Construction of Z-Scheme Heterojunction BiOCl/Bi2WO6 for Visible-Light Photocatalytic Degradation of Tetracycline Hydrochloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Bi2WO6, BiOCl, and BiOCl/Bi2WO6 Composites
2.2. Characterization of the Synthesized Samples
2.3. Photocatalytic Degradation Experiment
3. Results and Discussion
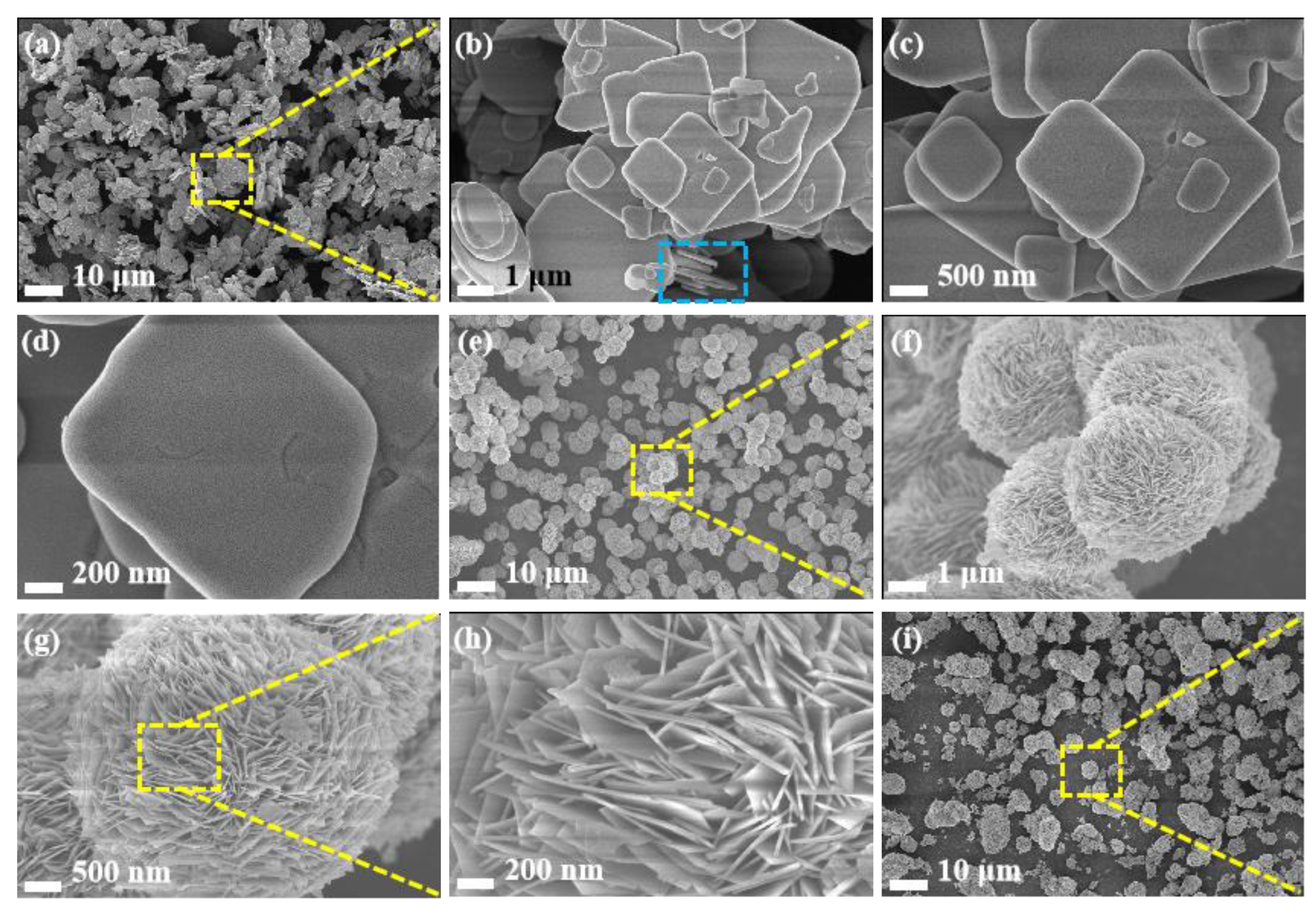
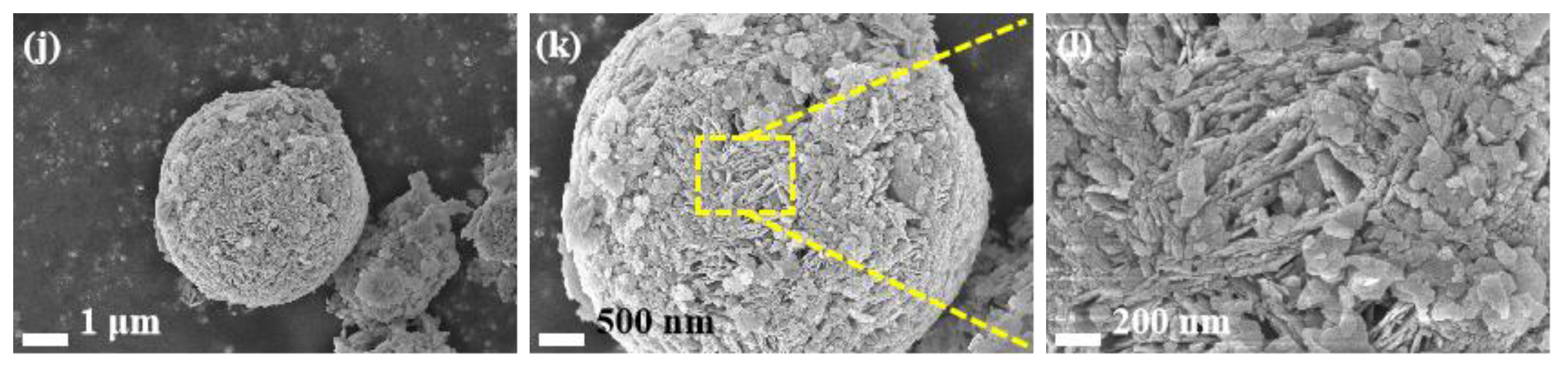
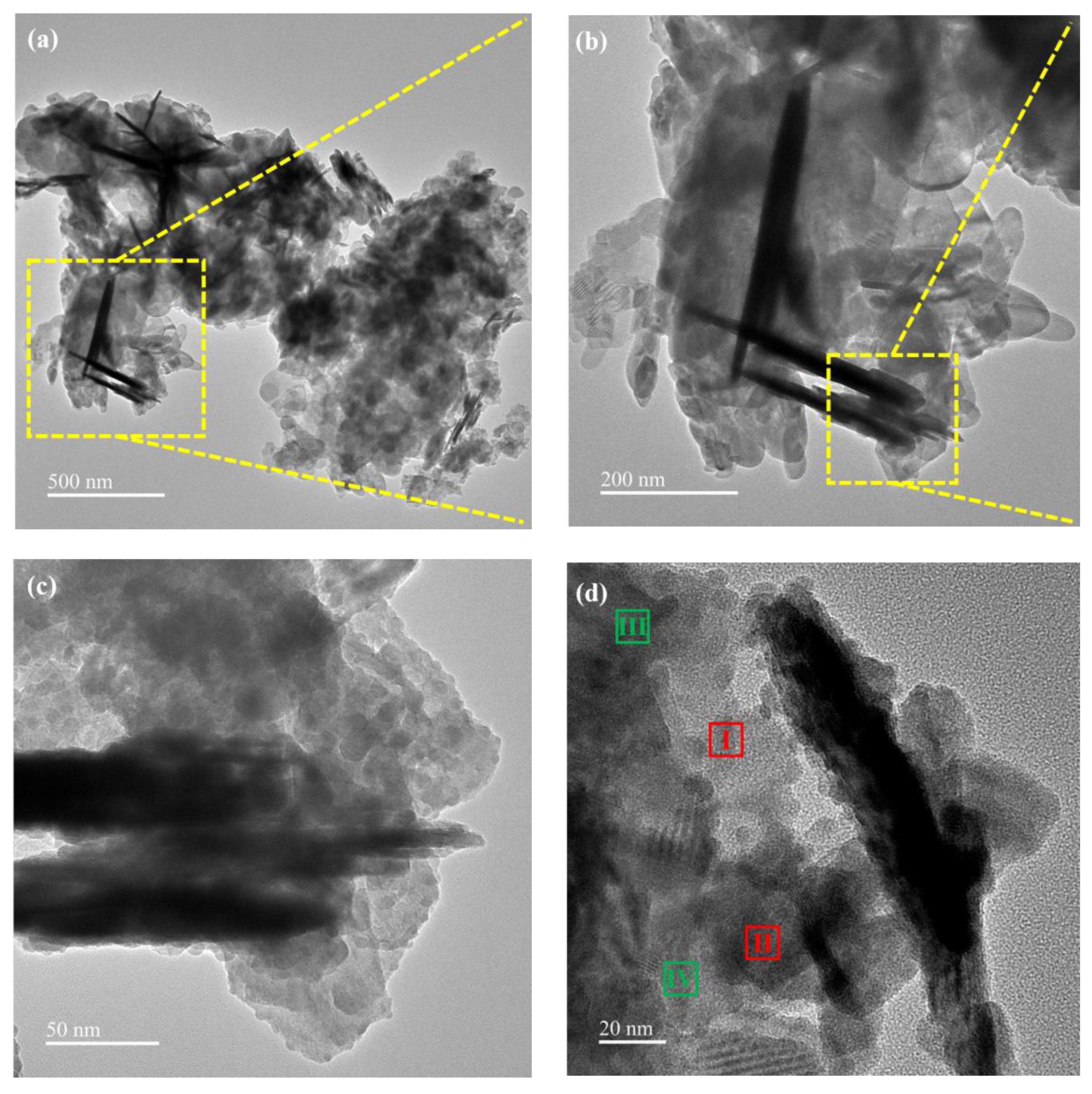
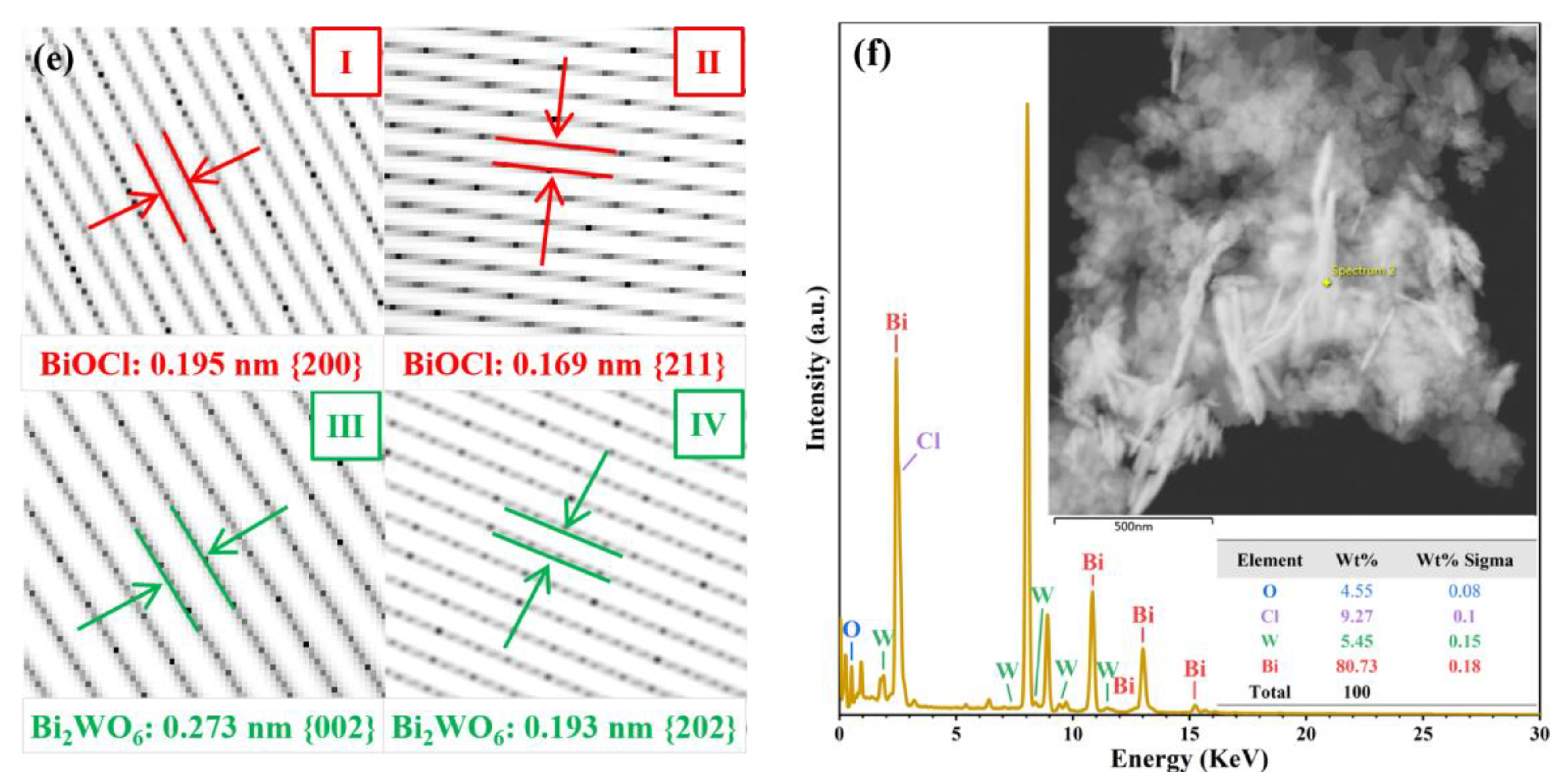
3.1. Characterization of Catalysts
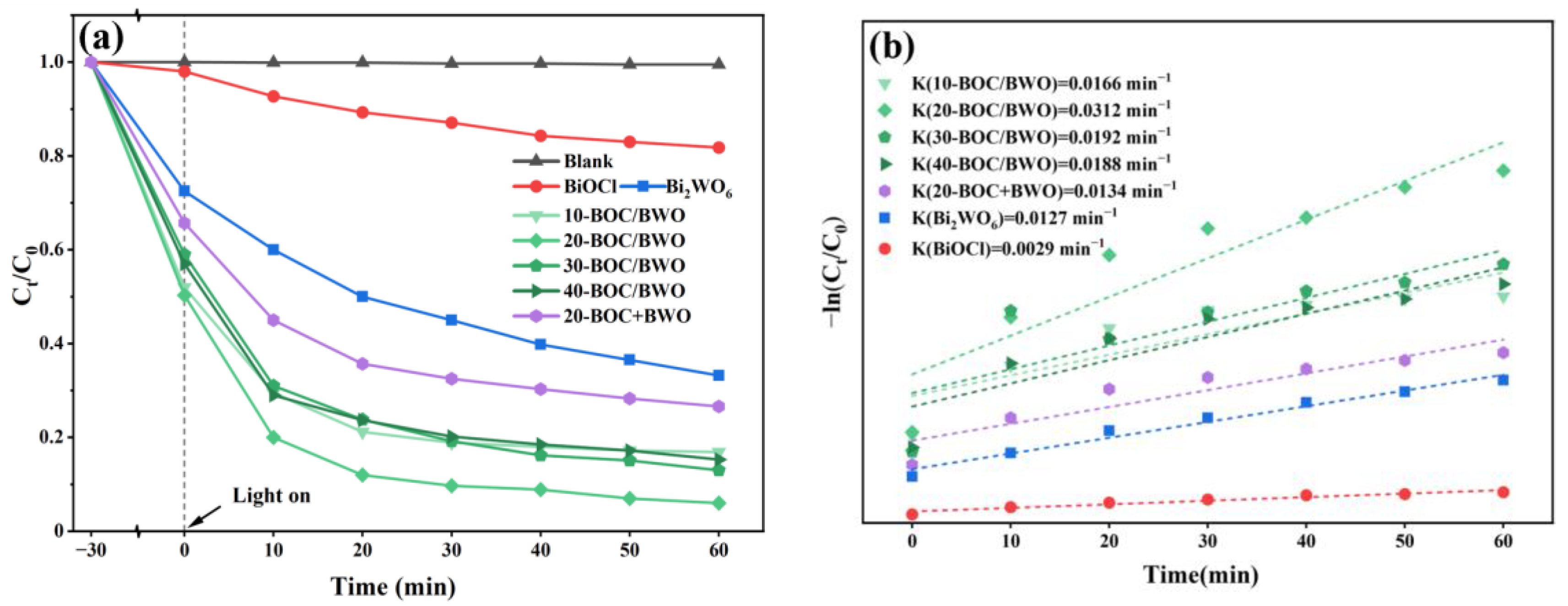
3.2. Photocatalytic Activity
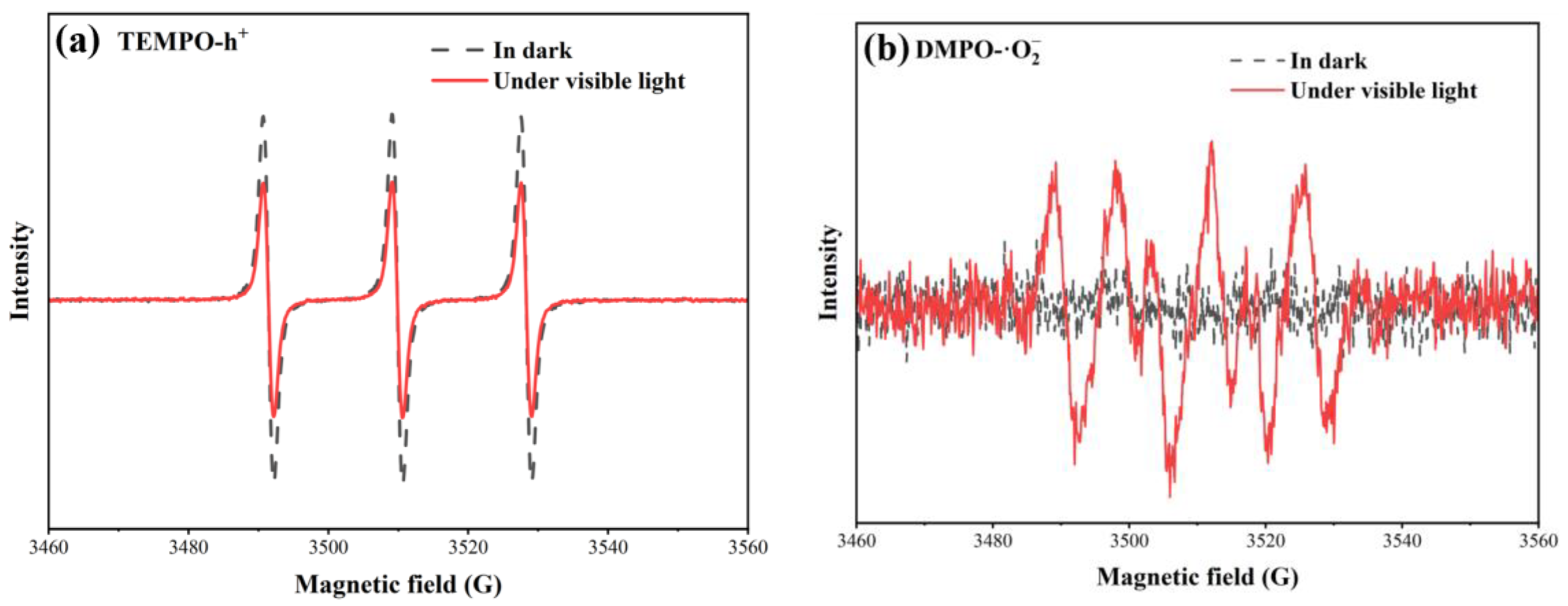
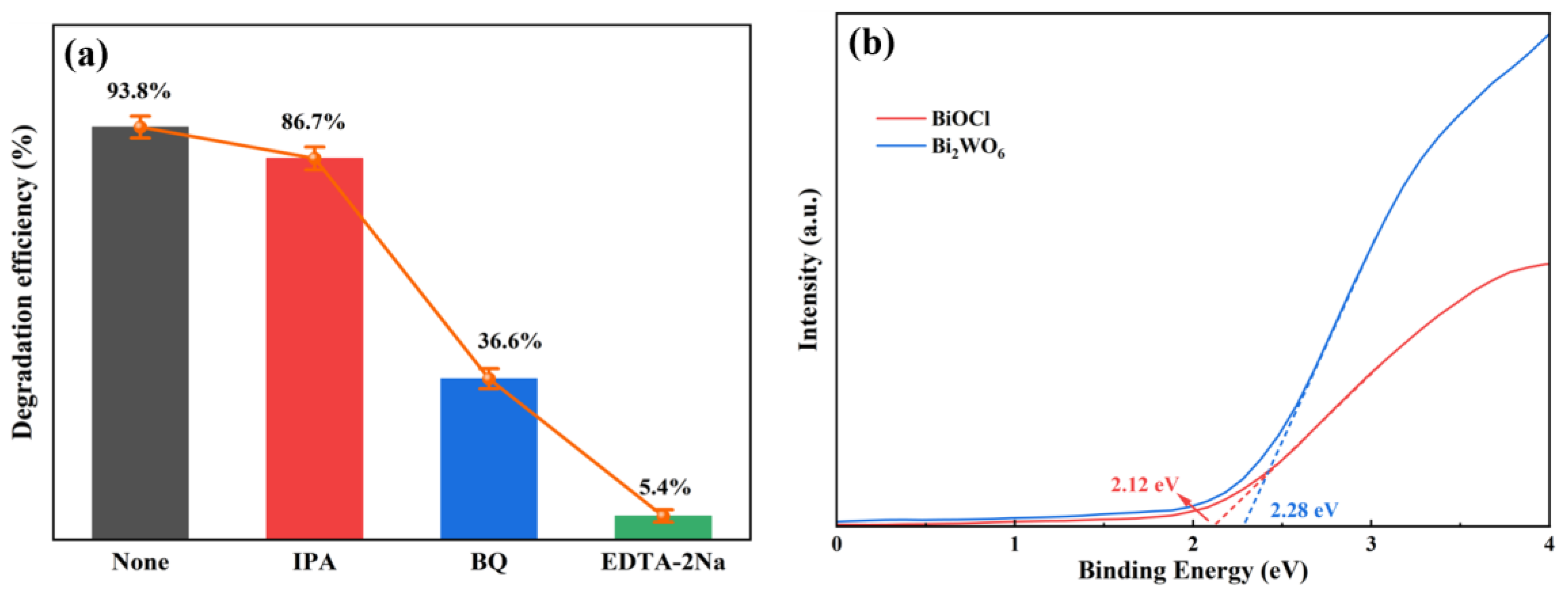
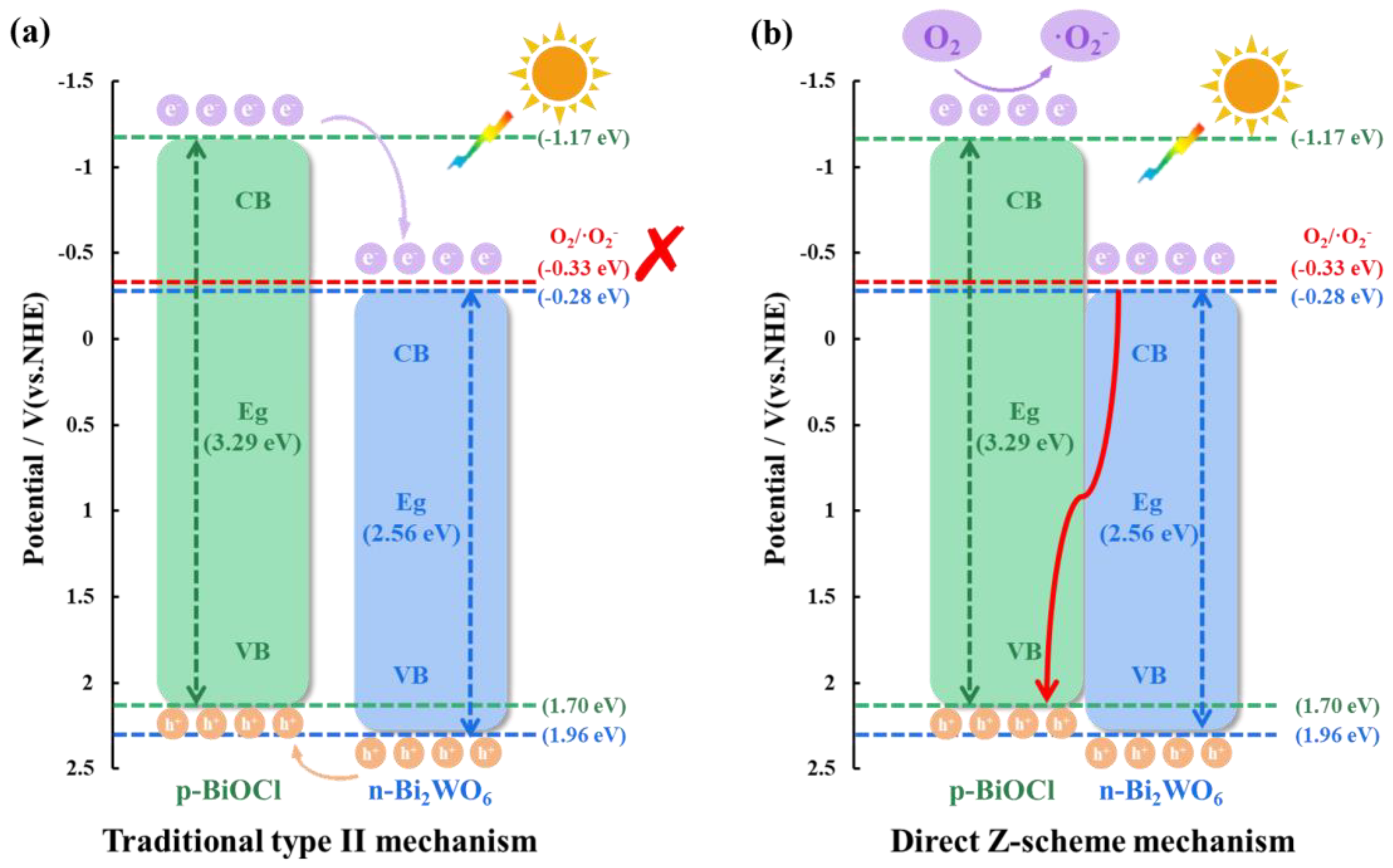
3.3. Mechanism of Enhanced Photoactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iskandar, K.; Murugaiyan, J.; Hammoudi Halat, D.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic discovery and resistance: The chase and the race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef]
- Hanna, N.; Tamhankar, A.J.; Stålsby Lundborg, C. Antibiotic concentrations and antibiotic resistance in aquatic environments of the WHO Western Pacific and South-East Asia regions: A systematic review and probabilistic environmental hazard assessment. Lancet Planet. Health 2023, 7, e45–e54. [Google Scholar] [CrossRef] [PubMed]
- Haciosmanoglu, G.G.; Mejias, C.; Martin, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review. J. Environ. Manag. 2022, 317, 115397. [Google Scholar] [CrossRef]
- Rathod, M.; Haldar, S.; Basha, S. Nanocrystalline cellulose for removal of tetracycline hydrochloride from water via biosorption: Equilibrium, kinetic and thermodynamic studies. Ecol. Eng. 2015, 84, 240–249. [Google Scholar] [CrossRef]
