Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction
Abstract
1. Introduction
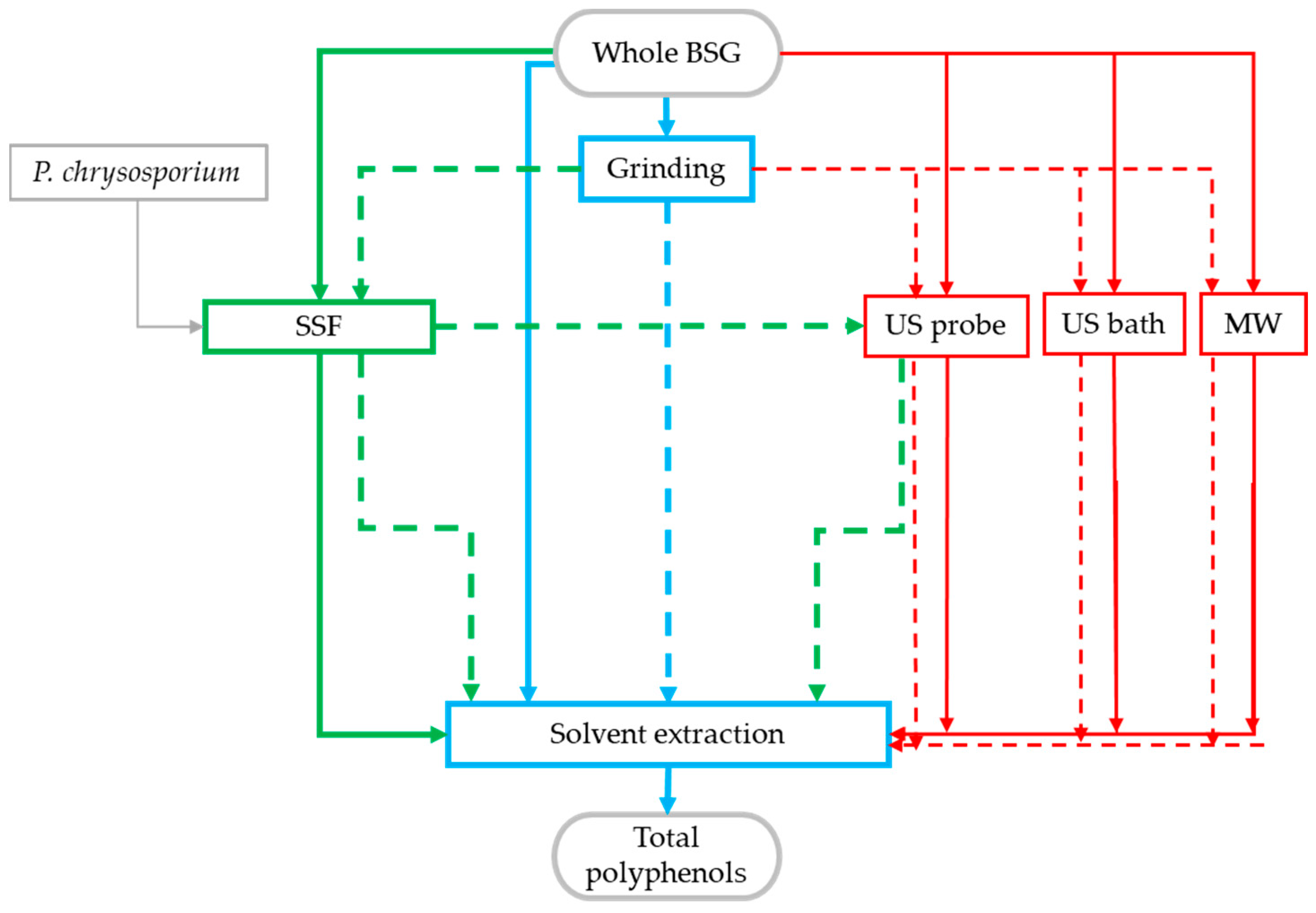
2. Materials and Methods
2.1. Chemicals
2.2. Microorganism
2.3. BSG
2.4. SSF
SSF Monitoring: Respirometric Determination
2.5. Total Polyphenol Recovery
2.5.1. Solvent Extraction
2.5.2. MW- and US-Assisted Extractions
2.6. BSG Water Extract
2.7. Analytical Determinations on BSG and BSG Extracts
2.7.1. Moisture% of BSG
2.7.2. pH
2.7.3. Total Proteins
2.7.4. Laccase Activity
2.7.5. Degrees Brix
2.7.6. D-Glucose Content
2.7.7. Total Phenolic Content (TPC)
2.8. Data Analysis and Statistics
3. Results
3.1. BSG Characterization
3.2. TPCs of Pre-Treated wBSG and gBSG
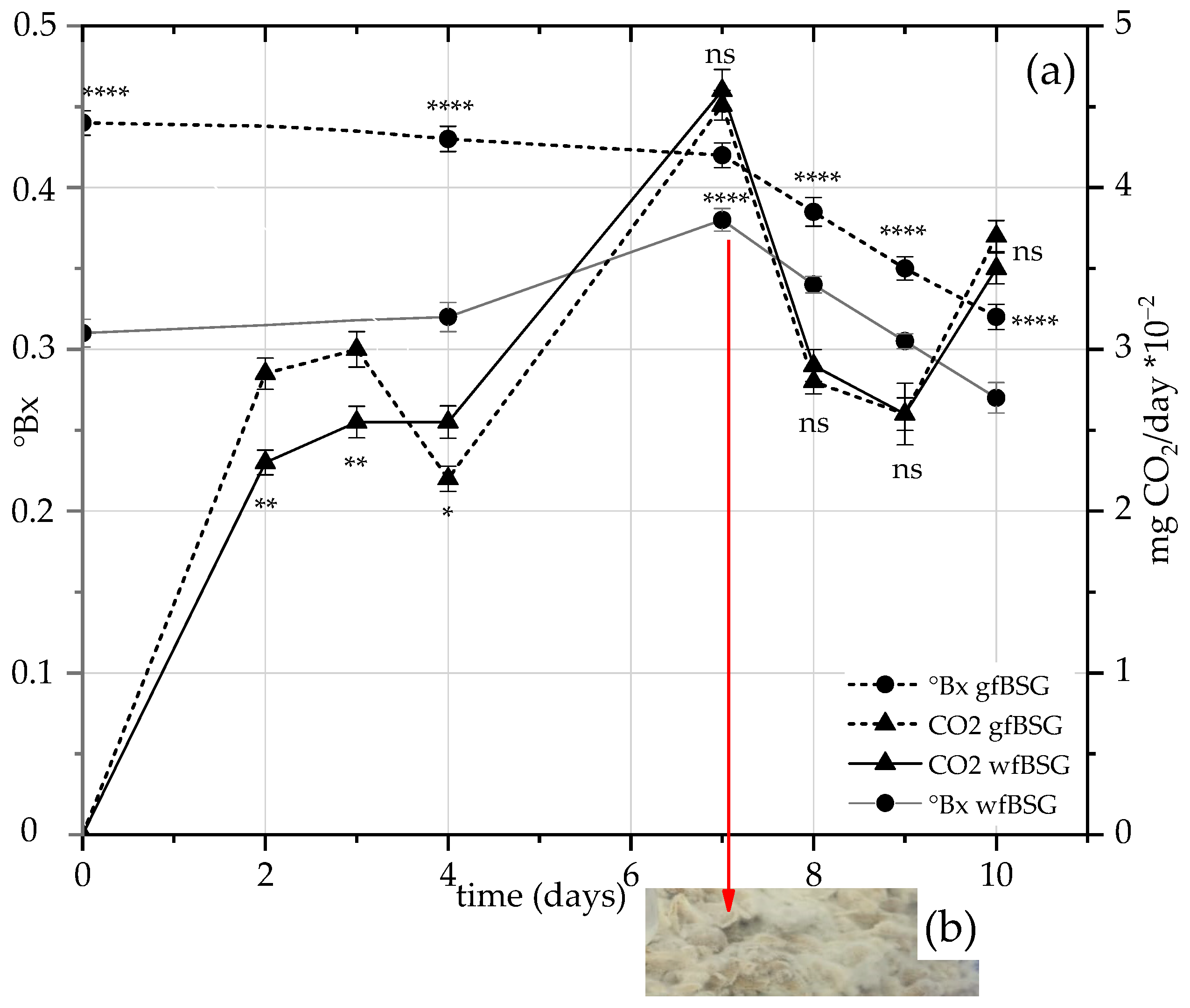
3.3. SSF in Microcosms with P. chrysosporium
3.3.1. TPCs of wfBSG and gfBSG: Test 1
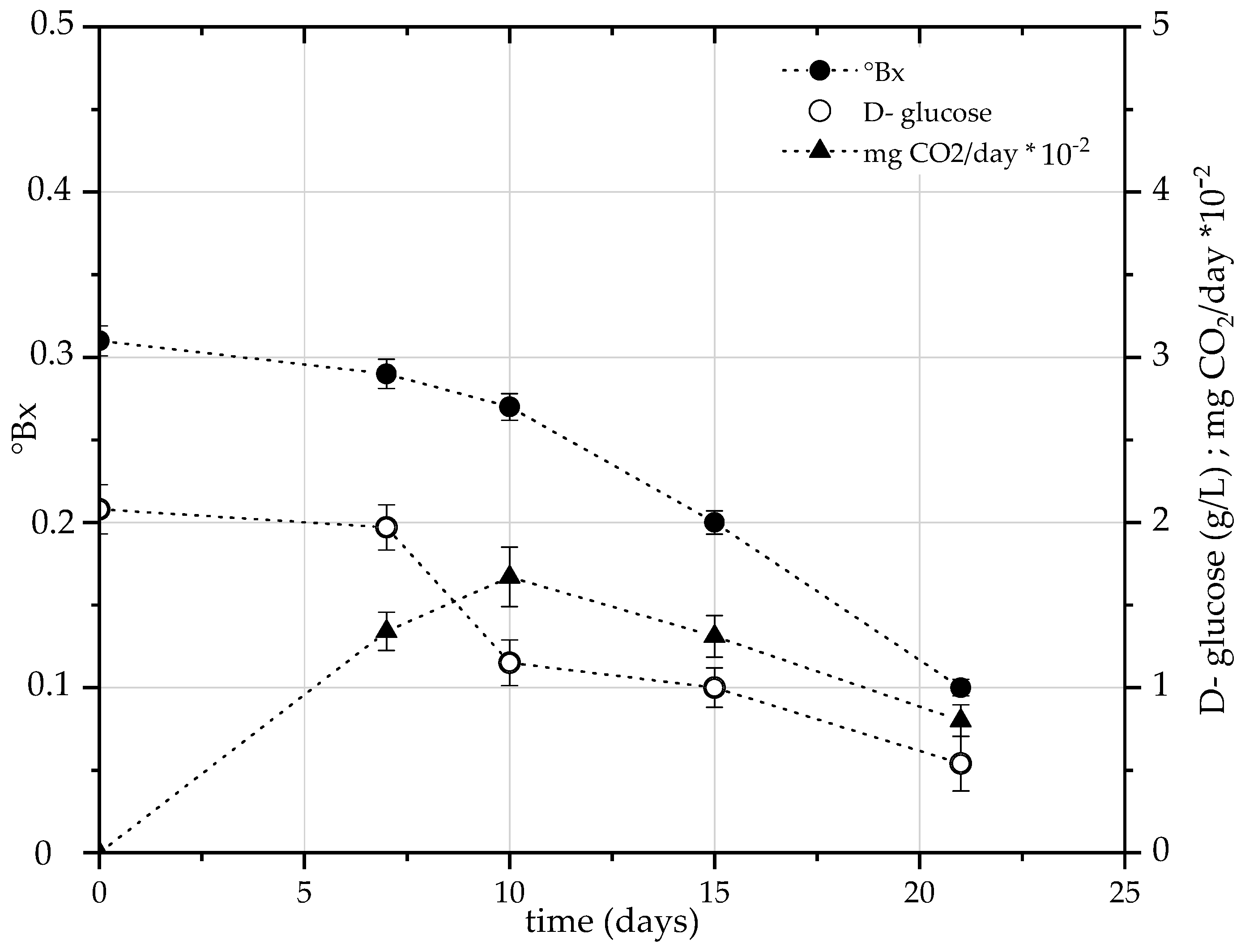
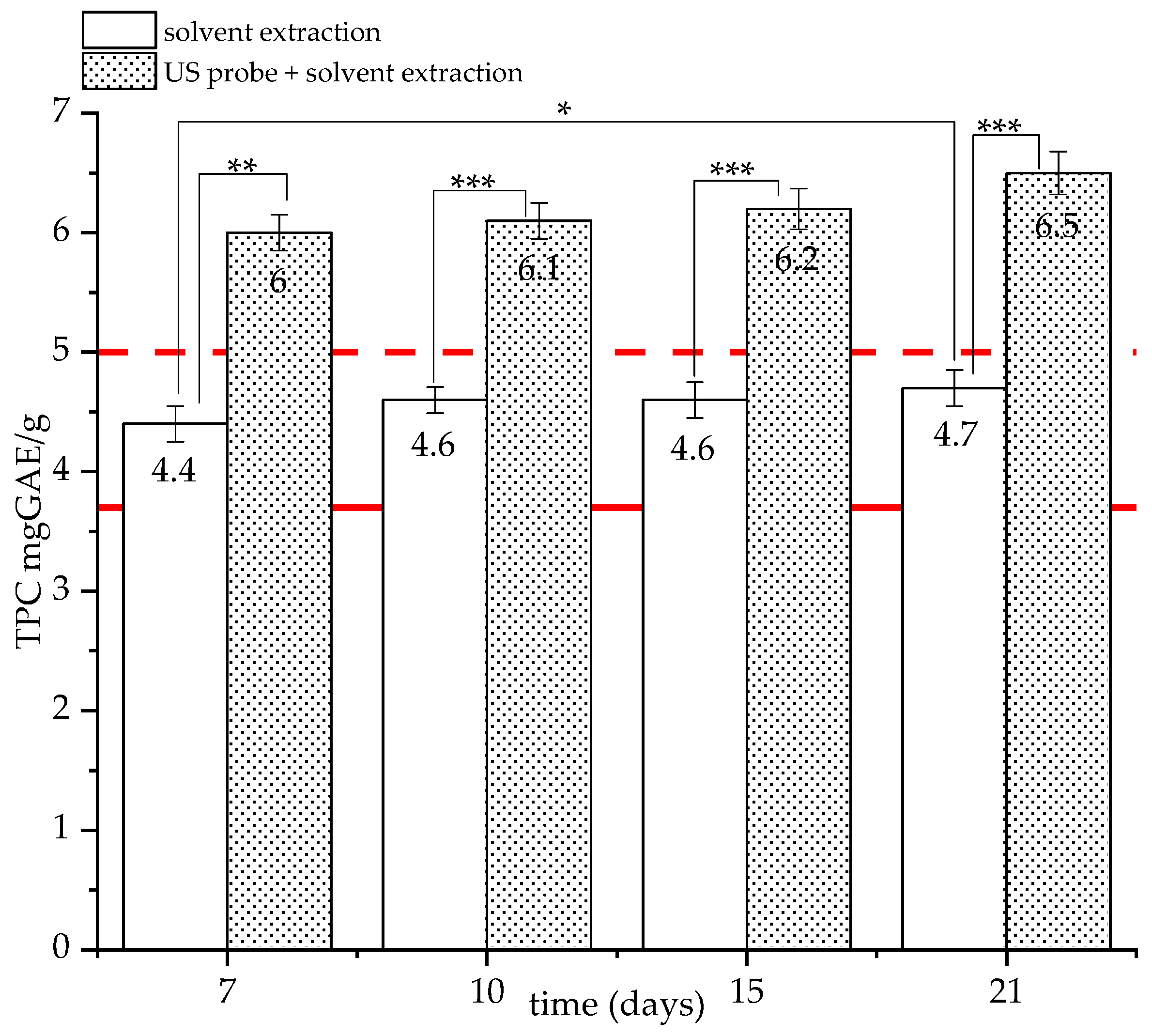
3.3.2. Effect of US Probe Pre-Treatment on TPC of gfBSG: SSF Test 2
4. Discussion
4.1. BSG Characterization
TPCs of wBSG and gBSG
4.2. BSG Physical Pre-Treatments for TPC Improvement
4.3. TPC of wfBSG and gfBSG: Test 1
4.4. Effect of US Probe Pre-Treatment on TPC of gfBSG: SSF Test 2
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| °Bx | Degrees Brix |
| BSG | Brewer’s spent grain |
| gBSG | Ground BSG |
| gfBSG | Ground fermented BSG |
| MEA | Malt extract agar |
| MW | Microwave |
| OD600 | Optical density at 600 nm |
| SSF | Solid-state fermentation |
| US | Ultrasound |
| wBSG | Whole BSG |
| wfBSG | Whole fermented BSG |
References
