Adsorption and Absorption Techniques for the Separation of Gaseous C2–C5 Olefins
Abstract
1. Introduction
2. Adsorption Techniques for Volatile Olefins
2.1. Major Adsorbent Material for Volatile Olefins
2.1.1. Carbon Material
2.1.2. Zeolites
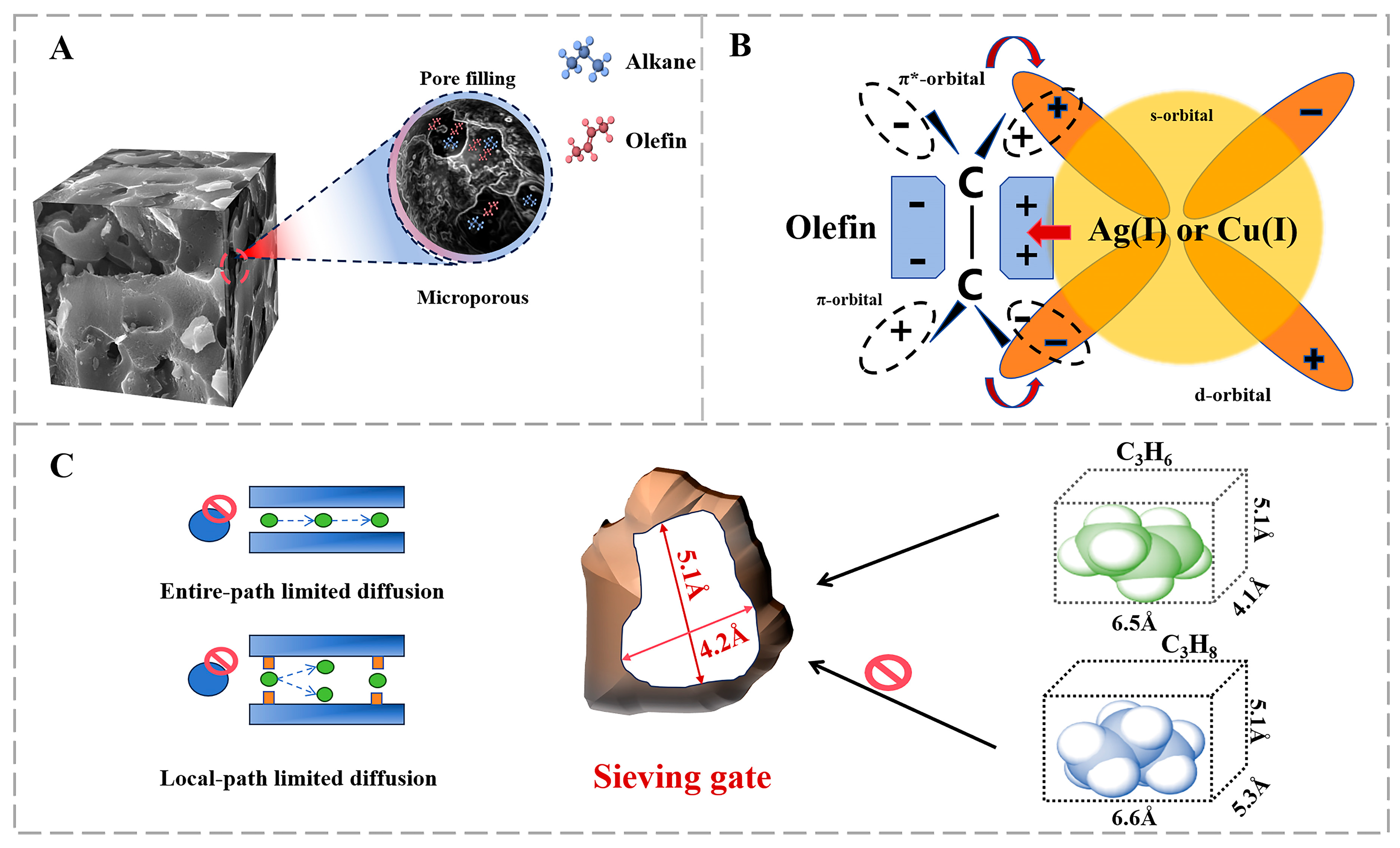
2.1.3. MOF Materials
2.2. Adsorption Separation of Representative Olefins
2.2.1. Adsorption Separation of Volatile Ethylene and Propylene
2.2.2. Adsorption of C4 Olefins
2.2.3. Adsorption of Isoprene
3. Liquid Absorption Techniques for Volatile Olefins
3.1. Absorbents for Volatile Olefins
3.1.1. Organic Liquids
3.1.2. Ionic Liquids
3.2. Liquid Absorption of Representative Olefins
3.2.1. Ethylene Absorption
3.2.2. C4 Olefin Absorption
3.2.3. Isoprene Absorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New trends in olefin production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Kusmiyati; Amin, N.A.S. Production of gasoline range hydrocarbons from catalytic reaction of methane in the presence of ethylene over W/HZSM-5. Catal. Today 2005, 106, 271–274. [Google Scholar] [CrossRef]
- Hulea, V. Toward platform chemicals from bio-based ethylene: Heterogeneous catalysts and processes. ACS Catal. 2018, 8, 3263–3279. [Google Scholar] [CrossRef]
- Zacharopoulou, V.; Lemonidou, A.A. Olefins from biomass intermediates: A review. Catalysts 2017, 8, 2. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Vu, K.B.; Busca, G. (Bio) Propylene production processes: A critical review. J. Environ. Chem. Eng. 2021, 9, 105673. [Google Scholar] [CrossRef]
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Appl. Petrochem. Res. 2015, 5, 377–392. [Google Scholar] [CrossRef]
- Jasper, S.; El-Halwagi, M.M. A techno-economic comparison between two methanol-to-propylene processes. Processes 2015, 3, 684–698. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwon, Y.S.; Kim, D.H.; Park, H.; Park, Y.I. A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation. Membranes 2021, 11, 482. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Zhou, X.; Xiao, J.; Li, Z. Novel C-PDA adsorbents with high uptake and preferential adsorption of ethane over ethylene. Chem. Eng. Sci. 2016, 155, 338–347. [Google Scholar] [CrossRef]
- Janke, C.; Gaida, S.; Jennewein, S. The production of isoprene from cellulose using recombinant Clostridium cellulolyticum strains expressing isoprene synthase. Microbiologyopen 2020, 9, e1008. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, Z.; Liu, S.; Cui, L.; Dai, Q.; He, J.; Dong, W.; Bai, C. Synthesis of 1, 3-butadiene and its 2-substituted monomers for synthetic rubbers. Catalysts 2019, 9, 97. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degreve, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef]
- Heracleous, E.; Pachatouridou, E.; Louie, L.; Dugar, D.; Lappas, A.A. Efficient route for the production of isoprene via decarboxylation of bioderived mevalonolactone. ACS Catal. 2020, 10, 9649–9661. [Google Scholar] [CrossRef]
- Zhilyaeva, N.; Lytkina, A.; Mironova, E.Y.; Ermilova, M.; Orekhova, N.; Shevlyakova, N.; Tverskoy, V.; Yaroslavtsev, A. Polyethylene with radiation-grafted sulfonated polystyrene membranes for butane and butenes separation. Chem. Eng. Res. Des. 2020, 161, 253–259. [Google Scholar] [CrossRef]
- Völksch, B.; Weingart, H. Comparison of ethylene-producing Pseudomonas syringae strains isolated from kudzu (Pueraria lobata) with Pseudomonas syringae pv. phaseolicola and Pseudomonas syringae pv. glycinea. Eur. J. Plant Pathol. 1997, 103, 795–802. [Google Scholar] [CrossRef]
- Lynch, S.; Eckert, C.; Yu, J.; Gill, R.; Maness, P.-C. Overcoming substrate limitations for improved production of ethylene in E. coli. Biotechnol. Biofuels Bioprod. 2016, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Ogawa, T.; Ishihara, K.; Fujii, T.; Nagahama, K.; Omata, T.; Inoue, Y.; Tanase, S.; Morino, Y. Molecular cloning in Escherichia coli, expression, and nucleotide sequence of the gene for the ethylene-forming enzyme of Pseudomonas syringae pv. phaseolicola PK2. Biochem. Biophys. Res. Commun. 1992, 188, 826–832. [Google Scholar] [CrossRef]
