Abstract
In this study, three fungal steroids (1–3) were isolated from the fruiting bodies of the poisonous mushroom Gymnopilus orientispectabilis, based on bioactivity-guided isolation methods. The chemical structures of the isolates (1–3) were determined using NMR spectroscopic methods. Compounds 1–3 exhibited inhibition activity against E. coli, and their interactions with several bacterial drug targets were studied via in silico molecular docking, where the lowest binding energies were observed for penicillin binding protein 3 (PBP3) (−62.89, −75.89 and −74.47 kcal/mol, for compounds 1, 2 and 3, respectively). An MD simulation was performed to examine the conformational stability, motion and flexibility of protein–ligand complexes. In conclusion, this study investigates fungal steroids from G. orientaspectabilis as potential sources for new antimicrobial agents, encouraging further research to develop novel therapies.
1. Introduction
Pathogens are microorganisms that have harmful effects on organisms, including humans, that can trigger immune responses [1]. Antimicrobial activity denotes the capacity to combat microorganisms by either inhibiting their growth or inducing their destruction [2]. This characteristic is vital for health maintenance and infection prevention, as it targets bacteria, viruses, fungi, and other potentially harmful microbes. By eliminating these pathogens, antimicrobial agents help reduce the risk of diseases and support the body’s immune defense mechanisms. Various types of antimicrobial agents have been developed and utilized based on the specific characteristics of different microorganisms. Among these, penicillin is one of the most well-known and widely used agents, particularly effective in combating bacterial infections [3]. Streptomycin and kanamycin are also among the prominent antimicrobial agents known for their effectiveness against bacterial growth [4], while nystatin is used to fight fungal infections [5]. These agents have been instrumental in controlling the spread of infectious diseases caused by bacteria, fungi, algae, and other microorganisms, contributing significantly to advancements in medical and agricultural fields.
Antimicrobial agents function by inducing cytotoxicity in harmful microorganisms, thereby inhibiting their growth and suppressing their reproduction, effectively controlling their population and contributing to infection management [6,7]. These mechanisms have made such agents indispensable in clinical applications, helping to treat a wide range of microbial infections. However, their use comes with several significant limitations. Key challenges include potential toxicity to mammalian hosts [8], restricted efficacy against certain microbial species [9,10], and the growing problem of antibiotic resistance [11]. These issues underscore the need for ongoing research and development to improve antimicrobial agents, ensuring their safety, expanding their spectrum of activity, and overcoming resistance mechanisms to maintain their effectiveness in clinical and environmental settings.
Mushrooms serve as an excellent source of functional foods and medicine, containing vital bioactive secondary metabolites. These offer various therapeutic benefits, including antioxidant, antimicrobial, anticancer, and immunomodulatory properties [12,13,14]. Poisonous mushrooms, in particular, possess valuable secondary metabolites that have potential pharmaceutical applications [15]. Gymnopilus orientispectabilis, formerly known as G. spectabilis (Fr.) Singer or G. junonius [16], has not been extensively studied for its chemical composition. Gymnopilins and psilocybin are identified as the primary components responsible for the neurotoxic properties of G. orientispectabilis [17,18]. Most research on the chemical composition of this mushroom has focused on acetylenic compounds and oligoisoprenoids (gymnopilins) [17,18,19], and our group earlier identified cytotoxic sesquiterpenes, a unique ergosterol derivative and triterpenoid with various biological activities [20,21,22]. With the persistent challenges associated with conventional antimicrobial agents, medicinal chemists have become increasingly interested in naturally derived antimicrobials. Recently, fungal steroids have gained recognition for their wide range of biological activities [23,24,25], and numerous studies have focused on their antimicrobial properties [26,27,28].
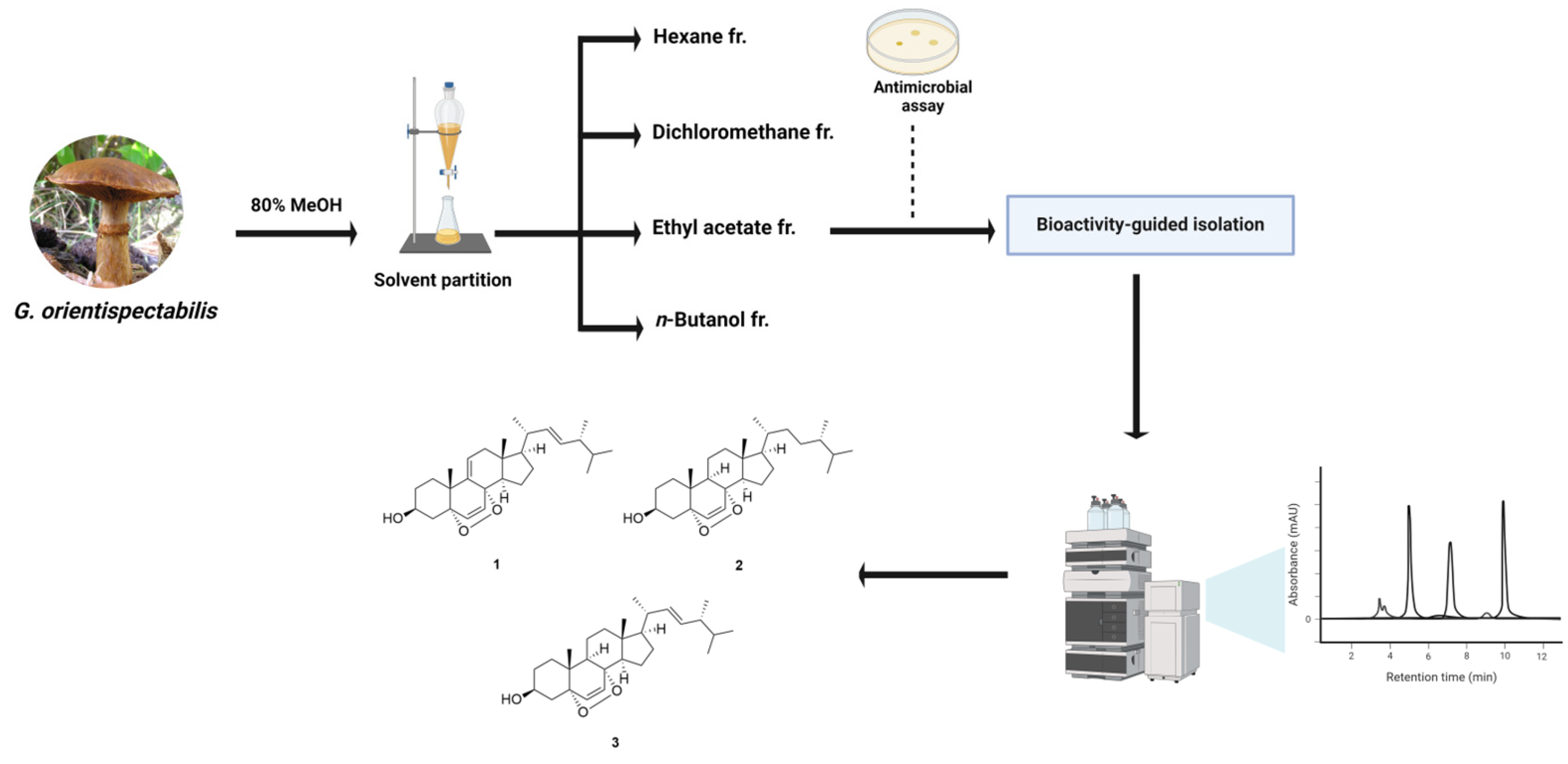
As part of our project exploring natural products with significant antimicrobial activities, we performed a bioactivity-guided isolation of a poisonous mushroom G. orientispectabilis. As a result of repetitive column chromatography and evaluation of antimicrobial activity, active fungal steroids (1–3) were successfully identified (Figure 1). Molecular docking was performed to investigate the binding orientation of 1–3 with bacterial drug targets, and post-binding interactions with key residues and potential structural changes were analyzed to predict the ligand’s behavior in physiological environments. The results of this study underscore the potential of fungal steroids as antimicrobial agents and provide valuable insights for advancing the development of novel antibiotics in the drug design process.
