1. Introduction
Wastewater discharge from industrial areas, such as textile, paper, and food sectors, which contain dyes, poses a significant environmental problem contributing to water pollution [
1]. Currently, there are over ten thousand types of synthetic dyes, with the majority being used by the textile industry, making it a major polluter, primarily due to the composition and quantity of the effluent [
2]. In the textile dyeing process, 10–15% of unfixed dye is discharged into the environment as wastewater, primarily into water bodies [
3]. For instance, in Ethiopia, pollution of water bodies by textile industries, from which textile dye effluents emerge, is detected mostly in lakes [
4,
5]. The presence of dyes in effluents is highly noticeable, predominantly basic ones, which affect the aesthetic quality of the environment, hinder light penetration, and diminish the amount of dissolved oxygen content in water bodies, thereby blocking photosynthesis and affecting the growth of aquatic species [
6]. Furthermore, the blockage of soil pores by dye effluent may result in a decrease in soil productivity [
7]. Overall, the low biodegradability of dyes and their harmful impacts on humans and ecosystems raise concerns about water sources. Therefore, dye-bearing industrial effluents need to undergo adequate treatment before being released into the environment [
2].
In recent times, the removal of dyes has been investigated by several methods such as physicochemical and biological techniques, adsorption technology, and hybrid treatment systems [
8,
9]. However, these approaches have a number of drawbacks, including poor performance, high expense, the production of hazardous by-products, operational delays, and ineffectiveness in removing certain contaminants [
9,
10,
11,
12]. Among them, adsorption is the most commonly employed method for treating textile effluents contaminated with dyes because of its affordability, effectiveness, and simplicity in altering adsorbents [
8,
13,
14]. Activated carbon has gained popularity as an adsorbent and is frequently used to depollute dye-laden wastewater [
15,
16]. However, due to its high cost and regeneration issues, researchers are exploring more cost-effective substitute adsorbents [
17,
18].
Termite mounds (TM) are natural formations created by termites using a mixture of organic matter and mineral elements sourced from the surrounding soil. These mounds play a significant role in enhancing the quality of soil by increasing its carbon content, clay content, and overall nutrient levels [
19,
20]. The termite nest has a better nutrient composition and chemical makeup than the nearby soil, both physically and chemically [
21]. The mound mainly contains silicon, aluminium, iron, and titanium oxides, which are essential elements for adsorption [
22]. The structural properties, as well as the mechanical and chemical stability of the termite mound, establish it as a highly promising adsorbent for the treatment of industrial wastewater [
20]. Previous applications have demonstrated its effectiveness in removing inorganic pollutants such as arsenic, cadmium, fluoride, and chromium [
19,
22,
23,
24]. Furthermore, termite mound composites have been utilized as a support for heterogeneous catalysts with zinc oxide and
nanoparticles to remove anionic and cationic dyes [
20,
25]. Nevertheless, one of the disadvantages of using bare termite mounds as adsorbents is the phase separation problem. After adsorption of the pollutant, filtration or centrifugation—both expensive, time-consuming, and inefficient separation techniques—were used to separate the termite mound from the pollutant in aqueous media.
In the present study, these issues were addressed by coating the termite mound with magnetic iron oxide to improve its magnetic characteristics, which in turn improved the sorption capabilities and solved the separation problem.
is a non-toxic, readily produced, water-insoluble substance with a large specific surface area and greater saturation magnetization [
26,
27]. Despite of these advantages,
adsorbents are very much susceptible to agglomeration and chemical dissolution [
28,
29]. In order to prevent this, the
adsorbent was coated with HTM (heat-activated termite mound). Thus, the limitations of both adsorbents, namely, termite mound and
, were addressed by synthesizing a composite from
and termite mound by chemical coprecipitation. The co-precipitation route, in which
and
aqueous salt solutions are precipitated by the addition of a base, is usually adopted for the synthesis of
due to its simplicity and capacity for large-volume production of
[
30]. In the coprecipitation route, nitrogen gas is introduced into the reaction mixture to prevent oxidation of
to
, and
is usually preferred as the precipitating agent as it does not leave ionic residues. Up to now, there has not been any published research on the use of a
–HTM composite to remove BB41 dyes from textile effluent. Therefore, this study aimed to synthesize a magnetic
–HTM composite using coprecipitation of iron oxide salts and heat-activated termite mound. The study involved characterization of the
–HTM composite through BET, SEM, FTIR, and XRD. Additionally, the study aimed to determine the adsorption performance of the composite on BB41, a representative cationic dye commonly used in textile industries, using RSM optimization based on the Box–Behnken design. Furthermore, the study explored the applicability of the adsorbent in real textile wastewater and the reusability of the adsorbent.
2. Materials and Methods
2.1. Materials
The sample of termite mound in the study was sourced from a termite mound located in Mojo, Ethiopia. Before treatment, the raw termite mound sample was washed with distilled water several times to get a neutral pH, after which it was dried at 105 °C for 24 h. Subsequently, it was pounded with a mortar and pestle and sieved through a 75 μm pore size mesh. Using this method, 200 g of dried termite mounds was heated to 450 °C for three hours, the result of which was labeled as H-TM. The reagents and chemicals employed in this experiment were all of analytical grade. In the adsorption investigations, BB41 dye was used, which is an azo-cationic dye. Preparation of a 1000
, stock solution was conducted by dissolving weighed volumes of BB41 dye in 1000 mL of deionized water. The required initial pH was achieved using NaOH or HCl solutions. The characteristics of the Basic Blue 41 dye are expressed in
Table 1.
