1. Introduction
Sinomenine is a bioactive alkaloid extracted from the root and stem of medicinal plant sinomenium acutum (Thunb.) Rehd et Wils. [
1], which has anti-inflammatory, immunosuppressive, anti-arrhythmic, and other curative effects [
2,
3]. It has been widely used in clinical medicine to treat rheumatoid arthritis, malignant tumor, nephropathy, and other diseases in China, Japan, and other Asian countries [
4,
5,
6,
7,
8] and has a remarkable clinical effect and high cure rate. At present, Sinomenine Hydrochloride, which is the hydrochloride form of sinomenine with better water solubility, is used as the active pharmaceutical ingredient (API) to make pharmaceutical preparations of tablets, pills, capsules, or injections [
9,
10], and other new dosage forms have also been developed in recent years [
11,
12,
13]. The structural formula of Sinomenine Hydrochloride (CAS No. 6080-33-7) is shown in
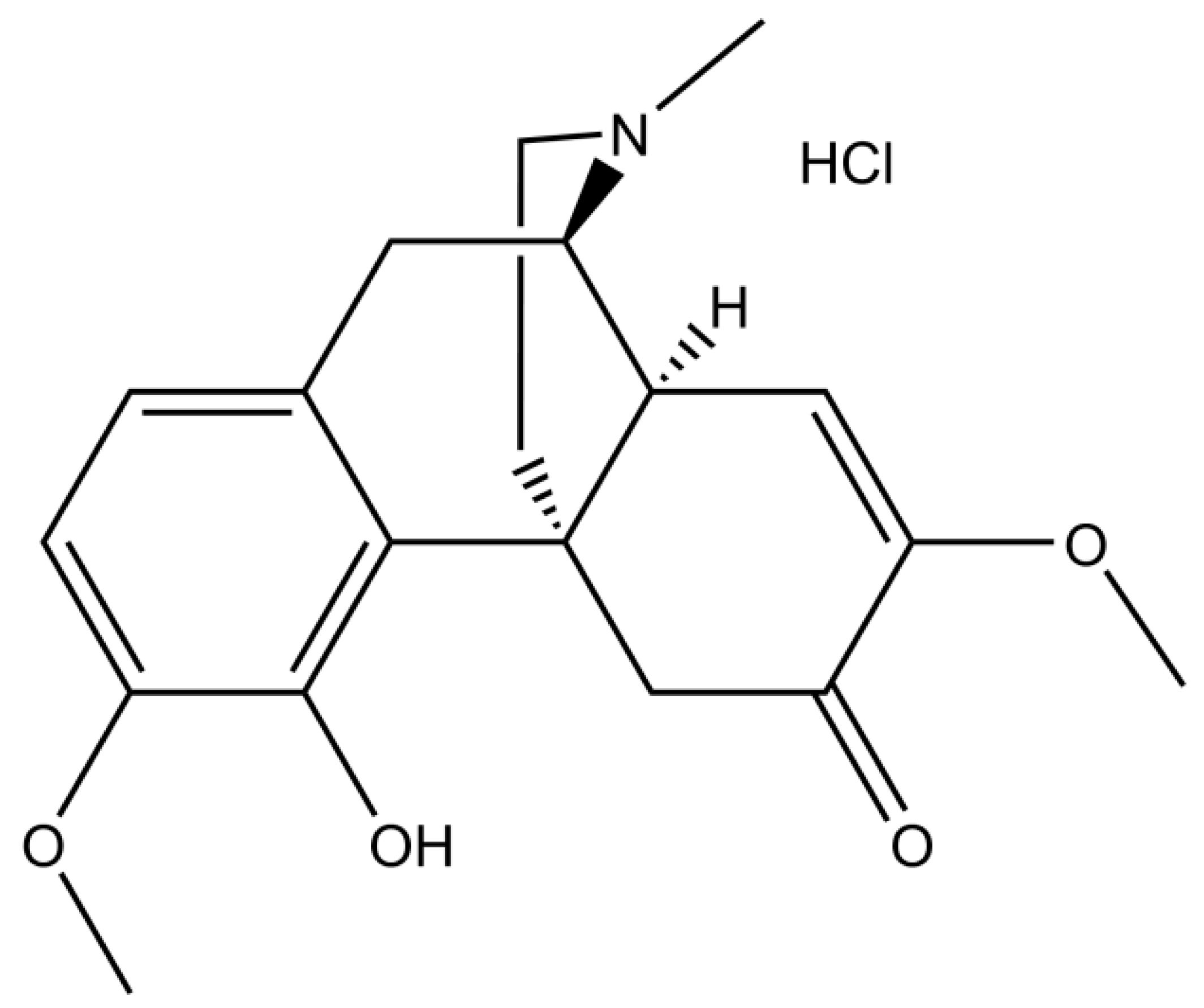
Figure 1 [
14].
Clinical application has shown that Sinomenine Hydrochloride can improve the curative effect when combined with a variety of drugs. However, some toxic reactions to Sinomenine Hydrochloride have been reported in recent years, in particular, anaphylactic shock. The safety of drugs depends not only on the toxicological properties of APIs, but also on the toxicological properties of impurities in the drug substance. The purity of Sinomenine Hydrochloride is also an important factor in its quality control. It is essential to choose a proper solvent in the process of crystallization that has a significant influence on the purity and productivity. Moreover, solubility data is an important factor to be able to optimize the crystallization process [
15]. Ethanol is usually used as a solvent in the crystallization process of Sinomenine Hydrochloride. Relevant studies were consulted and the solubility data of Sinomenine Hydrochloride in ethanol–water mixed solvent have not been reported. Therefore, it is necessary to determine the solubility of Sinomenine Hydrochloride under the conditions of different ratios of ethanol–water mixed solvent and temperature.
In this study, the solubility of Sinomenine Hydrochloride in ethanol–water mixed solvent systems with different proportions was determined at different temperatures (283.15, 288.15, 293.15, 298.15, 303.15, 308.15 K). The CNIBS/R-K model, Modified Apelblat model, Yaws model, and Apelblat–Jouyban–Acree model were used to fit the solubility data. Compared to the activity coefficient method, the empirical equations do not require many thermodynamic parameters. These characteristics make it suitable for the solubility simulation calculation of some substances for which thermodynamic parameters are not easily obtained. The thermodynamic parameters of Sinomenine Hydrochloride in these solvents were calculated from the measured solubility data using the modified version of the Van’t Hoff model. The determined data can provide a reference for the industrial purification of Sinomenine Hydrochloride.
