Extraction of Terpenoids from Pine Needle Biomass Using Dimethyl Ether
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Micropyrolysis
2.3. 2D-GC Separation
2.4. MS Detection
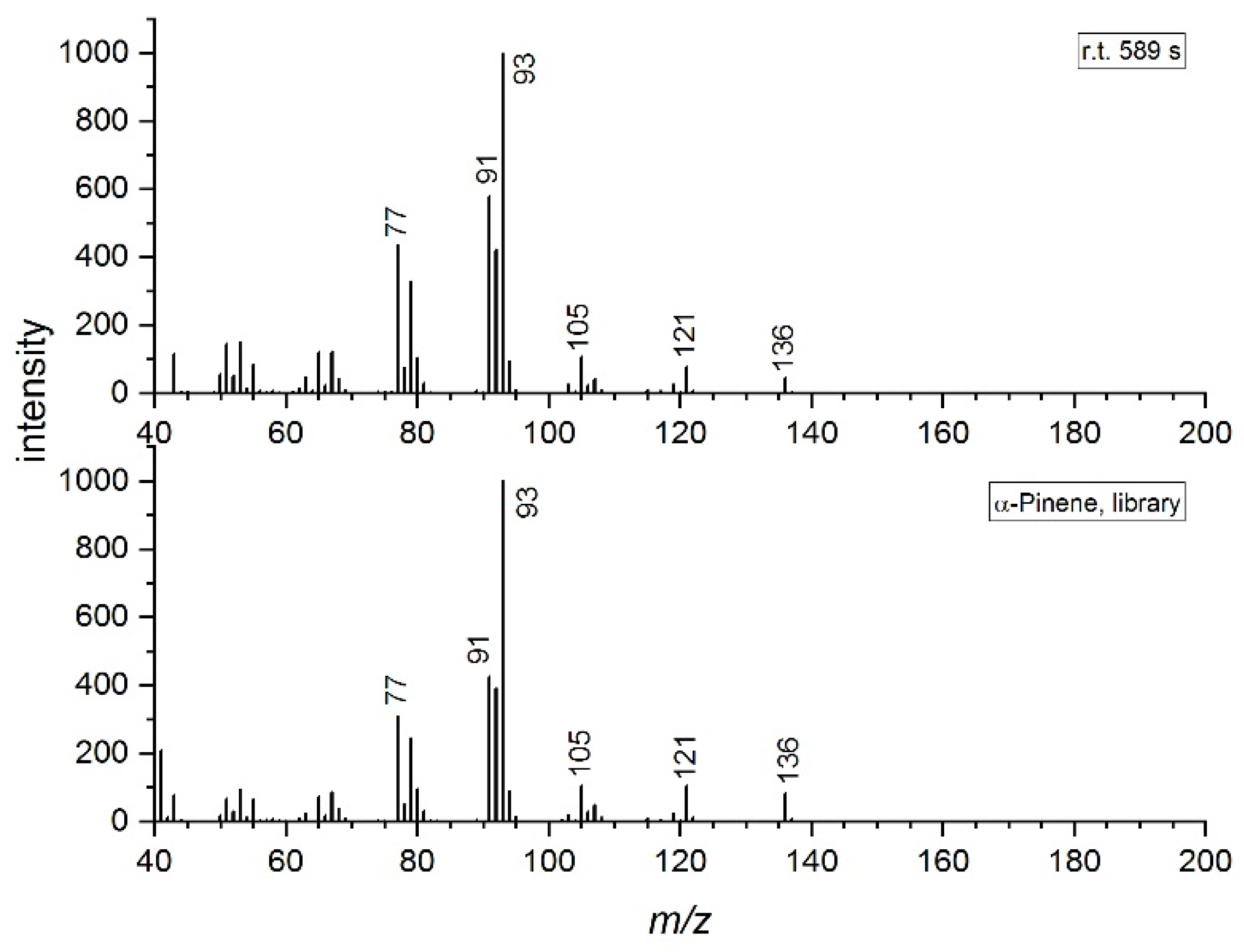
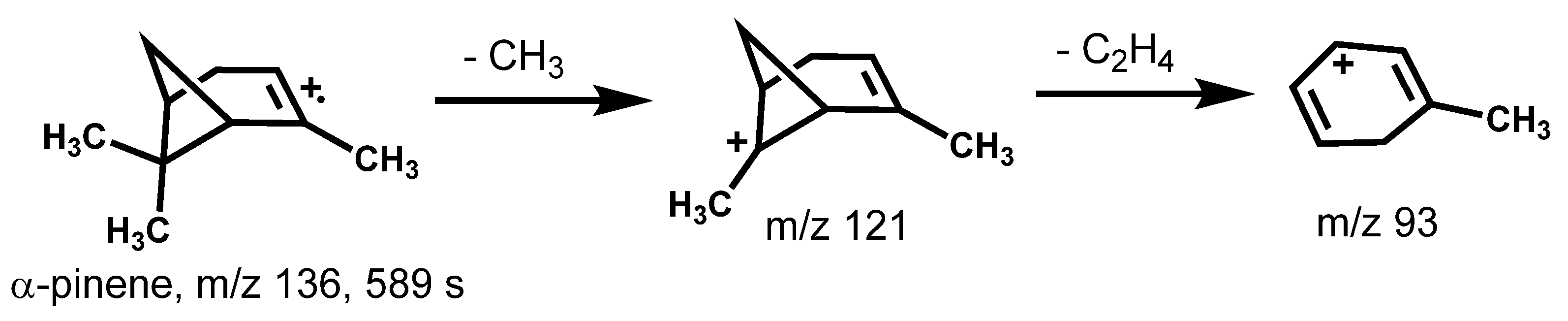
2.5. Compound Identification
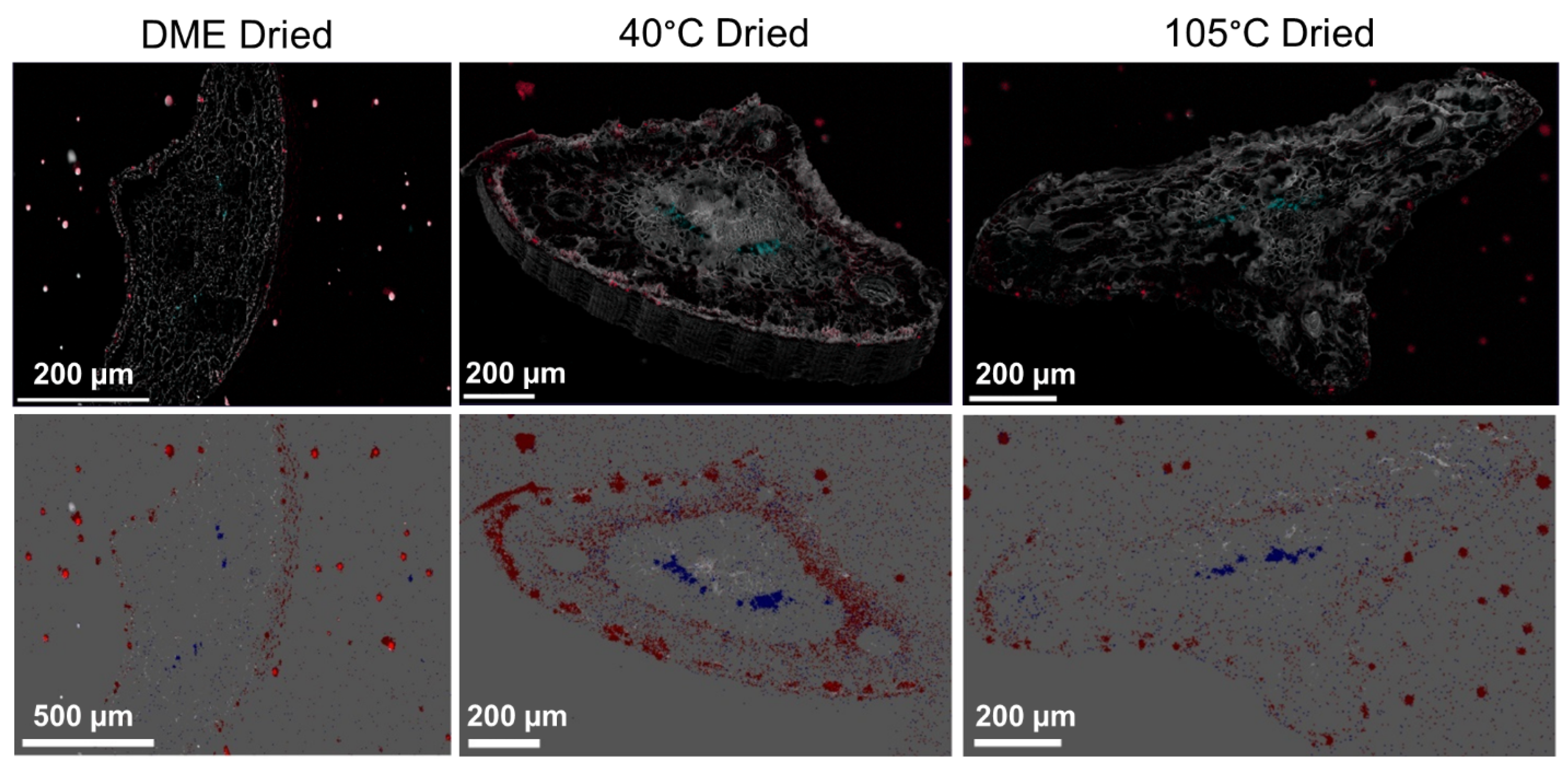
2.6. Sample Preparation and SEM-EDS Imaging
3. Results
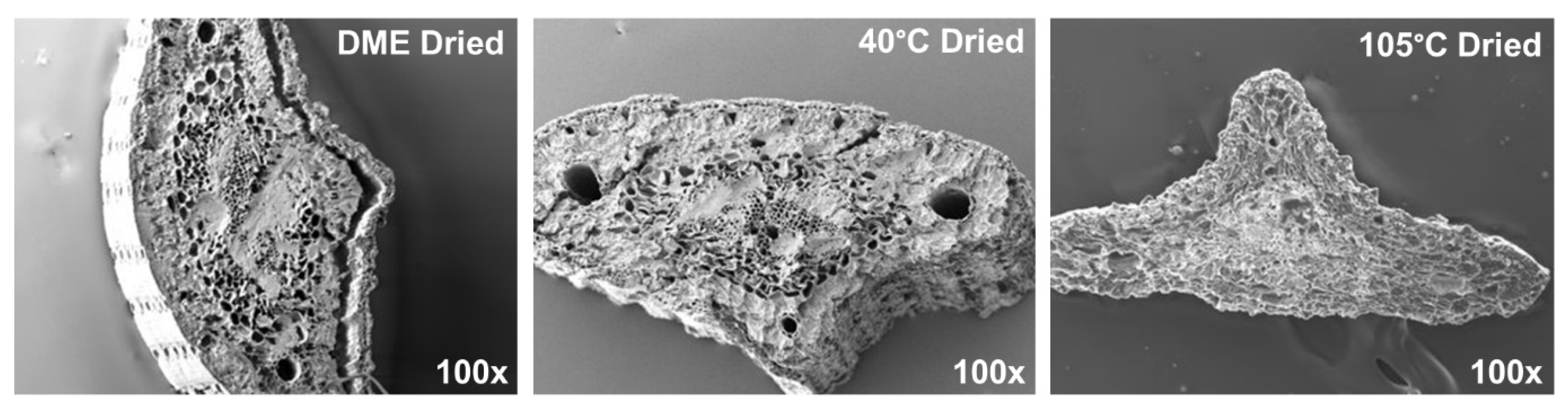
3.1. Structural Changes to Pine Needle Material
3.2. Analysis of the THF-Soluble Terpenoids
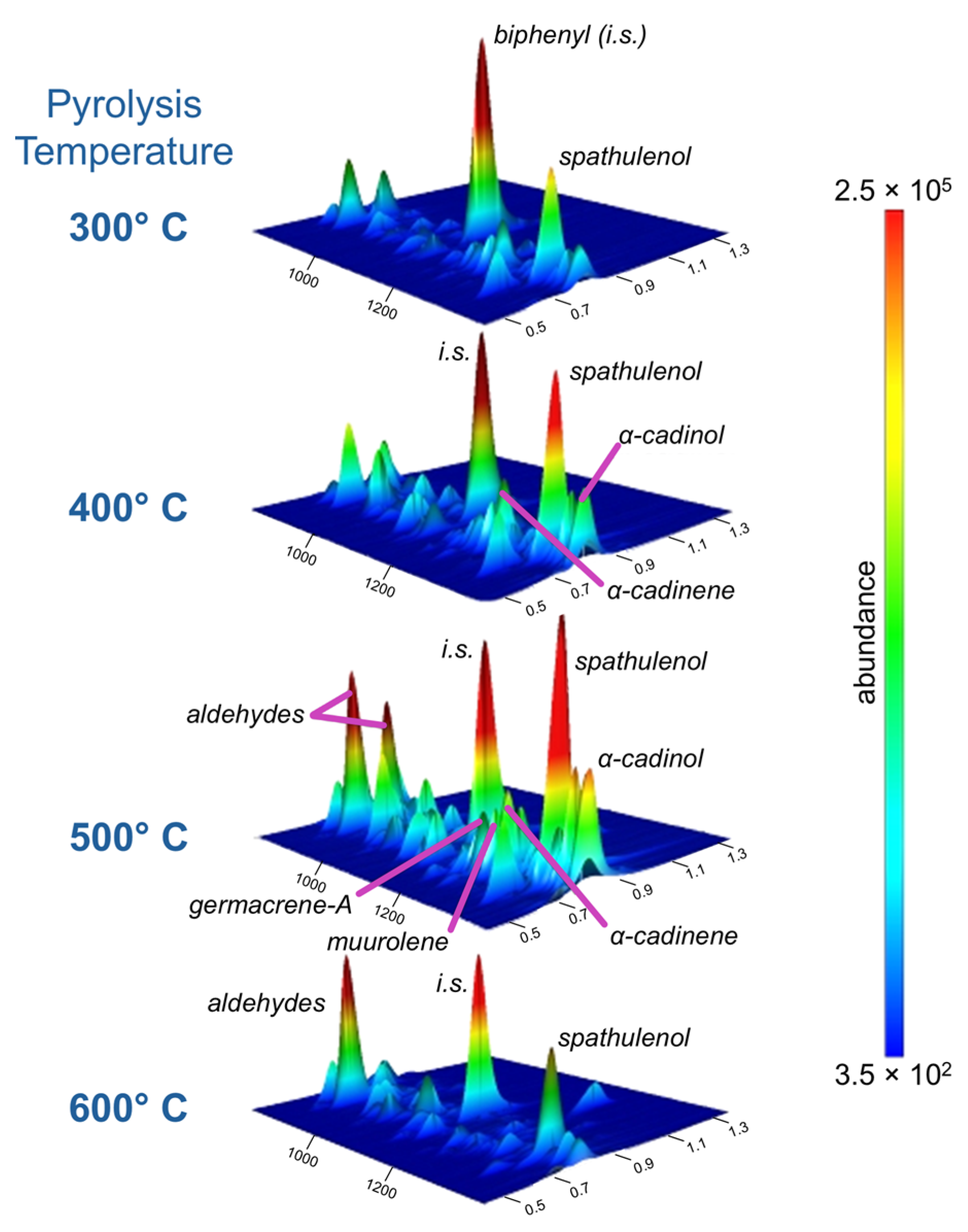
3.3. Residual Paste Water Leach and Thermal Desorption/Pyrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DME | Dimethyl ether |
| SPME | Solid-phase microextraction |
| 2D-GC/MS | Two-dimensional gas chromatography/mass spectrometry |
| SEM | Scanning electron microscopy |
| EDS | Energy dispersive X-ray spectroscopy |
| THF | Tetrahydrofuran |
| 2D-GC | Two-dimensional gas chromatography |
| PEG | Polyethylene glycol |
References
- Ryan, M.G.; Richardson, D.M. The Complete Pine. BioScience 1999, 49, 1023–1024. [Google Scholar] [CrossRef]
- William, I.; Roberts, I. American Potash Manufacture before the American Revolution. In Proceedings of the American Philosophical Society; American Philosophical Society: Philadelphia, PA, USA, 1972; Volume 116, pp. 383–395. Available online: http://www.jstor.org/stable/986069 (accessed on 1 May 2025).
