Antimicrobial Steroids from Poisonous Mushroom Gymnopilus orientispectabilis and Their Molecular Docking Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. Chromatographic Techniques
2.3. Spectroscopy
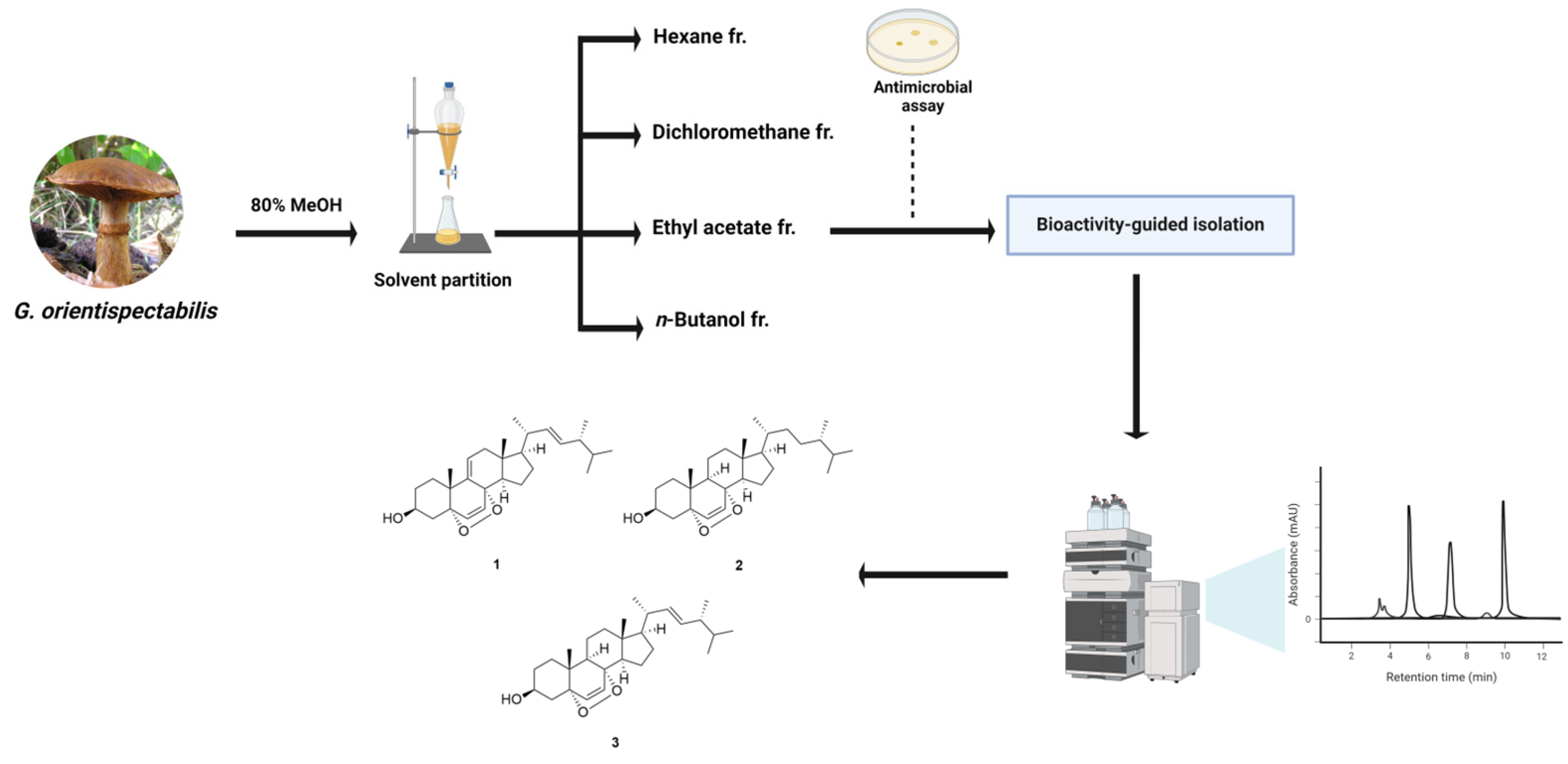
2.4. Extraction and Isolation
2.5. Antimicrobial Assay
2.6. Ligand and Protein Preparation
2.7. In Silico ADMET Analysis
2.8. Molecular Docking
2.9. Molecular Dynamics Simulation Trajectory
3. Results and Discussion
3.1. Isolation of Compounds 1–3
3.2. Reported Bioactivities of Compounds 1–3
3.3. Antimicrobial Effects
3.4. ADMET Analysis
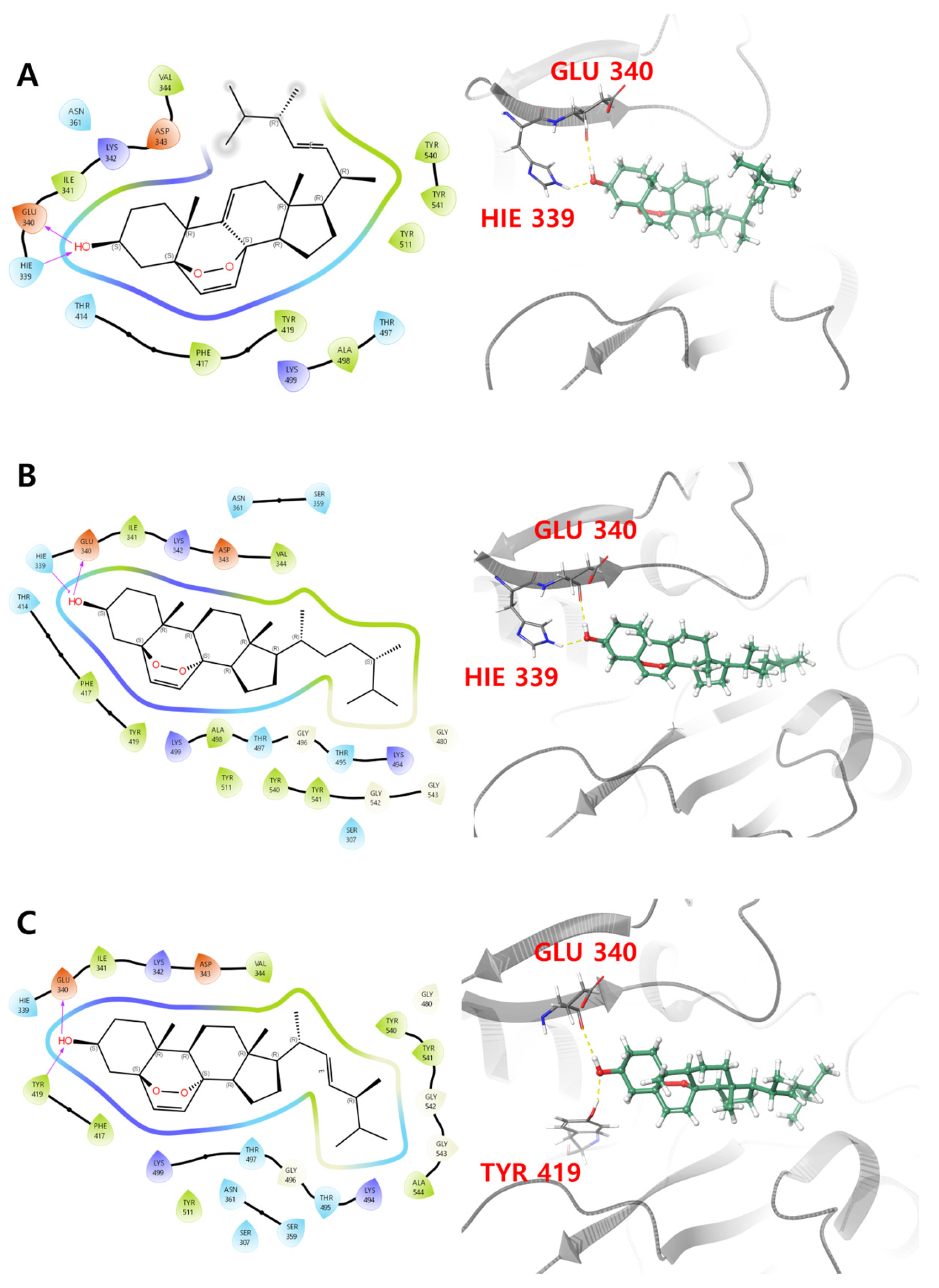
3.5. Molecular Docking Analysis
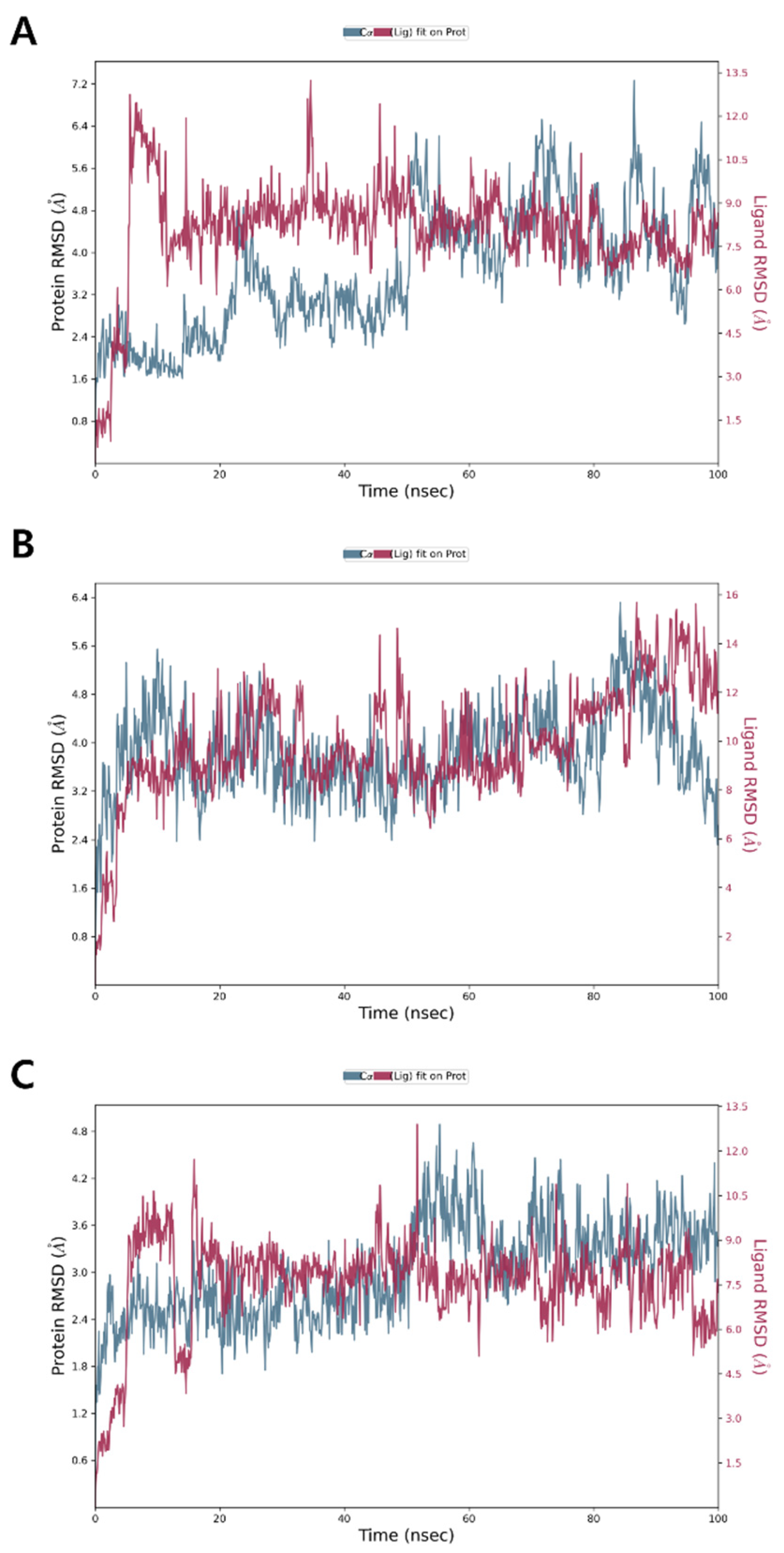
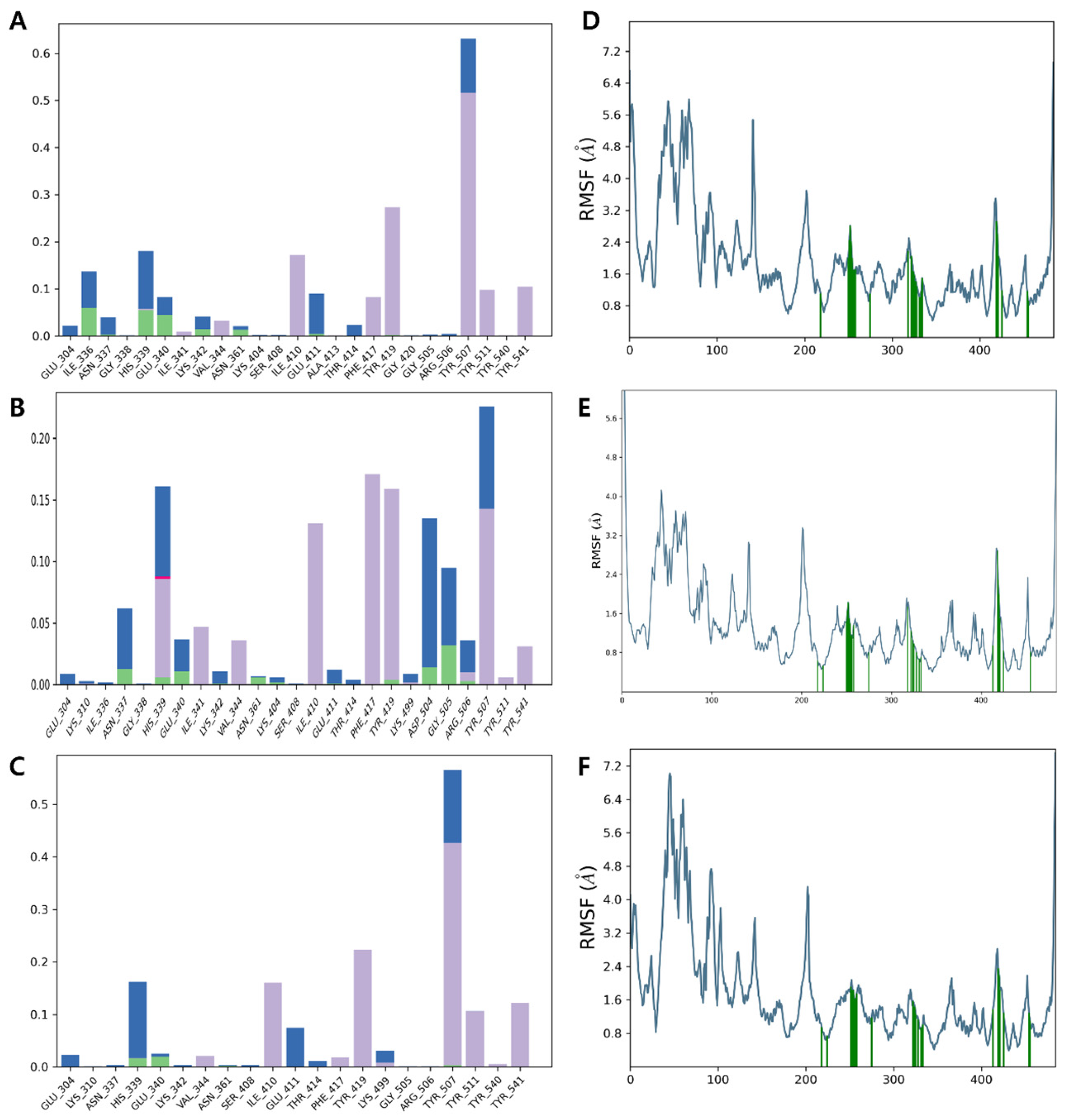
3.6. Molecular Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liévin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. Penicillin. Br. Med. J. 1941, 2, 386. [Google Scholar] [CrossRef]
- Umezawa, H.; Ueda, M.; Maeda, K.; Yagishita, K.; Kondō, S.; Okami, Y.; Utahara, R.; Ōsato, Y.; Nitta, K.; Takeuchi, T. Production and isolation of a new antibiotic, kanamycin. J. Antibiot. Ser. A 1957, 10, 181–188. [Google Scholar]
- Lyu, X.; Zhao, C.; Yan, Z.-m.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des. Devel. Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef]
- Brown, M.; Collier, P.J.; Gilbert, P. Influence of growth rate on susceptibility to antimicrobial agents: Modification of the cell envelope and batch and continuous culture studies. Antimicrob. Agents Chemother. 1990, 34, 1623–1628. [Google Scholar] [CrossRef]