2. Experimental
2.1. Materials
Sinomenine Hydrochloride standard was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China) with a purity of 98.0%. Sinomenine Hydrochloride was provided by Hunan Zhengqing Pharmaceutical Group Co., Ltd. (Huaihua, China) with a minimum purity of 98.0%. Purified water was prepared by a Milli-Q water purifier (Millipore, Bedford, MA, USA). The pH of the water was measured at 298.15 K using a sartorius PB-30 pH meter (Beijing, China) with three-point calibration immediately prior to use, giving pH = [6.98 ± 0.005]. All reagents used, like ethanol and acetonitrile, were of analytical grade. Sodium dihydrogen phosphate was purchased from Chemart Tianjin Chemical Technology Co., Ltd. (Tianjin, China).
2.2. Instruments
The following instruments were used to conduct the experiments: Intelligent Thermostatic Culture Oscillator (HNY- 2102C, manufactured by Tianjin Ounuo Instrument Co., Ltd., Tianjin, China), Digital Display Thermostatic Water Bath (XMTD- 204, manufactured by Shanghai Yuejin Medical Device Co., Ltd., Shanghai, China), Cryogenic Coolant Circulation Pump (DLSB- 5 L/25, manufactured by Tianjin Konuo Instrument Equipment Co., Ltd., Tianjin, China), 1/100 K Electronic Balance (METTLER AE 240, manufactured by Mettler Toledo, Columbus, OH, USA), High-performance liquid chromatography apparatus (Waters ACQuity Arc, manufactured by Waters, Milford, MA, USA), and a Liquid Chromatography Column (Plus C18-A, 4.6 × 250 mm, 5 μm, manufactured by Diamonsil, Beijing, China).
2.3. Solubility Measurements
The solubility of Sinomenine Hydrochloride in mixture solvents (water–ethanol) was measured by the equilibrium method, similarly to previous reports in the literature [
16,
17,
18]. For each target composition, 10.00 g of the ethanol–water binary mixture was prepared gravimetrically to achieve an ethanol mole fraction
(
Table 1). The Sinomenine Hydrochloride samples were added to the mixed solvent in a 10 mL stoppered tube. They were placed in a thermostatic shaking box and shaken for 24 h to obtain a supersaturated solution. Then, the supersaturated solutions were put in a constant temperature water bath for 5 days, and the sample solution reached the solid–liquid equilibrium state at this temperature (the preliminary results showed that 24 h + 5 d was sufficient to reach the equilibrium condition). The supernatant was passed through a pre-warmed 0.22 μm PTFE syringe filter to obtain a particle-free, saturated solution of Sinomenine Hydrochloride. The saturated solutions were diluted 100 times, and then the High-Performance Liquid Chromatography (HPLC) was used to measure the concentration of Sinomenine Hydrochloride in the saturated solution.
The experiment was repeated three times under the same conditions to minimize the experimental errors, and the mean value was used as the final result to calculate the mole fraction solubility. The mole fraction solubility of Sinomenine Hydrochloride in different mixed solvents was expressed by
, and was calculated according to Equation (1). The initial molar concentration of ethanol in the mixed solvent was expressed by
, and was calculated according to Equation (2).
where
m1,
m2, and
m3, respectively, represent the mass of Sinomenine Hydrochloride, ethanol, and water; and
M1,
M2, and
M3, respectively, represent the molar mass of Sinomenine Hydrochloride, ethanol, and water.
2.4. Analytical Methods
The contents of Sinomenine Hydrochloride in the equilibrium mixture were determined by HPLC on a Diamonsil Plus C18-A, (4.6 mm × 250 mm, 5 μm) chromatographic column. Referring to the content determination method of Sinomenine Hydrochloride in the Chinese Pharmacopoeia (2020 Ed), with 0.78% sodium dihydrogen phosphate (mass ratio) as the mobile phase A and acetonitrile as the mobile phase B, it was eluted with mobile phase A/mobile phase B (88:12, volume percent) for 15 min [
19]. The flow rate was 1 mL/min, the injection volume was 5 μL, the column temperature was 303 K, and the detection wavelength was 265 nm.
2.5. Powder X-Ray Diffraction
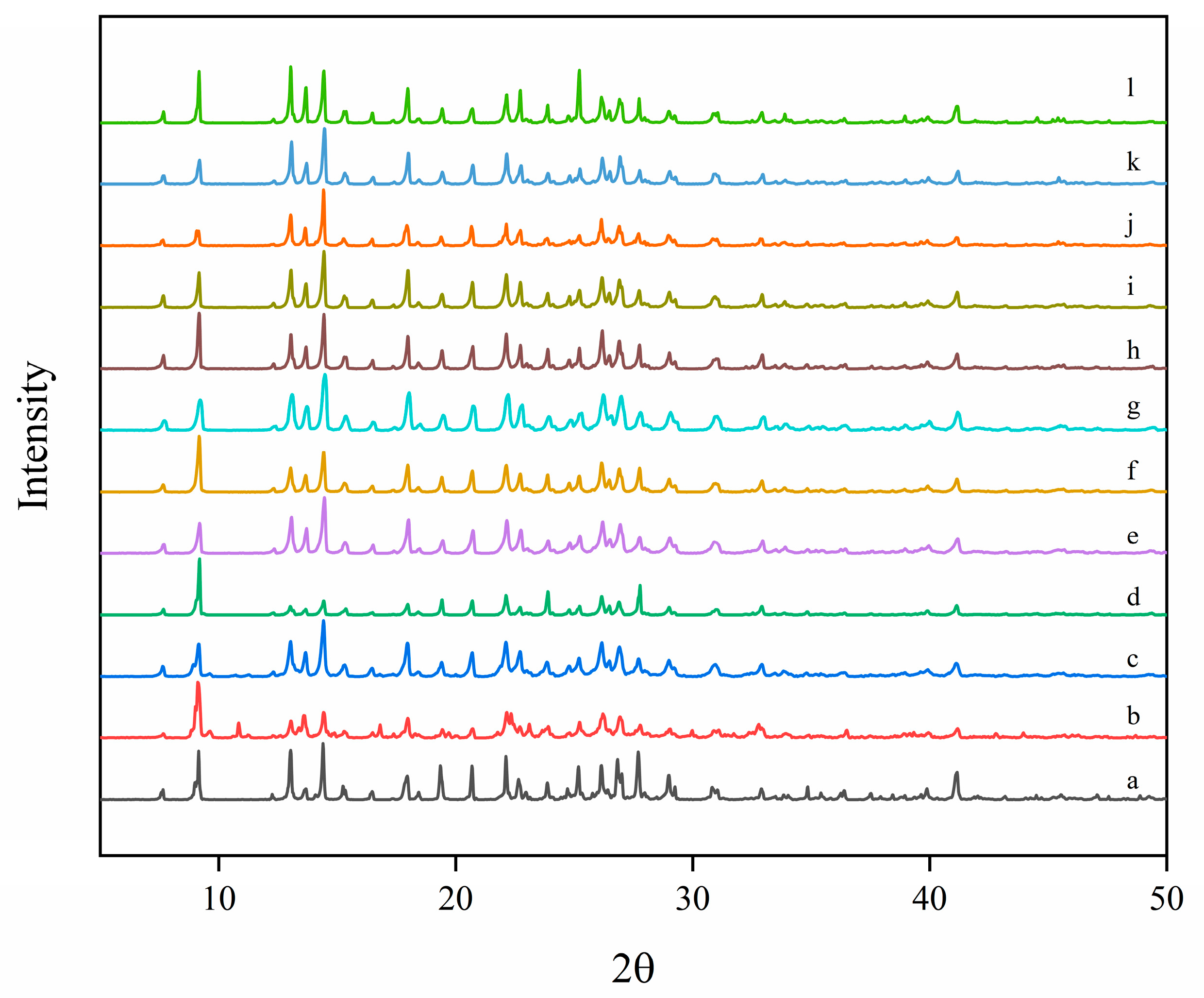