- Özgüven, F.; Vursavuş, K. Some physical, mechanical and aerodynamic properties of pine (Pinus pinea) nuts. J. Food Eng. 2005, 68, 191–196. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Chung, H.-J. Flavor Compounds of Pine Sprout Tea and Pine Needle Tea. J. Agric. Food Chem. 2000, 48, 1269–1272. [Google Scholar] [CrossRef]
- Elfvinga, B.; Ericsson, T.; Rosvall, O. The Introduction of Lodgepole Pine for Wood Production in Sweden: A Review. For. Ecol. Manag. 2001, 141, 15–29. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- Oasmaa, A.; Kuoppala, E.; Gust, S.; Solantausta, Y. Fast Pyrolysis of Forestry Residue. 1. Effect of Extractives on Phase Separation of Pyrolysis Liquids. Energy Fuels 2003, 17, 1–12. [Google Scholar] [CrossRef]
- Wiyono, B.; Tachibana, S.; Tinambunan, D. Chemical Compositions of Pine Resin, Rosin and Turpentine Oil from West Java. J. For. Res. 2006, 3, 7–17. [Google Scholar] [CrossRef]
- Rissanen, K.; Hölttä, T.; Barreira, L.F.M.; Hyttinen, N.; Kurtén, T.; Bäck, J. Temporal and Spatial Variation in Scots Pine Resin Pressure and Composition. Front. For. Glob. Change 2019, 2, 23. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A. Ponderosa pine resin defenses and growth: Metrics matter. Tree Physiol. 2015, 35, 1223–1235. [Google Scholar] [CrossRef]
- Clark, E.L.; Pitt, C.; Carroll, A.L.; Lindgren, B.S.; Huber, D.P. Comparison of lodgepole and jack pine resin chemistry: Implications for range expansion by the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Curculionidae). PeerJ 2014, 2, e240. [Google Scholar] [CrossRef]
- Ribeiro, A.; Bernardo-Gil, G. Densities and refractive indices of components of pine resin. J. Chem. Eng. Data 1990, 35, 204–206. [Google Scholar] [CrossRef]
- Bernardo-Gil, M.G.; Ribeiro, M.A. Vapor-Liquid Equilibria of Binary Systems based on Pine Resin. FIuidPhaseEquilibria 1989, 53, 15–22. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, Z.; Ding, B. Study on the antimutagenic effect of pine needle extract. Mutat. Res. 1995, 347, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef]
- Singha, A.S.; Thakur, V.K. Study of Mechanical Properties of Ureaformaldehyde Theromsets Reinforced by Pine Needle Powder. BioResources 2009, 4, 292–308. [Google Scholar] [CrossRef]
- Singha, A.S.; Thakur, V.K. Mechanical, Morphological and Thermal Properties of Pine Needle-Reinforced Polymer Composites. Int. J. Polym. Mater. Polym. Biomater. 2008, 58, 21–31. [Google Scholar] [CrossRef]
- Chang, R.; Rohindra, D.; Lata, R.; Kuboyama, K.; Ougizawa, T. Development of poly(ε-caprolactone)/pine resin blends: Study of thermal, mechanical, and antimicrobial properties. Polym. Eng. Sci. 2019, 59, E32–E41. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Physicochemical characterization and kinetic study of pine needle for pyrolysis process. J. Therm. Anal. Calorim. 2015, 124, 487–497. [Google Scholar] [CrossRef]
- Pandey, S.; Dhakal, R.P. Pine Needle Briquettes: A Renewable Source of Energy. Int. J. Energy Sci. 2013, 3. Available online: https://www.researchgate.net/publication/265783688_Pine_Needle_Briquettes_A_Renewable_Source_of_Energy (accessed on 1 May 2025).
- Kim, E.-J.; Kim, S.-M. Bread Properties Utilizing Extracts of Pine Needle according to Preparation Method. Korean J. Food Sci. Technol. 1998, 30, 542–547. [Google Scholar]
- Eriksson, G.; Jensen, S.; Kylin, H.; Strachan, W. The pine needle as a monitor of atmospheric pollution. Nature 1989, 341, 42–44. [Google Scholar] [CrossRef]
- Lamppu, J.; Huttunen, S. Relations between Scots pine needle element concentrations and decreased needle longevity along pollution gradients. Environ. Polution 2003, 122, 119–126. [Google Scholar] [CrossRef]