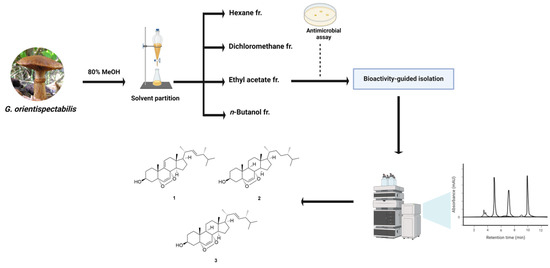
Figure 1.
Schematic representation of bioactivity-guided isolation of G. orientispectabilis.
2. Materials and Methods
2.1. Fungal Material
Fresh fruiting bodies of G. orientispectabilis were collected from the Hongneung Experimental Forest in Seoul, Korea, in September 2018. A voucher specimen (NIFoS 180914-01) of the mushroom was authenticated by one of the authors (K. H. Kim) and was deposited at the Forest Bioresources Department, National Institute of Forest Science, Korea.
2.2. Chromatographic Techniques
Semi-preparative HPLC was conducted on YL 9100 HPLC system (Young Lin, Anyang, South Korea) equipped with a UV/Vis detector using an Alltech reversed-phase YMC-Pak C-18 column (10 μm, 20 mm × 250 mm) and a normal-phase YMC-Pack SIL-HG column (S-10 μm, 12 nm, 20 mm × 250 mm). Column chromatography was performed using silica gel (230–400 mesh, Merck, Karlsruhe, Germany) and C-18 (YMC·GEL ODS-A, 12 nm, S-150 μm). Thin-layer chromatography (TLC) was conducted using silica gel 60 F254 (0.25 mm, Merck) plates and reverse-phase (RP)-18 F254s plates (Merck). Spots on TLC were detected using UV and heating after dipping in 20% sulfuric acid in H2O.
2.3. Spectroscopy
Ultraviolet (UV) spectra were obtained through an Agilent 8453 UV–visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). NMR spectra were measured using a Bruker AVANCE III operating at 600 MHz (1H) and 150 MHz (13C) (Bruker, Billerica, MA, USA). Chemical shifts are reported in δ (ppm) values relative to tetramethylsilane (TMS).
2.4. Extraction and Isolation
Air-dried and chopped fruiting bodies of G. orientispectabilis (1.2 kg) were subjected to extraction three times with 80% aqueous MeOH (each 3 L × 24 h) at room temperature. The resulting extracts were filtered, and the filtrate was evaporated under reduced pressure using a rotary evaporator to obtain a crude MeOH extract (143.3 g). The MeOH extract was suspended in a mixture of distilled water (700 mL) and MeOH (30 mL) and successively solvent-partitioned with hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol to yield soluble layers of hexane, CH2Cl2, EtOAc, and n-butanol at 11.0, 6.6, 14.0, and 19.0 g, respectively. Hexane fraction was subjected to silica gel column chromatography (CC) (Hexane/EtOAc, 10:1 to1:1) to yield nine fractions (Fr. A1–A9). Fr. A3 and A4 (1.4 g) were combined and subjected to reverse-phase CC (MeOH/H2O, 95:5 to 100:0) to yield three fractions (Fr. A31–A33). Fr. A32 (397.0 mg) was further fractionated using semi-preparative HPLC (MeOH/H2O, 95:5 to 100:0), which yielded subfractions A321–A324, compounds 1 (tR 76.0 min, 26.0 mg) and 2 (tR 97.0 min, 20.0 mg). Subfraction A324 (72.0 mg) was purified using semi-preparative HPLC (EtOAc/hexane, 50:50 to 100:0), and compound 3 (tR 56.0 min, 48.0 mg) was obtained.
9,11-Dehydroergosterol peroxide (1): White powder; C28H42O3; 1H-NMR (600 MHz, CDCl3) δ 6.60 (1H, d, J = 8.6 Hz), 6.29 (1H, d, J = 8.5 Hz), 5.43 (1H, dd, J = 6.1, 1.9 Hz), 5.24 (1H, dd, J = 15.3, 7.7 Hz), 5.16 (1H, dd, J = 15.5, 8.4 Hz), 4.02 (1H, m), 2.26 (1H, dd, J = 17.0, 6.1 Hz), 2.14 (1H, m), 2.10 (1H, m), 1.09 (3H, s), 1.00 (3H, d, J = 6.6 Hz), 0.91 (3H, d, J = 6.8 Hz), 0.84 (3H, d, J = 6.8 Hz), 0.82 (3H, d, J = 6.8 Hz), 0.74 (3H, s); 13C NMR (150 MHz, CDCl3) δ 142.52, 135.45, 135.11, 132.42, 130.74, 119.74, 82.71, 78.36, 66.38, 55.85, 48.15, 43.60, 42.76, 41.17, 39.91, 37.95, 36.02, 33.06, 32.56, 31.91, 30.55, 25.54, 20.88, 20.71, 19.94, 19.64, 17.55, 12.96 (Figures S1 and S2).
(3β,5α,8α)-5,8-Epidioxyergost-6-en-3-ol (2): White powder; C28H46O3; 1H-NMR (600 MHz, CDCl3) δ 6.51 (1H, d, J = 8.5 Hz), 6.24 (1H, d, J = 8.5 Hz), 4.00 (1H, m), 2.11 (1H, ddd, J = 13.8, 5.0, 1.9 Hz), 0.91 (3H, d, J = 6.5 Hz), 0.88 (3H, s), 0.85 (3H, d, J = 6.8 Hz), 0.80 (3H, s), 0.78 (3H, d, J = 3.3 Hz), 0.77 (3H, d, J = 3.3 Hz); 13C NMR (150 MHz, CDCl3) δ 135.40, 130.77, 82.16, 79.46, 66.47, 56.26, 51.56, 51.05, 44.72, 39.41, 39.01, 36.94, 36.91, 35.63, 34.69, 33.50, 31.42, 30.55, 30.10, 28.21, 23.41, 20.63, 20.51, 18.75, 18.17, 17.58, 15.44, 12.62 (Figures S3 and S4).
