Simultaneous Determination of 23 Trans Fatty Acids in Common Edible Oils by Gas Chromatography-Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Sample Preparation
2.4. GC-MS Detection
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chromatographic Separation of 23 Kinds of TFAMEs
3.2. Method Validation Results
3.3. The Profile of TFAs in Edible Oil
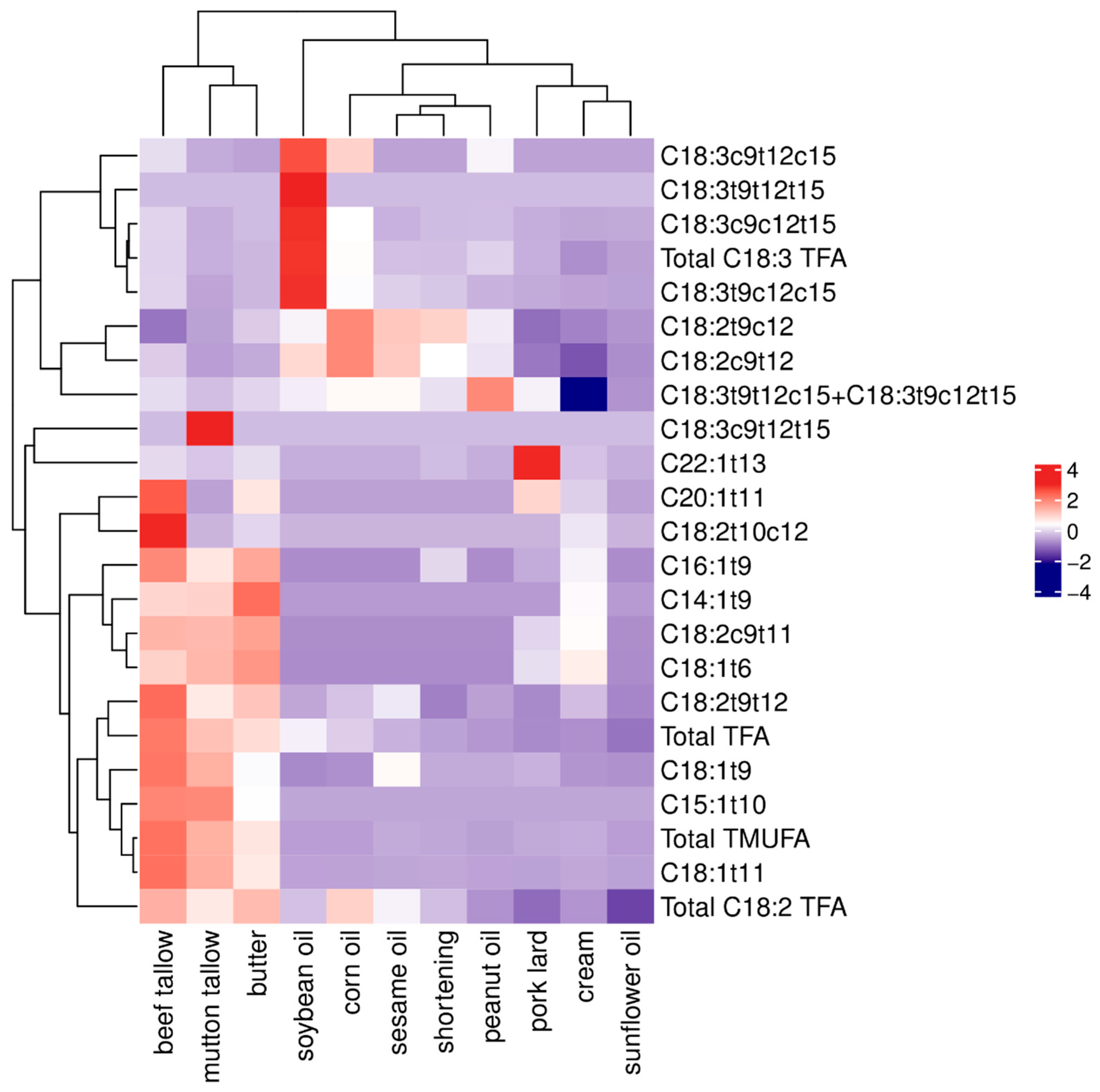
3.4. Cluster Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans Fat Intake and Its Dietary Sources in General Populations Worldwide: A Systematic Review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Enjalbert, F.; Zened, A.; Cauquil, L.; Meynadier, A. Integrating Data from Spontaneous and Induced trans-10 Shift of Ruminal Biohydrogenation Reveals Discriminant Bacterial Community Changes at the OTU Level. Front. Microbiol. 2023, 13, 1012341. [Google Scholar] [CrossRef]
- Niforou, A.; Magriplis, E.; Klinaki, E.; Niforou, K.; Naska, A. On Account of Trans Fatty Acids and Cardiovascular Disease Risk—There Is Still Need to Upgrade the Knowledge and Educate Consumers. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1811–1818. [Google Scholar] [CrossRef]
- Oteng, A.B.; Kersten, S. Mechanisms of Action of Trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Harvey, K.A.; Arnold, T.; Rasool, T.; Antalis, C.; Miller, S.J.; Siddiqui, R.A. Trans-Fatty Acids Induce Pro-Inflammatory Responses and Endothelial Cell Dysfunction. Br. J. Nutr. 2008, 99, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Pase, C.S.; Bürger, M.E. Trans Fat Intake and Behavior. In The Molecular Nutrition of Fats; Academic Press: Cambridge, MA, USA, 2019; pp. 189–197. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Guo, J.; Zhao, T.; Tang, H.; Jin, K.; Li, L.; Xue, Y.; Yang, R.; Chen, J.; et al. Abnormal Erythrocyte Fatty Acid Composition in First-Diagnosed, Drug-Naïve Patients with Depression. J. Affect. Disord. 2022, 318, 414–422. [Google Scholar] [CrossRef]
- Song, G.; Zhang, M.; Peng, X.; Yu, X.; Dai, Z.; Shen, Q. Effect of Deodorization Method on the Chemical and Nutritional Properties of Fish Oil during Refining. LWT-Food Sci. Technol. 2018, 96, 560–567. [Google Scholar] [CrossRef]