- Li, S.J.; Peng, L.; Yang, C.G.; Song, S.X.; Xu, Y.F. Cometabolic biodegradation of antibiotics by ammonia oxidizing microorganisms during wastewater treatment processes. J. Environ. Manag. 2022, 305, 114336. [Google Scholar] [CrossRef] [PubMed]
- Bueno, I.; He, H.; Kinsley, A.C.; Ziemann, S.J.; Degn, L.R.; Nault, A.J.; Beaudoin, A.L.; Singer, R.S.; Wammer, K.H.; Arnold, W.A. Biodegradation, photolysis, and sorption of antibiotics in aquatic environments: A scoping review. Sci. Total Environ. 2023, 897, 165301. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Peng, L.; Gu, Y.; Liang, C.Z.; Pott, R.W.M.; Xu, Y.F. Insights into biodegradation of antibiotics during the biofilm-based wastewater treatment processes. J. Clean Prod. 2023, 393, 136321. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Wang, Y.Y.; Zhou, S.; Jiang, X.X.; Ma, X.; Liu, C. Robust performance of a membrane bioreactor for removing antibiotic resistance genes exposed to antibiotics: Role of membrane foulants. Water Res. 2018, 130, 139–150. [Google Scholar] [CrossRef]
- Gu, W.Y.; Huang, X.Y.; Tian, Y.H.; Cao, M.; Zhou, L.; Zhou, Y.; Lu, J.; Lei, J.Y.; Zhou, Y.B.; Wang, L.Z.; et al. High-efficiency adsorption of tetracycline by cooperation of carbon and iron in a magnetic Fe/porous carbon hybrid with effective Fenton regeneration. Appl. Surf. Sci. 2021, 538, 147813. [Google Scholar] [CrossRef]
- Yang, Q.X.; Yan, Y.; Yang, X.F.; Liao, G.Y.; He, J.; Wang, D.S. The effect of complexation with metal ions on tetracycline degradation by Fe2+/3+ and Ru3+ activated peroxymonosulfate. Chem. Eng. J. 2022, 429, 132178. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. The performance of bismuth-based compounds in photocatalytic applications. Surf. Interfaces 2021, 23, 100927. [Google Scholar] [CrossRef]
