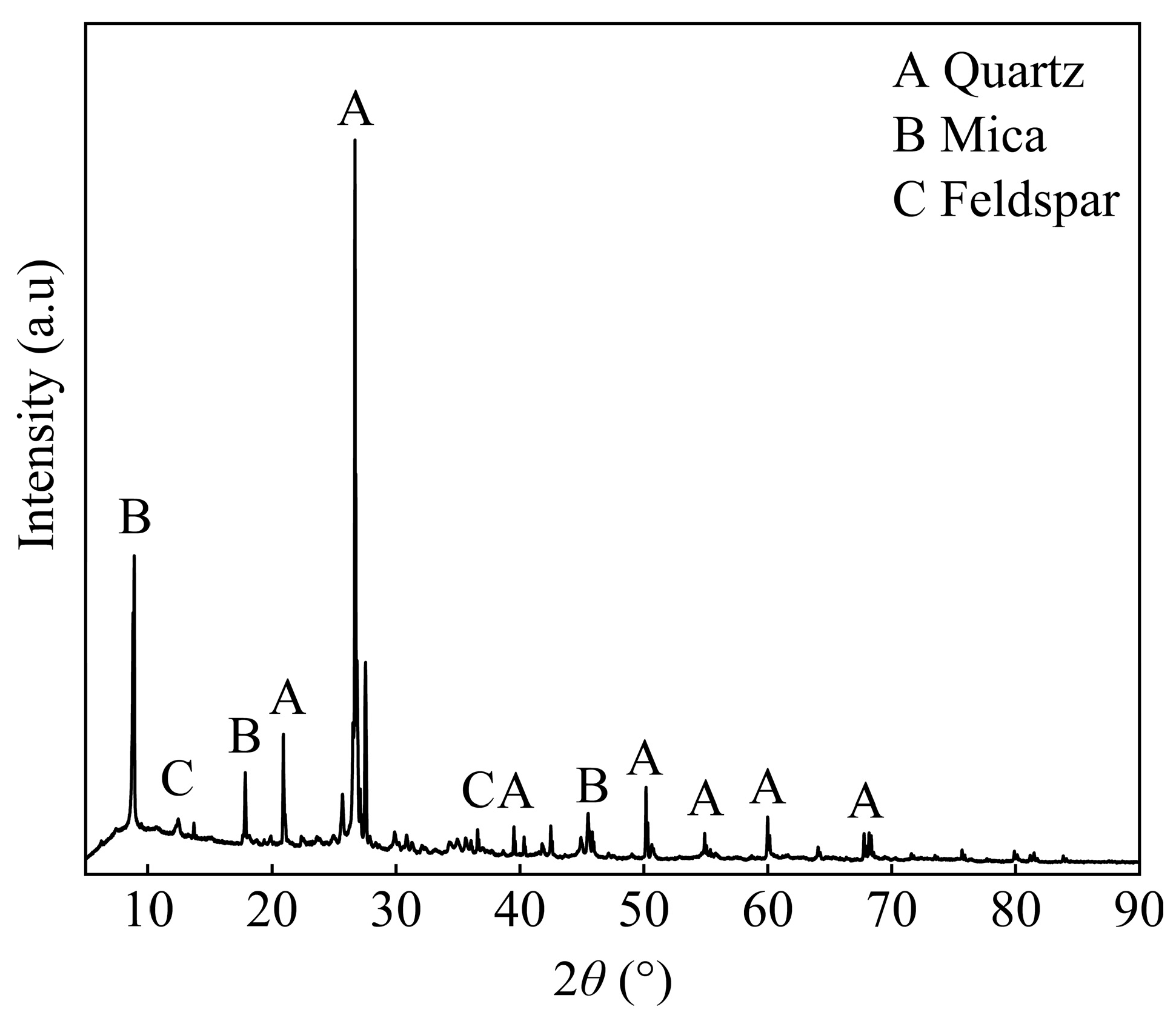
3.1. Mineralogical Characterization of Raw Ore
3.2. Study on Leaching Characteristics of Raw Ore
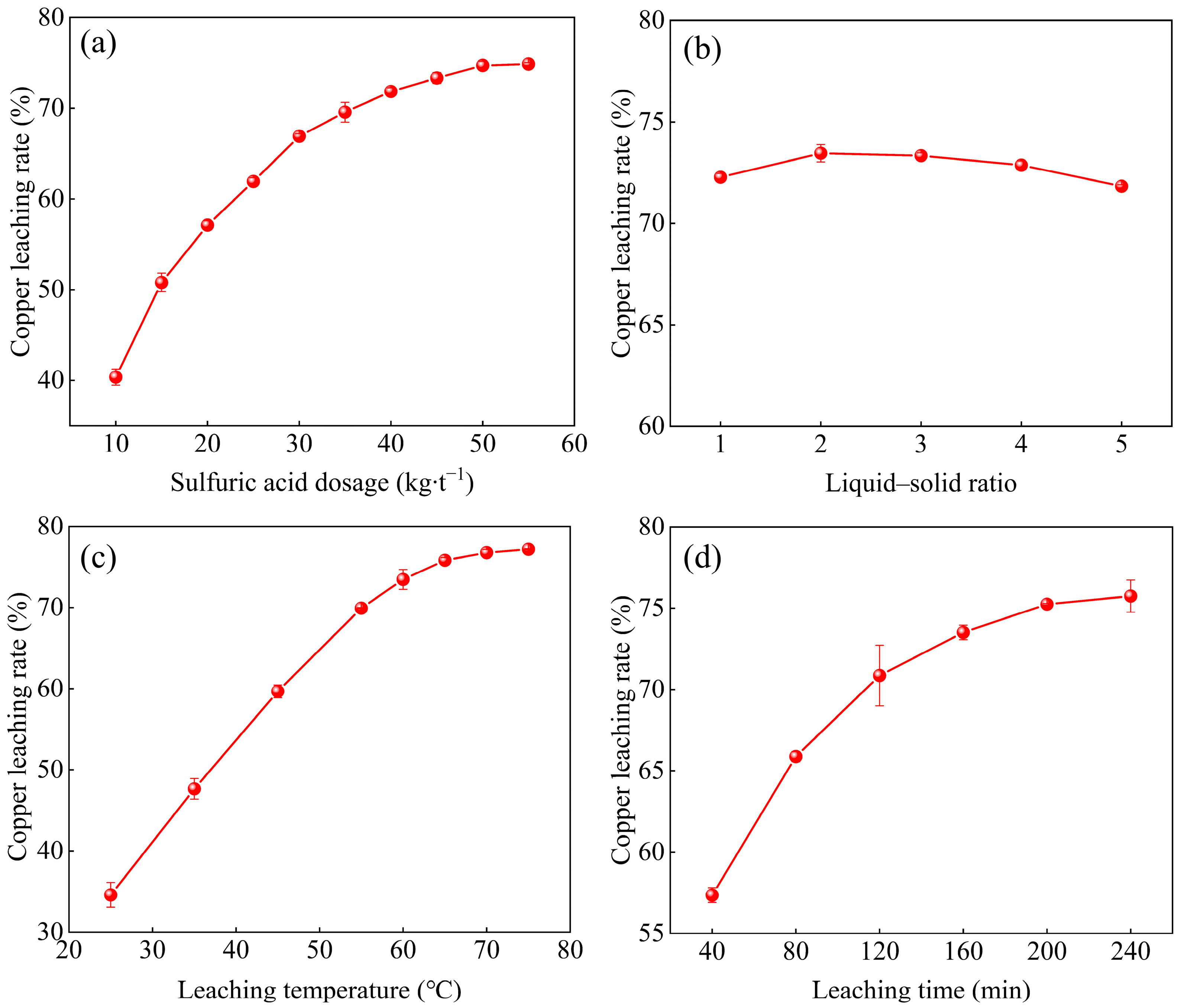
3.2.1. Sulfuric Acid Dosage
3.2.2. Liquid–Solid Ratio
3.2.3. Leaching Temperature
3.2.4. Leaching Time
3.2.5. Analysis of the Reasons for the Difficulty in Leaching of Raw Ore
3.3. Enhanced Leaching Behavior of Ore by the Organic Cationic Surfactant
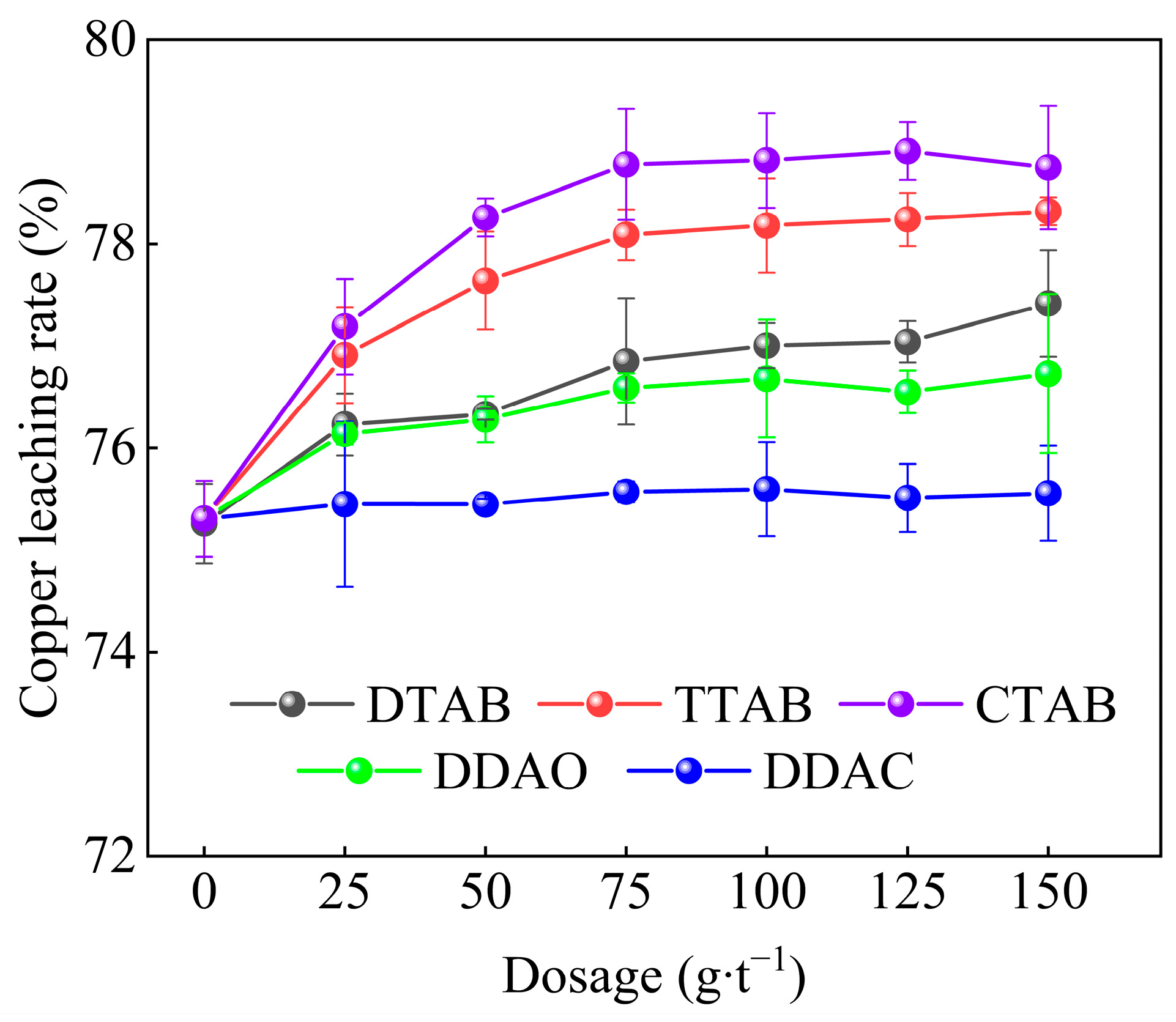
3.3.1. Effect of Type and Dosage of Organic Cationic Surfactant on Copper Leaching Rate
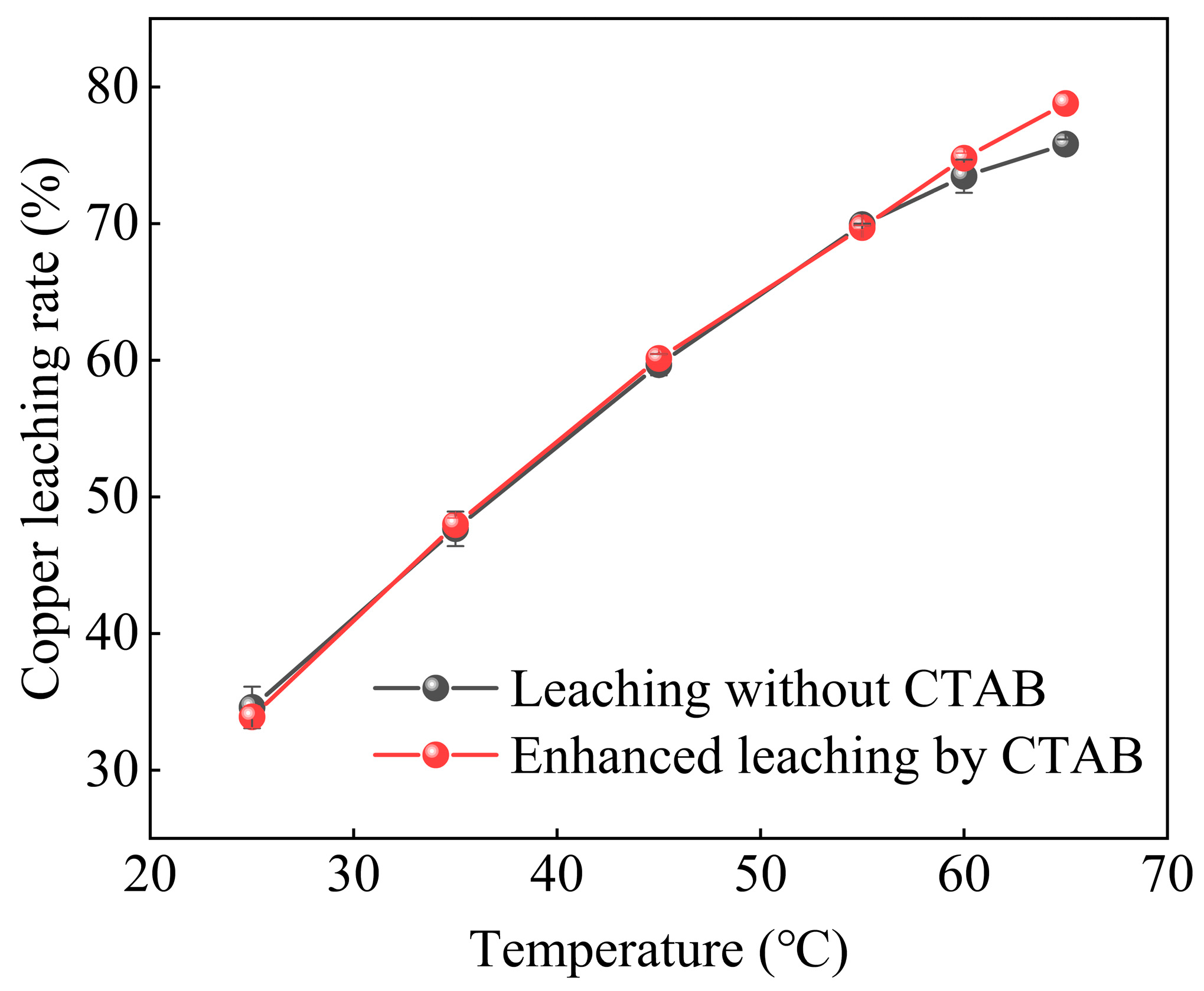
3.3.2. Effect of Organic Cationic Surfactant on Leaching Temperature