- Gootz, T.D. Discovery and development of new antimicrobial agents. Clin. Microbiol. Rev. 1990, 3, 13–31. [Google Scholar] [CrossRef]
- Feingold, D.S. Antimicrobial chemotherapeutic agents: The nature of their action and selective toxicity. N. Engl. J. Med. 1963, 269, 900–907. [Google Scholar] [CrossRef]
- Khelaifia, S.; Drancourt, M. Susceptibility of archaea to antimicrobial agents: Applications to clinical microbiology. Clin. Microbiol. Infect. 2012, 18, 841–848. [Google Scholar] [CrossRef]
- Schwarz, S.; Cavaco, L.; Shen, J.Z.; Aarestrup, F.M. Antimicrobial Resistance in Bacteria from Livestock and Companion Animals; ASM Press: Washington, DC, USA, 2018; Chapter 4; pp. 51–82. [Google Scholar]
- Larsson, D.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Cho, S.-M.; Seok, S.-J.; Yun, B.-S. Chemical constituents of Gymnopilus spectabilis and their antioxidant activity. Mycobiology 2008, 36, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Dašić, M. Mushrooms as possible antioxidant and antimicrobial agents. Iran. J. Pharm. Res. 2012, 11, 1095. [Google Scholar]
- Lee, S.; Yu, J.S.; Lee, S.R.; Kim, K.H. Non-peptide secondary metabolites from poisonous mushrooms: Overview of chemistry, bioactivity, and biosynthesis. Nat. Prod. Rep. 2022, 39, 512–559. [Google Scholar] [CrossRef]
- Thorn, R.G.; Malloch, D.W.; Saar, I.; Lamoureux, Y.; Nagasawa, E.; Redhead, S.A.; Margaritescu, S.; Moncalvo, J.-M. New species in the Gymnopilus junonius group (Basidiomycota: Agaricales). Botany 2020, 98, 293–315. [Google Scholar] [CrossRef]
- Findlay, J.A.; He, Z.-Q. Minor constituents of Gymnopilus spectabilis. J. Nat. Prod. 1991, 54, 184–189. [Google Scholar] [CrossRef]