2.2. Synthesis of Magnetic–HTM Composite
Magnetic
–HTM composite was synthesized through the co-precipitation of 9.5 g (
), 3.6 g (
), along with heat-activated termite mound sample (H-TM) ranging from 2–200 g, the solution of which was dissolved in 400 mL of deionized water. The resulting solution was agitated using a magnetic stirrer at 80 °C under a nitrogen gas atmosphere without the presence of oxygen. After one hour, 50 mL of
(25
) was added, and the mixture was agitated for an extra 60 min. The solution was then cooled to 25 °C, decanted with a magnet, and rinsed five times with hot water (80 °C). The resulting composite was dried at 70 °C for a day and kept in a desiccator and for adsorption studies [
31].
2.3. Characterizations of Magnetic Iron Oxide–HTM Composite
Determination of the specific surface area and total pore volume of were magnetic –HTM composite were evaluated via the Brunauer–Emmett–Teller (BET) (SA-9600 Series, Tokyo, Japan) device. A Fourier-transform infrared (FT-IR) spectrometer (Perkin Elmer, Annapolis, MD, USA) was used to recognize the functional groups existing in magnetic–HTM composite using a KBR disk. The magnetic–HTM composite’s crystalline phase was identified through the application of the XRD (XRD 7000, Tokyo, Japan). The morphological analysis of the magnetic –HTM composite was studied using scanning electron microscopy (SEM) (Thermo Fisher Scientific, Hillsboro, OR, USA). The –HTM composite’s internal structure was examined using a working distance of 10–50 μm and a voltage of 10–20 kV.
2.4. Adsorption Experiments
The batch sorption tests involved diluting the stock solution (
) to obtain the required concentrations for the BB41 dye solution. The initial dye concentration, adsorbent dosage, contact time, and solution temperature for the adsorption tests were set at 10–100
, 10 to 90 min, 40–60 °C, and 1–3
, respectively, with the pH maintained at 5. A digital pH meter was utilized to precisely measure and adjust the pH levels of the solutions using either 0.1 M NaOH or 0.1 M HCl solution. The solutions were agitated in a temperature-controlled shaker at 121 rpm. Afterward, the adsorbent was separated from the solution by applying an external magnet. After equilibrium time, the amount of BB41 dye left in solution was analyzed using a UV-visible spectrophotometer (Hitachi 100-40 spectrometer (10040), Hitachi, Tokyo, Japan) at a maximum wavelength of 617 nm. The percentage of dye removal (R %) was calculated using Equation (1).
where
(
) and
(
) are BB41 dye concentrations initially and at equilibrium, respectively.
The BB41 dye amount in the adsorbent phase was calculated using Equation (2), where
is the amount of dye uptake (
) at equilibrium,
and
are the initial and equilibrium concentrations (
) of BB41 dye in solution, V is the volume of the solution (L), and M is the mass of the adsorbent (g).
is the amount of dye uptake () at time t, BB41 dye concentration () at time t.
A solution of 50
BB41 dye was prepared in distilled water. The absorption of various concentrations of BB41 dye was measured at the dye’s maximum wavelength (
= 617 nm). The calibration curve shown in
Figure 1 was obtained by plotting absorbance versus concentration of BB41 at
. The samples’ concentrations were estimated using a linear regression equation, Equation (4),
where A is absorbance and C
e is BB41 dye concentrations at equilibrium, with
= 0.997, derived by drawing a calibration curve for BB41 dye across a concentration range (8–20
). All adsorption tests were conducted in triplicate, and the average results were used to analyze the data.
2.5. Experimental Design Using BBD
The removal of BB41 dye from aqueous solution was optimized using
–HTM composite and Box–Behnken experimental design [
32]. The study examined independent variables, which included temperature (40–60 °C), dye concentration (10–100
), adsorbent dose (1–3
), and contact time (10–90 min), with pH maintained constantly at 5, as maximum adsorption was found at this pH. Three codes were assigned to the factor levels: 0 for the medium or central point, 1 for high, and −1 for low. The BBD, with levels and independent variables, is shown in
Table 2. These parameters and ranges were determined through the literature review and preliminary investigations. The BBD model, which considers factorial and the center point, resulted in a total of 29 combinations of runs. Equation (5) presents a second-order polynomial model developed to correlate the dependent and independent variables.
where
Y is the predicted dependent variable,
is the coefficient of intercept,
is coefficient of the linear variable
,
is the quadratic coefficient of
,
is the interaction coefficient of
and
, and
is the residual term.
ANOVA (R2, adjusted R2, F-test, and VIF), residual analysis, and normal plots were employed to assess the statistical significance. To evaluate the significance of the regression coefficients, the F and p values were used at the confidence level of 95.
