Efficient Separation of Hydroxylamine from Metal Ions by PIM-ED Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Synthesis of SPEEK
2.3. Membrane Preparation
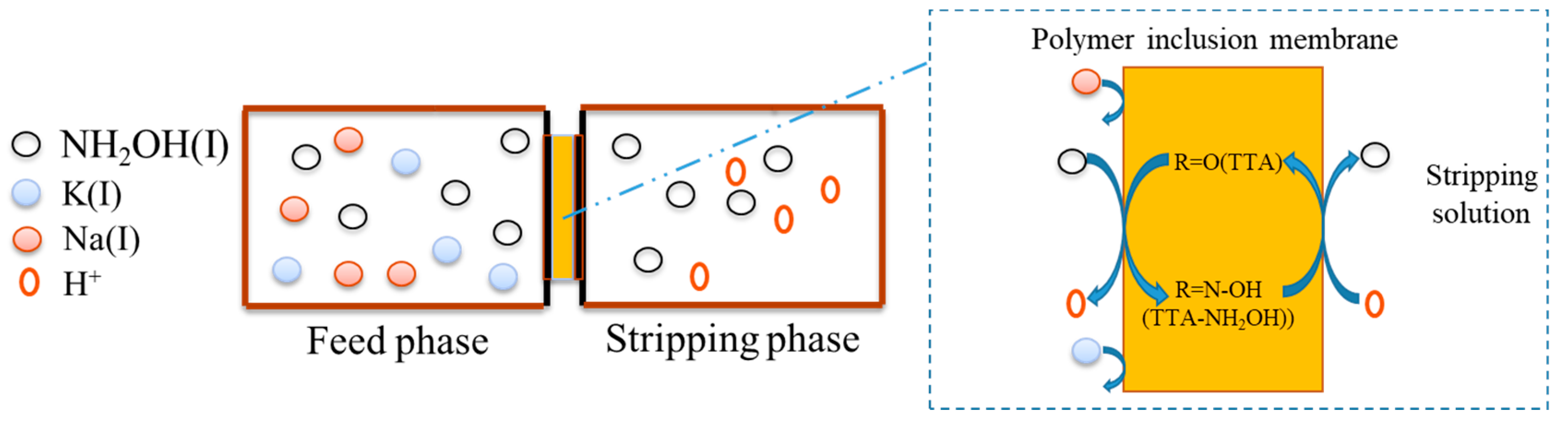
2.4. Transport Experiment
2.5. PIM-ED Process Transport Experiment
3. Results and Discussion
3.1. PIM Process for HA Separation
3.1.1. Screening of Carrier Types
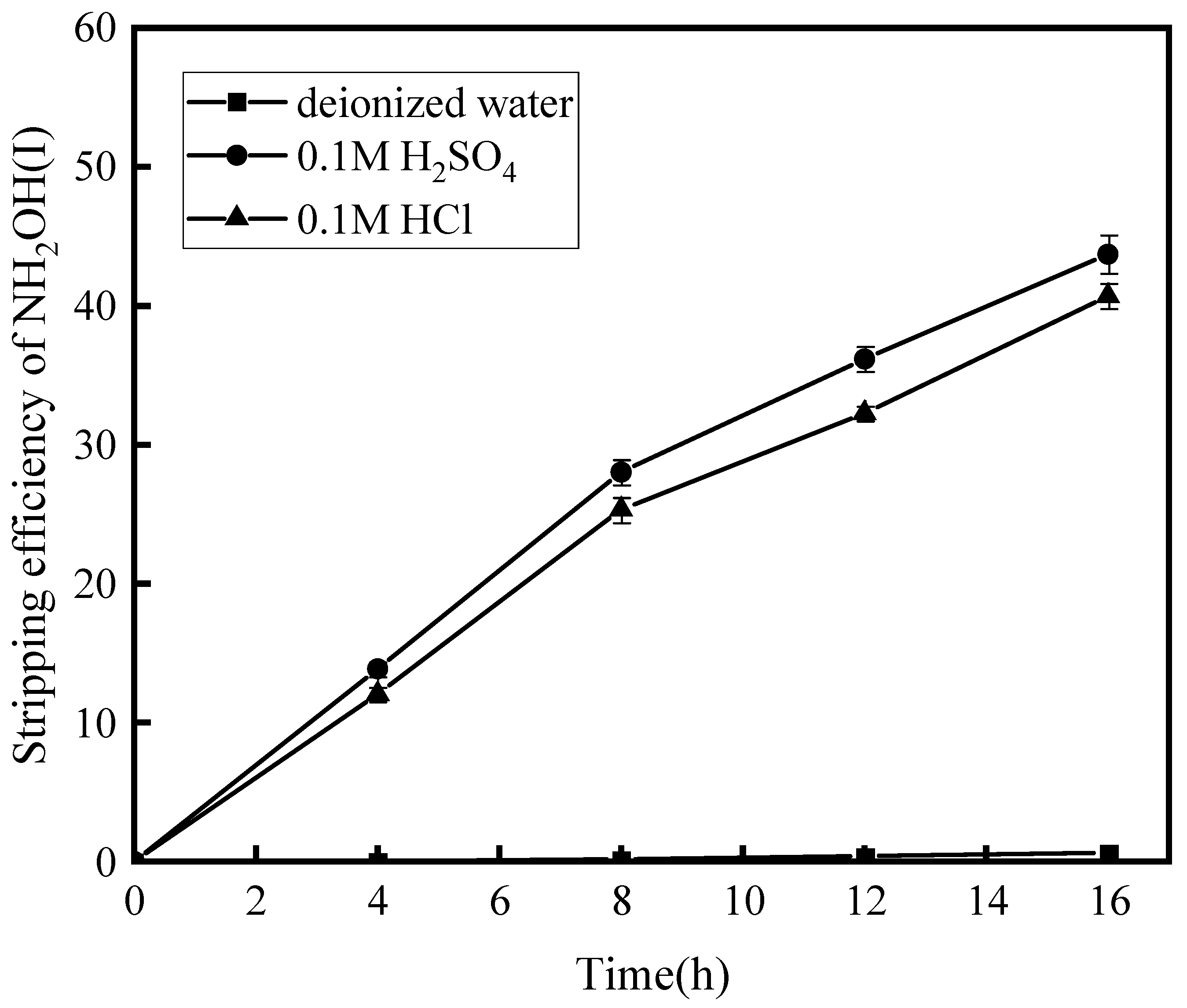
3.1.2. Effect of Feed and Stripping Condition
3.2. PIM-ED Process for HA Separation
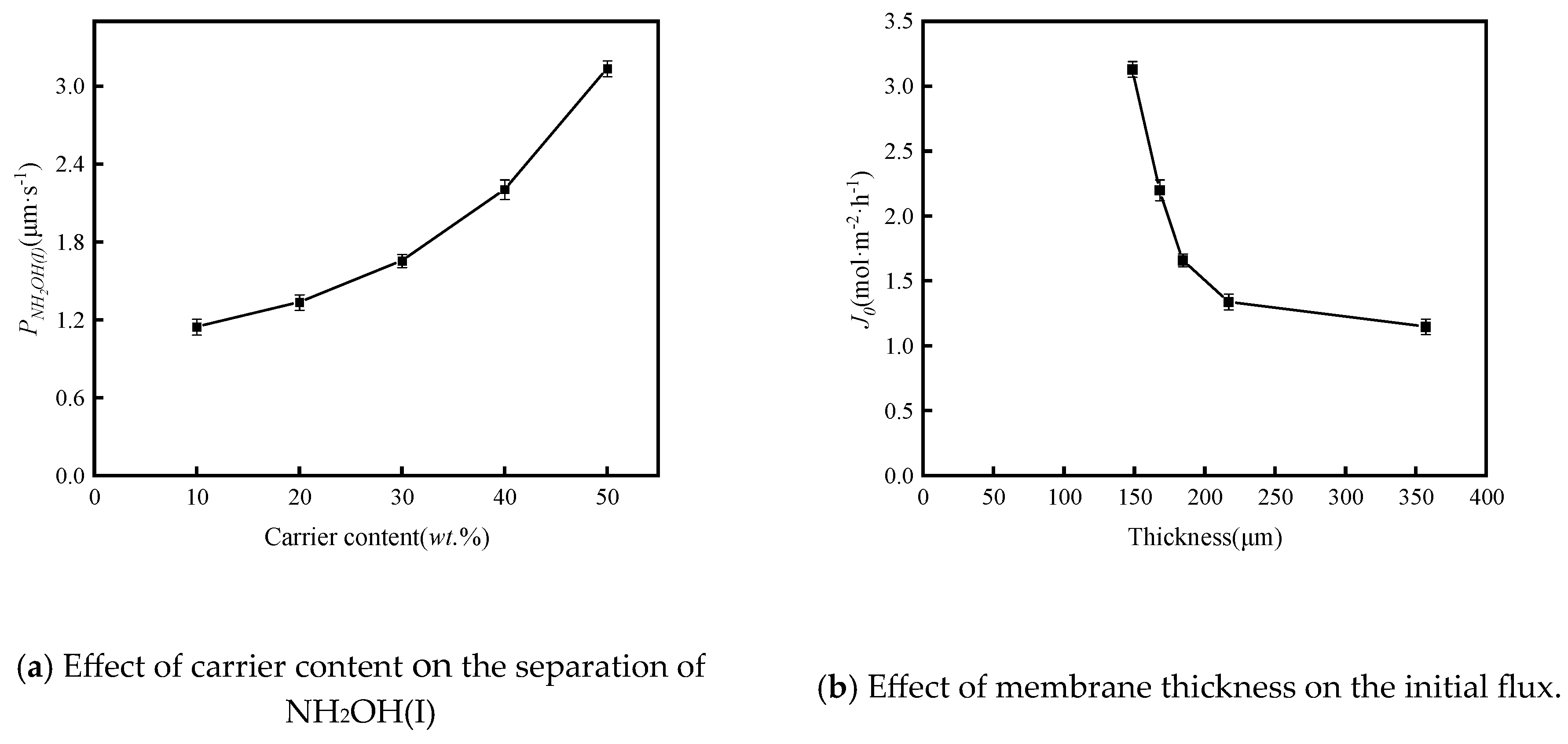
3.2.1. Effect of Carrier Content
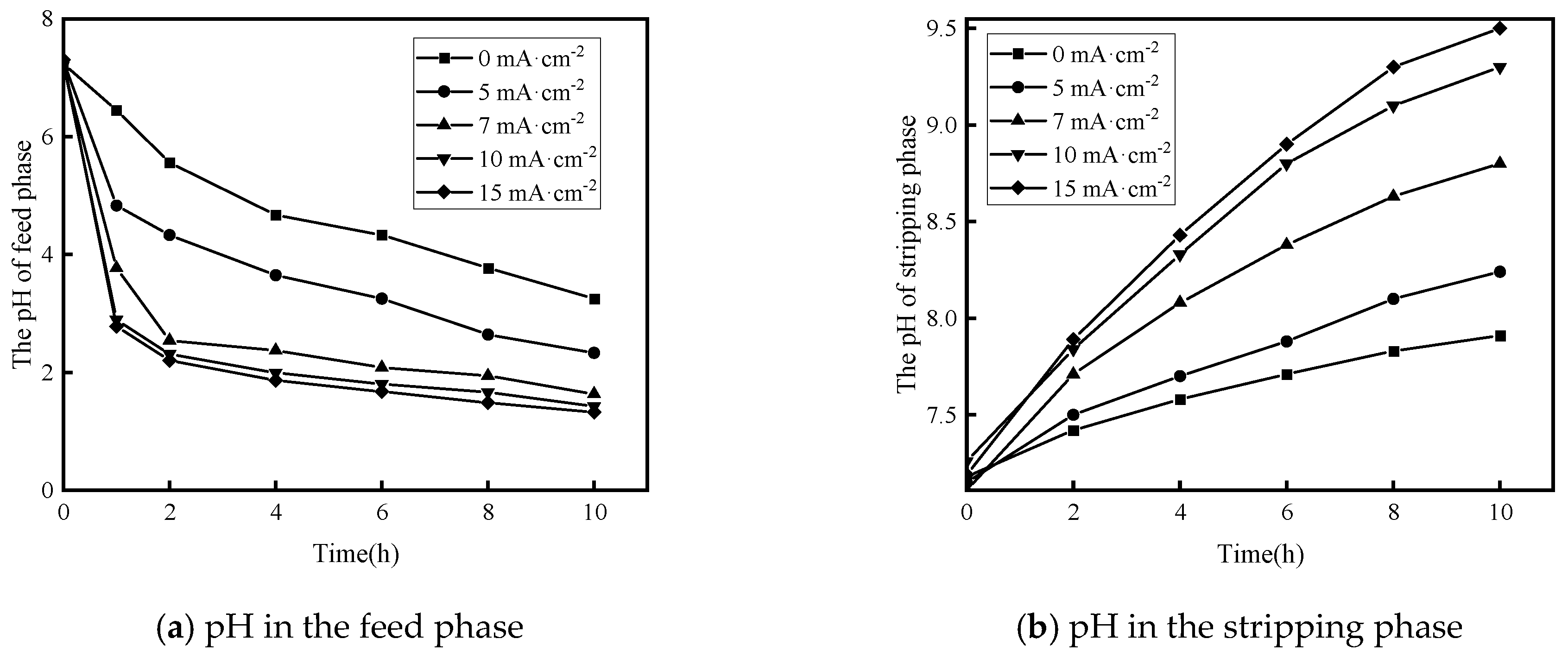
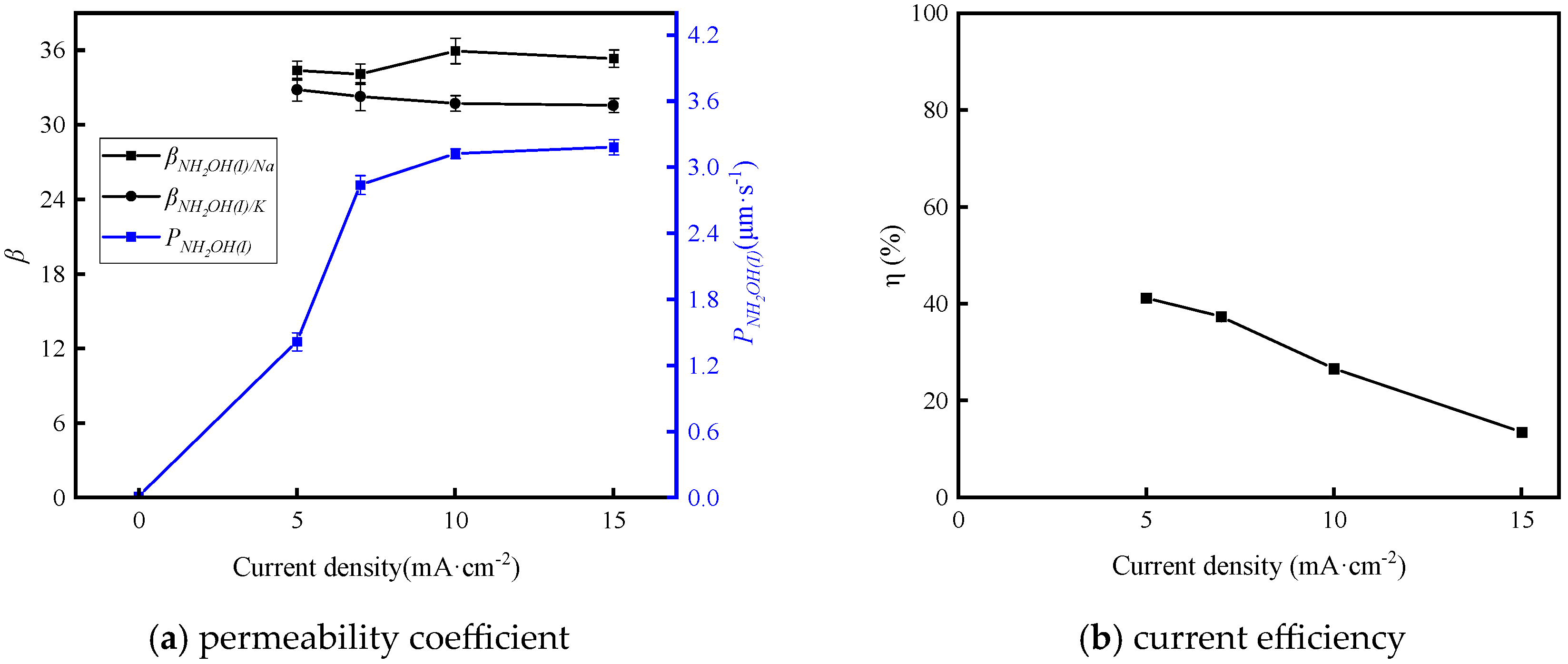
3.2.2. Effect of Current Density
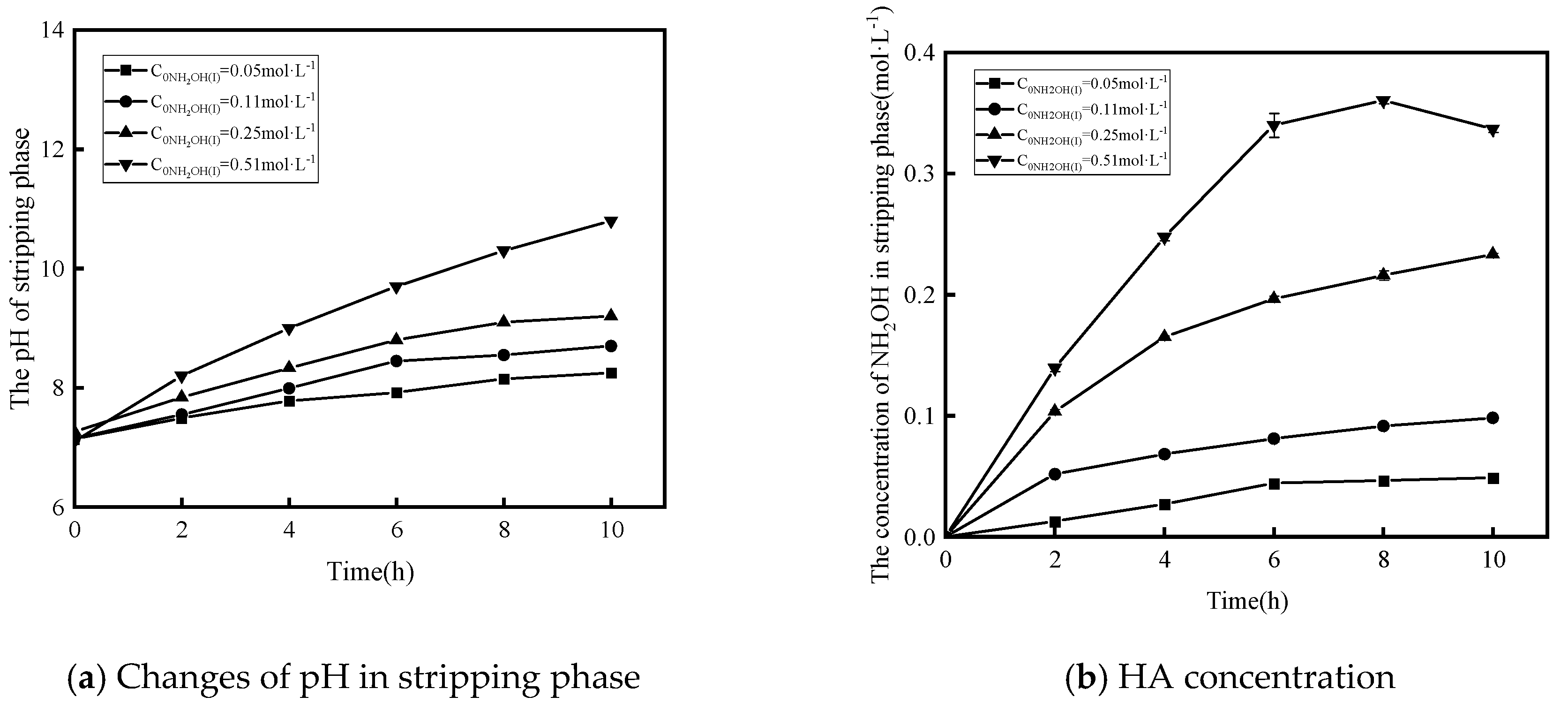
3.2.3. Effect of Concentration of NH2OH(I) in Feed Phase
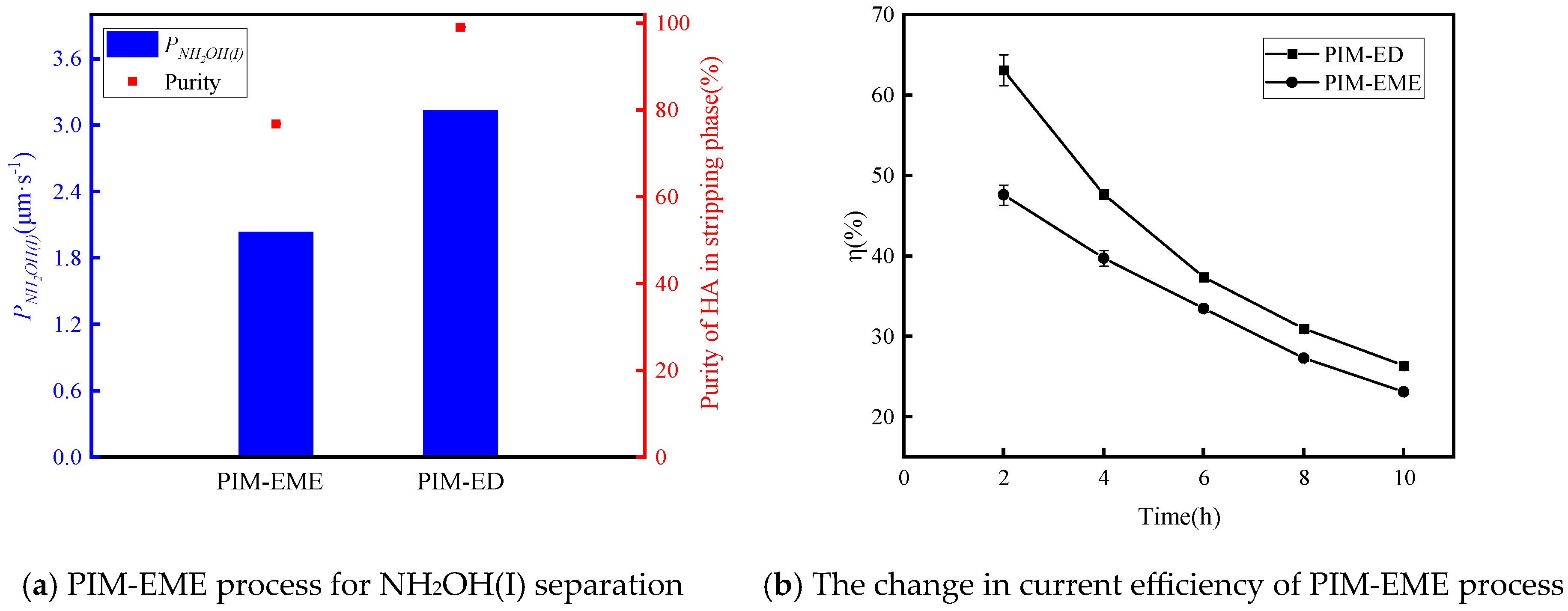
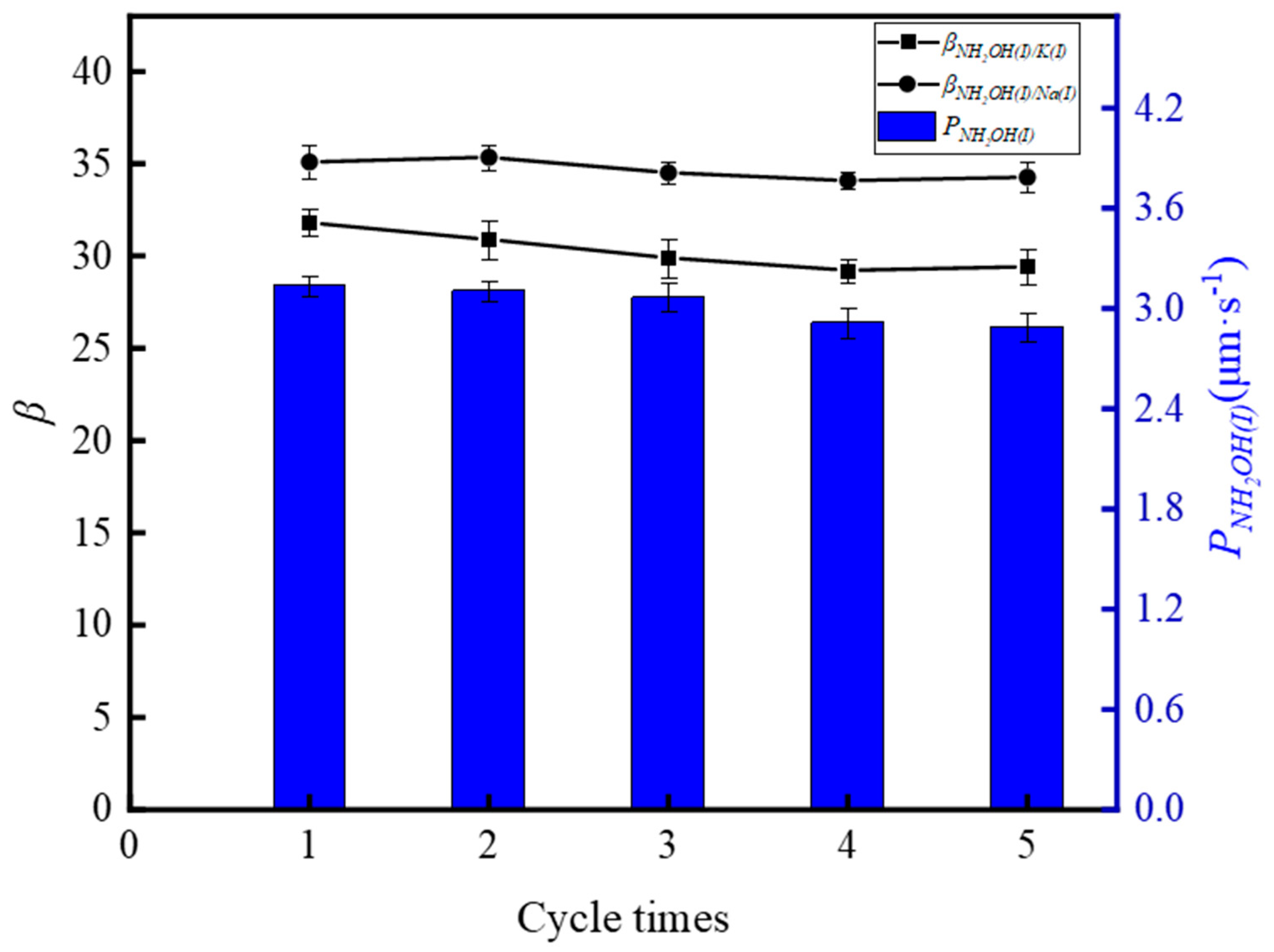
3.3. Stability Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, F.; You, K.; Peng, C.; Tan, S.; Li, R.; Liu, P.; Wu, J.; Luo, H. A simple and efficient approach for preparation of hydroxylamine sulfate from the acid-catalyzed hydrolysis reaction of cyclohexanone oxime. Chem. Eng. J. 2015, 272, 102–107. [Google Scholar] [CrossRef]
- Zhang, W.; Su, X.; Hao, Z.; Qin, S.; Qing, W.; Xia, C. Pervaporation membrane reactor for producing hydroxylamine chloride via an oxime hydrolysis reaction. Ind. Eng. Chem. Res. 2014, 54, 100–107. [Google Scholar] [CrossRef]
