Efficient Exploitation of Lepidolite Resources: A Review on Beneficiation Techniques, Extraction Methods, and Synergistic Optimization
Abstract
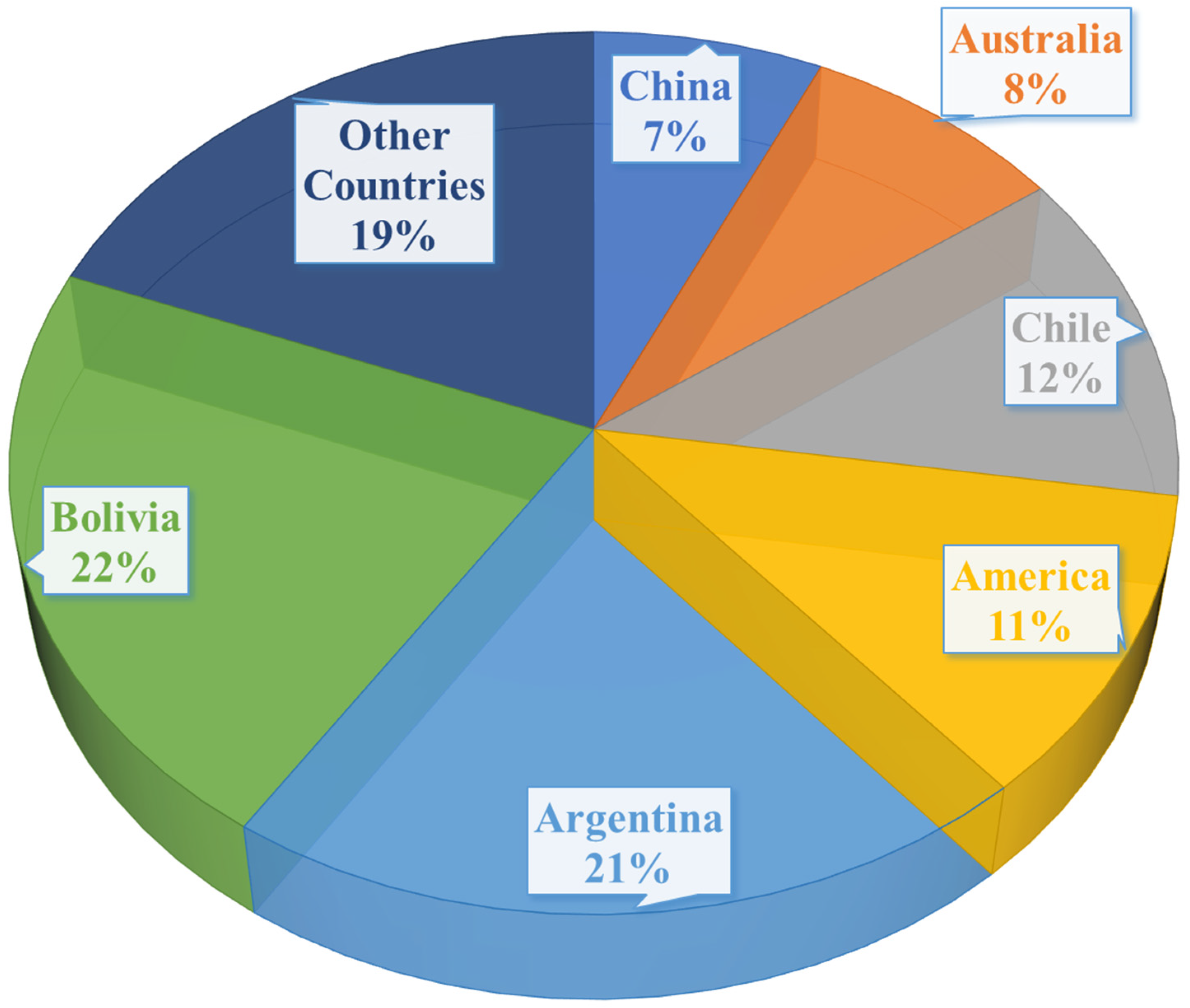
1. Introduction
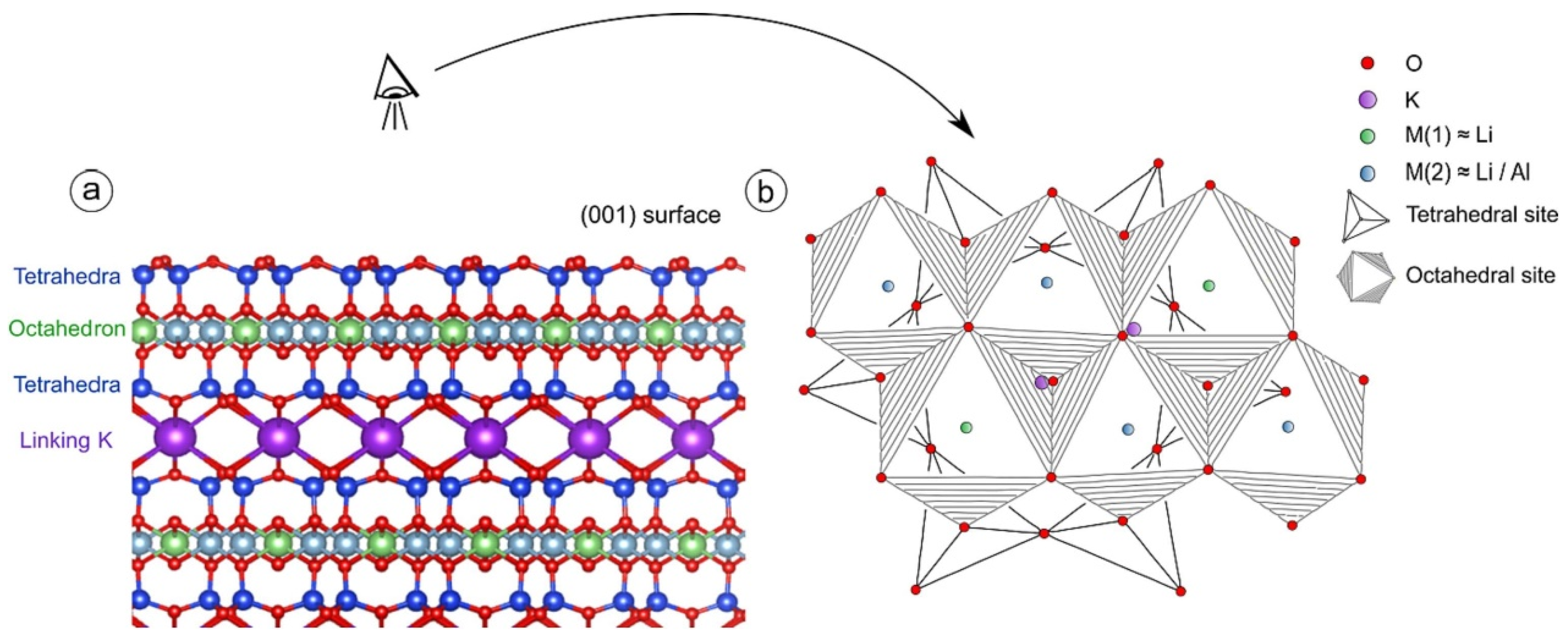
2. Mineralogical Characteristics
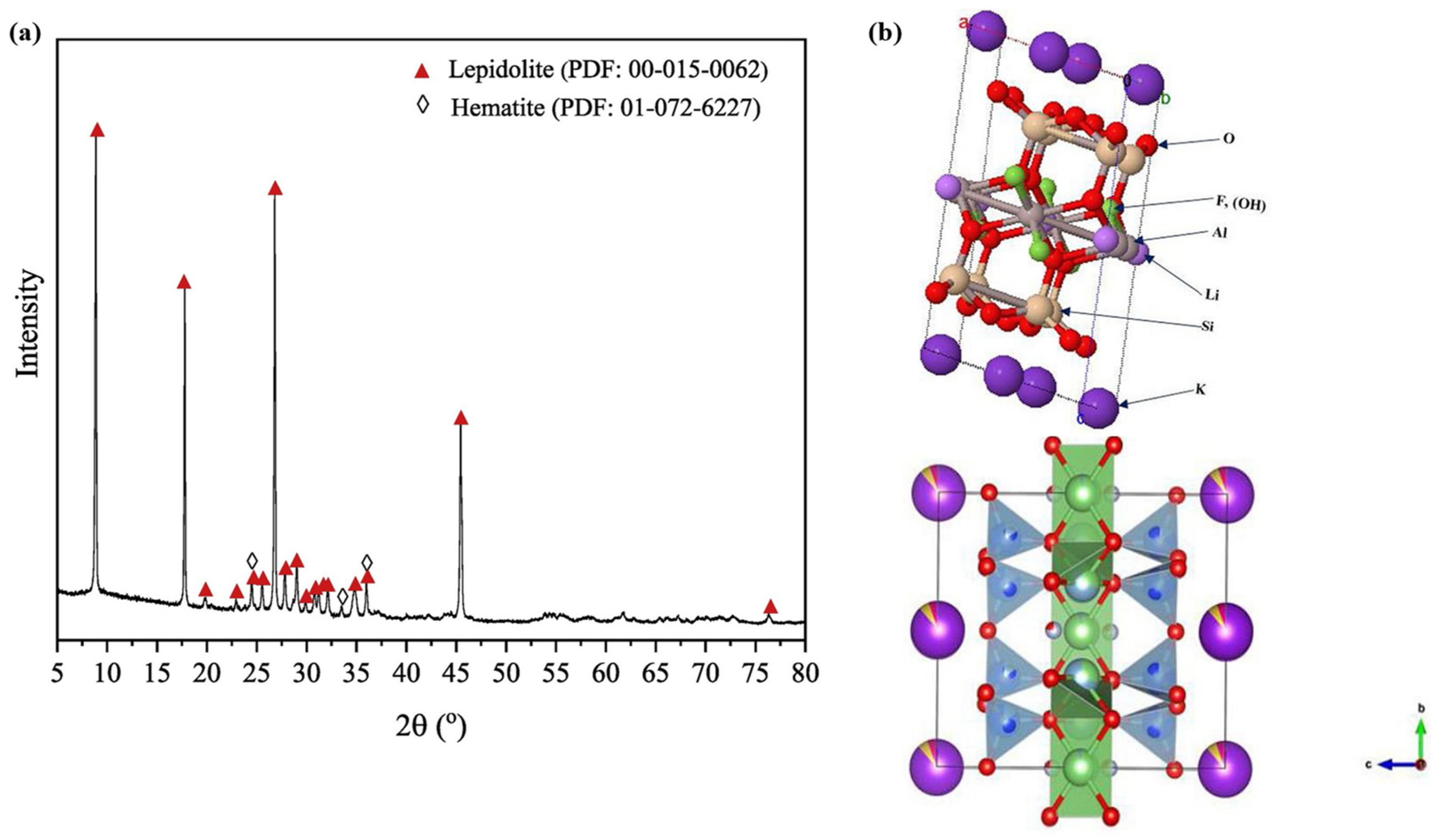
2.1. Chemical Composition and Crystal Structure
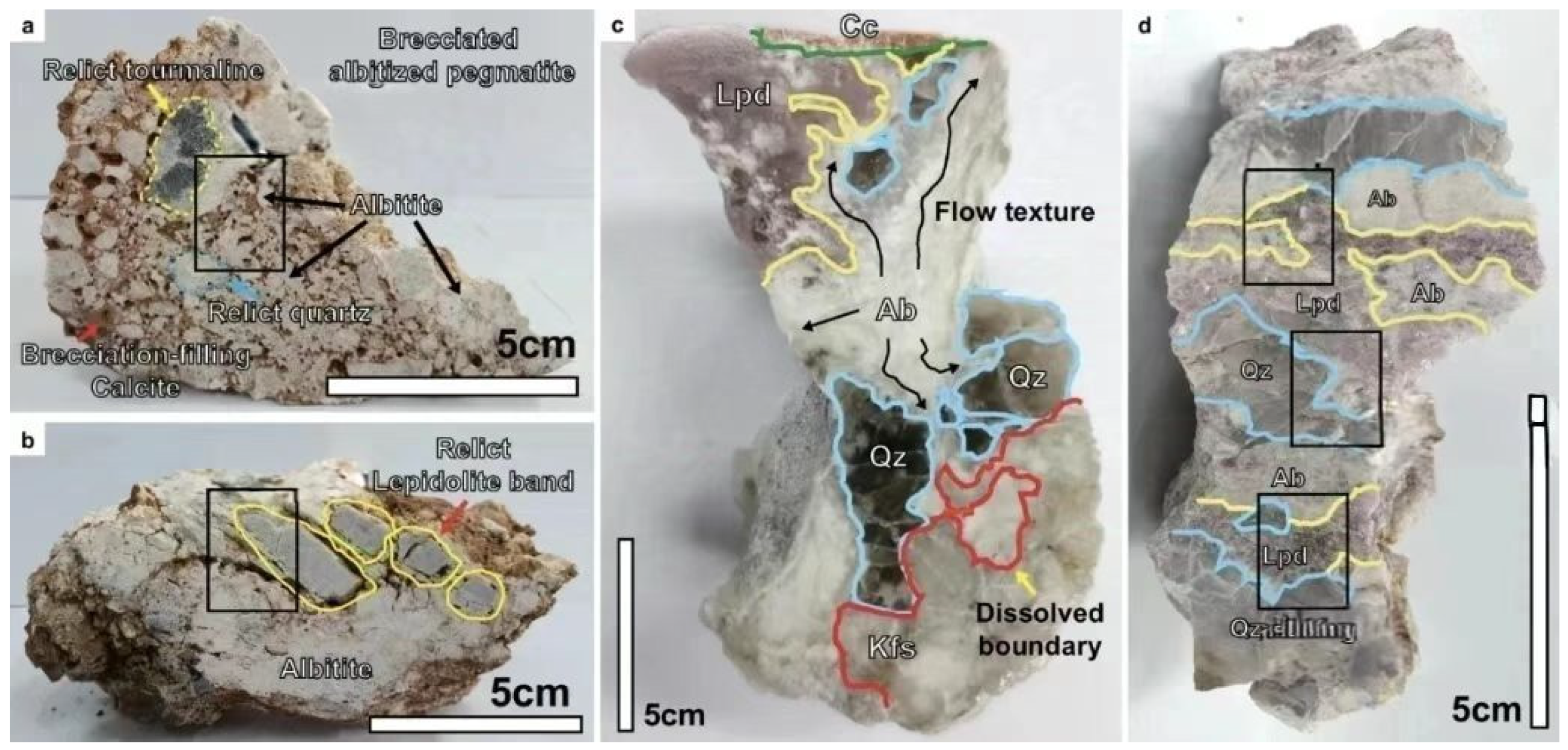
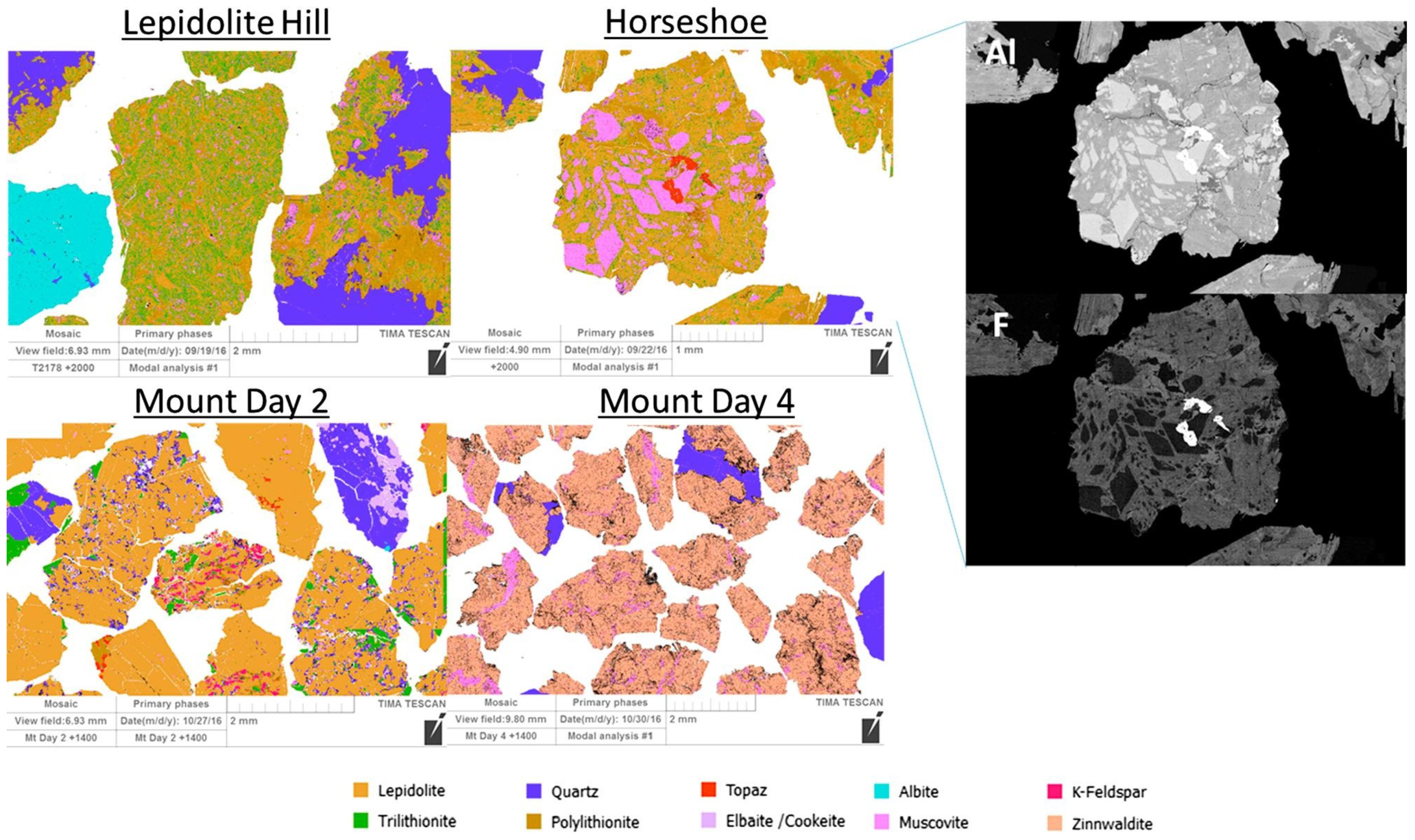
2.2. Gangue Mineral Associations
3. Beneficiation Techniques for Lepidolite
3.1. Froth Flotation
3.1.1. Fundamental Mechanism of Lepidolite Flotation
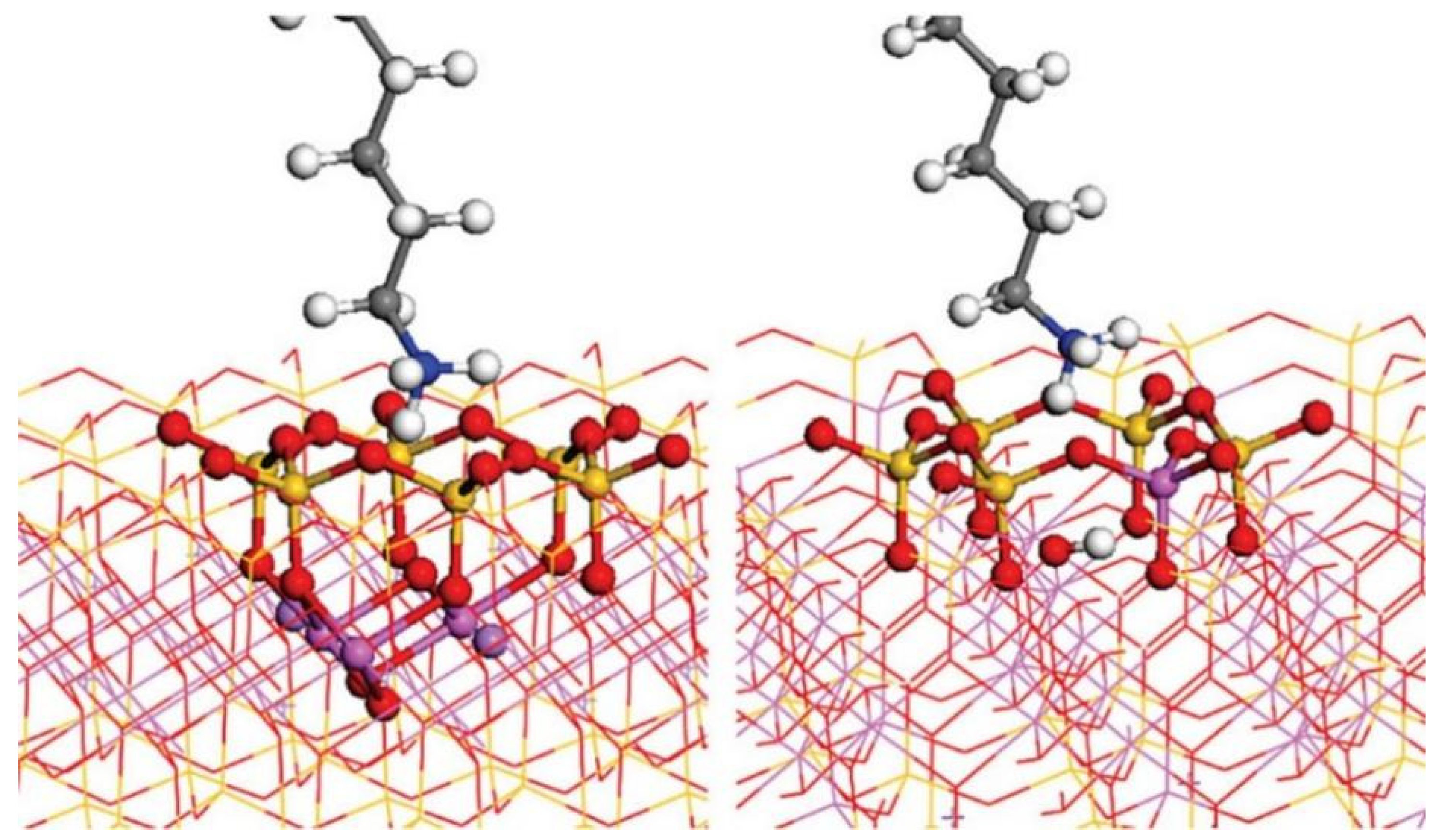
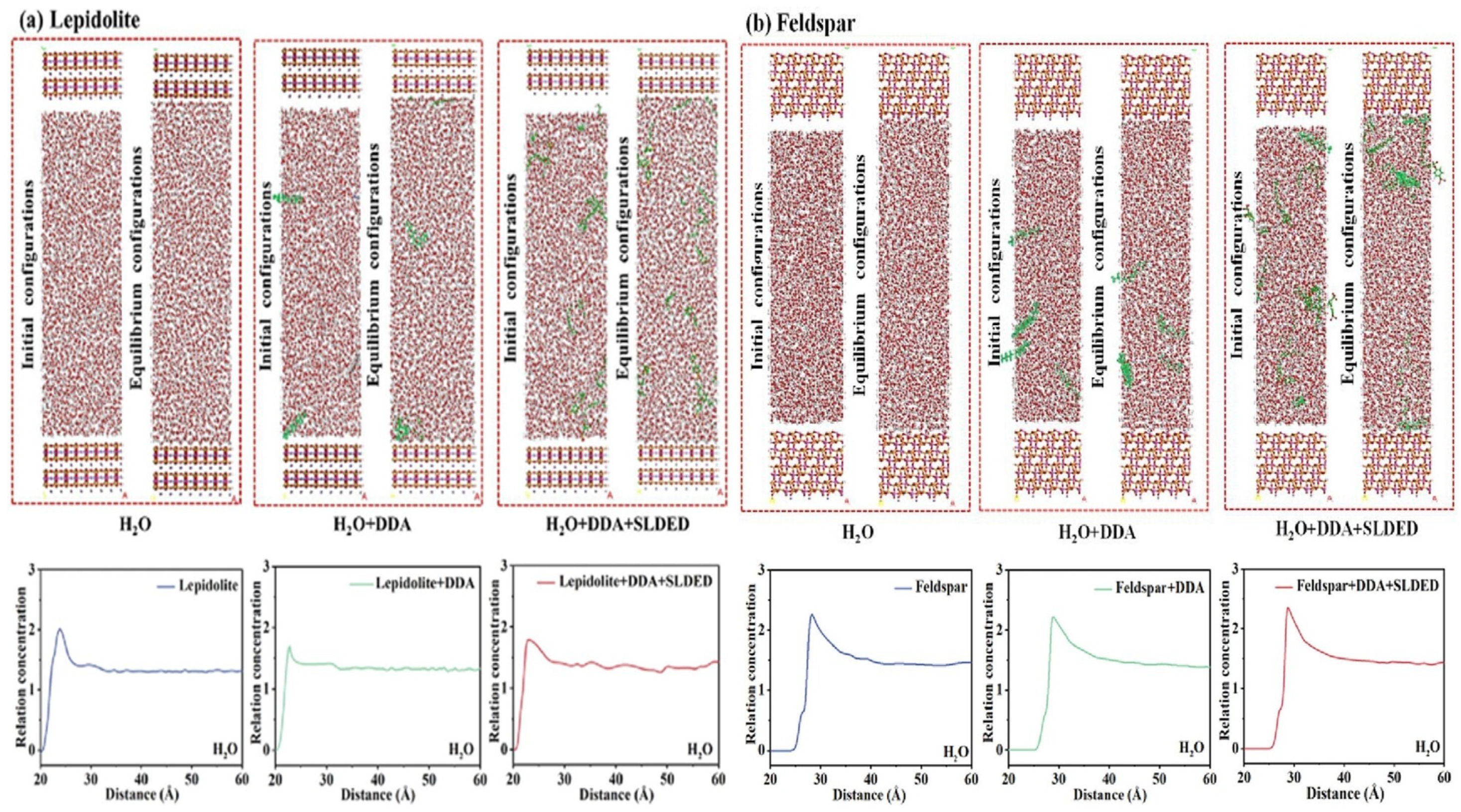
3.1.2. Flotation Collectors
- ▪
- Primary Amines
- ▪
- Secondary Amines and Quaternary Ammonium Salts
- ▪
- Ether Amines
- ▪
- Anionic collector
- ▪
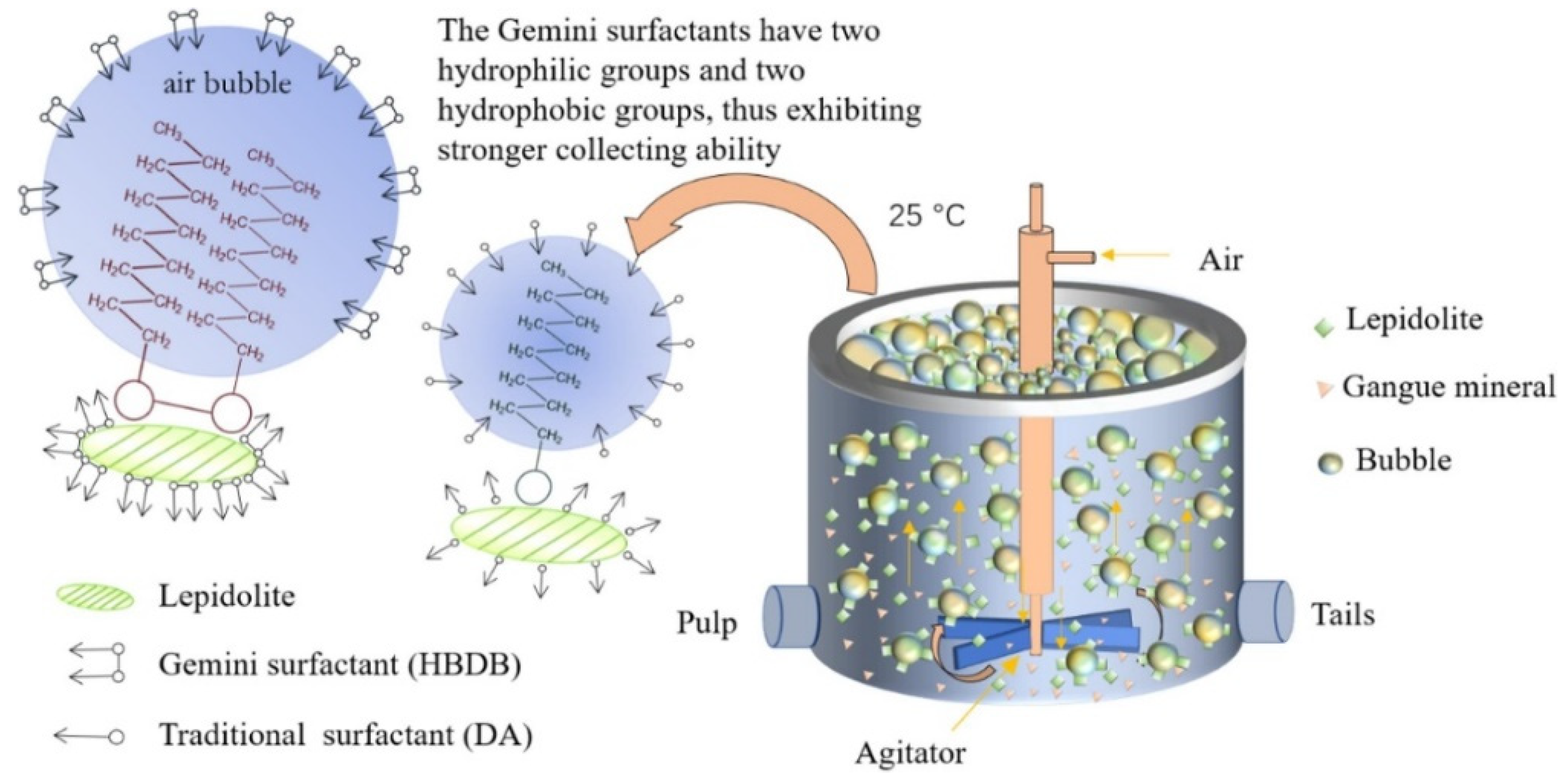
- Mixed Collectors
- (1)
- Enhanced Selectivity: The combined action of mixed collectors boosts the hydrophobicity of lepidolite while minimizing the adhesion of unwanted gangue minerals.
- (2)
- Broad pH Tolerance: The effectiveness of mixed collectors across a range of neutral-to-mild pH levels lessens the reliance on extreme acidic or alkaline conditions.
- (3)
- Efficiency and Sustainability: By improving adsorption efficiency, the need for high collector dosages is reduced, which also alleviates equipment corrosion and mitigates the environmental impacts associated with single collectors.
3.1.3. Modifiers and Frothers
3.1.4. Challenges and Future Prospects
3.2. Optical Sorting
