Microextraction and Eco-Friendly Techniques Applied to Solid Matrices Followed by Chromatographic Analysis
Abstract
1. Introduction
2. Environmental Pollutants
3. Eco-Friendly Sample Preparation
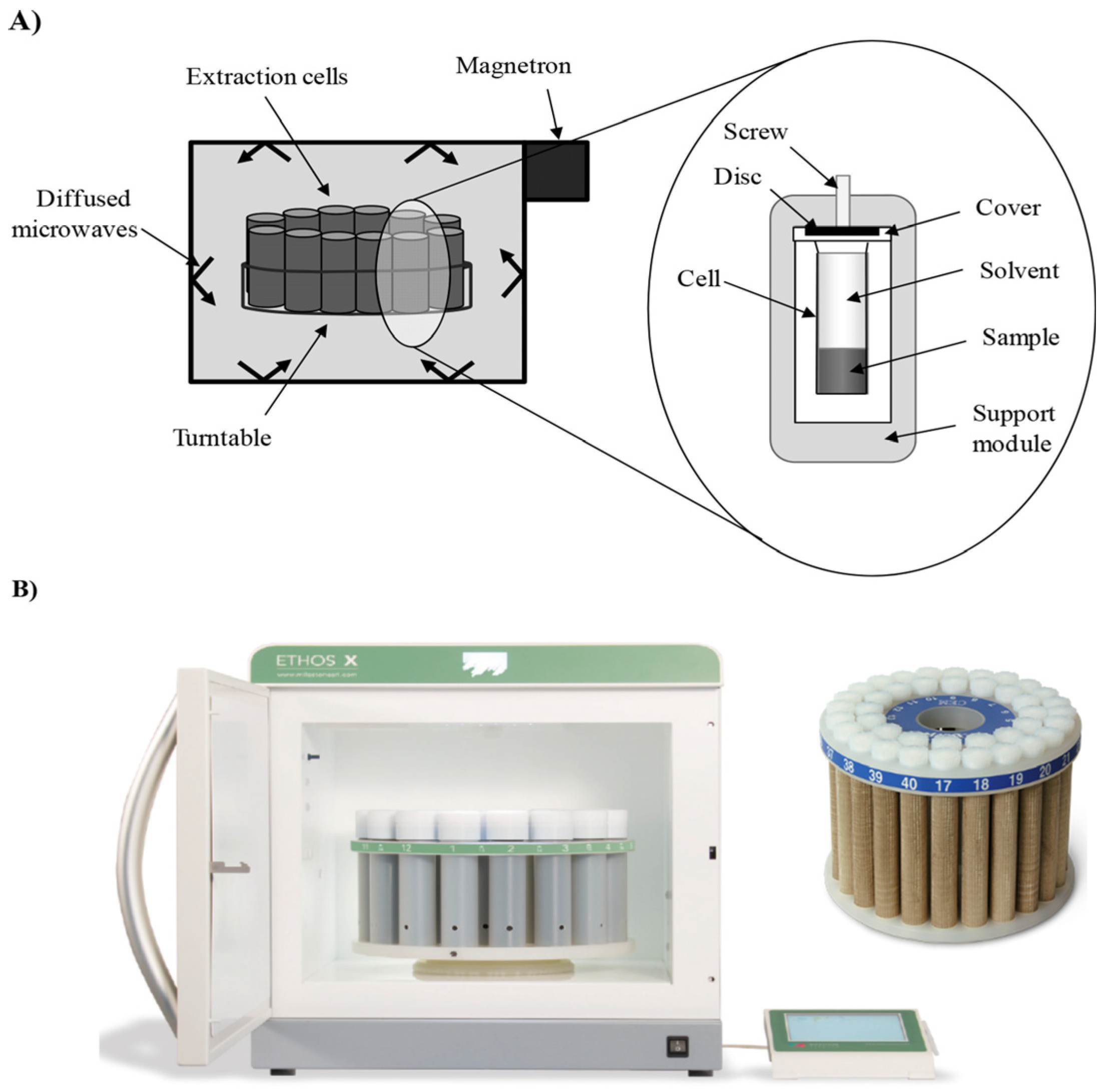
3.1. Microwave-Assisted Extraction
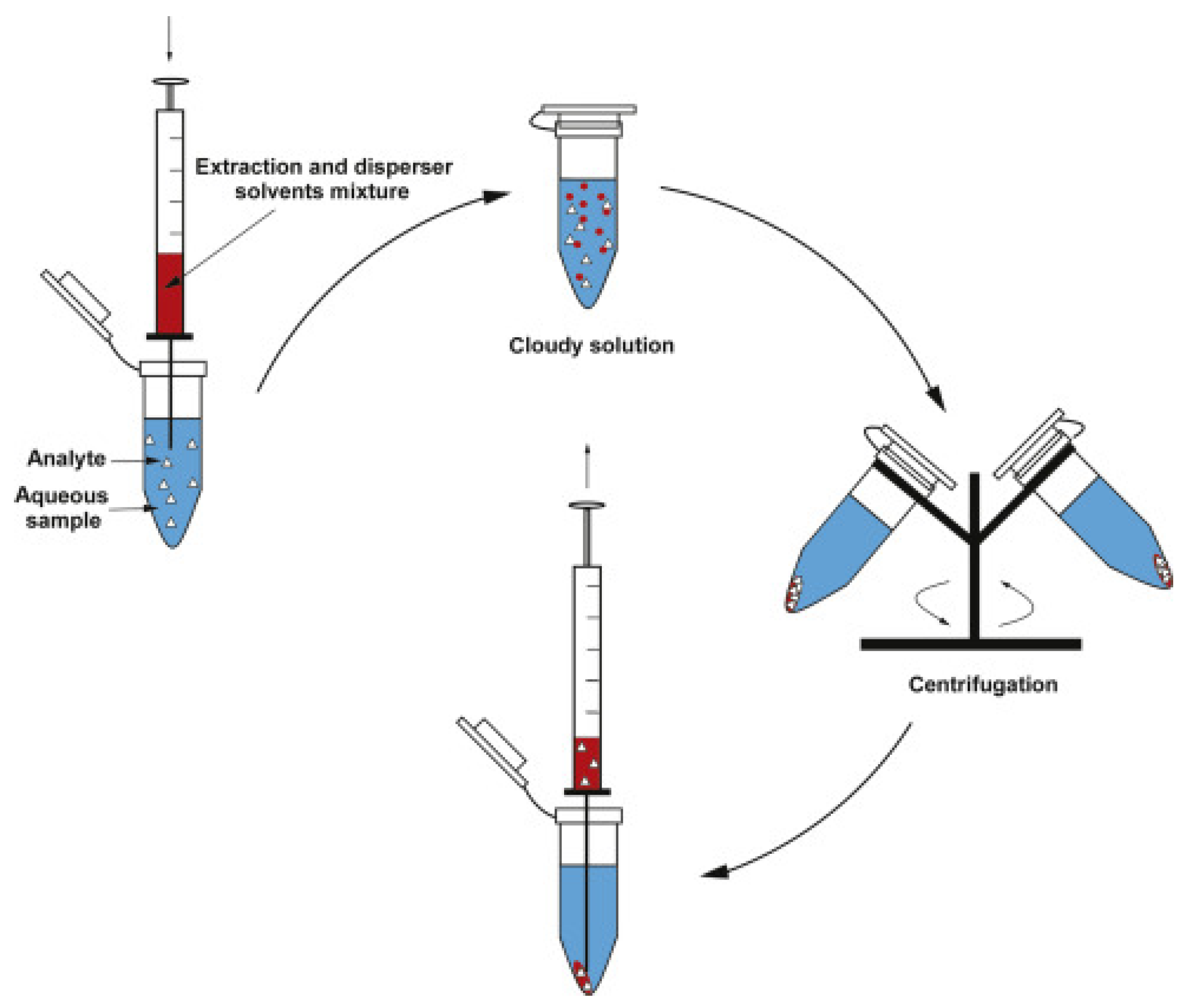
3.2. Ultrasound-Assisted Extraction
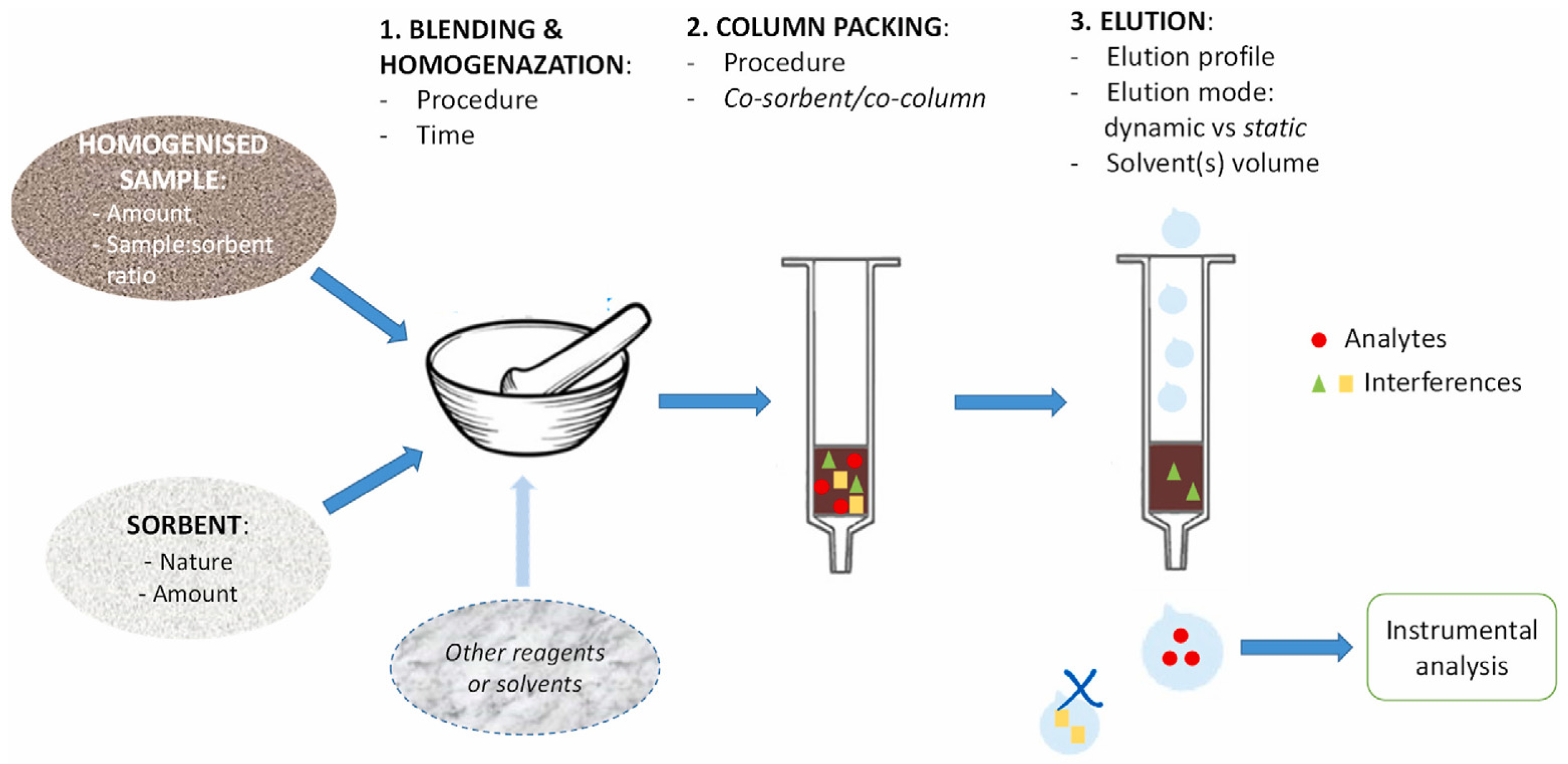
3.3. Matrix Solid-Phase Dispersion
| Analytes | Matrix | Extraction Approach | Volume and Type of Organic Solvent | Instrumentation | LOD | Linear Range | [Ref.]—Publishing Year |
|---|---|---|---|---|---|---|---|
| PAHs | Sediment and sludge | MAE + SPE as clean-up | 50 mL n-hexane:acetone 1:1 (v/v) | GC-MS | 0.025–1.211 µg/kg | 0.01–0.8 mg/L | [44]—2023 |
| PAHs | Soil and sediment | MAE | 15 mL hexane:acetone 1:1 (v/v) | GC-MS | 7.8–15.6 µg/kg (sediment) 15.6–31.3 µg/kg (soil) | 0.5–10 mg/kg | [45]—2023 |
| Cytostatic compounds | Sludge and sediment | MAE | 14 mL MeOH | UHPLC-MS/MS | 0.42–79.8 ng/g (sludge) 0.10–87.46 ng/g (sediment) | 0.2–12 µg/g | [47]—2020 |
| Biocides | Particulate fractions of urban and surface waters | MAE | 20 mL of MeOH/DCM 60:40 (v/v) | HPLC-MS/MS | 0.4–200 ng/g | 0.05–250 µg/L | [48]—2020 |
| NPAHs and OPAHs | PM2.5 | UAE + SPE clean-up | 5 mL of MeOH | LC-MS/MS | 0.001–0.042 µg/L | 0.025–10 µg/L | [50]—2023 |
| OPEs and organophosphate hydroxylated degradation products | Sediment | UAE + SPE clean-up | OP triesters and hydroxylated degradation products: 30 mL of ACN (UAE) and 9 mL of ACN (SPE); OP diesters: 30 mL MeOH (UAE) and 9 mL of MeOH (SPE) | LC-ESI-MS/MS | - | 0.05–50 ng/g | [51]—2022 |
| BTs, BTRs, and BSAs | PM2.5 and PMcoarse | UAE | 5 mL of ethyl acetate | GC-MS | 0.001–0.08 ng/m3 (PM2.5) 0.002–0.14 ng/m3 (PMcoarse) | 0.01–10 ng/µL | [52]—2020 |
| Herbicides | Soils | UAE | 10 mL of H2O/MeOH 40:60 (v/v) | LC-MS/MS | 0.010–0.097 ng/g | 0.1–100 µg/L | [53]—2023 |
| Plastic additives (PAs) | Soil | UAE + dSPE and UAE + QuEchERS | 20 mL H2O/MeOH 80:20 (v/v) and 20 mL n-hexane (UAE); 3 mL of ethyl acetate (dSPE); 10 mL of H2O + 10 mL of ethyl acetate (UAE + QuEchERS) | UHPLC-MS/MS | - | 0.1–100 ng/mL | [54]—2024 |
| PAHs and PASHs | Marine sediment | UAE-MSD | 500 µL of DCM:MeOH 65:35 (v/v) | GC-MS | 8.8–30.2 ng/g | 0.5–200 µg/L | [55]—2021 |
| Sulfonamides | Soil | MSPD | 3 g of C18 (dispersive sorbent); 12 mL of ACN (elution solvent) | HPLC-MS/MS | 0.024–0.058 µg/kg | 0.01–0.5 µg/mL | [60]—2024 |
| OPFRs | Sewage sludge | MSPD | 2 g of C18 (dispersive sorbent) 5 mL of acetone (elution solvent) | UPLC-ESI-MS/MS | - | 0.02–150 ng/mL | [61]—2023 |
| PPCPs and booster biocides | Sediment | VA-MSPD | 5 mL of MeOH (elution solvent) | HPLC-(QqLIT)-MS-MS | 0.13–11.06 ng/g | - | [62]—2021 |
| Chlorophenols | River sediment | µMSPD | 100 mg of Celite AZO + 150 µL of TDES (dispersive sorbent); 450 µL of ACN (elution solvent) | HPLC-PDA | 1.04–2.48 µg/g | 10–150 µg/g | [63]—2023 |
| PAHs and PCBs | Sediment | MSPD | 1 g of florisil + 0.5 g of 3-chloropropyl-bonded silica particles (dispersive sorbent); 5 mL acetone/hexane 50:50 (v/v) (elution solvent) | GC-MS | 0.06–1.1 ng/g | 0.05–3 mg/L | [64]—2021 |
3.4. Solid-Phase Microextraction
3.4.1. Development of New Coatings
3.4.2. Application of Commercial Devices and Fiber Coatings
3.4.3. SPME Coupled with Green Solid–Liquid Extraction Technique
3.4.4. Development of Novel Configurations
3.5. Stir Bar Sorptive Extraction
| Analytes | Matrix | Extraction Approach | Coating Material | Volume and Type of Organic Solvent | Instrumentation | LOD | Linear Range | [Ref.]—Publishing Year |
|---|---|---|---|---|---|---|---|---|
| Benzene | Soil, vegetables | HS-SPME | nano-activated carbon/ionic liquid (NAC/IL) | - | GC-FID | - | 0.1–3 mg/L | [69]—2021 |
| Nitroaromatics | Soil | HS-CF-SPME | poly 3,4-ethylenedioxythiophene and gold nanoparticles composite coating on a gold wire (AuNPs/PEDOT@Au) | - | GC-FID | 0.5–3 ng/g | 0.5–250 ng/g | [70]—2023 |
| Nitrated polycyclic aromatic hydrocarbons (NPAHs) | Sediments | Solid–liquid extraction + DI-SPME | zeolite imidazolate framework-8/hexagonal boron nitride (ZIF-8/h-BN) | - | GC-MS | 0.42–0.61 ng/g | 5–500 ng/g | [71]—2023 |
| Triacetone triperoxide | Soil, paper | HS-SPME | metal–organic frameworks (MOFs), including IRMOF-8, MOF-5, UIO-66, ZIF-8, and MIL-101(Cr) | - | GC-MS | 13 ng/mL | 50–5000 ng/L | [72]—2023 |
| Phenoxycarboxylic acids herbicides | Soil | Solid–liquid extraction + DI-MF-SPME | monolith/aminated carbon nanotubes composite (MACN) | 1.99 mL of ACN + 498 µL MeOH | HPLC/DAD | 0.20–0.61 µg/kg | 2–500 µg/kg | [73]—2021 |
| Sulfonylurea herbicides | Soil | UAE + IT-SPME | porous monolith-based magnetism-reinforced | 1.96 mL of ACN | HPLC/DAD | 0.30–1.5 µg/kg | 1–300 µg/kg | [74]—2020 |
| Polychlorinated biphenyls | Soil | UAE + DI-SPME | Nitrogen-rich carbon nitride | 20.5 mL of acetone | GC-FID | 3.1–11.1 pg/mL | 0.01–1000 pg/mL | [75]—2021 |
| Polycyclic aromatic hydrocarbons | Seabed sediment | UAE + HS-SPME | UiO-67/perfluorooctanoic acid (UiO-67/PFOA) | 31 mL of acetone | GC-FID | 0.003–0.008 ng/mL | 0.01–20 ng/mL | [76]—2024 |
| Polycyclic aromatic hydrocarbons | Soil | Solid–liquid extraction + HS-SPME | Porphyrin-based covalent organic framework | - | GC-FID | 0.25–5 ng/mL | 1–150 ng/mL | [77]—2021 |
| Phenols | Soil | Solid–liquid extraction + HS-SPME | 1,3,5-trimethylphloroglucinol-benzidine (TpBD) COF | - | GC-MS | 0.39–0.72 ng/L | 2–10,000 ng/L | [78]—2023 |
| Polycyclic aromatic hydrocarbons, oxygenated polycyclic aromatic hydrocarbons, nitrated polycyclic aromatic hydrocarbons | Particulate (PM2.5) | UAE + DI-CF-SPME | 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) | 150 µL of ACN | GC-MS | 0.001–0.129 ng/m3 | 0.32–94.68 ng/m3 | [79]—2020 |