- Faldt, J.; Solheim, H.; Langstrom, B.; Borg-Karlson, A.K. Influence of fungal infection and wounding on contents and enantiomeric compositions of monoterpenes in phloem of Pinus sylvestris. J. Chem. Ecol. 2006, 32, 1779–1795. [Google Scholar] [CrossRef]
- Szmigielski, R.; Cieslak, M.; Rudzinski, K.J.; Maciejewska, B. Identification of volatiles from Pinus silvestris attractive for Monochamus galloprovincialis using a SPME-GC/MS platform. Environ. Sci. Pollut. Res. 2012, 19, 2860–2869. [Google Scholar] [CrossRef][Green Version]
- Backlund, I.; Arshadi, M.; Hunt, A.J.; McElroy, C.R.; Attard, T.M.; Bergsten, U. Extractive profiles of different lodgepole pine (Pinus contorta) fractions grown under a direct seeding-based silvicultural regime. Ind. Crops Prod. 2014, 58, 220–229. [Google Scholar] [CrossRef]
- Kacik, F.; Vel’kova, V.; Smira, P.; Nasswettrova, A.; Kacikova, D.; Reinprecht, L. Release of Terpenes from Fir Wood during Its Long-Term Use and in Thermal Treatment. Molecules 2012, 17, 9990–9999. [Google Scholar] [CrossRef] [PubMed]
- Achotegui-Castells, A.; Llusia, J.; Hodar, J.A.; Penuelas, J. Needle terpene concentrations and emissions of two coexisting subspecies of Scots pine attacked by the pine processionary moth (Thaumetopoea pityocampa). Acta Physiol. Plant. 2013, 35, 3047–3058. [Google Scholar] [CrossRef]
- Piccand, M.; Bianchi, S.; Halaburt, E.I.; Mayer, I. Characterization of extractives from biomasses of the alpine forests and their antioxidative efficacy. Ind. Crops Prod. 2019, 142, 111832. [Google Scholar] [CrossRef]
- Bukhanko, N.; Attard, T.; Arshadi, M.; Eriksson, D.; Budarin, V.; Hunt, A.J.; Geladi, P.; Bergsten, U.; Clark, J. Extraction of cones, branches, needles and bark from Norway spruce (Picea abies) by supercritical carbon dioxide and soxhlet extractions techniques. Ind. Crops Prod. 2020, 145, 112096. [Google Scholar] [CrossRef]
- da Silva, M.; Mateus, E.P.; Munha, J.; Drazyk, A.; Farrall, M.H.; Paiva, M.R.; das Neves, H.J.C.; Mosandl, A. Differentiation of ten pine species from central Portugal by monoterpene enantiomer-selective composition analysis using multidimensional gas chromatography. Chromatographia 2001, 53, S412–S416. [Google Scholar] [CrossRef]
- Idzojtic, M.; Kajba, D.; Franjic, J. Differentiation of F-1 hybrids P nigra J. F. Arnold x P sylvestris L., P nigra J. F. Arnold x P densiflora Siebold et Zucc, P nigra J. F. Arnold x P thunbergiana Franco and their parental species by needle volatile composition. Biochem. Syst. Ecol. 2005, 33, 427–439. [Google Scholar] [CrossRef]
- Pfeifhofer, H.W.; Idzojtic, M.; Zebec, M. The Needle Volatile Composition of Pinus nigra J. F. Arnold, P. sylvestris L., R densiflora Siebold et Zucc. and P. thunbergiana Franco Trispecies Hybrids. Silvae Genet. 2008, 57, 221–226. [Google Scholar] [CrossRef]
- Korica, A.; Polis, O.; Spalvis, K.; Bartkevics, V. Quantitative and Qualitative Seasonal Changes of Scots Pine and Norway Spruce Foliage Essential Oils in Latvia, and the Extraction Dynamics Thereof. Balt. For. 2015, 21, 51–58. [Google Scholar]
- Orav, A.; Kailas, T.; Liiv, M. Analysis of terpenoic composition of conifer needle oils by steam distillation/extraction, gas chromatography and gas chromatography mass spectrometry. Chromatographia 1996, 43, 215–219. [Google Scholar] [CrossRef]
- Rezanka, P.; Fahnrich, J. Isolation of terpenes from Norway Spruce needles by distillation. Chem. Listy 2003, 97, 119–122. [Google Scholar]