- Constant, A.; Coppens, P.; Baele, J.; Ziad, H.; Novak, T.; Kostelnik, P.; Pestel, F.D. Selective wet etching and hydrolysis of polycrystalline AlN films grown by metal organic chemical vapor deposition. Mater. Sci. Semicond. Process. 2022, 137, 106157. [Google Scholar] [CrossRef]
- Watzenberger, O.; Wilfinger, H.J. Method for Preparing Highly Stabilized Hydroxylamine Solutions. German. Patent No. DE 19936594, 15 February 2001. [Google Scholar]
- Heinz, W.; Joachim, T.; Heinz, K.; Eckhard, S.; Markus, W.; Bernd, G.; Bernd, R.; Bernd, S.; Steffen, K. Method for Preparing Highly Stabilized Hydroxylamine Solutions. U.S. Patent No. DE 10482212, 2004. [Google Scholar]
- Kumasaki, K. Calorimetric study on the decomposition of hydroxylamine in the presence of transition metals. J. Hazard. Mater. 2004, 115, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Q.; Qi, X.; Xu, Y.; Zhang, D.; Wang, Y.; Zhao, X. A novel hydroxylamine ionic liquid salt resulting from the stabilization of NH2OH by a SO3H functionalized ionic liquid. Chem. Commun. 2015, 15, 1930–1932. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qi, X.; Gao, L.; Xu, Y.; Zhang, D.; Wang, S.; Zhao, X.; Wang, Y. Application of Hydroxylamine Ionic Liquid Salts in Hydroxylation of Benzene to Phenol with Ammonium Molybdate-Copper Chloride-Ionic Liquid System. Chem. Lett. 2016, 46, 289–292. [Google Scholar] [CrossRef]
