Exploring Chicken Feathers as a Cost-Effective Adsorbent for Aqueous Dye Removal
Abstract
1. Introduction
2. Materials and Methods
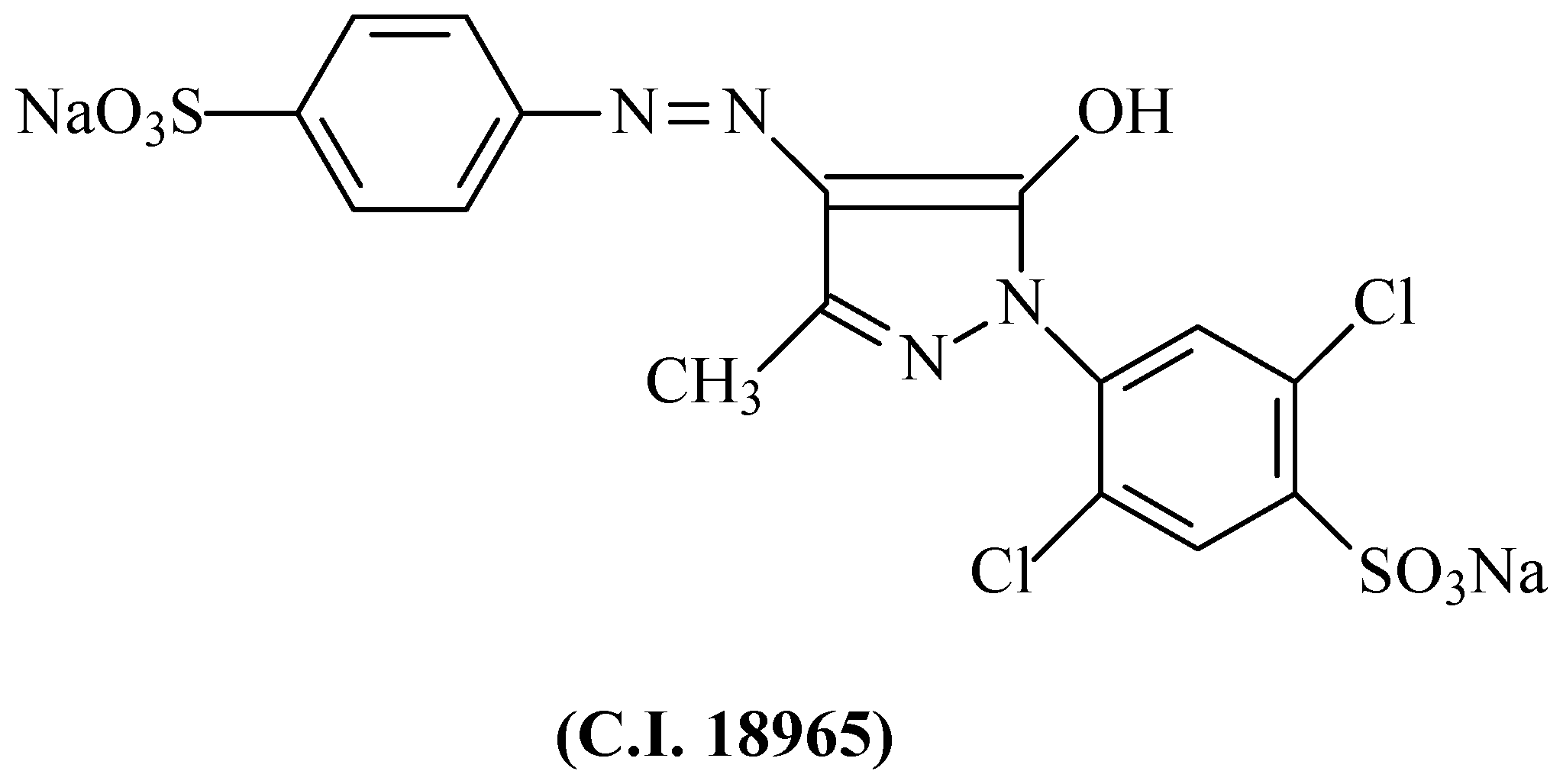
2.1. Dye
2.2. Adsorbent
2.3. Kinetic Experiments
2.4. Influence of Activation
2.5. Adsorption Experiments
2.6. Adsorption Isotherms
3. Results and Discussion
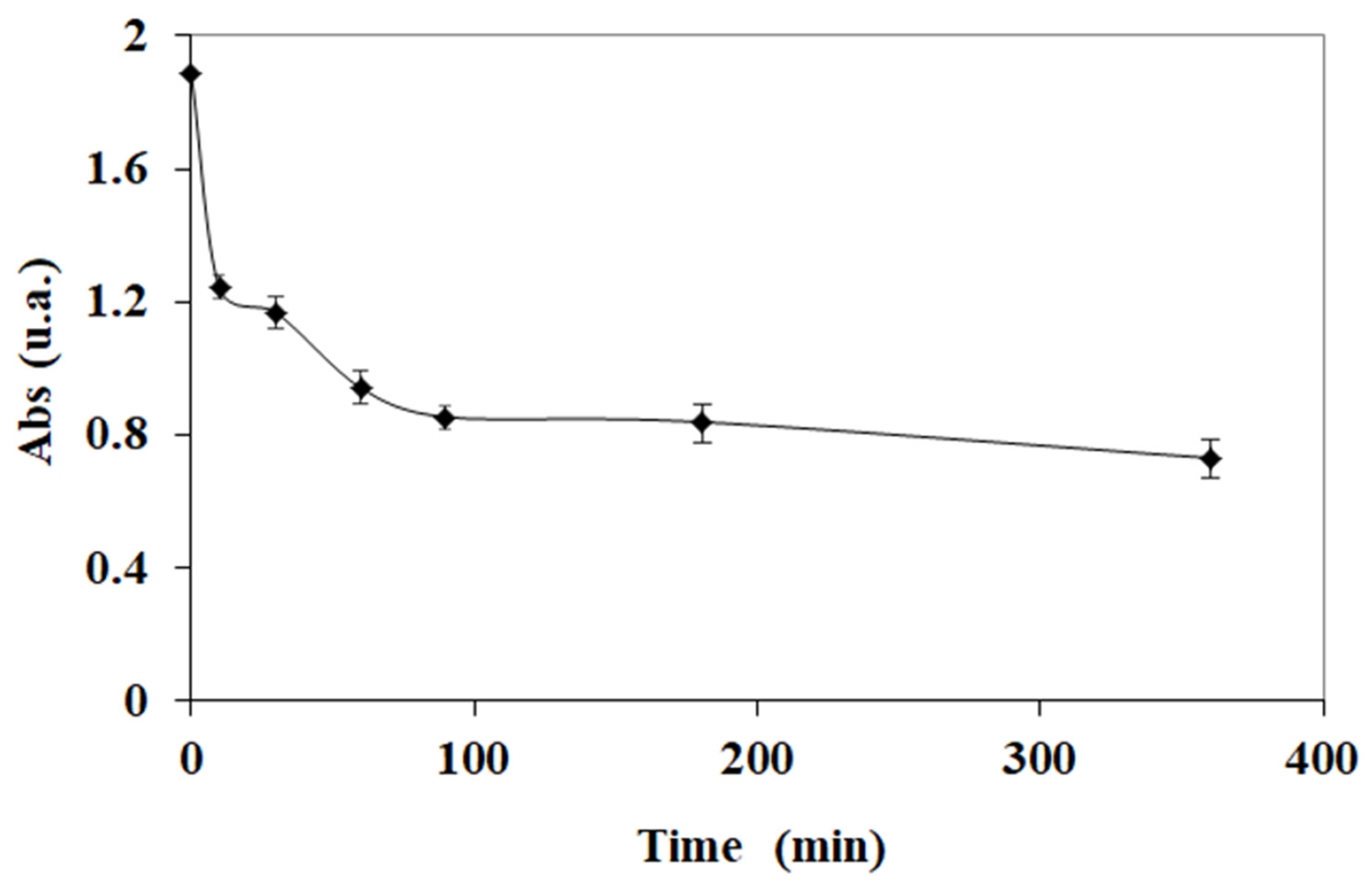
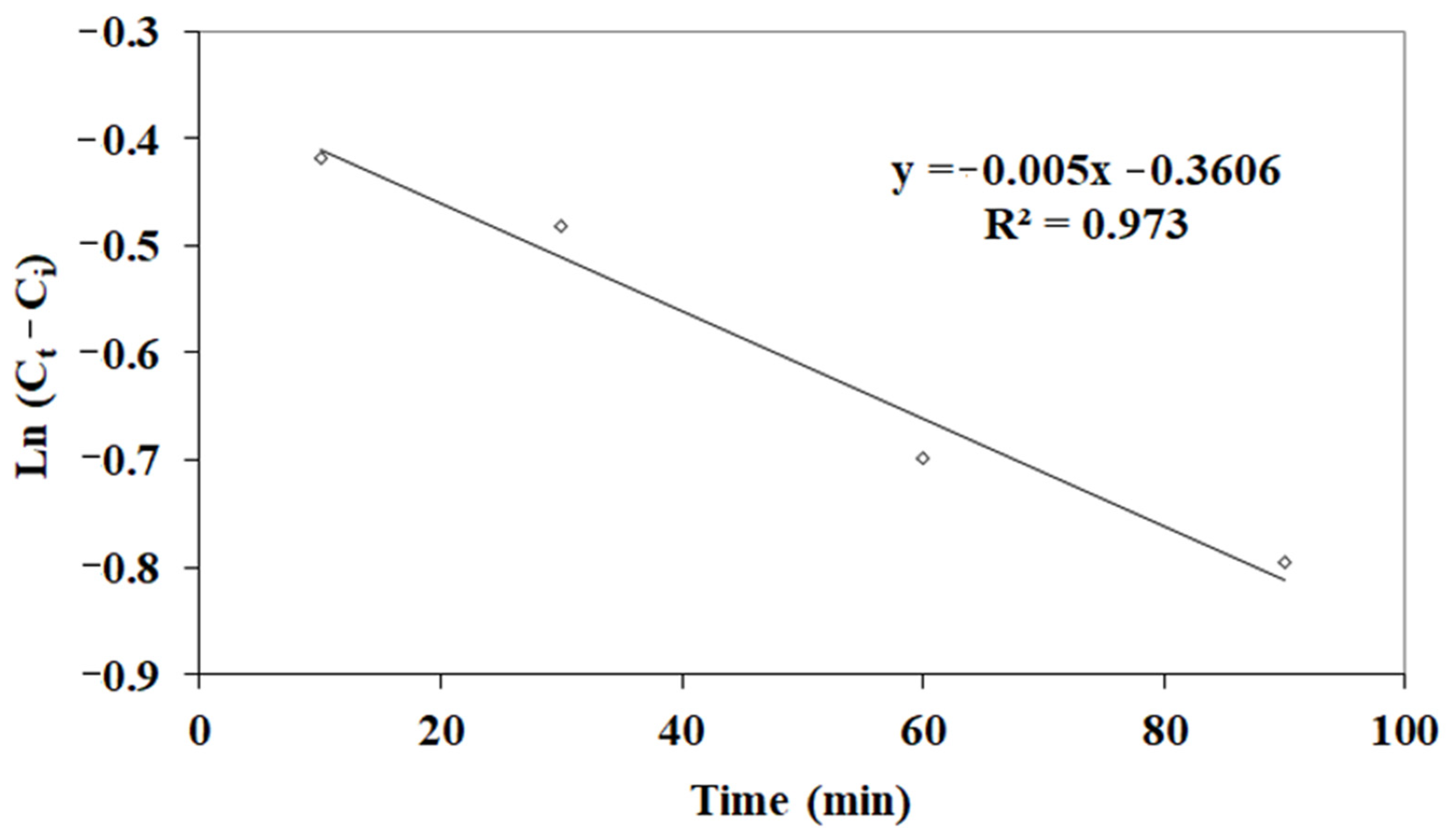
3.1. Kinetic Studies
3.2. Influence of Activation
3.3. Adsorption Tests: Evaluation of Temperature and pH
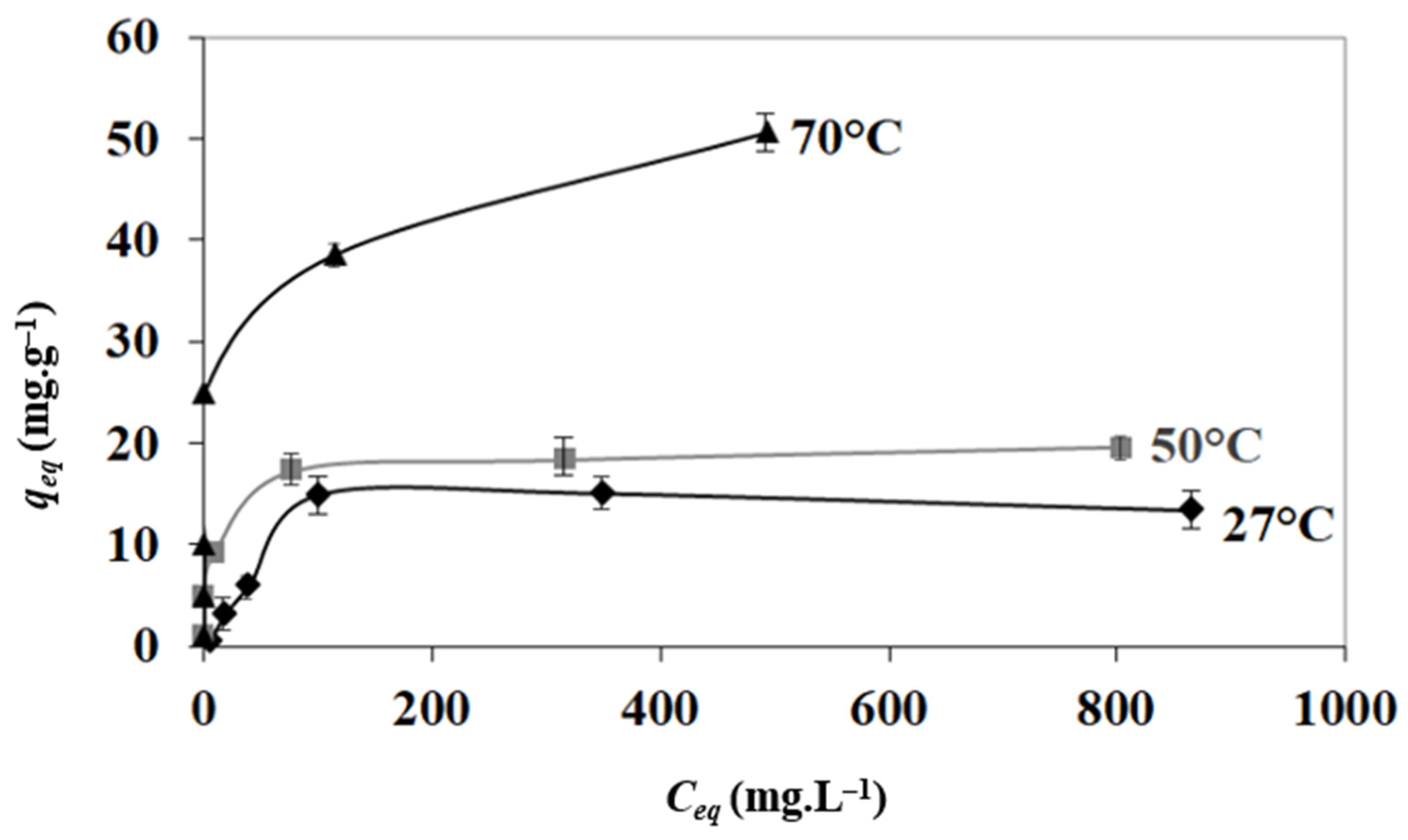
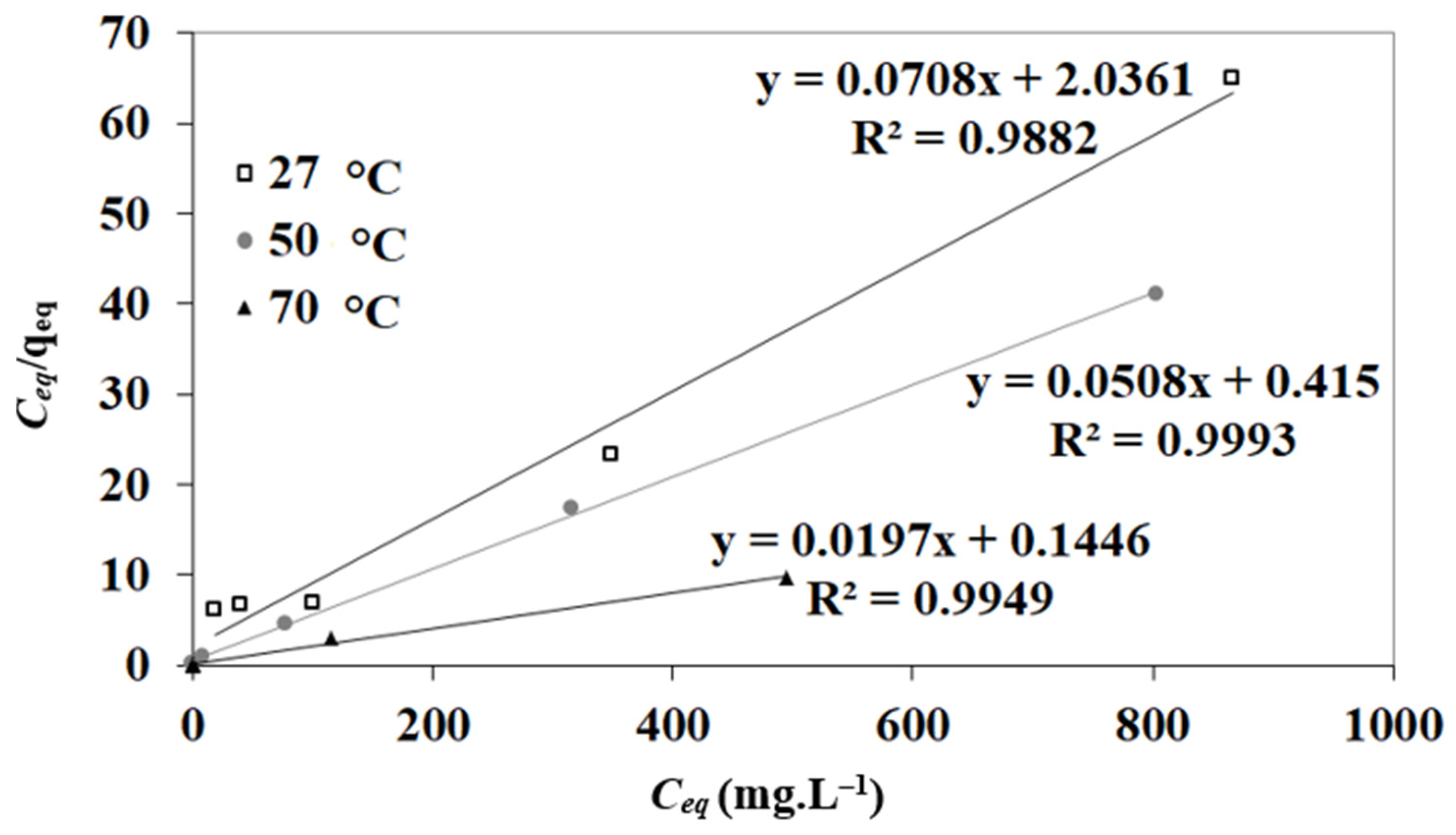
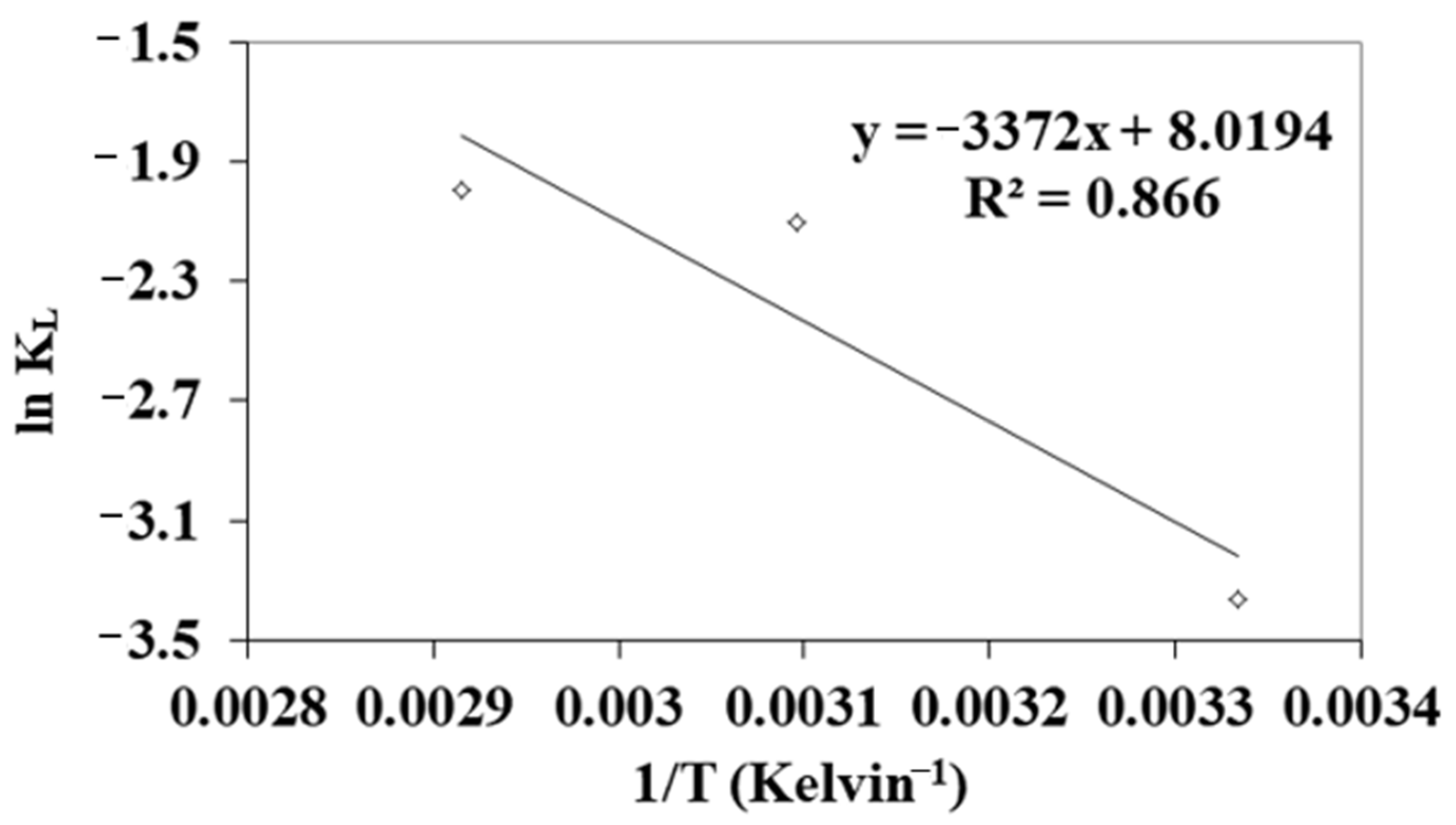
3.4. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, S.; Pandey, S.; Arotiba, O.A. Sol-Gel Derived Xanthan Gum/Silica Nanocomposite—A Highly Efficient Cationic Dyes Adsorbent in Aqueous System. Int. J. Biol. Macromol. 2017, 103, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of Pesticides from Water and Wastewater: Chemical, Physical and Biological Treatment Approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An Overview on the Removal