| Amines | Particulate (PM2.5) | HS-SPME | 85 µm polyacrylate (PA) | - | GC-MS/MS | 0.01–49 pg/m3 | 0.01–10 ng/µL | [80]—2020 |
| Geosmin and 2-methylisoborneol | Soil | HS-SPME | 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) | - | GC-MS | 0.16–0.72 ng/L | 0.05–20 µg/L | [81]—2021 |
| Semi-volatile organic compounds | Particulate (PM2.5) | Solid–liquid extraction + DI-SPME | 85 µm polyacrylate (PA) | 150 µL of ACN | GC×GC/Q-TOFMS | - | - | [82]—2025 |
| BTEX | Soil | HS-SPME | 75 µm carboxen/polydimethylsiloxane (Car/PDMS) and 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) | - | Portable GC-MS | 100–200 µg/m3 | - | [83]—2023 |
| Biogenic Volatile Organic Compounds (BVOCs) | Plant | Dynamic BVOC Sampling System (DBSS)-HS-SPME | 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) | - | GC-MS | - | - | [84]—2021 |
| VOCs | Soil | HS-SPME | 95 μm Carbon Wide Range/Polydimethylsiloxane (CWR/PDMS) | - | GC-MS | 0.039–1.20 µg/kg | - | [85]—2024 |
| Ferrocene and five derivatives | Soil | UAE + DI-SPME | 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) | - | gas chromatography–microwave-induced plasma with atomic emission detection (GC-MIP-AED) | 0.9–4 ng/g | 0.01–20 ng/mL | [86]—2020 |
| Herbicides | Soil | Solid–liquid extraction + IT-SPME | 35% diphenyl-65% dimethyl polysiloxane | 1 mL of MeOH | capillary liquid chromatography (capLC)-DAD | 0.05–0.1 µg/g | 0.5–4 µg/g | [87]—2024 |
| BVOCs | Soil | HS-SPME | 75 μm carboxen/polydimethylsiloxane (CAR/PDMS) | - | GC-MS | 0.01–0.30 µg/kg | - | [88]—2023 |
| Nitrated polycyclic aromatic hydrocarbons | Sediments | ASE + DI-SPME | 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) | 61.2 mL of dichloromethane | GC-MS/MS | 0.020–0.472 ng/g | 0.1–300 ng/g | [89]—2022 |
| Pesticides | Soil | Miniaturized solid–liquid extraction (MISOLEX) + DI-SPME | 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) | 5 mL of acetone + 10 mL of petroleum ether | GC-MS | 0.005–1.16 µg/kg | 0.01–25 µg/L | [90]—2022 |
| Poly(methyl Methacrylate) Micro/Nanoplastics | Soil | CA-SPME | 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) | - | GC-MS | 0.28 µg | 5–1000 µg | [91]—2024 |
| Trichloroethylene (TCE) | Soil | HS-SPME | 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) | - | Portable GC-MS | - | 0–100 µg/L | [92]—2021 |
| Pesticides | Soil | DI-SPME LC-Tips | C18 | 700 µL of MeOH | GC-MS/MS | 0.01–10 µg/kg | 0.1–50 µg/kg | [93]—2024 |
| VOCs | Gasoline-spiked soil, plant | HS-SPME | 100 μm polydimethylsiloxane (PDMS) | - | GC-MS | - | - | [94]—2024 |
| PAHs, PCBs, PAEs | Soil | UAE + gas-cycle-assisted (GCA) HS-SPME | 100 μm polydimethylsiloxane (PDMS) and 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) | - | GC-FID | 0.49–1.51 pg/mL | 0.002–100 ng/mL | [95]—2021 |
| PAHs, BTEX | Soil | In-syringe vacuum-assisted (ISV)-HS-SPME | hybrid of covalent triazine-based frameworks and metal–organic frameworks (COF/MOF) | - | GC-FID | 0.07–5 ng/g | 0.23–9000 ng/g | [96]—2023 |
| PAHs | Soil | low-pressure (LP)-HS-SPME | Nano-octadecylsilica/polyvinyl alcohol (NODS/PVA) | - | GC-FID | 3–50 ng/g | 0.01–1300 ng/g | [97]—2021 |
| PPCPs | Sewage sludge | MAE-DI-SPME-On-fiber derivatization with MTBSTFA | 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) | 24 mL of water/MeOH mixture 95:5 (v/v) (MAE) | GC-MS | 3.25–48.9 ng/g | 146–2466 ng/g | [98]—2020 |
| BTs, BTRs, BSAs | PM10 | MAE-DI-SPME | 85 µm polyacrylate (PA) | 15 mL of water/ethanol mixture 70/30 (v/v) | GC-MS/MS | 0.021–0.21 ng/mL | 17–100 ng/mL | [99]—2021 |
| BTEX | Soil | Ultrasound-assisted pressure-regulated SPME (UA-PR-SPME) | Graphene oxide/gamma-aminopropyltriethoxysilane coated fiber (GO-APTES) | 500 µL of MeOH (UAE) | GC-FID | 0.1–0.4 ng/g | 2.4–5000 ng/g | [100]—2020 |
| PAHs | Soil | MSPD-CA-SPME | 100 μm polydimethylsiloxane (PDMS) | - | GC-MS | 4.2–8.5 ng/g | 40–4000 ng/g | [101]—2020 |
| VOCs | Soil | HS-SBSE | Polydimethylsiloxane (PDMS) | - | GC-MS | - | - | [85]—2024 |
| Polychlorinated biphenyls, polybrominated diphenyl ethers, organochlorine compounds | Sediment | UAE + DI-SBSE | Polydimethylsiloxane (PDMS) | 2.2 mL of MeOH | GC-MS/MS | 0.029–6.5 ng/g | 0.1–3000 ng/g | [106]—2021 |
| Benzotriazole ultraviolet absorbers | Soil | UAE + DI-SBSE | Azo-linked porous organic polymers/polydimethylsiloxane (PP/PDMS) | 18 mL of MeOH | HPLC-DAD | 0.12–0.33 µg/L | 0.5–100 µg/L | [107]—2020 |
| Benzophenones | Soil and sunscreen | UAE + HF-SBSE | Covalent organic framework-V modified porous polypropylene | 20 mL of MeOH + 150 µL of ACN | HPLC-UV | 0.02–0.03 ng/mL | 0.1–200 ng/mL | [108]—2022 |
3.6. Liquid-Phase Microextraction
| Analytes | Matrix | Extraction Approach | Volume and Type of Organic Solvent | Instrumentation | LOD | Linear Range | [Ref.]—Publishing Year |
|---|---|---|---|---|---|---|---|
| Pesticides | Soil, sugarcane, and jaggery | Solid–liquid extraction + DLLME-SFO | 1 mL of ACN (Solid–liquid extraction) 50 μL of 1-Dodecanol (extractant solvent for DLLME-SFO) | GC-μECD | 0.868–2.522 ng/g | 6.25–100 ng/g | [110]—2022 |
| Phthalate esters and bisphenol A | Particulate (PM2.5) | UAE + DLLME + vortex-assisted micro-solid-phase extraction (VA-μ-SPE) | 3 mL of acetone (UAE) 400 μL of acetone (dispersive solvent for DLLME), 70 μL of chloroform (extractant solvent for DLLME) 100 µL of acetone (extractant solvent for VA-μ-SPE) | GC-MS/MS | 0.07–0.15 ng/mL | 0.3–100 ng/mL | [111]—2021 |
| Flame retardants | Sewage sludge | DLLME | 3 μL of ACN (dispersive solvent), 10 mg of ([P+6,6,6,14]2[MnCl42−]) MIL (extractant solvent), 20 μL of methanol (desorption solvent) | Py-GC–MS | 16.9–375 µg/L | 200–6000 µg/L | [113]—2024 |