- Color sensors distinguish minerals by surface color (e.g., separating diamonds from kimberlite).
- NIR sensors identify chemical composition via spectral absorption (e.g., quartz vs. feldspar).
- X-ray transmission (XRT) or laser-induced breakdown spectroscopy (LIBS) detect internal density or elemental composition.
4. Metallurgy
4.1. Direct Leaching Methods
4.1.1. Acid Leaching
| Acid System | Efficiency (Li Recovery) | Reagent Cost | Corrosion Control | Residue Handling | Industrial Viability |
|---|---|---|---|---|---|
| H2SO4 | 90–97% [54,66] | Moderate | Requires corrosion-resistant reactors (e.g., Hastelloy, glass-lined steel) | Slag valorization (e.g., cement substitution); SOx scrubbing (NaOH wet scrubbers) | High (mature technology) |
| HCl | 85–95% [68,72] | High | Limited material compatibility (titanium or tantalum required) | High chloride waste; requires neutralization (Ca(OH)2) | Moderate (niche applications) |
| HF/H2SO4 | 95–98% [69,70] | Very High | PTFE-lined reactors mandatory; HF handling increases operational complexity | Fluoride sludge (CaF2 precipitation); hazardous waste disposal | Low (environmental risks) |
| H2SiF6 | 95–98% [70] | High | Moderate corrosion (stainless steel acceptable with short exposure) | Fluorosilicate residues; requires alkaline neutralization | Emerging (needs scaling) |
4.1.2. Alkali Leaching
4.1.3. Bio-Leaching
- Hybrid Bio-Chemical Process