- Wang, C.C.; Yan, R.Y.; Cai, M.J.; Liu, Y.P.; Li, S.J. A novel organic/inorganic S-scheme heterostructure of TCPP/Bi12O17Cl2 for boosting photodegradation of tetracycline hydrochloride: Kinetic, degradation mechanism, and toxic assessment. Appl. Surf. Sci. 2023, 610, 155346. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Tran, H.H.; Huynh, T.C.; Le, K.T.; Cao, T.M.; Pham, V.V. Harnessing potassium peroxymonosulfate activation of WO3/diatomite composites for efficient photocatalytic degradation of tetracycline. RSC Adv. 2024, 14, 25019–25030. [Google Scholar] [CrossRef]
- Xu, M.; Yang, J.K.; Sun, C.Y.; Liu, L.; Cui, Y.; Liang, B. Performance enhancement strategies of bi-based photocatalysts: A review on recent progress. Chem. Eng. J. 2020, 389, 124402. [Google Scholar] [CrossRef]
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent advancements in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M = W, Mo, Cr) for environmental remediation and clean energy production. J. Ind. Eng. Chem. 2021, 95, 1–15. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.Z.; Hu, C.; Huang, H.W. Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin. J. Catal. 2021, 42, 1413–1438. [Google Scholar] [CrossRef]
- Wu, S.J.; Sun, J.G.; Li, Q.; Hood, Z.D.; Yang, S.; Su, T.M.; Peng, R.; Wu, Z.; Sun, W.W.; Kent, P.R.C.; et al. Effects of surface terminations of 2D Bi2WO6 on photocatalytic hydrogen evolution from water splitting. ACS Appl. Mater. Interfaces 2020, 12, 20067–20074. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Liang, X.Z.; Zheng, H.L.; Liu, Y.Y.; Wang, Z.Y.; Wang, P.; Zhang, X.Y.; Qin, X.Y.; Dai, Y.; Whangbo, M.H.; et al. Photocatalytic reduction of CO2 to methanol by three-dimensional hollow structures of Bi2WO6 quantum dots. Appl. Catal. B-Environ. 2017, 219, 209–215. [Google Scholar] [CrossRef]
