Free Fatty Acid Determination in Alcoholic and Non-Alcoholic Beers via Liquid Chromatography–High-Resolution Mass Spectrometry Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Stock and Working Solutions
2.3. Instrumentation
2.4. Sample Preparation
2.5. Method Validation
2.6. Sampling
2.7. Statistical Analysis
3. Results
3.1. Sample Preparation and Method Validation
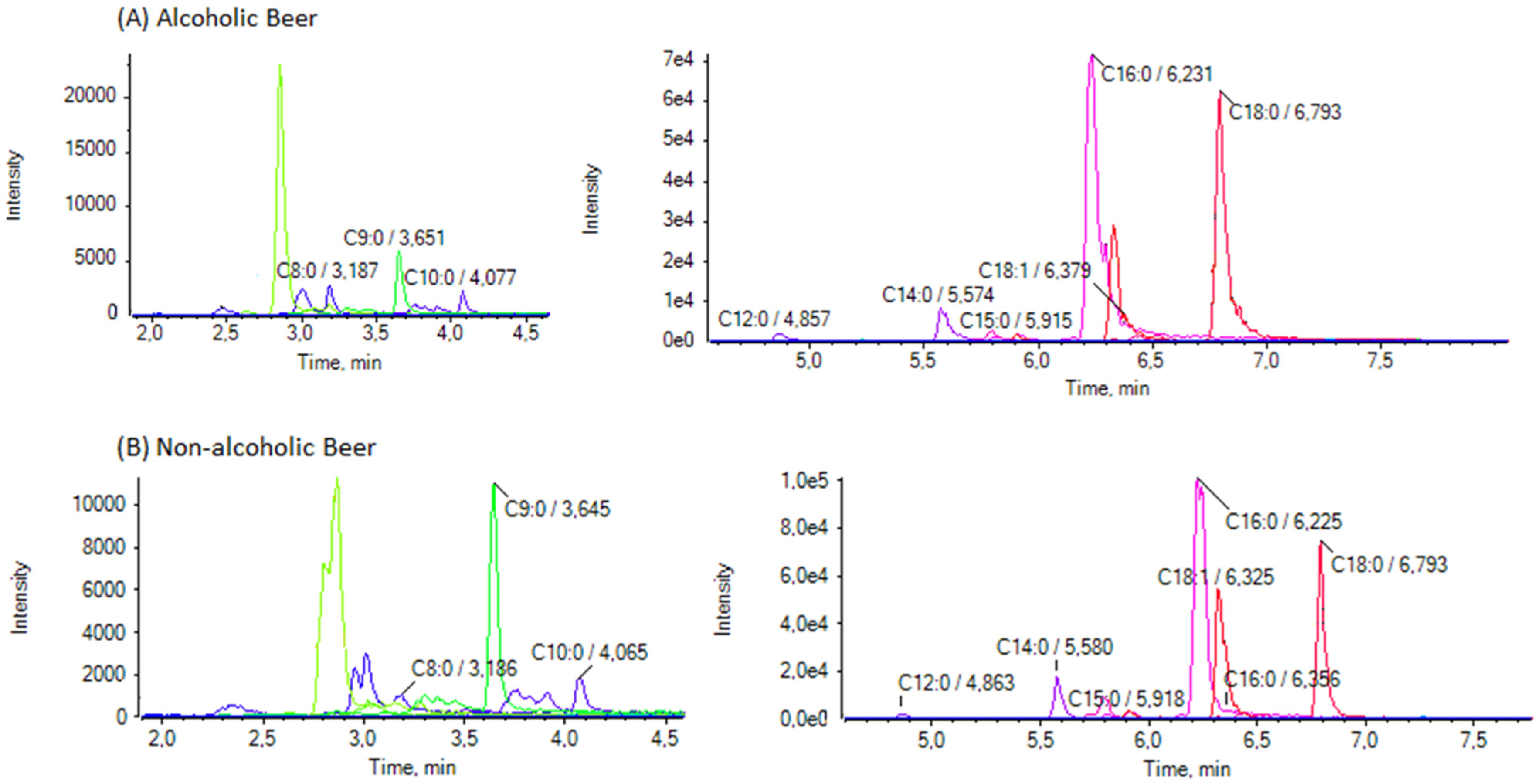
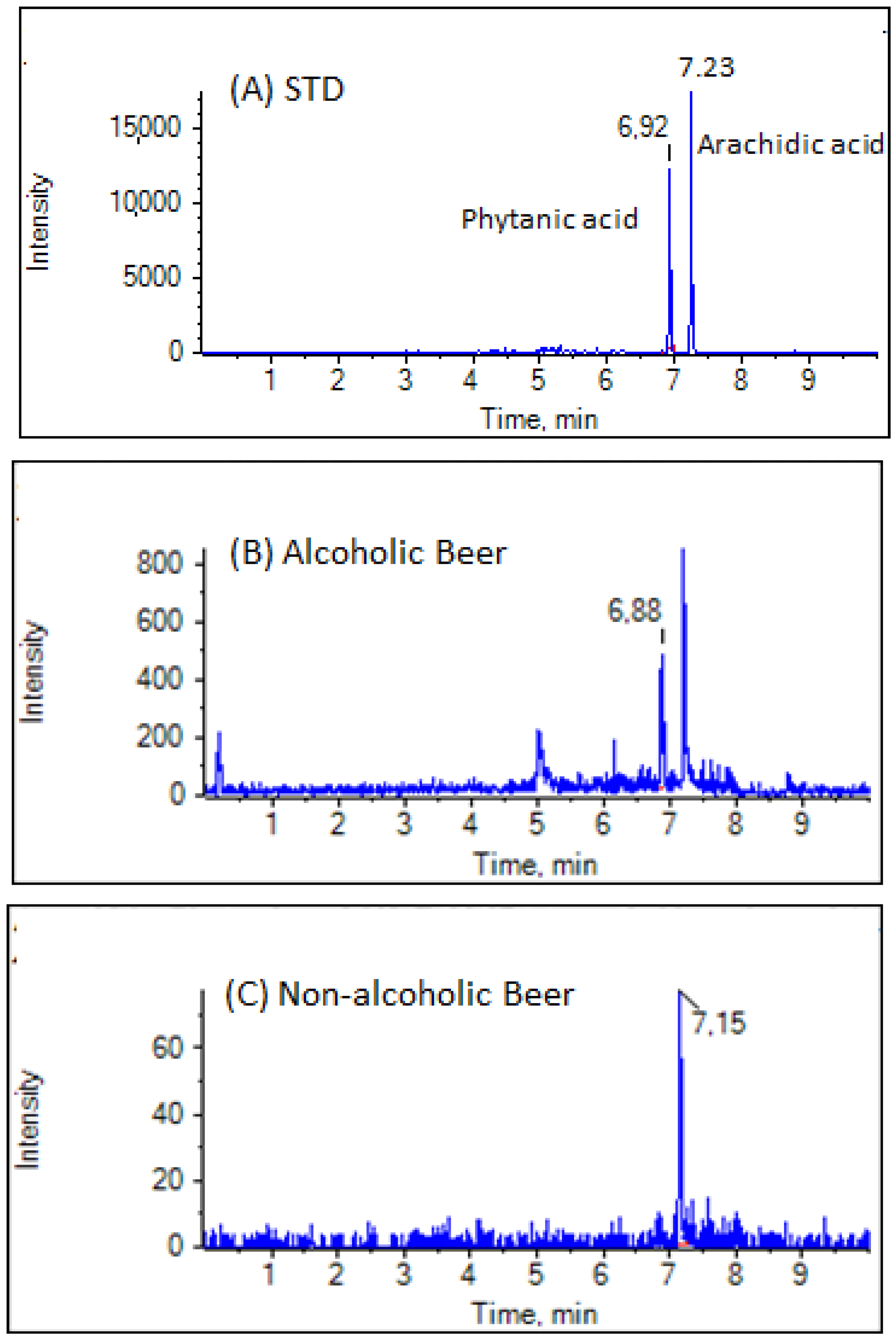
3.2. Analysis of Samples
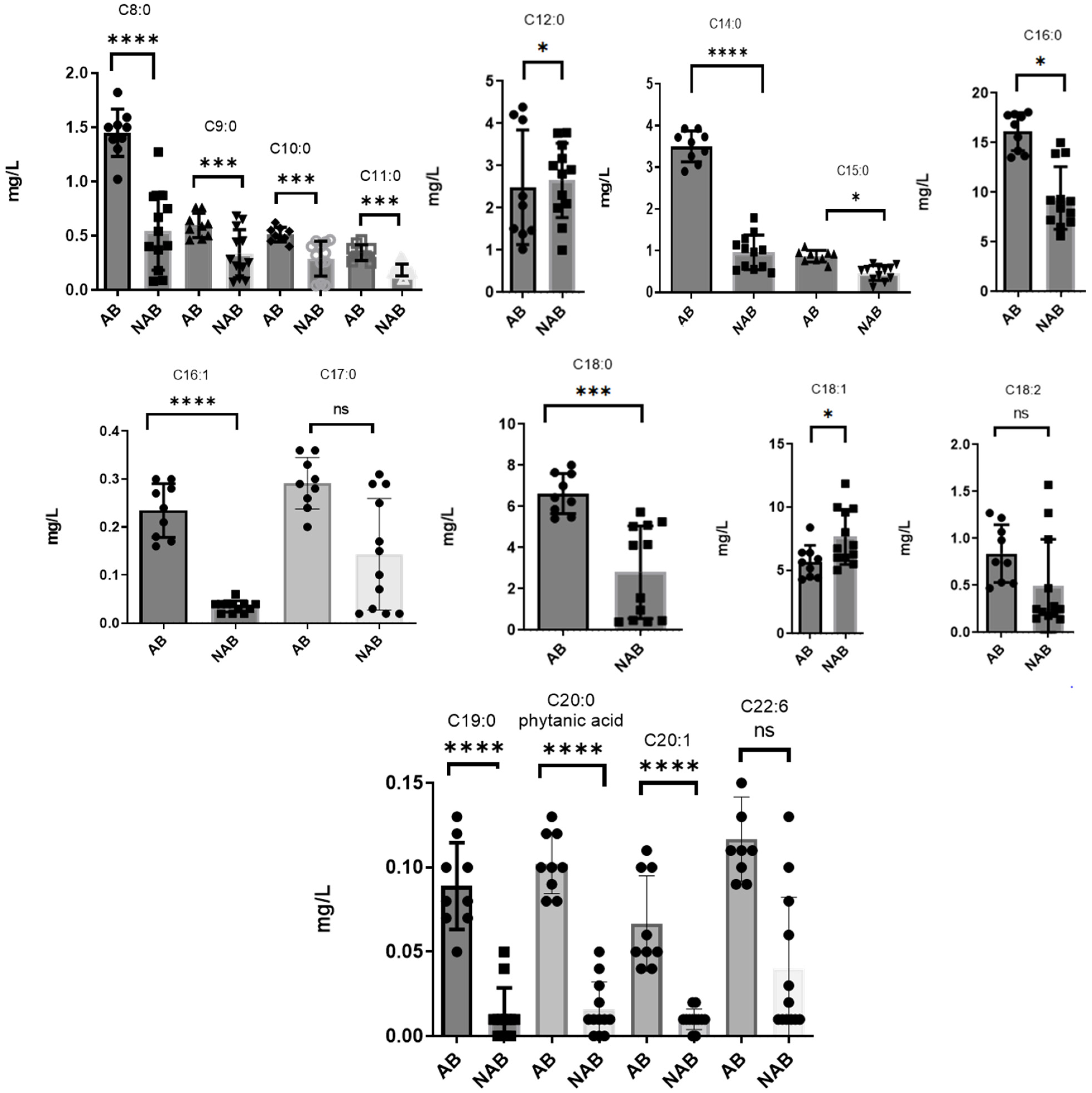
3.3. Comparison of FFA Levels in Alcoholic and Non-Alcoholic Beers
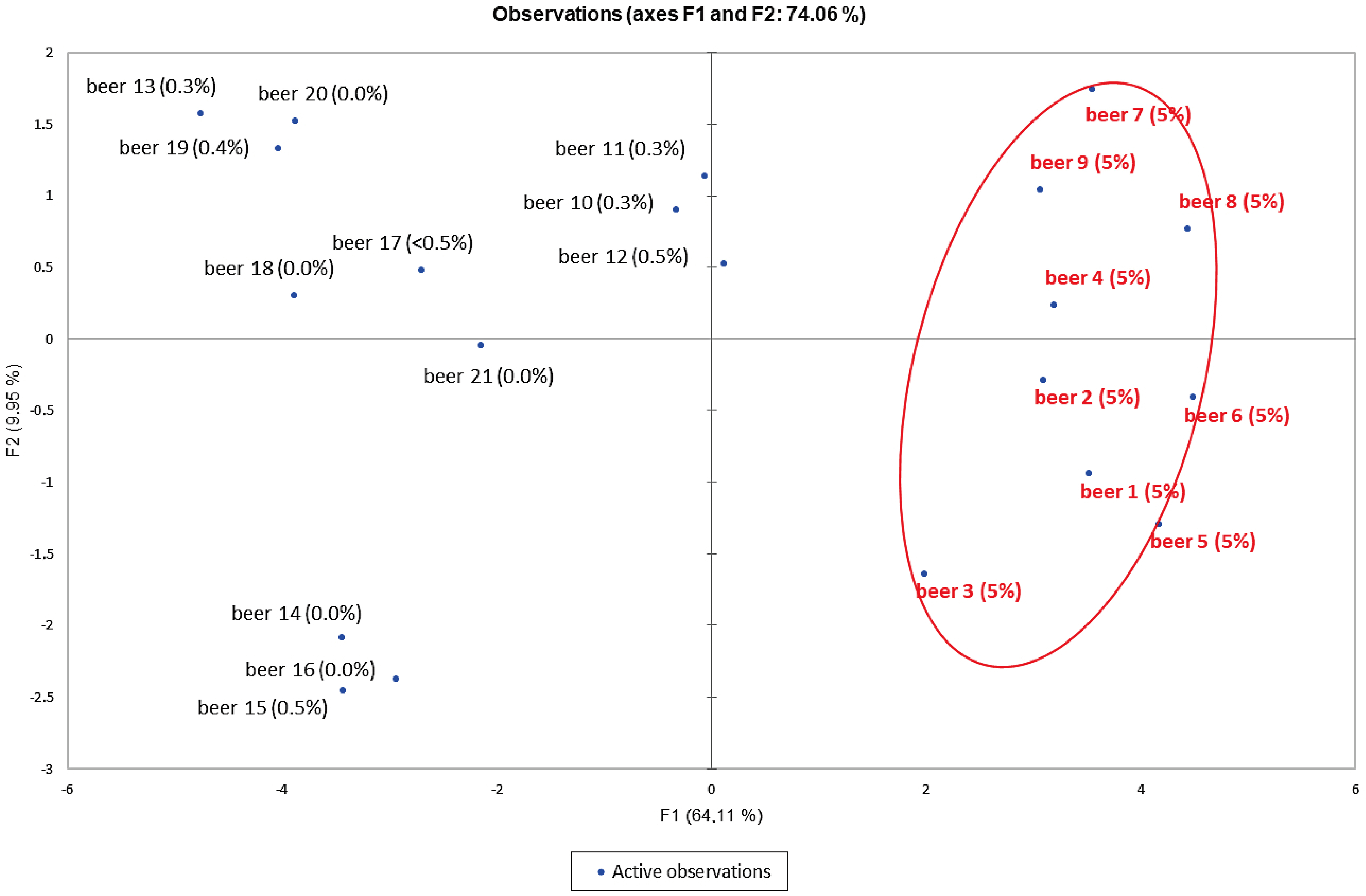
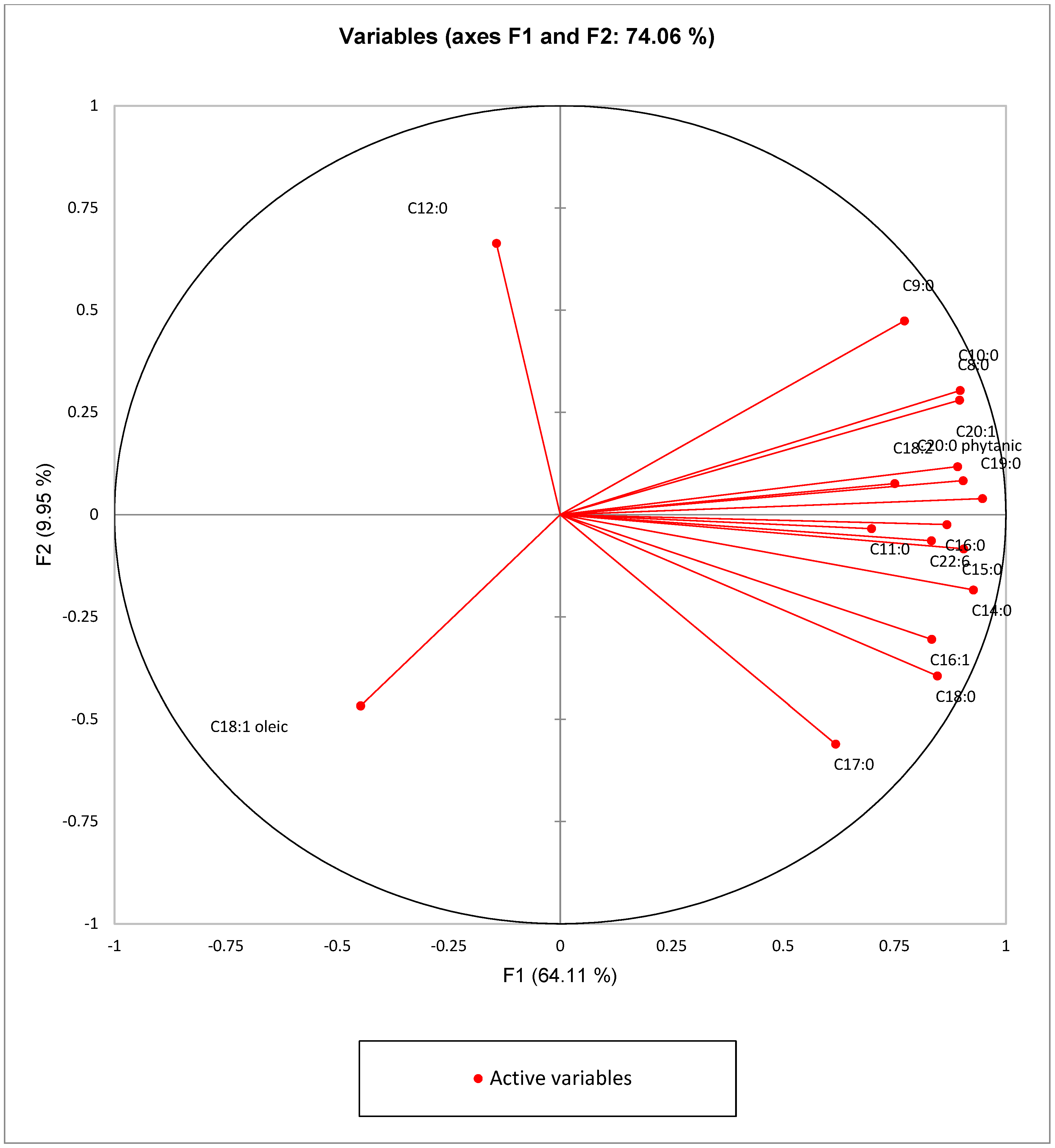
3.4. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, D.K. Beer: Production, Sensory Characteristics and Sensory Analysis. In Alcoholic Beverages; Elsevier: Amsterdam, The Netherlands, 2012; pp. 133–158. ISBN 978-0-85709-051-5. [Google Scholar]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Anjos, O.; Zhao, H. Functionality of Special Beer Processes and Potential Health Benefits. Processes 2020, 8, 1613. