1. Introduction
Malpighiaceae is a botanical family comprising 72 genera and approximately 1500 species, of which 46 genera and 599 species occur in Brazil [
1]. This family ranks among the most diverse groups of Neotropical shrubs, trees, and lianas [
2]. Various genera and species within this family exhibit diverse chemical and biological activities, primarily attributed to the presence of secondary metabolites in their leaves, stems, trunks, and aerial parts [
3]. Thus, several studies have highlighted the chemical and biological significance of species within this botanical family.
The antioxidant potential, antiprotozoal activity and antifungal activity against
Candida species exhibited by
Diplopterys pubipetala [
4,
5], the occurrence of bioactive polyphenols in
Hiraea leaves [
6], and the antitumor properties of
Byrsonima crassifolia fruits [
7] underscore the diverse functional attributes within the Malpighiaceae family.
Two of the largest lianescent genera of this botanical family in the Neotropics are
Banisteriopsis and
Stigmaphyllon [
8].
Banisteriopsis is a genus with 62 endemic species to the Neotropical region, showing varied phytochemical activities [
8,
9]. The occurrence of nucleoside hydrolase inhibitor metabolites against
Leishmania donovani in
Banisteriopsis laevifolia [
10], along with alkaloids and psychoactive compounds in
Banisteriopsis caapi [
11] and the antifungal potential of
B. laevifolia leaves and flowers, underscores the remarkable functional diversity of
Banisteriopsis species.
In addition to these phytochemical findings in
Banisteriopsis, other genera, particularly
Stigmaphyllon, also exhibit notable phytochemical diversity.
Stigmaphyllon comprises 120 species widely distributed in the Americas, Africa, Asia, and Oceania [
2,
8]. Approximately 48
Stigmaphyllon species are cultivated in northern and southern Brazil [
12]. The presence of saponins, flavonoids, and other metabolites, combined with the antioxidant activity of essential oils from
Stigmaphyllon blanchetii leaves and the antitumor effect of
Stigmaphyllon ovatum on melanoma cell lines, highlights the multifunctional potential of this genus [
12].
From a botanical standpoint, the anatomical and morphological aspects of
Banisteriopsis and
Stigmaphyllon are well characterized [
2,
13]; however, the chemical differences between these genera remain poorly understood. This gap is particularly significant when considering differences among plant classes cultivated in tropical regions with contrasting moisture regimes (dry vs. humid) and distinct growth habits (liana vs. shrub). To date, no studies have reported metabolomic differences in leaf composition between
Banisteriopsis and
Stigmaphyllon at the levels of genus, cultivation environment, or plant habit. Thus, metabolomic analyses can provide compound annotations that enable the assessment of interspecific chemical variation according to chemotaxonomic parameters.
Among chemical profiling techniques, metabolomic analysis has emerged as a cornerstone of modern plant science. Plant metabolites play fundamental roles in growth, development, environmental adaptation, and nutritional quality. As a key discipline within systems biology, plant metabolomics enables a comprehensive examination of metabolite composition, variability, and functional significance [
14]. In this context, untargeted metabolomic analyses using liquid chromatography coupled with mass spectrometry have confirmed the presence of defense-related compounds, facilitated the discrimination of metabolites among species within and across genera, and contributed to new phylogenetic classifications [
6,
15,
16].
Given the vast amount of metabolomic data in plants, chemometric approaches are essential for uncovering patterns and relationships within complex datasets [
17]. The need for robust discriminant tools becomes particularly critical when dealing with metabolomic profiles generated by chromatography coupled with mass spectrometry, due to the high complexity and large number of detected peaks [
17]. The application of multivariate statistical tools, such as Partial Least Squares Discriminant Analysis (PLS-DA), has proven effective in distinguishing species and predicting bioactive potential based on chemical profiles [
6,
9,
18]. Comparative metabolomic studies across related genera provide valuable insights into evolutionary divergence, adaptive strategies, and the distribution of bioactive metabolites.
In this context, the present study aimed to compare the metabolomic profiles of leaves from 15 Banisteriopsis and 26 Stigmaphyllon species (Malpighiaceae) using UHPLC-QTOF-MS/MS. Partial Least Squares Discriminant Analysis (PLS-DA) was applied to assess variations at multiple levels, including genus identity, environmental occurrence (dry vs. humid habitats), and plant growth habit (liana vs. shrub).
2. Materials and Methods
2.1. Plant Material
A total of 64 leaf samples were analyzed, comprising 29 from
Banisteriopsis species and 35 from
Stigmaphyllon species, collected across different regions of Brazil (
Table S1). The leaves were collected between 2014 and 2018 from fully developed adult plants. Following collection, the leaves were dried at 40 °C for three days and subsequently stored in the HUEFS herbarium at 18 °C under continuous air conditioning (24 h/day) until extract preparation. Species identification was confirmed by botanist R. F. de Almeida (C. E. Moss Herbarium, School of Animal, Plant and Environment, University of Witwatersrand, Johannesburg, 2092, South Africa). Details on species names, collection locations, plant habits, and herbarium identification numbers are provided in
Table S1 of the Supplementary Materials.
All plant samples (
Table S1) were collected under natural field conditions, without control of environmental parameters such as temperature, humidity, or precipitation. Consequently, the dataset reflects the natural variability inherent to the native habitats of each species across Brazil. In this study, the terms “dry” and “humid” refer to the predominant ecological characteristics of the biomes from which the samples were obtained. Accordingly, species collected in the Cerrado and Caatinga biomes were classified as originating from dry environments, whereas those collected in the Amazon Rainforest and Atlantic Forest were classified as originating from humid environments. This ecological classification provides a consistent and biologically meaningful framework for interpreting metabolomic differences associated with contrasting environmental conditions.
2.2. Preparation of Extracts
To prepare the extracts, the collected leaves (
Table S1) were initially dried in a desiccator, frozen using liquid nitrogen, and ground in a knife mill. The powdered material was accurately weighed and subjected to extraction with ethanol (Sigma-Aldrich, St. Louis, MO, USA) and water (Fisher Scientific, Fair Lawn, NJ, USA). For each 20 mg of sample, 1 mL of an ethanol/water solution (4:1,
v/
v) was added.