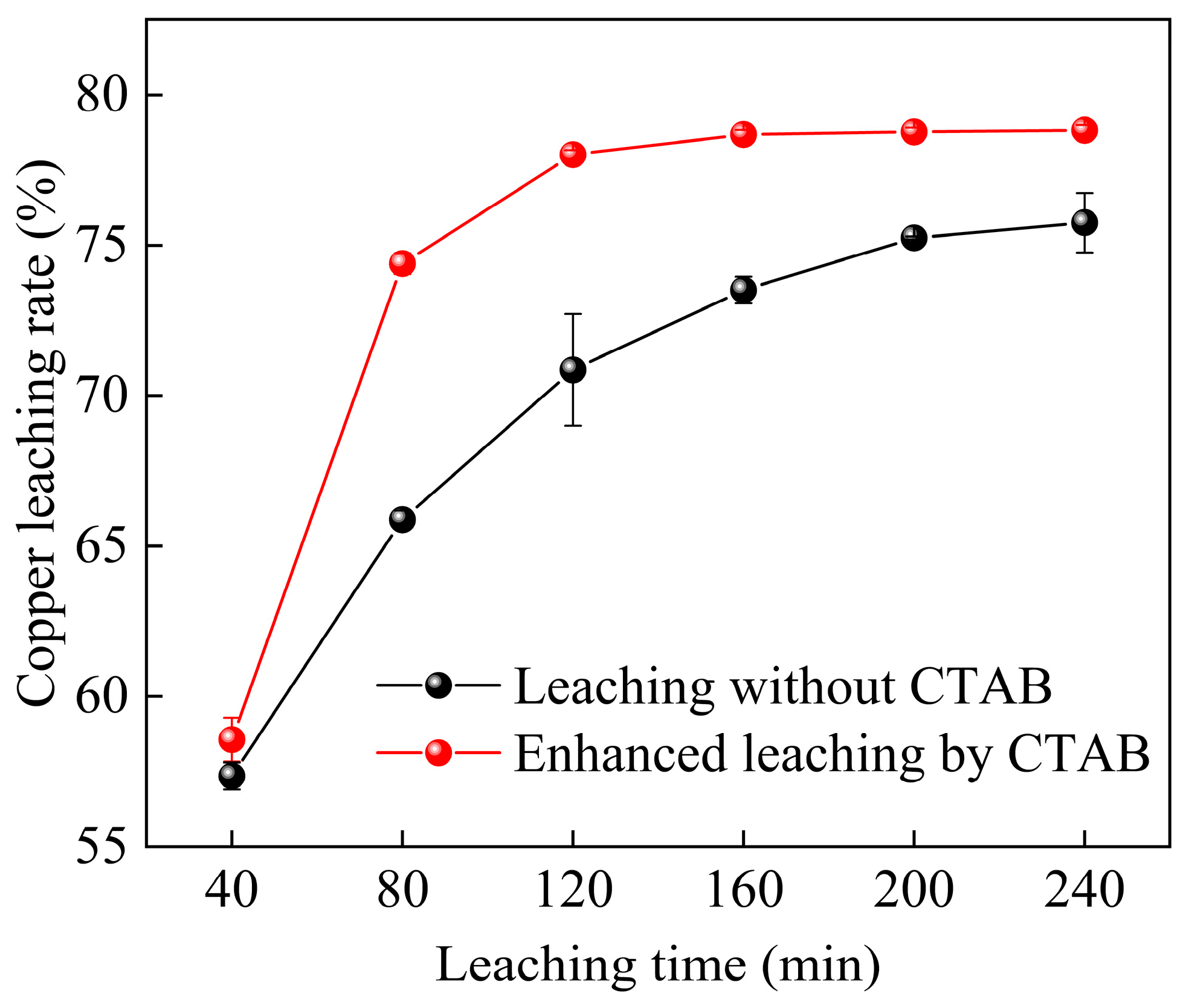
3.3.3. Effect of Organic Cationic Surfactant on Leaching Time
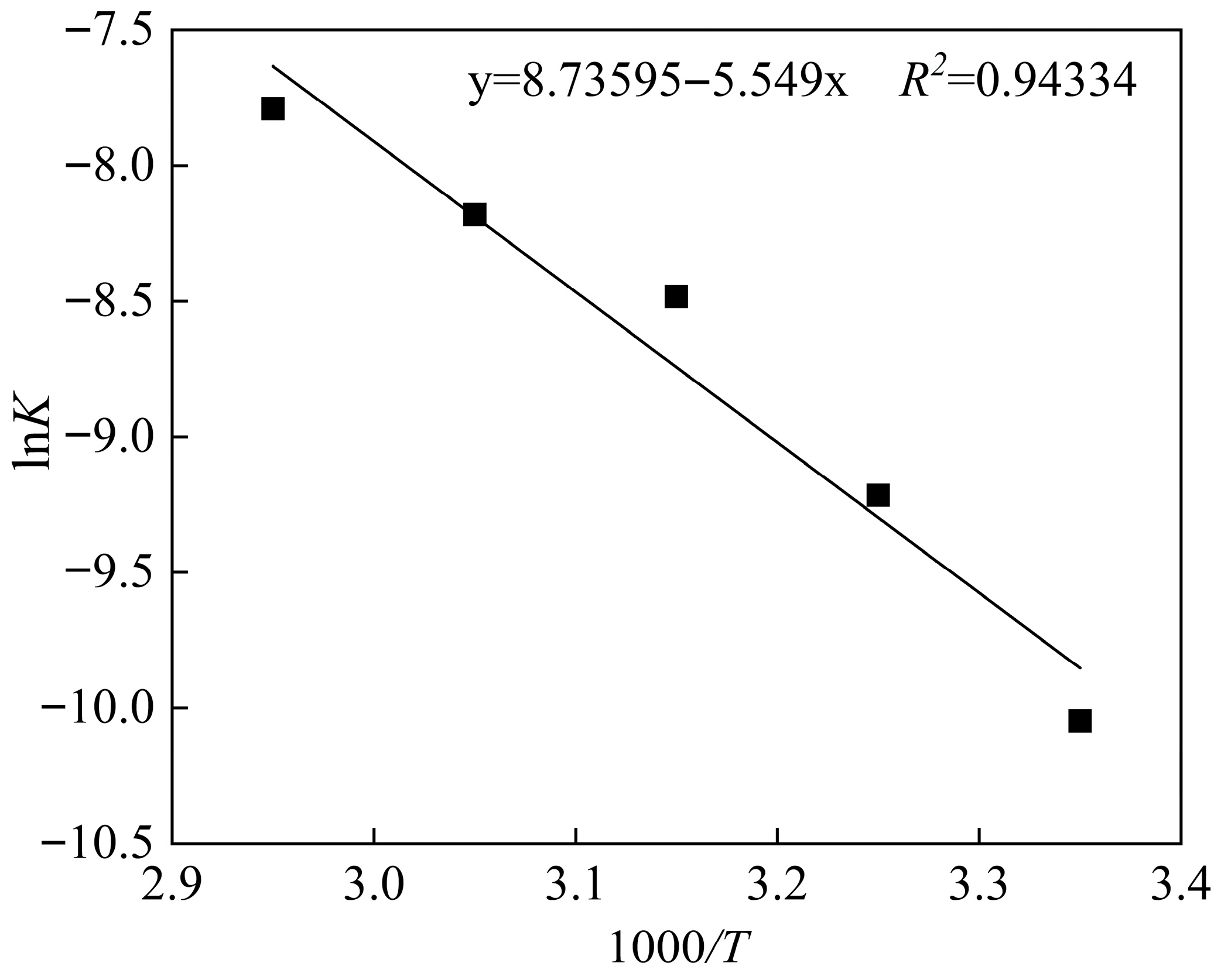
3.4. Kinetic Analysis of Leaching
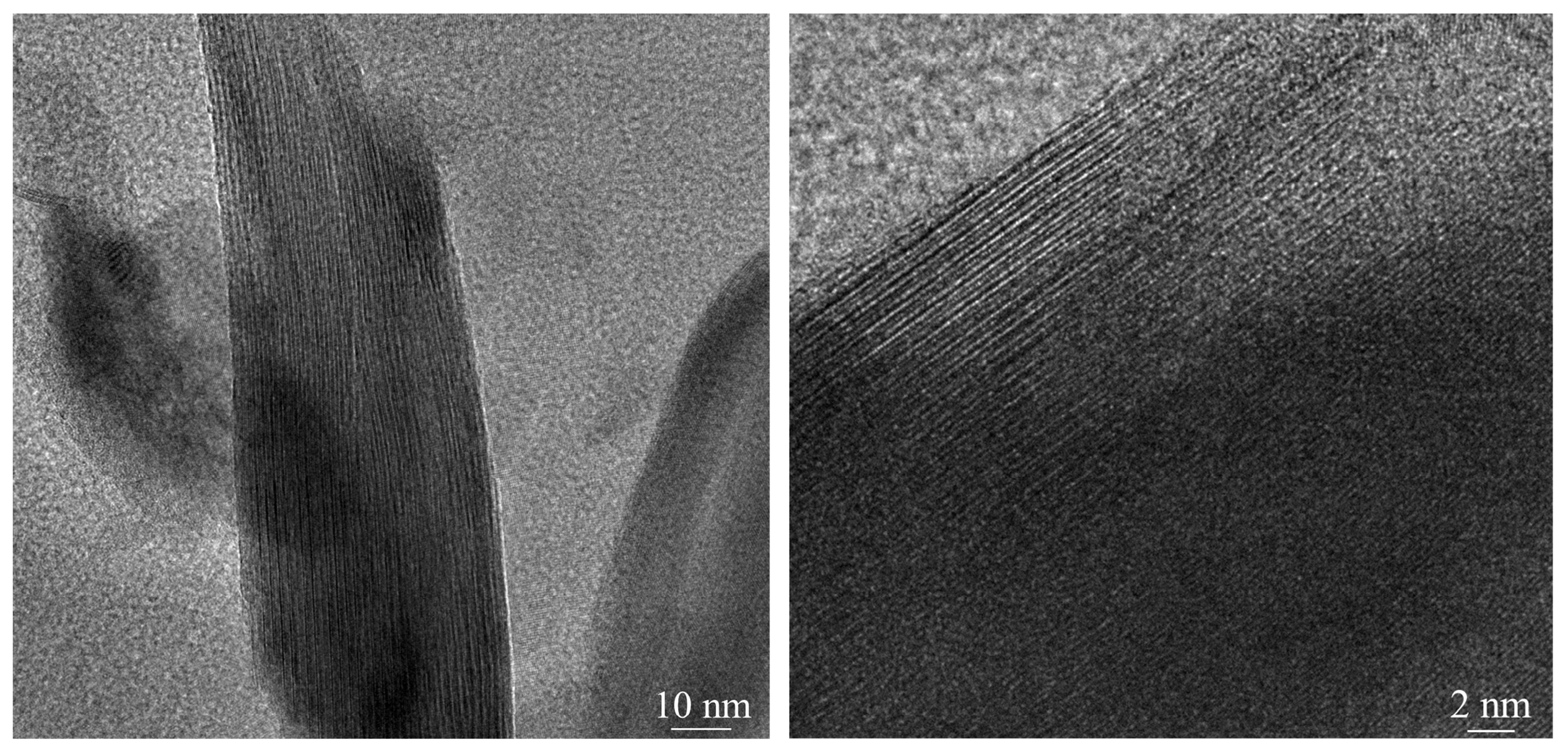
3.5. Mechanism of CTAB-Enhanced Leaching
3.5.1. Analysis of FTIR
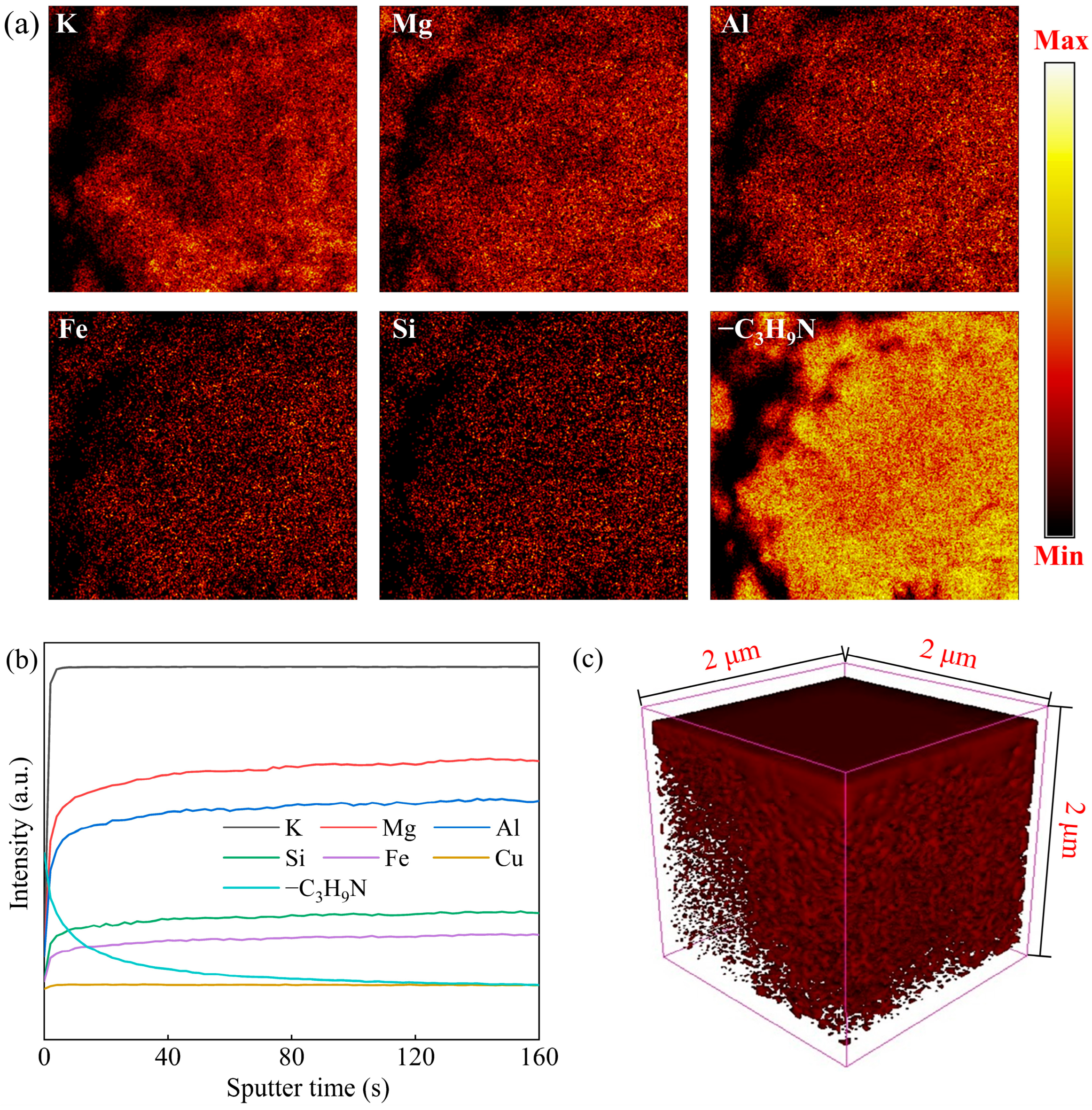
3.5.2. Analysis of TOF-SIMS
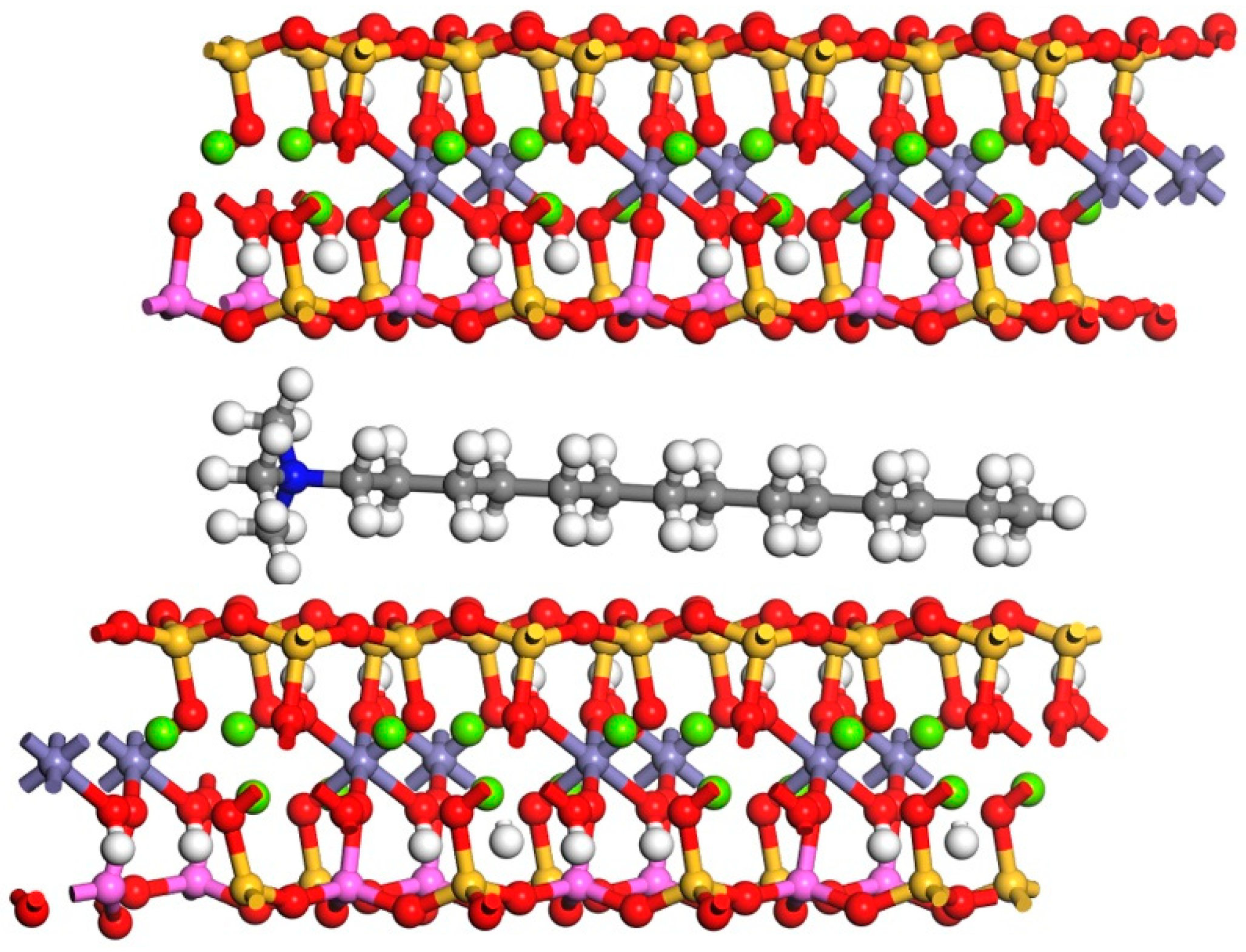
3.5.3. Analysis of Molecular Dynamics Simulation
3.5.4. XRD Analysis of Leaching Residues
3.6. Energy Consumption, Economy, and Potential Merit and Demerit Analysis
4. Conclusions
- (1)