- Trubetskaya, A.; Budarin, V.; Arshadi, M.; Magalhaes, D.; Kazanc, F.; Hunt, A.J. Supercritical extraction of biomass as an effective pretreatment step for the char yield control in pyrolysis. Renew. Energy 2021, 170, 107–117. [Google Scholar] [CrossRef]
- Bojovic, S.; Jurc, M.; Ristic, M.; Popovic, Z.; Matic, R.; Vidakovic, V.; Stefanovic, M.; Jurc, D. Essential-Oil Variability in Natural Populations of Pinus mugo Turra from the Julian Alps. Chem. Biodivers. 2016, 13, 181–187. [Google Scholar] [CrossRef]
- Kupcinskiene, E.; Stikliene, A.; Judzentiene, A. The essential oil qualitative and quantitative composition in the needles of Pinus sylvestris L. growing along industrial transects. Environ. Pollut. 2008, 155, 481–491. [Google Scholar] [CrossRef]
- Judzentiene, A.; Stikliene, A.; Kupcinskiene, E. Changes in the essential oil composition in the needles of Scots pine (Pinus sylvestris L.) under anthropogenic stress. Sci. World J. 2007, 7, 141–150. [Google Scholar] [CrossRef]
- Nikolic, B.; Todosijevic, M.; Ratknic, M.; Dordevic, I.; Stankovic, J.; Cvetkovic, M.; Marin, P.D.; Tesevic, V. Terpenes and n-Alkanes in Needles of Pinus cembra. Nat. Prod. Commun. 2018, 13, 1035–1037. [Google Scholar] [CrossRef]
- Pollien, P.; Chaintreau, A. Simultaneous Distillation−Extraction: Theoretical Model and Development of a Preparative Unit. Anal. Chem. 1997, 69, 3285–3292. [Google Scholar] [CrossRef]
- Tanner, R.L.; Zielinska, B. Determination of the Biogenic Emission Rates of Species Contributing to VOC in the San-Joaquin Valley of California. Atmos. Environ. 1994, 28, 1113–1120. [Google Scholar] [CrossRef]
- Tammela, P.; Nygren, M.; Laakso, I.; Hopia, A.; Vuorela, H.; Hiltunen, R. Volatile compound analysis of ageing Pinus sylvestris L. (Scots pine) seeds. Flavour. Fragr. J. 2003, 18, 290–295. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Vinogorova, V.T.; Rafałowski, K. HS-SPME analysis of volatile organic compounds of coniferous needle litter. Atmos. Environ. 2003, 37, 4645–4650. [Google Scholar] [CrossRef]
- Thanh, K.L.; Commandre, J.M.; Valette, J.; Volle, G.; Meyer, M. Detailed identification and quantification of the condensable species released during torrefaction of lignocellulosic biomasses. Fuel Process. Technol. 2015, 139, 226–235. [Google Scholar] [CrossRef]
- Karaogul, E.; Alma, M.H. Solvent-free Microwave and Hydro-distillation Extraction of Essential Oils from the Sawdust of Pines: Correlation with Heat-map. Bioresources 2019, 14, 8229–8240. [Google Scholar] [CrossRef]
- Bier, M.C.J.; Medeiros, A.B.P.; de Oliveira, J.S.; Cocco, L.C.; Costa, J.D.; de Carvalho, J.C.; Soccol, C.R. Liquefied gas extraction: A new method for the recovery of terpenoids from agroindustrial and forest wastes. J. Supercrit. Fluids 2016, 110, 97–102. [Google Scholar] [CrossRef]
- Levine, I. Method of Simultaneously Defatting, Dehydrating, and Eliminating Bacteria from Foodstuffs. US3795750A, 2 May 1974. [Google Scholar]
- Levine, I.E. Methoxymethane Sterilization Method. US3900288A, 13 August 1975. [Google Scholar]
- Levine, I.E. Methoxymethane Sterilization Method. US4048343A, 13 September 1977. [Google Scholar]
- Bauer, M.C.; Kruse, A. The use of dimethyl ether as an organic extraction solvent for biomass applications in future biorefineries: A user-oriented review. Fuel 2019, 254, 115703. [Google Scholar] [CrossRef]
- Zheng, Q.; Watanabe, M. Advances in low-temperature extraction of natural resources using liquefied dimethyl ether. Resour. Chem. Mater. 2022, 1, 16–26. [Google Scholar] [CrossRef]
- Kanda, H. Super-energy-saving Dewatering Method for High-specific-surface-area Fuels by Using Dimethyl Ether. Adsorpt. Sci. Technol. 2008, 26, 345–349. [Google Scholar] [CrossRef]