- El-Aal, Y.A.A.; Abdel-Fattah, D.M.; Ahmed, K.E.-D. Some Biochemical Studies on Trans Fatty Acid-Containing Diet. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Fat Intake and Risk of Cardiovascular Disease and All-Cause Mortality in a Population at High Risk of Cardiovascular Disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty Acid Profiles of Milk from Holstein Cows, Jersey Cows, Buffalos, Yaks, Humans, Goats, Camels, and Donkeys Based on Gas Chromatography–Mass Spectrometry. J. Dairy Sci. 2022, 105, 1687–1700. [Google Scholar] [CrossRef]
- Fang, H.; Cao, M.; Zhang, X.; Wang, K.; Deng, T.; Lin, J.; Liu, R.; Wang, X.; Liu, A. The Assessment of Trans Fatty Acid Composition in Edible Oil of Different Brands and Regions in China in 2021. Food Chem. 2023, 121, 105394. [Google Scholar] [CrossRef]
- Amores, G.; Virto, M. Total and Free Fatty Acids Analysis in Milk and Dairy Fat. Separations 2019, 6, 14. [Google Scholar] [CrossRef]
- Pojjanapornpun, S.; Nakornsadet, A.; Sombutsuwan, P.; Chumsantea, S.; Krisnangkura, K.; Aryusuk, K. Simulation of Fatty Acid Peak Profile on a 100-m CP-Sil 88 Column and Application for Analysis of Commercial Trans Fat Alternatives in Thailand. J. Food Compos. Anal. 2020, 91, 103525. [Google Scholar] [CrossRef]
- Bravo-Lamas, L.; Aldai, N.; Kramer, J.K.G.; Barron, L.J.R. Case Study Using Commercial Dairy Sheep Flocks: Comparison of the Fat Nutritional Quality of Milk Produced in Mountain and Valley Farms. LWT-Food Sci. Technol. 2018, 89, 374–380. [Google Scholar] [CrossRef]
- Momchilova, S.M.; Nikolova-Damyanova, B.M. Advances in Silver Ion Chromatography for the Analysis of Fatty Acids and Triacylglycerols—2001 to 2011. Anal. Sci. 2012, 28, 837–844. [Google Scholar] [CrossRef]
- Precht, D.; Molkentin, J.; Destaillats, F.; Wolff, R.L. Comparative Studies on Individual Isomeric 18:1 Acids in Cow, Goat, and Ewe Milk Fats by Low-Temperature High-Resolution Capillary Gas-Liquid Chromatography. Lipids 2001, 36, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alcala, L.M.; Alonso, L.; Fontecha, J. Stability of Fatty Acid Composition after Thermal, High Pressure, and Microwave Processing of Cow Milk as Affected by Polyunsaturated Fatty Acid Concentration. J. Dairy Sci. 2014, 97, 7307–7315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, X.; Zhao, H.T.; Dong, A.J.; Wang, J.; Liu, G.Y.; Wang, P.; Cheng, C.; Zhang, H. A Quick Method for Routine Analysis of C18 Trans Fatty Acids in Non-Hydrogenated Edible Vegetable Oils by Gas Chromatography–Mass Spectrometry. Food Control 2015, 57, 293–301. [Google Scholar] [CrossRef]