- Ishihara, K.; Matsuoka, M.; Inoue, Y.; Tanase, S.; Ogawa, T.; Fukuda, H. Overexpression and in vitro reconstitution of the ethylene-forming enzyme from Pseudomonas syringae. J. Ferment. Bioeng. 1995, 79, 205–211. [Google Scholar] [CrossRef]
- Pirkov, I.; Albers, E.; Norbeck, J.; Larsson, C. Ethylene production by metabolic engineering of the yeast Saccharomyces cerevisiae. Metab. Eng. 2008, 10, 276–280. [Google Scholar] [CrossRef]
- Ishihara, K.; Matsuoka, M.; Ogawa, T.; Fukuda, H. Ethylene production using a broad-host-range plasmid in Pseudomonas syringae and Pseudomonas putida. J. Ferment. Bioeng. 1996, 82, 509–511. [Google Scholar] [CrossRef]
- Tao, L.; Dong, H.J.; Chen, X.; Chen, S.F.; Wang, T.-H. Expression of ethylene-forming enzyme (EFE) of Pseudomonas syringae pv. glycinea in Trichoderma viride. Appl. Microbiol. Biotechnol. 2008, 80, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, Y.; Hua, J.; Tao, L.; Qin, W.; Chen, S. Overexpression of bacterial ethylene-forming enzyme gene in Trichoderma reesei enhanced the production of ethylene. Int. J. Biol. Sci. 2010, 6, 96. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.T.; Yuan, X.J.; Zhang, M.; Mo, X.H.; Tan, L.L.; Zhu, L.P.; Chen, W.J.; Yao, M.D.; Hu, B. Metabolic engineering of Methylobacterium extorquens AM1 for the production of butadiene precursor. Microb. Cell Factories 2018, 17, 194. [Google Scholar] [CrossRef]
- Mori, Y.; Noda, S.; Shirai, T.; Kondo, A. Direct 1, 3-butadiene biosynthesis in Escherichia coli via a tailored ferulic acid decarboxylase mutant. Nat. Commun. 2021, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Qin, B.; Li, Y.; Sun, Y.; Su, S.; Xian, M. Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl. Microbiol. Biotechnol. 2011, 90, 1915–1922. [Google Scholar] [CrossRef]
- Zou, S.; Di, Z.; Li, H.; Liu, Y.; Ji, Z.; Li, H.; Chen, C.; Wu, M.; Hong, M. Stable fluorinated hybrid microporous material for the efficient separation of C2–C3 alkyne/alkene mixtures. Inorg. Chem. 2022, 61, 7530–7536. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Guo, Y.; Zhu, T.; Xu, W. Adsorption and desorption characteristics of hydrophobic hierarchical zeolites for the removal of volatile organic compounds. Chem. Eng. J. 2021, 411, 128558. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Kujawska, A.; Kujawski, J.K.; Bryjak, M.; Cichosz, M.; Kujawski, W. Removal of volatile organic compounds from aqueous solutions applying thermally driven membrane processes. 2. Air gap membrane distillation. J. Membr. Sci. 2016, 499, 245–256. [Google Scholar] [CrossRef]
- Mansour, E.; Curling, S.; Stéphan, A.; Ormondroyd, G. Absorption of volatile organic compounds by different wool types. Green Mater. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Hao, X.; Xiafan, X.; Liubiao, C.; Jia, G.; Junjie, W. A novel cryogenic condensation system based on heat-driven refrigerator without power input for volatile organic compounds recovery. Energy Convers. Manag. 2021, 238, 114157. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Jiang, B.; Xiao, H. Experimental study on ethylene recovery using the solvent absorption method. Chem. Eng. Technol. 2011, 34, 1353–1358. [Google Scholar] [CrossRef]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review of indoor air treatment technologies. Rev. Environ. Sci. Biotechnol 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Wang, Y.; Yang, H.; Song, Q. Characteristics and mechanism of low-temperature NO adsorption by activated carbon. Chem. Eng. J. 2024, 495, 153639. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Natarajan, T.S. Bael fruit shell–derived activated carbon adsorbent: Effect of surface charge of activated carbon and type of pollutants for improved adsorption capacity. Biomass Convers. Biorefin. 2024, 14, 8761–8774. [Google Scholar] [CrossRef]
- Zhen, H.; Jang, S.M.; Teo, W.; Li, K. Modified silicone–PVDF composite hollow-fiber membrane preparation and its application in VOC separation. J. Appl. Polym. Sci. 2006, 99, 2497–2503. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012, 73, 261–284. [Google Scholar] [CrossRef]