2.6. Determination of the Point of Zero Charge of the Composite
To determine the point of zero charge (pHPZC) of the composite, a 50 mL (0.1 M NaCl solution) was prepared first into five flasks (each 100 mL). Then, 0.1 M HCl or NaOH was added to each flask to adjust the pH values to 3, 5, 7, 9, and 11. This adjusted pH is called the initial pH (pHi). Subsequently, 0,2 g of the composite was added to each flask where the pHi was adjusted. The solutions were stirred for 24 h, after which the pH was measured and labeled as (pHf). The pHZPC of the adsorbent was the point at which the intersection on the pHi axis, where the pH is equal to zero, was drawn against the difference between (pHi) versus (pHf)
2.7. Real Textile Wastewater Sample
The effectiveness of the synthesized –HTM composite was thoroughly evaluated for its ability to remove the BB41 dye from contaminated wastewater samples. This investigation aimed to assess the material’s potential as an efficient adsorbent in wastewater treatment applications, particularly in the context of dye pollution. A sample of wastewater was taken from the inlet of the DH GEDA blanket factory, yarn dyeing, and textile wastewater treatment plant in Ethiopia. This sampling aimed to evaluate the purification potential of the synthesized adsorbent in isolation, without the influence of the existing treatment facility. The analysis seeks to provide insight into the effectiveness of the new adsorbent in addressing the specific contaminants present in the wastewater generated from the dyeing process, and to check the purification potential of the synthesized adsorbent without a treatment plant. Before analysis, the sample was kept at 4 °C in an amber glass bottle. It was characterized for total suspended solids, EC, pH, and chemical oxygen demand before and after treatment.
2.8. Desorption and Regeneration Study
The regenerative property of the BB41 dye-loaded magnetic iron oxide–HTM was evaluated in an aqueous solution with the following conditions: 50 BB41 dye solution, 2 –HTM dose, shaking at 121 rpm, pH 5 for 47.5 min. The composite was dried in an oven at 80 °C for 24 h after being separated by magnetic decantation. The dried BB41 dye-loaded –HTM was shaken at 121 rpm for 90 min in 400 mL of a 0.15, 0.2, and 0.35 M NaOH solution in order to conduct desorption. The amount of BB41 dye desorbed from the used adsorbent was determined by measuring the concentration of BB41 dye present in the supernatant solution. Adsorption and desorption of –HTM adsorbent to treat BB41 dye were continued for four cycles. The result was recorded to determine the regeneration efficiency of –HTM.
3. Results and Discussion
3.1. Characterization of Magnetic Iron Oxide–HTM Composite
According to the results of the BET analysis, the specific surface area of magnetic iron oxide–HTM was determined to be 60.05 m
2/g. This measurement indicates the surface area available for chemical reactions or interactions per gram of the material, highlighting its potential applications for adsorption. The specific surface areas of the composite were significantly higher than those of the natural termite mound (31.5 m
2g
−1), likely due to magnetic particles on the mound’s surface [
33]. The total pore volume has increased due to the higher iron oxide content in the composite (0.03
) compared to the natural termite mound (0.01
). This may be due to the development of a secondary pore structure during the precipitation of iron oxide particles. FTIR analysis, which is shown in
Figure 2, was conducted to thoroughly investigate and identify the various functional groups present on the surface of magnetic iron oxide–HTM. The presence of 2800
and 3700
indicates
-OH stretching [
34]. The broad bands observed at 3616
in the termite mound are indicative of the presence of water molecules associated with the stretching modes of hydroxyl (O–H) groups. The absorption bands observed at 1010.00
and 910.49
can be attributed to the stretching vibrations of the Si–O bond. These characteristic peaks are indicative of the presence of silicon–oxygen linkages, which play a crucial role in various chemical structures and materials. The wavenumbers 752.07
and 782.41
signify the presence of silicon–oxygen (Si–O) bonds in quartz, while the band observed at 689.03
is attributed to the bending of silicon–oxygen–silicon (Si–O–Si) linkages within the termite mound [
35]. A significant reduction of this band was noted for the modified materials following the formation of the composite. Furthermore, the FT-IR spectra indicate the presence of three distinct bands located at 535
, 1630
, and 3359
in the FT-IR spectrum of magnetic iron oxide–HTM composite [
30]. The spectral band at 535
is attributed to the vibrational modes of the Fe–O bonds within the crystalline lattice of
. Additionally, the FT-IR spectrum exhibits characteristic bands corresponding to hydroxyl groups at 1630
and 3359
[
36]. The characteristic bands of both the termite mound
were also identified in the spectrum of the magnetic iron oxide–HTM composite, revealing that the preparation of the composite by means of the chemical co-precipitation process was successful.
The X-ray diffraction (XRD) patterns of the magnetic iron oxide–HTM composite samples are presented in
Figure 3, offering a comprehensive analysis of their crystalline structures and phase characteristics. The crystalline structures of the HTM sample, identified through XRD analysis (ICDD PDF-2 247 Release 2016 RDB include tridymite (59.92°), aluminosilicate (25.53°), kaolinite (13.68°), cristobalite (20.07° and 68.15°), quartz (26.67° and 50.15°), illite (8.76°), and alumina (60.16°). A similar composition has also been reported for termite mounds [
33]. The presence of tridymite, quartz, and cristobalite structures signifies the existence of
[
37].
