Synergistic Effects of SDS and Non-Ionic Surfactants on Ceramic Membrane Cleaning Performance Under Acidic Conditions
Abstract
1. Introduction
2. Experiment
2.1. Materials and Methods
2.1.1. Surfactants Used in the Study
2.1.2. Ceramic Membranes and Pre-Treatment
2.1.3. Instrumentation
2.2. Experimental Procedures
2.2.1. Methods of Analysis
2.2.2. Formulation and Optimization of Surfactant Combinations
2.2.3. Surface Tension and Membrane Flux Measurements
3. Results and Discussion
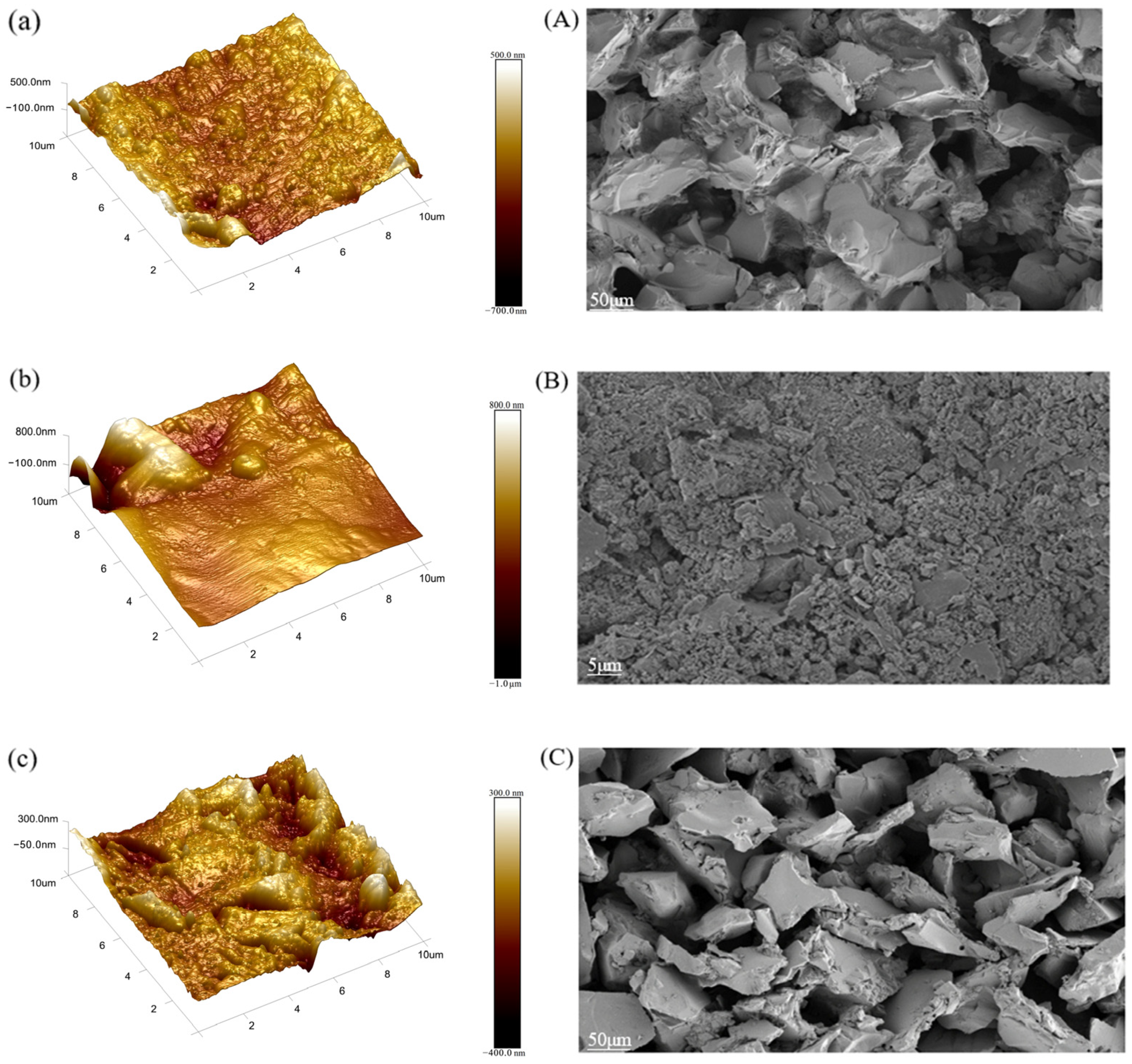
3.1. Analysis of Ceramic Membrane Contaminants
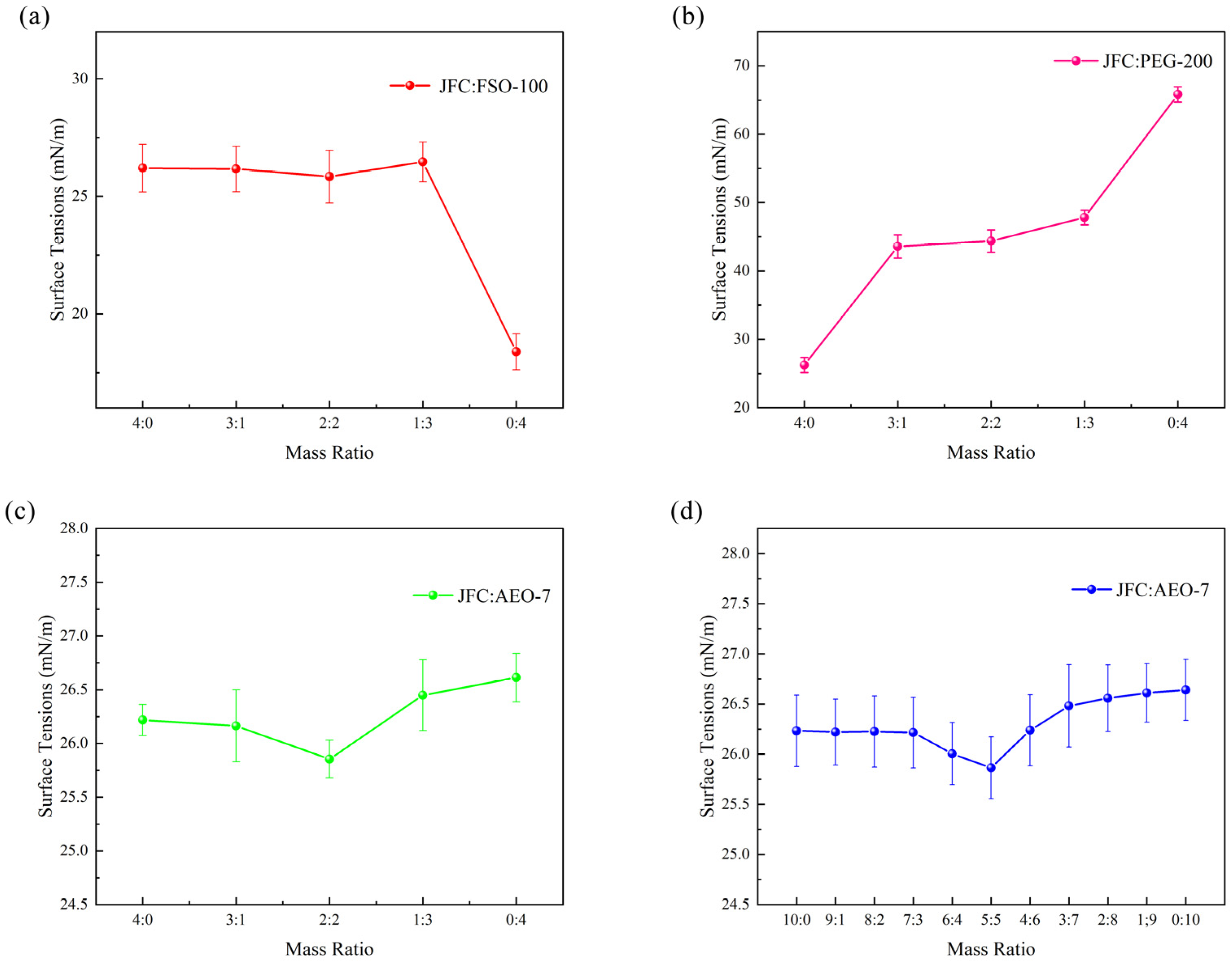
3.2. Surface Tension of Composite Solution
3.3. R0 of Composite Solution
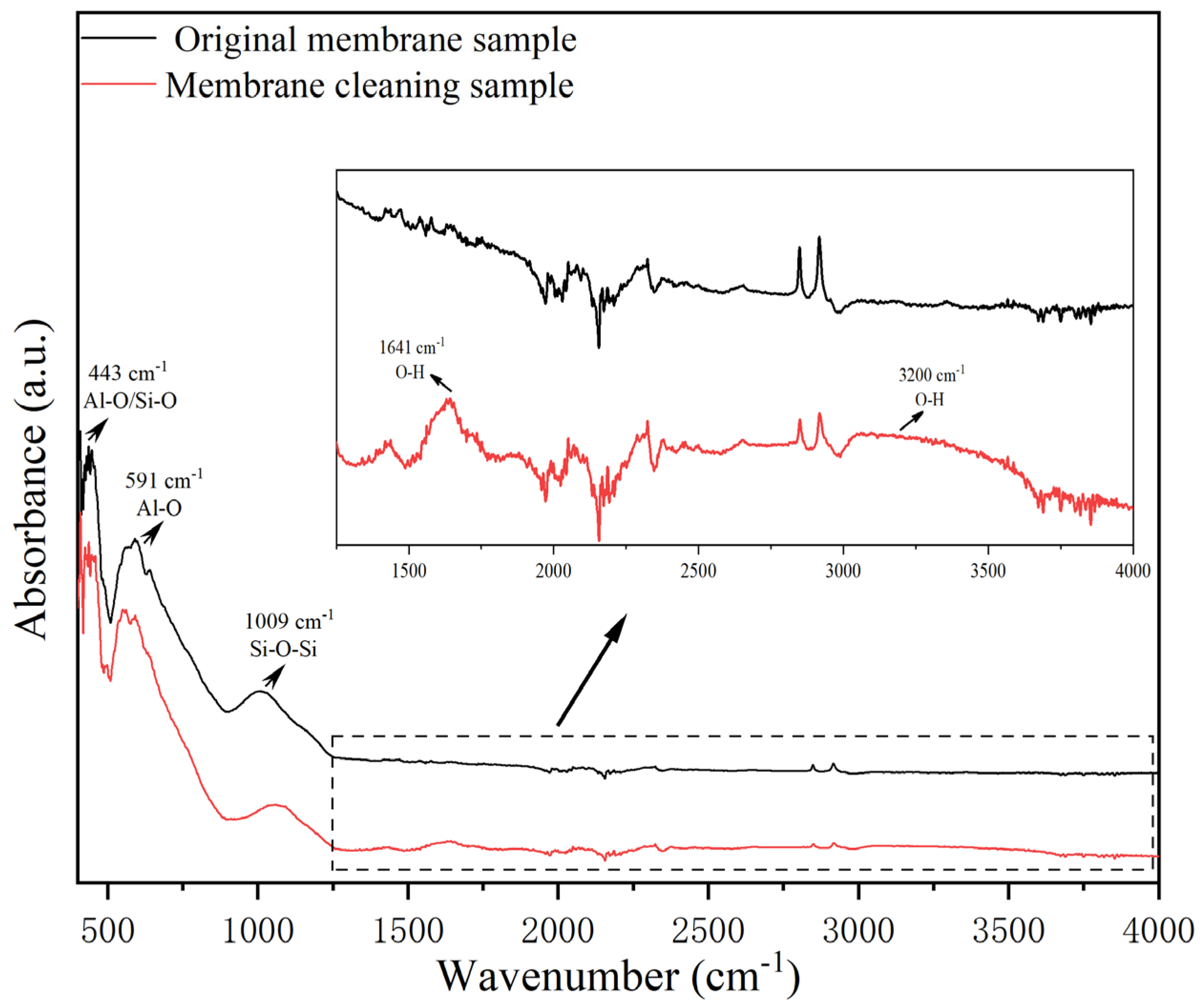
3.4. Effect of Cleaning Agent Treatment on Surface Groups and Structure of Membrane
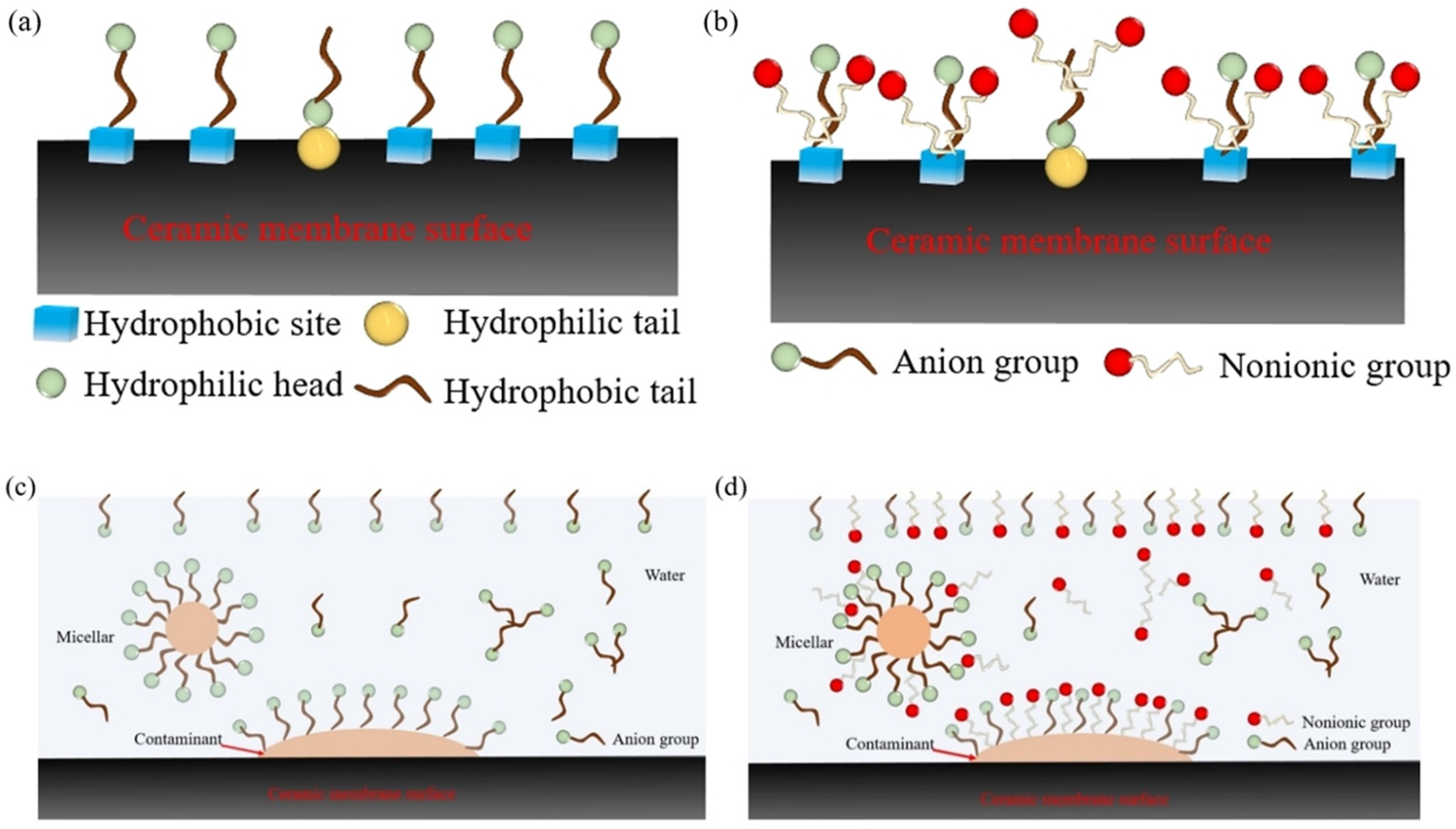
3.5. Study on Synergistic Cleaning Mechanism of Composite SFT Under Acidic Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, C.; Xu, W.; Tian, Y.; Liang, Q.; Pan, W.-X.; Mei, G.-J. Experimental study on direct-reverse flotation of a refractory siliceous collophanite in Guizhou. Nonferrous Met. Min. Process Sect. 2024, 02, 29–35. [Google Scholar]
- Derqaoui, M.; Aarab, I.; Abidi, A.; Yaacoubi, A.; El Amari, K.; Etahiri, A.; Baçaoui, A. Review of the reagents used in the direct flotation of phosphate ores. Arab. J. Geosci. 2021, 15, 49. [Google Scholar] [CrossRef]
- Chen, A.-A.; Zhang, Q. Influence of measuring process properties on phosphate rock slurry rheology based on Brookfield method. Physicochem. Probl. Miner. Process. 2022, 56, 156202. [Google Scholar] [CrossRef]