Ergosterol peroxide (3): White powder; C28H44O3; 1H-NMR (600 MHz, CDCl3): δ 6.50 (1H, d, J = 8.5 Hz), 6.24 (1H, d, J = 8.5 Hz), 5.22 (1H, dd, J = 15.3, 7.7 Hz), 5.14 (1H, dd, J = 15.4, 8.4 Hz), 3.94 (1H, m), 2.11 (1H, ddd, J = 13.8, 5.0, 1.9 Hz), 2.02 (1H, ddd, J = 9.6, 8.5, 6.7 Hz), 1.00 (3H, d, J = 6.6 Hz), 0.91 (3H, d, J = 6.8 Hz), 0.88 (3H, s), 0.83 (3H, d, J = 6.8 Hz), 0.82 (3H, s), 0.81 (3H, s) (Figure S5).
2.5. Antimicrobial Assay
The antimicrobial activities were tested with 3 microorganisms in a 96-well-plate: Escherichia coli KCTC 1682 (Korea Collection for Type Cultures) representing Gram-negative bacteria, Staphylococcus aureus KCTC 3881 representing Gram-positive bacteria, and Candida albicans KCTC 27242 representing fungi. Cell culture (95 μL) was diluted to 0.5 McFarland Standard scale in each well. Extracts or compounds dissolved in DMSO were added up to final concentrations (0.5, 1, 2, 5, 10, 20, 50, and 100 μg/mL). Total culture (100 μL) was incubated at 37 °C for 16 h. Cell inhibition was measured at 600 nm using MultiskanTM GO Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA). The IC50 value was calculated using the exponential trend line in Excel (Microsoft, Redmond, WA, USA). Kanamycin and nystatin were used as positive control against the bacterium and yeast, respectively. All measurements were performed in triplicates.
2.6. Ligand and Protein Preparation
Dihydropteroate synthase (DHPS), OmpC, penicillin binding protein (PBP), transpeptidase, DNA gyrase subunit B, topoisomerase Ⅳ, dihydrofolate synthase, and dehydratase were chosen as target proteins, which are potential antibiotic targets based on their important roles in the life cycles of bacteria and fungi [29]. The X-ray crystal structures of the proteins were obtained from RCSB PDB database (PDB, https://www.rcsb.org/) (accessed on 4 December 2024). The bioactive ligands, compounds 1–3, were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) (access date: 4 December 2024) as 3D Standard Data Format (3D SDF). The proteins were prepared and refined through removing water molecules, filling in missing side chains and optimizing hydrogen bonds. Ligand preparation involved optimizing tautomeric and ionization states, as well as ring conformation, using the OPLS4 force field. Schrodinger Ligprep and Protein Preparation Workflow tool (Schrödinger Maestro, Release 2024-2, Platform Windows-x64) was used for protein and ligand preparation.
2.7. In Silico ADMET Analysis
Therapeutic compounds are expected to demonstrate optimal bioavailability, minimal toxicity, and efficient delivery to therapeutic targets within the organism at appropriate concentrations. These characteristics, encompassing pharmacokinetics, were assessed through the evaluation of ADME parameters (Absorption, Distribution, Metabolism, and Excretion). The key physicochemical, pharmacokinetic drug-likeness were computed using Web tool SwissADME (http://www.swissadme.ch) (accessed on 4 December 2024) [30,31]. Additionally, the safety profiles of compounds were evaluated using in silico Web tool ProTox-Ⅱ (https://tox.charite.de/protox3) (accessed on 4 December 2024) and StopTox. This tool predicts toxicity based on molecular similarity, pharmacophores, fragment propensities and a machine learning model [32].
2.8. Molecular Docking
Molecular docking analysis of compounds 1–3 was performed using Schrodinger docking suite (Schrödinger Maestro, Release 2024-2, Platform Windows-x64). Docking studies were conducted against the following targets: OmpC (PDB ID 3UU2), DNA gyrase subunit B (PDB ID 4DUH), topoisomerase Ⅳ (PDB ID 4HZ0), dihydrofolate synthase (PDB ID 6XG5), dihydropteroate synthase (PDB ID 5V7A), dehydratase (PDB ID 1U1Z), penicillin binding protein 3 (PDB ID 7ONW), penicillin binding protein 2 (PDB ID 6G9S) and transpeptidase (PDB ID 6NTW). Druggable pockets, representing highly potential binding sites, were identified using the SiteMap tool. Grid boxes with dimension 20 Å × 20 Å × 20 Å were generated using the Glid Receptor Grid Generation. The grid centers were determined based on the centroid of the ligand, and the binding site was identified using SiteMap tool. Ligand conformations were refined in torsional space using a distance-dependent dielectric model within the OPLS4 field during Glide XP docking. Docking poses were optimized within the field, allowing full flexibility of the ligand through post-docking minimization. The binding energy of the optimized free protein–ligand complex was calculated using Molecular Mechanics-Generalized Born Surface Area (MM-GBSA).
2.9. Molecular Dynamics Simulation Trajectory
Molecular dynamics (MD) simulation is a computational approach used to model and examine the interactions between a protein and a ligand within a simulated biological environment [33]. To assess the stability of the ligand–protein complex, a 100 ns MD simulation was performed on the complex with the highest binding affinity. Desmond, integrated within the Maestro platform, was utilized for these simulations. The ligand–protein complex was solvated using TIP3P water molecules within an orthorhombic box (dimensions: a = 10.0 Å, b = 10.0 Å, c = 10.0 Å; angles: α = 90.0°, β = 90.0°, γ = 90.0°). To neutralize the system, sodium and chloride ions were added to the simulation box. The OPLS4 force field was applied to ensure accurate stability analysis of the ligand–protein complex. Upon completion of the simulation, several metrics, including RMSD (root mean square deviation) and RMSF (root mean square fluctuation), were analyzed to characterize the protein–ligand interactions.
3. Results and Discussion
3.1. Isolation of Compounds 1–3
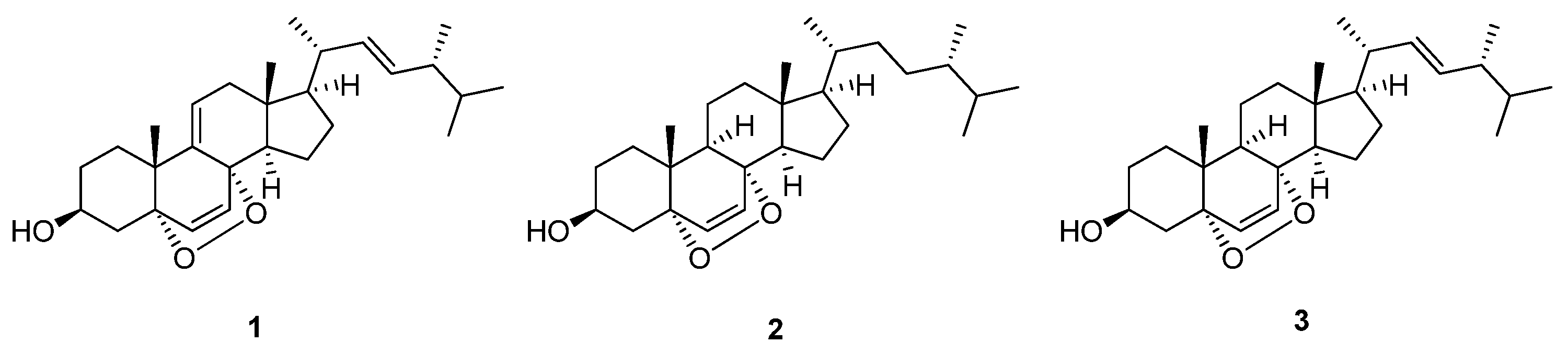
Air-dried G. orientispectabilis was extracted with 80% methanol (MeOH), which provided the resultant MeOH extract. The extract was subjected to fractionation and isolation, where column chromatography and HPLC purification techniques were used, resulting in the isolation of three fungal steroids (1–3) (Figure 2). These were identified to be 9,11-dehydroergosterol peroxide (1) [34], (3β,5α,8α)-5,8-epidioxyergost-6-en-3-ol (2) [35], and ergosterol peroxide (3) [36] by comparing their NMR spectroscopic and physical data with those previously reported.