, aluminium oxide, brookite (
Goethite (FeOOH),
(
), CaO (
), and (
were also found in trace amount in HTM sample from XRD analysis. The diffraction peaks observed at 2θ = 43.60, 35.67, 57.40, 29.85, and 54.14 correspond to the characteristic patterns identified in the maghemite standard γ-
and Fe
3O
4. Distinguishing the overlapping X-ray diffraction (XRD) peaks of α-Fe
2O
3 (hematite) and Fe
3O
4 (magnetite) can be difficult because of their comparable structures. Nevertheless, certain key peaks can efficiently differentiate between the two minerals. The 24.2° (104), 49.5° (024) peaks are typically a strong giveaway for α-Fe
2O
3, but the 30.1° peak is not present in hematite and is key for Fe
3O
4, and the 43.1° peak (400) is also strong and mostly unique to Fe
3O
4. These findings contribute to a comprehensive understanding of the magnetism exhibited by the composite material [
38]. The X-ray diffraction analysis of pure iron oxide, presented in
Figure 3, revealed the presence of a cubic iron oxide phase characterized by diffraction peaks at d-values of 2.56, 2.52, 2.93, 2.09, and 1.61 Å. This analysis suggests the presence of maghemite, which is identified by a lattice parameter of Ao = 0.835 nm [
39]. The XRD analyses show the presence of maghemite (γ-
) and magnetite (
), making the prepared iron oxides magnetic. This indicates the magnetic properties of iron oxide have remained unchanged.
The morphology of the magnetic iron oxide–HTM composite and heat-activated termite mound (HTM) was studied using an SEM. Naked HTM possesses a smooth surface structure as depicted in
Figure 4a. As shown in
Figure 4b, the surface of magnetic iron oxide–HTM became rough, indicating its surface is heterogeneous. On the surface of magnetic iron oxide–HTM composite tiny pores can be seen, which likely have a significant role in adsorption of BB41 dye from the aqueous solution. The small white spots were indicative of iron oxide particles that were dispersed across the surface of the termite mound.
3.2. Effect of HTM Amount on Preparation of Magnetic Iron Oxide–HTM Composite
Magnetic iron oxide–HTM composite was synthesized by co-precipitation of 9.5 g (
), 3.6 g (
), and 50 mL
(25%) with 2, 4, 8, 16, and 20 g heat-activated termite mound (HTM) dissolved in 400 mL of deionized water. To determine the effect of the amount of termite mound employed in the preparation of the magnetic iron oxide–HTM adsorbent, adsorption of BB41 dye was carried out in a batch process. Batch adsorption was conducted under the following conditions: an initial concentration of 50
, an adsorbent dosage of 0.2 g, room temperature, a contact time of 3 h, and a shaking speed of 121 rpm. The results indicate that as the quantity of termite mound used in the synthesis of the composite increased from 2 g to 16 g, the removal of BB41 dye (%) increased from 59.23% to 96.89%, after which the figure remained almost unchanged (
Figure 5). The percentage of dye removal with a small amount of termite mound was very low, indicating the presence of aggregation of iron oxide, leading to inadequate dispersion and implying low adsorption [
23]. When an equal amount of bare iron oxide adsorbent alone was utilized, the removal percentage was quite low (29.35%), attributed to the aggregation of the iron oxide. On the other hand, when the amount of termite mound was increased for synthesizing magnetic iron oxide–HTM, the removal of BB41 dye (%) increased, meaning Fe
3O
4 was efficiently dispersed on the surface of the termite mound, signifying higher adsorption [
40]. Therefore, this study overcomes the disadvantages of naked
adsorbent, namely, agglomeration [
31]. However, it was noted that the magnetic properties of the magnetic iron oxide–HTM, prepared by using 20 g of the termite mound, declined. Consequently, the optimal quantity of termite mound required for the synthesis of magnetic iron oxide–HTM was determined to be 16 g. The magnetic iron oxide–HTM utilized in the subsequent phase of the study was prepared using this specified amount. Thus, the amount of termite mound suitable for the synthesis of magnetic iron oxide–HTM was determined to be 16 g, and the magnetic iron oxide–HTM used in the next step of the study was prepared using this amount.
3.3. Magnetization Property
The magnetic properties of the dried magnetic iron oxide–HTM adsorbent are depicted in
Figure 6a, demonstrating its attraction to an external magnet. According to the result, magnetic iron oxide–HTM composite was synthesized through the co-precipitation of 9.5 g (
), 3.96 g, (
) (molar ratio 1.75:1), 16 g termite mound, and 50 mL (
in 400 mL of deionized water. The result yielded 20 g of magnetic iron oxide–HTM adsorbent, of which 16 g accounted for heat-activated termite mound. This resulted in the termite mound constituting 80% of the magnetic iron oxide–HTM by mass, highlighting the economic feasibility of the study [
41]. Notably, despite the termite mound making up 80% of the adsorbent’s mass, it still exhibits attraction to an external magnet. This is attributed to the presence of iron oxide in natural termite mounds, as verified by X-ray diffraction (XRD) results and a prior study indicating that 26.08
of the termite mounds consists of iron oxide (
) [
19].
Figure 6b illustrates the magnetic properties of magnetic iron oxide–HTM adsorbent after adsorbing the BB41 dye molecule, showing the efficient separation of treated water from the spent magnetic iron oxide–HTM adsorbent loaded with BB41 dye. Furthermore, the bulk magnetization was observed to decline with an increase in the weight of the termite mound in the composite, in the order of 2 < 4 < 8 < 16 < 20 g. This decline is attributed to the presence of other foreign matter within the termite mound composition.