- A sequential bio-pre-leaching step can be employed to soften mineral structures via silicate decomposition by Acidithiobacillus ferrooxidans (A.F.), followed by a short-duration acid leaching process to enhance efficiency. For instance, Zhao et al. demonstrated that organic acids (e.g., lactic acid) secreted by Raoultella sp. Z107 synergistically accelerate lithium dissolution by inducing lattice distortion and acid etching.
- Intelligent Surfactant DesignDeveloping bio-compatible chemical surfactants (e.g., SLG-Gemini hybrid formulations) could reduce interfacial tension while promoting microbial adhesion. This approach mimics the rhamnolipid mechanism reported by Xu et al., where biosurfactants significantly lowered mineral surface hydrophobicity (contact angle reduction from 75.22° to 6.8°), thereby optimizing bacterial–mineral interactions.
- Genetically Engineered Microbial Strains
- Enhancing the Fe/S oxidation capacity of native strains (e.g., A.F. via CRISPR-Cas9 editing) could expedite leaching kinetics. Duan et al. observed that Fe2+ oxidation by AF contributed to 50% of lithium release from jadarite, suggesting that engineered strains with amplified oxidative pathways could significantly improve process efficiency.
- Green Hydrometallurgical SystemsReplacing traditional strong acids with ionic liquids (e.g., imidazolium-based solvents) or low-toxicity lixiviants (e.g., citric acid) could balance extraction efficiency with environmental sustainability. Wang et al. highlighted that heterotrophic bacteria like Bacillus mucilaginosus (BM) secrete polysaccharides and organic acids, providing a biochemical blueprint for designing eco-friendly leaching agents.