- Xie, Z.; Dan, M.; Zhao, G.; Wang, D. Recent advances in microbial high-value utilization of brewer’s spent grain. Biores. Technol. 2024, 408, 131197. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.B.; Wu, J. Food-based uses of brewers spent grains: Current applications and future possibilities. Food Biosci. 2023, 54, 102774. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afric. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- McCarthy, A.L.; O’Callaghan, Y.C.; Connolly, A.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Phenolic extracts of brewers’ spent grain (BSG) as functional ingredients—Assessment of their DNA protective effect against oxidant-induced DNA single strand breaks in U937 cells. Food Chem. 2012, 134, 641–646. [Google Scholar] [CrossRef]
- Guido, L.F.; Moreira, M.M. Techniques for Extraction of Brewer’s Spent Grain Polyphenols: A Review. Food Bioproc. Technol. 2017, 10, 1192–1209. [Google Scholar] [CrossRef]
- Moirangthem, K.; Koirala, P.; Maina, H.N.; Rai, D.K.; Coda, R. Impact of pre-treatments and bioprocessing on the carbohydrate and polyphenol profile of brewers’ spent grain. Food Bioprod. Process. 2024, 148, 62–71. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of Polyphenols from Brewer’s Spent Grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Morais, S.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. A novel application of microwave-assisted extraction of polyphenols from brewer’s spent grain with HPLC-DAD-MS analysis. Anal. Bioanal. Chem. 2012, 403, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Sologubik, C.A.; Fernández, M.B.; Manrique, G.D.; D’Alessandro, L.G. Extraction of Antioxidant Phenolic Compounds from Brewer’s Spent Grain: Optimization and Kinetics Modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of Phenolic Compound Extraction from Brewers’ Spent Grain Using Ultrasound Technologies Coupled with Response Surface Methodology. Sustainability 2022, 14, 3309. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Ramos, C.; Trigueros, E.; Beltrán, S.; Sanz, M.T. Study of subcritical water scale-up from laboratory to pilot system for brewer’s spent grain valorization. Ind. Crops Prod. 2023, 191, 115927. [Google Scholar] [CrossRef]
- Lock, T.J.; Mah, S.H.; Lai, Z.W. Versatile Applications of Brewer’s Spent Grain: Solid-State Fermentation and Nutritional Added Value. Appl. Biochem. Biotechnol. 2024, 196, 5508–5532. [Google Scholar] [CrossRef] [PubMed]
- da Costa Maia, I.; Thomaz dos Santos D’Almeida, C.; Guimarães Freire, D.M.; d’Avila Costa Cavalcanti, E.; Cameron, L.C.; Furtado Dias, J.; Larraz Ferreira, M.S. Effect of solid-state fermentation over the release of phenolic compounds from brewer’s spent grain revealed by UPLC-MSE. LWT 2020, 133, 110136. [Google Scholar] [CrossRef]
- Lonsane, B.K.; Ghildyal, N.P.; Budiatman, S.; Ramakrishna, S.V. Engineering aspects of solid state fermentation. Enzym. Microb. Technol. 1985, 7, 258–265. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Cebrián, M.; Ibarruri, J. Filamentous fungi processing by solid-state fermentation. In Current Developments in Biotechnology and Bioengineering; Taherzadeh, M.J., Ferreira, J.A., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 251–292. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, A. A biorefinery approach for the production of xylitol, ethanol and polyhydroxybutyrate from brewer’s spent grain. AIMS Agric. Food 2016, 1, 52–66. [Google Scholar] [CrossRef]