To assess potential solid-state transformations and the crystallinity of Sinomenine Hydrochloride during the experiments, the excess solid remaining in the suspension was characterized by powder X-ray diffraction (PXRD). PXRD patterns were collected on a Bruker D8 Advance instrument (Bruker AXS GmbH, Karlsruhe, Germany) using Cu Kα radiation (λ = 1.5406 Å) operated at 40 kV and 40 mA. Data were acquired over 2θ = 10–50° with a step size of 0.02° and a scan rate of 10° min−1.
3. Result and Discussion
3.1. Experimental Results
3.1.1. PXRD Analysis
The PXRD patterns of the equilibrated bottom-phase solid of Sinomenine Hydrochloride and the neat material are shown in
Figure 2. In both cases, peaks occur at the same characteristic 2θ positions (
Figure 2). The coincidence of peak positions indicates that Sinomenine Hydrochloride remained in the same crystalline form throughout the experiments, with no transformation to polymorphic or amorphous phases.
3.1.2. Linear Relation Investigation
Sinomenine Hydrochloride was precisely weighed and dissolved in water, and the standard solutions of 2.00 × 103 μg/ mL, 1.00 × 103 μg/ mL, 5.00 × 102 μg/ mL, 2.50 × 102 μg/ mL, 1.25 × 102 μg/ mL, 62.50 μg/ mL, 31.25 μg/ mL were obtained. The peak area of Sinomenine Hydrochloride was detected by HPLC. Taking the peak area as Y and the concentration of Sinomenine Hydrochloride as X to carry out linear regression with Origin 9.0 software, the regression equation of Sinomenine Hydrochloride was obtained, Y = 3.000 × 106 X − 7.426 × 103 (r = 0.999). The results showed that the method could accurately quantify Sinomenine Hydrochloride in the concentration range of 0.03 mg/mL–2.00 mg/mL.
3.1.3. Solubility Data of Sinomenine Hydrochloride
The mole fraction solubility of Sinomenine Hydrochloride at different temperatures (283.15 K, 288.15 K, 293.15 K, 298.15 K, 303.15 K, 308.15 K) and different proportions of ethanol–water (0:10–10:0) mixed solvents is shown in
Table 1. The results show that the mole fraction solubility of Sinomenine Hydrochloride in ethanol–water mixed solvent was affected by solvent composition and temperature change.
The solubility of Sinomenine Hydrochloride in pure water higher was than that in pure ethanol, and the solubility of Sinomenine Hydrochloride increased first and then decreased with the increase in the molar mass of ethanol in the ethanol–water solvent system. When the ratio of ethanol–water was 5:5 (by mass), the solubility value reached its maximum. The underlying mechanism may be that Sinomenine Hydrochloride is an ionic, strongly polar solute. Introducing a small amount of ethanol into water weakens bulk water structuring and modifies the local solvent microenvironment around the solute. Ethanol is enriched in the first solvation shell (preferential solvation), which lowers the solute activity coefficient and thus increases the mole fraction solubility. As the ethanol mole fraction increases further, the mixture dielectric constant drops and ionic hydration becomes less favorable; ion–dipole stabilization and dissociation are weakened, the activity coefficient rises, and the mole fraction solubility decreases. The interplay of these effects produces the observed maximum at ≈ 0.24–0.32 and is consistent with the minimum Gibbs free energy of dissolution (ΔGd) inferred from our correlations.
In addition, the equilibrium solubility of the Sinomenine Hydrochloride was also affected by the temperature. It is clear that Sinomenine Hydrochloride had the lowest solubility value when the temperature was 283.15 K, and the solubility gradually increased as the temperature increased. These results indicate that the proper ratio of ethanol–water and temperature improved the solubility of Sinomenine Hydrochloride in the recrystallization process.
3.2. Solubility Data Correlation
Compared to the activity coefficient method, empirical equations do not require many thermodynamic parameters. These characteristics make them suitable for the solubility simulation calculation of some substances for which thermodynamic parameters are not easily obtained [
20,
21]. When the solubility is not known, the equilibrium solubility simulation with a high degree of fit is helpful to accurately predict the solubility of compounds.