of Synthetic Dyes from Water by Electrochemical Advanced Oxidation Processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- García-Sabido, D.; López-Mesas, M.; Carrillo-Navarrete, F. Chicken Feather Fibres Waste as a Low-Cost Biosorbent of Acid Blue 80 Dye. Desalin. Water Treat. 2016, 57, 3732–3740. [Google Scholar] [CrossRef]
- Pradhan, P.; Bajpai, A. Preparation and Characterization of Films from Chicken Feathers for Dye Adsorption. Mater. Today Proc. 2020, 29, 1204–1212. [Google Scholar] [CrossRef]
- Christina, B.; Thanigaimani, K.; Sudhakaran, R.; Mohan, S.; Arumugam, N.; Almansour, A.I. Karthikeyan perumal Green Waste Immobilized Ag/Cu Feather like Bi-Matrix on Garment Dye Decomposes and Their Bio-Efficacy. Environ. Res. 2024, 242, 117761. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Gonçalves, M.; Oliveira, D.Q.L.; Guerreiro, M.C.; Guilherme, L.R.G.; Dallago, R.M. Solid Waste from Leather Industry as Adsorbent of Organic Dyes in Aqueous-Medium. J. Hazard. Mater. 2007, 141, 344–347. [Google Scholar] [CrossRef]