- Sarwar, S.; Shaheen, N.; Ashraf, M.M.; Bahar, N.; Akter, F.; Hossain, M.; Mridha, M.K.; Kajal, M.A.M.; Bin Rasul, S.S.; Khondker, R.; et al. Fatty Acid Profile Emphasizing Trans-Fatty Acids in Commercially Available Soybean and Palm Oils and Its Probable Intake in Bangladesh. Food Chem. Adv. 2024, 4, 100611. [Google Scholar] [CrossRef]
- Ganguly, R.; Pierce, G.N. The Toxicity of Dietary Trans Fats. Food Chem. Toxicol. 2015, 78, 170–176. [Google Scholar] [CrossRef]
- Baltacıoğlu, C. Effect of Different Frying Methods on the Total Trans Fatty Acid Content and Oxidative Stability of Oils. J. Am. Oil Chem. Soc. 2017, 94, 923–934. [Google Scholar] [CrossRef]
- Field, C.J.; Blewett, H.H.; Proctor, S.; Vine, D. Human Health Benefits of Vaccenic Acid. Appl. Physiol. Nutr. Metab. 2009, 34, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.M.; Edel, A.L.; Patenaude, A.F.; McCullough, R.S.; Blackwood, D.P.; Chouinard, P.Y.; Paquin, P.; Lamarche, B.; Pierce, G.N. Dietary Vaccenic Acid Has Antiatherogenic Effects in LDLr-/- Mice. J. Nutr. 2010, 140, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Wang, F.; Zheng, N.; Wang, J. Simultaneous Determination of C18 Fatty Acids in Milk by GC-MS. Separations 2021, 8, 118. [Google Scholar] [CrossRef]
- Teng, F.; Wang, P.; Yang, L.; Ma, Y.; Day, L. Quantification of Fatty Acids in Human, Cow, Buffalo, Goat, Yak, and Camel Milk Using an Improved One-Step GC-FID Method. Food Anal. Methods 2017, 10, 2881–2891. [Google Scholar] [CrossRef]
- Mavlanov, U.; Czaja, T.P.; Nuriddinov, S.; Dalimova, D.; Dragsted, L.O.; Engelsen, S.B.; Khakimov, B. The Effects of Industrial Processing and Home Cooking Practices on Trans-Fatty Acid Profiles of Vegetable Oils. Food Chem. 2025, 469, 142571. [Google Scholar] [CrossRef]

| No. | Trans Fatty Acid (TFA) | Windows (No.) | Retention Time (min) | Quantitative Ion (m/z) | Qualitative Ion (m/z) | Dwell Time (ms) | LOQ (ppb) | Linearity Range (ppb) | Standard Curve Regression Equation (ppb) | R2 | FAME-FA Conversion Coefficient | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | C10:1 c4 | 1 | 25.8 | 74 | 110 | 152 | 96 | 12 | / | / | / | / | / |
| 1 | C14:1 t9 | 42.9 | 166 | 87 | 74 | 208 | 12 | 10 | 5–250 | Y = 2.1238x − 22.6676 | 0.9998 | 0.9417 | |