3.4. Development of BBD Model Equation and Statistical Analysis
The sorption of BB41 dye by magnetic iron oxide–HTM adsorbent was maximized using four key independent variables: adsorbent dosage, solution temperature, initial dye concentration, and contact time. The BBD model was used to examine the individual and interacting effects of these independent variables.
Table 3 shows the experimental and predicted removal percentages of BB41 dye.
Equation (6) depicts the empirical model in which the relationship between the BB41 dye removal percentage (dependent factor) by magnetic iron oxide–HTM adsorbent and independent factors in the form of coded factors was generated by software.
The adequacy of the quadratic model of BB41 dye removal by magnetic iron oxide–HTM was determined using model summary statistics and analysis of variance (ANOVA).
Table 4 illustrates the adequacy of the quadratic model, confirmed by the F-value of ANOVA and the R
2 value of the model summary statistics. Overall, the model is significant because of a larger F (139.27) value, checked by its associated
p-value
[
42]. This proposes that the model is compatible and sufficient for explaining how magnetic iron oxide–HTM adsorbs BB41 dye (
Table 3). In addition, the effectiveness of the model was evaluated using model summary statistics in which the predicted removal percentage (
) of BB41 dye accounts for 98
of the variations, indicated by high correlation coefficients (R
2 = 0.98 for magnetic iron oxide–HTM). The adjusted R
2 (0.98) is acceptable for showing good agreement with the predicted R
2 (0.96) as their difference is
0.2. Moreover, the adequacy of the model was appraised by assessing the predicted versus actual plot and the normal plot of residuals. A graph was plotted to compare predicted response values with actual response values in order to identify any values that the model does not easily predict. In
Figure 7 the actual versus predicted responses were plotted, showing that the values are around the center line of the graph, the majority of which are easily predicted through the model. The normal plot of the residuals of magnetic iron oxide–HTM, shown in
Figure 8, indicates that the residual points are normally distributed, resembling a straight line [
42].
The simplified fitted quadratic equation of BB41 dye % with insignificant terms are avoided shown in Equation (7).
3.4.1. Significance Level of Model Terms
The coefficient estimates (shown in
Table 5) of magnetic iron oxide–HTM show that the predicted change in response for each unit increases in factor value, assuming all other factors remain constant. The table displays the coefficient values, standard errors, F-values, and significance levels. It is indicated that the linear coefficients A (adsorbent dose), B (concentration), C (contact time), and D (temperature), the quadratic coefficients A
2, B
2, and C
2, and the interaction coefficients BC (concentration and contact time), CD (contact time and temperature), and AB (dose and concentration) were all found to be statistically significant at a 95
confidence level.
As shown in
Table 5, model terms (A, B, C, D, AB, BC, CD, A
2, B
2, and C
2) are significant because the
p-values are
0.05. In addition, the lack of fit value is insignificant, with a
p-value 0.62, being
0.05, because the model was intended to be to fitted [
43].
3.4.2. The Effect of Different Factors and Their Interactions on the Process of Dye Adsorption
The key variables that need to must be accounted for in order to achieve the maximum removal percentage of BB41 dye—A (adsorbent dose), B (concentration), C (contact duration), and D (temperature)—are shown in
Figure 9. The effects of these four parameters are compared at a specific position in the design space using a perturbation plot [
44]. Overall, as shown in
Figure 9, the BB41 dye removal percentage increased with increased contact time, adsorbent dose, and temperature. However, with an increase in the initial BB41 dye amount (
), the removal percentage declined over the ranges of value given. The highest BB41 dye removal percentage is seen with the adsorbent dose, while the lowest is seen with the contact time. The steeper slope of the dose of magnetic iron oxide–HTM compared to the other factors suggests that the result, or dye removal, is particularly prone to even slight changes in this element. By contrast, the contact time showed a much flattened out curve, indicating that this factor had less of an effect on the response. Moreover, the moderate curve and reverse direction of the initial BB41 dye amount curves suggested that the factor had a lesser effect and a converse relationship with the response.
Figure 10 depicts 3D and contour plots showing the interaction effect of temperature and time on the percentage removal of BB41 dye, with dye concentration and dose set at 70
and 2.02
, respectively. Overall, as illustrated in
Figure 10, it is evident that there was a substantial increase in the percentage of BB41 dye removal with rising temperature up to 60 °C, as well as with increased contact time (min). However, it is noteworthy that temperature had a greater impact compared to contact time. The percentage of BB41 dye removal started at a low level but steadily increased over the given ranges of values. As the solution’s temperature increased from 40 °C to 58 °C and contact time increased (5–65 min), while keeping dye concentration and dose stable at 70
and 2.02
, removal of BB41 dye increased considerably from 77.93% to reach a peak of 95.34%. The increase in temperature resulted in a corresponding increase in the removal percentage of the dye. This could be attributed to the higher temperature enhancing the movement of the BB41 dye ions, leading to faster diffusion towards the magnetic iron oxide–HTM adsorbent. This, in turn, accelerated the adsorption rate and contributed to a higher dye removal percentage. In contrast, the effect of temperature was greater than that of contact time on the removal percentage of dye, as tested by the F-test, since the F-value for temperature (174.41) is higher than that of contact time (57.45), as depicted in
Table 5 [
45].