- Padmapriya, M.; Ramesh, S.T.; Biju, V.M. Synthesis of Seawater Based Geopolymer: Characterization and Adsorption Capacity of Methylene Blue from Wastewater. Mater. Today Proc. 2021, 51, 1770–1776. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of Methylene Blue Dye from Artificially Contaminated Water Using Citrus Limetta Peel Waste as a Very Low Cost Adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Santos, S.C.R.; Oliveira, Á.F.M.; Boaventura, R.A.R. Bentonitic Clay as Adsorbent for the Decolourisation of Dyehouse Effluents. J. Clean. Prod. 2016, 126, 667–676. [Google Scholar] [CrossRef]
- da Cunha, I.C.; Brandelli, A.; Braga, A.R.C.; Sala, L.; Kalil, S.J. Feather Meal as a Source of Peptides with Antioxidant Activity from Enzymatic Hydrolysis. Waste Biomass Valorization 2023, 14, 421–430. [Google Scholar] [CrossRef]
- Bezus, B.; Ruscasso, F.; Garmendia, G.; Vero, S.; Cavello, I.; Cavalitto, S. Revalorization of Chicken Feather Waste into a High Antioxidant Activity Feather Protein Hydrolysate Using a Novel Psychrotolerant Bacterium. Biocatal. Agric. Biotechnol. 2021, 32, 101925. [Google Scholar] [CrossRef]