| Pesticides, bisphenols, musks and UV filters | Sediment | QuEChERS + DLLME | 10 mL of ACN (QuEChERS) 85 μL of carbon tetrachloride (extractant solvent for DLLME)) | GC-MS | 0.005–2.5 ng/mL | 0.01–40 ng/mL | [114]—2022 |
| Pyrethroid insecticides | Soil | UAE + SALLE + DLLME-SFO | 17 mL of ACN (UAE) 300 μL of 1-undecanol (extractant solvent for DLLME-SFO) | GC-MS | 1.5–6.1 ng/mL | 5–5000 ng/mL | [116]—2020 |
| Pesticides | Soil | Solid–liquid extraction + HF-LPME | 20.0 μL of octanol (acceptor solvent) | LC-MS | 66.1–198.1 µg/L | 500–1000 µg/L | [117]—2023 |
| Herbicides and metabolites | Soil | Solid–liquid extraction + HF-LPME | 1 mL of di-hexyl ether (acceptor solvent) | HPLC-UV | 0.1–0.3 µg/kg | 2–60 µg/kg | [118]—2023 |
| Endocrine-disrupting compounds and pesticides | Moss, rock–soil | UAE + SADF-LPME | 25 g of ACN (UAE) 0.1326 g of a mixture dichlormethane: 1,2-dichloroethane (1:1 v/v) (SADF-LPME) | GC-MS | 1.0–6.6 ng/g | 3.8–205 ng/g | [119]—2024 |
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Wieczorek, M.N.; Zhou, W.; Pawliszyn, J. Perspective on sample preparation fundamentals. Adv. Sample Prep. 2024, 10, 100114. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef] [PubMed]
- Khuman, S.N.; Lee, H.-Y.; Cho, I.-G.; Chung, D.; Lee, S.Y.; Lee, J.; Oh, J.-K.; Choi, S.-D. Monitoring of organochlorine pesticides using pine needle, pine bark, and soil samples across South Korea: Source apportionment and implications for atmospheric transport. Chemosphere 2025, 370, 144043. [Google Scholar] [CrossRef] [PubMed]
- Peneva, S.; Le, Q.N.P.; Munhoz, D.R.; Wrigley, O.; Wille, F.; Doose, H.; Halsall, C.; Harkes, P.; Sander, M.; Braun, M.; et al. Microplastic analysis in soils: A comparative assessment. Ecotoxicol. Environ. Saf. 2025, 289, 117428. [Google Scholar] [CrossRef]
- Mitra, S. Sample Preparation Techniques in Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC-Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- López-Lorente, A.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC-Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Kokosa, J.M.; Przyjazny, A. Green microextraction methodologies for sample preparations. Green Anal. Chem. 2022, 3, 100023. [Google Scholar] [CrossRef]
- Schettino, L.; Peris-Pastor, G.; Benedé, J.L.; Chisvert, A. A comprehensive review on the use of microextraction techniques in the analysis of cosmetic products. Adv. Sample Prep. 2022, 3, 100024. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep e Analytical greenness metric for sample preparation. TrAC-Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A new tool for evaluating and/or selecting analytical methods: Summarizing the information in a hexagon. TrAC-Trends Anal. Chem. 2019, 118, 538–547. [Google Scholar] [CrossRef]
- Nowak, P.M.; Kościelniak, P. What Color Is Your Method? Adaptation of the RGB Additive Color Model to Analytical Method Evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC-Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- González-Martín, R.; Gutiérrez-Serpa, A.; Pino, V.; Sajid, M. A tool to assess analytical sample preparation procedures: Sample preparation metric of sustainability. J. Chromatogr. A 2023, 1707, 464291. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Richardson, S.D. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2009, 81, 4645–4677. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. In-cell clean-up pressurized liquid extraction and gaschromatography–tandem mass spectrometry determination ofhydrophobic persistent and emerging organic pollutants in coastal sediments. J. Chromatogr. A 2016, 1429, 107–118. [Google Scholar] [CrossRef]
- Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Simultaneous in-vial acetylation solid-phase microextraction followed by gas chromatography tandem mass spectrometry for the analysis of multiclass organic UV filters in water. J. Hazard. Mater. 2017, 323, 45–55. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Anderson, J.L. Expanding the use of polymeric ionic liquids in headspace solid-phase microextraction: Determination of ultraviolet filters in water samples. J. Chromatogr. A 2018, 1540, 11–20. [Google Scholar] [CrossRef]
- Mei, M.; Huang, X. Online analysis of five organic ultraviolet filters in environmental water samples using magnetism-enhanced monolith-based in-tube solid phase microextraction coupled with high-performance liquid chromatography. J. Chromatogr. A 2017, 1525, 1–9. [Google Scholar] [CrossRef]
- Fumes, B.H.; Lanças, F.M. Use of graphene supported on aminopropyl silica for microextraction of parabens from water samples. J. Chromatogr. A 2017, 1487, 64–71. [Google Scholar] [CrossRef]
- Naccarato, A.; Elliani, R.; Sindona, G.; Tagarelli, A. Multivariate optimization of a microextraction by packed sorbent-programmed temperature vaporization-gas chromatography–tandem mass spectrometry method for organophosphate flame retardant analysis in environmental aqueous matrices. Anal. Bioanal. Chem. 2017, 409, 7105–7120. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Chahkandi, M.; Targhoo, A. Synthesis of nano-hydroxyapatite sorbent for microextraction in packed syringe of phthalate esters in water samples. Anal. Chim. Acta 2017, 950, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Ghaemi, F. Microextraction in packed syringe by using a three-dimensional carbon nanotube/carbon nanofiber–graphene nanostructure coupled to dispersive liquid-liquid microextraction for the determination of phthalate esters in water samples. Microchim. Acta 2017, 184, 3851–3858. [Google Scholar] [CrossRef]