- The binding rate of copper in the refractory copper oxide ore studied in this research is high. Copper is predominantly present in biotite, forming significant amounts of cupriferous biotite, which results in a low leaching rate by regular heating–agitation acid leaching. Cupriferous biotite has poor grindability, and it is mainly distributed in the coarse fraction of grinding products.
- (2)
- Leaching tests indicated that when the dosage of sulfuric acid was 45 kg/t, the liquid–solid ratio was 2:1, the leaching temperature was 65 °C, with a leaching time of 200 min, the copper leaching rate was 75.26%. When the organic cationic surfactant CTAB was used as the leaching agent at a dosage of 75 g/t, the copper leaching rate increased to 78.32%. Compared to the optimal result of the regular heating–agitation acid leaching test, the leaching rate of copper increased by 3.06% and the leaching time was shortened by 80 min. The leaching process is aptly characterized by a mixed control model, wherein the reaction rate is governed by both chemical reaction and diffusion. The activation energy of the leaching process is 46.13 kJ/mol.
- (3)
- The results of the mechanistic study indicate that the organic cations in CTAB can replace potassium ions within the biotite interlayer, neutralizing excess anions and weakening the electrostatic Coulomb forces between the interlayer cations and the hexagonal structure, increasing the interlayer spacing. During the process of increasing the distance between the biotite layers, the organic cations will gradually shift from the horizontal direction to the vertical direction, and play a “pillaring” role between the biotite layers. The expanded interlayer spacing generates more vacancies, providing additional sites for organic cations and H+. This facilitates the entry of H+ from the sulfuric acid solution into the biotite interlayer, where they react with copper within the biotite, enhancing the copper leaching rate, accelerating copper extraction, and shortening the leaching time.
