Comparison and Validation of QuEChERS Extraction Methods Coupled with UHPLC/Orbitrap HR-MS for the Determination of Antibiotics and Related Compounds in Fish and Fish Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection and Pretreatment
2.3. QuEChERS Extraction
2.4. UHPLC/LTQ Orbitrap MS Analysis
2.5. Validation Study
2.6. Green Analytical Chemistry of the Extraction Procedures
3. Results and Discussion
3.1. Optimization of QuEChERS Extraction Method
3.2. Validation Results
3.3. Evaluation of the Greenness of the Studied Methods
3.4. Comparison of the Validated Methods with Other Studies
3.5. Application to Real Fish and Fish Feed Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manjarrés-López, D.P.; Vitale, D.; Callejas-Martos, S.; Usuriaga, M.; Picó, Y.; Pérez, S.; Montemurro, N. An Effective Method for the Simultaneous Extraction of 173 Contaminants of Emerging Concern in Freshwater Invasive Species and Its Application. Anal. Bioanal. Chem. 2023, 415, 7085–7101. [Google Scholar] [CrossRef]
- Peña-Herrera, J.M.; Montemurro, N.; Barceló, D.; Pérez, S. Development and Validation of an Analytical Method for Determination of Pharmaceuticals in Fish Muscle Based on QuEChERS Extraction and SWATH Acquisition Using LC-QTOF-MS/MS System. Talanta 2019, 199, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Baesu, A.; Ballash, G.; Mollenkopf, D.; Wittum, T.; Sulliván, S.M.P.; Bayen, S. Suspect Screening of Pharmaceuticals in Fish Livers Based on QuEChERS Extraction Coupled with High Resolution Mass Spectrometry. Sci. Total Environ. 2021, 783, 146902. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; Arthur, J.R. Improving Biosecurity Through Prudent and Responsible Use of Veterinary Medicines in Aquatic Food Production; FAO: Rome, Italy, 2012; p. 226. ISBN 978-92-5-106975-2. [Google Scholar]
- Mukota, A.K.; Gondam, M.F.K.; Tsafack, J.J.T.; Sasanya, J.; Reybroeck, W.; Ntale, M.; Nyanzi, S.A.; Tebandeke, E. Primary Validation of Charm II Tests for the Detection of Antimicrobial Residues in a Range of Aquaculture Fish. BMC Chem. 2020, 14, 32. [Google Scholar] [CrossRef]
- Dabestani-Rahmatabad, A.; Nava-Guajardo, L.; Nicol, E.; Stephane, B. A Review on the Main Antibiotic Drugs Used in Fish Farming: Ecotoxicity, Characterization and Remediation. Aquac. Fish. Stud. 2021, 3, 1–11. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Hoogenveen, R.; Rosenlund, G.; Garlito, B.; Zeilmaker, M.J. Do Background Levels of the Pesticide Pirimiphosmethyl in Plant-Based Aquafeeds Affect Food Safety of Farmed Atlantic Salmon? Food Addit. Contam. Part A 2020, 37, 2109–2122. [Google Scholar] [CrossRef]
- Koloka, O.; Boti, V.; Hela, D.; Albanis, T.; Konstantinou, I. PFAS Residue Analysis in Fish and Fish Feed by Applying a Modified QuEChERS Method in Combination with LC-HRMS Detection. Emerg. Contam. 2025, 11, 100467. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/documents/card/en/c/cc0461en (accessed on 15 March 2023).