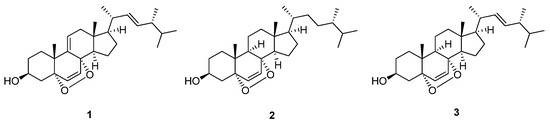
Figure 2.
Chemical structures of compounds 1–3 isolated from G. orientispectabilis.
3.2. Reported Bioactivities of Compounds 1–3
The primary chemical components of mushroom are sterols, with epidioxyergosterols being particularly recognized for their ability to inhibit specific cell growth and induce apoptosis. Compounds 1–3 have demonstrated the ability to induce cell cycle arrest and apoptosis in various cell lines, including HeLa, A375 human malignant melanoma, HL-60 leukemia, and HT-29 colorectal cancer cells, displaying significant antitumor activity [37,38,39]. Notably, compound 3 has been reported to exhibit anti-inflammatory [39], anti-obesity [40], immunosuppressive [41], and antitubercular properties [42]. Given that no additional activities have been identified for compounds 1 and 2, it would be beneficial to explore the antimicrobial activities of all three epidioxyergosterol compounds. Furthermore, complementing this investigation with in silico studies could provide additional valuable insights.
3.3. Antimicrobial Effects
The MeOH extract of G. orientispectabilis was evaluated for antimicrobial activities against E. coli, S. aureus and C. albicans. The extract only showed significant activity against E. coli, and among the solvent-partitioned fractions (hexane, CH2Cl2, EtOAc, n-butanol), hexane fraction (HX) exhibited notable inhibition with the IC50 value of 0.383 mg/mL. Eight subfractions of HX were further evaluated for inhibition against E. coli, where subfractions A3 and A4 showed the most significant inhibition, with IC50 values of 1.060 and 1.396 mg/mL, respectively. Among the subfractions resulting from A3 and A4, subfraction A32 exhibited effective inhibition with an IC50 value of 0.3466 mg/mL. Compounds 1–3 isolated from subfraction A32 all exhibited significant inhibition against E. coli, with IC50 values of 0.241, 0.424, 0.213 mg/mL, respectively, compared to the positive control (Kanamycin, IC50 = 5 μg/mL). These indicate comparatively weak antimicrobial activity compared to conventional steroidal drugs (ethinylestradiol and quinestrol each showed inhibition against E. coli with the IC50 values of 47 ± 28.9 and 0.2 ± 0.04 µg/mL) [43]. However, when comparing the IC50 values of compounds 1–3 with those of the reported antimicrobial natural steroids, chenodeoxycholic acid and ursodeoxycholic acid (IC50 = 543.4 and 547.5 μg/mL, respectively) [44], it suggests that compounds 1–3 could potentially serve as natural antimicrobial agents. Nonetheless, further experimental studies are necessary to clearly understand their mechanisms of action.
3.4. ADMET Analysis
Pharmacokinetic characteristics and toxicity often pose significant challenges during the development of drug candidates, as interactions between compounds can impact the pharmacokinetics of other drugs [45]. To address this, compounds 1–3 were analyzed using Swiss ADME software [31]. Water solubility was classified as poorly soluble in ESOL and Ali models but as moderately soluble in the SILICOS-IT model for all compounds. This suggests that 1–3 may have low bioavailability in water, highlighting the need to enhance their water solubility. Compounds 1–3 exhibited favorable lipophilicity with XLogP values below 5, and all compounds satisfied the criteria of HBA < 10 and HBD < 5, indicating that they possess drug-likeness as evaluated by Lipinski’s rule [46]. These results implied that improving water solubility could enhance the overall potential of compounds 1–3 for drug development. The TPSA values of 1–3 were below 140 Å, indicating their potential for enhanced oral bioavailability. This is further supported by their high gastrointestinal absorption, making them suitable for oral administration. Additionally, the inability to cross the BBB, the lack of interaction as a P-gp substrate, and the absence of CYP450 inhibition indicate favorable pharmacokinetic properties, suggesting minimal impact on drug metabolism. In silico toxicity evaluation of compounds 1–3 revealed that all compounds exhibit immunotoxicity and respiratory toxicity as a common characteristic. Additionally, compounds 2 and 3 demonstrated mutagenic effects, highlighting potential concerns for their safety profiles. On the other hand, acute toxicities, including inhalation, oral, dermal exposure, and eye irritation and corrosion, were not observed in any of the compounds tested using StopTox, with confidence levels ranging from 50 to 90% (Table 1). These findings offer valuable insights into the pharmacokinetic and toxicological properties of compounds 1–3, which will aid future efforts to enhance their safety and efficacy in drug development.

Table 1.
ADMET profile of compounds 1–3.
3.5. Molecular Docking Analysis
In molecular docking analysis, the binding affinity (MMGBSA dG binding energy) of compounds 1–3 was evaluated against selected bacterial drug targets, including OmpC, DNA gyrase subunit B, topoisomerase Ⅳ, dihydrofolate synthase, dihydropteroate synthase, dehydratase, penicillin-binding protein 3, penicillin-binding protein 2 and transpeptidase (Table 2).

Table 2.
Molecular docking target and binding energy of compounds 1–3.