- Song, S.J.; Xing, Z.P.; Zhao, H.A.; Li, Z.Z.; Zhou, W. Recent advances in bismuth-based photocatalysts: Environment and energy applications. Green Energy Environ. 2023, 8, 1232–1264. [Google Scholar] [CrossRef]
- Yu, G.L.; Sun, Q.F.; Yang, Y.; Chen, S.; Long, Y.N.; Li, Y.F.; Ge, S.Y.; Zheng, D. BiOCl-based composites for photocatalytic degradation of antibiotics: A review of synthesis method, modification, factors affecting photodegradation and toxicity assessment. J. Alloy. Compd. 2024, 981, 173733. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Jasso-Salcedo, A.B.; Hedin, N.; Church, T.L.; Aizpuru, A.; Escobar-Barrios, V.A. Semiconducting nanocrystalline bismuth oxychloride (BiOCl) for photocatalytic reduction of CO2. Catalysts 2020, 10, 998. [Google Scholar] [CrossRef]
- Cao, J.Y.; Li, J.J.; Chu, W.; Cen, W.L. Facile synthesis of Mn-doped BiOCl for metronidazole photodegradation: Optimization, degradation pathway, and mechanism. Chem. Eng. J. 2020, 400, 125813. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, Y.J.; Wang, W.K.; Pei, D.N.; Huang, G.X.; Chen, J.J.; Zhang, X.; Yu, H.Q. Enhanced photocatalytic degradation of bisphenol A by Co-doped BiOCl nanosheets under visible light irradiation. Appl. Catal. B-Environ. 2018, 221, 320–328. [Google Scholar] [CrossRef]
- Cao, J.Y.; Cen, W.L.; Jing, Y.; Du, Z.X.; Chu, W.; Li, J.J. P-doped BiOCl for visible light photodegradation of tetracycline: An insight from experiment and calculation. Chem. Eng. J. 2022, 435, 134683. [Google Scholar] [CrossRef]
- Yang, B.; He, L.P.; Guo, Y.; Zhao, T.; Shi, Z.Z.; Liu, C.X.; Yang, M.; Yang, Q.; Sun, S.D.; Cui, J. Enhanced catalysis for degradation of antibiotic by hydroxyl-functionalized S-doped BiOCl with high capacity of local spatial charge separation. Colloid Surf. A-Physicochem. Eng. Asp. 2023, 669, 131448. [Google Scholar] [CrossRef]