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond Plucking: Feathers Bioprocessing into Valuable Protein Hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Gaur, B.; Mittal, J.; Hassan, H.; Mittal, A.; Baker, R.T. Removal of Hazardous Aniline Blue Dye Using a Potential Biosorbent—Hen Feather. J. Indian Chem. Soc. 2024, 101, 101322. [Google Scholar] [CrossRef]
- Pasquali, E.A.; Demaman Oro, C.E.; Bernardi, J.L.; Venquiaruto, L.D.; Treichel, H.; Mossi, A.J.; Dallago, R.M. Adsorption of Cr(VI) by Wet Blue Leather: Sustainable Solution for Leather Industry Effluents. J. Water Process Eng. 2025, 69, 106807. [Google Scholar] [CrossRef]
- Mittal, A. Adsorption Kinetics of Removal of a Toxic Dye, Malachite Green, from Wastewater by Using Hen Feathers. J. Hazard. Mater. 2006, 133, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.W.; Lee, Y.S.; Ahmad, S.A.; Ooi, P.T.; Phang, L.Y. Chicken Feather Valorization by Thermal Alkaline Pretreatment Followed by Enzymatic Hydrolysis for Protein-Rich Hydrolysate Production. Waste Manag. 2018, 79, 658–666. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of Keratin Protein Extraction from Waste Chicken Feathers Using Hybrid Pre-Treatment Techniques. Sustain. Chem. Pharm. 2020, 17, 100267. [Google Scholar] [CrossRef]
- Santos, M.M.F.; Grisi, C.V.B.; de Souza, E.G.T.; de Morais Lima, J.; da Silva Ferreira, V.C.; Kurozawa, L.E.; Madruga, M.S.; da Silva, F.A.P. Biotransformation of Free-Range Chicken Feather into Functional Protein Hydrolysates Using Microwave Alkaline Pretreatment. Food Biosci. 2024, 59, 103897. [Google Scholar] [CrossRef]
- Raydan, N.D.V.; Loquet, A.; Habenstein, B.; Kauffmann, B.; Charrier, B.; Chatel, G.; Robles, E. A Comprehensive Comparative Study of Ultrasound-Alkaline and Thermal-Alkaline Hydrolysis of Duck Feather. J. Clean. Prod. 2024, 467, 142927. [Google Scholar] [CrossRef]
- Mittal, A.; Thakur, V.; Mittal, J.; Vardhan, H. Process Development for the Removal of Hazardous Anionic Azo Dye Congo Red from Wastewater by Using Hen Feather as Potential Adsorbent. Desalin. Water Treat. 2014, 52, 227–237. [Google Scholar] [CrossRef]