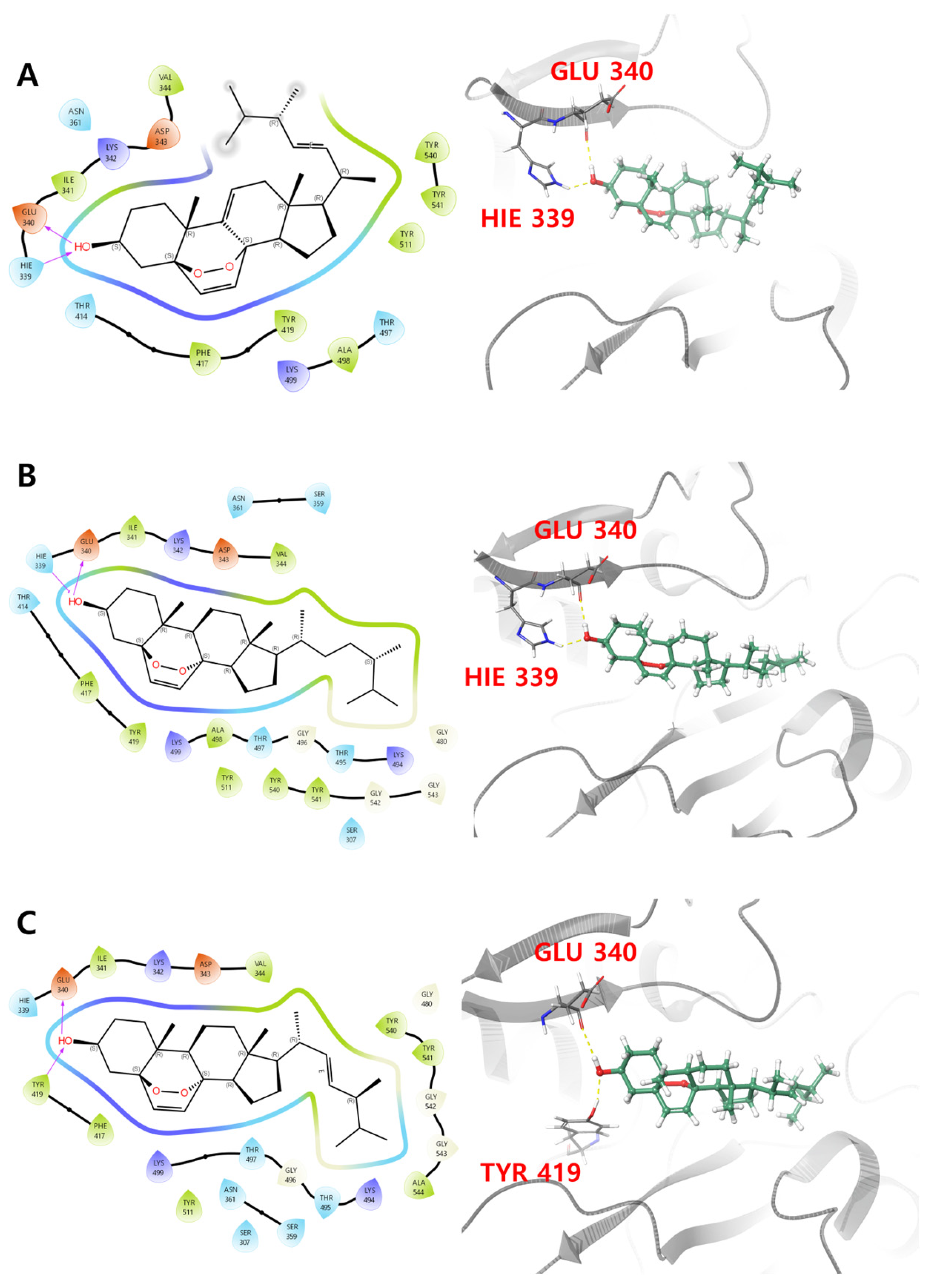
Compounds 1–3 all exhibited the highest binding affinity with penicillin binding protein 3 (PDB ID: 7ONW), with values of −62.89, −75.94 and −74.47 kcal/mol, respectively, where binding affinities with other proteins ranged from −40 to −60 kcal/mol. PBP3 contains a transpeptidase module with an active site that drives its transpeptidase activity. This is primarily dependent on eight key residues, Ser 307, Lys 310, Ser 359, Asn 361, Lys 494, Thr 495, Gly 496 and Thr 497, which are crucial for the binding of β-lactam antibiotics to the active site of PBPs [47]. As shown in Figure 3A and Table 3, primary interactions between PBP3 with 3-OH of compound 1 were observed, via H-bonds with Glu 340 and Hie 339. Identical H-bond interactions were observed for compound 2, and H-bonds between Glu 340 and Tyr 419 residues with the 3-OH of compound 3 were observed (Figure 3B,C). Additionally, the prime energy, which reflects structural stability, was observed to be −19,514.4, −19,488.1 and −19,511.0 kcal/mol, respectively, for compounds 1–3, respectively. Compound 2 showed slightly higher prime energy than compounds 1 and 3, suggesting the possibility of adopting a less stable pose with the protein.
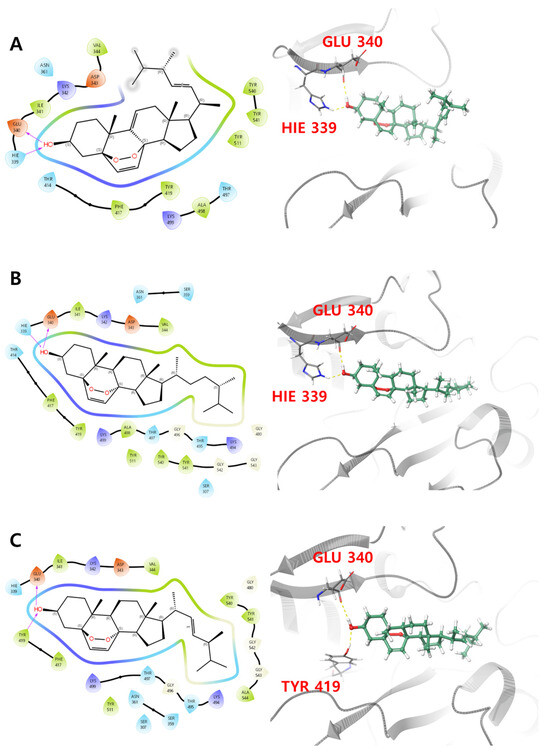
Figure 3.
Two- and three-dimensional interactions of (A) compound 1, (B) compound 2 and (C) compound 3 with PBP3 (PDB ID: 7ONW).

Table 3.
Residues of PBP3 (PDB ID: 7ONW) interacting with ligand and binding evaluation metrics.
3.6. Molecular Dynamics Simulation
Molecular dynamics (MD) simulations serve as a powerful tool for evaluating the motions and flexibility of proteins or protein–ligand complexes, providing valuable understanding of molecular behavior at an atomic level. These simulations have been extensively applied in various research fields, including drug discovery and development [33]. Although molecular docking provides valuable understanding of protein–ligand binding, it is inherently limited in capturing the dynamic nature of protein–ligand interactions. To overcome this, MD simulations were conducted. The metrics derived from the MD simulation trajectory include the protein–ligand interaction histogram, root mean square deviation (RMSD) of ligand atoms, RMSD of protein Cα atoms, and protein root mean square fluctuation (RMSF).