| 2 | C15:1 t10 | 2 | 48.4 | 74 | 69 | 180 | 222 | 12 | 10 | 5–250 | Y = 11.5450x − 131.148 | 0.9996 | 0.9449 |
| 3 | C16:1 t9 | 3 | 54.43 | 194 | 69 | 74 | 236 | 12 | 10 | 5–250 | Y = 6.5063x − 250.9835 | 0.9997 | 0.9477 |
| 4 | C17:1 t10 | 4 | 62.9 | 208 | 74 | 69 | 250 | 12 | 20 | 10–500 | Y = 1.5149x − 76.8526 | 0.9991 | 0.9503 |
| 5 | C18:1 t6 | 5 | 72.7 | 74 | 69 | 222 | 264 | 12 | 20 | 10–500 | Y = 9.0349x − 405.8 | 0.9993 | 0.9527 |
| 6 | C18:1 t9 | 73.0 | 74 | 69 | 222 | 264 | 12 | 10 | 5–250 | Y = 38.7466x − 763.8356 | 0.9998 | ||
| 7 | C18:1 t11 | 73.7 | 74 | 69 | 222 | 264 | 12 | 30 | 15–3000 | Y = 3.5880x − 174.2697 | 0.9991 | ||
| 8 | C18:2 t9t12 | 6 | 82.4 | 294 | 67 | 81 | 263 | 10 | 10 | 125–5000 | Y = 11.9951x − 6754.5248 | 0.9994 | 0.9524 |
| 9 | C18:2 c9t12 | 85.3 | 294 | 67 | 81 | 263 | 10 | 10 | 50–2000 | Y = 10.3814x − 3425.0548 | 0.9991 | ||
| 10 | C18:2 t9c12 | 86.9 | 294 | 67 | 81 | 263 | 10 | 10 | 50–2000 | Y = 9.6048x + 3268.9916 | 0.9990 | ||
| 11 | C19:1 t7 | 87.1 | 278 | 74 | 236 | 194 | 10 | 10 | 5–250 | Y = 0.7035x + 0.4895 | 0.9991 | 0.9548 | |
| 12 | C19:1 t10 | 88.0 | 278 | 69 | 236 | 194 | 10 | 20 | 10–500 | Y = 0.9160x − 65.8606 | 0.9994 | ||
| 13 | C18:3 t9t12t15 | 7 | 97.7 | 79 | 67 | 121 | 292 | 8 | 60 | 75–1500 | Y = 12.0283x − 8181.0863 | 0.9995 | 0.9520 |
| 14 | C18:3 t9t12c15 | 101.2 | 79 | 67 | 121 | 292 | 8 | 60 | 75–1500 | Y = 10.2026x − 649.0786 | 0.9990 | ||
| 15 | C18:3 t9c12t15 | ||||||||||||
| 16 | C18:3 c9c12t15 | 102.6 | 79 | 67 | 121 | 292 | 8 | 20 | 17.5–3500 | Y = 10.2953x − 1140.8669 | 0.9994 | ||
| 17 | C18:3 c9t12t15 | 103.2 | 79 | 67 | 121 | 292 | 8 | 40 | 37.5–750 | Y = 7.3719x − 1904.6952 | 0.9993 | ||
| 18 | C18:3 c9t12c15 | 106.0 | 79 | 67 | 121 | 292 | 8 | 20 | 17.5–350 | Y = 8.0243x − 1368.4318 | 0.9991 | ||
| 19 | C18:3 t9c12c15 | 106.8 | 79 | 67 | 121 | 292 | 8 | 20 | 17.5–3500 | Y = 10.2532x − 1452.9265 | 0.9994 | ||
| 20 | C20:1 t11 | 107.2 | 250 | 69 | 208 | 292 | 8 | 20 | 20–1000 | Y = 0.3553x − 1.5752 | 0.9994 | 0.9568 | |
| 21 | C18:2 c9t11 | 8 | 113.0 | 294 | 67 | 81 | 149 | 8 | 20 | 10–2000 | Y = 0.6747x − 718.5914 | 0.9991 | 0.9524 |
| 22 | C18:2 t10c12 | 115.8 | 294 | 67 | 81 | 149 | 8 | 20 | 10–2000 | Y = 0.5267x − 467.0668 | 0.9995 | ||
| 23 | C22:1 t13 | 9 | 134.4 | 74 | 69 | 320 | 236 | 8 | 10 | 5–250 | Y = 0.5267x − 467.0668 | 0.9995 | 0.9602 |