- Kayano, T.; Kitamura, N.; Miyazaki, S.; Ichiyanagi, T.; Shimomura, N.; Shibuya, I.; Aimi, T. Gymnopilins, a product of a hallucinogenic mushroom, inhibit the nicotinic acetylcholine receptor. Toxicon 2014, 81, 23–31. [Google Scholar] [CrossRef]
- Tanaka, M.; Hashimoto, K.; Okuno, T.; Shirahama, H. Neurotoxic oligoisoprenoids of the hallucinogenic mushroom, Gymnopilus spectabilis. Phytochemistry 1993, 34, 661–664. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a styrylpyrone-fused steroid with a hexacyclic 6/5/6/6/6/5 skeleton from a mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.-K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharmacal Res. 2024, 47, 272–287. [Google Scholar] [CrossRef]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.-H.; Kim, S.-H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharmacal Res. 2020, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.A.; Kinghorn, A.D.; Rakotondraibe, H.L. Bioactive and unusual steroids from Penicillium fungi. Phytochemistry 2023, 209, 113638. [Google Scholar] [CrossRef] [PubMed]
- Zhabinskii, V.N.; Drasar, P.; Khripach, V.A. Structure and biological activity of ergostane-type steroids from fungi. Molecules 2022, 27, 2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, Z.; Qiu, P.; Wang, Q.; Yan, J.; Lu, Y.; Wasu, P.-A.; Hong, K.; She, Z. Two new bioactive steroids from a mangrove-derived fungus Aspergillus sp. Steroids 2018, 140, 32–38. [Google Scholar] [CrossRef]