- Leite, P.; Cátia Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Bošnjaković, A.; Planinić, M.; Parlov Vuković, J.; Novak, P.; Jednačak, T.; Tišma, M. Improvement of the Nutraceutical Profile of Brewer’s Spent Grain after Treatment with Trametes versicolor. Microorganisms 2022, 10, 2295. [Google Scholar] [CrossRef]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes versicolor in lignocellulose-based bioeconomy: State of the art, challenges and opportunities. Biores. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Ramirez, J.G.; Vasquez-Lara, F.; Sanchez-Estrada, A.; Troncoso-Rojas, R.; Heredia-Olea, E.; Islas-Rubio, A.R. Arabinoxylans Release from Brewers’ Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium oxysporum and the Antioxidant Capacity of the Extracts. Foods 2022, 11, 1415. [Google Scholar] [CrossRef]
- Mollea, C.; Bosco, F. Natural rubber biodegradation by Alternaria alternata and Penicillium chrysogenum isolates. Biorem. J. 2020, 24, 112–128. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of Phenolic Acid and Enzyme Production from Corn Silage Biologically Treated by Trametes versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Biores. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef]
- Nagai, M.; Kawata, M.; Watanabe, H.; Ogawa, M.; Saito, K.; Takesawa, T.; Kanda, K.; Sato, T. Important role of fungal intracellular laccase for melanin synthesis: Purification and characterization of an intracellular laccase from Lentinula edodes fruit bodies. Microbiology 2003, 149 Pt 9, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Samaras, T.S.; Camburn, P.A.; Chandra, S.X.; Gordon, M.H.; Ames, J.M. Antioxidant properties of kilned and roasted malts. J. Agric. Food Chem. 2005, 53, 8068–8074. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Lan, Y.; Ohm, J.B.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical composition, fermentable sugars, free amino acids, phenolics, and minerals in brewers’ spent grains obtained from craft brewing operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Liziad, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromat. 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Mojzer, B.E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2010, 31, 227–249. [Google Scholar] [CrossRef]
- Santi Stefanello, F.; Obem dos Santos, C.; Bochi, V.C.; Burin Fruet, A.P.; Bromenberg Soquetta, M.; Dörr, A.C.; Nörnberg, J.L. Analysis of polyphenols in brewer’s spent grain and its comparison with corn silage and cereal brans commonly used for animal nutrition. Food Chem. 2018, 239, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Ince, A.E.; Sahin, S.; Sumnu, G. Comparison of microwave and ultrasound-assisted extraction techniques for leaching of phenolic compounds from nettle. J. Food Sci. Technol. 2014, 51, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Zalazar-García, D.; Torres, E.; Rodriguez-Ortiz, L.; Deng, Y.; Soria, J.; Bucalá, V.; Rodriguez, R.; Mazza, G. Cleaner and sustainable processes for extracting phenolic compounds from bio-waste. J. Environ. Manag. 2020, 273, 111154. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.; Fox, G. Fungal Biovalorization of a Brewing Industry Byproduct, Brewer’s Spent Grain: A Review. Foods 2021, 10, 2159. [Google Scholar] [CrossRef] [PubMed]