- (4)
- Although CTAB exhibits a certain level of toxicity, it possesses excellent degradability and the feasibility of being recycled, and generally does not pose a significant threat to the environment. When used as a leaching aid, CTAB can contribute to substantial energy savings. Under ideal industrial conditions, its application in the heating–agitation acid leaching at the local hydrometallurgical plant can reduce the cost by approximately USD 6.11–9.36 per ton of ore.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Wang, G.R.; Yang, H.Y.; Tong, L.L.; Liu, Y.Y. Research on technological mineralogy of copper oxide ore in Luanshya, Zambia. J. Northeast. Univ. Nat. Sci. 2019, 40, 350–355. (In Chinese) [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevič, M.; Drahota, P.; Kříbek, B.; Nyambe, I.; Vaněk, A.; Penížek, V.; Sracek, O.; Natherová, V. Cobalt-bearing copper slags from Luanshya (Zambian Copperbelt): Mineralogy, geochemistry, and potential recovery of critical metals. J. Geochem. Explor. 2022, 237, 106987. [Google Scholar] [CrossRef]
- Yu, B.Q.; Sun, C.B.; Kou, J. Process mineralogical study on refractory copper oxide ore in Zambia. Met. Min. 2021, 2, 110–114. [Google Scholar] [CrossRef]
- Wang, G.R.; Yang, H.Y.; Liu, Y.Y.; Tong, L.L.; Auwalu, A. The alteration mechanism of copper-bearing biotite and leachable property of copper-bearing minerals in Mulyashy copper mine, Zambia. Sci. Rep. 2019, 9, 15040. [Google Scholar] [CrossRef]
- Wang, G.R.; Liu, Y.Y.; Tong, L.L.; Jin, Z.N.; Chen, G.B.; Yang, H.Y. Effect of temperature on leaching behavior of copper minerals with different occurrence states in complex copper oxide ores. Trans. Nonferrous Met. Soc. China 2019, 29, 2192–2201. [Google Scholar] [CrossRef]
- Liu, Y.P. Application of novel technology in zambia luanshya Muliashi copper hydrometallurgical plant. Nonferr. Met. Eng. 2015, 5, 36–40. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.P.; Fu, Y.H.; Liu, Z.L.; Wang, P.L.; Yin, J.; Kou, J.; Sun, C.B.; Liu, W.X. Enhanced leaching of copper from refractory oxidized copper ore by calcium fluoride: Behavior and mechanism. Green Smart Min. Eng. 2024, 1, 85–95. [Google Scholar] [CrossRef]
- Liu, Y.P. Study on the test of enhanced agitation leaching of Muliashi low-grade complex copper oxide ore. Nonferr. Min. Metall. 2018, 34, 32–37. [Google Scholar]
- Manchisi, J.; Mhandu, T.J.; Kane, J.; Ndlovu, S. A hybrid leaching process to enhance the dissolution of cupriferous micas in the Chingola refractory ore. Hydrometallurgy 2019, 186, 151–161. [Google Scholar] [CrossRef]
- Whyte, R.M.; Schoeman, N.; Bowes, K.G. Processing of Konkola copper concentrates and Chingola refractory ore in a fully integrated hydrometallurgical pilot plant circuit. J. South. Afr. Inst. Min. Metall. 2001, 101, 427–436. [Google Scholar]
- Yu, B.Q.; Kou, J.; Sun, C.B.; Xing, Y. Extraction of copper from copper-bearing biotite by ultrasonic-assisted leaching. Int. J. Miner. Metall. Mater. 2022, 29, 212–217. [Google Scholar] [CrossRef]
- Yan, J.W.; Pan, D.A.; Li, B. Application of ultrasonic intensification in hydrometallurgy leaching process. Chin. J. Process. Eng. 2020, 20, 1241–1247. [Google Scholar] [CrossRef]
- Sun, G.R.; Jiang, M.Q.; Wang, S.X.; Fu, L.K.; Zuo, J.G.; Zhang, G.W.; Hu, Z.K.; Zhang, L.B. Enhancement of copper metal dissolution in sulfuric acid solution with oxygen and ultrasound. J. Mater. Res. Technol. 2023, 16, 5016–5027. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.M.; Huang, J.; Liu, T.; Zhao, J.; Zhang, G.B. Acid-Leaching of vanadium from stone coal with calcium fluoride addition. Chin. J. Rare Met. 2013, 37, 628–632. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Liu, J.E.; Zhou, J.W. Research progress on the influence of impurity ions on the corrosion resistance of anodes in zinc electrodeposition. Chin. Nonferrous Met. 2024, 53, 24–32. [Google Scholar]
- Zhou, F.; Zhang, L.S.; Wang, Z.W.; Zhang, Y.X.; Chi, R.A. Effect of surfactant addition on leaching process of weathered crust elution-deposited rare earth ores with magnesium sulfate. Int. J. Min. Sci. Technol. 2023, 33, 1045–1053. [Google Scholar] [CrossRef]
- Cai, Z.L.; Wang, Y.; Zhang, Y.M.; Tian, H.Q. Effects of surfactant on Bacillus mucilaginosus adsorption characteristics during vanadium bioleaching process. J. Environ. Chem. Eng. 2022, 10, 108961. [Google Scholar] [CrossRef]
- Ai, C.M.; Wu, A.X.; Wang, Y.M.; Hou, C.L. Optimization and mechanism analysis of surfactant accelerating leaching test. J. Cent. South. Univ. Technol. 2016, 23, 1032–1039. [Google Scholar] [CrossRef]
- Huang, Y.K.; Wang, D.S.; Liu, H.T.; Fan, G.X.; Peng, W.J.; Cao, Y.J. Selective complexation leaching of copper from copper smelting slag with the alkaline glycine solution: An effective recovery method of copper from secondary resource. Sep. Purif. Technol. 2023, 326, 124619. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wang, Y.Y.; Ke, Y.; Sun, Z.M.; Li, Y.; Peng, C.; Zhu, M.F.; Luo, Y.J.; Min, X.B. Facile and complete sulfuric acid leaching of zinc ferrite assisted by copper powder: Leaching mechanism and kinetics investigation. Sep. Purif. Technol. 2024, 328, 125090. [Google Scholar] [CrossRef]
- Li, Y.K.; Hou, D.S.; Ding, X.Y.; Kang, X.J.; Liu, Q.F. Influence of coal-measure kaolinite with different types on the preparation of kaolinite nanotube. Appl. Clay Sci. 2023, 246, 107179. [Google Scholar] [CrossRef]