- Keller, A.C.; Maillard, M.P.; Hostettmann, K. Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry 1996, 41, 1041–1046. [Google Scholar] [CrossRef]
- Dos Santos Dias, A.C.; Couzinet-Mossion, A.; Ruiz, N.; Lakhdar, F.; Etahiri, S.; Bertrand, S.; Ory, L.; Roussakis, C.; Pouchus, Y.F.; Nazih, E.-H. Steroids from marine-derived fungi: Evaluation of antiproliferative and antimicrobial activities of eburicol. Mar. Drugs 2019, 17, 372. [Google Scholar] [CrossRef]
- Nadaraia, N.S.; Amiranashvili, L.S.; Merlani, M.; Kakhabrishvili, M.L.; Barbakadze, N.N.; Geronikaki, A.; Petrou, A.; Poroikov, V.; Ciric, A.; Glamoclija, J. Novel antimicrobial agents’ discovery among the steroid derivatives. Steroids 2019, 144, 52–65. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J.D.; McCammon, J.A. Molecular dynamics simulations and drug discovery. BMC Biol. 2011, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-K.; Kuo, Y.-H.; Chiang, B.-H.; Lo, J.-M.; Sheen, L.-Y. Cytotoxic activities of 9, 11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009, 57, 5713–5719. [Google Scholar] [CrossRef]
- Pan, M.-H.; Huang, Y.-T.; Chang, C.-I.; Ho, C.-T.; Pan, B.S. Apoptotic-inducing epidioxysterols identified in hard clam (Meretrix lusoria). Food Chem. 2007, 102, 788–795. [Google Scholar] [CrossRef]
- Krzyczkowski, W.; Malinowska, E.; Suchocki, P.; Kleps, J.; Olejnik, M.; Herold, F. Isolation and quantitative determination of ergosterol peroxide in various edible mushroom species. Food Chem. 2009, 113, 351–355. [Google Scholar] [CrossRef]