- Hu, L.J.; Ding, Z.C.; Yan, F.; Li, K.; Feng, L.; Wang, H.Q. Construction of hexagonal prism-like defective BiOCl hierarchitecture for photocatalytic degradation of tetracycline hydrochloride. Nanomaterials 2022, 12, 2700. [Google Scholar] [CrossRef]
- He, X.H.; Kai, T.H.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Li, S.J.; Cai, M.J.; Liu, Y.P.; Wang, C.C.; Yan, R.Y.; Chen, X.B. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr(VI) reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Fenelon, E.; Bui, D.P.; Tran, H.H.; You, S.J.; Wang, Y.F.; Cao, T.M.; Van Pham, V. Straightforward synthesis of SnO2/Bi2S3/BiOCl-Bi24O31Cl10 composites for drastically enhancing rhodamine B photocatalytic degradation under visible light. ACS Omega 2020, 5, 20438–20449. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, Z.; Wu, C.; Zuo, H.; Liu, J.; Chang, X.; Yan, Q. Construction of BiOCl/Bi2WO6 Z-scheme heterojunction with close interfacial contact using CNT as electron medium. Colloid Surf. A-Physicochem. Eng. Asp. 2024, 681, 132847. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Y. Construction of a flower-like S-scheme Bi2WO6/BiOCl nano-heterojunction with enhanced visible-light photocatalytic properties. New J. Chem. 2022, 46, 21418–21427. [Google Scholar] [CrossRef]
- Xu, M.; Lu, M.; Yang, Y.; Ai, L.; Fan, H.; Guo, N.; Wang, L. Efficient degradation of pollutants by Bi2WO6/BiOCl heterojunction activated peroxymonosulfate: Performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 112156. [Google Scholar] [CrossRef]
- Sun, Z.H.; Guo, J.J.; Zhu, S.M.; Mao, L.; Ma, J.; Zhang, D. A high-performance Bi2WO6-graphene photocatalyst for visible light-induced H2 and O2 generation. Nanoscale 2014, 6, 2186–2193. [Google Scholar] [CrossRef]