- Abdelmjid, B.; Mohamed, O.; Abdellah, A.; Najib, T.; Saad, A.Y. Novel low-cost bentonite-based membranes for microfiltration, ultrafiltration, and nanofiltration applications. Nov. Mater. Environ. Remediat. Appl. 2023, 13, 247–275. [Google Scholar]
- Meltem, A.; Mehmet, D.; İsmail, K. Ceramic membrane overview and applications in textile industry: A review. Water Sci. Technol. 2021, 84, 1059–1078. [Google Scholar]
- Gao, P.; Xu, S.-J.; Xu, Z.-L.; Li, P.; Wu, Y.-Z.; Li, L.-Q.; Zhang, H.-Z. High-Flux Fine Hollow Fiber Nanofiltration Membranes for the Purification of Drinking Water. Ind. Eng. Chem. Res. 2021, 60, 1817–1828. [Google Scholar] [CrossRef]
- Arndt, F.; Roth, U.; Nirschl, H.; Schütz, S.; Guthausen, G. New insights into sodium alginate fouling of ceramic hollow fiber membranes by NMR imaging. AlChE J. 2016, 62, 2459–2467. [Google Scholar] [CrossRef]
- Wang, Y.N.; Tang, C.Y. Fouling of nanofiltration, reverse osmosis, and ultrafiltration membranes by protein mixtures: The role of inter-foulant-species interaction. Environ. Sci. Technol. 2011, 45, 6373–6379. [Google Scholar] [CrossRef]
- Cai, C.; Sun, W.-J.; He, S.-Y.; Zhang, Y.-N.; Wang, X.-L. Ceramic membrane fouling mechanisms and control for water treatment. Front. Environ. Sci. Eng. 2023, 17, 126. [Google Scholar] [CrossRef]
- Atallah, C.; Mortazavi, S.; Tremblay, A.; Doiron, A. In-Process Steam Cleaning of Ceramic Membranes Used in the Treatment of Oil Sands Produced Water. Ind. Eng. Chem. Res. 2019, 58, 15232–15243. [Google Scholar] [CrossRef]