- Rungta, M.; Zhang, C.; Koros, W.J.; Xu, L. Membrane-based ethylene/ethane separation: The upper bound and beyond. AIChE J. 2013, 59, 3475–3489. [Google Scholar] [CrossRef]
- Ohlrogge, K.; Brockmöller, J.; Wind, J.; Behling, R. Engineering aspects of the plant design to separate volatile hydrocarbons by vapor permeation. Sep. Sci. Technol. 1993, 28, 227–240. [Google Scholar] [CrossRef]
- Heymes, F.; Manno-Demoustier, P.; Charbit, F.; Fanlo, J.L.; Moulin, P. A new efficient absorption liquid to treat exhaust air loaded with toluene. Chem. Eng. J. 2006, 115, 225–231. [Google Scholar] [CrossRef]
- Biard, P.F.; Coudon, A.; Couvert, A.; Giraudet, S. A simple and timesaving method for the mass-transfer assessment of solvents used in physical absorption. Chem. Eng. J. 2016, 290, 302–311. [Google Scholar] [CrossRef]
- Wang, X.; Daniels, R.; Baker, R. Recovery of VOCs from high-volume, low-VOC-concentration air streams. AIChE J. 2001, 47, 1094–1100. [Google Scholar] [CrossRef]
- Le Minh, N.; Sivret, E.C.; Shammay, A.; Stuetz, R.M. Factors affecting the adsorption of gaseous environmental odors by activated carbon: A critical review. Crit. Rev. Environ. Sci. 2018, 48, 341–375. [Google Scholar] [CrossRef]
- Gan, G.; Fan, S.; Li, X.; Zhang, Z.; Hao, Z. Adsorption and membrane separation for removal and recovery of volatile organic compounds. J. Environ. Sci. 2023, 123, 96–115. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Liu, X.; Shang, J.-X.; Yu, R.; Shui, J. Non-classical hydrogen storage mechanisms other than chemisorption and physisorption. Appl. Phys. Rev. 2022, 9, 021315. [Google Scholar] [CrossRef]
- Saha, D.; Kim, M.-B.; Robinson, A.J.; Babarao, R.; Thallapally, P.K. Elucidating the mechanisms of Paraffin-Olefin separations using nanoporous adsorbents: An overview. iScience 2021, 24, 103042. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, H.; Niu, Q.; Fu, M.; Huang, H.; Ye, D. Microbial targeted degradation pretreatment: A novel approach to preparation of activated carbon with specific hierarchical porous structures, high surface areas, and satisfactory toluene adsorption performance. Environ. Sci. Technol. 2019, 53, 7632–7640. [Google Scholar] [CrossRef]
- Chiang, H.L.; Chiang, P.; Huang, C. Ozonation of activated carbon and its effects on the adsorption of VOCs exemplified by methylethylketone and benzene. Chemosphere 2002, 47, 267–275. [Google Scholar] [CrossRef]
- Kim, K.J.; Kang, C.S.; You, Y.J.; Chung, M.C.; Woo, M.W.; Jeong, W.J.; Park, N.C.; Ahn, H.G. Adsorption–desorption characteristics of VOCs over impregnated activated carbons. Catal. Today 2006, 111, 223–228. [Google Scholar] [CrossRef]
- Villacañas, F.; Pereira, M.F.R.; Órfão, J.J.; Figueiredo, J.L. Adsorption of simple aromatic compounds on activated carbons. J. Colloid Interface Sci. 2006, 293, 128–136. [Google Scholar] [CrossRef]
- Qian, Q.; Gong, C.; Zhang, Z.; Yuan, G. Removal of VOCs by activated carbon microspheres derived from polymer: A comparative study. Adsorption 2015, 21, 333–341. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Yüzak, Y.; Thomas, K.M. Adsorption and desorption kinetics for hydrophilic and hydrophobic vapors on activated carbon. Carbon 2006, 44, 989–1004. [Google Scholar] [CrossRef]
- Liu, H.B.; Yang, B.; Xue, N.D. Enhanced adsorption of benzene vapor on granular activated carbon under humid conditions due to shifts in hydrophobicity and total micropore volume. J. Hazard. Mater. 2016, 318, 425–432. [Google Scholar] [CrossRef]
- Jia, L.; Shi, J.; Long, C.; Lian, F.; Xing, B. VOCs adsorption on activated carbon with initial water vapor contents: Adsorption mechanism and modified characteristic curves. Sci. Total Environ. 2020, 731, 139184. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, J.; Yu, G.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. Hydrophobic design of adsorbent for VOC removal in humid environment and quick regeneration by microwave. Microporous Mesoporous Mater. 2020, 294, 109869. [Google Scholar] [CrossRef]