- Almeida, M.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Luo, H.; Yao, H.; Wang, X.; Liang, X.; Li, B.; Liu, H.; Li, Y. Selective recovery of lithium from mother liquor of Li2CO3 by synergistic hydrophobic deep eutectic solvents: Performance and mechanistic insight. Sep. Purif. Technol. 2023, 313, 123353. [Google Scholar] [CrossRef]
- Mahanty, B.N.; Mohapatra, P.K.; Raut, D.R.; Das, D.K.; Behere, P.G.; Afzal, M.; Verboom, W. Polymer inclusion membrane containing a diglycolamide-functionalized calix[4]arene for actinide ion uptake and transport. J. Membr. Sci. 2016, 516, 194–201. [Google Scholar] [CrossRef]
- Cai, C.; Yang, F.; Zhao, Z.; Liao, Q.; Bai, R.; Guo, W.; Chen, P.; Zhang, Y.; Zhang, H. Promising transport and high-selective separation of Li(I) from Na(I) and K(I) by a functional polymer inclusion membrane (PIM) system. J. Membr. Sci. 2019, 579, 1–10. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Cattrall, R.W.; Truong, Y.B.; Kyratzis, I.L.; Kolev, S.D. The use of poly(vinylidenefluoride-co-hexafluoropropylene) for the preparation of polymer inclusion membranes. Application to the extraction of thiocyanate. J. Membr. Sci. 2016, 510, 481–488. [Google Scholar] [CrossRef]