| Trans Fatty Acid (TFA) | Concentration Multiple | Sunflower Oil | Pork Lard | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 mg/kg | 100 mg/kg | 200 mg/kg | 25 mg/kg | 100 mg/kg | 200 mg/kg | ||||||||
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | Recovery (%) | CV (%) | Recovery (%) | CV (%) | Recovery (%) | CV (%) | Recovery (%) | CV (%) | ||
| C14:1 t9 | 1 | 82.5 | 5.1 | 87.8 | 4.5 | 89.2 | 6.4 | 83.4 | 7.7 | 84.2 | 3.5 | 89.7 | 4.9 |
| C15:1 t10 | 1 | 83.2 | 4.2 | 85.3 | 4.6 | 89.8 | 7.5 | 81.2 | 6.2 | 83.4 | 4.3 | 88.4 | 7.2 |
| C16:1 t9 | 1 | 86.5 | 8.5 | 86.4 | 6.5 | 88.7 | 4.6 | NA | |||||
| C17:1 t10 | 2 | 85.4 | 4.6 | 85.4 | 4.6 | 90.2 | 5.5 | 81.5 | 4.4 | 85.2 | 5.6 | 89.7 | 8.9 |
| C18:1 t6 | 2 | 89.7 | 8.9 | 83.6 | 4.4 | 91.4 | 4.1 | NA | |||||
| C18:1 t9 | 1 | NA | NA | ||||||||||
| C18:1 t11 | 3 | NA | NA | ||||||||||
| C18:2 t9t12 | 5 | NA | NA | ||||||||||
| C18:2 c9t12 | 2 | NA | NA | ||||||||||
| C18:2 t9c12 | 2 | NA | NA | ||||||||||
| C19:1 t7 | 1 | 88.7 | 3.8 | 89.7 | 6.9 | 90.2 | 8.6 | 79.3 | 3.5 | 81.2 | 7.2 | 89.9 | 3.6 |
| C19:1 t10 | 2 | 83.5 | 4.5 | 88.2 | 7.1 | 89.7 | 3.5 | 78.5 | 4.7 | 82.2 | 6.9 | 91.3 | 4.7 |
| C18:3 t9t12t15 | 4 | 84.3 | 7.3 | 88.6 | 5.4 | 91.5 | 7.4 | 81.2 | 5.1 | 83.6 | 4.6 | 91.3 | 7.3 |
| C18:3 t9t12c15 | 4 | NA | NA | ||||||||||
| +C18:3 t9c12t15 | |||||||||||||
| C18:3 c9c12t15 | 1 | NA | NA | ||||||||||
| C18:3 c9t12t15 | 2 | 79.6 | 5.4 | 83.5 | 3.5 | 91.2 | 6.2 | 83.6 | 7.4 | 88.1 | 2.3 | 90.2 | 6.7 |
| C18:3 c9t12c15 | 1 | 88.1 | 5.5 | 89.7 | 2.4 | 90.6 | 9.1 | 82.4 | 3.8 | 85.7 | 3.8 | 92.6 | 3.8 |
| C18:3 t9c12c15 | 1 | NA | NA | ||||||||||
| C20:1 t11 | 1 | 75.4 | 5.3 | 79.8 | 5.3 | 86.5 | 3.7 | NA | |||||
| C18:2 c9t11 | 4 | 81.5 | 6.6 | 85.5 | 4.3 | 85.4 | 5.9 | NA | |||||
| C18:2 t10c12 | 4 | 82.3 | 3.8 | 83.7 | 2.9 | 86.4 | 8.6 | 83.3 | 4.0 | 86.9 | 3.9 | 90.6 | 3.7 |
| C22:1 t13 | 1 | 79.6 | 4.2 | 81.3 | 3.7 | 85.1 | 4.9 | NA | |||||
| Trans Fatty Acid (TFA) | Soybean Oil n = 13 | Peanut Oil n = 22 | Coil Oil n = 15 | Sunflower Oil n = 14 | Sesame Oil n = 17 | Pork Lard n = 18 | Beef Tallow n = 11 | Mutton Tallow n = 12 | Butter n = 20 | Cream n = 17 | Shortening n = 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:1 t9 | ND | ND | ND | ND | ND | ND | 0.009 ± 0.002 | 0.009 ± 0.004 | 0.017 ± 0.008 | 0.006 ± 0.003 | ND |