- Hunter-Sellars, E.; Tee, J.; Parkin, I.P.; Williams, D.R. Adsorption of volatile organic compounds by industrial porous materials: Impact of relative humidity. Microporous Mesoporous Mater. 2020, 298, 110090. [Google Scholar] [CrossRef]
- Ge, X.; Wu, Z.; Wu, Z.; Yan, Y.; Cravotto, G.; Ye, B.C. Microwave-assisted modification of activated carbon with ammonia for efficient pyrene adsorption. J. Ind. Eng. Chem. 2016, 39, 27–36. [Google Scholar] [CrossRef]
- Martínez, F.; Pariente, I.; Brebou, C.; Molina, R.; Melero, J.A.; Bremner, D.; Mantzavinos, D. Chemical surface modified-activated carbon cloth for catalytic wet peroxide oxidation of phenol. J. Chem. Technol. Biotechnol. 2014, 89, 1182–1188. [Google Scholar] [CrossRef]
- Carvajal-Bernal, A.M.; Gómez-Granados, F.; Giraldo, L.; Moreno-Piraján, J.C.; Balsamo, M.; Erto, A. Kinetic and thermodynamic study of n-pentane adsorption on activated carbons modified by either carbonization or impregnation with ammonium hydroxide. Microporous Mesoporous Mater. 2020, 302, 110196. [Google Scholar] [CrossRef]
- Hao, P.; Shi, Y.; Li, S.; Cai, N. Hydrophobic activated carbon for elevated-temperature pressure swing adsorption. Adsorption 2020, 26, 1093–1100. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Kvasova, E.S.; Kolokolov, D.I.; Gorbunov, D.E.; Nizovtsev, A.S.; Lashchinskaya, Z.N.; Stepanov, A.G. Understanding Alkene Interaction with Metal-Modified Zeolites: Thermodynamics and Mechanism of Bonding in the π-Complex. Inorg. Chem. 2024, 63, 5083–5097. [Google Scholar] [CrossRef] [PubMed]
- Assen, A.H.; Virdis, T.; De Moor, W.; Moussa, A.; Eddaoudi, M.; Baron, G.; Denayer, J.F.; Belmabkhout, Y. Kinetic separation of C4 olefins using Y-fum-fcu-MOF with ultra-fine-tuned aperture size. Chem. Eng. J. 2021, 413, 127388. [Google Scholar] [CrossRef]
- Fu, L.; Zuo, J.; Liao, K.; Shao, M.; Si, W.; Zhang, H.; Gu, F.; Huang, W.; Li, B.; Shao, Y. Preparation of adsorption resin and itas application in VOCs adsorption. J. Polym. Res. 2023, 30, 167. [Google Scholar] [CrossRef]
- Wang, S.; Huang, L.; Zhang, Y.; Li, L.; Lu, X. A mini-review on the modeling of volatile organic compound adsorption in activated carbons: Equilibrium, dynamics, and heat effects. Chin. J. Chem. Eng. 2021, 31, 153–163. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Linares-Solano, A.; Rand, B. Water adsorption on activated carbons: Study of water adsorption in micro-and mesopores. J. Phys. Chem. B 2001, 105, 7998–8006. [Google Scholar] [CrossRef]
- Laskar, I.I.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. Modeling the effect of relative humidity on adsorption dynamics of volatile organic compound onto activated carbon. Environ. Sci. Technol. 2019, 53, 2647–2659. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Zhang, J.; Zheng, Y.; Zhang, H.; Liu, J.; Cui, Y. Fabrication of hydrophobic regenerated activated carbon with high specific surface area. J. Mater. Sci. 2021, 56, 19969–19982. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; He, Z.; Wang, P.; Yan, Y.; Ran, J. Hydrophobic modified activated carbon using PDMS for the adsorption of VOCs in humid condition. Sep. Purif. Technol. 2020, 239, 116517. [Google Scholar] [CrossRef]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.; Cazorla-Amorós, D.; Linares-Solano, A. Benzene and toluene adsorption at low concentration on activated carbon fibres. Adsorption 2011, 17, 473–481. [Google Scholar] [CrossRef]
- Liu, Y.; Mallouk, K.; Emamipour, H.; Rood, M.J.; Liu, X.; Yan, Z. Isobutane adsorption with carrier gas recirculation at different relative humidities using activated carbon fiber cloth and electrothermal regeneration. Chem. Eng. J. 2019, 360, 1011–1019. [Google Scholar] [CrossRef]
- Mekki, A.; Boukoussa, B. Structural, textural and toluene adsorption properties of microporous–mesoporous zeolite omega synthesized by different methods. J. Mater. Sci. 2019, 54, 8096–8107. [Google Scholar] [CrossRef]