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, Z.; Gamez, G.; Law, W.S.; Chen, H.; Yang, S.; Chingin, K.; Balabin, R.M.; Wang, R.; Zhang, T.; et al. Simultaneous Sampling of Volatile and Non-Volatile Analytes in Beer for Fast Fingerprinting by Extractive Electrospray Ionization Mass Spectrometry. Anal. Bioanal. Chem. 2010, 398, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J.K. The Role of Total Lipids and Fatty Acids Profile on the Water Uptake of Barley Grain during Steeping. Food Chem. 2014, 151, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Bravi, E.; Marconi, O.; Sileoni, V.; Perretti, G. Determination of Free Fatty Acids in Beer. Food Chem. 2017, 215, 341–346. [Google Scholar] [CrossRef]
- Horák, T.; Čulík, J.; Jurková, M.; Čejka, P.; Kellner, V. Determination of Free Medium-Chain Fatty Acids in Beer by Stir Bar Sorptive Extraction. J. Chromatogr. A 2008, 1196–1197, 96–99. [Google Scholar] [CrossRef]
- Battistutta, F.; Buiatti, S.; Zenarola, C.; Zironi, R. Rapid Analysis of Free Medium-chain Fatty Acids and Related Ethyl Esters in Beer Using SPE and HRGC. J. High Resolut. Chromatogr. 1994, 17, 662–664. [Google Scholar] [CrossRef]