- Xiao, T.; Zhu, Z.-H.; Li, L.-C.; Shi, J.-X.; Li, Z.-X.; Zuo, X.-J. Membrane fouling and cleaning strategies in microfiltration/ultrafiltration and dynamic membrane. Sep. Purif. Technol. 2023, 318, 123977. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kim, Y.-H.; Jung Jeon, M.; Jang, A.; Kim, J.; Kim, H. The effects of physical cleaning and chemical backwashing on foulant formation in a microfiltration membrane intended for the reuse of wastewater. Desalination Water Treat. 2016, 57, 26586–26594. [Google Scholar] [CrossRef]
- Ang, W.; Yip, N.; Tiraferri, A.; Elimelech, M. Chemical cleaning of RO membranes fouled by wastewater effluent: Achieving higher efficiency with dual-step cleaning. J. Membr. Sci. 2011, 382, 100–106. [Google Scholar] [CrossRef]
- Gül, A.; Hruza, J.; Dvořák, L.; Yalcinkaya, F. Chemical Cleaning Process of Polymeric Nanofibrous Membranes. Polymers 2022, 14, 1102. [Google Scholar] [CrossRef]
- Leister, N.; Pfaff, D.; Karbstein, H.P. Coalescence of Inner Water Droplets in Double Emulsions Due to Surfactant Transport through Oil. Chem. Ing. Tech. 2022, 94, 365–373. [Google Scholar] [CrossRef]
- Tartaro, G.; Le Mouee, G.; Loon, S.; Palazzo, G. Modelling the partitioning equilibria of nonionic surfactant mixtures within the HLD framework. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130648. [Google Scholar] [CrossRef]
- Lu, Y.; Li, R.; Manica, R.; Liu, Q.-X.; Xu, Z.-H. Enhancing oil–solid and oil–water separation in heavy oil recovery by CO2-responsive surfactants. AlChE J. 2021, 67, e17033. [Google Scholar] [CrossRef]