- El-Bindary, M.A.; El-Desouky, M.G.; El-Bindary, A.A. Adsorption of Industrial Dye from Aqueous Solutions onto Thermally Treated Green Adsorbent: A Complete Batch System Evaluation. J. Mol. Liq. 2022, 346, 117082. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of Adsorption, Adsorption Energy and Activation Energy in Adsorption and Ion Exchange Systems. Desalin. Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Fil, B.A.; Yilmaz, M.T.; Bayar, S.; Elkoca, M.T. Investigation of Adsorption of the Dyestuff Astrazon Red Violet 3rn (Basic Violet 16) on Montmorillonite Clay. Braz. J. Chem. Eng. 2014, 31, 171–182. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Verma, R.; Sankarasubramanian, S.; Yadav, S.; Carabineiro, S.A.C.; Susan, M.A.B.H. Chicken Feathers Derived Materials for the Removal of Chromium from Aqueous Solutions: Kinetics, Isotherms, Thermodynamics and Regeneration Studies. J. Dispers. Sci. Technol. 2022, 43, 446–460. [Google Scholar] [CrossRef]
| Treatment | Adsorption Capacity (mg g−1) |
|---|---|
| Natural feather | 3.18 ± 0.12 |
| Feather treated with HCl 0.1 mol L−1 | 5.00 ± 0.15 |
| Feather treated with HCl 1.0 mol L−1 | 5.79 ± 0.10 |