The mixture was homogenized using a Qiagen TissueLyzer II (Qiagen, Hilden, Germany) at 25 MHz for 5 min, followed by an additional homogenization step lasting 30 min. After centrifugation for 15 min, 300 μL of the resulting supernatant was transferred to a 96-well plate. The solvent was subsequently evaporated in a Labconco CentriVap concentrator (Labconco Corporation, Kansas City, MO, USA), and the plates were stored at −80 °C until chromatographic analysis. This methodology, as well as the selection of ethanol and water as suitable solvents for the extraction of compounds from the Malpighiaceae family, was previously tested by Mannochio-Russo et al. (2022) [
16].
2.3. Chromatographic Analysis by UHPLC-MS/MS
For UHPLC-MS/MS analysis, the extracts were solubilized in 200 µL of methanol/water (4:1,
v/
v) containing 2 µM sulfachloropyridazine, which was used as an internal standard to monitor injection performance and retention time consistency [
16]. The chromatographic separation employed a binary solvent system consisting of water (solvent A) and acetonitrile (solvent B), both acidified with 0.1% (
v/
v) formic acid.
The flow rate was maintained at 0.5 mL·min−1. The gradient elution program was as follows: 5% solvent B for 1 min, followed by a linear increase from 5% to 100% solvent B over 5 min, a wash step with 100% solvent B for 2 min, a return to 5% solvent B over 1 min, and equilibration at 5% solvent B for an additional 1 min.
Chromatographic analyses were performed using an UltiMate™ 3000 ultra-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled to a Maxis Impact quadrupole time-of-flight (Q-TOF) mass spectrometer (Bruker Daltonics GmbH & Co. KG, Billerica, MA, USA). Compound separation was carried out on a Kinetex® C18 reversed-phase UHPLC column (Phenomenex Inc., Torrance, CA, USA) with a particle size of 1.7 µm and dimensions of 50 × 2.1 mm.
The mass spectrometer, equipped with an electrospray ionization (ESI) source and a Q-TOF mass analyzer, was operated under the following conditions: source temperature, 200 °C; nitrogen as the nebulizing gas at 2 bar; and capillary voltage set to 4200 V.
Mass spectra were acquired in data-dependent acquisition (DDA) mode, whereby the most intense precursor ions in each scan were automatically selected for subsequent tandem mass spectrometry (MS/MS) fragmentation. See a representative chromatogram in
Figure S1. All original chromatographic profiles obtained by UHPLC-MS/MS are available on the MassIVE platform (MSV000085119).
The UHPLC-MS/MS chromatographic data were processed using MZmine software version 2.53 with the following parameters: block mass correction based on hexakis(1H,1H,2H-difluoroethoxy)phosphazene (Synquest Laboratories Inc., Alachua, FL, USA); mass detection thresholds of 1.0 × 103 for MS1 and 1.0 × 101 for MS2 (centroid mode); peak deconvolution with a minimum peak duration of 0.01 min; baseline level of 1.0 × 103; and median m/z centering. Baseline and signal-to-noise ratio adjustments were optimized for each ionization mode independently. Multivariate statistical analysis was subsequently conducted using Partial Least Squares-Discriminant analysis (PLS-DA) for each ionization mode.
2.4. Chemometric Analysis by PLS-DA
The chromatographic data were organized in a matrix to compare the chromatographic profiles of
Banisteriopsis and
Stigmaphyllon (Malpighiaceae). The samples (
Table S1) were added in columns, and the peak intensities were compiled in the rows. For each comparison (genus, occurrence environment levels, and plant habit), a PLS-DA analysis was performed.
A data matrix with 23,173 rows and 64 columns (positive ionization mode) and a data matrix with 19,289 rows and 64 columns (negative ionization mode) were analyzed by PLS-DA to identify the discriminating metabolites between the compared classes.
The individual analysis of ionization modes (positive and negative) is justified by the chromatographic processing that each mode requires, such as signal/noise ratio and number of ionized compounds, which vary depending on the type of ionization.
For each retention time and its mass-charge value, identification values (ID) were assigned. Before multivariate analysis, data were sum-normalized, log-transformed (base10), and autoscaled. After pre-processing, PLS-DA analyses were performed. All chemometrics analyses were executed using the Metaboanalyst 6.0 software with 95% confidence. The loading graphs were projected using Origin 8.0 software.
PLS-DA model validation used R2 (fit) and Q2 (predictive ability) from cross-validation. High Q2 and R2 indicate accurate and robust models. These parameters validated the PLS-DA model of this research.
The annotation of discriminant metabolites was performed through a comprehensive analysis of their MS/MS fragmentation patterns, combined with database-assisted spectral matching. Experimental spectra were then compared against reference spectra available in curated spectral libraries, including the National Institute of Standards and Technology (NIST) Mass Spectral Library and MassBank, to ensure high-confidence identification. Matching was based on criteria such as mass accuracy, fragment ion correspondence, and spectral similarity scores, thereby reducing the likelihood of false positives and improving annotation reliability. Therefore, the confidence level of the annotations was restricted to MS/MS information [
19].
3. Results and Discussion
Hydroethanolic extracts from 64 Malpighiaceae species were analyzed by UHPLC-MS/MS, and the resulting datasets (positive and negative ionization modes) were compared using PLS-DA. This multivariate approach revealed metabolomic patterns and discriminant metabolites across genera, occurrence environments, and plant habits.