In this study, the CNIBS/R-K model, Modified Apelblat model, Yaws model, and Apelblat–Jouyban–Acree model were used to correlate the equilibrium solubility data of Sinomenine Hydrochloride. The prediction ability of models was represented by the values of relative average deviation (RAD) and root mean square deviation (RMSD) [
22]. The calculation of these values is represented in Equations (3) and (4).
where
represents the predicted value of the model;
represents the measured value of the experiment; and N represents the number of data.
3.2.1. CNIBS/ R-K Model
When the solution is in an ideal state, the CNIBS/R-K model can be used to investigate the relationship between the solubility of solute and binary solvent systems at the same thermodynamic temperature, and was first proposed by Acree et al. [
23,
24]. The formula of the equation is shown in Equation (5).
where
is the mole fraction composition of ethanol in the solvent mixture and
is the mole fraction composition of water in the solvent mixture.
is the mole fraction solubility of Sinomenine Hydrochloride in ethanol.
is the mole fraction solubility of Sinomenine Hydrochloride in water.
is the mole fraction solubility of Sinomenine Hydrochloride in the solvent mixture.
is the model constant and N refers to the number of “curve-fit” parameters. The value of N is 2 and
. By substituting them into Equation (5), a new equation can be obtained [
25].
By introducing a constant term to Equation (6), it can be further simplified to produce Equation (7):
where
,
,
,
, and
are the model parameters calculated through regression analysis.
By substituting the solubility data of Sinomenine Hydrochloride into Equation (7), some model parameters were obtained through precise calculation, and the specific values are listed in
Table 2. The smaller the values of RAD and RMSD, the better the predictive ability of the model. The R
2 of this model was not less than 0.990. All RAD values were less than 4.622 × 10
−3, and all RMSD values were less than 4.079 × 10
−3. They indicate that the model has a good predictive ability and can be used to predict the solubility of Sinomenine Hydrochloride in ethanol–water mixed solvents.
3.2.2. Modified Apelblat Model
For a binary mixed solid–liquid equilibrium system, the solubility can be expressed by Equation (8).
is the amount of solute in the solution;
is the activity coefficient of the solute; and
is the standard fugacity. The fugacity ratio of the pure solute can be calculated by thermodynamic equation that is shown in Equation (9).
T is the absolute temperature; Tt is the triple point temperature of the solute, and the melting temperature is often used to replace the triple point temperature in practical calculation; is the Melting enthalpy of the solute; is the hot melt difference of solid–liquid equal pressure; and R is the ideal gas constant.
When the solution is relatively dilute,
can be regarded as the infinite dilution activity coefficient
. The relationship between the infinite dilution activity coefficient and temperature is shown in Equation (10):
Equations (8)–(10) are combined to obtain Equation (11).
Equation (11) can also be expressed as Equation (12) [
26].
where T is the thermodynamic temperature; and A, B, and C are the model parameters of Modified Apelblat model, and can be obtained by fitting the solubility data.
The Modified Apelblat model is mainly used to investigate the relationship between the solubility and thermodynamic temperature of a solute in an ideal solution.
The values of the model parameters obtained through calculation are shown in
Table 3. The R
2 of this model was not less than 0.939, which indicated that the result was strong. In addition, the RAD was less than 7.708 × 10
−2, and the RMSD was less than 5.168 × 10
−2, indicating that the prediction ability of the Modified Apelblat model was poorer than that of the CNIBS/R-K model.
3.2.3. Yaws Model
Like the Modified Apelblat model, the Yaws model is also used to investigate the relationship between the solubility and thermodynamic temperature of a solute in an ideal solution. A
1, B
1, and C
1 are the parameters of the model [
27]. The equation is shown in Equation (13).
The values of model parameters obtained through calculation are shown in
Table 4. The R
2 of this model was not less than 0.964, the RAD was less than 2.440 × 10
−2, and the RMSD was less than 1.955 × 10
−2. The prediction ability of the Yaws model is not significantly different from that of the Modified Apelblat model.
3.2.4. Apelblat–Jouyban–Acree Model
The Apelblat–Jouyban–Acree model is obtained by combining the Modified Apelblat model and the Jouyban–Acree model. It is a three-dimensional model of solute solubility in binary solvents, solvent composition, and dissolution temperature [
28] The equation is shown in Equation (14), where A
2, B
2, C
2, A
3, B
3, C
3, S
0, S
1, and S
2 are the parameters of the model.
After the equation fitting, the values of the parameters A
2, B
2, C
2, A
3, B
3, C
3, S
0, S
1, and S
2 are −86.24, 3121.73, 13.21, 43.08, −2170.28, −6.33, 0.98, −0.03, and 0.64, respectively. The r is 0.991. The RAD and RMSD values are 1.16 × 10
2 and 1.47 × 10
2, respectively. The prediction ability of the Apelblat–Jouyban–Acree model is poorer than that of the CNIBS/R-K model, when the solubility of Sinomenine Hydrochloride was measured in different ethanol–water mixed solvents within the temperature range of 283.15 K–308.15 K.