4.2. Thermal Activation Methods
4.2.1. Sulfation Roasting
4.2.2. Salt Roasting
4.2.3. Carbonation Roasting
4.3. Microwave-Assisted Roasting
5. Conclusions and Future Prospects
- For the beneficiation of lepidolite, mixed-collector flotation shows high selectivity and recovery under neutral pH, but fine-particle recovery is low, and collector costs are high. Molecular dynamics simulations need to be strengthened to reveal the interactions between collectors and mineral surfaces.
- For the extraction of lepidolite, the integration of pyrometallurgical and hydrometallurgical methods has emerged as a promising strategy for enhancing lithium recovery rates, reducing reagent consumption, and minimizing energy costs. Efforts to recover co-occurring metals (Rb, Cs) and valorize tailings through industrial symbiosis (e.g., silica for solar panels) are critical for achieving zero-waste goals. The use of microwave-assisted roasting, bioleaching, and advanced flotation techniques has shown potential in improving the sustainability and efficiency of lithium extraction from lepidolite. However, challenges remain in fine-particle recovery, collector optimization, and the need for more sustainable and cost-effective reagents.
- Innovation in Beneficiation Techniques: Developing advanced flotation technologies that can selectively separate fine-grained lepidolite from gangue minerals with high efficiency. This includes the optimization of mixed collectors and the exploration of nanobubble technology to enhance fine-particle recovery.
- Sustainable Reagent Development: The design and synthesis of biodegradable and cost-effective collectors that can replace traditional chemicals, reducing the environmental footprint of lepidolite processing.
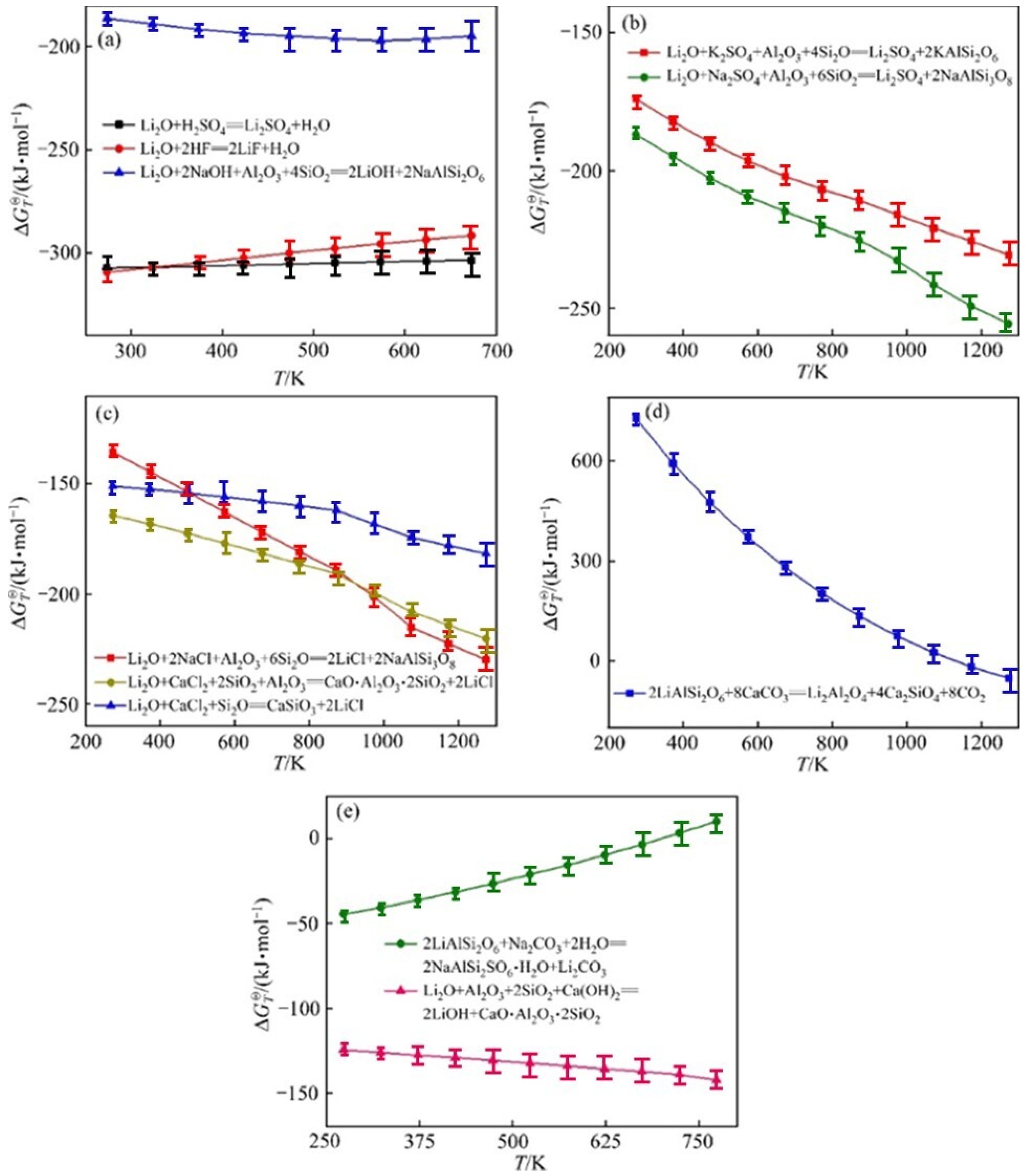
- Thermodynamic Optimization: Further investigation into the thermodynamics of lithium extraction processes to identify conditions that maximize lithium recovery while minimizing energy consumption and environmental impact.
- Adopting green chemistry principles—such as replacing HF with biodegradable ligands or recycling process water via membrane technologies—fosters eco-efficient operations. Furthermore, collaboration with downstream industries (e.g., ceramics, batteries) to utilize tailings as raw materials exemplifies circular economy practices, ensuring resource efficiency across the lithium value chain.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, T.; Fan, N.; Chen, W.; Dai, T. Lithium Extraction from Hard Rock Lithium Ores (Spodumene, Lepidolite, Zinnwaldite, Petalite): Technology, Resources, Environment and Cost. China Geol. 2023, 6, 137–153. [Google Scholar]
- Zhang, J.; Zhang, X.; Tan, X.; Yi, Y.; Zhang, L. A Review on Beneficiation and Lithium Extraction of Lepidolite. Conserv. Util. Miner. Resour. 2024, 44, 68–74. [Google Scholar]
- Zeng, G.; Liu, Y.; Chen, D. Natural Lepidolite Enables Fast Polysulfide Redox for High-Rate Lithium Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2102058. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Zhang, J.; Cao, Y.; He, J.; Li, G. Selective Inhibition Mechanisms of Fe (III) in the Flotation of Lepidolite. Minerals 2024, 14, 851. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Merigot, K.; Quadir, Z.; Rickard, W.D.A.; Evans, N.J.; McDonald, B.J.; Catovic, E.; Spitalny, P. Applications of Advanced Analytical and Mass Spectrometry Techniques to the Characterisation of Micaceous Lithium-Bearing Ores. Miner. Eng. 2018, 116, 182–195. [Google Scholar] [CrossRef]
- Gu, G.; Gao, T. Sustainable Production of Lithium Salts Extraction from Ores in China: Cleaner Production Assessment. Resour. Policy 2021, 74, 102261. [Google Scholar] [CrossRef]
- Bai, Y.; Cui, W.; Gao, Y.; Wen, W.; Sun, Y.; Yan, P. Synergistic Mechanism of Mixed Cationic/Anionic Collectors on Lepidolite Flotation from the Perspective of Improving the Performance of Flotation Foam. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130354. [Google Scholar] [CrossRef]
- Levinson, A.A. Studies in the Mica Group; Relationship Between Polymorphism and Composition in the Muscovite-Lepidolite Series. Am. Mineral. 1953, 38, 88–107. [Google Scholar]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Guo, H.; Hu, Q.; Peng, W.; Wang, J. Extraction of Valuable Metals from Lepidolite. Hydrometallurgy 2012, 117, 116–118. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Yin, Z.; Wang, Z.; Guo, H.; Peng, W.; Hu, Q. A Novel Process for Extracting Lithium from Lepidolite. Hydrometallurgy 2012, 121, 54–59. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.J.; An, J.W.; Kim, M.J.; Tran, T. Factors Affecting the Extraction of Lithium from Lepidolite. Hydrometallurgy 2013, 134, 54–61. [Google Scholar] [CrossRef]
- Liu, K. Research Progress in Flotation Collectors for Lepidolite Mineral: An Overview. Miner. Process. Extr. Metall. Rev. 2023, 45, 728–742. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, Z.; Wei, Q.; Qin, W. Key Technologies and Development Trends for Efficient Flotation Recovery of Lepidolite. Green Smart Min. Eng. 2024, 1, 273–288. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Han, Y.; Zhang, S. Review on the Beneficiation of Li, Be, Ta, Nb-Bearing Polymetallic Pegmatite Ores in China. Minerals 2023, 13, 865. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Cheng, C.; Wang, H.; Liu, R.; Hu, Y.; He, G.; Yu, X.; Fu, W. Recycling Lepidolite from Tantalum–Niobium Mine Tailings by a Combined Magnetic–Flotation Process Using a Novel Gemini Surfactant: From Tailings Dams to the ‘Bling’ Raw Material of Lithium. ACS Sustain. Chem. Eng. 2020, 8, 18206–18214. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2019, 42, 123–141. [Google Scholar] [CrossRef]