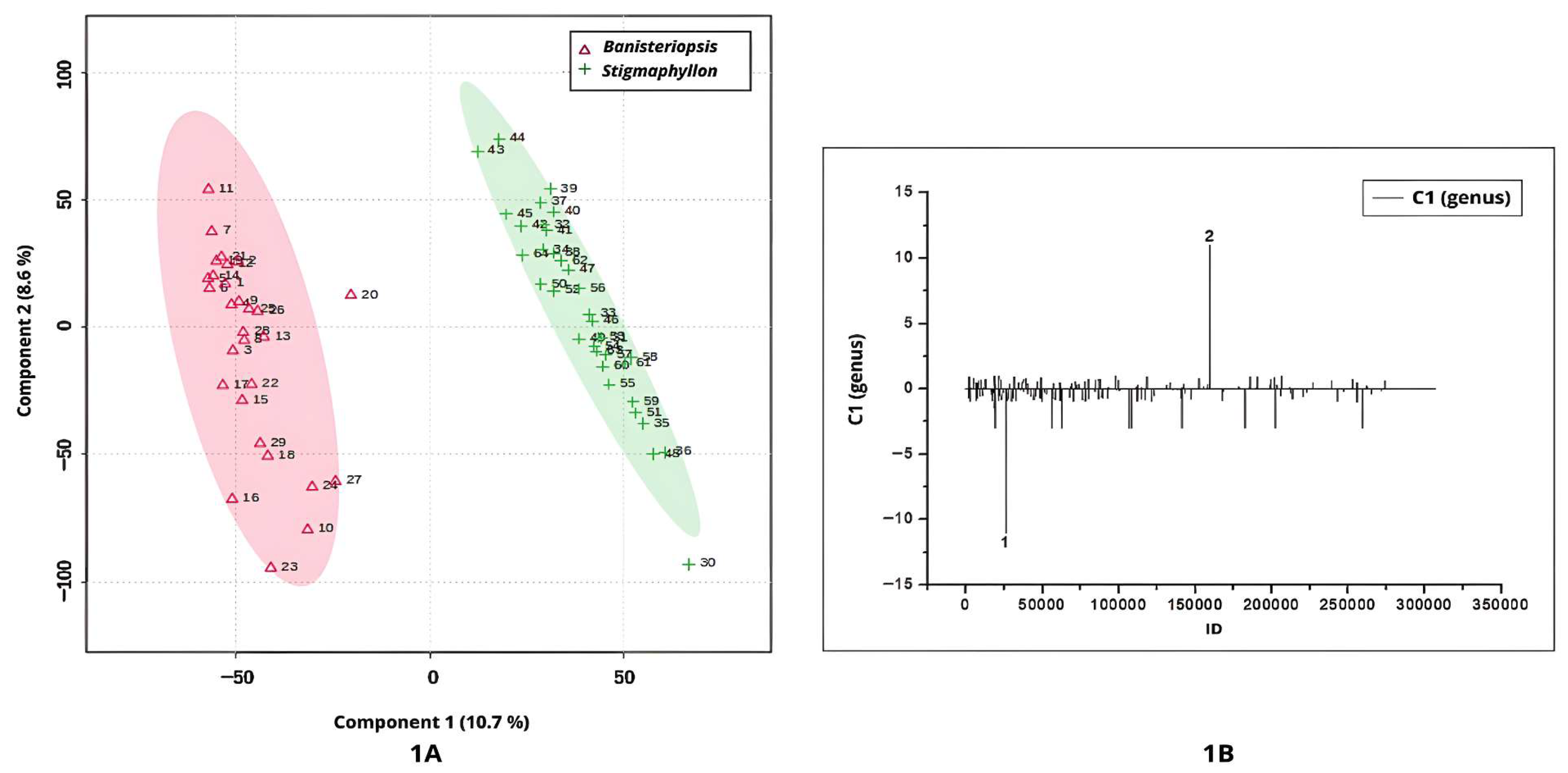
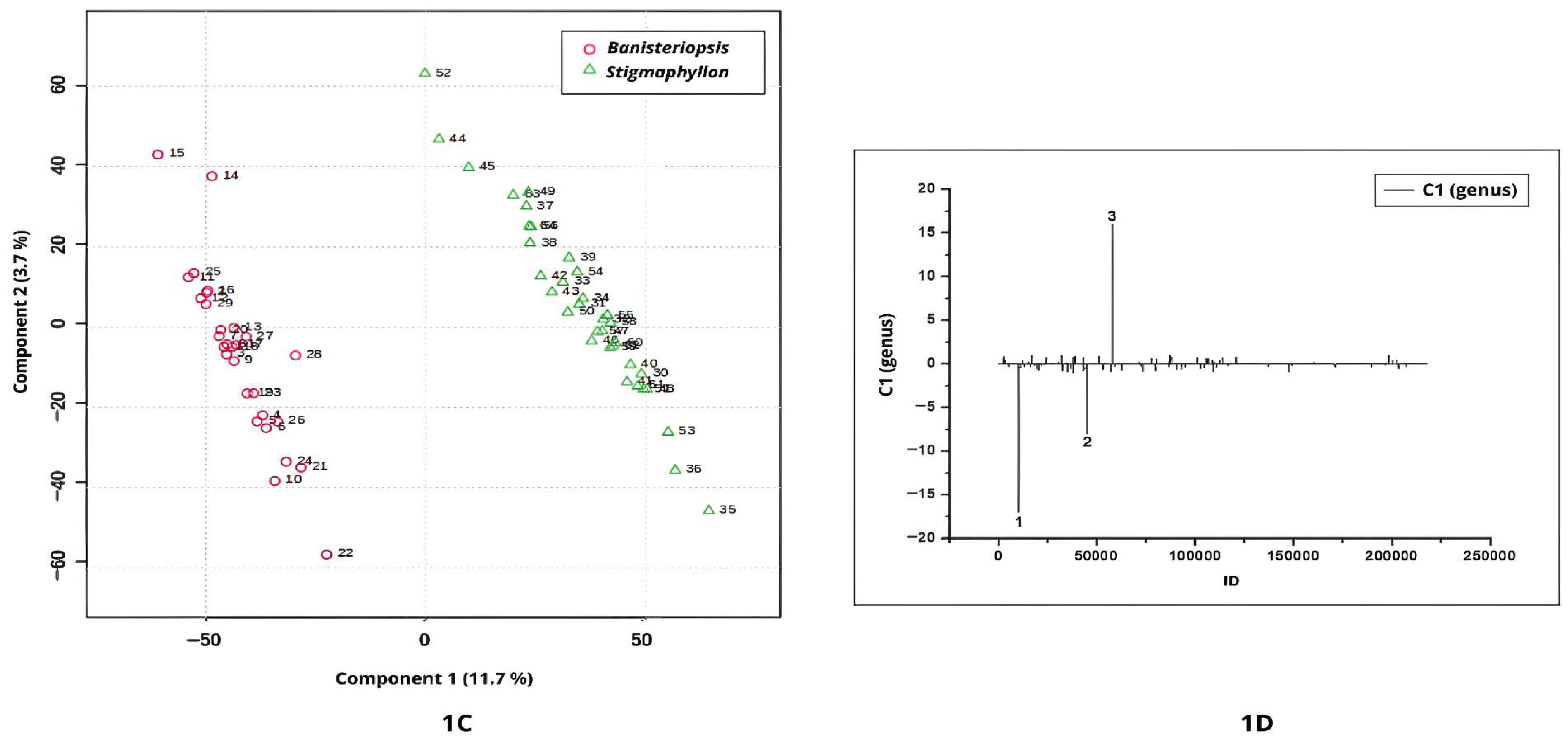
Figure 1A–D illustrate the discrimination between
Banisteriopsis and
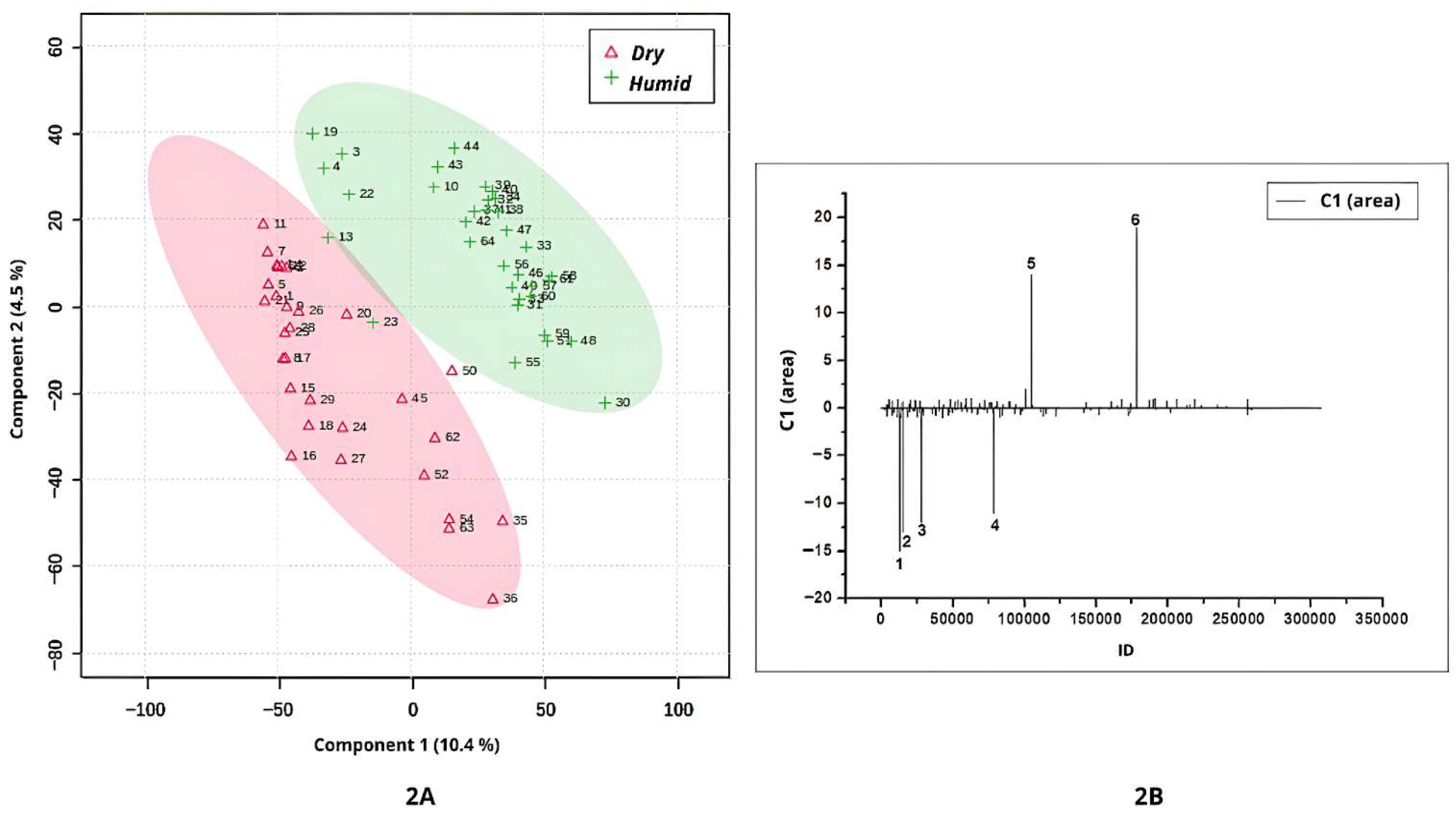
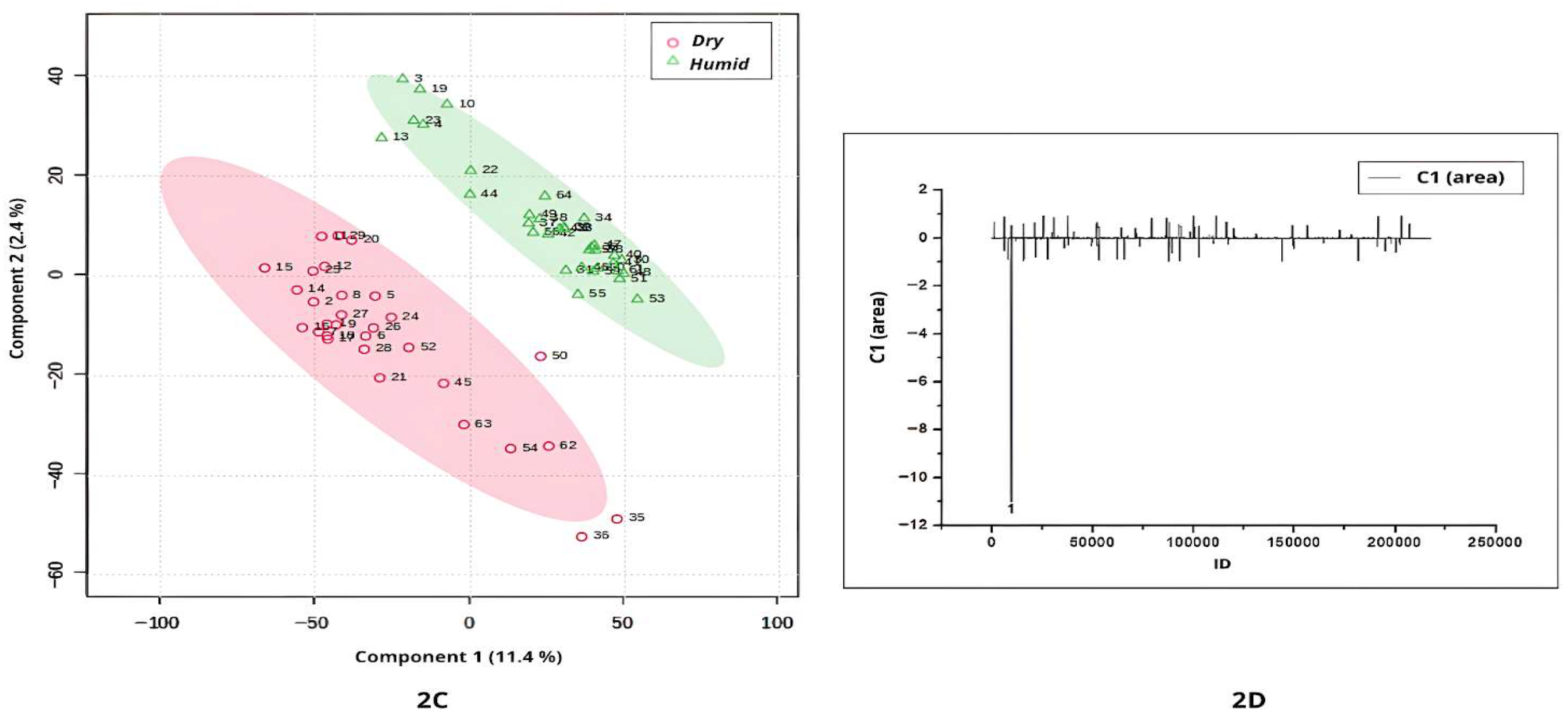
Stigmaphyllon under both ionization modes. The analysis based on cultivation conditions (dry vs. humid) is shown in
Figure 2A–D, while
Figure 3A–D depict the separation according to plant habit.
3.1. Genus-Level Discrimination in Metabolomic Profiles by PLS-DA Using UHPLC-MS/MS in Positive and Negative Ionization Modes
PLS-DA applied to the UHPLC-MS/MS chromatographic profiles under positive ionization mode yielded key insights into the discrimination between
Banisteriopsis and
Stigmaphyllon genus. The score plot for Component 1 clearly delineated two distinct clusters corresponding to the two genera, with a 95% confidence interval (
Figure 1A).
Samples from
Banisteriopsis were positioned on the negative side of Component 1, indicating distinct metabolomic characteristics relative to
Stigmaphyllon (
Figure 1A). According to the loadings plot (
Figure 1B), peak 1 (ID 26087;
m/
z = 481.0950; [M+H]
+; C
21H
21O
13) differentiates
Banisteriopsis from
Stigmaphyllon genus. The ion at
m/
z = 481.0950 is likely myricetin 3-galactoside (
Table 1).