- Baruah, A.; Pathak, A.K.; Ojha, K. Study on rheology and thermal stability of mixed (nonionic–anionic) surfactant based fracturing fluids. AlChE J. 2016, 62, 2177–2187. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, S.; Jiao, W.; Zuo, X.; Zhang, Y.; Liu, Y. High-efficiency cleaning technology and lifespan prediction for the ceramic membrane treating secondary treated effluent. Water Sci. Technol. 2023, 88, 321–338. [Google Scholar] [CrossRef]
- Mousavi, S.-P.; Hemmati-Sarapardeh, A.; Norouzi-Apourvari, S.; Jalalvand, M.; Schaffie, M.; Ranjbar, M. Toward mechanistic understanding of wettability alteration in calcite and dolomite rocks: The effects of resin, asphaltene, anionic surfactant, and hydrophilic nanoparticles. J. Mol. Liq. 2020, 321, 114672. [Google Scholar] [CrossRef]
- Wang, X.-M.; Wu, G.; Yuan, C.-T.; Zhu, Q.-Q.; Li, C.-L.; Sun, S.-Q.; Hu, S.-Q. Molecular dynamics simulations of aggregation behavior of sodium dodecyl sulfate on SiO2 and CaCO3 surfaces. Surf. Interface Anal. 2017, 50, 284–289. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Yan, J.-L.; Wang, C.-Y.; Diao, D.-L.; Zhang, Y.; Zhang, L.-L. Dual-responsive emulsion system: Unraveling pH and host-guest interactions for emulsion stability and enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133253. [Google Scholar] [CrossRef]
- Liao, Y.; Niu, Y.-B.; Pan, Y.-Q.; Yu, L. Modeling the effects of mixed surfactants on the behaviors and properties of the oil-water interface with molecular dynamics. CIESC J. 2022, 73, 4003–4014. [Google Scholar]
- Shi, G.-Q.; Han, C.; Wang, Y.-M.; Wang, H.-T. Experimental study on synergistic wetting of a coal dust with dust suppressant compounded with noncationic surfactants and its mechanism analysis. Powder Technol. 2019, 356, 1077–1086. [Google Scholar] [CrossRef]