- Wu, Y.H.; Ma, Y.L.; Sun, Y.G.; Ji, W.X.; Lin, F.; Yang, Y.P.; Ma, L.J.; Zhu, C.H.; Xu, Y.J.; Miao, Q. Effects of acid ionization on the formation mechanism of bimodal mesoporous Al-MCM-41s from coal gasification fine residue and evaluation of adsorption capabilities. J. Hazard. Mater. 2021, 417, 126052. [Google Scholar] [CrossRef] [PubMed]
- El Haskouri, J.; Cabrera, S.; Caldés, M.; Alamo, J.; Beltrán-Porter, A.; Marcos, M.; Amorós, P.; Beltrán-Porter, D. Ordered mesoporous materials: Composition and topology control through chemistry. Int. J. Inorg. Mater. 2001, 3, 1157–1163. [Google Scholar] [CrossRef]
- Valdés, H.; Riquelme, A.L.; Solar, V.A.; Azzolina-Jury, F.; Thibault-Starzyk, F. Removal of chlorinated volatile organic compounds onto natural and Cu-modified zeolite: The role of chemical surface characteristics in the adsorption mechanism. Sep. Purif. Technol. 2021, 258, 118080. [Google Scholar] [CrossRef]
- Kang, S.; Ma, J.; Wu, Q.; Deng, H. Adsorptive removal of dichloromethane vapor on FAU and MFI zeolites: Si/Al ratio effect and mechanism. J. Chem. Eng. Data 2018, 63, 2211–2218. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and photocatalytic removal of Persistent Organic Pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Lopez, Y.C.; Viltres, H.; Gupta, N.K.; Acevedo-Pena, P.; Leyva, C.; Ghaffari, Y.; Gupta, A.; Kim, S.; Bae, J.; Kim, K.S. Transition metal-based metal–organic frameworks for environmental applications: A review. Environ. Chem. Lett. 2021, 19, 1295–1334. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.H.; Kwon, E.E.; Szulejko, J.E. Metal–organic frameworks for the control and management of air quality: Advances and future direction. J. Mater. Chem. A 2016, 4, 345–361. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, P.; Tong, Z.; Zhao, Z.; Zhao, Z. Enhanced hydrophobic MIL (Cr) metal-organic framework with high capacity and selectivity for benzene VOCs capture from high humid air. Chem. Eng. J. 2017, 313, 1122–1131. [Google Scholar] [CrossRef]
- Chauvel, A.; Lefebvre, G. Petrochemical Processes; Editions OPHRYS: Paris, France, 2001; Volume 80. [Google Scholar]
- Jayaraman, A.; Yang, R.T.; Munson, C.L.; Chinn, D. Deactivation of π-complexation adsorbents by hydrogen and rejuvenation by oxidation. Ind. Eng. Chem. Res. 2001, 40, 4370–4376. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kuah, W.C.; Effendy, S.; Farooq, S. Industrial scale propylene/propane separation using pressure vacuum swing adsorption. Ind. Eng. Chem. Res. 2018, 57, 6451–6463. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3 (TMA)2 (H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Wang, Q.M.; Shen, D.; Bülow, M.; Lau, M.L.; Deng, S.; Fitch, F.R.; Lemcoff, N.O.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Microporous Mesoporous Mater. 2002, 55, 217–230. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.-Y.; Seo, Y.-K.; Chang, J.-S.; Grenèche, J.-M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100 (Fe), an iron (III) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. [Google Scholar] [CrossRef]
- Yoon, J.W.; Seo, Y.K.; Hwang, Y.K.; Chang, J.S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E. Controlled reducibility of a metal–organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiu, W.; Quan, W.; Koros, W.J. Advanced carbon molecular sieve membranes derived from molecularly engineered cross-linkable copolyimide for gas separations. Nat. Mater. 2023, 22, 109–116. [Google Scholar] [CrossRef]
- Wang, L.; Xue, W.; Zhu, H.; Guo, X.; Huang, H.; Zhong, C. Stepwise engineering the pore aperture of a cage-like MOF for the efficient separation of isomeric C4 paraffins under humid conditions. Angew. Chem. Int. Ed. 2023, 135, e202218596. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Wang, W.; Yang, H.; Hong, A.N.; Bu, X.; Feng, P. Simultaneous control of flexibility and rigidity in pore-space-partitioned metal–organic frameworks. J. Am. Chem. Soc. 2023, 145, 10980–10986. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Z.; Yang, L.; Hu, J.; Jin, A.; Yang, Z.; Zhao, Y.; Meng, B.; Zhou, Y.; Wang, J. A molecular sieve with ultrafast adsorption kinetics for propylene separation. Science 2024, 383, 179–183. [Google Scholar] [CrossRef]