- Elliani, R.; Tagarelli, A.; Naccarato, A. Assessment of benzothiazoles, benzotriazoles and benzenesulfonamides in environmental waters using an optimized combination of microextraction by packed sorbent with programmed temperature vaporization-gas chromatography tandem-mass spectrometry. Talanta 2023, 258, 124410. [Google Scholar] [CrossRef]
- Naccarato, A.; Gionfriddo, E.; Sindona, G.; Tagarelli, A. Simultaneous determination of benzothiazoles, benzotriazoles and benzosulfonamides by solid phase microextraction-gaschromatography-triple quadrupole mass spectrometry in environmental aqueous matrices and human urine. J. Chromatogr. A 2014, 1338, 164–173. [Google Scholar] [CrossRef]
- Martín Santos, P.; Campo, L.; Olgiati, L.; Polledri, E.; del Nogal Sánchez, M.; Fustinoni, S. Development of a method to profile 2- to 4-ring polycyclic aromatic hydrocarbons in saliva samples from smokers and non-smokers by headspace-solid-phase microextraction-gas chromatography-triple quadrupole tandem mass spectrometry. J. Chromatogr. B 2020, 1152, 122273. [Google Scholar] [CrossRef]
- Elliani, R.; Naccarato, A.; Malacaria, L.; Tagarelli, A. A rapid method for the quantification of urinary phthalate monoesters: A new strategy for the assessment of the exposure to phthalate ester by solid-phase microextraction with gas chromatography and tandem mass spectrometry. J. Sep. Sci. 2020, 43, 3061–3073. [Google Scholar] [CrossRef]
- Naccarato, A.; Elliani, R.; Tagarelli, A. A protocol based on solid phase microextraction -gas chromatography-tandem mass spectrometry for the monitoring of parabens and bisphenols in human saliva. J. Chromatogr. A 2023, 1707, 464303. [Google Scholar] [CrossRef]
- Naccarato, A.; Elliani, R.; Tagarelli, A. A direct immersion-solid-phase microextraction method for the automated determination of 3- to 6-ring polycyclic aromatic hydrocarbons in saliva by gas-chromatography-tandem mass spectrometry. J. Chromatogr. A 2024, 1736, 465404. [Google Scholar] [CrossRef]
- Naccarato, A.; Gionfriddo, E.; Elliani, R.; Pawliszyn, J.; Sindona, G.; Tagarelli, A. Investigating the robustness and extraction performance of a matrix-compatible solid-phase microextraction coating in human urine and its application to assess 2–6-ring polycyclic aromatic hydrocarbons using GC–MS/MS. J. Sep. Sci. 2018, 41, 929–939. [Google Scholar] [CrossRef]
- González-Martín, R.; Trujillo-Rodríguez, M.J.; Freire, M.G.; Ayala, J.H.; Pino, V. Effervescence tablets based on magnetic ionic liquids as simple microdevices for the in situ dispersive liquid-liquid microextraction of urinary biomarkers. Anal. Chim. Acta 2024, 1328, 343187. [Google Scholar] [CrossRef] [PubMed]
- Lepri de Oliveira, M.; Alves Rocha, B.; de Oliveira Souza, V.C.; Barbosa, F., Jr. Determination of 17 potential endocrine-disrupting chemicals in human saliva by dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. Talanta 2019, 196, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Jain, R.; Singh, P.; Ch, R.; Mudiam, M.K.R. Determination of Urinary PAH Metabolites Using DLLME Hyphenated to Injector Port Silylation and GC–MS-MS. J. Anal. Toxicol. 2015, 39, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Alves Rocha, B.; Ruiz Brandão da Costa, B.; Perez de Albuquerque, N.C.; Moraes de Oliveira, A.R.; Oliveira Souza, J.M.; Al-Tameemi, M.; Dobal Campiglia, A.; Barbosa, F., Jr. A fast method for bisphenol A and six analogues (S, F, Z, P, AF, AP) determination in urine samples based on dispersive liquid-liquid Microextraction and liquid chromatography-tandem mass spectrometry. Talanta 2016, 154, 511–519. [Google Scholar] [CrossRef]
- Martín Santos, P.; Jiménez Carracedo, C.; del Nogal Sánchez, M.; Pérez Pavón, J.L.; Cordero, B.M. A sensitive and automatic method based on microextraction by packed sorbents for the determination of polycyclic aromatic hydrocarbons in saliva samples. Microchem. J. 2020, 152, 104274. [Google Scholar] [CrossRef]
- Silveira, R.S.; Rocha, B.A.; Rodrigues, J.L.; Barbosa, F., Jr. Rapid, sensitive and simultaneous determination of 16 endocrine disrupting chemicals (parabens, benzophenones, bisphenols, and triclocarban) in human urine based on microextraction by packed sorbent combined with liquid chromatography tandem mass spectrometry (MEPS-LC-MS/MS). Chemosphere 2020, 240, 124951. [Google Scholar] [CrossRef]
- Moreira Fernandez, M.A.; Coelho André, L.; de Lourdes Cardeal, Z. Hollow fiber liquid-phase microextraction-gas chromatography-masss pectrometry method to analyze bisphenol A and other plasticizer metabolites. J. Chromatogr. A 2017, 1481, 31–36. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Luo, X.; Wang, K.; Zhang, S.; Ji, Z.; Gao, Y.; You, J. A novel switchable solvent liquid-phase microextraction technique based on the solidification of floating organic droplets: HPLC-FLD analysis of polycyclic aromatic hydrocarbon monohydroxy metabolites in urine samples. New J. Chem. 2020, 44, 3038. [Google Scholar] [CrossRef]
- Gomes, J.M.; Araujo Almeida, T.F.; Aparecida da Silva, T.; de Lourdes Cardeal, Z.; Costa Menezes, H. Saliva biomonitoring using LPME-GC/MS method to assess dentistry exposure to plasticizers. Anal. Bioanal. Chem. 2020, 412, 7799–7810. [Google Scholar] [CrossRef]
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave extraction: A novel sample preparation method for chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Moretti, S.; Elliani, R.; Sprovieri, F.; Pirrone, N.; Tagarelli, A. A green approach for organophosphate ester determination in airborne particulate matter: Microwave-assisted extraction using hydroalcoholic mixture coupled with solid-phase microextraction gas chromatography tandem mass spectrometry. Talanta 2018, 189, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC-Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Ndwab, S.; Malungana, M.; Mahlambi, P. Comparison of Ultra-Sonication and Microwave Extraction Followed by Filtration or Filtration and Solid-Phase Extraction Clean-Up for PAH Determination from Sediment and Sludge: Human Health and Ecological Risk Assessment. Appl. Sci. 2023, 13, 5619. [Google Scholar] [CrossRef]