- Kanda, H.; Makino, H.; Miyahara, M. Energy-saving drying technology for porous media using liquefied DME gas. Adsorption 2008, 14, 467–473. [Google Scholar] [CrossRef]
- Kanda, H.; Makino, H. Energy-efficient coal dewatering using liquefied dimethyl ether. Fuel 2010, 89, 2104–2109. [Google Scholar] [CrossRef]
- Oshita, K.; Takaoka, M.; Nakajima, Y.; Morisawa, S.; Kanda, H.; Makino, H.; Takeda, N. Sewage Sludge Dewatering Process Using Liquefied Dimethyl Ether as Solid Fuel. Dry. Technol. 2011, 29, 624–632. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P. Simple extraction method of green crude from natural blue-green microalgae by dimethyl ether. Fuel 2011, 90, 1264–1266. [Google Scholar] [CrossRef]
- Kanda, H.; Morita, M.; Makino, H.; Takegami, K.; Yoshikoshi, A.; Oshita, K.; Takaoka, M.; Morisawa, S.; Takeda, N. Deodorization and Dewatering of Biosolids by Using Dimethyl Ether. Water Environ. Res. 2011, 83, 23–25. [Google Scholar] [CrossRef]
- Oshita, K.; Takaoka, M.; Nakajima, Y.; Morisawa, S.; Kanda, H.; Makino, H.; Takeda, N. Characteristics of Biosolids in Dimethyl Ether Dewatering Method. Water Environ. Res. 2012, 84, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Oshita, K.; Toda, S.; Takaoka, M.; Kanda, H.; Fujimori, T.; Matsukawa, K.; Fujiwara, T. Solid fuel production from cattle manure by dewatering using liquefied dimethyl ether. Fuel 2015, 159, 7–14. [Google Scholar] [CrossRef]
- Nerome, H.; Hoshino, R.; Ito, S.; Esaki, R.; Eto, Y.; Wakiyama, S.; Sharmin, T.; Goto, M.; Kanda, H.; Mishima, K. Functional Ingredients Extraction from Garcinia mangostana Pericarp by Liquefied Dimethyl Ether. Eng. J. 2016, 20, 155–162. [Google Scholar] [CrossRef][Green Version]
- Hoshino, R.; Murakami, K.; Wahyudiono, W.; Machmudah, S.; Okita, Y.; Ohashi, E.; Kanda, H.; Goto, M. Economical Wet Extraction of Lipid from labyrinthula Aurantiochytrium limacinum by Using Liquefied Dimethyl Ether. Eng. J. 2016, 20, 145–153. [Google Scholar] [CrossRef]
- Hoshino, R.; Ogawa, M.; Murakami, K.; Wahyudiono; Kanda, H.; Goto, M. Extraction of Lipids from Wet Arthrospira platensis by Liquefied Dimethyl Ether. Solvent Extr. Res. Dev. Jpn. 2017, 24, 47–60. [Google Scholar] [CrossRef]
- Boonnoun, P.; Shotipruk, A.; Kanda, H.; Goto, M. Optimization of rubber seed oil extraction using liquefied dimethyl ether. Chem. Eng. Commun. 2019, 206, 746–753. [Google Scholar] [CrossRef]
- Kanda, H.; Shirai, H. Method for Removing Water Contained in Solid Using Liquid Material. WO2003101579A1, 11 December 2003. [Google Scholar]
- Zheng, Q.; Zhang, Y.; Wahyudiono; Fouquet, T.; Zeng, X.; Kanda, H.; Goto, M. Room-temperature extraction of direct coal liquefaction residue by liquefied dimethyl ether. Fuel 2020, 262, 116528. [Google Scholar] [CrossRef]
- Machmudah, S.; Wicaksono, D.T.; Happy, M.; Winardi, S.; Wahyudiono; Kanda, H.; Goto, M. Water removal from wood biomass by liquefied dimethyl ether for enhancing heating value. Energy Rep. 2020, 6, 824–831. [Google Scholar] [CrossRef]
- Kanda, H.; Wahyudiono; Machmudah, S.; Goto, M. Direct Extraction of Lutein from Wet Macroalgae by Liquefied Dimethyl Ether without Any Pretreatment. ACS Omega 2020, 5, 24005–24010. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Zhou, Y.; Li, W.; Lee, Y.; Kanda, H.; Gao, X.-X.; Hu, R.; Brooks, K.G.; Zia, R.; et al. The Synergism of DMSO and Diethyl Ether for Highly Reproducible and Efficient MA0.5FA0.5PbI3 Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2001300. [Google Scholar] [CrossRef]