- Cui, Y.-J.; Guan, S.-H.; Feng, L.-X.; Song, X.-Y.; Ma, C.; Cheng, C.-R.; Wang, W.-B.; Wu, W.-Y.; Yue, Q.-X.; Liu, X. Cytotoxicity of 9, 11-dehydroergosterol peroxide isolated from Ganoderma lucidum and its target-related proteins. Nat. Prod. Commun. 2010, 5, 1934578X1000500806. [Google Scholar]
- Zheng, L.; Wong, Y.-S.; Shao, M.; Huang, S.; Wang, F.; Chen, J. Apoptosis induced by 9, 11-dehydroergosterol peroxide from Ganoderma lucidum mycelium in human malignant melanoma cells is Mcl-1 dependent. Mol. Med. Rep. 2018, 18, 938–944. [Google Scholar]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264. 7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef]
- Jeong, Y.-U.; Park, Y.-J. Ergosterol peroxide from the medicinal mushroom Ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. Int. J. Mol. Sci. 2020, 21, 460. [Google Scholar] [CrossRef]
- Kuo, Y.; Weng, S.; Chou, C.; Chang, T.; Tsai, W. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br. J. Pharmacol. 2003, 140, 895. [Google Scholar] [CrossRef]
- Duarte, N.; Ferreira, M.J.U.; Martins, M.; Viveiros, M.; Amaral, L. Antibacterial activity of ergosterol peroxide against Mycobacterium tuberculosis: Dependence upon system and medium employed. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.A.; Webster, C.M.; Shaw, L.N.; Torres, N.J.; Jobson, M.-E.; Totzke, B.C.; Jackson, J.K.; McGreig, J.E.; Wass, M.N.; Robinson, G.K. Steroid drugs inhibit bacterial respiratory oxidases and are lethal toward methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2024, 230, e149–e158. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, J.; Xing, X.; Jin, C.; Xiao, X.; Zhao, Y.; Zhang, P.; Zang, Q.; Li, Z. Screening for novel antibacterial agents based on the activities of compounds on metabolism of Escherichia coli: A microcalorimetric study. J. Hazard. Mater. 