- Almeida, M.; Silva, A.; Cattrall, R.W.; Kolev, S.D. A study of the ammonium ion extraction properties of polymer inclusion membranes containing commercial dinonylnaphthalene sulfonic acid. J. Membr. Sci. 2015, 478, 155–162. [Google Scholar] [CrossRef]
- Casadellà, A.; Schaetzle, O.; Loos, K. Ammonium across a Selective Polymer Inclusion Membrane: Characterization, Transport, and Selectivity. Macromol. Rapid Commun. 2016, 37, 858–864. [Google Scholar] [CrossRef]
- Mwakalesi, A.J.; Potter, I.D. Targeting of cationic organic pesticide residues using polymer inclusion membranes containing anacardic acid from cashew nut shell liquid as a green carrier. J. Water Process. Eng. 2021, 43, 102222. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Xie, H.; Tan, M.; Wang, L.; Liu, Y.; Zhan, Y. Mechanistic investigation of intensified separation of molybdenum (VI) and vanadium (V) using polymer inclusion membrane electrodialysis. J. Hazard. Mater. 2023, 456, 131671. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Cheng, P.; Li, Z.; Zhang, D.; Yang, Q.; Wang, Y. Application of an immobilized ionic liquid for the preparation of hydroxylamine via hydrolysis of cyclohexanone oxime. Z. Anorg. Allg. Chem. 2021, 647, 742–750. [Google Scholar] [CrossRef]