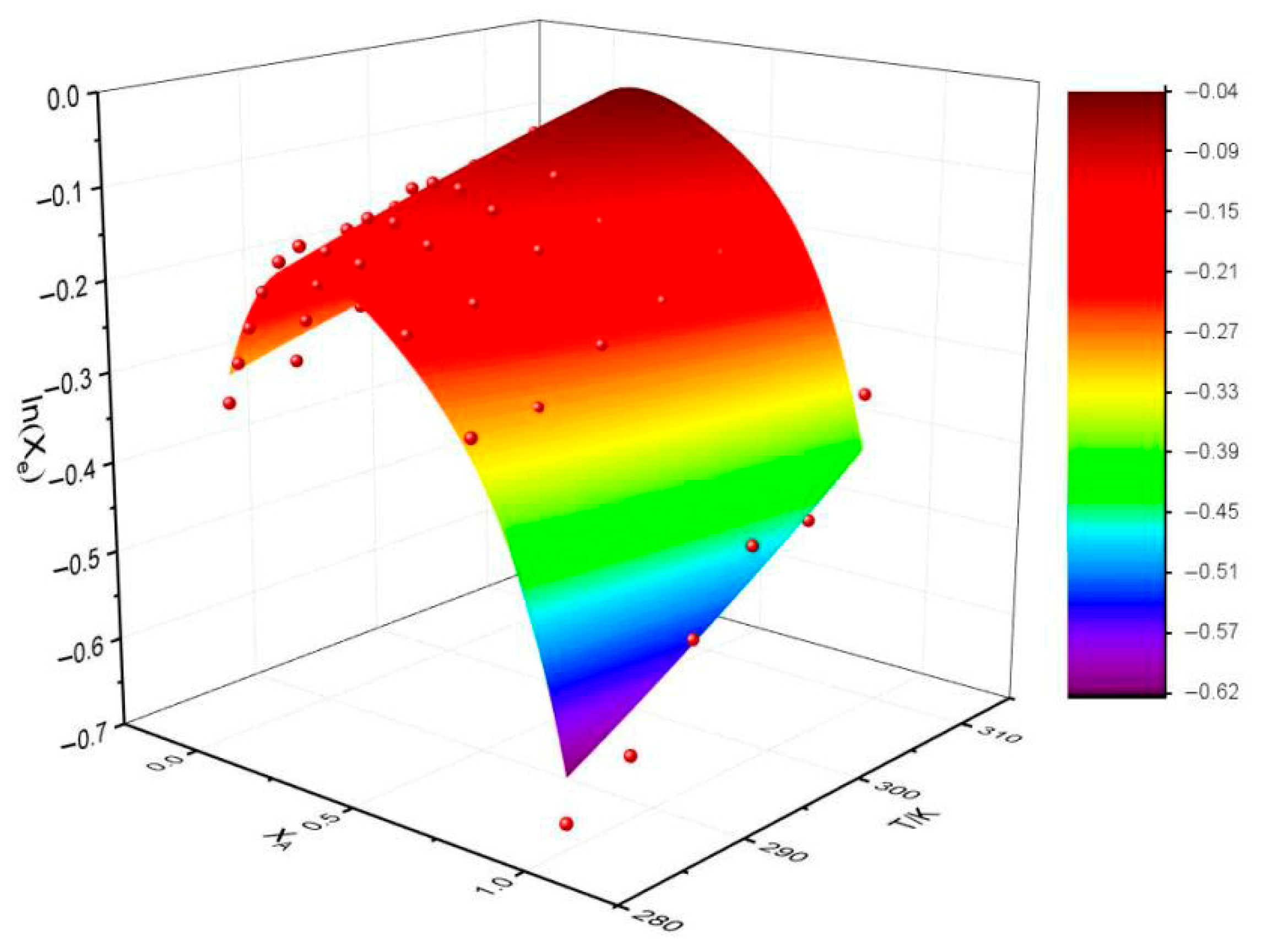
Figure 3 shows the relationship between the predicted values of the model and the actual values. When the temperature is 298.15 K, the solubility of Sinomenine Hydrochloride falls on the curved surface, and the fitting result is the best. However, the rest of the points are outside of the surface. This result is consistent with poor RAD and RMSD values. Overall, the prediction ability of the CNIBS/R-K model is the best.
3.3. Thermodynamic Parameter Calculation
Melting enthalpy (Δ
fusH) and melting entropy (Δ
fusS) can be obtained using the Van‘t Hoff model in ideal solution [
29], but the enthalpy and entropy of the mixtures cannot be ignored in non-ideal solution. Melting enthalpy is replaced by dissolving enthalpy (ΔH
d), and melting entropy is replaced by dissolving entropy (ΔS
d). The formula is shown in Equation (15) [
30].
where R is gas molar constant; ΔH
d is the dissolving enthalpy of Sinomenine Hydrochloride; ΔS
d is the dissolving entropy of Sinomenine Hydrochloride; and T is thermodynamic temperature.
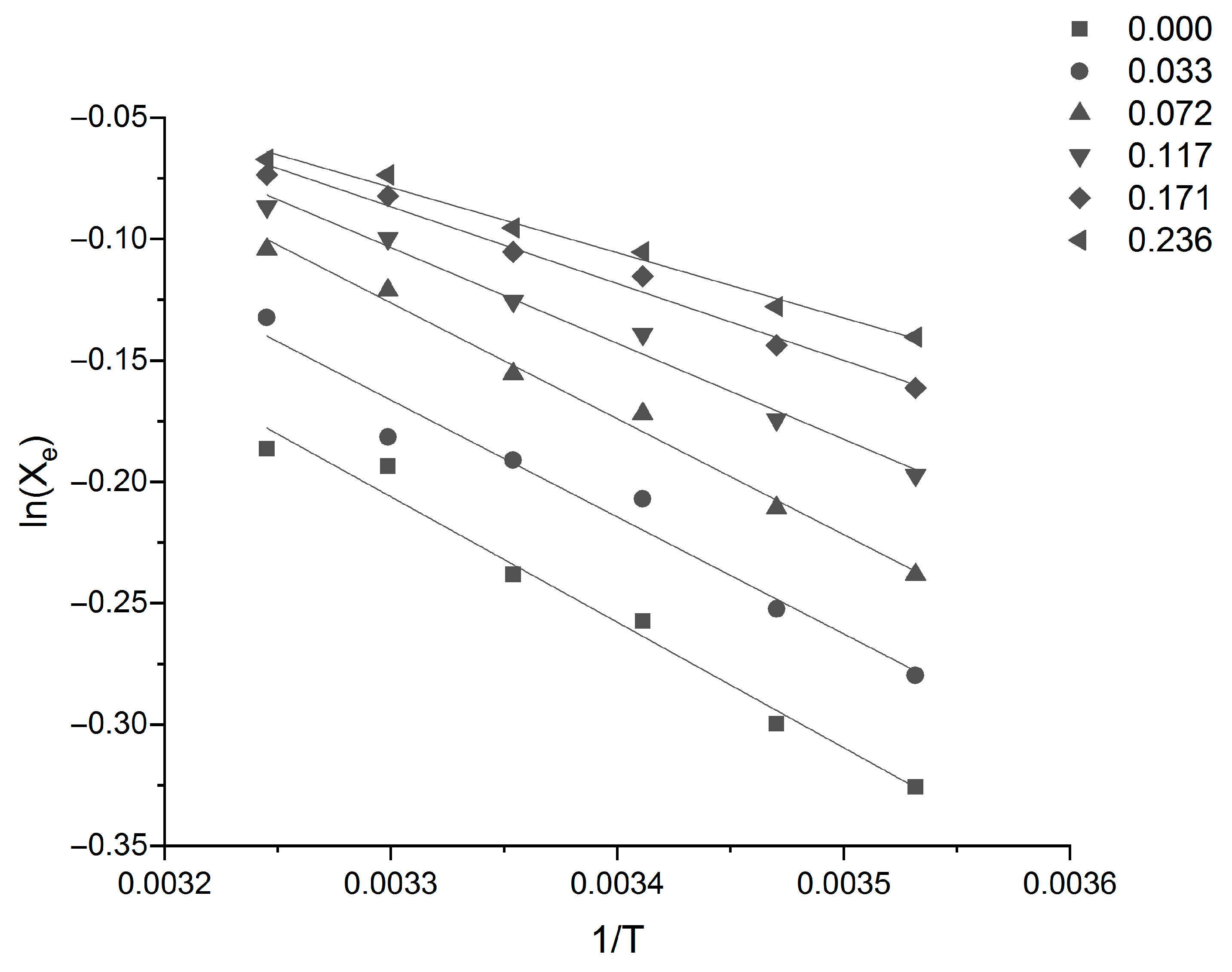
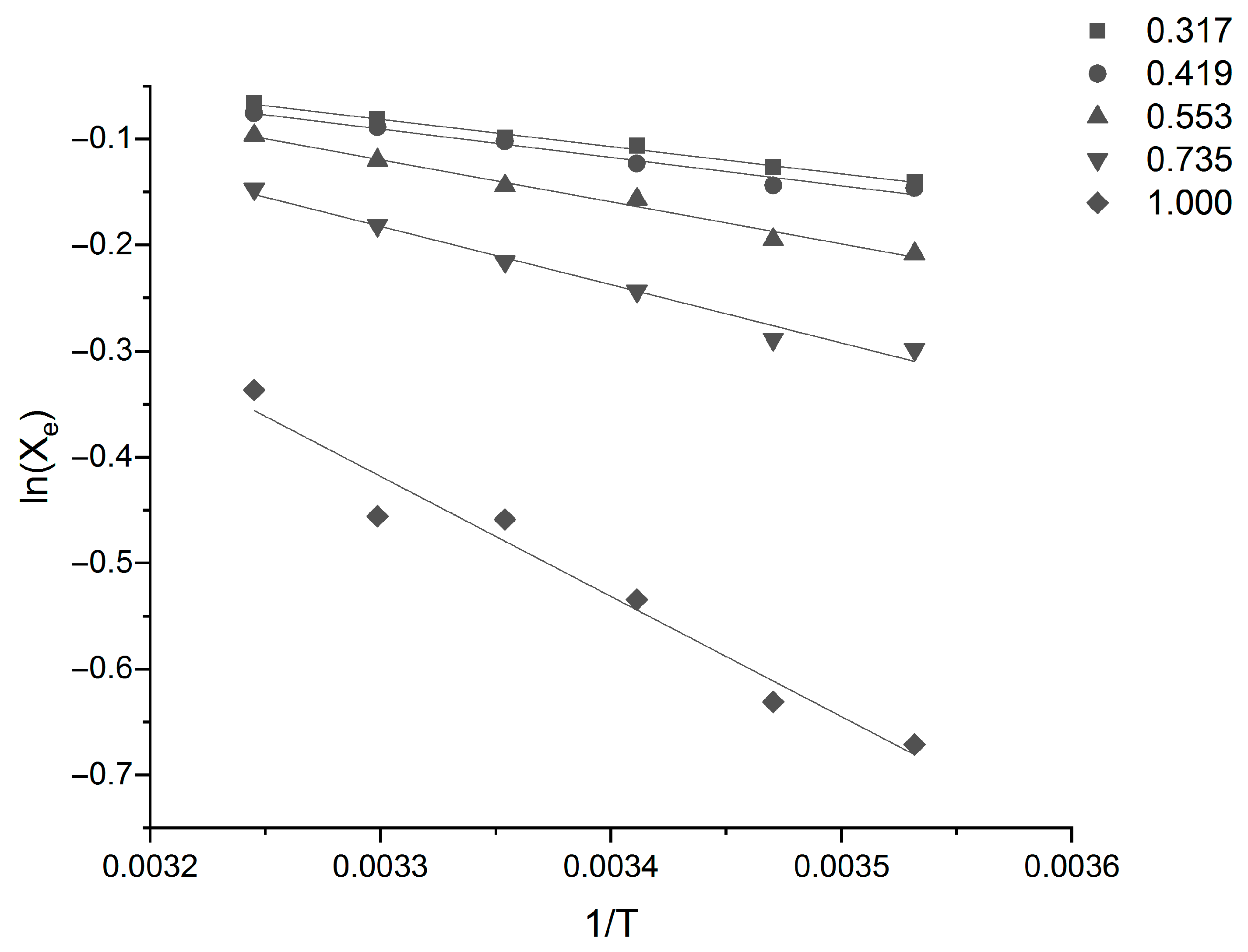
Equation (15) was used to fit the data, and the related thermodynamic parameters of Sinomenine Hydrochloride can be calculated from the linear fit of ln
Xe against 1/T in
Figure 4 and
Figure 5. The values of the parameters are listed in
Table 5.
The changes in Gibbs free energy (ΔG
d) for the dissolution of Sinomenine Hydrochloride in different solvents were also calculated using the Gibbs−Helmholtz equation shown in Equations (16) and (17) [
31].
where
represents the thermodynamic temperature; and n represents the number of points of temperature. This experiment was conducted at six temperatures, so