- Kariyawasam, T.; Doran, G.S.; Howitt, J.A.; Prenzler, P.D. Optimization and Comparison of Microwave-Assisted Extraction, Supercritical Fluid Extraction, and Eucalyptus Oil–Assisted Extraction of Polycyclic Aromatic Hydrocarbons from Soil and Sediment. Environ. Toxicol. Chem. 2023, 42, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Brums, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; Da Silva, E.G.P.; Portugal, L.A.; Dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Tuček, J.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Halko, R. Cytostatic compounds in sludge and sediment: Extraction and determination by a combination of microwave-assisted extraction and UHPLC–MS/MS. Anal. Bioanal. Chem. 2020, 412, 3639–3651. [Google Scholar] [CrossRef]
- Paijens, C.; Frère, B.; Caupos, E.; Moilleron, R.; Bressy, A. Determination of 18 Biocides in Both the Dissolved and Particulate Fractions of Urban and Surface Waters by HPLC-MS/MS. Water Air Soil Pollut. 2020, 231, 210. [Google Scholar] [CrossRef]
- Boinis, N.; Konomi, A.; Gkotsis, G.; Nika, M.-C.; Thomaidis, N.S. Trends in extraction techniques for the determination of organic micropollutants in liver tissues of vertebrates. Anal. Bioanal. Chem. 2025, 417, 535–553. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Shi, Z.; Li, J. A Sensitive Online SPE-LC–APCI–MS/MS Method for Simultaneous Determination of 17 Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Atmospheric Particulate Matter. Chromatographia 2023, 86, 677–688. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, L.; Wang, H.; Lu, D.; Luo, X. Development and validation of a liquid chromatography-tandem mass spectrometry method for comprehensive detection of organophosphate esters and their degradation products in sediment. J. Chromatogr. A 2022, 1665, 462826. [Google Scholar] [CrossRef]
- Nuñez, A.; Vallecillos, L.; Marcé, R.M.; Borrull, F. Occurrence and risk assessment of benzothiazole, benzotriazole and benzenesulfonamide derivatives in airborne particulate matter from an industrial area in Spain. Sci. Total Environ. 2020, 708, 135065. [Google Scholar] [CrossRef]
- Castiñeira-Landeira, A.; Vazquez, L.; Gonzalez-Leirado, H.; Llompart, M.; Dagnac, T. Ultrasound-assisted extraction followed by liquid chromatography coupled to tandem mass spectrometry for the simultaneous determination of multiclass herbicides in soil. Anal. Bioanal. Chem. 2023, 415, 7197–7209. [Google Scholar] [CrossRef]
- Dvorakova, D.; Tsagkaris, A.S.; Pulkrabova, J. Novel strategies for the determination of plastic additives derived from agricultural plastics in soil using ultrahigh-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS). Sci. Total Environ. 2024, 946, 174492. [Google Scholar] [CrossRef] [PubMed]
- Bahia, P.V.B.; Nascimento, M.M.; Hatje, V.; de Andrade, J.B.; Machado, M.E. Microscale extraction combined with gas chromatography/mass spectrometry for the simultaneous determination of polycyclic aromatic hydrocarbons and polycyclic aromatic sulfur heterocycles in marine sediments. J. Chromatogr. A 2021, 1653, 462414. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Long, A.R.; Short, C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. A 1989, 475, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L. Current trends in the determination of organic compounds in foodstuffs using matrix solid phase dispersion. TrAC-Trends Anal. Chem. 2024, 172, 117601. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. New insights into the application of MSPD in various fields of analytical chemistry. TrAC-Trends Anal. Chem. 2019, 112, 29–51. [Google Scholar] [CrossRef]
- Ramos, L. Use of new tailored and engineered materials for matrix solid-phase dispersion. TrAC-Trends Anal. Chem. 2019, 118, 751–758. [Google Scholar] [CrossRef]
- Yuan, J.P.; Liu, C.; Jiang, H.; Zhou, Z.; Xie, M.; Sun, Y. Rapid and Simultaneous Determination of 13 Sulfonamides in Soil by Matrix Solid-Phase Dispersion With High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Sep. Sci 2024, 47, e70015. [Google Scholar] [CrossRef]
- Castro, G.; Sørmo, E.; Yu, G.; Sait, S.T.L.; González, S.V.; Arp, H.P.H.; Asimakopoulos, A.G. Analysis, occurrence and removal efficiencies of organophosphate flame retardants (OPFRs) in sludge undergoing anaerobic digestion followed by diverse thermal treatments. Sci. Total Environ. 2023, 870, 161856. [Google Scholar] [CrossRef]
- Soares, K.L.; Sunyer-Caldú, A.; Barbosa, S.C.; Primel, E.G.; Fillmann, G.; Diaz Cruz, M.S. Rapid and cost-effective multiresidue analysis of pharmaceuticals, personal care products, and antifouling booster biocides in marine sediments using matrix solid phase dispersion. Chemosphere 2021, 267, 129085. [Google Scholar] [CrossRef] [PubMed]
- El-Deen, A.K.; Shimizu, K. Miniaturized ternary deep eutectic solvent-based matrix solid-phase dispersion: A green sample preparation method for the determination of chlorophenols in river sediment. J. Sep. Sci. 2023, 46, 2200717. [Google Scholar] [CrossRef]
- Portet-Koltalo, F.; Tian, Y.; Berger-Brito, I.; Benamar, A.; Boulangé-Lecomte, C.; Machour, N. Determination of multi-class polyaromatic compounds in sediments by a simple modified matrix solid phase dispersive extraction. Talanta 2021, 221, 121601. [Google Scholar] [CrossRef]