- Korbel, C.; Filippova, I.V.; Filippov, L.O. Froth Flotation of Lithium Micas—A Review. Miner. Eng. 2023, 192, 107986. [Google Scholar] [CrossRef]
- Timich, M.; Contessotto, R.; Ulsen, C. Process Mineralogy of Li-Enriched Pegmatite Combining Laboratory Mineral Separations and SEM-Based Automated Image Analysis. Minerals 2023, 13, 343. [Google Scholar] [CrossRef]
- Hien-Dinh, T.T.; Luong, V.T.; Gieré, R.; Tran, T. Extraction of Lithium from Lepidolite via Iron Sulphide Roasting and Water Leaching. Hydrometallurgy 2015, 153, 154–159. [Google Scholar] [CrossRef]
- Dong, X.; Li, C.; Li, J.; Wang, J.; Huang, W. A Game-Theoretic Analysis of Implementation of Cleaner Production Policies in the Chinese Electroplating Industry. Resour. Conserv. Recycl. 2010, 54, 1442–1448. [Google Scholar] [CrossRef]
- Ogorodova, L.P.; Kiseleva, I.A.; Melchakova, L.V.; Schuriga, T.N. Thermodynamic Properties of Lithium Mica: Lepidolite. Thermochim. Acta 2005, 435, 68–70. [Google Scholar] [CrossRef]
- Hu, F.; Liu, X.; He, S. Cesium-Rubidium Mineralization in Himalayan Leucogranites. Sci. China Earth Sci. 2023, 66, 2827–2852. [Google Scholar] [CrossRef]
- Haldar, S.K. Introduction to Mineralogy and Petrology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 32–56. [Google Scholar]
- Guggenheim, S. Cation Ordering in Lepidolite. Am. Mineral. 1981, 66, 1221–1232. [Google Scholar]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.; Altarawneh, M.; Dlugogorski, B.Z. Leaching of Lepidolite and Recovery of Lithium Hydroxide from Purified Alkaline Pressure Leach Liquor by Phosphate Precipitation and Lime Addition. Hydrometallurgy 2021, 201, 105538. [Google Scholar] [CrossRef]
- Li, G.; Yang, J.; Yang, J. Literature Review of Extracting Lithium from Lepidolite. Bull. Chin. Ceram. Soc. 2017, 36, 1599–1604. [Google Scholar]
- Garrett, D.E. Handbook of Lithium and Natural Calcium Chloride; Elsevier Ltd.: London, UK, 2004. [Google Scholar]
- Su, H.; Zhu, Z.; Wang, L.; Qi, T. Research Progress in Extraction and Recovery of Lithium from Hard-Rock Ores. CIESC J. 2019, 70, 10–23. [Google Scholar]
- Choi, W.; Park, C.; Heo, C. Magmatic to Aqueous Phase Transition in Li-Pegmatite: Microtextural and Geochemical Study of Muscovite–Lepidolite from Boam Mine Area, Uljin, South Korea. Miner. Depos. 2024, 59, 1641–1660. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.; Chae, W.; Kim, S.B.; Kim, H. Electrostatically Controlled Enrichment of Lepidolite via Flotation. Mater. Trans. 2012, 53, 2191–2194. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- He, J.; Xu, H.; Yang, B.; Gong, W.; Zhang, Z.; Shan, Y.; Song, T. Adsorption Properties of an Efficient Combined Collector and Its Enhanced Separation of Lepidolite from Feldspar. J. Ind. Eng. Chem. 2025; in press. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, M.; Wen, W.; Zhu, S.; Mo, W.; Yan, P. Synergistic Mechanism of Dodecylamine/Octanol Mixtures Enhancing Lepidolite Flotation from the Self-Aggregation Behaviors at the Air/Liquid Interface. Physicochem. Probl. Miner. Process. 2023, 59, 343–355. [Google Scholar] [CrossRef]
- Qin, W.; Li, T.; Wang, N.; Huang, Z.; Zhang, Y. Floatation Process of Improving Lepidolite Ore Concentrate Grade and Recovery. Foshan Ceram. 2018, 28, 27–31. [Google Scholar]
- Deng, Y.; Ou, L. Mechanism for the Selective Separation of Lepidolite from Albite by Sodium Lauroyl Glutamate as a Green Environment Collector. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134922. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z.; Yu, J.G. Investigation of Dodecylammonium Adsorption on Mica, Albite and Quartz Surfaces by QM/MM Simulation. Mol. Phys. 2015, 113, 3423–3430. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Hu, Y.; He, G.; Yu, X.; Fu, W. Froth Flotation Separation of Lepidolite Ore Using a New Gemini Surfactant as the Flotation Collector. Sep. Purif. Technol. 2022, 282, 119122. [Google Scholar] [CrossRef]
- Cho, J.; Jung, M.Y.; Lee, H.; An, J. Froth-Flotation Separation as an Alternative for the Treatment of Soil Enriched with Fluorine Derived from Mica. Int. J. Environ. Res. Public Health 2022, 19, 1775. [Google Scholar] [CrossRef]
- Isa, I.; Ajaka, E.O.; Adebayo, A.O.; Alabi, O.O. Beneficiation of Lithium from Spodumene Rich Pegmatite: A Review. Int. J. Adv. Eng. Manag. 2024, 6, 19–26. [Google Scholar] [CrossRef]
- Urade, S. Investigating the Feasibility of Using Physical Separations to Pre-Concentrate Lithium Sedimentary Claystones. Master’s Thesis, University of Nevada, Reno, NV, USA, 2023. [Google Scholar]
- Liu, Y.; Liu, J. The Flotation Process of Lepidolite in Jiangxi Province in China. Adv. Mater. Res. 2014, 1033, 1309–1312. [Google Scholar] [CrossRef]
- Zou, Y.W.; Zhang, J.; Ding, Y. Study on Comprehensive Recovery of a Low Grade Zinnwaldite Ore from Jiangxi. Nonferrous Met. (Miner. Process. Sect.) 2019, 5, 85–89. [Google Scholar]
- Cheng, Q.; Chen, W.; Liu, G. Review on Progress of Lepidolite Flotation Collectors and Depressants. Conserv. Util. Miner. Resour. 2023, 43, 11–19. [Google Scholar]