n = 6.
The results of the thermodynamic parameters, including ΔH
d, ΔG
d, and ΔS
d, for the dissolution of Sinomenine Hydrochloride in ethanol–water mixture at different temperatures, are presented in
Table 5. All of the ΔH
d values are positive values, and the ΔH
d of Sinomenine Hydrochloride first decreases and then increases with increasing ethanol mole fraction in the ethanol–water solvent system. This shows that the dissolution behavior of Sinomenine Hydrochloride in ethanol–water mixed solvent is endothermic, and the interactions between solvent molecules and Sinomenine Hydrochloride molecules are much stronger than those between the solvent–solvent and solute–solute molecules. This is also the reason why the solubility of Sinomenine Hydrochloride in the mixed solvent increases with the increase in temperature. The ΔS
d values are positive in different proportions of ethanol–water mixed solvents. This indicates that the dissolution process of Sinomenine Hydrochloride is entropically driven in the solvent. In addition, all of the ΔG
d values are also positive values. This indicates that the dissolution process of Sinomenine Hydrochloride is apparently not spontaneous in the range of temperature (283.15 K–308.15 K). The greater the ΔG
d value, the weaker the solubility. The value of ΔG
d first decreases and then increases with increasing ethanol mole fraction in the ethanol–water mixed solvent, and the change trend is the same as the solubility data of Sinomenine Hydrochloride in this study, which indicates that the data obtained in this study is consistent with the theoretical values.