2011, 185, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Hussain, E.A.; Shujaat, S.; Khan, M.U.; Ali, Q.; Malook, S.U.; Ali, D. Antibacterial potential of Propolis: Molecular docking, simulation and toxicity analysis. AMB Express 2024, 14, 81. [Google Scholar] [CrossRef]
- Adamovich, S.N.; Kondrashov, E.V.; Ushakov, I.A.; Shatokhina, N.S.; Oborina, E.N.; Vashchenko, A.V.; Belovezhets, L.A.; Rozentsveig, I.B.; Verpoort, F. Isoxazole derivatives of silatrane: Synthesis, characterization, in silico ADME profile, prediction of potential pharmacological activity and evaluation of antimicrobial action. Appl. Organomet. Chem. 2020, 34, e5976. [Google Scholar] [CrossRef]
- Sauvage, E.; Derouaux, A.; Fraipont, C.; Joris, M.; Herman, R.; Rocaboy, M.; Schloesser, M.; Dumas, J.; Kerff, F.; Nguyen-Disteche, M. Crystal structure of penicillin-binding protein 3 (PBP3) from Escherichia coli. PLoS ONE 2014, 9, e98042. [Google Scholar] [CrossRef]
- Liu, K.; Kokubo, H. Exploring the stability of ligand binding modes to proteins by molecular dynamics simulations: A cross-docking study. J. Chem. Inf. Model. 2017, 57, 2514–2522. [Google Scholar] [CrossRef]
- Paul, S.K.; Saddam, M.; Rahaman, K.A.; Choi, J.-G.; Lee, S.-S.; Hasan, M. Molecular modeling, molecular dynamics simulation, and essential dynamics analysis of grancalcin: An upregulated biomarker in experimental autoimmune encephalomyelitis mice. Heliyon 2022, 8, e11232. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]