- Yang, Z.; Xu, H.; Tang, X.; Zhou, H.; Xie, T.; Shen, L.; Luo, X. Application of a Novel Mixed Anionic/Cationic Collector in the Selective Flotation Separation of Lepidolite and Quartz. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134919. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, X.; Zhao, X.; Xu, C.; Zhang, D.; Gu, W.; Wang, R.; Lu, X. Study on Biogenic Acid-Mediated Enhanced Leaching of Lepidolite by Aspergillus Niger Based on Transcriptomics. Bioresour. Technol. 2025, 418, 131920. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Zhao, X.; Xu, C.; Zhang, D.; Gu, W.; Wang, R.; Lu, X. The Synergistic Interaction of Fungi with Different Structural Characteristics Improves the Leaching of Lithium from Lepidolite. Biochem. Eng. J. 2025, 213, 109564. [Google Scholar] [CrossRef]
- Sousa, R.; Ramos, V.; Guedes, A.; de Sousa, A.B.; Noronha, F.; Leite, M.M. Flotation of Lithium Ores to Obtain High-Grade Li2O Concentrates. Are There Any Mineralogical Limitations? Int. J. Min. Mater. Metall. Eng. 2019, 5, 7–18. [Google Scholar]
- Filippov, L.O.; Filippova, I.V.; Crumiere, G.; Sousa, R.; Leite, M.M.; de Sousa, A.B.; Korbel, C.; Tripathy, S.K. Separation of Lepidolite from Hard-Rock Pegmatite Ore via Dry Processing and Flotation. Miner. Eng. 2022, 187, 107768. [Google Scholar] [CrossRef]
- Wei, Q.; Feng, L.; Dong, L.; Jiao, F.; Qin, W. Selective Co-Adsorption Mechanism of a New Mixed Collector on the Flotation Separation of Lepidolite from Quartz. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125973. [Google Scholar] [CrossRef]
- Xiang, G.Y.; Fan, R.H.; Zhu, W.T.; Wei, S.U.N.; Zheng, R.J.; Gao, Z.Y. Synergistic Mechanism of Metal Ions and Sodium N-Oleoylsarcosinate on Flotation Separation of Lepidolite from Feldspar. Trans. Nonferrous Met. Soc. China 2025, 35, 296–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Q.; Jiao, F.; Qin, W. Role of Nanobubbles on the Fine Lepidolite Flotation with Mixed Cationic/Anionic Collector. Powder Technol. 2023, 427, 118785. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Q.; Yang, W.; Miao, Y.; Feng, Q. A Novel Combined Collector with Superior Selectivity for Flotation Separation of Lepidolite from Feldspar: Experimental Insight and MD Simulation. Sep. Purif. Technol. 2024, 347, 127627. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiao, F.; Wei, Q.; Liang, G.; Qin, W. Mitigating the Negative Impact of Feldspar Slime on Lepidolite Flotation by a Novel Anti-Slime Collector. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134811. [Google Scholar] [CrossRef]
- Huang, Z.; Li, W.; He, G.; Shen, L.; Chen, X.; Shuai, S.; Fu, W. Adsorption Mechanism of Amidoxime Collector on the Flotation of Lepidolite: Experiment and DFT Calculation. Langmuir 2022, 38, 15858–15865. [Google Scholar] [CrossRef]
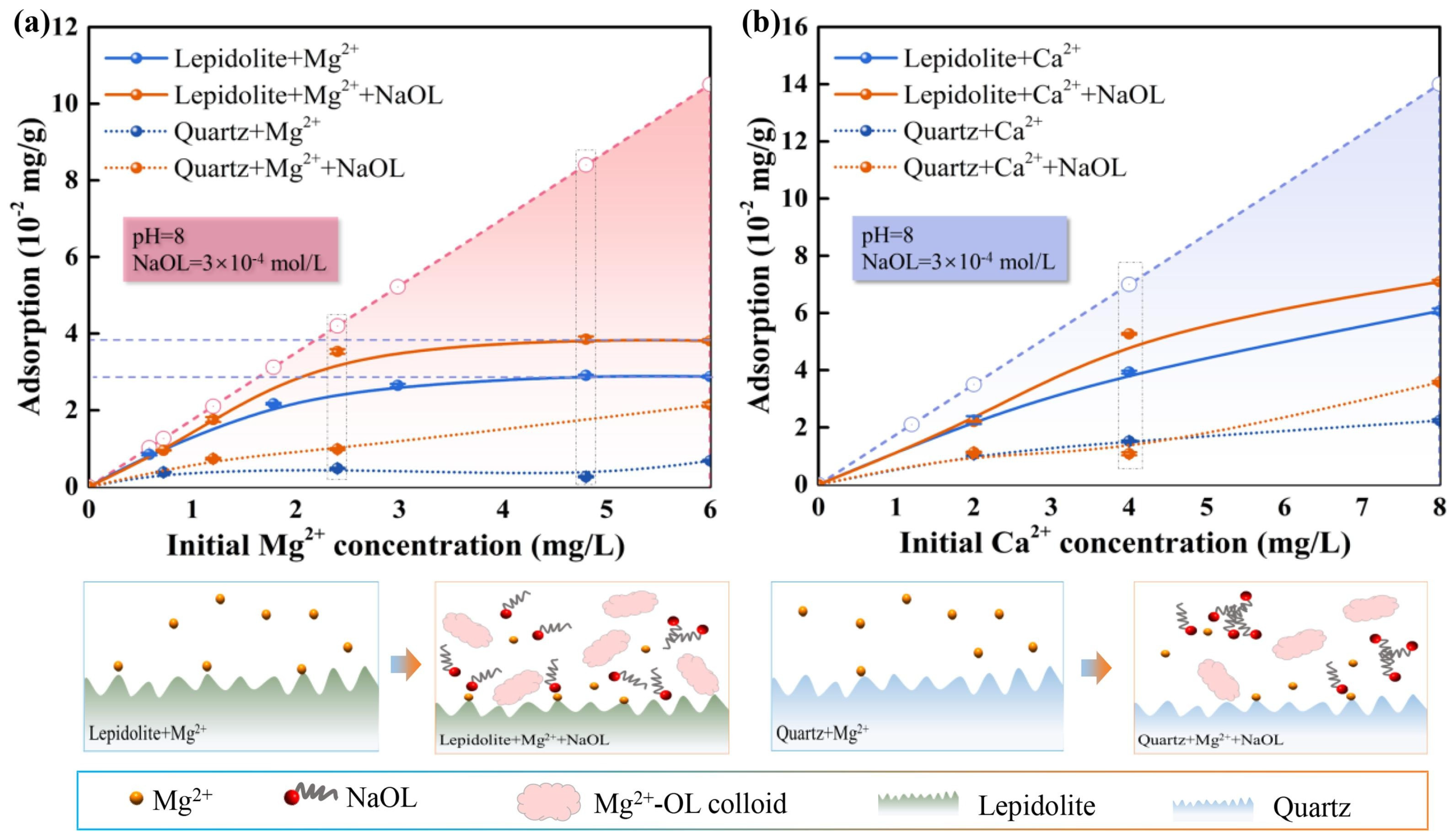
- Xu, R.; Liu, Y.; Sun, N.; Kang, J.; Sun, W.; Tang, H.; Wang, L. The Activation Role of Mg2+ in the Lepidolite Flotation Using NaOl. Sep. Purif. Technol. 2024, 351, 128035. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Tripathy, S.K.; Nayak, A.; Hembrom, K.C.; Dey, S.; Rath, R.K.; Mohanta, M.K. Beneficiation of Lithium Bearing Pegmatite Rock: A Review. Miner. Process. Extr. Metall. Rev. 2024, 45, 1–27. [Google Scholar] [CrossRef]
- Cook, B.K.; Gibson, C.E. A Review of Fatty Acid Collectors: Implications for Spodumene Flotation. Minerals 2023, 13, 212. [Google Scholar] [CrossRef]
- Fleet, M.E.; Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Micas; Geological Society of London: London, UK, 2003; pp. 34–41. [Google Scholar]
- Retamal, F.; Solar, C.; Saavedra, J.H.; Quezada, G.R.; Orvalho, S.; Toledo, P.G. Effect of Aliphatic Alcohol-Based and Polyglycol Polymer-Based Foaming Agents on the Water-Liquid-Vapor Interface by Means of Molecular Dynamics. J. Mol. Liq. 2024, 407, 125279. [Google Scholar] [CrossRef]
- Kundu, T.; Rath, S.S.; Das, S.K.; Parhi, P.K.; Angadi, S.I. Recovery of Lithium from Spodumene-Bearing Pegmatites: A Comprehensive Review on Geological Reserves, Beneficiation, and Extraction. Powder Technol. 2023, 415, 118142. [Google Scholar] [CrossRef]
- Pashkevich, D.; Li, R.; Waters, K. Temperature and Climate-Induced Fluctuations in Froth Flotation: An Overview of Different Ore Types. Can. Metall. Q. 2023, 62, 511–548. [Google Scholar] [CrossRef]
- Liu, J.L.; Yin, Z.L.; Li, X.H.; Hu, Q.Y.; Liu, W. Recovery of Valuable Metals from Lepidolite by Atmosphere Leaching and Kinetics on Dissolution of Lithium. Trans. Nonferrous Met. Soc. China 2019, 29, 641–649. [Google Scholar] [CrossRef]
- Leng, M.; Liu, Q. Study on the Extraction of Lithium from Lepidolite Ore by Composite Sulfate Roasting and First-Principle Computation. J. Sustain. Metall. 2024, 10, 2768–2780. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Thorough Extraction of Lithium and Rubidium from Lepidolite via Thermal Activation and Acid Leaching. Miner. Eng. 2022, 178, 107407. [Google Scholar] [CrossRef]
- Yang, H.; Zhong, W.; Zhong, F.; Zhao, J.; Li, D.; Zhang, L.; Guo, X. Research Progress of Lithium Extraction Technology from Lepidolite. Chin. J. Process. Eng. 2022, 24, 1251–1262. [Google Scholar]
- Lei, Z.; Zhong, H.; Wang, S.; Cao, Z.; Zhou, Z. Composite Salt Roasting-Leaching Performance and Mechanism of Lepidolite Containing Rubidium and Cesium. Multipurpose Util. Miner. Resour. 2019, 3, 152–156. [Google Scholar]