- Abderrazek, E.; Younes, D.; Mohamed, D.; Abdelmjid, B.; Hajar, E.M.; Soukaina, E.A.; Najib, T. Experimental study of phenol removal from aqueous solution by adsorption onto synthesized Faujasite-type Y zeolite. Desalination Water Treat. 2022, 277, 144–154. [Google Scholar]
- Zhan, M.-Y.; Sun, L.-L.; Cheng, W.-M.; Lv, X.-W.; Shi, Q.-L.; Huang, Q.-M.; Wang, H.-S. Experimental study on coal seam moisturizing inhibitor and mechanism of preventing coal spontaneous combustion. Energy 2024, 291, 130312. [Google Scholar] [CrossRef]
- Wang, X.-W.; Jia, L.; Dang, C. The wetting transition of low surface tension droplet on the special-shaped microstructure surface. Colloid Interface Sci. Commun. 2022, 50, 100649. [Google Scholar] [CrossRef]
- Wang, H.-T.; Du, Y.-H.; Wei, X.-B.; He, X.-X. An experimental comparison of the spray performance of typical water-based dust reduction media. Powder Technol. 2019, 345, 580–588. [Google Scholar] [CrossRef]
- Avornyo, A.; Thanigaivelan, A.; Krishnamoorthy, R.; Hassan, S.W.; Banat, F. Ag-CuO-Decorated Ceramic Membranes for Effective Treatment of Oily Wastewater. Membranes 2023, 13, 176. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Momtahin, A.; Tasannum, N.; Faria, N.T.; Mofijur, M.; Hoang, A.T.; Vo, D.N.; Mahlia TM, I. Strategies to improve membrane performance in wastewater treatment. Chemosphere 2022, 306, 135527. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Gao, X.-L.; Xu, L.; Wang, J. Investigation of synchronous arsenic and salinity rejection via nanofiltration system and membrane cleaning. Desalination Water Treat. 2015, 57, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, M.-Y.; Yang, H.; Zhao, D.-W.; Zhang, K.-M. Study on the influence of new compound reagents on the functional groups and wettability of coal. Fuel 2021, 302, 121113. [Google Scholar] [CrossRef]