- Gordon, R.; Power, A.; Chapman, J.; Chandra, S.; Cozzolino, D. A Review on the Source of Lipids and Their Interactions during Beer Fermentation That Affect Beer Quality. Fermentation 2018, 4, 89. [Google Scholar] [CrossRef]
- DeVries, K. Determination of Free Fatty Acids in Wort and Beer. J. Am. Soc. Brew. Chem. 1990, 48, 13–17. [Google Scholar] [CrossRef]
- Müller, M.P.; Schmid, F.; Becker, T.; Gastl, M. Impact of Different Hop Compounds on the Overfoaming Volume of Beer Caused by Primary Gushing. J. Inst. Brew. 2010, 116, 459–463. [Google Scholar] [CrossRef]
- Bravi, E.; Marconi, O.; Perretti, G.; Fantozzi, P. Influence of Barley Variety and Malting Process on Lipid Content of Malt. Food Chem. 2012, 135, 1112–1117. [Google Scholar] [CrossRef]
- Bravi, E.; Benedetti, P.; Marconi, O.; Perretti, G. Determination of Free Fatty Acids in Beer Wort. Food Chem. 2014, 151, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt, F.; Lindtner, S.; Gastl, M. Profiling of Free Fatty Acids in Wort Using an Isolator Column-Assisted LC-MS/MS Approach. J. Food Compos. Anal. 2025, 140, 107215. [Google Scholar] [CrossRef]
- Lordan, R.; O’Keeffe, E.; Dowling, D.; Mullally, M.; Heffernan, H.; Tsoupras, A.; Zabetakis, I. The in Vitro Antithrombotic Properties of Ale, Lager, and Stout Beers. Food Biosci. 2019, 28, 83–88. [Google Scholar] [CrossRef]