| C15:1 t10 | ND | ND | ND | ND | ND | ND | 0.008 ± 0.003 | 0.008 ± 0.003 | 0.003 ± 0.003 | ND | ND |
| C16:1 t9 | ND | ND | ND | ND | ND | 0.014 ± 0.003 | 0.122 ± 0.051 | 0.067 ± 0.035 | 0.105 ± 0.085 | 0.046 ± 0.054 | 0.034 ± 0.011 |
| C17:1 t10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| C18:1 t6 | ND | ND | ND | ND | ND | 0.034 ± 0.014 | 0.074 ± 0.081 | 0.090 ± 0.063 | 0.107 ± 0.105 | 0.058 ± 0.045 | ND |
| C18:1 t9 | 0.027 ± 0.016 | 0.056 ± 0.026 | 0.033 ± 0.014 | 0.033 ± 0.022 | 0.143 ± 0.043 | 0.063 ± 0.024 | 0.303 ± 0.136 | 0.235 ± 0.126 | 0.131 ± 0.143 | 0.037 ± 0.022 | 0.056 ± 0.019 |
| C18:1 t11 | 0.022 ± 0.013 | 0.022 ± 0.019 | 0.012 ± 0.004 | 0.016 ± 0.008 | 0.041 ± 0.025 | 0.019 ± 0.021 | 2.538 ± 1.050 | 1.840 ± 0.708 | 1.120 ± 0.620 | 0.059 ± 0.039 | 0.055 ± 0.045 |
| C18:2 t9t12 | 0.078 ± 0.009 | 0.077 ± 0.007 | 0.086 ± 0.010 | 0.069 ± 0.009 | 0.097 ± 0.031 | 0.070 ± 0.004 | 0.158 ± 0.083 | 0.112 ± 0.015 | 0.125 ± 0.032 | 0.085 ± 0.045 | 0.068 ± 0.002 |
| C18:2 c9t12 | 0.474 ± 0.252 | 0.329 ± 0.131 | 0.670 ± 0.539 | 0.171 ± 0.105 | 0.509 ± 0.120 | 0.129 ± 0.084 | 0.285 ± 0.106 | 0.199 ± 0.061 | 0.222 ± 0.095 | 0.060 ± 0.045 | 0.380 ± 0.063 |
| C18:2 t9c12 | 0.258 ± 0.089 | 0.246 ± 0.102 | 0.482 ± 0.421 | 0.135 ± 0.093 | 0.373 ± 0.086 | 0.082 ± 0.019 | 0.092 ± 0.015 | 0.151 ± 0.055 | 0.206 ± 0.155 | 0.110 ± 0.085 | 0.357 ± 0.107 |
| C19:1 t7 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| C19:1 t10 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| C18:3 t9t12t15 | 0.083 ± 0.008 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| C18:3 t9t12c15 + C18:3 t9c12t15 | 0.115 ± 0.012 | 0.180 ± 0.025 | 0.125 ± 0.019 | 0.081 ± 0.045 | 0.125 ± 0.014 | 0.116 ± 0.027 | 0.108 ± 0.019 | 0.097 ± 0.007 | 0.106 ± 0.011 | 0.022 ± 0.042 | 0.110 ± 0.009 |
| C18:3 c9c12t15 | 0.613 ± 0.191 | 0.057 ± 0.096 | 0.171 ± 0.244 | 0.019 ± 0.024 | 0.035 ± 0.028 | 0.029 ± 0.015 | 0.096 ± 0.074 | 0.029 ± 0.018 | 0.054 ± 0.028 | 0.016 ± 0.010 | 0.055 ± 0.025 |
| C18:3 c9t12t15 | ND | ND | ND | ND | ND | ND | ND | 0.025 ± 0.037 | ND | ND | ND |
| C18:3 c9t12c15 | 0.094 ± 0.031 | 0.025 ± 0.009 | 0.045 ± 0.020 | ND | ND | ND | 0.018 ± 0.016 | 0.003 ± 0.005 | ND | ND | ND |
| C18:3 t9c12c15 | 0.466 ± 0.162 | 0.024 ± 0.011 | 0.123 ± 0.211 | 0.003 ± 0.007 | 0.063 ± 0.152 | 0.013 ± 0.038 | 0.068 ± 0.058 | 0.006 ± 0.011 | 0.031 ± 0.026 | 0.005 ± 0.010 | 0.053 ± 0.016 |