- Wang, Q.; Tuo, L.; Zhou, G.; Zhang, Y.; Geng, X.; Zhang, F.; Li, Y. Effect of silicones and polymers on the wetting and foaming properties of anionic and nonionic hydrocarbon surfactants. Environ. Sci. Pollut. Res. Int. 2022, 29, 81713–81725. [Google Scholar] [CrossRef] [PubMed]
- Madaeni, S.S.; Samieirad, S. Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination 2010, 257, 80–86. [Google Scholar] [CrossRef]
- Xu, J.; Cui, J.-Y.; Sun, H.-T.; Wu, Y.-T.; Xue, C.-G.; Xie, A.; Li, C.-C. Facile preparation of hydrophilic PVDF membrane via tea polyphenols modification for efficient oil-water emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 657, 130639. [Google Scholar] [CrossRef]
- Yang, L.; Yin, D.; Zhang, W.-Q.; Han, T.-C.; Zhao, P.; Wang, T.-J.; Cheng, L.-M. Composite surfactant based on AEO and ADS for colloidal silica particles removal in post CMP cleaning of copper interconnection. Mater. Sci. Semicond Process 2023, 164, 107620. [Google Scholar] [CrossRef]
- Ji, B.; Jiang, B.-Y.; Yuan, L.; Yu, C.-F.; Zhou, G.; Zhao, Y.; Wang, S.-J.; Wang, X.-H. Experimental and molecular dynamics simulation study on the influence of SDS and JFC composite ratios on bituminous coal wettability. Process Saf. Environ. Prot. 2023, 174, 473–484. [Google Scholar] [CrossRef]




| Type | Reagent Name | Abbreviation | Molecular Formula |
|---|---|---|---|