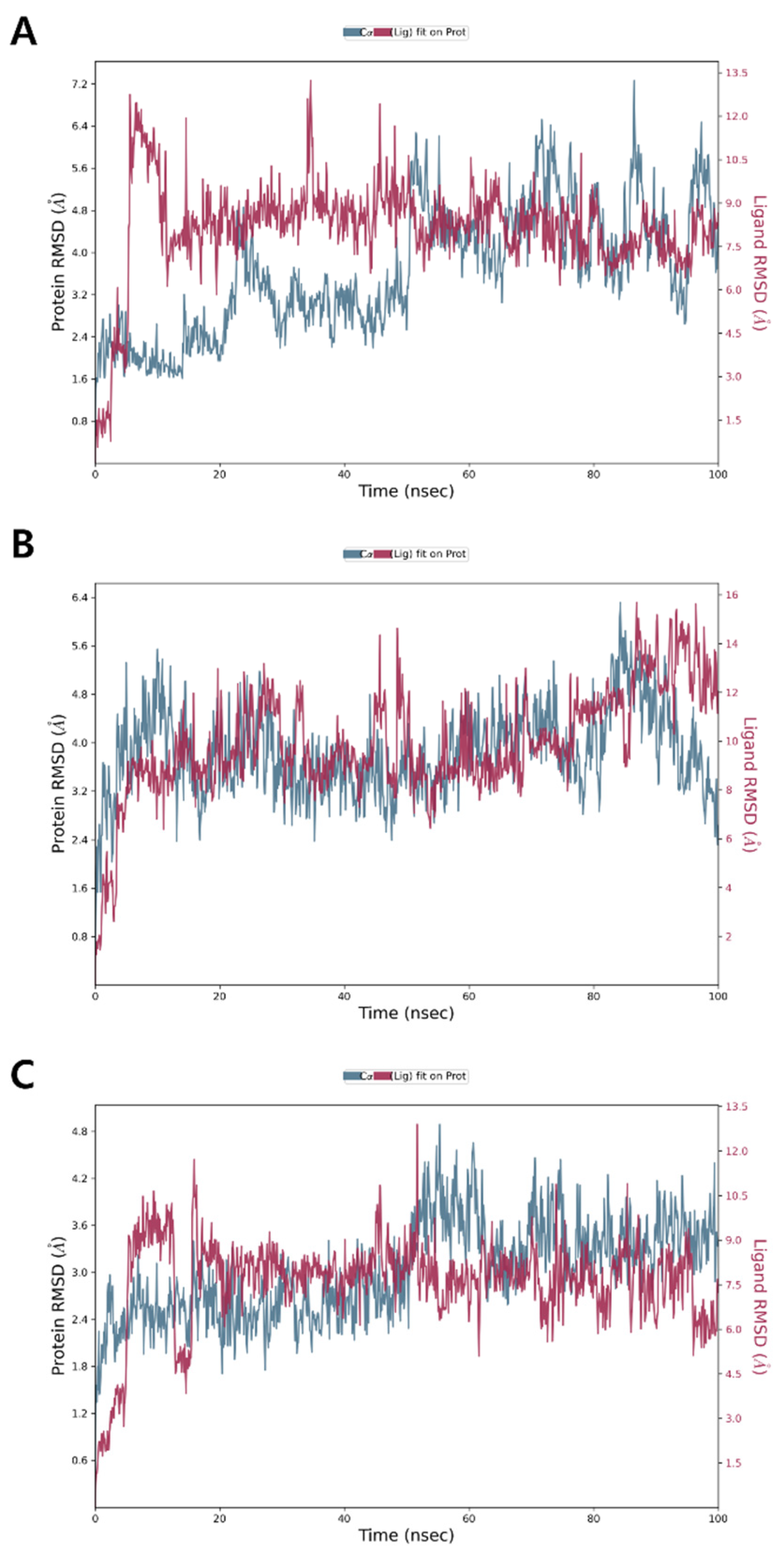
The RMSD of the PBP3 Cα backbone complex with 1 showed an initial increase during the first 10 ns, after which it stabilized over the subsequent 50 ns. However, further fluctuations were observed between 60 ns and 100 ns, suggesting a dynamic equilibrium with minor instability toward the end (Figure 4A). For compounds 2 and 3, the RMSD showed an initial increase during the first 10 ns, followed by stabilization with minimal dynamic fluctuations throughout the remaining simulation period, indicating relatively stable binding interactions (Figure 4B,C). The average RMSD values for the backbone of PBP3 complexes with compounds 1, 2 and 3 were approximately 3.6, 3.8 and 3.0 Å, respectively. An RMSD of around 3.0 Å suggests that the ligand–protein complex adopts a conformational state with partial structural alignment to the native pose [48]. This indicates significant conformational flexibility, reflecting a non-ideal yet not fully unstable interaction of compounds 1–3 with PBP3.
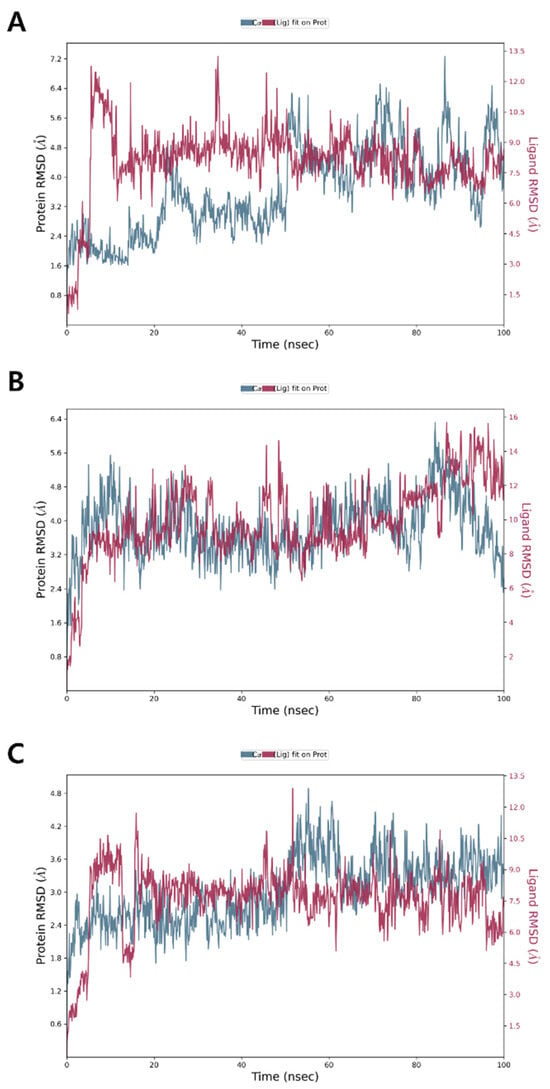
Figure 4.
MD simulation RMSD of PBP3 complexes with (A) compound 1, (B) compound 2 and (C) compound 3.
In the protein–ligand interaction analysis, compounds 1 and 3 showed stronger interactions, forming H-bonds with His 339 and Glu 340 (<10%) and hydrophobic interactions with Tyr 419 and Tyr 507 (30% and 50%, respectively). Compound 2 exhibited weaker interactions, contributing less than 3% to hydrogen bonds and less than 15% to hydrophobic interactions (Figure 5A–C), which agrees with the relatively weaker binding affinity of compound 2 with the binding pocket of PBP3. The root mean square fluctuation (RMSF) reflects the positional variability of ligand atoms, with lower values indicating greater stability of the complex [49]. Residues interacting with the ligand, marked by green vertical bars, exhibited RMSF values around 1.5 Å, indicating a stable interaction profile. The average RMSF values of the active site residues in PBP3, including Ser 307, Lys 310, Ser 359, Asn 361, Lys 494, Thr 495, Gly 496, and Thr 497, in a complex with compounds 1–3, were approximately 0.78 Å, 0.58 Å and 0.84 Å, respectively (Figure 5D–F). The reduced RMSF values of the active site residues suggest that the ligand may restrict the conformational flexibility required for catalytic activity, thereby contributing to enzyme inhibition.