| C20:1 t11 | ND | ND | ND | ND | ND | 0.024 ± 0.023 | 0.049 ± 0.056 | ND | 0.020 ± 0.030 | 0.007 ± 0.021 | ND |
| C18:2 c9t11 | ND | ND | ND | ND | ND | 0.234 ± 0.124 | 0.690 ± 0.278 | 0.668 ± 0.322 | 0.767 ± 0.613 | 0.382 ± 0.276 | ND |
| C18:2 t10c12 | ND | ND | ND | ND | ND | ND | 0.164 ± 0.023 | ND | 0.016 ± 0.050 | 0.024 ± 0.069 | ND |
| C22:1 t13 | ND | ND | ND | ND | ND | 0.017 ± 0.012 | 0.002 ± 0.002 | 0.001 ± 0.002 | 0.002 ± 0.003 | 0.001 ± 0.002 | 0.001 ± 0.001 |
| ∑TMUFA | 0.048 ± 0.029 | 0.079 ± 0.034 | 0.045 ± 0.018 | 0.049 ± 0.029 | 0.185 ± 0.056 | 0.172 ± 0.036 | 3.106 ± 1.155 | 2.250 ± 0.747 | 1.506 ± 0.746 | 0.214 ± 0.123 | 0.147 ± 0.043 |
| ∑ C18:2 TFA | 0.811 ± 0.327 | 0.651 ± 0.231 | 1.238 ± 0.954 | 0.376 ± 0.103 | 0.979 ± 0.191 | 0.515 ± 0.160 | 1.388 ± 0.349 | 1.130 ± 0.363 | 1.337 ± 0.651 | 0.660 ± 0.417 | 0.804 ± 0.154 |
| ∑ C18:3 TFA | 1.371 ± 0.334 | 0.286 ± 0.107 | 0.464 ± 0.484 | 0.103 ± 0.033 | 0.222 ± 0.146 | 0.162 ± 0.054 | 0.290 ± 0.146 | 0.160 ± 0.054 | 0.191 ± 0.055 | 0.044 ± 0.052 | 0.218 ± 0.039 |
| ∑ TFA | 2.216 ± 0.595 | 1.016 ± 0.319 | 1.748 ± 1.169 | 0.528 ± 0.103 | 1.386 ± 0.257 | 0.849 ± 0.195 | 4.784 ± 1.282 | 3.540 ± 0.863 | 3.034 ± 1.216 | 0.917 ± 0.537 | 1.169 ± 0.208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Li, X.; Wang, K.; Wu, X.; Zhang, J.; Wang, F. Simultaneous Determination of 23 Trans Fatty Acids in Common Edible Oils by Gas Chromatography-Mass Spectrometry. Separations 2025, 12, 171. https://doi.org/10.3390/separations12070171
Cao Y, Li X, Wang K, Wu X, Zhang J, Wang F. Simultaneous Determination of 23 Trans Fatty Acids in Common Edible Oils by Gas Chromatography-Mass Spectrometry. Separations. 2025; 12(7):171. https://doi.org/10.3390/separations12070171
Chicago/Turabian StyleCao, Yanping, Xia Li, Kun Wang, Xianpeng Wu, Jie Zhang, and Fengen Wang. 2025. "Simultaneous Determination of 23 Trans Fatty Acids in Common Edible Oils by Gas Chromatography-Mass Spectrometry" Separations 12, no. 7: 171. https://doi.org/10.3390/separations12070171
APA StyleCao, Y., Li, X., Wang, K., Wu, X., Zhang, J., & Wang, F. (2025). Simultaneous Determination of 23 Trans Fatty Acids in Common Edible Oils by Gas Chromatography-Mass Spectrometry. Separations, 12(7), 171. https://doi.org/10.3390/separations12070171