Myricetin-3-galactoside is a flavonol glycoside previously identified in other plant materials, such as the leaves of
Myrtus communis [
20]. No reports of myricetin-3-galactoside in the
Banisteriopsis genus have been found. Therefore, we report here for the first time the annotation of this compound in the
Banisteriopsis species analyzed (
Table 1). As this compound was annotated in the leaves of
Banisteriopsis and exhibited the highest importance in the loadings analysis, it may serve as a potential biomarker for the sampled
Banisteriopsis species. Antioxidant and antigenotoxic activities have been reported for this compound [
20,
21]. Consequently, the
Banisteriopsis samples analyzed may exhibit antioxidant and antigenotoxic activities attributable to myricetin-3-galactoside.
Peak 2 in
Figure 1B (ID 159789;
m/
z = 303.0490; [M+H]
+; C
15H
11O
7), tentatively annotated as quercetin, exhibited positive values in Component 1, indicating its potential as a chemical recurring for
Stigmaphyllon genus. Within the Malpighiaceae family, quercetin has been reported across various genera, including
Byrsonima,
Camarea,
Diplopterys,
Galphimia, and
Malpighia [
3,
5].
Quercetin is associated with diverse bioactivities, such as antioxidant and antibacterial effects, as well as potential applications in oncology and cardiovascular therapies [
22]. Beyond its pharmacological relevance, quercetin also plays a crucial ecological role by mitigating herbivory while preserving the integrity of pollinators and natural enemies [
23].
Metabolomic analyses of
Banisteriopsis and
Stigmaphyllon species have revealed the presence of quercetin in both genera, including
Banisteriopsis anisandra,
B. harleyi, and
B. quadriglandula species. However, a markedly higher quercetin signal intensity was detected in
Stigmaphyllon, indicating greater concentrations of this flavonoid. The elevated levels of quercetin in
Stigmaphyllon may be associated with its thin and delicate leaves, which, due to their increased vulnerability, likely require enhanced synthesis of quercetin as a defense mechanism against herbivory and insect predation, as well as protection from solar radiation [
23,
24].
The UHPLC-MS/MS chromatographic profiles acquired in negative ionization mode revealed distinct separation between the genera, with
Banisteriopsis exhibiting negative scores along Component 1 (
Figure 1C). The negative peaks in
Figure 1D are compounds relevant to the Banisteriopsis genus. According to the loadings plot (
Figure 1D), negative peaks 1 and 2 represent key discriminant metabolites for the genus
Banisteriopsis.