| Compound | Chronic Toxicity by Pro Tox II | Pharmacokinetics by Swiss ADME | ||
|---|---|---|---|---|
| 1 | Cytotoxicity | No | GI absorption | High |
| Respiratory toxicity | Yes | BBB | No | |
| Immunotoxicity | Yes | P-gp substrate | No | |
| 2 | Mutagenic | No | CYP450 inhibitor | No |
| Cytotoxicity | No | GI absorption | High | |
| Respiratory toxicity | Yes | BBB | No | |
| Immunotoxicity | Yes | P-gp substrate | No | |
| 3 | Mutagenic | Yes | CYP450 inhibitor | No |
| Cytotoxicity | No | GI absorption | High | |
| Respiratory toxicity | Yes | BBB | No | |
| Immunotoxicity | Yes | P-gp substrate | No | |
| Mutagenic | Yes | CYP450 inhibitor | No | |
| Key Targets | Target Protein (PDB ID) | Organism | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|---|---|
| Cytoplasmic membrane structure | OmpC (PDB ID: 3UU2) | Salmonella enterica sub sp. | −55.89 | −60.25 | −71.14 |
| DNA | DNA gyrase subunit B (PDB ID: 4DUH) | Escherichia coli K-12 | −56.56 | −41.39 | −34.25 |
| Topoisomerase IV (PDB ID: 4HZ0) | Escherichia coli K-12 | −51.79 | −58.65 | −60.58 | |
| Metabolism | Dihydrofolate synthase (PDB ID: 6XG5) | Escherichia coli K-12 | −62.21 | −59.44 | −54.36 |
| Dihydropteroate synthase (PDB ID: 5V7A) | Escherichia coli CFT073 | −57.70 | −57.59 | −56.11 | |
| Dehydratase (PDB ID: 1U1Z) | Pseudomonas aeruginosa | −38.88 | −46.37 | −51.34 | |
| Cell wall synthesis | Penicillin binding protein 3 (PDB ID: 7ONW) | Escherichia coli | −62.89 | −75.94 | −74.47 |
| Penicillin binding protein 2 (PDB ID: 6G9S) | Escherichia coli K-12 | −58.86 | −61.94 | −48.92 | |