- Mahdi, H.I.; Muraza, O. An exciting opportunity for zeolite adsorbent design in separation of C4 olefins through adsorptive separation. Sep. Purif. Technol. 2019, 221, 126–151. [Google Scholar] [CrossRef]
- Kim, J.N.; Park, J.H.; Lee, S.J.; Ko, M.S.; Beum, H.T.; Park, J.; Ko, C.H.; Han, S.S.; Cho, S.-H. Separation of Olefins from OLEFINS/Paraffins Mixed Gas. U.S. Patent 8,436,223, 7 May 2013. [Google Scholar]
- Kim, H.; Jung, Y. Can Metal–Organic Framework Separate 1-Butene from Butene Isomers? J. Phys. Chem. Lett. 2014, 5, 440–446. [Google Scholar] [CrossRef]
- Gehre, M.; Guo, Z.; Rothenberg, G.; Tanase, S. Sustainable Separations of C4-Hydrocarbons by Using Microporous Materials. ChemSusChem 2017, 10, 3947–3963. [Google Scholar] [CrossRef] [PubMed]
- Tijsebaert, B.; Varszegi, C.; Gies, H.; Xiao, F.S.; Bao, X.; Tatsumi, T.; Müller, U.; Vos, D.D. Liquid phase separation of 1-butene from 2-butenes on all-silica zeolite RUB-41. Chem. Commun. 2008, 21, 2480–2482. [Google Scholar] [CrossRef]
- Gücüyener, C.; Johan, V.D.B.; Joaristi, A.M.; Magusin, P.C.M.M.; Hensen, E.J.M.; Gascon, J.; Kapteijn, F. Facile synthesis of the DD3R zeolite: Performance in the adsorptive separation of buta-1,3-diene and but-2-ene isomers. J. Mater. Chem. 2011, 45, 18386–18397. [Google Scholar] [CrossRef]
- Takahashi, A.; Yang, R.T.; Munson, C.L.; Chinn, D. Cu(I)−Y-Zeolite as a Superior Adsorbent for Diene/Olefin Separation. Langmuir 2001, 17, 8405–8413. [Google Scholar] [CrossRef]
- Yang, R.T.; Kikkinides, E.S. New sorbents for olefin/paraffin separations by adsorption via π -complexation. AIChE J. 1995, 41, 509–517. [Google Scholar] [CrossRef]
- Takahashi, A.; Yang, R.T.; Munson, C.L.; Chinn, D. Influence of Ag content and H2S exposure on 1,3-butadiene/1-butene adsorption by Ag ion-exchanged Y-zeolites (Ag-Y). Ind. Eng. Chem. Res. 2001, 40, 3979–3988. [Google Scholar] [CrossRef]
- Bagreev, A.; Menendez, J.A.; Dukhno, I.; Tarasenko, Y.; Bandosz, T.J. Bituminous coal-based activated carbons modified with nitrogen as adsorbents of hydrogen sulfide. Carbon 2004, 42, 469–476. [Google Scholar] [CrossRef]
- Ye, P.; Fang, Z.; Su, B.; Xing, H.; Yang, Y.; Su, Y.; Ren, Q. Adsorption of propylene and ethylene on 15 activated carbons. J. Chem. Eng. Data 2010, 55, 5669–5672. [Google Scholar] [CrossRef]
- Ruhl, M.J. Recover VOCs via adsorption on activated carbon. Chem. Eng. Prog. 1993, 89, 37–41. [Google Scholar]
- Gupta, V.K.; Verma, N. Removal of volatile organic compounds by cryogenic condensation followed by adsorption. Chem. Eng. Sci. 2002, 57, 2679–2696. [Google Scholar] [CrossRef]
- Huibin, Z.; Liu, H.; Aboulnaga, E.; Liu, H.; Cheng, T.; Xian, M. Microbial production of isoprene: Opportunities and challenges. Industrial Biotechnology: Products and Processes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Mcauliffe, J.C.; Paramonov, S.E.; Sanford, K.J. Fuel Compositions Comprising Isoprene Derivatives. U.S. Patent No. 8,450,549, 28 May 2013. [Google Scholar]
- Pierucci, S.; Del Rosso, R.; Bombardi, D.; Concu, A.; Lugli, G. An innovative sustainable process for VOCs recovery from spray paint booths. Energy 2005, 30, 1377–1386. [Google Scholar] [CrossRef]
- Luna-Triguero, A.; Vicent-Luna, J.M.; Poursaeidesfahani, A.; Vlugt, T.J.H.; Sánchez-de-Armas, R.; Gómez-álvarez, P.; Calero, S. Improving olefin purification using metal organic frameworks with open metal sites. ACS Appl. Mater. Interfaces 2018, 10, 16911–16917. [Google Scholar] [CrossRef]
- Sui, H.; Zhang, T.; Cui, J.; Li, X.; Crittenden, J.; Li, X.; He, L. Novel off-gas treatment technology to remove volatile organic compounds with high concentration. Ind. Eng. Chem. Res. 2016, 55, 2594–2603. [Google Scholar] [CrossRef]
- Adhi, T.P.; Situmorang, Y.A.; Winoto, H.P.; Ariono, D.; Septiana, D.; Imanuela, P.; Indarto, A. H2S–CO2 gas separation with ionic liquids on low ratio of H2S/CO2. Heliyon 2021, 7, e08611. [Google Scholar] [CrossRef]
- McPhee, D. Microbial Derived Isoprene and Methods for Making the Same. U.S. Patent No. 8,492,605, 23 July 2013. [Google Scholar]