| Feather treated with NaOH 0.1 mol L−1 | 1.35 ± 0.08 |
| Feather treated with NaOH 1.0 mol L−1 | 0.86 ± 0.06 |
| Run | pH | T (°C) | mgdye g−1feather |
|---|---|---|---|
| 1 | −1 (3.0) | −1 (30) | 4.72 ± 0.10 |
| 2 | 1 (7.0) | −1 (30) | 5.90 ± 0.03 |
| 3 | −1 (3.0) | 1 (50) | 8.43 ± 0.00 |
| 4 | 1 (7.0) | 1 (50) | 9.53 ± 0.21 |
| 5 | 0 (5.0) | 1.41 (54) | 8.15 ± 0.18 |
| 6 | 0 (5.0) | −1.41 (26) | 4.96 ± 0.05 |
| 7 | 1.41 (7.8) | 0 (40) | 5.62 ± 0.02 |
| 8 | −1.41 (2.2) | 0 (40) | 6.65 ± 0.12 |
| 9 | 0 (5.0) | 0 (40) | 4.80 ± 0.05 |
| 10 | 0 (5.0) | 0 (40) | 4.94 ± 0.00 |
| 11 | 0 (5.0) | 0 (40) | 4.82 ± 0.02 |
| T (°C) | N (mg g−1) | KL (L mg−1) | R2 |
|---|---|---|---|
| 27 | 14.12 | 0.0347 | 0.9982 |
| 50 | 19.68 | 0.122 | 0.9993 |
| 70 | 50.76 | 0.136 | 0.9949 |
| T (°C) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J Kmol−1) |
|---|---|---|---|
| 27 | 8.01 | 28.04 | 66.62 |
| 50 | 6.46 | ||
| 70 | 5.