Peak 1 (ID = 10218;
m/
z = 325.0938; [M–H]
−; C
15H
18O
8) was annotated as coumaroyl hexoside, a phenylpropanoid detected in leaf extracts of
B. campestris,
B. argyrophylla,
B. harleyi,
B. megaphylla,
B. membranifolia,
B. laevifolia, and
B. stellaris (
Table 1). While compounds containing the coumaroyl moiety have previously been reported in leaves of
B. laevifolia and
B. stellaris [
9], the occurrence of coumaroyl hexoside is reported here for the first time in the
Banisteriopsis species listed in
Table 1.
We found no evidence in the literature regarding the phytochemical role of coumaroyl hexoside, nor any reports of its restricted occurrence or phylogenetic association with Banisteriopsis species. Thus, the identification of coumaroyl hexoside as a discriminant metabolite in the comparison between Banisteriopsis and another genus of the Malpighiaceae represents a relevant finding, contributing to the understanding of metabolomic differentiation within this family. Moreover, this result expands the chemical diversity known for Malpighiaceae, suggesting unexplored biosynthetic capacities within the group. In addition, it provides a potential chemotaxonomic marker that may assist in clarifying evolutionary relationships among closely related taxa.
Peak 2 (
Figure 1D), with
m/
z = 613.1142, corresponds to a metabolite detected in
Banisteriopsis leaf extracts. However, this ion has not been previously reported in the literature, nor does it match any entries in the NIST or MassBank spectral databases. The absence of spectral correspondence in the consulted libraries indicates a high probability that the detected compound represents a previously unreported natural product. This finding not only underscores the importance of advancing structural elucidation and functional characterization but also highlights the critical need to expand and refine spectral databases, particularly by incorporating high-quality MS/MS reference spectra. Such efforts are essential to improve metabolite annotation reliability, reduce the rate of unidentified features in untargeted metabolomics, and foster the discovery of novel bioactive molecules.
The most positive values of Component 1 were attributed to the genus
Stigmaphyllon (
Figure 1C). The positive signal in the loading plot indicates that the single positive feature observed in
Figure 1D (peak 3, ID 57760;
m/
z = 619.4214) is associated with
Stigmaphyllon leaves. Peak 3 could not be annotated based on available spectral libraries.
3.2. Occurrence Environment-Level Discrimination in Metabolomic Profiles by PLS-DA Using UHPLC-MS/MS in Positive and Negative Ionization Modes
In this study, the term humidity refers to the general environmental conditions associated with the natural occurrence of each species, rather than to direct quantitative measurements of climatic humidity. Specifically, the classification of “dry” and “humid” environments was based on the predominant ecological characteristics of the Brazilian biomes where the species naturally occur. This ecological classification is widely recognized in the Brazilian literature and provides a consistent framework for distinguishing metabolomic patterns across contrasting habitats.
Based on occurrence environment levels, the PLS-DA analysis revealed clear discrimination between leaf samples cultivated in dry and humid environments (
Figure 2A). Samples from dry regions exhibited the highest negative scores along Component 1 in the score plot (
Figure 2A). The corresponding loadings plot (
Figure 2B) identified four compounds (peaks 1, 2, 3, and 4) that were more abundantly synthesized under dry conditions.
Peak 1 shown in
Figure 2B (ID = 12982;
m/
z = 611.1611; [M+H]
+; C
27H
31O
16) was annotated as quercetin-3-
O-robinobioside, a bioactive flavonoid with well-documented medicinal properties, previously reported in other plant species such as
Abelmoschus manihot (Malvaceae) [
25]. Although this compound is associated with medicinal properties and offers benefits to humans [
25], flavonoids are also recognized for their role in enhancing plant survival under abiotic stress conditions, such as low water availability in cultivated environments [
26]. Therefore, although no studies have directly linked quercetin-3-
O-robinobioside to a metabolomic response to water scarcity, our analyses indicate that it serves as a potential biomarker for
Banisteriopsis and
Stigmaphyllon species developed in environments of similar occurrence.
Table 1 presents the
Banisteriopsis and
Stigmaphyllon species in a dry occurrence environment where quercetin-3-
O-robinobioside was detected. To our knowledge, the occurrence of this metabolite in all these species has not been previously reported in the literature.
In
Figure 2B, peak 2 (ID = 15392;
m/
z = 269.1983), peak 3 (ID = 28196;
m/
z = 592.4046), and peak 4 (ID = 78676;
m/
z = 131.9824) were identified as discriminant compounds in leaves cultivated in dry regions, although they were not annotated.
The highest positive values in Component 1 of
Figure 2A correspond to samples cultivated in a humid occurrence environment. Two compounds (peaks 5 and 6;
Figure 2B) were characteristic of
Banisteriopsis and
Stigmaphyllon leaves from these environments. Peak 5 (ID = 105117;
m/
z = 785.4863) and peak 6 (ID = 178588;
m/
z = 223.1160) were not annotated in the available spectral libraries.
When comparing samples from humid and dry occurrence environments using UHPLC-MS/MS profiles in negative ionization mode, the lowest scores were associated with those cultivated in dry regions (
Figure 2C). A single compound (peak 1, ID = 9604;
m/
z = 937.6092; not annotated) was responsible for the separation between dry- and humid-occurrence environment samples (
Figure 2D).
Dry environments such as the Cerrado and Caatinga are characterized by pronounced seasonality, extended dry periods, high solar radiation, and annual rainfall typically below 1800 mm. In contrast, humid biomes like the Amazon Rainforest and Atlantic Forest experience high and well-distributed precipitation throughout the year, often exceeding 2000 mm, and maintain relative humidity levels above 80% [
2]. These strongly contrasting climatic regimes exert significant influence on plant physiology, adaptive strategies, and the biosynthesis of secondary metabolites. Such environmental pressures likely contribute to the metabolomic patterns observed in this study, as reflected in the PLS-DA separations shown in
Figure 2A–D.
In summary, the results of the comparison between Banisteriopsis and Stigmaphyllon leaves from dry and humid environments showed that, based on the metabolomic profile (positive and negative ionization modes), the environmental occurrence will affect metabolomic synthesis. These findings are important because they provide evidence that environmental conditions act as key modulators of secondary metabolism, influencing the qualitative and quantitative diversity of specialized metabolites. They also suggest that metabolomic plasticity may contribute to the ecological success and adaptive strategies of Malpighiaceae species across heterogeneous habitats. Furthermore, the identification of environment-dependent chemical signatures offers potential biomarkers for studying plant–environment interactions and may guide future investigations into the ecological and pharmacological relevance of these metabolites.