- Feher, F.J.; Kan, J.K.; McAuliffe, J.C.; McCall, T.F.; Pickert, L.J.; Ploetz, C.D.; Rodewald, S.; Sabo, T.A.; Wong, T.H. Purification of Isoprene from Renewable Resources. U.S. Patent No. 8,569,562, 29 October 2013. [Google Scholar]
- Lei, Y.; Guo, Z.; Du, L.; Meng, X.; Liu, X.; Wu, X.; Chen, Y. Replacing DMF with ionic liquid in isoprene/n-pentane separation in C5 plants: Solvent design, process optimization, and industrial-scale assessment. Fuel 2024, 357, 130006. [Google Scholar] [CrossRef]
- Yousefi, M.; Azizi, S.; Peyghambarzadeh, S.; Azizi, Z. Intensification of ethylene and ethane absorption in N-methyl-2-pyrrolidone (NMP) by adding silver nanoparticles. Chem. Eng. Process. 2020, 158, 108184. [Google Scholar] [CrossRef]
- Hariz, R.; del Rio Sanz, J.; Mercier, C.; Valentin, R.; Dietrich, N.; Mouloungui, Z.; Hébrard, G. Absorption of toluene by vegetable oil–water emulsion in scrubbing tower: Experiments and modeling. Chem. Eng. Sci. 2017, 157, 264–271. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Luo, H.; Liang, C.; Jiang, D.-e.; Dai, S. Advanced liquid membranes based on novel ionic liquids for selective separation of olefin/paraffin via olefin-facilitated transport. Ind. Eng. Chem. Res. 2008, 47, 881–888. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, D.B.; Choi, D.K.; Ahn, B.S.; gon Kim, H.; Kim, H.S.; Lee, C.H.; Sung, J.Y. Highly selective facilitated transport membranes for isoprene/n-pentane separation. J. Membr. sci. 2006, 279, 403–409. [Google Scholar] [CrossRef]
- Salar-García, M.; Ortiz-Martínez, V.; Hernández-Fernández, F.; de Los Ríos, A.; Quesada-Medina, J. Ionic liquid technology to recover volatile organic compounds (VOCs). J. Hazard. Mater. 2017, 321, 484–499. [Google Scholar] [CrossRef]
- Geng, H.; Zhao, H.; Huang, J.; Gan, L.; Li, D. Effect of highly active oxygen on methane catalytic removal at low temperatures using a core–shell structural catalyst. Chem. Eng. J. 2023, 469, 143913. [Google Scholar] [CrossRef]
- Comyns, A.E. Encyclopedic Dictionary of Named Processes in Chemical Technology; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Wu, Q.R.; Li, G.L.; Cheng, Y.Y.; Fan, B.Y.; Bai, Y.E.; Zhang, N.; Wang, Z.Z.; Zhang, X.P.; Zhang, S.J. Efficient separation of ethylene/ethane by incorporation of silver salts into protic imidazole ionic liquids. Chem. Eng. J. 2023, 461, 141942. [Google Scholar] [CrossRef]
- Moura, L.; Darwich, W.; Santini, C.C.; Gomes, M.F.C. Imidazolium-based ionic liquids with cyano groups for the selective absorption of ethane and ethylene. Chem. Eng. J. 2015, 280, 755–762. [Google Scholar] [CrossRef]
- Immisch, O. Extractive distillation: Recent advances in operation strategies. Rev. Chem. Eng. 2015, 31, 13–26. [Google Scholar] [CrossRef]
- Pavlov, O.S.; Karsakov, S.A.; Pavlov, S.Y. Development of processes for C4 hydrocarbons separation and 1,3-butadiene purification. Theor. Found. Chem. Eng. 2011, 45, 858–867. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.Y.; Lee, B. Simulation of 1,3-butadiene extractive distillation process using N-methyl-2-pyrrolidone solvent. Korean J. Chem. Eng. 2012, 29, 1493–1499. [Google Scholar] [CrossRef]
- Cao, M.; Luan, C.; Yu, P.; Du, Y.; Xu, W.; Tian, H. Data Determination and Process of Vapor-Liquid Equilibrium of Butane-Butene System by Complex Extractive Distillation Using Salt. Ind. Eng. Chem. Res. 2022, 61, 14132–14139. [Google Scholar] [CrossRef]
- Xing, H.B.; Zhao, X.; Yang, Q.W.; Su, B.G.; Bao, Z.B.; Yang, Y.W.; Ren, Q.L. Molecular Dynamics Simulation Study on the Absorption of Ethylene and Acetylene in Ionic Liquids. Ind. Eng. Chem. Res. 2013, 52, 9308–9316. [Google Scholar] [CrossRef]
- Kilaru, P.K.; Scovazzo, P. Correlations of low-pressure carbon dioxide and hydrocarbon solubilities in imidazolium, phosphonium-, and ammonium-based room-temperatuire ionic liquids. Part 2. using activation energy of viscosity. Ind. Eng. Chem. Res. 2008, 47, 910–919. [Google Scholar] [CrossRef]