- Muller dos Santos, M.; Souza da Rosa, A.; Dal’Boit, S.; Mitchell, D.A.; Krieger, N. Thermal denaturation: Is solid-state fermentation really a good technology for the production of enzymes? Biores. Technol. 2004, 93, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Biotransformation of lignocellulosic materials into value-added products—A review. Int. J. Biol. Macromol. 2017, 98, 447–458. [Google Scholar] [CrossRef]



| (A) | ||||
| SSF Test 1 | ||||
| Abiotic Controls | Biotic Samples | |||
| BSG Type (40 g) | Control wBSG | Control gBSG | wfBSG | gfBSG |
| Inoculum | water (3 mL) | standardized inoculum (3 mL) | ||
| Temperature | 39 °C (till the 2nd day), shift to 30 °C (till the 10th day) | |||
| Duration | 10 days | |||
| (B) | ||||
| SSF Test 2 | ||||
| Abiotic Controls | Biotic Samples | |||
| BSG type (40 g) | Control gBSG | gfBSG | ||
| Inoculum | water (3 mL) | standardized inoculum (3 mL) | ||
| Water added to gBSG | 2 mL | 2 mL | ||
| Temperature | 30 °C | |||
| Duration | 21 days | |||
| Parameters | wBSG | gBSG |
|---|---|---|
| Moisture% | 62 ± 2% | 74.3 ± 3% |
| pH * | 5.4 ± 0.3 | 5.8 ± 0.2 |
| Total proteins *, g/gDW | 0.009 ± 0.001 | 0.01 ± 0.001 |
| °Bx * | 0.26 ± 0.2 | 0.30 ± 0.1 |
| D-glucose *, g/L | 2.05 | 2.08 |
| TPC **, mg GAE/gDW | 3.4 ± 0.2 | 4 ± 0.2 |
| wBSG | gBSG | |||
|---|---|---|---|---|
| Without Pre-Treatment | 3.4 ± 0.41 | 4.0 ± 0.52 | ||
| Pre-Treatment | Power (W) | Time (Min) | ||
| MW | 400 | 1 | 3.7 ± 0.45 | 4.0 ± 0.56 |
| 800 | 4.0 ± 0.51 | 4.8 ± 0.61 | ||
| US bath | 550 | 20 | 3.6 ± 0.42 | 4.0 ± 0.49 |
| 40 | 3.5 ± 0.41 | 4.1 ± 0.50 | ||
| US probe | 250 | 15 | 4.8 ± 0.50 | 5.8 ± 0.70 |
| 30 | 5.0 ± 0.60 | 5.9 ± 0.66 | ||
| (A) | Moisture% | pH | ||
| Day | wfBSG | gfBSG | wfBSG | gfBSG |
| 0 | 62.3 ± 3 | 77.3 ± 4 | 6.01 ± 0.27 | 6.12 ± 0.29 |