| Transpeptidase (PDB ID: 6NTW) | Escherichia coli K-12 | −47.72 | −46.31 | −45.06 |
| Compound | Glide Emodel | Glide Gscore | Prime Energy | MMGBSA dG Bind | Residues Primarily Interacting with Ligand |
|---|---|---|---|---|---|
| 1 | −40.219 | −5.853 | −19,514.4 | −62.89 | H-bond interaction: HIE 339, GLU 340 |
| 2 | −41.396 | −4.837 | −19,488.1 | −75.94 | H-bond interaction: GLU 340, TYR 419 |
| 3 | −35.906 | −4.743 | −19,511.0 | −74.47 | H-bond interaction: HIE 339, GLU 340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, B.; Heo, E.J.; Nguyen, D.L.; Youn, U.J.; Kim, K.H.; Son, B.; Lee, S. Antimicrobial Steroids from Poisonous Mushroom Gymnopilus orientispectabilis and Their Molecular Docking Studies. Separations 2025, 12, 23. https://doi.org/10.3390/separations12020023
Jung B, Heo EJ, Nguyen DL, Youn UJ, Kim KH, Son B, Lee S. Antimicrobial Steroids from Poisonous Mushroom Gymnopilus orientispectabilis and Their Molecular Docking Studies. Separations. 2025; 12(2):23. https://doi.org/10.3390/separations12020023
Chicago/Turabian StyleJung, Bowon, Eun Jin Heo, Dieu Linh Nguyen, Ui Joung Youn, Ki Hyun Kim, Boram Son, and Seulah Lee. 2025. "Antimicrobial Steroids from Poisonous Mushroom Gymnopilus orientispectabilis and Their Molecular Docking Studies" Separations 12, no. 2: 23. https://doi.org/10.3390/separations12020023
APA StyleJung, B., Heo, E. J., Nguyen, D. L., Youn, U. J., Kim, K. H., Son, B., & Lee, S. (2025). Antimicrobial Steroids from Poisonous Mushroom Gymnopilus orientispectabilis and Their Molecular Docking Studies. Separations, 12(2), 23. https://doi.org/10.3390/separations12020023