- Öhrman, O.G.W.; Pettersson, E. Dewatering of Biomass Using Liquid Bio Dimethyl Ether. Dry. Technol. 2013, 31, 1267–1273. [Google Scholar] [CrossRef]
- McNally, J.S.; Foo, Z.H.; Deshmukh, A.; Orme, C.J.; Lienhard, J.H.; Wilson, A.D. Solute Displacement in the Aqueous Phase of Water-NaCl-Organic Ternary Mixtures Relevant to Solvent-Driven Water Treatment. RSC Adv. 2020, 10, 29516–29527. [Google Scholar] [CrossRef]
- Wilson, A.D.; Wendt, D.S.; Orme, C.J.; Adhikari, B.; Ginosaur, D.M. Methods and Systems for Treating an Aqueous Solution. WO2019191553A1, 3 October 2019. [Google Scholar]
- Wilson, A.D.; Wendt, D.S.; Orme, C.J.; Adhikari, B.; Ginosaur, D.M. Methods and Systems for Treating an Aqueous Solution. US11261111B2, 1 March 2022. [Google Scholar]
- Stetson, C.; Lee, H.; Orme, C.J.; Ginosar, D.M.; Wilson, A.D. Selective Precipitation of Metal Ion Salts from Saturated Aqueous Solutions. PCT/US2022/072792, 2022. [Google Scholar]
- Wilson, A.D.; Lienhard, J.; Deshmukh, A.; Foo, Z.H. Switchable System for High-Salinity Brine Desalination and Fractional Precipitation. US20220212957A1, 2022. [Google Scholar]
- Stetson, C.; Prodius, D.; Lee, H.; Orme, C.; White, B.; Rollins, H.; Ginosar, D.; Nlebedim, I.C.; Wilson, A.D. Solvent-Driven Fractional Crystallization for Atom-Efficient Separation of Metal Salts from Permanent Magnet Leachates. Nat. Commun. 2022, 13, 3789. [Google Scholar] [CrossRef]
- Foo, Z.H.; Stetson, C.; Dach, E.; Deshmukh, A.; Lee, H.; Menon, A.; Prasher, R.; Yip, N.Y.; Lienhard, J.H.; Wilson, A.D. Solvent-Driven Aqueous Separations for Hypersaline Brine Concentration and Resource Recovery. Trends Chem. 2022, 4, 1078–1093. [Google Scholar] [CrossRef]
- Deshmukh, A.; Foo, Z.H.; Stetson, C.; Lee, H.; Orme, C.J.; Wilson, A.D.; Lienhard, J.H. Thermodynamics of solvent-driven water extraction from hypersaline brines using dimethyl ether. Chem. Eng. J. 2022, 434, 134391. [Google Scholar] [CrossRef]
- Ji, W.S.; Ji, X.Y. Comparative Analysis of Volatile Terpenes and Terpenoids in the Leaves of Species-A Potentially Abundant Renewable Resource. Molecules 2021, 26, 5244. [Google Scholar] [CrossRef] [PubMed]
- Mitic, Z.S.; Jovanovic, S.C.; Zlatkovic, B.K.; Nikolic, B.M.; Stojanovic, G.S.; Marin, P.D. Needle Terpenes as Chemotaxonomic Markers in Pinus: Subsections Pinus and Pinaster. Chem. Biodivers. 2017, 14, e1600453. [Google Scholar] [CrossRef] [PubMed]
- Butkiene, R.; Nivinskiene, O.; Mockute, D.; Miliute, A. Variety of the essential oils composition of wood, needles (leaves), unripe and ripe berries of var. communis growing wild in Druskininkai district. Chemija 2007, 18, 35–40. [Google Scholar]
- Nikolic, B.M.; Ballian, D.; Mitic, Z.S. Diversity of Needle Terpenes Among Pinus Taxa. Forests 2025, 16, 623. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Lee, H.; Stetson, C.; Orme, C.J.; Kuns, M.W.; Lacey, J.A.; Vega-Montoto, L.; Snyder, S.W.; Wilbanks, J.R.; Bowen, J.L.; Wilson, A.D. Class-based separations of mixed solid-liquid systems with condensable solvent washing and extraction: The dilemma of pizza box recycling. J. Clean. Prod. 2023, 426, 139080. [Google Scholar] [CrossRef]
- Deshmukh, A.; Wilson, A.D.; Lienhard, J.H. Electrically Powered High-Salinity Brine Separation Using Dimethyl Ether. Ind. Eng. Chem. Res. 2024, 63, 8341–8356. [Google Scholar] [CrossRef]
- Gierlinger, N.; Keplinger, T.; Harrington, M. Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 2012, 7, 1694–1708. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.C.; Li, W.Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Schwob, I.; Bessiere, J.-M.; Masotti, V.; Viano, J. Changes in essential oil composition in Saint John’s wort (Hypericum perforatum L.) aerial parts during its phenological cycle. Biochem. Syst. Ecol. 2004, 32, 735–745. [Google Scholar] [CrossRef]