| Nonionic SFT | Primary alcohol ethoxylate | Penetrating agent JFC | RO(CH2CH2O)5H, R = C7-9 |
| Fatty alcohol polyoxyethylene ether-7 | AEO-7 | CH3(CH2)n(EO)7, n = 11 | |
| Fluorocarbon SFT | FSO-100 | RfCH2CH2O(CH2CH2O)nH, Rf = F(CF2CF2)m n = 0–15 m = 1–7 | |
| Polyethylene glycol 200 | PEG-200 | [CH2-O-CH2]n | |
| Anionic SFT | Sodium dodecyl sulfate | SDS | C12H25SO4Na |
| Substances | P2O5 (%) | MgO (%) | Fe2O3 (%) | Al2O3 (%) | MER Value (%) | SiO2 (%) | CaO (%) | F (%) |
|---|---|---|---|---|---|---|---|---|
| Slurry | 34.32 | 1.08 | 0.98 | 0.83 | 8.71 | 6.72 | 47.74 | 8.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Tian, M.; Liu, H.; An, Y.; Wu, M.; Lu, H. Synergistic Effects of SDS and Non-Ionic Surfactants on Ceramic Membrane Cleaning Performance Under Acidic Conditions. Separations 2025, 12, 112. https://doi.org/10.3390/separations12050112
Deng Y, Tian M, Liu H, An Y, Wu M, Lu H. Synergistic Effects of SDS and Non-Ionic Surfactants on Ceramic Membrane Cleaning Performance Under Acidic Conditions. Separations. 2025; 12(5):112. https://doi.org/10.3390/separations12050112
Chicago/Turabian StyleDeng, Yang, Mengkui Tian, Hai Liu, Yan An, Mingkun Wu, and Hongpeng Lu. 2025. "Synergistic Effects of SDS and Non-Ionic Surfactants on Ceramic Membrane Cleaning Performance Under Acidic Conditions" Separations 12, no. 5: 112. https://doi.org/10.3390/separations12050112
APA StyleDeng, Y., Tian, M., Liu, H., An, Y., Wu, M., & Lu, H. (2025). Synergistic Effects of SDS and Non-Ionic Surfactants on Ceramic Membrane Cleaning Performance Under Acidic Conditions. Separations, 12(5), 112. https://doi.org/10.3390/separations12050112