4. Conclusions
The solubilities of Sinomenine Hydrochloride in ethanol–water mixed solvent systems were determined at different temperatures (from 283.15 K to 308.15 K) under atmospheric pressure. The experimental results indicated that the solubility of Sinomenine Hydrochloride in pure water was higher than that in pure ethanol. When the proportion of ethanol in the mixed solvent increases, the solubility first increases and then decreases. Further, the solubility was the largest when the ethanol–water ratio was 5:5 (by mass), and the solubility increased with the increase in temperature. The results indicated that a proper ratio of ethanol–water and temperature improved the solubility of Sinomenine Hydrochloride in the process of Sinomenine Hydrochloride recrystallization. Sinomenine Hydrochloride can be dissolved at ≈ 0.24–0.32 and T = 308.15 K to exploit the solubility maximum, and then crystallization can be induced by cooling to 283.15 K and/or by shifting the solvent composition away from the maximum toward ethanol-richer mixtures. However, practical industrial implementation should still take all relevant conditions into comprehensive consideration. The CNIBS/R-K model, Modified Apelblat model, Yaws model, and Apelblat–Jouyban–Acree model were applied to mathematically describe the solubility of Sinomenine Hydrochloride. The CNIBS/R-K model has the best fitting effect, the maximum values of RAD and RMSD were 4.622 × 10−3 and 4.079 × 10−3, respectively. The dissolution of Sinomenine Hydrochloride ethanol–water mixed solvent systems was confirmed to be endothermic and not spontaneous based on the obtained thermodynamic parameters. This study can be regarded as the basic data of the separation and purification process of Sinomenine Hydrochloride.
Author Contributions
Conceptualization, Y.B. and W.L.; Methodology, H.W.; Validation, H.W.; Investigation, Y.B.; Resources, H.W.; Data curation, Y.B.; Writing—original draft, H.W.; Writing—review & editing, W.L.; Supervision, Y.B. and W.L.; Project administration, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Project of Tianjin Natural Science Foundation grant number (23JCZDJC00720), and the S&T Project of Haihe Laboratory of Modern Chinese Medicine (22HHZYSS00004).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would also like to thank the anonymous reviewers for their hard work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ng, J.P.L.; Coghi, P.; Law, B.Y.K.; Liu, L.; Wong, V.K.W. The present and future synthetic strategies of structural modifications of sinomenine. Org. Chem. Front. 2020, 7, 4089–4107. [Google Scholar] [CrossRef]
- Yin, N.N.; Xiong, Y.; Tao, W.T.; Chen, J.J.; Wang, Z.G. Sinomenine alleviates lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. Immunopharmacol. Immunotoxicol. 2020, 42, 147–155. [Google Scholar] [CrossRef]
- Li, H.; Wei, F.; Li, S.W.; Yan, L.; Lu, P.R. The effect of sinomenine eye drops on experimental dry eye in mice. Cutan. Ocul. Toxicol. 2020, 39, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kambhampati, S.P.; Zhang, Z.; Sharma, A.; Chen, S.; Duh, E.I.; Kannan, S.; Tso, M.O.M.; Kannan, R.M. Dendrimer mediated targeted delivery of sinomenine for the treatment of acute neuroinflammation in traumatic brain injury. J. Control. Release 2020, 323, 361–375. [Google Scholar] [CrossRef]
- Shen, Q.Y.; Zhang, X.Z.; Qi, J.; Shu, G.F.; Du, Y.Z.; Ying, X.Y. Sinomenine hydrochloride loaded thermosensitive liposomes combined with microwave hyperthermia for the treatment of rheumatoid arthritis. Int. J. Pharm. 2020, 576, 119001. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.B.; Jin, L.; Gong, T.T.; Pan, S.P.; Zheng, S.Y.; Zhang, X.W.; Yang, T. Effect of sinomenine hydrochloride on radiosensitivity of esophageal squamous cell carcinoma cells. Oncol. Rep. 2018, 39, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Mai, C.T.; Pan, H.D.; Liu, L.; Zhou, H.; Xie, Y. Targeting immunometabolism by active ingredients derived from traditional Chinese medicines for treatment of rheumatoid arthritis. Chin. Herb. Med. 2021, 13, 451–460. [Google Scholar] [CrossRef]
- Ramazi, S.; Fahanik-Babaei, J.; Mohamadi-Zarch, S.M.; Tashakori-Miyanroudi, M.; Nourabadi, D.; Nazari-Serenjeh, M.; Roghani, M.; Baluchnejadmojarad, T. Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: Involvement of oxidative stress, inflammation and pyroptosis. J. Chem. Neuroanat. 2020, 108, 101800. [Google Scholar] [CrossRef]
- Li, Z.Q.; Tian, S.; Gu, H.; Wu, Z.G.; Nyagblordzro, M.; Feng, G.; He, X. In Vitro-In Vivo Predictive Dissolution-Permeation-Absorption Dynamics of Highly Permeable Drug Extended-Release Tablets via Drug Dissolution/Absorption Simulating System and pH Alteration. AAPS PharmSciTech 2018, 19, 1882–1893. [Google Scholar] [CrossRef]
- Liu, W.G.; Ling, P.X.; Lin, X.K.; Chen, J.Y.; Wang, S.J.; Li, P.; Wu, X.J.; Zhao, D.M.; Liu, S.H. Therapeutic effect of an injectable sustained-release sinomenine hydrochloride and sodium hyaluronate compound in a rabbit model of osteoarthritis. Chin. Med. J. 2012, 125, 2543–2547. [Google Scholar]