- Shahvar, A.; Naccarato, A.; Saraji, M.; Lucena, R.; Cárdenas, S. Solid-phase microextraction. In Analytical Sample Preparation with Nano- and Other High-Performance Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–77. [Google Scholar] [CrossRef]
- Bonacci, T.; Mazzei, A.; Naccarato, A.; Elliani, R.; Tagarelli, A.; Brandmayr, P. Beetles “in red”: Are the endangered flat bark beetles Cucujus cinnaberinus and C. haematodes chemically protected? (Coleoptera: Cucujidae). Eur. Zool. J. 2018, 85, 129–137. [Google Scholar] [CrossRef]
- Naccarato, A.; Elliani, R.; Cavaliere, B.; Sindona, G.; Tagarelli, A. Development of a fast and simple gas chromatographic protocol based on the combined use of alkyl chloroformate and solid phase microextraction for the assay of polyamines in human urine. J. Chromatogr. A 2018, 1549, 1–13. [Google Scholar] [CrossRef]
- Giglio, A.; Brandmayr, P.; Dalpozzo, R.; Sindona, G.; Tagarelli, A.; Talarico, F.; Zetto, T.; Ferrero, E.A. The Defensive Secretion of Carabus lefebvrei Dejean 1826 Pupa (Coleoptera, Carabidae): Gland Ultrastructure and Chemical Identification. Microsc. Res. Tech. 2009, 72, 351–361. [Google Scholar] [CrossRef]
- Afzali, A.; Vahidi, H.; Fakhraie, S. Benzene extraction in environmental samples based on the mixture of nanoactivated carbon and ionic liquid coated on fused silica fiber before determination by headspace solid-phase microextraction-gas chromatography. Anal. Method Environ. Chem. J. 2021, 4, 68–78. [Google Scholar] [CrossRef]
- Akbarian, M.; Gholamalizadeh, A.; Ahmar, H.; Banitaba, M.H. Application of poly 3,4-ethylenedioxythiophene and gold nanoparticles composite on a gold wire as a coating for determination of nitroaromatics in soil using cold fiber solid phase microextraction combined with gas chromatography. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100804. [Google Scholar] [CrossRef]
- Kong, J.; Cao, X.; Huang, W.; Li, C.; Xian, Q.; Yang, S.; Li, S.; Sun, C.; He, H. Predicting the bioavailability of nitro polycyclic aromatic hydrocarbons in sediments: ZIF-8/h-BN solid-phase microextraction versus Tenax extraction. Environ. Poll. 2023, 318, 120896. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, X.; Mei, H.; Guo, H.; Li, H.; Wang, P.; Li, Y.; Deng, X.; Zhu, J.; Hu, C. Metal-organic frameworks as solid-phase microextraction adsorbents for the determination of triacetone triperoxide by gas chromatography-mass spectrometry. Forensic Sci. Int. 2023, 352, 111852. [Google Scholar] [CrossRef]
- Chen, H.; Luo, S.; Huang, X. Development of monolith/aminated carbon nanotubes composite-based solid-phasemicroextraction of phenoxycarboxylic acids herbicides in water and soil samples. J. Sep. Sci. 2021, 44, 4284–4294. [Google Scholar] [CrossRef]
- Pang, J.; Song, X.; Huang, X.; Yuan, D. Porous monolith-based magnetism-reinforce d in-tub e solid phase microextraction of sulfonylurea herbicides in water and soil samples. J. Chromatogr. A 2020, 1613, 460672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, L.; Li, M.; Qin, P.; Li, D.; Zhou, Q.; Lu, M.; Cai, Z. Nitrogen-rich carbon nitride as solid-phase microextraction fiber coating for high-efficient pretreatment of polychlorinated biphenyls from environmental samples. J. Chromatogr. A 2021, 1659. [Google Scholar] [CrossRef]
- Li, D.; Qin, M.; Lou, X.; Zhu, J.; Ma, W.; Zhang, N.; Lu, M. Constructing perfluorinated UiO-67 for enrichment of polycyclic aromatic hydrocarbons in seawater and seabed sediments. J. Chromatogr. A 2024, 1737, 465463. [Google Scholar] [CrossRef]
- Yu, C.; Wu, F.; Luo, X.; Zhang, J. Porphyrin-based covalent organic framework coated stainless steel fiber for solid-phase microextraction of polycyclic aromatic hydrocarbons in water and soil samples. Microchem. J. 2021, 168, 106364. [Google Scholar] [CrossRef]
- Guo, W.; Tao, H.; Shuai, Q.; Huang, L. Architectural engineering inspired in situ growth of covalent organic frameworks as outstanding fiber coating for solid-phase microextraction of phenols. Microchem. J. 2023, 189, 108564. [Google Scholar] [CrossRef]
- dos Santos, R.R.; de Lourdes Cardeal, Z.; Menezes, H.C. Phase distribution of polycyclic aromatic hydrocarbons and their oxygenated and nitrated derivatives in the ambient air of a Brazilian urban area. Chemosphere 2020, 250, 126223. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.R.; Lee, J.Y.; Ahn, Y.G.; Kim, Y.P. Determination of atmospheric amines at Seoul, South Korea via gas chromatography/tandem mass spectrometry. Chemosphere 2020, 258, 127367. [Google Scholar] [CrossRef]
- Conrady, M.W.; Bauer, M.; Jo, K.D.; Cropek, D.M.; Busby, R.R. Solid-phase microextraction (SPME) for determination of geosmin and 2-methylisoborneol in volatile emissions from soil disturbance. Chemosphere 2021, 284, 131333. [Google Scholar] [CrossRef]
- Batista, J.M.; Valenzuela, E.F.; Costa Menezes, H.; Cardeal, Z.L. An exploratory study of volatile and semi-volatile organic compounds in PM2.5 atmospheric particles from an outdoor environment in Brazil. Environ. Sci. Pollut. Res. Int. 2025, 32, 657–676. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Wu, C.; Luo, F.; Lin, Z.; Naidu, R. Rapid on-site detection of underground petroleum pipeline leaks and risk assessment using portable gas chromatography-mass spectrometry and solid phase microextraction. J. Chromatogr. A 2023, 1696, 463980. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, D.; Gori, A.; Ferrini, F.; Brunetti, C. An Improvement of SPME-Based Sampling Technique to Collect Volatile Organic Compounds from Quercus ilex at the Environmental Level. Metabolites 2021, 11, 388. [Google Scholar] [CrossRef]
- Kaikiti, K.; Omirou, M.; Ioannides, I.M.; Agapiou, A. Mapping soil VOCs using three green sample extraction techniques and GC-MS. Microchem. J. 2024, 207, 111851. [Google Scholar] [CrossRef]