Figure 11 shows the 3D and contour plots of BB41 dye removal over the change of the initial dye concentration (10 to 100
) and contact time (5 to 90 min). At the same time, the adsorbent dosage and temperature of the solution were held constant at 2.2
and 55 °C, respectively. Overall, there was a slight increase in the proportion of BB41 dye removal with increasing contact time over the 90 min given, while, with regard to increasing the amount of initial BB41 dye
, it dropped. As dye concentration increased from 10
to 65
and time increased (5–65 min), while the adsorbent dosage and temperature of the solution were held at 2.2
and 55 °C, removal of BB41 dye increased from 83.94% to reach a peak of 91.04%. On increasing the initial BB41 dye concentration from 10 to 100
, the sorption percentage of the dye gradually declined owing to the saturation of adsorptive sites in the magnetic iron oxide–HTM adsorbent. However, as the contact time increased, adsorption efficiency also increased until all magnetic iron oxide–HTM sites were fully occupied, after which the figure remained stable [
46].
Figure 12 illustrates the interactive effect of the initial dye concentration (10 to 100
) and the adsorbent dosage (1 to 3
) on the dye removal (%), while the contact time and the temperature of the solution were kept constant at 47.5 min and 71 °C, respectively. Overall, there was a considerable increase in the percentage of BB41 dye removal with increasing magnetic iron oxide–HTM dose over the 3
, while in contrast, with regard to increasing the amount of initial BB41 dye
, it declined. The highest percentage of BB41 dye removal, with contact time and the temperature kept constant at 47.5 min and 71 °C (<temperature of real textile effluent) respectively, at an initial concentration of BB41 dye 75
(>real textile effluent concentration) and an adsorbent dose 2.5
, reached a peak of 92.34%. As the initial concentration of BB41 dye increased from 10
to 75
and adsorbent dose increased (1–2.5
) simultaneously, removal of BB41 dye increased from 46.94% to 92.34%. As the initial concentrations of BB41 dye rose from 10 to 100
, the adsorption percentage decreased. This drop in the adsorption percentage with high-level BB41 dye concentrations was attributed to the saturation of the adsorbent’s adsorptive sites [
33]. Overall, the findings showed that BB41 dye removal by magnetic iron oxide–HTM depended on the range of dye concentration from 10 to 100
.
The removal of dye (
) rose with a higher adsorbent dose from 1 to 3
, as shown in
Figure 12. With the increased dosage of magnetic iron oxide–HTM, more binding and reactive sites were accessible, leading to enhanced BB41 uptake for dye removal (
) [
47]. This noticeable improvement in dye removal (
) was assessed by the higher F-value [
48]. Overall, the findings showed that BB41 dye removal by magnetic iron oxide–HTM was dependent on the adsorbent dosage across the range of 1 to 3
.
3.4.3. Process Parameters Optimization and Model Validation
The objective of this study was to determine the maximum removal of BB41 dye within a specific range of independent parameters. Numerical optimization was utilized to determine the maximum dye removal efficiency (
) and targets of input parameter values defined as in range, using quadratic models. To achieve this goal, the lower and maximum boundaries were set between −1 and +1. The results of the numerical optimization for the magnetic iron oxide–HTM adsorption of BB41 dye are shown in
Table 6. Magnetic iron oxide–HTM numerical optimization findings show that the maximum efficiency of removal (
) is 98.34
when the input parameters are set in the range of the initial BB41 dye concentration of 100
, the adsorbent dosage of 2.6
, the contact time of 47.5 min, and the solution temperature of 60 °C. Three tests were conducted at optimal levels of the process variables, after which the average values were recorded (96.89
) and compared with the predicted value (99.34%) in
Table 6. The BB41 dye removal percentages from validation experiments were found to be within 95% of the predicted values, indicating the model’s reliability.
3.5. Application in Real Textile Wastewater
Adsorption studies were carried out under optimal circumstances identified in earlier stages of the investigation to assess the applicability of the suggested adsorption process to real wastewater samples. The pH, EC, total suspended solids, and chemical oxygen demand of the wastewater sample were 7.6, 1840 , 1660 , and 610 , respectively. According to the results obtained, the COD amount was reduced from 610 to 118.95610 , achieving over 80% COD removal for all experimented concentrations of BB41 dye. The resultant COD value is below the Ethiopian Environmental Protection COD discharge standard (250 ). A slight reduction in adsorption efficiency was observed compared to synthetic wastewater. This finding is attributed to the low competition of ions present in the real wastewater sample with BB41 dye.
3.6. Isothermal Study
The purpose of equilibrium adsorption is to determine the sorption capacity and to study the mechanisms of adsorption. To investigate these aspects, experiments were conducted using initial dye concentrations of 20, 40, 60, 80, 100, 120, 140, 160, 180, and 200 mg/L, at a dosage of 2 g/L, room temperature, and a pH of 5. All series of batch adsorption isotherms experiments were conducted in triplicate. The average value of the three measurements was recorded. When the dye concentration increased from 20 to 80 mg/L, the adsorption capacity also rose from 11.8 to 36.695 mg/g as a result of an increase in the driving force of mass transfer between the adsorbent and adsorbate. However, the adsorbed amount of dye did not show an increase after 80
dye concentration due to the adsorbent sites being occupied with dye molecules, indicating equilibrium was attained. The graphical representation of the effect of the initial concentration of BB41 dye on the results, along with the nonlinear models of the Langmuir and Freundlich isotherms, is shown in
Figure 13. The two widely recognized models, Langmuir and Freundlich isotherms, are employed to model the wastewater treatment. Nonlinear equations of the Langmuir and the Freundlich isotherms were expressed in Equations (8) and (9).
where
Ce (mg/L) is the concentration of BB41 dye in the aqueous phase at equilibrium;
qe (mg/g) is the amount of BB41 dye adsorbed;
Qmax (mg/g) is the adsorption capacity based on the Langmuir equation;
b (L/mg) is the Langmuir constant;
is the adsorption coefficient based on the Freundlich equation; 1/
n is the adsorption intensity based on the Freundlich equation.