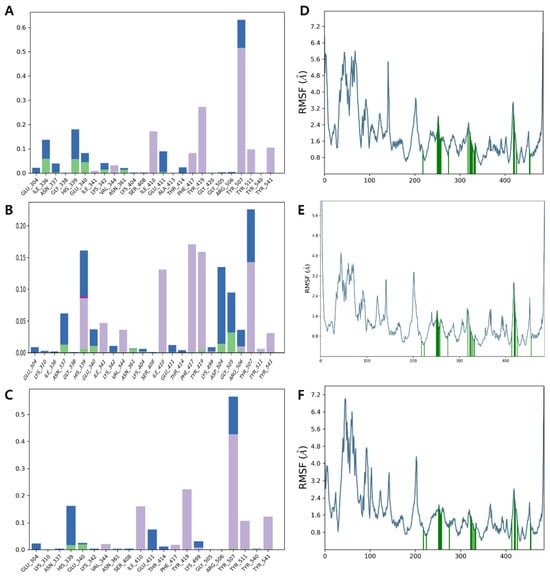
Figure 5.
Protein–ligand interaction histogram indicating residues in direct contact with (A) compound 1, (B) compound 2 and (C) compound 3 (green, purple, pink, and blue bars represent H-bonds, hydrophobic contacts, ionic interactions, and water bridges, respectively), and RMSF profile for (D) compound 1, (E) compound 2 and (F) compound 3, with green vertical bars highlighting ligand-contacting residues.
4. Conclusions
In the current study, 9,11-dehydroergosterol peroxide (1), (3β,5α,8α)-5,8-epidioxyergost-6-en-3-ol (2), and ergosterol peroxide (3) were isolated from G. orientispectabilis via bioactivity-guided separation. The extract of G. orientispectabilis, its active fractions, and the isolated fungal steroids exhibited significant antimicrobial activity against E. coli. The isolated steroids were examined in silico to identify their mode of action in exhibiting antimicrobial activity. This is based on the understanding that antibiotics operate through various mechanisms, such as inhibition of cell wall synthesis, membrane depolarization, protein synthesis inhibition, nucleic acid synthesis inhibition, and metabolic pathway disruption [50]. Based on molecular docking analysis, it was predicted that the fungal steroids (1–3) may exhibit antimicrobial activity by inhibiting bacterial cell wall synthesis, since all compounds exhibited the lowest binding energy with PBP3. The hydroxyl group of compounds 1–3 formed H-bonds with Glu 340, Hie 339 and Tyr 419 residues, indicating a potential inhibitory interaction that could disrupt the activity of the binding site. MD simulations further validated the docking results, where the RMSD of the protein–ligand complex remained stable at approximately 3 Å for all compounds. Furthermore, RMSF analysis revealed restricted movement of key residues in PBP3, suggesting that compounds 1–3 may inhibit the activity by indirectly modulating its dynamics. The ADMET analysis indicated low water solubility, favorable bioavailability, and some toxicity concerns for compounds 1–3, emphasizing areas for enhancement to improve their safety and efficacy in future drug development. This study provides important insights into the potential therapeutic application of G. orientispectabilis and its fungal steroids, highlighting their potential as antimicrobial agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations12020023/s1, Figure S1: 1H NMR spectrum (600 MHz, CDCl3) of 1; Figure S2: 13C NMR spectrum (150 MHz, CDCl3) of 1; Figure S3: 1H NMR spectrum (600 MHz, CDCl3) of 2; Figure S4: 13C NMR spectrum (150 MHz, CDCl3) of 2; Figure S5: 1H NMR spectrum (600 MHz, CDCl3) of 3.
Author Contributions
Conceptualization, B.S. and S.L.; methodology, B.J. and E.J.H.; formal analysis, B.J., E.J.H. and D.L.N.; investigation, B.J., E.J.H. and D.L.N.; resources, U.J.Y.; writing—original draft preparation, B.J. and E.J.H.; writing—review and editing, K.H.K., B.S. and S.L.; visualization, B.J. and E.J.H.; supervision, B.S. and S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-00212670).
Data Availability Statement
All data supporting the results of this study are included in the manuscript and the Supplementary Materials, and the datasets are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liévin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. Penicillin. Br. Med. J. 1941, 2, 386. [Google Scholar] [CrossRef]
- Umezawa, H.; Ueda, M.; Maeda, K.; Yagishita, K.; Kondō, S.; Okami, Y.; Utahara, R.; Ōsato, Y.; Nitta, K.; Takeuchi, T. Production and isolation of a new antibiotic, kanamycin. J. Antibiot. Ser. A 1957, 10, 181–188. [Google Scholar]
- Lyu, X.; Zhao, C.; Yan, Z.-m.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des. Devel. Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef]
- Brown, M.; Collier, P.J.; Gilbert, P. Influence of growth rate on susceptibility to antimicrobial agents: Modification of the cell envelope and batch and continuous culture studies. Antimicrob. Agents Chemother. 1990, 34, 1623–1628. [Google Scholar] [CrossRef]
- Gootz, T.D. Discovery and development of new antimicrobial agents. Clin. Microbiol. Rev. 1990, 3, 13–31. [Google Scholar] [CrossRef]
- Feingold, D.S. Antimicrobial chemotherapeutic agents: The nature of their action and selective toxicity. N. Engl. J. Med. 1963, 269, 900–907. [Google Scholar] [CrossRef]
- Khelaifia, S.; Drancourt, M. Susceptibility of archaea to antimicrobial agents: Applications to clinical microbiology. Clin. Microbiol. Infect. 2012, 18, 841–848. [Google Scholar] [CrossRef]
- Schwarz, S.; Cavaco, L.; Shen, J.Z.; Aarestrup, F.M. Antimicrobial Resistance in Bacteria from Livestock and Companion Animals; ASM Press: Washington, DC, USA, 2018; Chapter 4; pp. 51–82. [Google Scholar]
- Larsson, D.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Cho, S.-M.; Seok, S.-J.; Yun, B.-S. Chemical constituents of Gymnopilus spectabilis and their antioxidant activity. Mycobiology 2008, 36, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Dašić, M. Mushrooms as possible antioxidant and antimicrobial agents. Iran. J. Pharm. Res. 2012, 11, 1095. [Google Scholar]
- Lee, S.; Yu, J.S.; Lee, S.R.; Kim, K.H. Non-peptide secondary metabolites from poisonous mushrooms: Overview of chemistry, bioactivity, and biosynthesis. Nat. Prod. Rep. 2022, 39, 512–559. [Google Scholar] [CrossRef]
- Thorn, R.G.; Malloch, D.W.; Saar, I.; Lamoureux, Y.; Nagasawa, E.; Redhead, S.A.; Margaritescu, S.; Moncalvo, J.-M. New species in the Gymnopilus junonius group (Basidiomycota: Agaricales). Botany 2020, 98, 293–315. [Google Scholar] [CrossRef]
- Findlay, J.A.; He, Z.-Q. Minor constituents of Gymnopilus spectabilis. J. Nat. Prod. 1991, 54, 184–189. [Google Scholar] [CrossRef]
- Kayano, T.; Kitamura, N.; Miyazaki, S.; Ichiyanagi, T.; Shimomura, N.; Shibuya, I.; Aimi, T. Gymnopilins, a product of a hallucinogenic mushroom, inhibit the nicotinic acetylcholine receptor. Toxicon 2014, 81, 23–31. [Google Scholar] [CrossRef]