- Zhang, X.; Aldahri, T.; Tan, X.; Liu, W.; Zhang, L.; Tang, S. Efficient Co-Extraction of Lithium, Rubidium, Cesium and Potassium from Lepidolite by Process Intensification of Chlorination Roasting. Chem. Eng. Process. Process Intensif. 2020, 147, 107777. [Google Scholar] [CrossRef]
- Liu, J.; Yin, Z.; Liu, W.; Li, X.; Hu, Q. Treatment of Aluminum and Fluoride During Hydrochloric Acid Leaching of Lepidolite. Hydrometallurgy 2020, 191, 105222. [Google Scholar] [CrossRef]
- Gu, H.; Kuang, G.; Li, H.; Pei, W.; Wang, H. Enhanced Lithium Leaching from Lepidolite in Continuous Tubular Reactor Using H2SO4 + H2SiF6 as Lixiviant. Trans. Nonferrous Met. Soc. China 2021, 31, 2165–2173. [Google Scholar] [CrossRef]
- Guo, H.; Lv, M.; Kuang, G.; Cao, Y.; Wang, H. Stepwise Heat Treatment for Fluorine Removal on Selective Leachability of Li from Lepidolite Using HF/H2SO4 as Lixiviant. Sep. Purif. Technol. 2021, 259, 118194. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, J.; Fu, L.; Zuo, Y.; Zhang, G.; Wang, S.; Zhang, L. Microwave-Enhanced Sulfate Roasting for Lithium Extraction from Lepidolite: A Comprehensive Study. J. Clean. Prod. 2024, 434, 140248. [Google Scholar] [CrossRef]
- Kline, W.E.; Fogler, H.S. Dissolution Kinetics: Catalysis by Strong Acids. J. Colloid Interface Sci. 1981, 82, 93–102. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.Z.; Wang, H.D. Enhanced Acid Treatment to Extract Lithium from Lepidolite with a Fluorine-Based Chemical Method. Hydrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, Y.; Ma, B.; Wang, C.; Chen, Y. An Environmentally Friendly Improved Chlorination Roasting Process for Lepidolite with Reduced Chlorinating Agent Dosage and Chlorinated Waste Gas Emission. Sep. Purif. Technol. 2023, 310, 123173. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Selective Recovery and Efficient Separation of Lithium, Rubidium, and Cesium from Lepidolite Ores. Sep. Purif. Technol. 2022, 288, 120667. [Google Scholar] [CrossRef]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.C.; Altarawneh, M.; Dlugogorski, B.Z. Extraction of Lithium from Lepidolite by Sodium Bisulphate Roasting, Water Leaching and Precipitation as Lithium Phosphate from Purified Leach Liquors. Hydrometallurgy 2023, 222, 106139. [Google Scholar] [CrossRef]
- Park, T.; Shin, J.; Kim, S.; Ryu, T.; Kim, B.; Chang, H. An Effective Lithium Extraction Route from Lepidolite. Hydrometallurgy 2023, 222, 106202. [Google Scholar] [CrossRef]
- Sedlakova-Kadukova, J.; Marcincakova, R.; Luptakova, A.; Vojtko, M.; Fujda, M.; Pristas, P. Comparison of Three Different Bioleaching Systems for Li Recovery from Lepidolite. Sci. Rep. 2020, 10, 14594. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ju, J.; Zhang, J.; Yi, A.; Lei, Z.; Wang, L.; Zhu, Z.; Qi, T. Lithium Recovery from Lepidolite Roasted with Potassium Compounds. Miner. Eng. 2020, 145, 106087. [Google Scholar] [CrossRef]
- Swanson, T.H.; Bailey, S.W. Redetermination of the Lepidolite-2 M 1 Structure. Clays Clay Miner. 1981, 29, 81–90. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Zhou, Y.; Zhang, X.; Xu, C.; Duan, H.; Wang, R.; Lu, X. Research on the Decomposition Mechanisms of Lithium Silicate Ores with Different Crystal Structures by Autotrophic and Heterotrophic Bacteria. Sci. Total Environ. 2024, 925, 171762. [Google Scholar] [CrossRef]
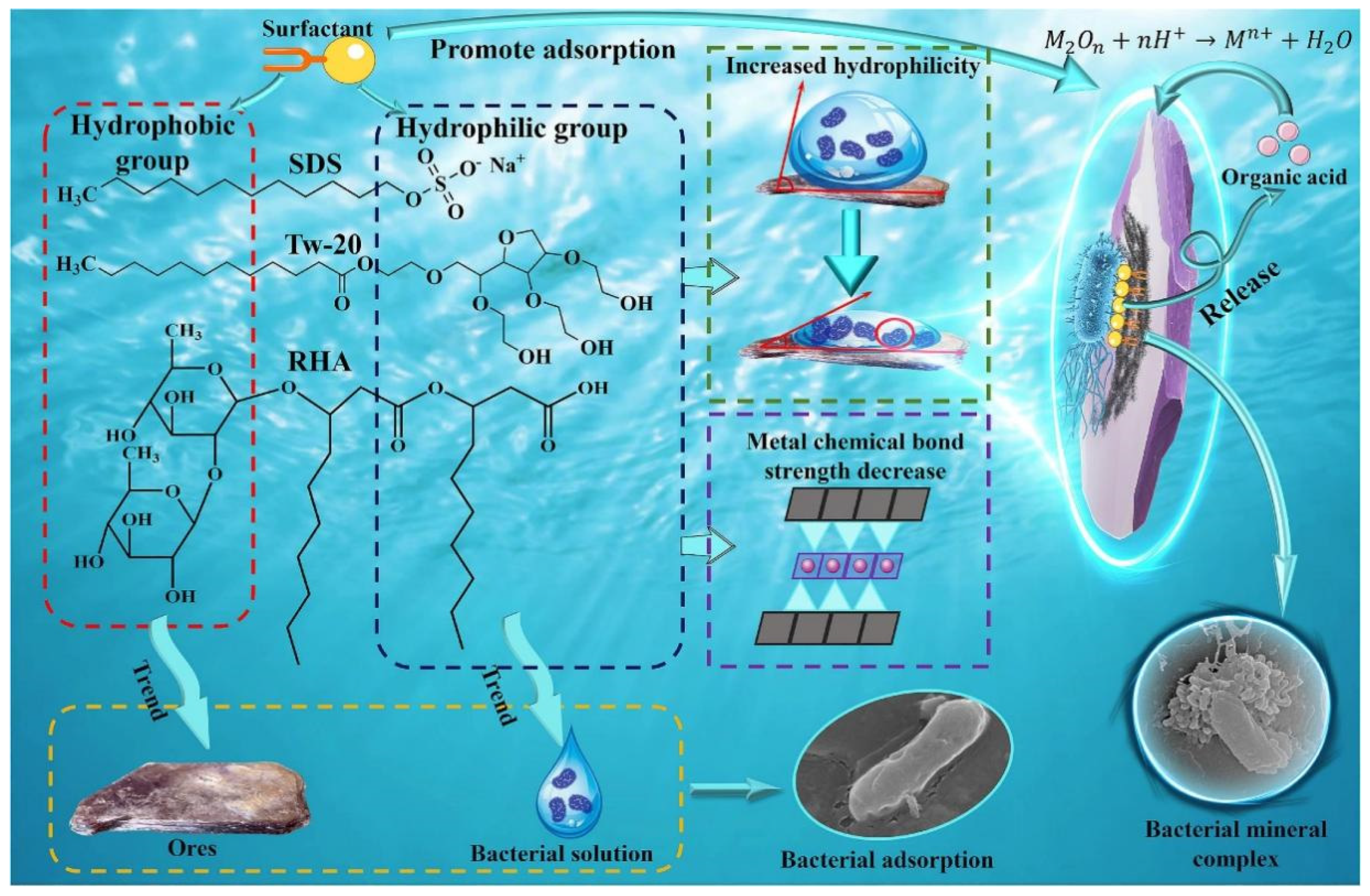
- Xu, C.; Zhao, X.; Duan, H.; Gu, W.; Zhang, D.; Wang, R.; Lu, X. Mechanism of Surfactant Effect on Bacterial Adsorption During Bioleaching of Lepidolite. Appl. Clay Sci. 2025, 264, 107646. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Ding, C.; Wang, X.; Zhang, X.; Wang, R.; Lu, X. Lithium Extraction from Typical Lithium Silicate Ores by Two Bacteria with Different Metabolic Characteristics: Experiments, Mechanism and Significance. J. Environ. Manag. 2023, 347, 119082. [Google Scholar] [CrossRef]
- Zhai, Q.; Liu, R.; Song, Y.; Mao, Z.; Sun, W. A Novel Technology for Lithium Extraction Through Low-Temperature Synergistic Roasting of α-Spodumene with Lepidolite. Sep. Purif. Technol. 2025, 355, 129667. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Li, C.; Yi, Y.; Liu, W.; Zhang, L. Energy-Efficient and Simultaneous Extraction of Lithium, Rubidium and Cesium from Lepidolite Concentrate via Sulfuric Acid Baking and Water Leaching. Hydrometallurgy 2019, 185, 244–249. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Wang, J.; Guo, H.; Hu, Q.; Peng, W. Extraction of Lithium from Lepidolite by Sulfation Roasting and Water Leaching. Int. J. Miner. Process. 2012, 110–111, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Z.; Aldahri, T.; Rohani, S.; Ren, S.; Liu, W. Separation and Recovery of Cesium Sulfate from the Leach Solution Obtained in the Sulfuric Acid Baking Process of Lepidolite Concentrate. Hydrometallurgy 2021, 199, 105537. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Leday, L.J.; Wang, D. Microwave-Assisted Extraction of Lithium from Hectorite Clay. Geosyst. Eng. 2024, 27, 108–117. [Google Scholar] [CrossRef]
- Setoudeh, N.; Nosrati, A.; Welham, N.J. Lithium Recovery from Mechanically Activated Mixtures of Lepidolite and Sodium Sulfate. Miner. Process. Extr. Metall. Rev. 2021, 130, 354–361. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Wang, J.; Liang, Y.; Xie, W.; Zhong, H.; He, Z. Advances and Promotion Strategies of Processes for Extracting Lithium from Mineral Resources. J. Ind. Eng. Chem. 2024, 140, 47–64. [Google Scholar] [CrossRef]