- Zhou, H.R.; Wen, Z.P.; Liu, J.; Ke, J.; Duan, X.G.; Wang, S.B. Z-scheme plasmonic Ag decorated WO3/Bi2WO6 hybrids for enhanced photocatalytic abatement of chlorinated-VOCs under solar light irradiation. Appl. Catal. B-Environ. 2019, 242, 76–84. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Z.; Qu, D.; Shi, J. Synthesis of chemically bonded BiOCl@Bi2WO6 microspheres with exposed (020) Bi2WO6 facets and their enhanced photocatalytic activities under visible light irradiation. Appl. Surf. Sci. 2016, 361, 63–71. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, J.J.; Yu, H.G.; Yu, J.G. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Song, J.M.; Mao, C.J.; Niu, H.L.; Shen, Y.H.; Zhang, S.Y. Hierarchical structured bismuth oxychlorides: Self-assembly from nanoplates to nanoflowers via a solvothermal route and their photocatalytic properties. Crystengcomm 2010, 12, 3875–3881. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, W.T.; Jie, H.N.; Tang, W.Y.; Chen, D.Y.; Ruan, T.; Bai, H.P. Degradation of norfloxacin by copper-doped Bi2WO6-induced sulfate radical-based visible light-Fenton reaction. RSC Adv. 2020, 10, 38024–38032. [Google Scholar] [CrossRef]
- Kan, L.; Chang, C.; Wang, Q.; Wang, X. Glycol assisted splitting BiOIO3 into plasmonic bismuth coupled with BiOI co-modified Bi2WO6 (BiOI/Bi/Bi2WO6) to form indirect Z-scheme heterojunction for efficient photocatalytic degradation of BPA. Sep. Purif. Technol. 2022, 297, 121537. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Fu, R.R.; Zeng, X.Q.; Ma, L.; Gao, S.M.; Wang, Q.Y.; Wang, Z.Y.; Huang, B.B.; Dai, Y.; Lu, J. Enhanced photocatalytic and photoelectrochemical activities of reduced TiO2-x/BiOCl heterojunctions. J. Power Sources 2016, 312, 12–22. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, W.; Tian, Z.; Zhao, Y.; Shen, Z. Facile fabrication of Bi2WO6/BiOCl hierarchical structure as adsorbents for methylene blue dye removal. Mater. Res. Express 2019, 6, 055034. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Fang, M.; Li, L.; Fu, F.; Xu, H.; Li, M.; Fan, X. A compact Z-scheme heterojunction of BiOCl/Bi2WO6 for efficiently photocatalytic degradation of gaseous toluene. J. Colloid Interface Sci. 2023, 631, 44–54. [Google Scholar] [CrossRef]
- Pham, M.-T.; Tran, H.-H.; Nguyen, T.-M.T.; Bui, D.-P.; Huang, Y.; Cao, J.; You, S.-J.; Van Viet, P.; Nam, V.H.; Wang, Y.-F. Revealing DeNOx and DeVOC reactions via the study of the surface and bandstructure of ZnSn(OH)6 photocatalysts. Acta Mater. 2021, 215, 117068. [Google Scholar] [CrossRef]
- Pham, M.T.; Nguyen, T.M.T.; Bui, D.P.; Wang, Y.F.; Tran, H.H.; You, S.J. Enhancing quantum efficiency at Ag/g-C3N4 interfaces for rapid removal of nitric oxide under visible light. Sustain. Chem. Pharm. 2022, 25, 9. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Li, M.Q.; Shuai, D.M.; Zhou, X.Y.; Xiong, X.Y.; Wang, C.; Hu, Q. Continuous photocatalysis via photo-charging and dark-discharging for sustainable environmental remediation: Performance, mechanism, and influencing factors. J. Hazard. Mater. 2021, 420, 126607. [Google Scholar] [CrossRef]
- Bognár, S.; Putnik, P.; Maksimovic, I.; Velebit, B.; Putnik-Delic, M.; Merkulov, D.S. Sustainable removal of tolperisone from waters by application of photocatalysis, nanotechnology, and chemometrics: Quantification, environmental toxicity, and degradation optimization. Nanomaterials 2022, 12, 4199. [Google Scholar] [CrossRef]
- Jovanović, D.; Orčić, D.; Šojić Merkulov, D.; Despotović, V.; Finčur, N. Study of factors impacting the light-driven removal of ciprofloxacin (Ciprocinal®) and identification of degradation products using LC–ESI–MS2. J. Photochem. Photobiol. A-Chem. 2025, 460, 116119. [Google Scholar] [CrossRef]