| 4 | 75.1 ± 3.6 | 78.3 ± 4.5 | 6.07 ± 0.30 | 6.14 ± 0.29 |
| 7 | 77.2 ± 4 | 78.2 ± 4.7 | 6.13 ± 0.28 | 6.44 ± 0.36 |
| 10 | 77.3 ± 4 | 79.0 ± 4 | 6.12 ± 0.29 | 6.22 ± 0.31 |
| (B) | Total Proteins (g/gDW) | Laccase (UI/L) | ||
| Day | wfBSG | gfBSG | wfBSG | gfBSG |
| 0 | 0.01 ± 0.0016 | 0.01 ± 0.0019 | 0 | 0 |
| 4 | 0.014 ± 0.0022 | 0.020 ± 0.0032 | 0.025 ± 0.0079 | 0.036 ± 0.0057 |
| 7 | 0.021 ± 0.0034 | 0.027 ± 0.0043 | 1.21 ± 0.0085 | 1.35 ± 0.0099 |
| 10 | 0.023 ± 0.0040 | 0.032 ± 0.0051 | 0.90 ± 0.0066 | 1.12 ± 0.0019 |
| Moisture% | pH | Total Proteins (g/gDW) | Laccase (UI/L) | |
|---|---|---|---|---|
| Day | ||||
| 0 | 71.4 ± 3.8 | 5.6 ±0.27 | 0.012 ± 0.0019 | 0 |
| 7 | 76.9 ± 2.8 | 4.6 ± 0.34 | 0.019 ± 0.0027 | 1.09 ± 0.0050 |
| 10 | 77.4 ± 2.9 | 4.9 ± 0.33 | 0.025 ± 0.0032 | 1.32 ± 0.0039 |
| 15 | 78.7 ± 3.7 | 4.7 ± 0.29 | 0.033 ± 0.0029 | 1.07 ± 0.0061 |
| 21 | 81.2 ± 2.5 | 5.8 ± 0.31 | 0.032 ± 0.0041 | 0.85 ± 0.0044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollea, C.; Bosco, F. Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction. Separations 2025, 12, 58. https://doi.org/10.3390/separations12030058
Mollea C, Bosco F. Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction. Separations. 2025; 12(3):58. https://doi.org/10.3390/separations12030058
Chicago/Turabian StyleMollea, Chiara, and Francesca Bosco. 2025. "Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction" Separations 12, no. 3: 58. https://doi.org/10.3390/separations12030058
APA StyleMollea, C., & Bosco, F. (2025). Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction. Separations, 12(3), 58. https://doi.org/10.3390/separations12030058