- Lordan, R.; O’Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, Neutral, and Polar Lipids of Brewing Ingredients, By-Products and Beer: Evaluation of Antithrombotic Activities. Foods 2019, 8, 171. [Google Scholar] [CrossRef]
- Hans, S.; Harishkumar, R.; Shiels, K.; Saha, S.K.; Toohey, H.; Cunneen, L.; Nalewajka, A.; Zabetakis, I.; Lordan, R. Bioactive Lipids Derived from Red Wine, Beers, and Their Dealcoholized Variants Inhibit Platelet-Activating Factor (PAF) Induced Platelet Activation in Vitro. Food Biosci. 2024, 59, 104053. [Google Scholar] [CrossRef]
- Piornos, J.A.; Koussissi, E.; Balagiannis, D.P.; Brouwer, E.; Parker, J.K. Alcohol-free and Low-alcohol Beers: Aroma Chemistry and Sensory Characteristics. Compr. Rev. Food Sci. Food Saf. 2023, 22, 233–259. [Google Scholar] [CrossRef]
- Food and Drug Administration—FDA. CPG Sec. 510.400 Dealcoholized Wine and Malt Beverages—Labeling USA: FDA. 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-510400-dealcoholized-wine-and-malt-beverages-labeling (accessed on 2 February 2025).