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Aissaoui, Y.; Jiménez-Skrzypek, G.; González-Sálamo, J.; Trabelsi-Ayadi, M.; Ghorbel-Abid, I.; Hernández-Borges, J. Determination of Multiclass Antibiotics in Fish Muscle Using a QuEChERS-UHPLC-MS/MS Method. Foods 2024, 13, 1081. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rambla-Alegre, M.; Carrasco, N.; Lopez De Alda, M.; Barceló, D. Fast Analysis of Relevant Contaminants Mixture in Commercial Shellfish. Talanta 2019, 205, 119884. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Jacobs, S.; Serra-Compte, A.; Cáceres, N.; Sioen, I.; Verbeke, W.; Barbosa, V.; Ferrari, F.; Fernández-Tejedor, M.; et al. Pharmaceuticals and Endocrine Disruptors in Raw and Cooked Seafood from European Market: Concentrations and Human Exposure Levels. Environ. Int. 2018, 119, 570–581. [Google Scholar] [CrossRef]
- Barros, S.C.; Silva, A.S.; Torres, D. Multiresidues Multiclass Analytical Methods for Determination of Antibiotics in Animal Origin Food: A Critical Analysis. Antibiotics 2023, 12, 202. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-Residue Determination of 115 Veterinary Drugs and Pharmaceutical Residues in Milk Powder, Butter, Fish Tissue and Eggs Using Liquid Chromatography–Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 880, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, A.; Fan, K.; Minh Tien, M.; Savoy, M.C.; Tarres, A.; Fuger, D.; Goyon, A.; Bessaire, T.; Mottier, P. Determination of 105 Antibiotic, Anti-Inflammatory, Antiparasitic Agents and Tranquilizers by LC-MS/MS Based on an Acidic QuEChERS-like Extraction. Food Addit. Contam. Part A 2018, 35, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, Y.; Mok, S.; Moon, H.B.; Jeon, J. Optimization of the QuEChERS Method for Multi-Residue Analysis of Pharmaceuticals and Pesticides in Aquaculture Products. Food Chem. 2023, 399, 133958. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, L.; Ziarrusta, H.; Zabaleta, I.; Usobiaga, A.; Olivares, M.; Zuloaga, O.; Etxebarria, N.; Prieto, A. Multiresidue Analytical Method for the Determination of 41 Multiclass Organic Pollutants in Mussel and Fish Tissues and Biofluids by Liquid Chromatography Coupled to Tandem Mass Spectrometry. ABC 2019, 411, 493–506. [Google Scholar] [CrossRef]
- Peña-Herrera, J.M.; Montemurro, N.; Barceló, D.; Pérez, S. Analysis of Pharmaceuticals in Fish Using Ultrasound Extraction and Dispersive Spe Clean-up on Que Z-Sep/C18 Followed by LC-QToF-MS Detection. MethodsX 2020, 7, 101010. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Lin, J.; Bao, R. A Modified QuEChERS-Based UPLC-MS/MS Method for Rapid Determination of Multiple Antibiotics and Sedative Residues in Freshwater Fish. Food Chem. X 2024, 22, 101268. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Communities 2010, 15, 1–72. [Google Scholar]
- Diallo, T.; Makni, Y.; Lerebours, A.; Thomas, H.; Guérin, T.; Parinet, J. Wide-Scope Screening of Multi-Class Contaminants in Seafood Using a Novel Sample Preparation (QuEChUP) Procedure Coupled with UHPLC-Q-TOF-MS: Application for Semi-Quantitation of Real Seafood Samples. Food Chem. 2023, 426, 136572. [Google Scholar] [CrossRef]
- Jung, H.N.; Park, D.H.; Yoo, K.H.; Cho, H.J.; Shim, J.H.; Shin, H.C.; Abd El-Aty, A.M. Simultaneous Quantification of 12 Veterinary Drug Residues in Fishery Products Using Liquid Chromatography-Tandem Mass Spectrometry. Food Chem. 2021, 348, 129105. [Google Scholar] [CrossRef]
- Turnipseed, S.B.; Storey, J.M.; Wu, I.L.; Andersen, W.C.; Madson, M.R. Extended Liquid Chromatography High Resolution Mass Spectrometry Screening Method for Veterinary Drug, Pesticide and Human Pharmaceutical Residues in Aquaculture Fish. Food Addit. Contam.—Part Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1501–1514. [Google Scholar] [CrossRef]
- Fernandez-Torres, R.; Lopez, B.A.B.; Consentino, M.O.; Mochon, M.C.; Payan, M.R. Enzymatic-Microwave Assisted Extraction and High-Performance Liquid Chromatography-Mass Spectrometry for the Determination of Selected Veterinary Antibiotics in Fish and Mussel Samples. J. Pharm. Biomed. Anal. 2011, 54, 1146–1156. [Google Scholar] [CrossRef]
- Subedi, B.; Mottaleb, M.A.; Chambliss, C.K.; Usenko, S. Simultaneous Analysis of Select Pharmaceuticals and Personal Care Products in Fish Tissue Using Pressurized Liquid Extraction Combined with Silica Gel Cleanup. J. Chromatogr. A 2011, 1218, 6278–6284. [Google Scholar] [CrossRef]