- Shaul, D.D. Solvent Extraction of Isoprene. U.S. Patent No. 3,607,967, 21 September 1971. [Google Scholar]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Kontogeorgis, G.M.; Woodley, J.M. Ionic-liquid-based bioisoprene recovery process design. Ind. Eng. Chem. Res. 2020, 59, 7355–7366. [Google Scholar] [CrossRef]
- Wang, Y.; Peh, S.B.; Zhao, D. Alternatives to cryogenic distillation: Advanced porous materials in adsorptive light olefin/paraffin separations. Small 2019, 15, e1900058. [Google Scholar] [CrossRef]
- Anson, A.; Lin, C.; Kuznicki, T.; Kuznicki, S. Separation of ethylene/ethane mixtures by adsorption on small-pored titanosilicate molecular sieves. Chem. Eng. Sci. 2010, 65, 807–811. [Google Scholar] [CrossRef]
- Xiao, F.S. Decorated zeolites for chemoselective alkyne/olefin separations. Sci. China. Chem. 2020, 63, 1177–1178. [Google Scholar] [CrossRef]

| Method | Temperature (°C) | Concentration (ppm) | Efficiency | Cost | Reference |
|---|---|---|---|---|---|
| Adsorption | −20–40 | 50–5000 | high | low | [35,36,37] |
| Membrane | 0–50 | 2000–50,000 | high | high | [38,39,40] |
| Condensation | <−30 | >10,000 | medium | high | [41] |
| Absorption | 20–40 | 500–5000 | high | low | [42,43,44] |
| Olefin | Source | Separation Technologies | Recovery Yield (%) | Purity (%) | Temperature (°C) | Energy Consumption | Description | Reference |
|---|---|---|---|---|---|---|---|---|
| Ethylene (C2) | Petroleum cracking | Cryogenic distillation | 95 | 99.9 | −100 | Separation process consumes 0.3% of total energy | High-purity product, mature technology, high energy cost, run at low temperature and high pressure | [138] |
| Liquid absorption | 90 | 99.9 | −40 | Saving 10% energy consumption compared to cryogenic distillation | n-butylene as solvent, low energy consumption but high solvent consumption | [34] | ||
| Adsorption | 90–95 | 99.9 | Room temperature | Low | Using molecular sieves, low energy consumption, higher adsorbent cost | [139,140] | ||
| Propylene (C3) | Petroleum cracking | Cryogenic distillation | 95 | 99.9 | −100 | USD 20 per ton of propylene | Capital- and energy-intensive process, mature process | [87] |
| Adsorption | >99 | 99.5 | Room temperature | USD 41 per ton of propylene | 4A zeolite as adsorbent, low separation cost, higher adsorbent cost | [87] | ||
| Butene (C4) | Petroleum cracking | Cryogenic distillation | - | - | - | High | Hard-to-separate C4 isomers | [99] |
| Adsorption | 93.5 | 99.9 | Room temperature | Low | Zeolites and MOFs as adsorbents, low separation cost, higher adsorbent cost | [97,99] | ||
| Liquid absorption | 99.7 | 99.7 | Room temperature | High | High separation efficiencies, high energy consumption | [132] | ||
| Isoprene (C5) | Petroleum cracking | Cryogenic distillation | - | - | - | High | Hard-to-separate C5 isomers | [137] |
| Liquid absorption | 95 | 99.5 | Room temperature | High | Conventional solvents demand higher concentrations of isoprene | [109] | ||
| Biological process | Adsorption | 80 | 99.9 | 40 | Low | Activated carbon as adsorbent, high adsorption capacity, water vapor inhibits isoprene adsorption | [110] | |
| Liquid absorption | 85 | 99.9 | Room temperature | Low | ILs as absorbing solvents, high recovery rate, and high stability, but high solvent costs | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Sun, C.; Xian, M.; Zou, H. Adsorption and Absorption Techniques for the Separation of Gaseous C2–C5 Olefins. Separations 2025, 12, 144. https://doi.org/10.3390/separations12060144
Guo F, Sun C, Xian M, Zou H. Adsorption and Absorption Techniques for the Separation of Gaseous C2–C5 Olefins. Separations. 2025; 12(6):144. https://doi.org/10.3390/separations12060144
Chicago/Turabian StyleGuo, Fengxiang, Chao Sun, Mo Xian, and Huibin Zou. 2025. "Adsorption and Absorption Techniques for the Separation of Gaseous C2–C5 Olefins" Separations 12, no. 6: 144. https://doi.org/10.3390/separations12060144
APA StyleGuo, F., Sun, C., Xian, M., & Zou, H. (2025). Adsorption and Absorption Techniques for the Separation of Gaseous C2–C5 Olefins. Separations, 12(6), 144. https://doi.org/10.3390/separations12060144