- Brányik, T.; Silva, D.P.; Baszczyňski, M.; Lehnert, R.; Almeida, E.; Silva, J.B. A Review of Methods of Low Alcohol and Alcohol-Free Beer Production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Kokole, D.; Jané Llopis, E.; Anderson, P. Non-alcoholic Beer in the European Union and UK: Availability and Apparent Consumption. Drug Alcohol Rev. 2022, 41, 550–560. [Google Scholar] [CrossRef]
- Hernández-Quiroz, F.; Nirmalkar, K.; Villalobos-Flores, L.E.; Murugesan, S.; Cruz-Narváez, Y.; Rico-Arzate, E.; Hoyo-Vadillo, C.; Chavez-Carbajal, A.; Pizano-Zárate, M.L.; García-Mena, J. Influence of Moderate Beer Consumption on Human Gut Microbiota and Its Impact on Fasting Glucose and β-Cell Function. Alcohol 2020, 85, 77–94. [Google Scholar] [CrossRef]
- Marques, C.; Dinis, L.; Barreiros Mota, I.; Morais, J.; Ismael, S.; Pereira-Leal, J.B.; Cardoso, J.; Ribeiro, P.; Beato, H.; Resende, M.; et al. Impact of Beer and Nonalcoholic Beer Consumption on the Gut Microbiota: A Randomized, Double-Blind, Controlled Trial. J. Agric. Food Chem. 2022, 70, 13062–13070. [Google Scholar] [CrossRef]
- Lamiquiz-Moneo, I.; Pérez-Calahorra, S.; Gracia-Rubio, I.; Cebollada, A.; Bea, A.M.; Fumanal, A.; Ferrer-Mairal, A.; Prieto-Martín, A.; Sanz-Fernández, M.L.; Cenarro, A.; et al. Effect of the Consumption of Alcohol-Free Beers with Different Carbohydrate Composition on Postprandial Metabolic Response. Nutrients 2022, 14, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, H.; Kano, Y.; Kamimura, M.; Osawa, T.; Kawakishi, S. Analysis of Long-Chain Fatty Acids in Beer by an HPLC-Fluorescence Detection Method. J. Agric. Food Chem. 1990, 38, 1363–1367. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Xiong, B. Preparation of Novel Solid-Phase Microextraction Fibers by Sol–Gel Technology for Headspace Solid-Phase Microextraction-Gas Chromatographic Analysis of Aroma Compounds in Beer. J. Chromatogr. A 2005, 1065, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the Aroma Profiles of Low-Alcohol Beers Using HS-SPME–GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Yang, M.; Zhai, X.; Huang, X.; Li, Z.; Shi, J.; Li, Q.; Zou, X.; Battino, M. Rapid Discrimination of Beer Based on Quantitative Aroma Determination Using Colorimetric Sensor Array. Food Chem. 2021, 363, 130297. [Google Scholar] [CrossRef]
- Horák, T.; Čulík, J.; Čejka, P.; Jurková, M.; Kellner, V.; Dvořák, J.; Hašková, D. Analysis of Free Fatty Acids in Beer: Comparison of Solid-Phase Extraction, Solid-Phase Microextraction, and Stir Bar Sorptive Extraction. J. Agric. Food Chem. 2009, 57, 11081–11085. [Google Scholar] [CrossRef]
- Kokotou, M.G. Fatty Acid Profiling in Greek Wines by Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HRMS). Separations 2024, 11, 321. [Google Scholar] [CrossRef]
- Cozzolino, D.; Degner, S. An Overview on the Role of Lipids and Fatty Acids in Barley Grain and Their Products during Beer Brewing. Food Res. Int. 2016, 81, 114–121. [Google Scholar] [CrossRef]
- Schwarz, P.; Stanley, P.; Solberg, S. Activity of Lipase during Mashing. J. Am. Soc. Brew. Chem. 2002, 60, 107–109. [Google Scholar] [CrossRef]