- Xia, M.Q.; Liu, L.; Tian, C.L.; Li, Q.; Hu, R.F.; Gui, S.Y.; Chu, X.Q. Pharmacokinetics of sinomenine hydrochloride cubic liquid crystal injection based on microdialysis technology. J. Drug Deliv. Sci. Technol. 2019, 52, 553–558. [Google Scholar] [CrossRef]
- Shu, Z.X.; Cao, Y.J.; Tao, Y.T.; Liang, X.; Wang, F.Y.; Li, Z.; Li, Z.B.; Gui, S.Y. Polyvinylpyrrolidone microneedles for localized delivery of sinomenine hydrochloride: Preparation, release behavior of in vitro & in vivo, and penetration mechanism. Drug Deliv. 2020, 27, 642–651. [Google Scholar] [CrossRef]
- Li, X.R.; Li, X.Y.; Zhou, Y.X.; Liu, Y.; Guo, M.; Zhu, Q.F.; Xie, Y.C.; Fan, Z.Y. Development of patch and spray formulations for enhancing topical delivery of sinomenine hydrochloride. J. Pharm. Sci. 2010, 99, 1790–1799. [Google Scholar] [CrossRef]
- Pan, H.M.; Lu, T.; Wu, X.D.; Gu, C.W.; Tao, N.L.; Zhang, B.; Wang, A.; Chen, G.M.; Zhang, K.H.; Cheng, J.; et al. Design and synthesis of sinomenine isoxazole derivatives via 1,3-dipolar cycloaddition reaction. Nat. Prod. Res. 2019, 35, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.M.J.; Vlaar, C.P.; Lopez-Mejias, V.; Stelzer, T. Solubility Measurements and Correlation of MBQ-167 in Neat and Binary Solvent Mixtures. J. Chem. Eng. Data 2021, 66, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Sandeepa, K.; Kumar, K.R.; Neeharika, T.S.V.R.; Satyavathi, B.; Thella, P.K. Solubility Measurement and Thermodynamic Modeling of Benzoic Acid in Monosolvents and Binary Mixtures. J. Chem. Eng. Data 2018, 63, 2028–2037. [Google Scholar] [CrossRef]
- Noubigh, A.; Jeribi, C.; Mgaidi, A.; Abderrabba, M. Solubility of gallic acid in liquid mixtures of (ethanol + water) from (293.15 to 318.15) K. J. Chem. Thermodyn. 2012, 55, 75–78. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Chen, G.Q.; Xu, R.J.; Zhao, H.K. Solubility Modeling and Solvent Effects of Allopurinol in 15 Neat Solvents. J. Chem. Eng. Data 2018, 63, 3551–3558. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume I, p. 204. [Google Scholar]
- Zhang, L.; Gong, X.C.; Wang, Y.F.; Qu, H.B. Solubilities of Protocatechuic Aldehyde, Caffeic Acid, D-Galactose, and D-Raffinose Pentahydrate in Ethanol-Water Solutions. J. Chem. Eng. Data 2012, 57, 2018–2020. [Google Scholar] [CrossRef]
- Ha, E.S.; Park, H.; Lee, S.K.; Sim, W.Y.; Jeong, J.S.; Kim, M.S. Equilibrium solubility and modeling of trans -resveratrol in dichloromethane and primary alcohol solvent mixtures at different temperatures. J. Mol. Liq. 2020, 311, 113363. [Google Scholar] [CrossRef]
- Sadeghi, M.; Rasmuson, A.C. Solubility of Salicylic Acid, Salicylamide, and Fenofibrate in Organic Solvents at Low Temperatures. J. Chem. Eng. Data 2020, 65, 4855–4861. [Google Scholar] [CrossRef]
- Acree, W.E.; Zvaigzne, A.I. Thermodynamic properties of non-electrolyte solutions: Part 4. Estimation and mathematical representation of solute activity coefficients and solubilities in binary solvents using the NIBS and Modified Wilson equations. Thermochim. Acta 1991, 178, 151–167. [Google Scholar] [CrossRef]
- Jouyban-Gharamaleki, A.; Hanaee, J. A novel method for improvement of predictability of the CNIBS/R-K equation. Int. J. Pharm. 1997, 154, 245–247. [Google Scholar] [CrossRef]
- Sun, S.J.; Wu, Y.F.; Yao, X.; Yang, S.P.; Liu, Y. Solubility Measurement and Modeling of Eszopiclone in Four Binary Solvents. J. Chem. Eng. Data 2020, 65, 2694–2702. [Google Scholar] [CrossRef]
- Wu, X.; Yang, S.S.; Xu, S.M.; Zhang, X.J.; Ren, Y.J. Measurement and correlation of the solubility of sodium acetate in eight pure and binary solvents. Chin. J. Chem. Eng. 2022, 44, 474–484. [Google Scholar] [CrossRef]
- Jia, Q.; Lei, D.; Zhang, S.J.; Zhang, J.Q.; Liu, N.; Kou, K.C. Solubility measurement and correlation for HNIW center dot TNT co-crystal in nine pure solvents from T = (283.15 to 318.15) K. J. Mol. Liq. 2021, 323, 114592. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, T.T.; Huang, Q.; Wang, L.Y.; Ban, C.L.; Shen, G.P. Solubility and thermodynamic properties of L-Pyroglutamic acid in pure and binary solvents. J. Mol. Liq. 2020, 320, 114361. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, C.; Xu, Z.; Wang, Y.L.; Zhao, H.P.; Hao, H.X. Determination and Correlation of Solubility Data and Dissolution Thermodynamic Data of L-Lactide in Different Pure Solvents. J. Chem. Eng. Data 2013, 58, 143–150. [Google Scholar] [CrossRef]
- Ahad, A.; Shakeel, F.; Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; AlJenoobi, F.I.; Al-Mohizea, A.M. Solubility and thermodynamic analysis of vinpocetine in various mono solvents at different temperatures. J. Therm. Anal. Calorim. 2022, 147, 3117–3126. [Google Scholar] [CrossRef]
- Garcia-Delgado, R.A.; Cotoruelo-Minguez, L.M.; Rodriguez, J.J. Equilibrium Study of Single-Solute Adsorption of Anionic Surfactants with Polymeric XAD Resins. Sep. Sci. Technol. 1992, 27, 1065–1076. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).