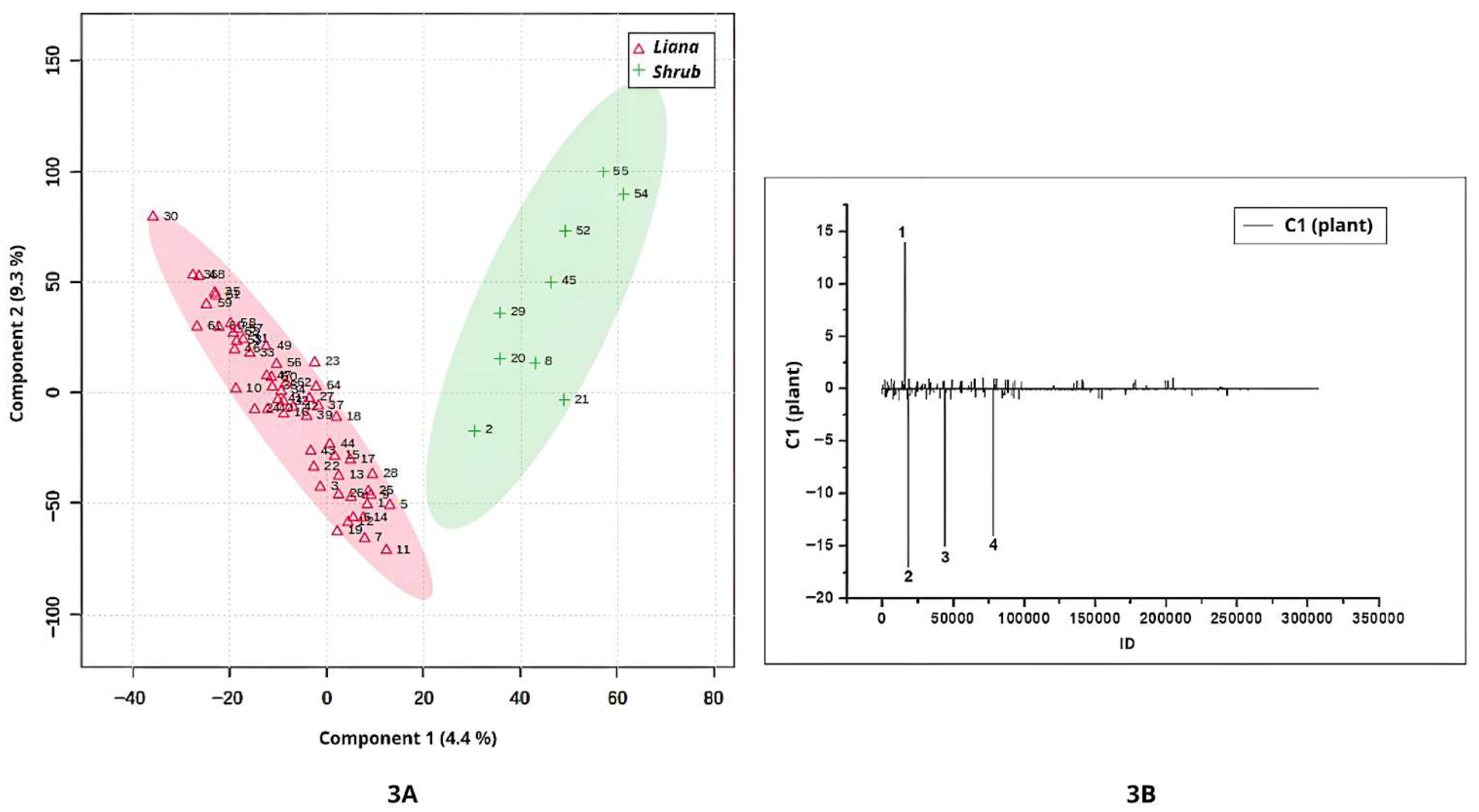
3.3. Habit Plant-Level Discrimination in Metabolomic Profiles by PLS-DA Using UHPLC-MS/MS in Positive and Negative Ionization Modes
The
Banisteriopsis and
Stigmaphyllon samples analyzed in this study included leaves from both lianas and shrubs (
Table S1). Component 1 of the analysis revealed a clear discrimination between the metabolomic profiles of liana and shrub leaves (
Figure 3A). The samples from shrubs had the highest positive values (
Figure 3A). According to the loadings (
Figure 3B), one compound, annotated as glucose (peak 1; ID = 15823;
m/
z = 383.1161; [2M+Na]
+; 2(C
6H
12O
6)Na, was predominantly associated with shrubs. Notably, samples with the highest positive values (
S. paralias, and
S. occidentale) corresponded to shrub leaves of the genus
Stigmaphyllon, suggesting that this growth habit in this genus may synthesize higher concentrations of glucose compared to lianas.
The presence of glucose in Malpighiaceae leaves has been reported in species such as
Banisteriopsis muricata and
Diplopterys pubipetala [
27,
28]. Optical and electron microscopy analyses suggest that glucose accumulation occurs in leaf glands that secrete sugar during development, functioning as extrafloral nectaries and attracting visitors for ecologically relevant interactions [
28]. Additionally, the identification of glucose as a metabolite associated with shrubs highlights its potential role in carbon allocation strategies, which may be linked to differences in energy storage and growth dynamics between lianas and shrubs.
The highest negative scores along Component 1 (
Figure 3A) were associated with liana samples. Three compounds contributed to the separation between lianas and shrubs: peak 2 (ID = 18390;
m/
z = 556.4688; not annotated), peak 3 (ID = 43785;
m/
z = 541.4069; not annotated), and peak 4 (ID = 77642;
m/
z = 967.5681; not annotated), all of which were detected at higher intensities in liana leaves.