- Bravi, E.; Perretti, G.; Buzzini, P.; Della Sera, R.; Fantozzi, P. Technological Steps and Yeast Biomass as Factors Affecting the Lipid Content of Beer during the Brewing Process. J. Agric. Food Chem. 2009, 57, 6279–6284. [Google Scholar] [CrossRef]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty Acid Synthesis and Elongation in Yeast. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2007, 1771, 255–270. [Google Scholar] [CrossRef]
- Moonjai, N.; Verstrepen, K.J.; Delvaux, F.R.; Derdelinckx, G.; Verachtert, H. The Effects of Linoleic Acid Supplementation of Cropped Yeast on Its Subsequent Fermentation Performance and Acetate Ester Synthesis. J. Inst. Brew. 2002, 108, 227–235. [Google Scholar] [CrossRef]
- Yu, J.; Huang, S.; Dong, J.; Fan, W.; Huang, S.; Liu, J.; Chang, Z.; Tian, Y.; Hao, J.; Hu, S. The Influence of LOX-Less Barley Malt on the Flavour Stability of Wort and Beer: Influence of LOX-Less Barley Malt on Flavour Stability. J. Inst. Brew. 2014, 120, 93–98. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Mantzourani, C.; Kokotos, G. Development of a Liquid Chromatography–High Resolution Mass Spectrometry Method for the Determination of Free Fatty Acids in Milk. Molecules 2020, 25, 1548. [Google Scholar] [CrossRef]
- Mantzourani, C.; Batsika, C.S.; Kokotou, M.G.; Kokotos, G. Free fatty acid profiling of Greek yogurt by liquid chromatography-high resolution mass spectrometry (LC-HRMS) analysis. Food Res. Int. 2022, 160, 111751. [Google Scholar] [CrossRef]
- Kokotou, M.G. Determination of Free Fatty Acids in Breast Milk Reveals the Presence of Hydroxypalmitic and Stearic Acids. Biomolecules 2024, 14, 1602. [Google Scholar] [CrossRef]
| Code | % ABV a | Country of Origin | Type |
|---|---|---|---|
| 1 | 5.0% | Greece | Lager |
| 2 | 5.0% | Greece | Lager |
| 3 | 5.0% | Greece | Lager |
| 4 | 5.0% | Greece | Lager |
| 5 | 5.0% | Greece | Lager |
| 6 | 5.0% | Greece | Lager |
| 7 | 5.0% | Greece | Lager |
| 8 | 5.0% | Belgium | Lager |
| 9 | 4.8% | Germany | Lager (Pilsner) |
| 10 | 0.3% | Netherlands | Lager |
| 11 | 0.3% | Germany | Lager (Pilsner) |
| 12 | 0.5% | Germany | Lager |
| 13 | 0.3% | Greece | Lager |
| 14 | 0.0% | Greece | Lager |
| 15 | 0.5% | Greece | Lager |
| 16 | 0.0% | Greece | Lager |
| 17 | <0.5% | Greece | Lager |
| 18 | 0.0% | Greece | Lager |
| 19 | 0.4% | Greece | Lager |
| 20 | 0.0% | Belgium | Lager |
| 21 | 0.0% | Germany | Lager (Pilsner) |
| Analyte | Spike Level 50 ng/mL | Spike Level 200 ng/mL | Spike Level 500 ng/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%R) | RSDr (%) | RSDR (%) | ME | Recovery (%R) | RSDr (%) | RSDR (%) | MF | Recovery (%R) | RSDr (%) | RSDR (%) | ME | |
| C6:0 | 87 | 3.9 | 6.2 | 0.9 | 85 | 11.8 | 14.2 | 0.8 | 82 | 2.8 | 3.3 | 0.8 |
| C7:0 | 92 | 2.5 | 6.4 | 1.0 | 99 | 6.4 | 6.6 | 0.9 | 86 | 0.8 | 12.2 | 0.9 |
| C8:0 | 95 | 9.6 | 6.4 | 0.9 | 93 | 6.4 | 5.6 | 0.8 | 91 | 10.7 | 10.5 | 0.8 |
| C9:0 | 90 | 3.2 | 7.5 | 0.9 | 94 | 13.2 | 1.7 | 0.9 | 89 | 2.9 | 6.4 | 0.9 |
| C10:0 | 85 | 2.6 | 12.7 | 0.9 | 88 | 0.1 | 8.6 | 0.8 | 85 | 4.9 | 9.0 | 0.8 |
| C11:0 | 91 | 10.9 | 11.3 | 0.9 | 105 | 15.8 | 10.7 | 0.8 | 103 | 12.5 | 2.4 | 0.8 |
| C12:0 | 89 | 5.2 | 12.0 | 0.8 | 91 | 6.2 | 8.6 | 0.9 | 88 | 2.9 | 3.1 | 0.8 |
| C13:0 | 84 | 5.7 | 9.3 | 0.8 | 87 | 4.4 | 10.4 | 0.9 | 85 | 8.2 | 8.7 | 0.8 |