- Wu, S.N.; Wu, P. Energetic and configurational mechanisms to facilitate mica nanosheets synthesis by organo-ammonium cation intercalation. Comput. Mater. Sci. 2023, 224, 112162. [Google Scholar] [CrossRef]
- Tamesue, S.; Saito, Y.; Toita, R. Salinity durable self-healing hydrogels as functional biomimetic systems based on the intercalation of polymer ions into mica. Polymer 2021, 228, 123870. [Google Scholar] [CrossRef]
- Alba, M.D.; Cota, A.; Osuna, F.J.; Pavón, E.; Perdigón, A.C.; Raffin, F. Bionanocomposites based on chitosan intercalation in designed swelling high-charged micas. Sci. Rep. 2019, 9, 10265. [Google Scholar] [CrossRef]
- Arslan, H.K.; Shekhah, O.; Wieland, D.C.F.; Paulus, M.; Sternemann, C.; Schroer, M.A.; Tiemeyer, S.; Tolan, M.; Fischer, R.A.; Wöll, C. Intercalation in layered metal-organic frameworks: Reversible inclusion of an extended π-system. J. Am. Chem. Soc. 2011, 13, 8158–8161. [Google Scholar] [CrossRef]
- Ismail, N.H.C.; Hashim, F.; Akil, H.M.; Salim, Z.A.S.A. Novel intercalation mechanism of cationic surfactants modified muscovite. Mater. Today Proc. 2022, 66, 4084–4087. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Z.L.; Li, Z.Q.; Huang, X.Y.; Xu, W.R.; Qi, T.G. Preparation and characterization of organic pillared hydromica. J. Wuhan Inst. Technol. 2017, 39, 243–247. [Google Scholar]
- Tomohiko, O.; Takumi, Y.; Taku, L. Kinetics of interlayer expansion of a layered silicate driven by caffeine intercalation in the water phase using transmission X-ray diffraction. J. Phys. Chem. 2017, 121, 6919–6925. [Google Scholar] [CrossRef]
- Sarkar, M.; Dana, K.; Ghatak, S. Evolution of molecular structure and conformation of n-alkylammonium intercalated iron rich bentonites. J. Mol. Struct. 2011, 1005, 161–166. [Google Scholar] [CrossRef]
- Smith, R.B.; Khoo, E.; Bazant, M.S. Intercalation kinetics in multiphase-layered materials. J. Phys. Chem. 2017, 121, 12505–12523. [Google Scholar] [CrossRef]
- Yao, D.H.; Huang, Z.L.; Li, Z.Q.; Wei, Y. Extracting potassium from hydromica by oxidation-pillar/ion exchange method. J. Wuhan Inst. Technol. 2017, 36, 607–610. [Google Scholar]
- Hu, M.W.; Wang, H.Q.; Xia, H.F.; Meng, P.; Luo, J.; Chen, Y.Q.; Chen, C.L.; Huang, Z.L. Potassium extraction from insoluble potassium mica mine with pillaring method. Non-Met. Min. 2014, 37, 14–18. [Google Scholar]
- Klimonda, A.; Kowalska, I. Application of polymeric membranes for the purification of solutions containing cationic surfactants. Water Sci. Technol. 2019, 79, 1241–1252. [Google Scholar] [CrossRef]
- Ye, X. Environmental harm of surfactant wastewater and its treatment technology. Sichuan Chem. Ind. 2019, 22, 11–13. [Google Scholar] [CrossRef]
- Srient, S.S.; Basak, A.; Ghosh, P.; Chatterjee, J. Separation of anionic surfactant in paste form from its aqueous solutions using foam fractionation. J. Environ. Chem. Eng. 2017, 5, 1586–1598. [Google Scholar] [CrossRef]
- Kumar, A.K.; Rawat, N.; Ghosh, P. Removal and recovery of a cationic surfactant from its aqueous solution by foam fractionation. J. Environ. Chem. Eng. 2020, 8, 103555. [Google Scholar] [CrossRef]
- Azar, E.; Blanc, C.; Mehdi, A.; Nobili, M.; Stocco, A. Mesoporous Silica Colloids: Wetting, Surface Diffusion, and Cationic Surfactant Adsorption. J. Phys. Chem. C 2019, 123, 26226–26235. [Google Scholar] [CrossRef]
- Liu, Y.P.; Fu, Y.H.; Yin, J.; Li, Y.; Deng, J.; Sun, C.B.; Kou, J. Study on leaching technology of acid and impurity control of refractory copper oxide from Luanshya. Met. Min. 2024, 8, 100–105. [Google Scholar] [CrossRef]
- Chen, A.Q.; Zhang, L.X.; Li, Q.; Zhu, J.X.; Li, S.Y.; He, H.P. Accuracy and error sources of the Rietveld Full Pattern Fitting method in quantitative analysis of illite ores. Rock. Miner. Anal. 2022, 41, 291–299. [Google Scholar] [CrossRef]
- Wei, Y. Research on Barium-Treatment and Organic Intercalation of Biotite. Master’s Dissertation, Wuhan Institute of Technology, Wuhan, China, 2018. [Google Scholar]
- Zhang, Y.; Wen, S.M. Copper Hydrometallurgy Theory and Practice, 1st ed.; Metallurgical Industry Press: Beijing, China, 2014. [Google Scholar]
- Zhu, R.L.; Zhu, L.Z.; Zhu, J.X.; Xu, L.H. Structure of cetyltrimethylammonium intercalated hydrobiotite. Appl. Clay Sci. 2007, 42, 224–231. [Google Scholar] [CrossRef]
- Li, Z.Q.; Wei, Y.; Meng, P.; Huang, Z.L. Effect of reaction temperature and time on biotite pillaring. J. Wuhan Inst. Technol. 2016, 38, 461–464. [Google Scholar] [CrossRef]
- Yu, B.Q. Mechanism Study on Heated Leaching of Cupriferous Mica-Type Copper Oxide Ore and Its Leaching Enhancement. Ph.D. Dissertation, University of Science and Technology Beijing, Beijing, China, 2022. [Google Scholar]
- Yuan, H.Y.; Dubbink, D.; Besselink, R.; Elshof, J.E. The rapid exfoliation and subsequent restacking of layered titanates driven by an acid–base reaction. Angew. Chem. Int. Ed. 2015, 54, 9239–9243. [Google Scholar] [CrossRef]
- Huang, Z.L.; Meng, P.; Li, Z.Q.; Chen, C.L.; Zhang, Z.L. Effect of different concentrations of pillared-reagent on biotite pillaring. J. Wuhan Inst. Technol. 2015, 37, 53–57. [Google Scholar]
- Meng, P.; Huang, Z.L.; Li, Z.Q.; Hu, M.W.; Chen, C.L.; Chi, R. Conditions and mechanism for extracting potassium from muscovite in potassium-bearing shale by the barium ion-exchange method. Int. J. Miner. Process. 2015, 142, 107–112. [Google Scholar] [CrossRef]
- Meng, P.; Huang, Z.L.; Li, Z.Q.; Wei, Y. Extraction of potassium from potassium-bearing hydromica by barium ion exchange/pillaring method, Collection. In Proceedings of the 15th Annual Symposium of Chinese Society of Mineral and Rock Geochemistry, Changchun, China, 23–29 June 2015; pp. 1–2. [Google Scholar]