Figure 13.
Nonlinear models of the Langmuir and Freundlich isotherms.
Figure 13.
Nonlinear models of the Langmuir and Freundlich isotherms.
3.7. Analysis of Isotherm Data
Equilibrium constants derived from nonlinear isotherm equations are presented in
Table 7. The Langmuir model exhibited superior fitting criteria, with coefficients of determination (
R2 > 0.98). The Langmuir isotherm posits a homogeneous adsorbent surface with uniform energy distribution across active sites. Adsorption is characterized as monolayer, reaching equilibrium when the adsorbate completely covers the surface [
49]. The dimensionless separation factor (
) reflects the characteristics of Langmuir isotherms, defined by the initial concentration of BB41 dye
(
) and the Langmuir constant
b (
).
The adsorption process is irreversible if = 0, favorable if 0 < < 1, linear if = 1, and unfavorable if > 1.
The Freundlich isotherm asserts that the adsorbent surface is heterogeneous, leading to non-uniform energy levels at active sites. This results in a reversible multilayer adsorption process, indicative of physisorption. The adsorption intensity, represented by 1/
n, indicates the type of isotherm: favorable when 0 < 1/
n < 1, irreversible when 1/
n = 1, and unfavorable when 1/
n > 1 [
50].
The equilibrium adsorption value of BB41 dye for 1/n was recorded at 0.403. This shows that the adsorption of BB41 dye on the magnetic composite is favorable, as the value of 1/n is located between 0 and 1. It also indicates that the adsorption is favorable, agreeing with the finding of the Langmuir isotherm.
Table 8 shows the composite’s adsorption capacity (
qe) for BB41 compared to standard adsorbents. The results indicate that the composite effectively removes the contaminant at a low cost.
3.8. Effect of pH and Adsorption Mechanism on the Percentage Removal of BB41 Dye
Figure 14 illustrates the effects of pH levels ranging from 3 to 11 on dye adsorption. The point of zero charge (pHpzc) was established by comparing the final pH (pHf) after dye adsorption with the percentage of dye removal, based on the initial pH. This analysis yielded a pHpzc of 7.85. The adsorption mechanism of the composite onto Basic Blue 41 at varying pH levels is influenced by both the characteristics of the dye and the chemical composition of the composite [
56]. The efficient removal of BB41 dye over a wide pH range may be attributed to the abundant presence of Al and Fe oxides, which function effectively in both acidic and basic environments. In order for a composite to work well in both acidic and basic environments, the pollutant’s properties are also important [
57]. The pH has an effect on the adsorption percentage of BB41 dye, with the maximum adsorption of 94.46% occurring at pH 5, which is the typical pH range (4.5–5, acidic dyeing conditions) used by textile industries to achieve maximum fixation of synthetic fibers with dye [
58].
The lowest adsorption is found at pH 11 due to the instability of the process. In an acidic medium with a pH below the point of zero charge (pHpzc) (7.85), the composite becomes protonated, reducing the rivalry between ions and dye cations, which is crucial for optimal results. However, the dissociated groups from Si–OH, as indicated by FT-IR results, attract dye cations. In a basic medium with a pH above the pHpzc (7.85), the existence of ions causes the surface of composite particles to become negatively charged, allowing the retention of dye cations through electrostatic attraction.