- Ren, H.T.; Qi, F.; Labidi, A.; Zhao, J.J.; Wang, H.; Xin, Y.; Luo, J.M.; Wang, C.Y. Chemically bonded carbon quantum dots/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic degradation: Interfacial engineering and mechanism insight. Appl. Catal. B-Environ. 2023, 330, 122587. [Google Scholar] [CrossRef]
- Guo, Y.; Ao, Y.H.; Wang, P.F.; Wang, C. Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocatalysts with enhanced visible-light-driven performance towards carbamazepine degradation. Appl. Catal. B-Environ. 2019, 254, 479–490. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Lin, Z.D.; Li, Z.S.; Pan, W.G. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, Z.; Huang, Y.; Yu, J.; Li, M. Construction of Z-Scheme Heterojunction BiOCl/Bi2WO6 for Visible-Light Photocatalytic Degradation of Tetracycline Hydrochloride. Separations 2025, 12, 111. https://doi.org/10.3390/separations12050111
Zhang H, Zhu Z, Huang Y, Yu J, Li M. Construction of Z-Scheme Heterojunction BiOCl/Bi2WO6 for Visible-Light Photocatalytic Degradation of Tetracycline Hydrochloride. Separations. 2025; 12(5):111. https://doi.org/10.3390/separations12050111
Chicago/Turabian StyleZhang, Hetian, Zengying Zhu, Yajie Huang, Jiaxing Yu, and Ming Li. 2025. "Construction of Z-Scheme Heterojunction BiOCl/Bi2WO6 for Visible-Light Photocatalytic Degradation of Tetracycline Hydrochloride" Separations 12, no. 5: 111. https://doi.org/10.3390/separations12050111
APA StyleZhang, H., Zhu, Z., Huang, Y., Yu, J., & Li, M. (2025). Construction of Z-Scheme Heterojunction BiOCl/Bi2WO6 for Visible-Light Photocatalytic Degradation of Tetracycline Hydrochloride. Separations, 12(5), 111. https://doi.org/10.3390/separations12050111