- Xu, L.; Ding, J.; Yang, Y.; Wu, P. Distinctions of hydroxylamine formation and decomposition in cyclohexanone ammoximation over microporous titanosilicates. J. Catal. 2014, 309, 1–10. [Google Scholar] [CrossRef]
- Altenburger, J.M.; Mioskowski, C.; d’Orchymont, H.; Schirlin, D.; Schalk, C.; Tarnus, C. Useful hydroxylamine derivatives for the synthesis of hydroxamic acids. Tetrahedron Lett. 1992, 33, 5055–5058. [Google Scholar] [CrossRef]
- Denmark, S.E.; Nguyen, S.T. Catalytic, Nucleophilic Allylation of Aldehydes with Allyl Acetate. Org. Lett. 2009, 11, 781–784. [Google Scholar] [CrossRef]
- Neuvonen, H.; Neuvonen, K.; Koch, A.; Kleinpeter, E.; Pasanen, P. Electron-withdrawing substituents decrease the electrophilicity of the carbonyl carbon. An investigation with the aid of (13)C NMR chemical shifts, nu(C[double bond]O) frequency values, charge densities, and isodesmic reactions to interpret substituent effects on Reactivity. J. Org. Chem. 2002, 67, 6995–7003. [Google Scholar] [CrossRef]
- Toh, Q.Y.; Mcnally, A.; Vera, S.; Erdmann, N.; Gaunt, M.J. Organocatalytic C-H bond arylation of aldehydes to bis-heteroaryl ketones. J. Am. Chem. Soc. 2013, 135, 3772–3775. [Google Scholar] [CrossRef]
- Nghiem, L.; Mornane, P.; Potter, I.; Perera, J.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Gyves, J.; Miguel, E. Metal ion separations by supported liquid membranes. Ind. Eng. Chem. Res. 1999, 38, 2182–2202. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wang, B.; Lang, Q.; Tan, M.; Lee, M.; Peng, C.; Zhang, Y. Insights into the facilitated transport mechanisms of Cr(VI) in ionic liquid-based polymer inclusion membrane-electrodialysis. Chem. Eng. J. 2020, 397, 125324. [Google Scholar] [CrossRef]
- Wu, N.; Almeida, M.; Simeonova, S.; Spassov, T.G.; Rangelov, A.; Cattrall, R.W.; Datcheva, M.; Kolev, S.D. Preparation and characterization of very thin polymer inclusion membranes (PIMs) and their application to the transport of thiocyanate. J. Membr. Sci. 2023, 668, 121249. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Zhang, F.; Tan, M.; Jiang, H.; Liu, Y.; Zhang, Y. Efficient separation and recovery of cobalt (II) and lithium (I) from spent lithium ion batteries (LIBs) by polymer inclusion membrane electrodialysis (PIMED). Chem. Eng. J. 2022, 430, 132924. [Google Scholar] [CrossRef]
- Iwata, Y.; Koseki, H. Risk evaluation of decomposition of hydroxylamine/water solution at various concentrations. Process Saf. Prog. 2002, 21, 136–141. [Google Scholar] [CrossRef]
- Andriani, G.; Pio, G.; Vianello, C.; Mocellin, P.; Salzano, E. Safety parameters and stability diagram of hydroxylamine hydrochloride and sulphate. Chem. Eng. J. 2024, 482, 148894. [Google Scholar] [CrossRef]