- Tanaka, M.; Hashimoto, K.; Okuno, T.; Shirahama, H. Neurotoxic oligoisoprenoids of the hallucinogenic mushroom, Gymnopilus spectabilis. Phytochemistry 1993, 34, 661–664. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a styrylpyrone-fused steroid with a hexacyclic 6/5/6/6/6/5 skeleton from a mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.-K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharmacal Res. 2024, 47, 272–287. [Google Scholar] [CrossRef]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.-H.; Kim, S.-H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharmacal Res. 2020, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.A.; Kinghorn, A.D.; Rakotondraibe, H.L. Bioactive and unusual steroids from Penicillium fungi. Phytochemistry 2023, 209, 113638. [Google Scholar] [CrossRef] [PubMed]
- Zhabinskii, V.N.; Drasar, P.; Khripach, V.A. Structure and biological activity of ergostane-type steroids from fungi. Molecules 2022, 27, 2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, Z.; Qiu, P.; Wang, Q.; Yan, J.; Lu, Y.; Wasu, P.-A.; Hong, K.; She, Z. Two new bioactive steroids from a mangrove-derived fungus Aspergillus sp. Steroids 2018, 140, 32–38. [Google Scholar] [CrossRef]
- Keller, A.C.; Maillard, M.P.; Hostettmann, K. Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry 1996, 41, 1041–1046. [Google Scholar] [CrossRef]
- Dos Santos Dias, A.C.; Couzinet-Mossion, A.; Ruiz, N.; Lakhdar, F.; Etahiri, S.; Bertrand, S.; Ory, L.; Roussakis, C.; Pouchus, Y.F.; Nazih, E.-H. Steroids from marine-derived fungi: Evaluation of antiproliferative and antimicrobial activities of eburicol. Mar. Drugs 2019, 17, 372. [Google Scholar] [CrossRef]
- Nadaraia, N.S.; Amiranashvili, L.S.; Merlani, M.; Kakhabrishvili, M.L.; Barbakadze, N.N.; Geronikaki, A.; Petrou, A.; Poroikov, V.; Ciric, A.; Glamoclija, J. Novel antimicrobial agents’ discovery among the steroid derivatives. Steroids 2019, 144, 52–65. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J.D.; McCammon, J.A. Molecular dynamics simulations and drug discovery. BMC Biol. 2011, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-K.; Kuo, Y.-H.; Chiang, B.-H.; Lo, J.-M.; Sheen, L.-Y. Cytotoxic activities of 9, 11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009, 57, 5713–5719. [Google Scholar] [CrossRef]
- Pan, M.-H.; Huang, Y.-T.; Chang, C.-I.; Ho, C.-T.; Pan, B.S. Apoptotic-inducing epidioxysterols identified in hard clam (Meretrix lusoria). Food Chem. 2007, 102, 788–795. [Google Scholar] [CrossRef]
- Krzyczkowski, W.; Malinowska, E.; Suchocki, P.; Kleps, J.; Olejnik, M.; Herold, F. Isolation and quantitative determination of ergosterol peroxide in various edible mushroom species. Food Chem. 2009, 113, 351–355. [Google Scholar] [CrossRef]
- Cui, Y.-J.; Guan, S.-H.; Feng, L.-X.; Song, X.-Y.; Ma, C.; Cheng, C.-R.; Wang, W.-B.; Wu, W.-Y.; Yue, Q.-X.; Liu, X. Cytotoxicity of 9, 11-dehydroergosterol peroxide isolated from Ganoderma lucidum and its target-related proteins. Nat. Prod. Commun. 2010, 5, 1934578X1000500806. [Google Scholar]
- Zheng, L.; Wong, Y.-S.; Shao, M.; Huang, S.; Wang, F.; Chen, J. Apoptosis induced by 9, 11-dehydroergosterol peroxide from Ganoderma lucidum mycelium in human malignant melanoma cells is Mcl-1 dependent. Mol. Med. Rep. 2018, 18, 938–944. [Google Scholar]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264. 7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef]
- Jeong, Y.-U.; Park, Y.-J. Ergosterol peroxide from the medicinal mushroom Ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. Int. J. Mol. Sci. 2020, 21, 460. [Google Scholar] [CrossRef]
- Kuo, Y.; Weng, S.; Chou, C.; Chang, T.; Tsai, W. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br. J. Pharmacol. 2003, 140, 895. [Google Scholar] [CrossRef]
- Duarte, N.; Ferreira, M.J.U.; Martins, M.; Viveiros, M.; Amaral, L. Antibacterial activity of ergosterol peroxide against Mycobacterium tuberculosis: Dependence upon system and medium employed. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.A.; Webster, C.M.; Shaw, L.N.; Torres, N.J.; Jobson, M.-E.; Totzke, B.C.; Jackson, J.K.; McGreig, J.E.; Wass, M.N.; Robinson, G.K. Steroid drugs inhibit bacterial respiratory oxidases and are lethal toward methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2024, 230, e149–e158. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, J.; Xing, X.; Jin, C.; Xiao, X.; Zhao, Y.; Zhang, P.; Zang, Q.; Li, Z. Screening for novel antibacterial agents based on the activities of compounds on metabolism of Escherichia coli: A microcalorimetric study. J. Hazard. Mater. 2011, 185, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Hussain, E.A.; Shujaat, S.; Khan, M.U.; Ali, Q.; Malook, S.U.; Ali, D. Antibacterial potential of Propolis: Molecular docking, simulation and toxicity analysis. AMB Express 2024, 14, 81. [Google Scholar] [CrossRef]
- Adamovich, S.N.; Kondrashov, E.V.; Ushakov, I.A.; Shatokhina, N.S.; Oborina, E.N.; Vashchenko, A.V.; Belovezhets, L.A.; Rozentsveig, I.B.; Verpoort, F. Isoxazole derivatives of silatrane: Synthesis, characterization, in silico ADME profile, prediction of potential pharmacological activity and evaluation of antimicrobial action. Appl. Organomet. Chem. 2020, 34, e5976. [Google Scholar] [CrossRef]
- Sauvage, E.; Derouaux, A.; Fraipont, C.; Joris, M.; Herman, R.; Rocaboy, M.; Schloesser, M.; Dumas, J.; Kerff, F.; Nguyen-Disteche, M. Crystal structure of penicillin-binding protein 3 (PBP3) from Escherichia coli. PLoS ONE 2014, 9, e98042. [Google Scholar] [CrossRef]
- Liu, K.; Kokubo, H. Exploring the stability of ligand binding modes to proteins by molecular dynamics simulations: A cross-docking study. J. Chem. Inf. Model. 2017, 57, 2514–2522. [Google Scholar] [CrossRef]
- Paul, S.K.; Saddam, M.; Rahaman, K.A.; Choi, J.-G.; Lee, S.-S.; Hasan, M. Molecular modeling, molecular dynamics simulation, and essential dynamics analysis of grancalcin: An upregulated biomarker in experimental autoimmune encephalomyelitis mice. Heliyon 2022, 8, e11232. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).