- Togunde, O.P.; Oakes, K.D.; Servos, M.R.; Pawliszyn, J. Optimization of Solid Phase Microextraction for Non-Lethal in Vivo Determination of Selected Pharmaceuticals in Fish Muscle Using Liquid Chromatography-Mass Spectrometry. J. Chromatogr. A 2012, 1261, 99–106. [Google Scholar] [CrossRef]
- Baduel, C.; Mueller, J.F.; Tsai, H.; Gomez Ramos, M.J. Development of Sample Extraction and Clean-up Strategies for Target and Non-Target Analysis of Environmental Contaminants in Biological Matrices. J. Chromatogr. A 2015, 1426, 33–47. [Google Scholar] [CrossRef]
- Huerta, B.; Rodríguez-Mozaz, S.; Barcelo, D. Analysis of Pharmaceutical Compounds in Biota. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 169–193. [Google Scholar] [CrossRef]
- DeAtley, A.; Zhao, L.; Lucas, D. Innovative Sample Prep Removes Lipids without Losing Analytes. Am. Lab. 2015, 47, 32–34. [Google Scholar]
- Drábová, L.; Dvořáková, D.; Urbancová, K.; Gramblička, T.; Hajšlová, J.; Pulkrabová, J. Critical Assessment of Clean-Up Techniques Employed in Simultaneous Analysis of Persistent Organic Pollutants and Polycyclic Aromatic Hydrocarbons in Fatty Samples. Toxics 2022, 10, 12. [Google Scholar] [CrossRef]
- Han, L.; Matarrita, J.; Sapozhnikova, Y.; Lehotay, S.J. Evaluation of a Recent Product to Remove Lipids and Other Matrix Co-Extractives in the Analysis of Pesticide Residues and Environmental Contaminants in Foods. J. Chromatogr. A 2016, 1449, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, Z.; Shi, Q.; Zhang, R.; Sun, A.; Zhao, J.; Wu, Y.; Shi, X. Rapid Determination and Risk Evaluation of Multi-Class Antibiotics in Aquatic Products by One-Step Purification Process Coupled with Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Talanta 2024, 277, 126421. [Google Scholar] [CrossRef]
- Gómez-Pérez, M.L.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Analysis of Veterinary Drug and Pesticide Residues in Animal Feed by High-Resolution Mass Spectrometry: Comparison between Time-of-Flight and Orbitrap. Food Addit. Contam. Part A 2015, 32, 1637–1646. [Google Scholar] [CrossRef]
- Petrović, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid Chromatography-Tandem Mass Spectrometry for the Analysis of Pharmaceutical Residues in Environmental Samples: A Review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef]
- Villagrasa, M.; López de Alda, M.; Barceló, D. Environmental Analysis of Fluorinated Alkyl Substances by Liquid Chromatography-(Tandem) Mass Spectrometry: A Review. Anal. Bioanal. Chem. 2006, 386, 953–972. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Zhang, X.; Ouyang, N.; Zhou, Y. Optimization of QuEChERS Method for Antibiotic Residue Analysis in Animal Foods via Response Surface Methodology. Separations 2023, 10, 459. [Google Scholar] [CrossRef]
- Kalogeropoulou, A.G.; Kosma, C.I.; Albanis, T.A. Simultaneous Determination of Pharmaceuticals and Metabolites in Fish Tissue by QuEChERS Extraction and UHPLC Q/Orbitrap MS Analysis. Anal. Bioanal. Chem. 2021, 413, 7129–7140. [Google Scholar] [CrossRef]
- Kosma, C.I.; Koloka, O.L.; Albanis, T.A.; Konstantinou, I.K. Accurate Mass Screening of Pesticide Residues in Wine by Modified QuEChERS and LC-Hybrid LTQ/Orbitrap-MS. Food Chem. 2021, 360, 130008. [Google Scholar] [CrossRef] [PubMed]
- Tran-Lam, T.-T.; Pham, P.T.; Bui, M.Q.; Dao, Y.H.; Le, G.T. Organophosphate Esters and Their Metabolites in Marine Fish from Vietnam: Analytical Method Development and Validation. J. Food Compos. Anal. 2024, 131, 106266. [Google Scholar] [CrossRef]
- Boti, V.; Martinaiou, P.; Gkountouras, D.; Albanis, T. Target and Suspect Screening Approaches for the Identification of Emerging and Other Contaminants in Fish Feeds Using High Resolution Mass Spectrometry. Environ. Res. 2024, 251, 118739. [Google Scholar] [CrossRef]
- Nannou, C.I.; Boti, V.I.; Albanis, T.A. Trace Analysis of Pesticide Residues in Sediments Using Liquid Chromatography-High-Resolution Orbitrap Mass Spectrometry. Anal. Bioanal. Chem. 2018, 410, 1977–1989. [Google Scholar] [CrossRef]
- European Commission Decision. Commission Decision 2021/808/EC the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC. 2021. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/808/oj/eng (accessed on 22 March 2021).