- Garcia, L.V.; Ho, Y.-C.; Myo Thant, M.M.; Han, D.S.; Lim, J.W. Lithium in a Sustainable Circular Economy: A Comprehensive Review. Processes 2023, 11, 418. [Google Scholar] [CrossRef]
- Fu, W.; Meng, L.; Qu, J. Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone. Metals 2024, 14, 1075. [Google Scholar] [CrossRef]
- Lan, R.; Wang, H.; Guo, Q.; Zhao, J.; Du, X.; Sun, J.; Dai, J.; Chen, K. A Preliminary Assessment of a Solid-State Lithium-Ion Battery in Radiation Environment by Geant4 Simulations. J. Appl. Phys. 2024, 136, 215901. [Google Scholar] [CrossRef]
- Murg, L.; Lee, S.C.; Grizzi, V.F. Enhanced Exploration of LiF–NaF Thermal Conductivity Through Transferable Equivariant Graph Neural Networks. J. Appl. Phys. 2025, 137, 064701. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, C.S.; Ye, X.J. Be2B Monolayer as a Well-Balanced Performance Anode for Lithium-Ion Batteries with Ultrahigh Theoretical Capacity and Low Diffusion Barrier. J. Appl. Phys. 2024, 136, 225001. [Google Scholar] [CrossRef]
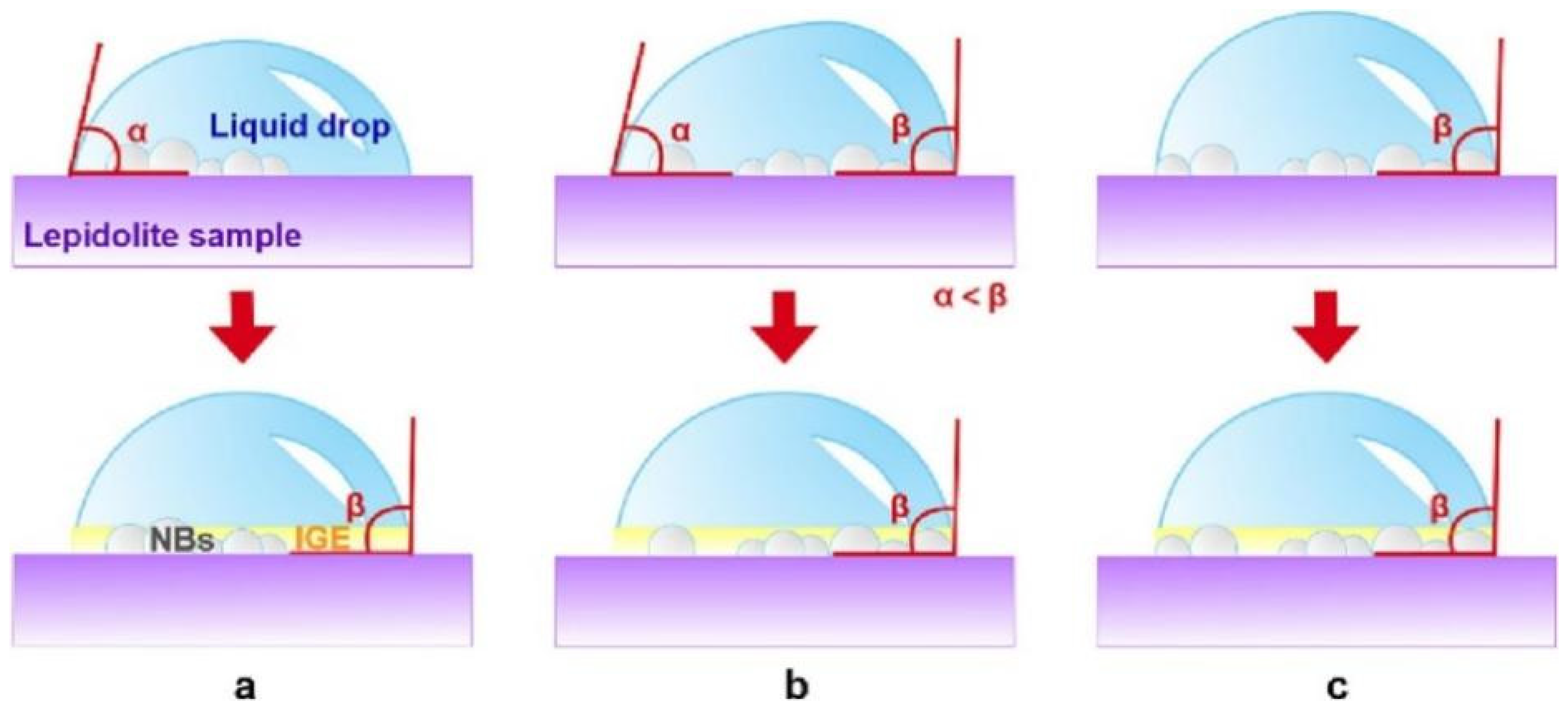
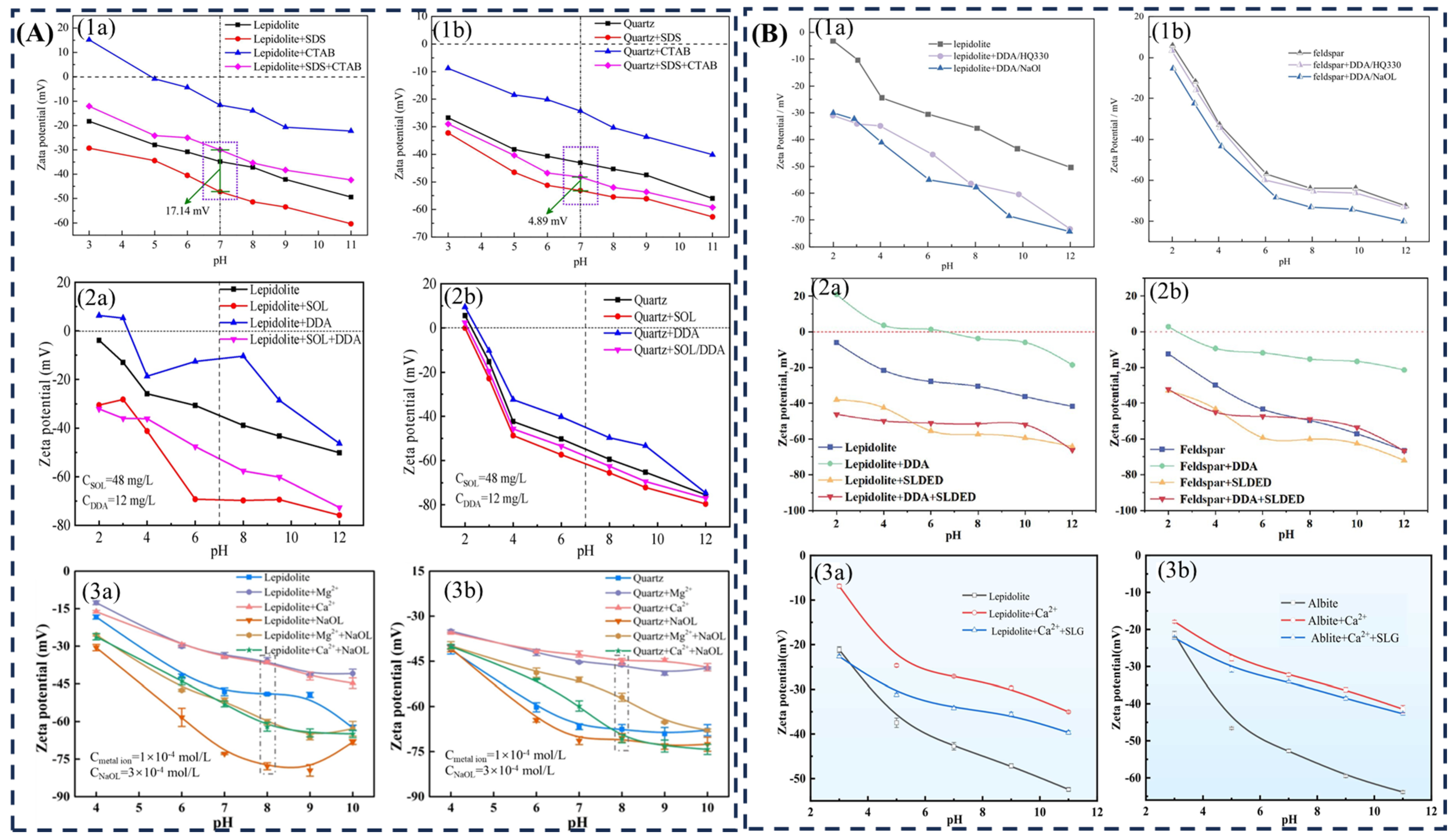
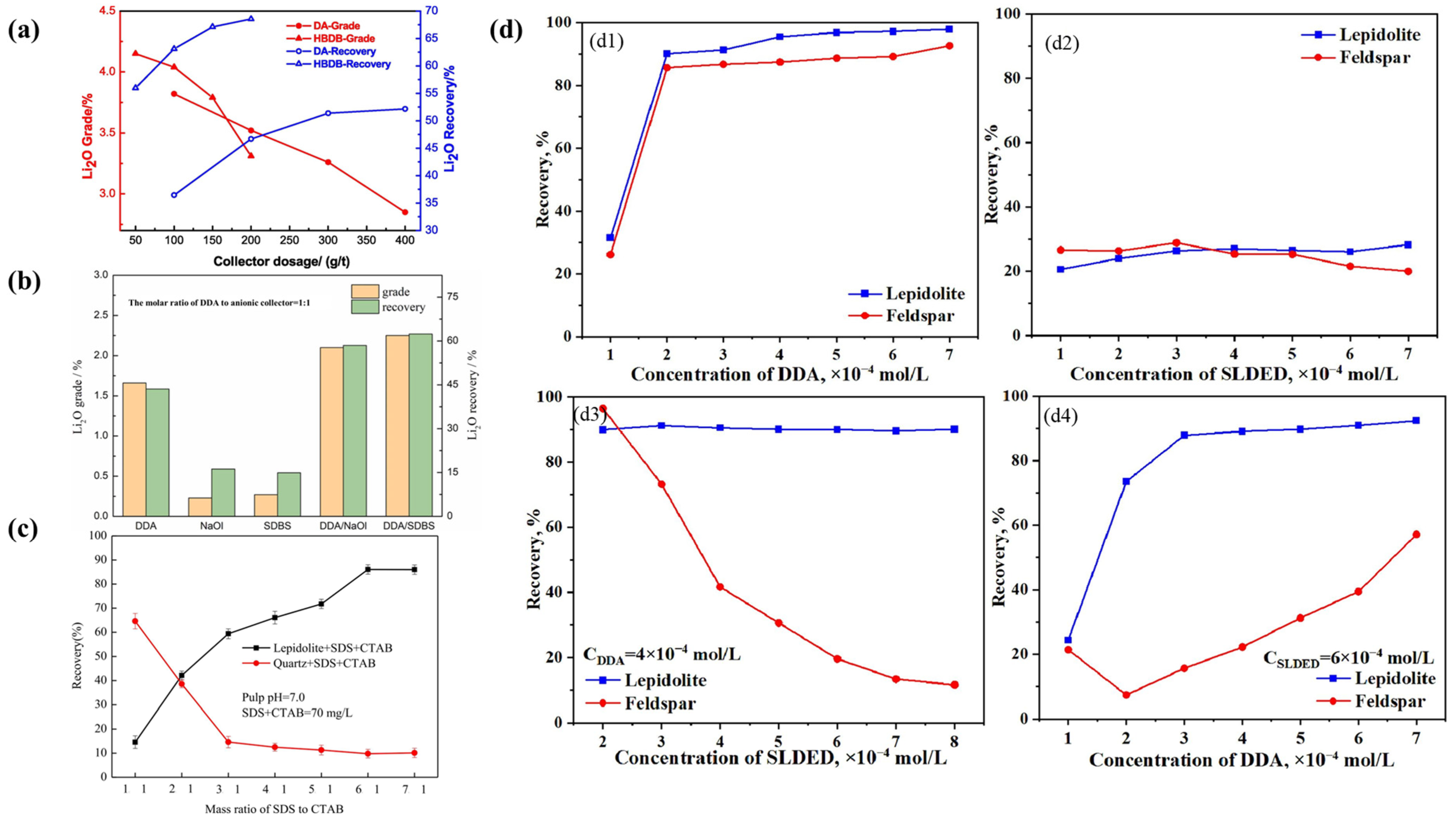
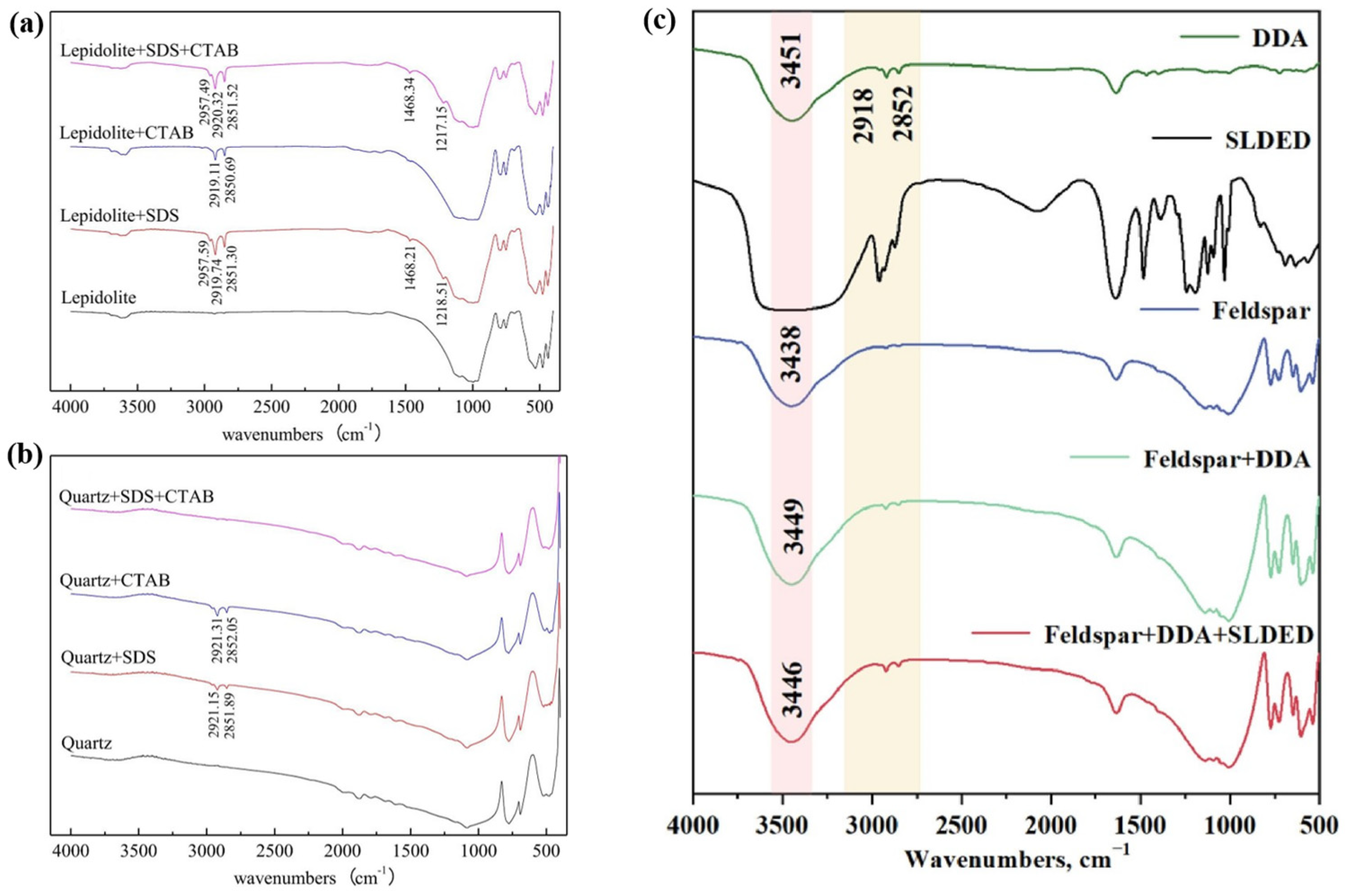
| Collector Type | Applicable pH | Li2O Grade (%) | Recovery (%) | Advantages | Limitations | Refs |
|---|---|---|---|---|---|---|
| Primary amines (DDA) | 3.5–11 | 3.77–5.55 | 76–98 | Broad applicability, high selectivity | High foam viscosity, equipment corrosion (under acidic conditions) | [34] |
| Gemini surfactants (HBDB) | 3–6.8 | 4.12–4.5 | 71–90 | High efficiency at low temperatures, reduced dosage | High synthesis cost | [33] |
| Mixed collectors (DDA/NaOl) | 6.5–8 | 4.2–4.99 | 86–95 | Synergistic effects, wide pH adaptability | Complex formulation optimization | [44] |
| Anionic collectors (SLG) | 5 | 5.28 | 87.95 | Eco-friendly, high selectivity | Requires Ca2+ activation, limited applicability | [54] |
| Method/Route | Condition | Efficiency | Environmental Concerns |
|---|---|---|---|
| Hydrochloric acid (HCl) [68] | 6.21 mol/L HCl at 381 K for 8 h, followed by calcination at 623 K | 95.7% of Li recovered | High HCl usage; manageable Al/F emissions |
| Stepwise heat treatment HF + H2SO4 adopting PTFE reactor | 120 °C for 3 h and 200 °C for 6 h | 98.6% of Li leached and 0.68% F in liquid phase | remove fluorine and unreacted sulfuric acid more effectively |
| HF + H2SO4 adopting stirred tank reactor [69] | 85 °C, 3 h, analytical pure HF and H2SO4 | 98% of Li leached | Toxic HF; fluorine control required |
| H2SO4+H2SiF6 adopting continuous tubular reactor [70] | 80 °C, 15 min, 15 wt.% H2SiF6, 70 wt.% H2SO4, Ore:H2SO4:H2SiF6 = 1:0.8:1.6 | 97.9% of Li leached | Fluorine residues; less hazardous than HF |
| H2SO4 baking and water leaching [54] | 200 °C, 4 h, 85 wt.% H2SO4, concentration: acid = 1.7:1, 85 °C leaching | 97.1% of Li, 96.0% of Rb and 95.1% of Cs leached | |
| H2SO4 baking, air roasting, water leaching, Li carbonation precipitation (CO2) reaction, Rb, Cs solvent extraction [71] | 300 °C, 5 h, 98 wt.% H2SO4 concentration: acid = 1.7:1, 800 °C, 2 h, 80 °C leaching | 90.5% of Li, 91.2% of Rb, and 89.4% of Cs leached | High-efficiency sulfuric acid recovery reduces alkali consumption and waste discharge |
| Chlorination roasting and water leaching [72] | 950 °C, 1 h, 25% CaCl2, 20% Ca(OH)2, 25 °C, 2 h, 2:1 mL/g, 300 rpm leaching | 85.5% of Li, 80.9% of K, 94.5% of Rb, 90.2% of Cs extracted | Reduce the dosage of chlorinating agent and the volatilization of chlorination |
| Metric | Bioleaching | Hydrometallurgy |
|---|---|---|
| Efficiency | Low (<20% Li recovery rate), slow process | High (>80% Li recovery rate), rapid reaction |
| Environmental Impact | High (low carbon footprint, biodegradable reagents) | Low (chemical pollution risks, high energy consumption) |
| Cost | High initial investment, low operational cost | High reagent/energy costs, mature infrastructure |
| Technological Maturity | Laboratory stage, industrial challenges remain | Widely industrialized, mature processes |
| Ore Compatibility | Prefers layered structures, low impurity content | High adaptability, suitable for complex ores |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, J.; Shi, X.; Wang, Q.; Lin, H.; Shang, H.; Shen, Z. Efficient Exploitation of Lepidolite Resources: A Review on Beneficiation Techniques, Extraction Methods, and Synergistic Optimization. Separations 2025, 12, 130. https://doi.org/10.3390/separations12050130
Ku J, Shi X, Wang Q, Lin H, Shang H, Shen Z. Efficient Exploitation of Lepidolite Resources: A Review on Beneficiation Techniques, Extraction Methods, and Synergistic Optimization. Separations. 2025; 12(5):130. https://doi.org/10.3390/separations12050130
Chicago/Turabian StyleKu, Jiangang, Xiao Shi, Qian Wang, Hanyu Lin, Hongliang Shang, and Zhengchang Shen. 2025. "Efficient Exploitation of Lepidolite Resources: A Review on Beneficiation Techniques, Extraction Methods, and Synergistic Optimization" Separations 12, no. 5: 130. https://doi.org/10.3390/separations12050130
APA StyleKu, J., Shi, X., Wang, Q., Lin, H., Shang, H., & Shen, Z. (2025). Efficient Exploitation of Lepidolite Resources: A Review on Beneficiation Techniques, Extraction Methods, and Synergistic Optimization. Separations, 12(5), 130. https://doi.org/10.3390/separations12050130