- Gardner, D.R.; James, L.F. Pine needle abortion in cattle: Analysis of isocupressic acid in North American gymnosperms. Phytochem. Anal. 1999, 10, 132–136. [Google Scholar] [CrossRef]
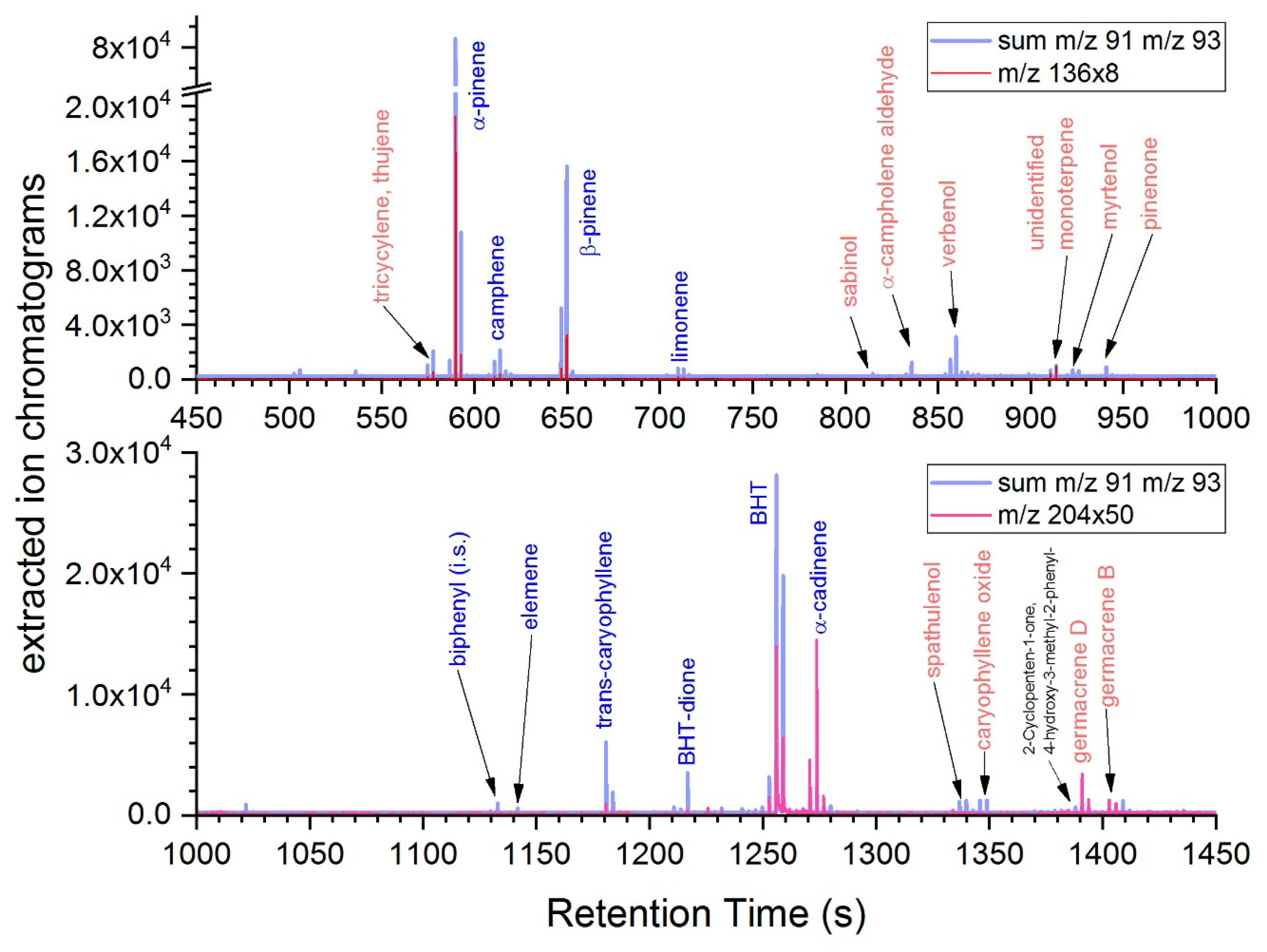
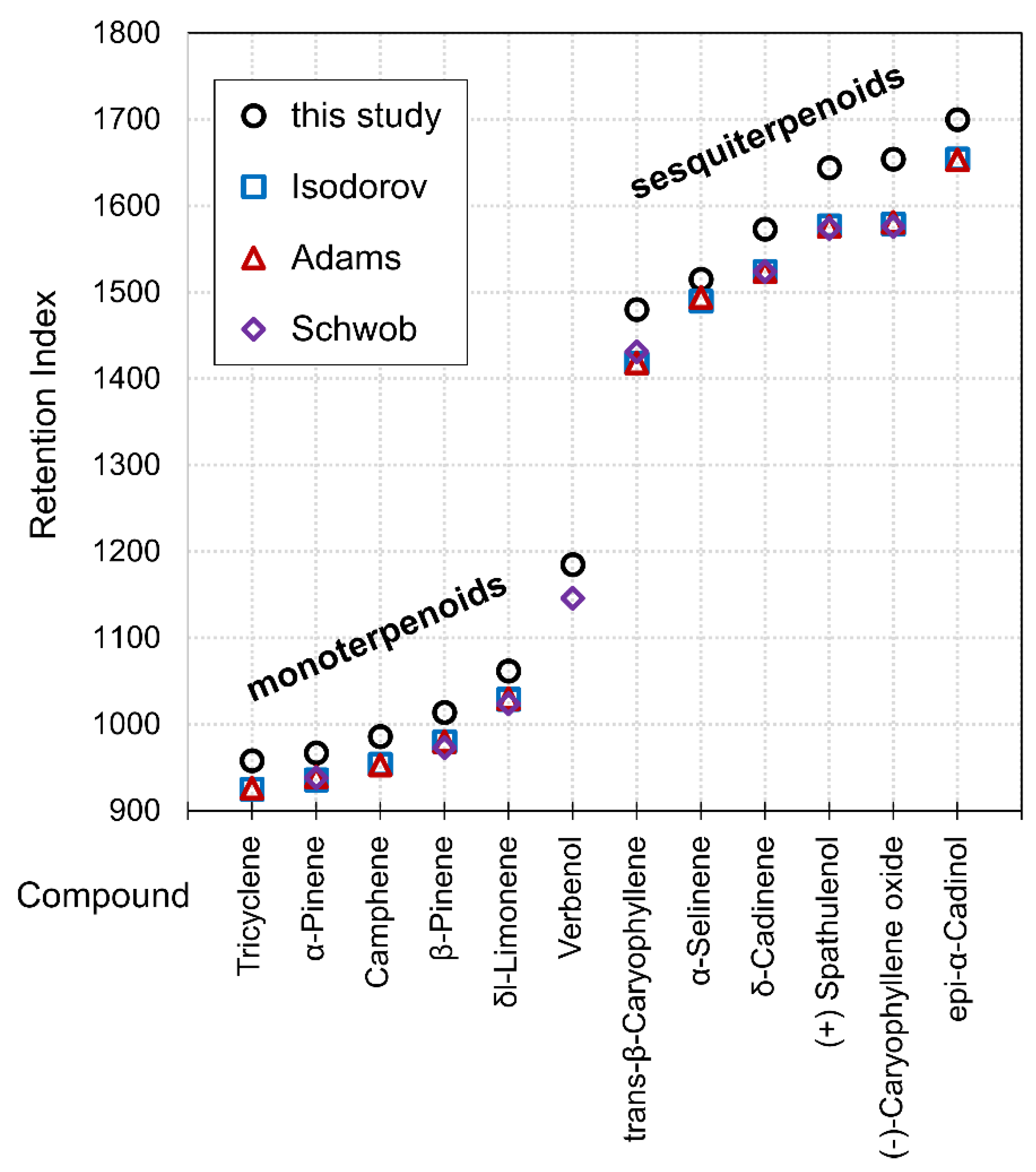
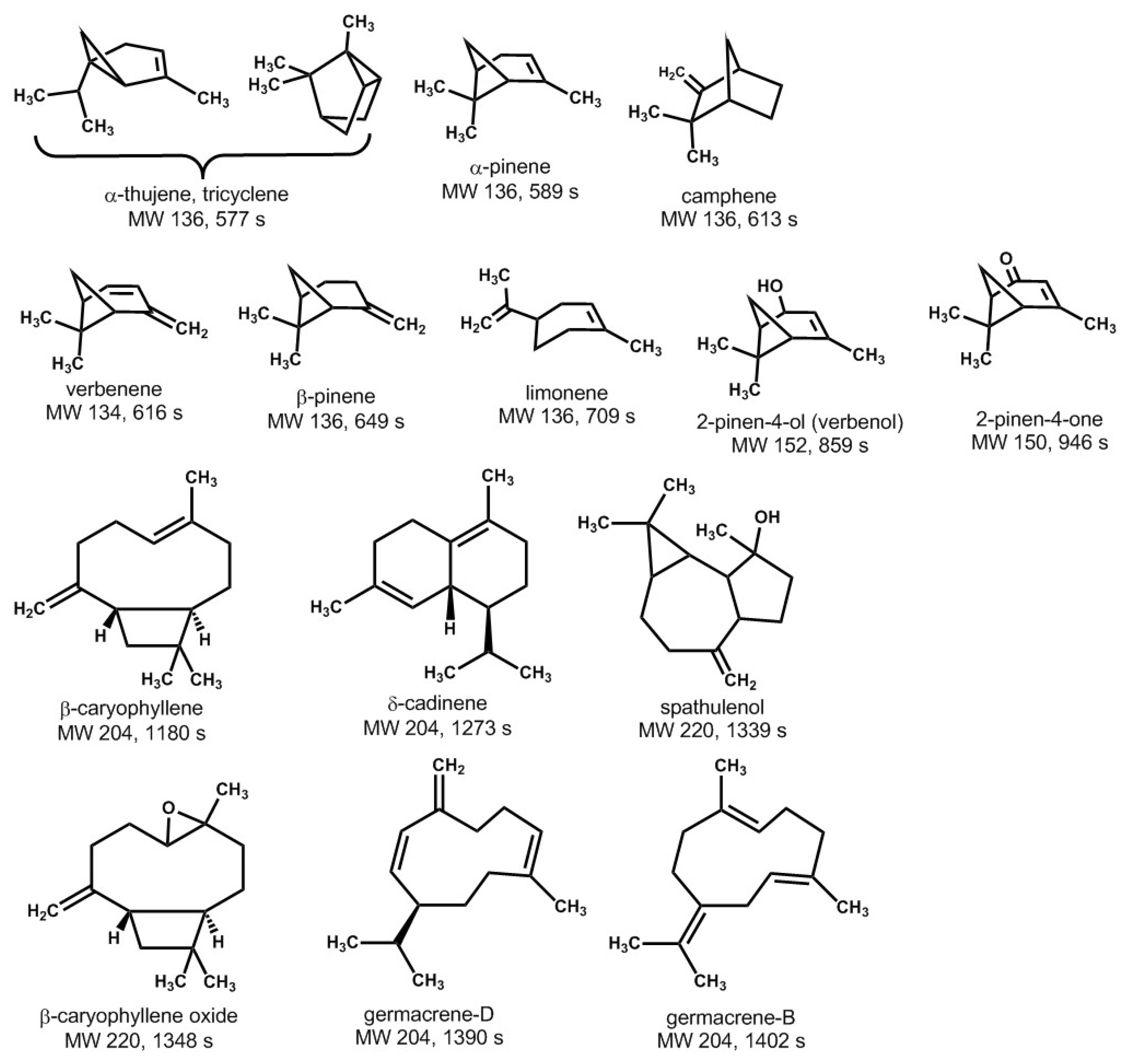

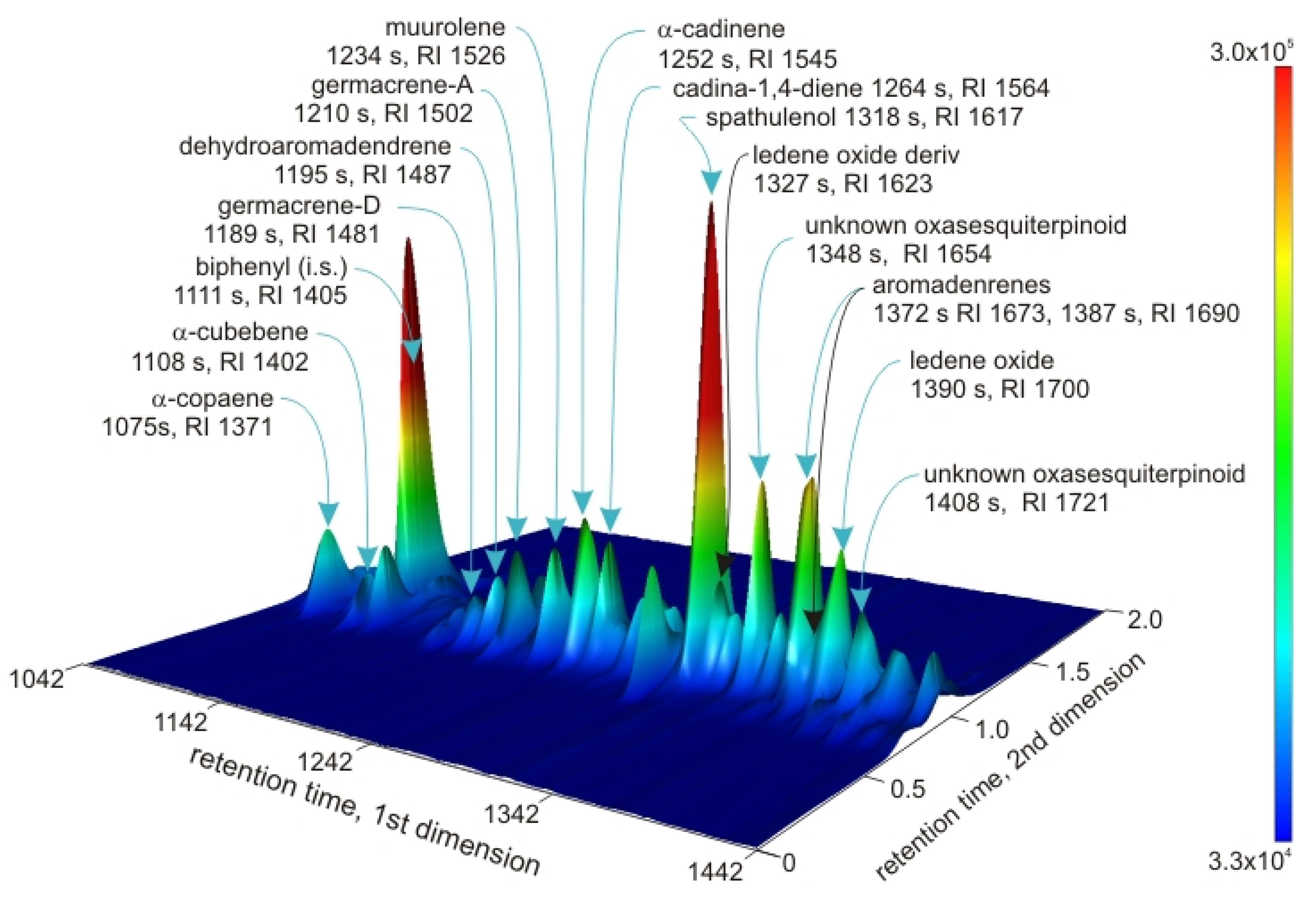
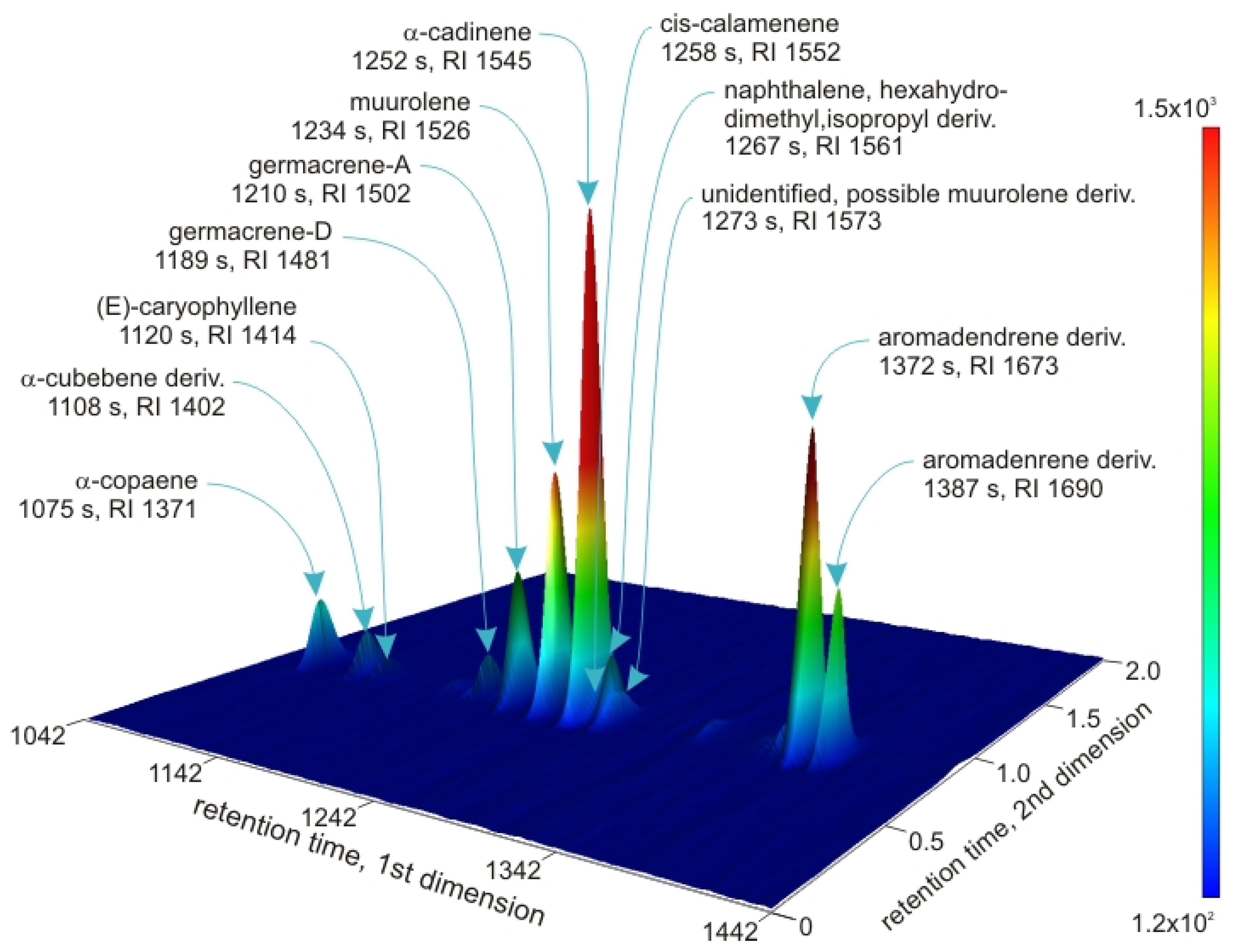
| Retention Index (s) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | This Study | Isodorov [45] | Adams [84] | Schwob [89] | First Dimension Retention Time | Mass Spectral Similarity Index | Molecular Weight (u) |
| Tricyclene | 958 | 844 | 926 | 577 | 844 | 136 | |
| α-Pinene | 967 | 936 | 939 | 938 | 589 | 936 | 136 |
| Camphene | 986 | 886 | 953 | 613 | 886 | 136 | |
| β-Pinene | 1014 | 911 | 980 | 973 | 649 | 911 | 136 |
| δl-Limonene | 1062 | 854 | 1030 | 1024 | 709 | 854 | 136 |
| Verbenol | 1185 | 838 | 1146 | 859 | 838 | 152 | |
| trans-β-Caryophyllene | 1480 | 950 | 1418 | 1431 | 1180 | 950 | 204 |
| α-Selinene | 1515 | 862 | 1494 | 1216 | 862 | 204 | |
| δ-Cadinene | 1573 | 870 | 1524 | 1524 | 1273 | 870 | 204 |
| (+) Spathulenol | 1644 | 836 | 1576 | 1574 | 1339 | 836 | 220 |
| (−)-Caryophyllene oxide | 1654 | 774 | 1581 | 1576 | 1348 | 774 | 220 |
| epi-α-Cadinol | 1700 | 815 | 1653 | 1390 | 815 | 222 | |
| Retention Index (s) | ||||||
|---|---|---|---|---|---|---|
| Compound | This Study | Isodorov [45] | Adams [84] | First Dimension Retention Time | Mass Spectral Similarity Index | Molecular Weight (u) |
| α-Copaene | 1374.3 | 1376 | 1377 | 1072 | 831 | 204 |
| Cubabene | 1405.9 | 1390 | 1390 | 1105 | 870 | 204 |
| 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene-, (Z)- | 1420.6 | 1430 | 1120 | 710 | 204 | |
| Germacrene D | 1485.4 | 1484 | 1186 | 796 | 204 | |
| Germacrene A | 1506.1 | 1508 | 1207 | 814 | 204 | |
| α-Muurolene | 1530.4 | 1499 | 1498 | 1231 | 775 | 204 |
| α-Cadinene | 1548.6 | 1537 | 1249 | 868 | 204 | |
| 1S,cis-Calamenene | 1554.7 | 1528 | 1255 | 898 | 202 | |
| Germacrene B | 1566.8 | 1559 | 1267 | 769 | 204 | |
| Cadina-1,4-diene | 1563.7 | 1533 | 1264 | 832 | 204 | |
| (−)-Caryophyllene oxide | 1591.0 | 1579 | 1582 | 1291 | 803 | 220 |
| (+) spathulenol | 1620.1 | 1576 | 1577 | 1318 | 869 | 220 |
| 10-epi-α-Cadinol | 1680.1 | 1372 | 687 | 222 | ||
| trans-Z-α-Bisabolene epoxide | 1696.8 | 1387 | 809 | 220 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groenewold, G.S.; Orme, C.; Stetson, C.; Brown, R.M.; Wendt, L.M.; Wilson, A.D. Extraction of Terpenoids from Pine Needle Biomass Using Dimethyl Ether. Separations 2025, 12, 169. https://doi.org/10.3390/separations12070169
Groenewold GS, Orme C, Stetson C, Brown RM, Wendt LM, Wilson AD. Extraction of Terpenoids from Pine Needle Biomass Using Dimethyl Ether. Separations. 2025; 12(7):169. https://doi.org/10.3390/separations12070169
Chicago/Turabian StyleGroenewold, Gary S., Christopher Orme, Caleb Stetson, Rebecca M. Brown, Lynn M. Wendt, and Aaron D. Wilson. 2025. "Extraction of Terpenoids from Pine Needle Biomass Using Dimethyl Ether" Separations 12, no. 7: 169. https://doi.org/10.3390/separations12070169
APA StyleGroenewold, G. S., Orme, C., Stetson, C., Brown, R. M., Wendt, L. M., & Wilson, A. D. (2025). Extraction of Terpenoids from Pine Needle Biomass Using Dimethyl Ether. Separations, 12(7), 169. https://doi.org/10.3390/separations12070169