- AOAC 2007.01; Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Gas Chromatography/Mass Spectrometry and Liquid Chromatography/Tandem Mass Spectrometry. AOAC International: Rockville, MD, USA, 2007. Available online: https://nucleus.iaea.org/sites/fcris/Shared%20Documents/SOP/AOAC_2007_01.pdf (accessed on 8 September 2025).
- Habibi, B.; Ghorbel-Abid, I.; Lahsini, R.; Chehimi Ben Hassen, D.; Trabelsi-Ayadi, M. Development and Validation of a Rapid Hplc Method for Multiresidue Determination of Erythromycin, Clarithromycin, and Azithromycin in Aquaculture Fish Muscles. Acta Chromatogr. 2019, 31, 109–112. [Google Scholar] [CrossRef]
- Horwitz, W. Evaluation of Analytical Methods Used for Regulation of Foods and Drugs. Anal. Chem. 1982, 54, 67A–76A. [Google Scholar] [CrossRef]
- Horwitz, W.; Albert, R. The Horwitz Ratio (HorRat): A Useful Index of Method Performance with Respect to Precision. J. AOAC Int. 2006, 89, 1095–1109. [Google Scholar] [CrossRef]
- Miserli, K.; Kosma, C.; Konstantinou, I. Determination of Pharmaceuticals and Metabolites in Sludge and Hydrochar after Hydrothermal Carbonization Using Sonication-QuEChERS Extraction Method and UHPLC LTQ/Orbitrap MS. Environ. Sci. Pollut. Res. Int. 2023, 30, 1686–1703. [Google Scholar] [CrossRef]
- Marinou, E.; Samanidou, V.F.; Papadoyannis, I.N. Development of a High Pressure Liquid Chromatography with Diode Array Detection Method for the Determination of Four Tetracycline Residues in Milk by Using QuEChERS Dispersive Extraction. Separations 2019, 6, 21. [Google Scholar] [CrossRef]
- Santos, L.; Rosa, J.; Freitas, A.; Leston, S.; Barbosa, J.; Ramos, F. Detection and Quantification of 47 Antibiotic Residues in Farmed European Sea Bass (Dicentrarchus labrax) Using a Multi-Class and Multi-Residue UHPLC-MS/MS Method. Food Addit. Contam. Part A 2019, 36, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Varenina, I.; Bilandžić, N.; Luburić, Đ.B.; Kolanović, B.S.; Varga, I. High Resolution Mass Spectrometry Method for the Determination of 13 Antibiotic Groups in Bovine, Swine, Poultry and Fish Meat: An Effective Screening and Confirmation Analysis Approach for Routine Laboratories. Food Control 2022, 133, 108576. [Google Scholar] [CrossRef]