| C14:0 | 86 | 8.7 | 9.9 | 0.9 | 103 | 14.2 | 9.0 | 0.8 | 109 | 5.7 | 8.5 | 1.1 |
| C14:1 | 84 | 3.2 | 1.3 | 0.8 | 85 | 1.7 | 4.6 | 0.9 | 87 | 2.6 | 8.6 | 0.8 |
| C15:0 | 83 | 5.8 | 4.3 | 0.9 | 94 | 4.2 | 2.7 | 0.8 | 91 | 6.8 | 10.9 | 1.0 |
| C16:0 | 82 | 4.2 | 11.3 | 1.1 | 109 | 1.6 | 8.1 | 0,9 | 103 | 8.0 | 7.4 | 1.1 |
| C16:1 | 81 | 6.2 | 3.1 | 0.8 | 89 | 8.2 | 14.0 | 0.9 | 92 | 9.0 | 8.7 | 1.0 |
| C17:0 | 81 | 5.2 | 1.5 | 0.9 | 80 | 3.1 | 1.4 | 1.1 | 80 | 9.3 | 5.6 | 1.1 |
| C17:1 | 82 | 4.8 | 1.7 | 0.9 | 82 | 2.8 | 2.2 | 0.9 | 82 | 6.6 | 1.6 | 1.1 |
| C18:0 | 87 | 1.6 | 10.3 | 1.1 | 106 | 0.2 | 5.9 | 1.1 | 105 | 2.6 | 14.7 | 1.0 |
| C18:1 oleic acid | 83 | 2.4 | 1.8 | 1.2 | 95 | 0.3 | 5.5 | 0.9 | 97 | 10.8 | 0.8 | 1.2 |
| C18:1 petroselinic acid | 83 | 2.0 | 3.2 | 1.0 | 91 | 1.2 | 3.2 | 1.0 | 92 | 2.0 | 0.9 | 1.1 |
| C18:2 | 84 | 6.5 | 10.2 | 0.8 | 87 | 4.1 | 3.9 | 0.9 | 85 | 8.1 | 9.2 | 1.1 |
| total-C18:3 | 81 | 3.2 | 6.6 | 0.9 | 84 | 0.6 | 0.8 | 0.9 | 85 | 9.0 | 6.2 | 1.0 |
| C19:0 | 86 | 2.0 | 6.6 | 0.8 | 86 | 1.2 | 6.0 | 1.0 | 86 | 1.6 | 14.7 | 1.0 |
| C20:0 arachidic acid | 83 | 3.2 | 4.0 | 0.9 | 81 | 2.6 | 0.7 | 0.8 | 84 | 8.0 | 13.1 | 0.9 |
| C20:0 phytanic acid | 86 | 1.3 | 1.1 | 0.8 | 84 | 3.6 | 7.2 | 1.1 | 85 | 3.6 | 8.9 | 1.1 |
| C20:1 | 85 | 1.3 | 6.1 | 0.9 | 87 | 0.2 | 4.2 | 1.1 | 85 | 2.2 | 7.2 | 1.1 |
| C20:3 bishomo-γ-linolenic acid | 85 | 5.6 | 7.6 | 1.3 | 86 | 1.7 | 5.9 | 1.0 | 87 | 10.9 | 7.3 | 1.2 |
| C20:3 5,8,11-eicosatrienoic acid | 85 | 6.9 | 10.9 | 0.8 | 89 | 3.1 | 4.9 | 1.0 | 94 | 14.53 | 7.3 | 1.1 |
| C20:4 | 84 | 5.2 | 5.9 | 0.9 | 89 | 1.9 | 6.0 | 0.9 | 87 | 6.67 | 8.0 | 1.1 |
| C20:5 | 82 | 6.3 | 12.8 | 0.9 | 82 | 3.7 | 3.8 | 0.8 | 87 | 13.32 | 5.7 | 1.1 |
| C21:0 | 86 | 5.2 | 10.0 | 1.0 | 89 | 0.5 | 0.5 | 0.8 | 86 | 9.21 | 12.7 | 0.8 |
| C22:0 | 84 | 9.6 | 9.8 | 1.0 | 85 | 2.7 | 1.4 | 0.8 | 85 | 12.50 | 13.2 | 0.8 |
| C22:1 | 83 | 5.7 | 8.6 | 1.0 | 84 | 8.8 | 3.4 | 0.9 | 108 | 5.86 | 1.1 | 1.0 |
| C22:4 | 82 | 6.5 | 3.2 | 0.8 | 84 | 5.2 | 10.2 | 0.8 | 89 | 11.24 | 7.6 | 1.2 |
| C22:5 | 82 | 3.2 | 1.0 | 0.9 | 89 | 2.1 | 1.6 | 0.8 | 84 | 6.67 | 1.2 | 0.9 |
| C22:6 | 84 | 6.4 | 7.9 | 1.0 | 83 | 1.2 | 7.9 | 0.9 | 89 | 10.62 | 4.6 | 1.0 |
| C23:0 | 85 | 4.2 | 6.7 | 0.9 | 85 | 6.2 | 11.6 | 0.8 | 86 | 5.41 | 12.8 | 0.9 |
| C24:0 | 84 | 4.2 | 6.6 | 0.9 | 84 | 5.2 | 12.5 | 0.8 | 100 | 2.44 | 13.7 | 0.8 |
| C24:1 | 85 | 6.8 | 5.8 | 0.9 | 85 | 7.6 | 2.1 | 1.0 | 85 | 14.82 | 2.5 | 1.1 |
| Free Fatty Acid | Alcoholic Beers (n = 9), Triplicates | Non-Alcoholic Beers (n = 12), Triplicates | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum Value (μg/mL) | Maximum Value (μg/mL) | Mean Value ± SD (μg/mL) | % | Minimum Value (μg/mL) | Maximum Value (μg/mL) | Mean Value ± SD (μg/mL) | % | |
| C6:0 | 0.09 | 0.17 | 0.12 ± 0.03 | 0.3 | 0.05 | 0.34 | 0.12 ± 0.09 | 0.4 |
| C7:0 | 0.13 | 0.38 | 0.24 ± 0.07 | 0.6 | 0.16 | 0.29 | 0.22 ± 0.04 | 0.8 |
| C8:0 | 1.0 | 1.8 | 1.4 ± 0.2 | 3.5 | 0.08 | 1.3 | 0.54 ± 0.36 | 2.0 |
| C9:0 | 0.46 | 0.76 | 0.59 ± 0.11 | 1.4 | 0.07 | 0.68 | 0.33 ± 0.22 | 1.2 |
| C10:0 | 0.40 | 0.62 | 0.51 ± 0.07 | 1.3 | 0.04 | 0.46 | 0.29 ± 0.16 | 1.1 |
| C11:0 | 0.25 | 0.46 | 0.34 ± 0.07 | 0.8 | 0.10 | 0.30 | 0.18 ± 0.05 | 0.7 |
| C12:0 | 1.0 | 4.4 | 2.5 ± 1.4 | 6.1 | 1.0 | 3.8 | 2.6 ± 0.9 | 9.7 |