- Wei, C.; Saraf, S.R.; Rogers, W.J.; Mannan, M.S. Thermal runaway reaction hazards and mechanisms of hydroxylamine with acid/base contaminants. Thermochim. Acta 2004, 421, 1–9. [Google Scholar] [CrossRef]
- Cisneros, L.O.; Wu, X.; Rogers, W.J.; Mannan, M.S.; Park, J.; North, S.W. Decomposition Products of 50 Mass% Hydroxylamine/Water Under Runaway Reaction Conditions. Process Saf. Environ. Prot. 2003, 81, 121–124. [Google Scholar] [CrossRef]
- Wachenberger, O.; Schölling, H.; Pfab, P.; Stöllfel, E. Preparation of Aqueous Hydroxylamine Solution of High Purity. CN 1260762A, 19 July 2000. [Google Scholar]
- Wostbrock, K.; Thiel, J.; Kruger, K.; Strofer, E.; Weber, M.; Gerber, B.; Rumpf, B.; Sachweh, B.; Kerth, S. Method for Producing an Aqueous Hydroxylamine Solution Devoid of Salt. U.S. Patent US20040149563A1, 30 December 2003. [Google Scholar]
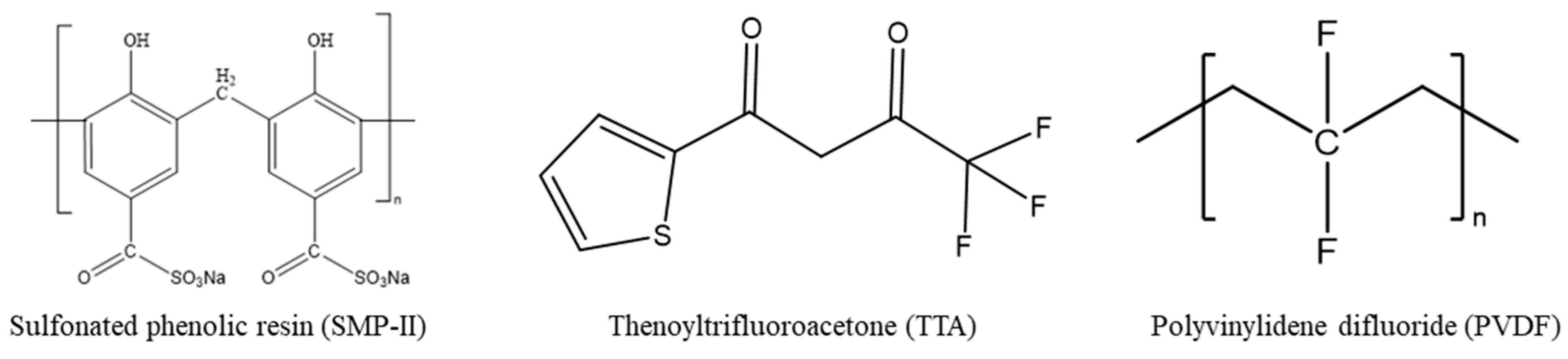
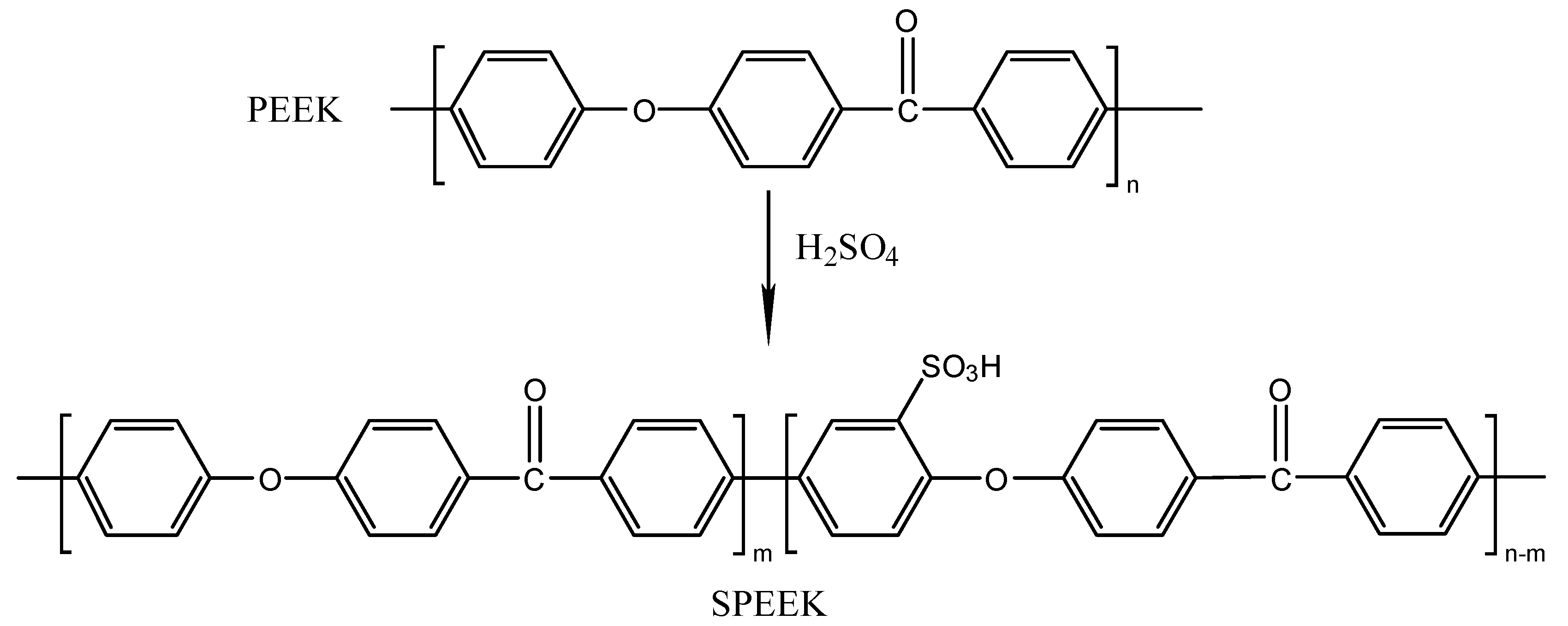
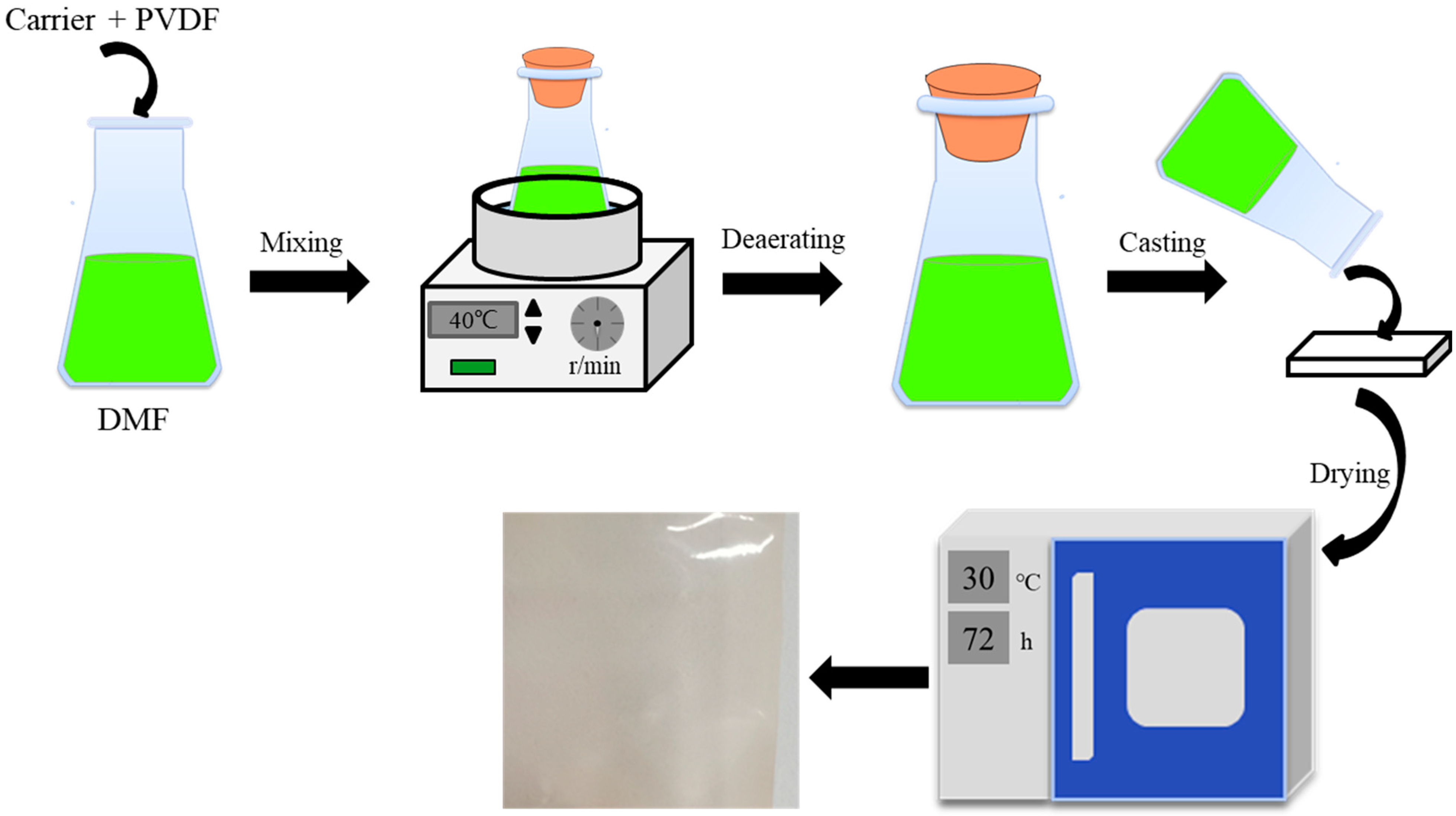
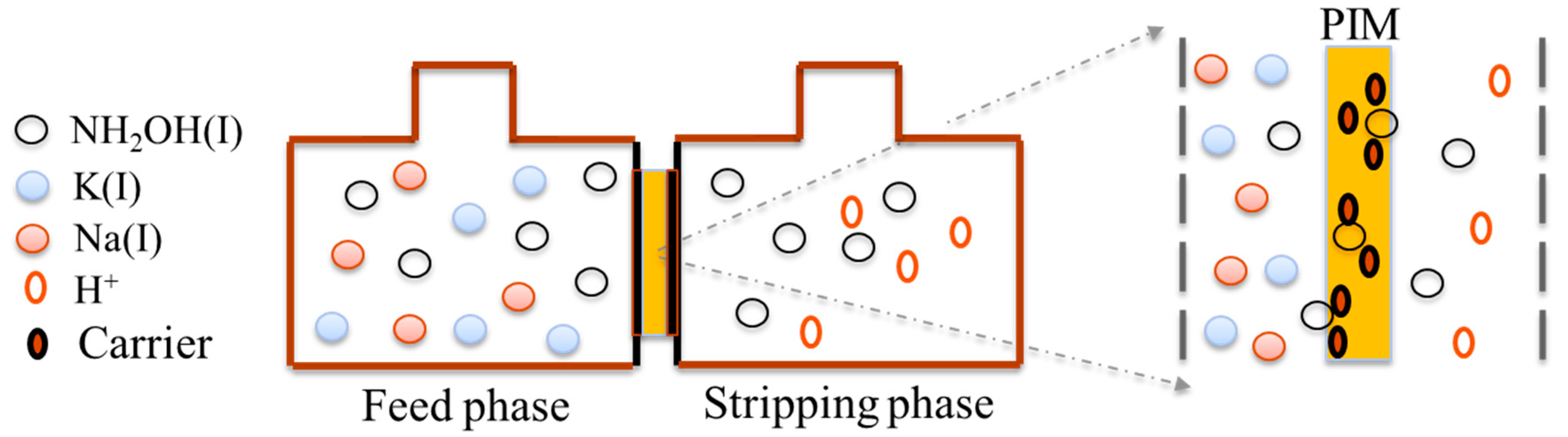



| Carrier | Carrier Content (wt.%) | Base Polymer | Base Polymer Content (wt.%) |
|---|---|---|---|
| SMP-II | 50 | PVDF | 50 |
| SPEEK | 50 | PVDF | 50 |
| TTA | 50 | PVDF | 50 |
| TTA | 40 | PVDF | 60 |
| TTA | 30 | PVDF | 70 |
| TTA | 20 | PVDF | 80 |
| TTA | 10 | PVDF | 90 |
| Carrier Types | J0NH2OH(I) (mol·m−2·h−1) | βNH2OH/K(I) | βNH2OH/Na(I) |
|---|---|---|---|