- Peñalver, R.; Campillo, N.; López-García, I.; Hernández-Córdoba, M. Solid-phase microextraction for the determination of iron organic compounds in seawaters and soils by gas chromatography coupled to microwave-induced plasma with atomic emission detection spectrometry. Microchem. J. 2020, 154, 104630. [Google Scholar] [CrossRef]
- Rodríguez-Palma, C.E.; Campíns-Falcó, P.; Herráez-Hernández, R. Assessing the Dissipation of Pesticides of Different Polarities in Soil Samples. Soil Syst. 2024, 8, 71. [Google Scholar] [CrossRef]
- Kaikiti, K.; Omirou, M.; Savvides, S.; Ioannides, I.M.; Agapiou, A. HS-SPME-GC-MS analysis of Cyprus vineyard soil for VOCs determination. Sustain. Chem. Environ. 2023, 2, 100021. [Google Scholar] [CrossRef]
- Kong, J.; Gao, Z.; Hu, G.; Huang, W.; Zhou, S.; He, H.; Xian, Q.; Sun, C. Solid-phase microextraction combined with gas chromatography/triple quadrupole tandem mass spectrometry for determination of nitrated polycyclic aromatic hydrocarbons in sediments. J. Sep. Sci. 2022, 45, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.P.; Knuth, D.; Böhm, L.; Wiltschka, K.; Schatz, M.; Düring, R.-A. A miniaturized method for fast, simple, and sensitive pesticide analysis in soils. J. Soils Sediments 2022, 22, 496–508. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Xiao, L.; Wang, M.; Feng, S.; Fan, J.; Pawliszyn, J. Quantitative Determination of Poly(methyl Methacrylate) Micro/Nanoplastics by Cooling-Assisted Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry: Theoretical and Experimental Insights. Anal. Chem. 2024, 96, 2227–2235. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Naidu, R.; Chadalavada, S.; Bekele, D.; Gell, P.; Donaghey, M.; Bowman, M. Application of portable gas chromatography–mass spectrometer for rapid field based determination of TCE in soil vapour and groundwater. Environ. Technol. Innov. 2021, 21, 101274. [Google Scholar] [CrossRef]
- Brinco, J.; Carvalho, R.; Gomes da Silva, M.; Guedes, P.; Ribeiro, A.B.; Mateus, E.P. Extraction of pesticides from soil using direct-immersion SPME LC-Tips followed by GC-MS/MS: Evaluation and proof-of-concept. J. Chromatogr. A 2024, 1735, 465295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.K.M.A.; Raynie, D.E. Development of Dry-herb vaporizer-assisted solid-phase Microextraction for the analysis of volatile Organic compounds. Adv. Sample Prep. 2024, 10, 100112. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, P.; Han, L.; Zhang, X.; Li, D.; Li, M.; Wang, Y.; Zhang, X.; Lu, M.; Cai, Z. Gas-cycle-assisted headspace solid-phase microextraction coupled with gas chromatography for rapid analysis of organic pollutants. Chem. Commun. 2021, 57, 8810. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Hashemi, P.; Adeli, M. A simple and portable vacuum assisted headspace solid phase microextraction device coupled to gas chromatography based on covalent organic framework/metal organic framework hybrid for simultaneous analysis of volatile and semi-volatile compounds in soil. J. Chromatogr. A 2023, 1705, 464195. [Google Scholar] [CrossRef]
- Derikvand, A.; Ghiasvand, A.; Dalvand, K.; Haddad, P.R. Fabrication and evaluation of a portable low-pressure headspace solid-phase microextraction device for on-site analysis. Microchem. J. 2021, 168, 106362. [Google Scholar] [CrossRef]
- Pérez-Lemus, N.; López-Serna, R.; Pérez-Elvira, S.I.; Barrado, E. Sample pre-treatment and analytical methodology for the simultaneous determination of pharmaceuticals and personal care products in sewage sludge. Chemosphere 2020, 258, 127273. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Martino, M.; Elliani, R.; Sprovieri, F.; Pirrone, N.; Tagarelli, A. An innovative green protocol for the quantification of benzothiazoles, benzotriazoles and benzosulfonamides in PM10 using microwave-assisted extraction coupled with solid-phase microextraction gas chromatography tandem-mass spectrometry. Environ. Pollut. 2021, 285, 117487. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, M.; Ghiasvand, A. An ultrasound-assisted pressure-regulated solid-phase microextraction setup for fast and sensitive analysis of volatile pollutants in contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 36306–36315. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Wu, H.; Xiao, L.; Dong, P.; Feng, S.; Fan, J. A facile cooling-assisted solid-phase microextraction device for solvent-free sampling of polycyclic aromatic hydrocarbons from soil based on matrix solid-phase dispersion technique. Anal. Chim. Acta 2020, 1115, 7–15. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2020, 152. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Quantitative extraction using an internally cooled solid phase microextraction device. Anal. Chem. 1995, 67, 34–43. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. New extraction media in microextraction techniques. A review of reviews. Microchem. J. 2020, 153, 104386. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Zhang, Q.; Zang, L.; Chen, B.; Hu, B. Stir bar sorptive extraction and its application. J. Chromatogr A 2021, 1637, 461810. [Google Scholar] [CrossRef] [PubMed]
- Tölgyessy, P.; Nagyová, S.; Roško, V.; Hucko, P. Simultaneous determination of short-chain chlorinated paraffins and other classes of persistent organic pollutants in sediment by gas chromatography–tandem mass spectrometry after ultrasonic solvent extraction combined with stir bar sorptive extraction. Chem. Pap. 2021, 75, 5645–5661. [Google Scholar] [CrossRef]