| C13:0 | 0.03 | 0.13 | 0.08 ± 0.03 | 0.2 | 0.01 | 0.11 | 0.04 ± 0.03 | 0.2 |
| C14:0 | 2.9 | 3.9 | 3.50 ± 0.37 | 8.5 | 0.47 | 1.8 | 0.96 ± 0.41 | 3.5 |
| C14:1 | 0.01 | 0.03 | 0.02 ± 0.01 | 0.1 | - | 0.04 | 0.02 ± 0.01 | 0.1 |
| C15:0 | 0.6 | 1.1 | 0.87 ± 0.14 | 2.1 | 0.12 | 0.69 | 0.47 ± 0.18 | 1.7 |
| C16:0 | 13.5 | 18.1 | 16.1 ± 1.9 | 39.4 | 5.6 | 15.0 | 9.4 ± 3.2 | 34.6 |
| C16:1 | 0.16 | 0.30 | 0.24 ± 0.06 | 0.6 | 0.02 | 0.06 | 0.03 ± 0.01 | 0.1 |
| C17:0 | 0.20 | 0.36 | 0.29 ± 0.05 | 0.7 | 0.02 | 0.31 | 0.14 ± 0.12 | 0.5 |
| C17:1 | 0.03 | 0.05 | 0.03 ± 0.01 | 0.1 | 0.00 | 0.02 | 0.01 ± 0.01 | 0.0 |
| C18:0 | 5.4 | 8.0 | 6.6 ± 1.0 | 16.2 | 0.40 | 5.7 | 2.8 ± 2.2 | 10.3 |
| C18:1 oleic acid | 4.3 | 8.4 | 5.7 ± 1.3 | 13.8 | 5.0 | 11.9 | 7.6 ± 2.2 | 28.1 |
| C18:1 petroselinic acid | - | - | - | - | - | - | - | - |
| C18:2 | 0.47 | 1.3 | 0.84 ± 0.31 | 2.1 | 0.14 | 1.6 | 0.50 ± 0.49 | 1.8 |
| total-C18:3 | - | 0.01 | 0.01 ± 0.00 | 0.0 | - | - | - | 0.0 |
| C19:0 | 0.05 | 0.13 | 0.09 ± 0.03 | 0.2 | - | 0.05 | 0.01 ± 0.01 | 0.0 |
| C20:0 arachidic acid | 0.03 | 0.06 | 0.04 ± 0.01 | 0.1 | - | 0.11 | 0.04 ± 0.04 | 0.2 |
| C20:0 phytanic acid | 0.08 | 0.13 | 0.10 ± 0.02 | 0.2 | - | 0.05 | 0.02 ± 0.02 | 0.1 |
| C20:1 | 0.04 | 0.11 | 0.07 ± 0.03 | 0.2 | - | 0.02 | 0.01 ± 0.01 | 0.0 |
| C20:3 Bishomo-γ-linolenic acid | - | - | - | - | - | - | - | - |
| C20:3 5,8,11-eicosatrienoic acid | - | - | - | - | - | - | - | - |
| C20:4 | - | - | - | - | - | - | - | - |
| C20:5 | 0.01 | 0.24 | 0.05 ± 0.07 | 0.1 | - | 0.06 | 0.02 ± 0.02 | 0.1 |
| C21:0 | 0.02 | 0.08 | 0.05 ± 0.02 | 0.1 | - | 0.01 | 0.01 ± 0.00 | 0.0 |
| C22:0 | 0.02 | 0.05 | 0.03 ± 0.01 | 0.1 | 0.01 | 0.21 | 0.07 ± 0.09 | 0.3 |
| C22:1 | 0.02 | 0.08 | 0.05 ± 0.02 | 0.1 | - | - | - | 0.0 |
| C22:4 | 0.01 | 0.01 | 0.01 ± 0.00 | 0.0 | - | 0.02 | 0.01 ± 0.01 | 0.0 |
| C22:5 | - | - | - | 0.0 | - | 2.2 | 0.31 ± 0.68 | 1.1 |
| C22:6 | 0.09 | 0.16 | 0.12 ± 0.02 | 0.3 | 0.01 | 0.13 | 0.04 ± 0.04 | 0.2 |
| C23:0 | - | 0.06 | 0.02 ± 0.02 | 0.1 | - | 0.02 | 0.01 ± 0.00 | 0.0 |
| C24:0 | 0.17 | 0.37 | 0.29 ± 0.08 | 0.7 | 0.09 | 0.79 | 0.33 ± 0.26 | 1.2 |
| C24:1 | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzourani, C.; Kokotou, M.G. Free Fatty Acid Determination in Alcoholic and Non-Alcoholic Beers via Liquid Chromatography–High-Resolution Mass Spectrometry Analysis. Separations 2025, 12, 114. https://doi.org/10.3390/separations12050114
Mantzourani C, Kokotou MG. Free Fatty Acid Determination in Alcoholic and Non-Alcoholic Beers via Liquid Chromatography–High-Resolution Mass Spectrometry Analysis. Separations. 2025; 12(5):114. https://doi.org/10.3390/separations12050114
Chicago/Turabian StyleMantzourani, Christiana, and Maroula G. Kokotou. 2025. "Free Fatty Acid Determination in Alcoholic and Non-Alcoholic Beers via Liquid Chromatography–High-Resolution Mass Spectrometry Analysis" Separations 12, no. 5: 114. https://doi.org/10.3390/separations12050114
APA StyleMantzourani, C., & Kokotou, M. G. (2025). Free Fatty Acid Determination in Alcoholic and Non-Alcoholic Beers via Liquid Chromatography–High-Resolution Mass Spectrometry Analysis. Separations, 12(5), 114. https://doi.org/10.3390/separations12050114