| SMP-II | 0.14 ± 0.01 | 2.4 ± 0.4 | 0.7 ± 0.3 |
| SPEEK | 0.19 ± 0.01 | 1.1 ± 0.1 | 2.1 ± 0.1 |
| TTA | 0.44 ± 0.03 | 37.8 ± 0.7 | 39.9 ± 0.8 |
| C0NH2OH(I)(mol·L−1) | J0 (mol·m2·h−1) | Extraction Efficiency (%) | Stripping Efficiency (%) | HA Purity (%) |
|---|---|---|---|---|
| 0.05 | 0.72 ± 0.02 | 95.2 ± 1.2 | 90.3 ± 2.4 | 98.0 ± 0.2 |
| 0.11 | 1.31 ± 0.03 | 96.4 ± 1.2 | 94.0 ± 1.2 | 98.2 ± 0.3 |
| 0.25 | 2.84 ± 0.06 | 93.7 ± 0.9 | 92.3 ± 0.5 | 99.4 ± 0.1 |
| 0.51 | 3.79 ± 0.09 | 82.9 ± 0.5 | 65.7 ± 0.5 | 79.8 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Ding, Z.; Zhu, Z.; Zhang, W. Efficient Separation of Hydroxylamine from Metal Ions by PIM-ED Process. Separations 2025, 12, 24. https://doi.org/10.3390/separations12020024
Yang L, Ding Z, Zhu Z, Zhang W. Efficient Separation of Hydroxylamine from Metal Ions by PIM-ED Process. Separations. 2025; 12(2):24. https://doi.org/10.3390/separations12020024
Chicago/Turabian StyleYang, Lilei, Zhongwei Ding, Zhengtao Zhu, and Weidong Zhang. 2025. "Efficient Separation of Hydroxylamine from Metal Ions by PIM-ED Process" Separations 12, no. 2: 24. https://doi.org/10.3390/separations12020024
APA StyleYang, L., Ding, Z., Zhu, Z., & Zhang, W. (2025). Efficient Separation of Hydroxylamine from Metal Ions by PIM-ED Process. Separations, 12(2), 24. https://doi.org/10.3390/separations12020024