- Zeng, G.M.; Bao, H.J.; Zhou, H.Y.; Huang, R.Q.; Chen, C.; Xu, Y.Q.; Ning, A. Study on the photo-degradation of cetyltrimethylammonium bromide. Chin. J. Pestic. Sci. 2012, 14, 229–232. [Google Scholar] [CrossRef]
- Wang, X.K.; Cheng, S.; Chen, B.M.; He, Y.P.; Huang, H.; Guo, Z.C. Study Status of Energy Saving Anodes and Electrolyte Ions for Copper Electrowinning. Mater. Prot. 2012, 14, 117–121. [Google Scholar] [CrossRef]
- Kuosa, M.; Ekberg, B.; Tanttu, L.; Häkkinen, A. Performance comparison of anthracite filter media of different origin in the removal of organic traces from copper electrolyte. Int. J. Miner. Process. 2017, 163, 24–34. [Google Scholar] [CrossRef]


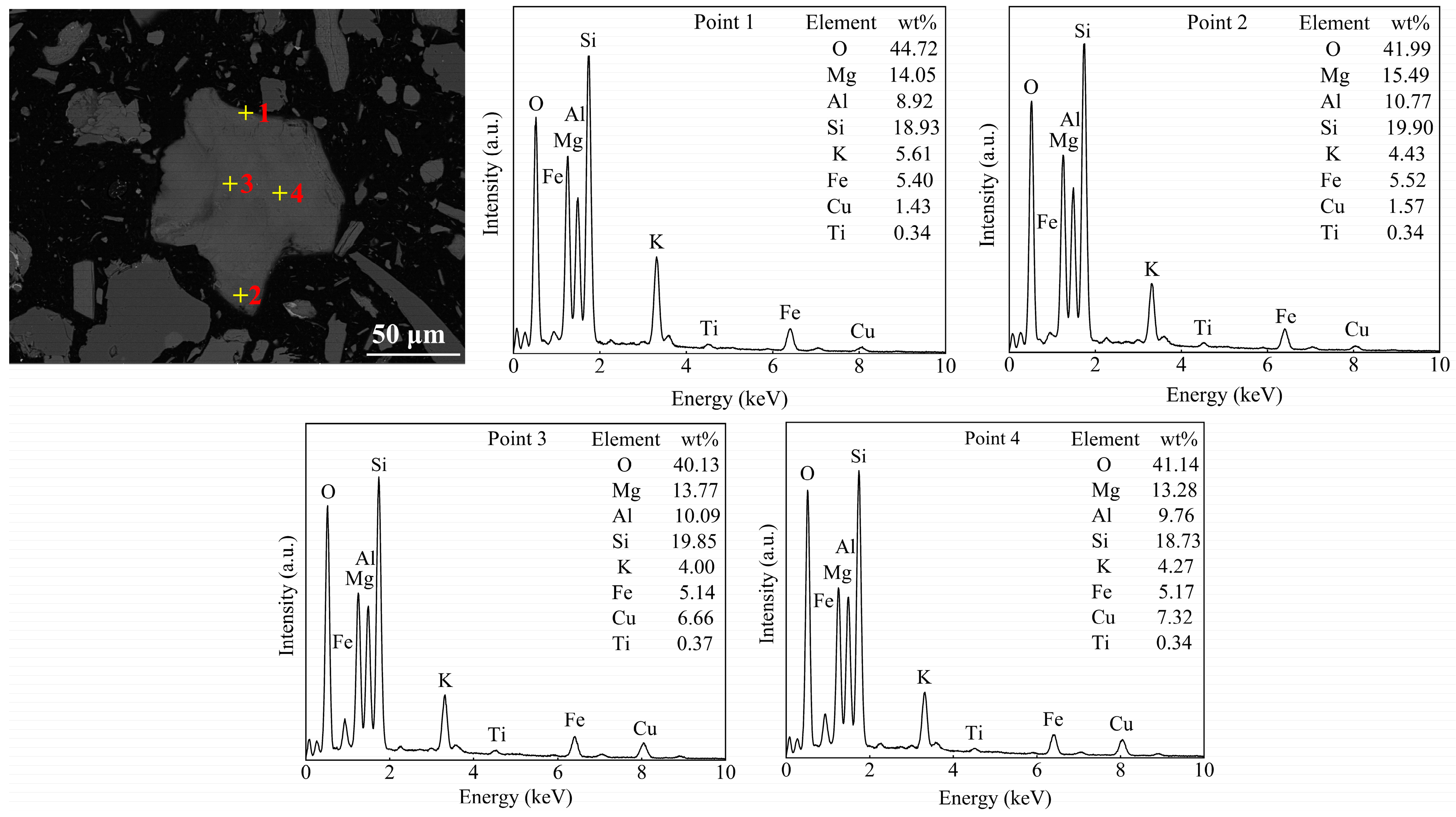



| Cu | Fe | Si | Al | K | Mg |
| 1.54 | 4.34 | 25.86 | 7.82 | 5.97 | 3.61 |
| Mn | Ti | Ca | Co | Na | S |
| 0.44 | 0.58 | 0.30 | 0.06 | 0.02 | 0.12 |
| Existing State | Content | Distribution Rate |
|---|---|---|
| Copper sulfate | 0.003 | 0.19 |
| Free copper oxide | 0.42 | 27.20 |
| Combined copper oxide | 0.91 | 58.94 |
| Primary copper sulfide | 0.08 | 5.12 |
| Secondary copper sulfide | 0.13 | 8.55 |
| Total copper | 1.54 | 100.00 |
| Reagents | Molecular Formula | Solubility | Cation Branched Chain Structure | Source |
|---|---|---|---|---|
| DTAB | C15H34NBr | Soluble in water | 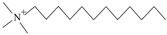 | Komio. Tianjin, China |
| TTAB | C17H38NBr | Soluble in water after heating | 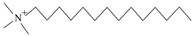 | Komio. Tianjin, China |
| CTAB | C19H42NBr | Soluble in water after heating | 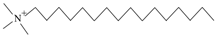 | Komio. Tianjin, China |
| DDAC | C22H48NCl | Soluble in water | 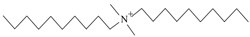 | Rhawn. Shanghai, China |
| DDAO | C14H31NO | Soluble in water | 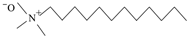 | Usolf. Shangdong, China |
| Quartz | Biotite | Feldspar | Sericite | Vermiculite | Kaolinite | Chlorite | Garnet | Hornblende |
| 35.44 | 25.83 | 12.65 | 9.41 | 2.25 | 3.20 | 2.98 | 0.59 | 0.52 |
| Sepiolite | Fayalite | Iron oxide | Tenorite | Cuprite | Libethenite | Brochantite | Sulfide copper | Other minerals |
| 0.51 | 0.68 | 1.80 | 0.46 | 0.01 | 0.11 | 0.09 | 0.10 | 3.37 |
| Existing State | Content | Distribution Rate |
|---|---|---|
| Free copper oxide | 0.02 | 5.13 |
| Combined copper oxide | 0.24 | 61.54 |
| Primary copper Sulfide | 0.05 | 12.82 |
| Secondary copper sulfide | 0.08 | 20.51 |
| Total copper | 0.39 | 100.00 |
| Item | a (Å) | b (Å) | c (Å) | α (deg) | β (deg) | γ (deg) |
|---|---|---|---|---|---|---|
| Biotite | 5.38 | 10.76 | 20.39 | 99.97 | 85.43 | 119.97 |
| Biotite with CTAB | 5.63 | 10.53 | 23.43 | 100.6 | 84.28 | 118.22 |
| Conditions and Item | Enhanced Leaching | Regular Leaching |
|---|---|---|
| Dosage of sulfuric acid (kg∙t−1) | 45 | |
| Leaching temperature (°C) | 65 | |
| Copper leaching rate (%) | 78.32 | 75.26 |
| Leaching time (min) | 120 | 200 |
| Dosage of CTAB (g∙t−1) | 75 | / |
| CTAB cost (USD∙t−1) | 3.75–7.00 | / |
| Heating power (kWh∙t−1) | 81.91 | |
| Total energy consumption (kWh∙t−1) | 163.82 | 273.03 |
| Heating power cost (USD∙t−1) | 19.65 | 32.76 |
| Total heating cost-saving (USD∙t−1) | 6.11–9.36 | / |
| Merits | Demerits |
|---|---|
| (1) CTAB markedly accelerates the leaching rate of cupriferous biotite-type copper oxide ore, substantially diminishing the necessary leaching period. (2) CTAB demonstrates excellent biodegradability and photodegradation, easily decomposes in the natural environment, especially suitable for the African environment with strong light, thereby facilitating environmentally sustainable treatment of leaching residue [49]. (3) In comparison to leaching adds such as calcium fluoride, CTAB demands a considerably lower dosage-less than 0.01 wt% of the total raw ore mass [7]. | (1) CTAB itself is toxic and introduces Br− into the system. Although the corrosiveness of Br− toward stainless-steel equipment is less severe than that of Cl− and F−, it remains non-negligible [50]. (2) Moreover, the organic cations in CTAB may compete with Cu2+ for extractants during the solvent extraction, potentially resulting in increased extra consumption of the extractant. (3) Additionally, in the context of oil removal from copper electrowinning solutions, CTAB is generally regarded as a dissolved organic contaminant once solubilized in water. Its presence in the electrowinning system may detrimentally impact the quality of the cathode copper [51]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Kou, J.; Sun, C.; Wang, P.; Wang, X. Enhancing Copper Leaching from Refractory Copper Oxide Ore Using Organic Cationic Surfactant. Separations 2025, 12, 212. https://doi.org/10.3390/separations12080212
Li Y, Kou J, Sun C, Wang P, Wang X. Enhancing Copper Leaching from Refractory Copper Oxide Ore Using Organic Cationic Surfactant. Separations. 2025; 12(8):212. https://doi.org/10.3390/separations12080212
Chicago/Turabian StyleLi, Yang, Jue Kou, Chunbao Sun, Peilong Wang, and Xiaoli Wang. 2025. "Enhancing Copper Leaching from Refractory Copper Oxide Ore Using Organic Cationic Surfactant" Separations 12, no. 8: 212. https://doi.org/10.3390/separations12080212
APA StyleLi, Y., Kou, J., Sun, C., Wang, P., & Wang, X. (2025). Enhancing Copper Leaching from Refractory Copper Oxide Ore Using Organic Cationic Surfactant. Separations, 12(8), 212. https://doi.org/10.3390/separations12080212