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caovilla, M.; Oro, C.E.D.; Mores, R.; Venquiaruto, L.D.; Mignoni, M.L.; Di Luccio, M.; Treichel, H.; Dallago, R.M.; Tres, M.V. Exploring Chicken Feathers as a Cost-Effective Adsorbent for Aqueous Dye Removal. Separations 2025, 12, 39. https://doi.org/10.3390/separations12020039
Caovilla M, Oro CED, Mores R, Venquiaruto LD, Mignoni ML, Di Luccio M, Treichel H, Dallago RM, Tres MV. Exploring Chicken Feathers as a Cost-Effective Adsorbent for Aqueous Dye Removal. Separations. 2025; 12(2):39. https://doi.org/10.3390/separations12020039
Chicago/Turabian StyleCaovilla, Marcela, Carolina E. Demaman Oro, Rúbia Mores, Luciana D. Venquiaruto, Marcelo L. Mignoni, Marco Di Luccio, Helen Treichel, Rogério Marcos Dallago, and Marcus V. Tres. 2025. "Exploring Chicken Feathers as a Cost-Effective Adsorbent for Aqueous Dye Removal" Separations 12, no. 2: 39. https://doi.org/10.3390/separations12020039
APA StyleCaovilla, M., Oro, C. E. D., Mores, R., Venquiaruto, L. D., Mignoni, M. L., Di Luccio, M., Treichel, H., Dallago, R. M., & Tres, M. V. (2025). Exploring Chicken Feathers as a Cost-Effective Adsorbent for Aqueous Dye Removal. Separations, 12(2), 39. https://doi.org/10.3390/separations12020039