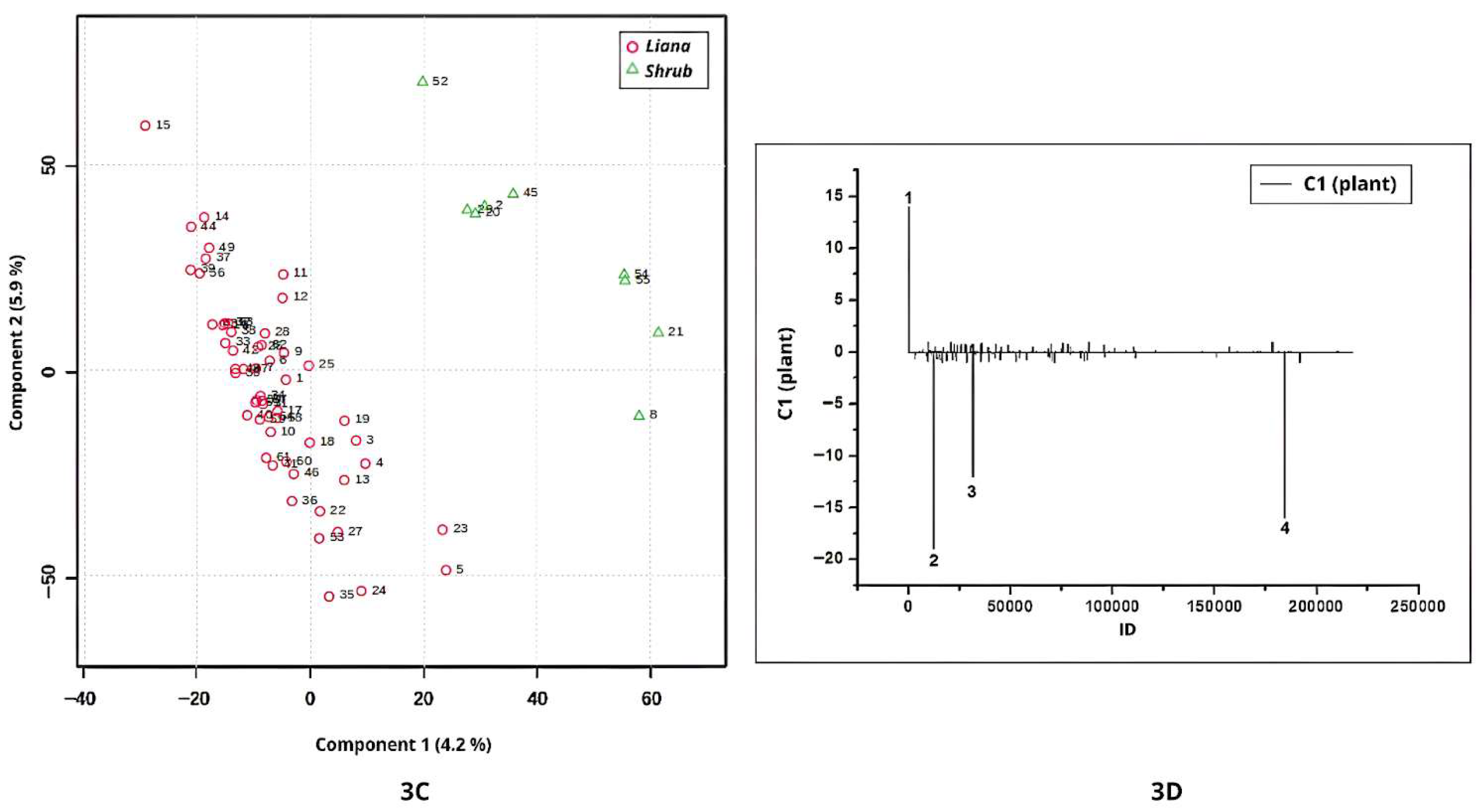
The chromatographic profiles (negative ionization mode) of shrub and liana leaves were also discriminated by PLS-DA (
Figure 3C). Samples from shrubs were associated with positive scores on Component 1 (
Figure 3C), with the corresponding positive loadings observed in
Figure 3D. In this plot, Peak 1 (ID = 78;
m/
z = 374.9905) was detected but remained unannotated.
Conversely, liana samples were characterized by negative scores on Component 1, with negative loadings in
Figure 3D representing metabolites more prevalent in lianas. This pattern indicates that, despite taxonomic differences among the liana species analyzed, their chemical profiles are similar due to the presence of these discriminant signals. Peaks 2 (ID = 12245;
m/
z = 769.4048), peak 3 (ID = 31584;
m/
z = 515.3509), and peak 4 (ID = 184231;
m/
z = 518.1523) contributed to this separation but were not annotated (
Figure 3D).
The classes of lianas and shrubs are metabolomically distinct, as evidenced by the PLS-DA analysis. In conclusion, these results emphasize that metabolomic differentiation between growth forms reflects intrinsic physiological and developmental traits rather than solely environmental influences.
4. Conclusions
PLS-DA analyses of the metabolomic profiles from 64 hydroethanolic leaf extracts of Banisteriopsis and Stigmaphyllon revealed distinct chemical markers that discriminate samples according to genus, environmental occurrence, and plant growth habit (lianas vs. shrubs). Through comparison with spectral libraries, five metabolites were identified as potential chemotaxonomic markers: glucose (a primary carbohydrate), coumaroyl hexoside (a phenylpropanoid), myricetin-3-galactoside, quercetin, and quercetin-3-O-robinobioside (all flavonoids). These metabolites contribute to the differentiation of leaf chemical composition between the two genera.
To our knowledge, this is the first comparative metabolomic assessment of these two Neotropical genera, providing insights into how both taxonomic and ecological factors shape metabolite composition, such as genus identity, water availability, and plant growth habit. The results indicate that metabolomic variation in Banisteriopsis and Stigmaphyllon is influenced by a combination of phylogenetic, environmental, and physiological factors, reflecting their adaptive responses in tropical habitats. Beyond their chemotaxonomic relevance, the identified metabolites represent promising candidates for future ecological, pharmacological, and biotechnological studies.
Overall, this study demonstrates the utility of UHPLC-QTOF-MS/MS combined with multivariate analysis in exploring chemical diversity within complex plant groups. Future research integrating untargeted metabolomics with transcriptomic and ecological datasets will be valuable for elucidating biosynthetic pathways and adaptive mechanisms in Malpighiaceae.