- Wang, Z.; He, M.; Chen, B.; Hu, B. Azo-linked porous organic polymers/polydimethylsiloxane coated stir bar for extraction of benzotriazole ultraviolet absorbers from environmental water and soil samples followed by high performance liquid chromatography-diode array detection. J. Chromatogr. A 2020, 1616, 460793. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Hong, Y.; Hu, W.; Li, W.; Chen, Z. Covalent organic framework-V modified porous polypropylene hollow fiber with detachable dumbbell-shaped structure for stir bar sorptive extraction of benzophenones. J. Chromatogr. A 2022, 1664, 462798. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M.; Olszowy, M. Miniaturized methods of sample preparation. In Handbook on Miniaturization in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–125. [Google Scholar]
- Yadav, P.; Singh, R.; Gupta, N.; Kumar, S.; Singh Thakur, R.; Khan, A.H.; Ghazi Ansari, N.; Kumar Patel, D. Modified DLLME-SFO approach for evaluation of multiclass agrochemicals and its associated risk assessment: Soil, Saccharum officinarum and Jaggery. Food Chem. Adv. 2022, 1, 100032. [Google Scholar] [CrossRef]
- Naing, N.N.; Xue Yi Goh, E.; Kee Lee, H. Enhanced microextraction of endocrine disrupting chemicals adsorb e d on airborne fine particulate matter with gas chromatography–tandem mass spectrometric analysis. J. Chromatogr. A 2021, 1637, 461828. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Martino, M.; Moretti, S.; Macagnano, A.; Zampetti, E.; Papa, P.; Avossa, J.; Pirrone, N.; Nerentorp, M.; et al. A Field Intercomparison of Three Passive Air Samplers for Gaseous Mercury in Ambient Air. Atmos. Meas. Tech. 2021, 14, 3657–3672. [Google Scholar] [CrossRef]
- Rodrigues, T.; Chibana Ferreira, K.; Isquibola, G.; Faza Franco, D.; Anderson, J.L.; de Oliveira Merib, J.; Clairmont Feitosa de Lima Gomes, P. Investigating a new approach for magnetic ionic liquids: Dispersive liquid-liquid microextraction coupled to pyrolysis gas-chromatography-mass spectrometry to determine flame retardants in sewage sludge samples. J. Chromatogr. A 2024, 1730, 465038. [Google Scholar] [CrossRef]
- Cunha, S.C.; Ferreira, R.; Marmelo, I.; Vieira, L.R.; Anacleto, P.; Maulvault, A.; Marques, A.; Guilhermino, L.; Fernandes, J.O. Occurrence and seasonal variation of several endocrine disruptor compounds (pesticides, bisphenols, musks and UV-filters) in water and sediments from the estuaries of Tagus and Douro Rivers (NE Atlantic Ocean coast). Sci. Total Environ. 2022, 838, 155814. [Google Scholar] [CrossRef] [PubMed]
- Pour, P.H.; Daryanavard, S.M.; Memar, M.; Naccarato, A. Development of Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction Based on Solidification of Floating Organic Droplets and Deep Eutectic Solvents for Multi-Class Pesticide Analysis in Agricultural Waters. Microchem. J. 2025, 212, 113404. [Google Scholar] [CrossRef]
- Zhu, M.; Niu, Z.; Zhang, W.; Zhang, J.; Wen, Y. The combination of two microextraction methods coupled with gas chromatography for the determination of pyrethroid insecticides in multimedia environmental samples: Air, water, soil, urine and blood. Water Environ. J. 2020, 34, 503–515. [Google Scholar] [CrossRef]
- Oliveira Martins, R.; Guimarães Souza, G.; Santos Machado, L.; Lopes de Araújo, G.; Costa Simas, R.; Gonçalves da Silva, B.J.; Damin, V.; Rodrigues Chaves, A. Hollow fiber liquid-phase microextraction of multiclass pesticides in soil samples: A green analytical approach for challenging environmental monitoring analysis. Microchem. J. 2023, 193, 109028. [Google Scholar] [CrossRef]
- Moret, S.; Hidalgo, M.; Sanchez, J.M. Hollow-Fiber Liquid-Phase Microextraction (HF-LPME) Coupled On-Line to Liquid Chromatography for the Determination of the Herbicides 2,4-Dichlorophenoxyacetic Acid and 2-Methyl-4-chlorophenoxyacetic Acid and Their Main Metabolites in Soil Samples. Separations 2023, 10, 273. [Google Scholar] [CrossRef]
- Tuğba Zaman, B.; Dalgıç Bozyiğit, G.; Şaylan, M.; Seda Koçoğlu, E.; Kartoğlu, B.; Aydın, E.S.; Girgin, A.; Borahan, T.; Oflu, S.; Kılınç, Y.; et al. Implementation of simple and effective fine droplet formation-based spray-assisted liquid phase microextraction for the simultaneous determination of twenty-nine endocrine disruptor compounds and pesticides in rock, soil, water, moss, and feces samples from antarctica using gas chromatography-mass spectrometry. Environ. Sci. Pollut. Res. 2024, 31, 10920–10933. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.-R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of Organic Compounds in Water Using Dispersive Liquid-Liquid Microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Rasmussen, K.E. Liquid-Liquid-Liquid Microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal. Chem. 1999, 71, 2650–2656. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naccarato, A.; Elliani, R.; Tagarelli, A. Microextraction and Eco-Friendly Techniques Applied to Solid Matrices Followed by Chromatographic Analysis. Separations 2025, 12, 124. https://doi.org/10.3390/separations12050124
Naccarato A, Elliani R, Tagarelli A. Microextraction and Eco-Friendly Techniques Applied to Solid Matrices Followed by Chromatographic Analysis. Separations. 2025; 12(5):124. https://doi.org/10.3390/separations12050124
Chicago/Turabian StyleNaccarato, Attilio, Rosangela Elliani, and Antonio Tagarelli. 2025. "Microextraction and Eco-Friendly Techniques Applied to Solid Matrices Followed by Chromatographic Analysis" Separations 12, no. 5: 124. https://doi.org/10.3390/separations12050124
APA StyleNaccarato, A., Elliani, R., & Tagarelli, A. (2025). Microextraction and Eco-Friendly Techniques Applied to Solid Matrices Followed by Chromatographic Analysis. Separations, 12(5), 124. https://doi.org/10.3390/separations12050124