3.9. Reusability of Composite
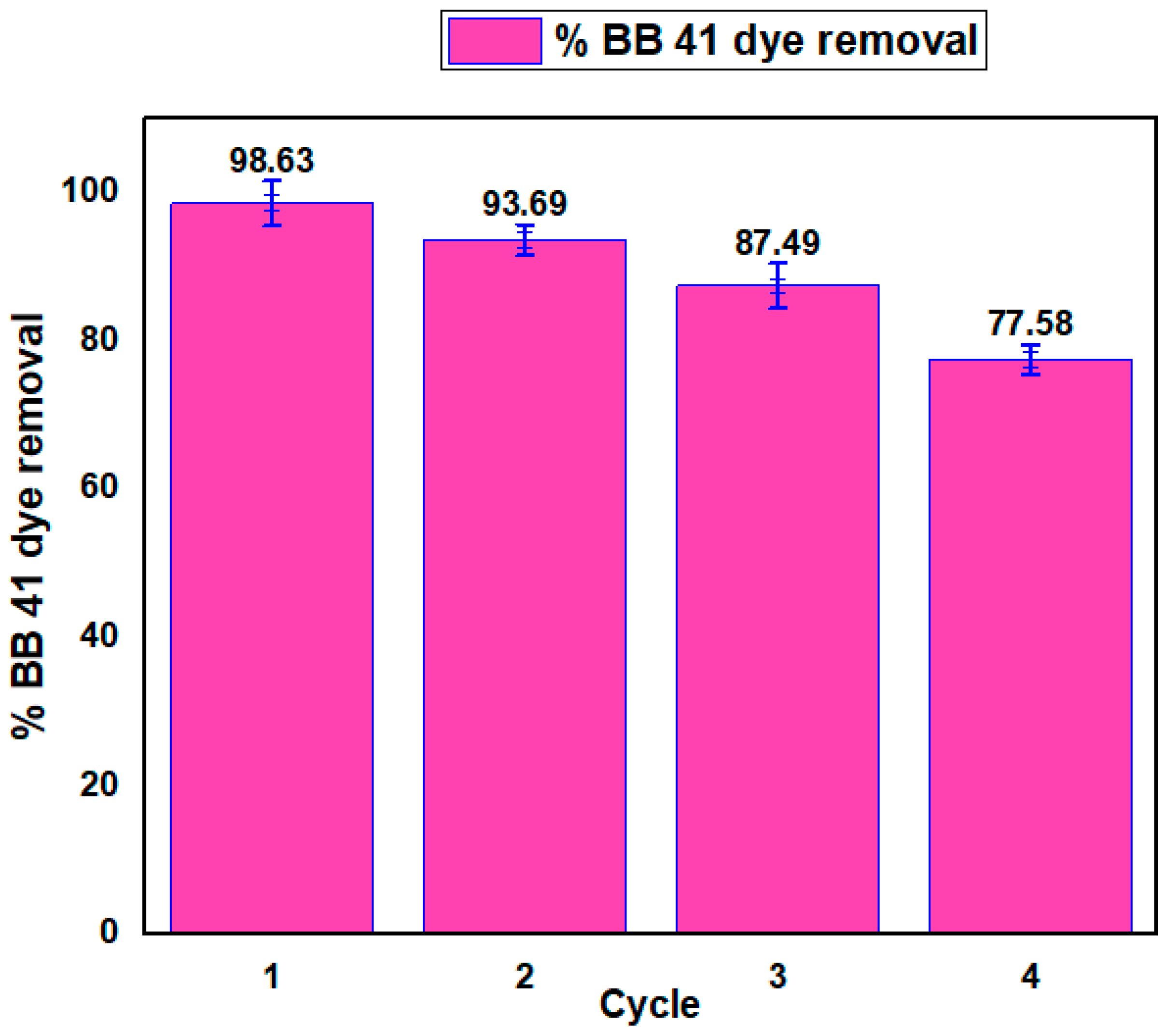
The stability of the magnetic iron oxide–HTM adsorbent was evaluated through four consecutive studies using a BB41 dye solution with a concentration of 50
, a temperature of 60 °C, a dosage of 2.6
, and a pH of 5 for 70 min. The removal percentages for the first and last cycles were 98.63
and 77.58
, respectively (as seen in
Figure 15). The reduced effectiveness of the reused adsorbent may be due to inadequate adhesion to the adsorbent structure, which could lead to the washing away of active functional species [
23]. However, the adsorbent showed good chemical stability and reusability for adsorption without any loss in performance. The result suggests enhancement of the bare
adsorbent, i.e., chemical dissolution was attained by coating it with heat-activated termite mound (HTM). The overall result indicates great potential for the magnetic iron oxide–HTM adsorbent as an effective adsorbent for BB41 dye molecules in an aqueous solution.
4. Conclusions
The study aimed to synthesize magnetic iron oxide–HTM composite for sorptive removal of BB41 dyes from real textile wastewater effluent. The synthesized magnetic iron oxide–HTM composite, 80% of which by mass is heat-activated termite mound, was used for adsorptive removal of BB41 dye, which suggests the economic feasibility of the composite. XRD, SEM, and FT-IR analyses verified the presence of iron oxide particles in the magnetic iron oxide–HTM composite and validated the presence of the magnetite phase in composites. The study used a Box–Behnken design to investigate the adsorption performance of magnetic iron oxide–HTM magnetic composites for the maximum removal of Basic Blue 41 (BB41) dye from aqueous solution. Regression analysis indicated a good agreement with the experimental data to the quadratic model, with an R2 value of 0.97. The adsorption process was found to be dependent on the magnetic iron oxide–HTM dose, contact time, temperature, and initial dye concentration. Adsorption isotherm studies revealed that the Langmuir model best fit the experimental data, indicating a maximum adsorption capacity of 36.695 mg/g for the composite. Magnetic iron oxide–HTM demonstrated the best dye removal performance, effectively eliminating over 80 of COD containing BB41 dye from a real wastewater sample. The magnetic iron oxide–HTM adsorbent exhibited adequate magnetic properties, enabling the successful separation of dye-loaded magnetic iron oxide–HTM adsorbent from the treated liquid phase solution using an external magnet and the recovery of magnetic iron oxide–HTM. Reusability analysis showed that the magnetic iron oxide–HTM composite successfully removed BB41 dye from aqueous solution after four consecutive applications without losing its magnetic nature. Overall, the limitation of separation of spent pristine termite mound adsorbent from the treated solution, and the shortcomings of adsorbent (agglomeration and chemical dissolution), were overcome by synthesizing magnetic iron oxide–HTM composite. The results of the study suggest further exploration of magnetic iron oxide–HTM for practical applications in the treatment of BB41 dye from the textile industry.