- Essam, H.M.; Saad, M.N.; Elzanfaly, E.S.; Amer, S.M. Optimization and Validation of Eco-Friendly RP-HPLC and Univariate Spectrophotometric Methods for the Simultaneous Determination of Fluorometholone and Tetrahydrozoline Hydrochloride. Acta Chromatogr. 2021, 33, 216–227. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for Assessing the Greenness of Analytical Procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Kowtharapu, L.P.; Katari, N.K.; Muchakayala, S.K.; Marisetti, V.M. Green Metric Tools for Analytical Methods Assessment Critical Review, Case Studies and Crucify. TrAC Trends Anal. Chem. 2023, 166, 117196. [Google Scholar] [CrossRef]
- Mansour, F.R.; Omer, K.M.; Płotka-Wasylka, J. A Total Scoring System and Software for Complex Modified GAPI (ComplexMoGAPI) Application in the Assessment of Method Greenness. Green Anal. Chem. 2024, 10, 100126. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary Green Analytical Procedure Index (ComplexGAPI) and Software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Yin, L.; Yu, L.; Guo, Y.; Wang, C.; Ge, Y.; Zheng, X.; Zhang, N.; You, J.; Zhang, Y.; Shi, M. Green Analytical Chemistry Metrics for Evaluating the Greenness of Analytical Procedures. J. Pharm. Anal. 2024, 14, 101013. [Google Scholar] [CrossRef] [PubMed]
- Ayasinghe, G.D.T.M.; Szpunar, J.; Lobinski, R.; Edirisinghe, E.M.R.K.B. Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Fishes 2023, 8, 154. [Google Scholar] [CrossRef]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Multiresidue Determination of Veterinary Drugs in Aquaculture Fish Samples by Ultra High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. B 2012, 895–896, 39–47. [Google Scholar] [CrossRef]
- Dai, J.; Lin, H.; Pan, Y.; Sun, Y.; Wang, Y.; Qiao, J.; Lian, H.; Xu, C. Determination of Chlorpromazine and Its Metabolites in Animal-Derived Foods Using QuEChERS-Based Extraction, EMR-Lipid Cleanup, and UHPLC-Q-Orbitrap MS Analysis. Food Chem. 2023, 403, 134298. [Google Scholar] [CrossRef]
- Konak, Ü.İ.; Yatmaz, H.A.; Nilüfer, Ş.; Erkaymaz, T.; Certel, M. Multiresidue Method for the Simultaneous Analysis of Antibiotics and Mycotoxins in Feeds by Ultra-High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Acta Aliment. 2021, 50, 74–82. [Google Scholar] [CrossRef]
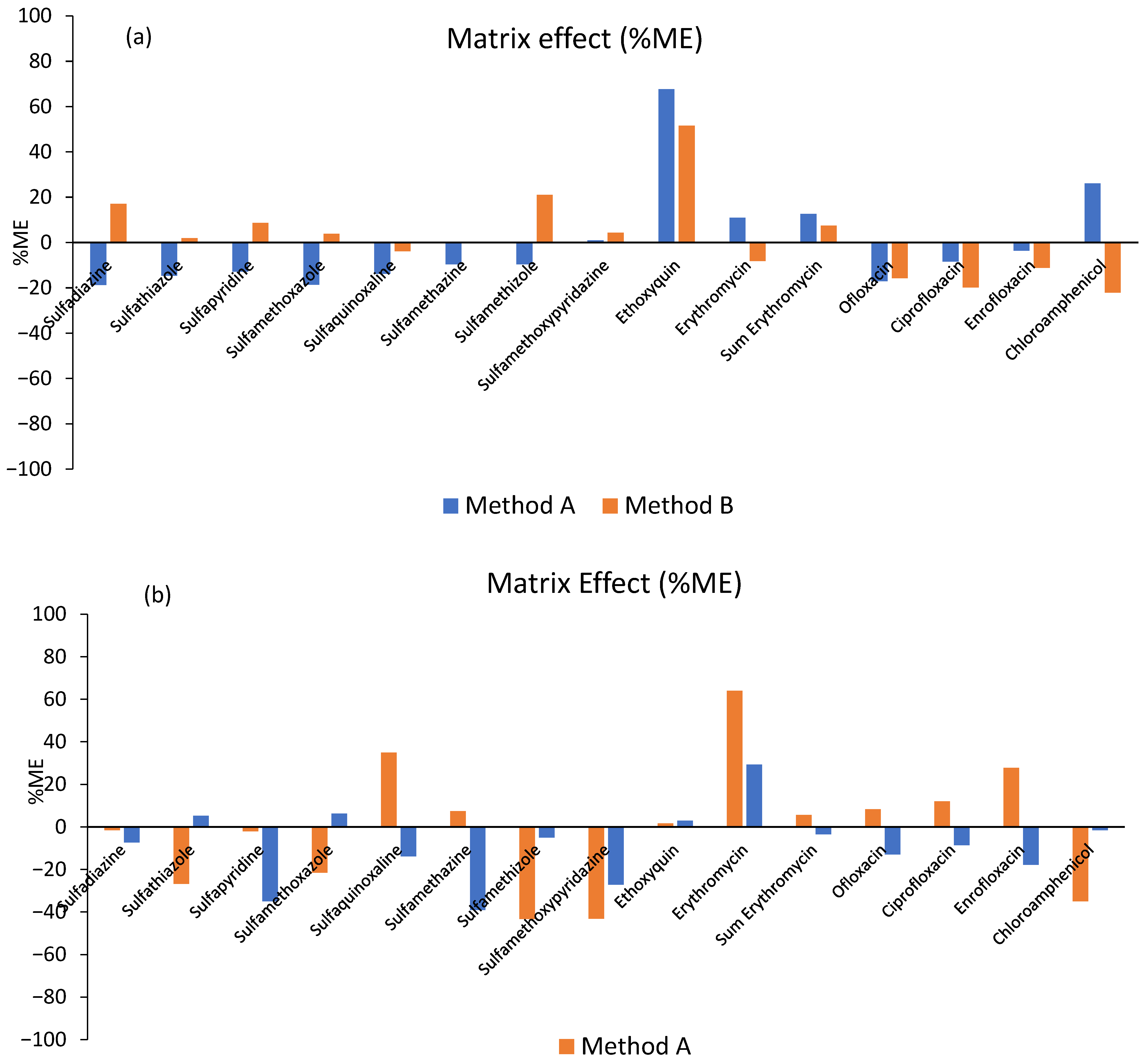
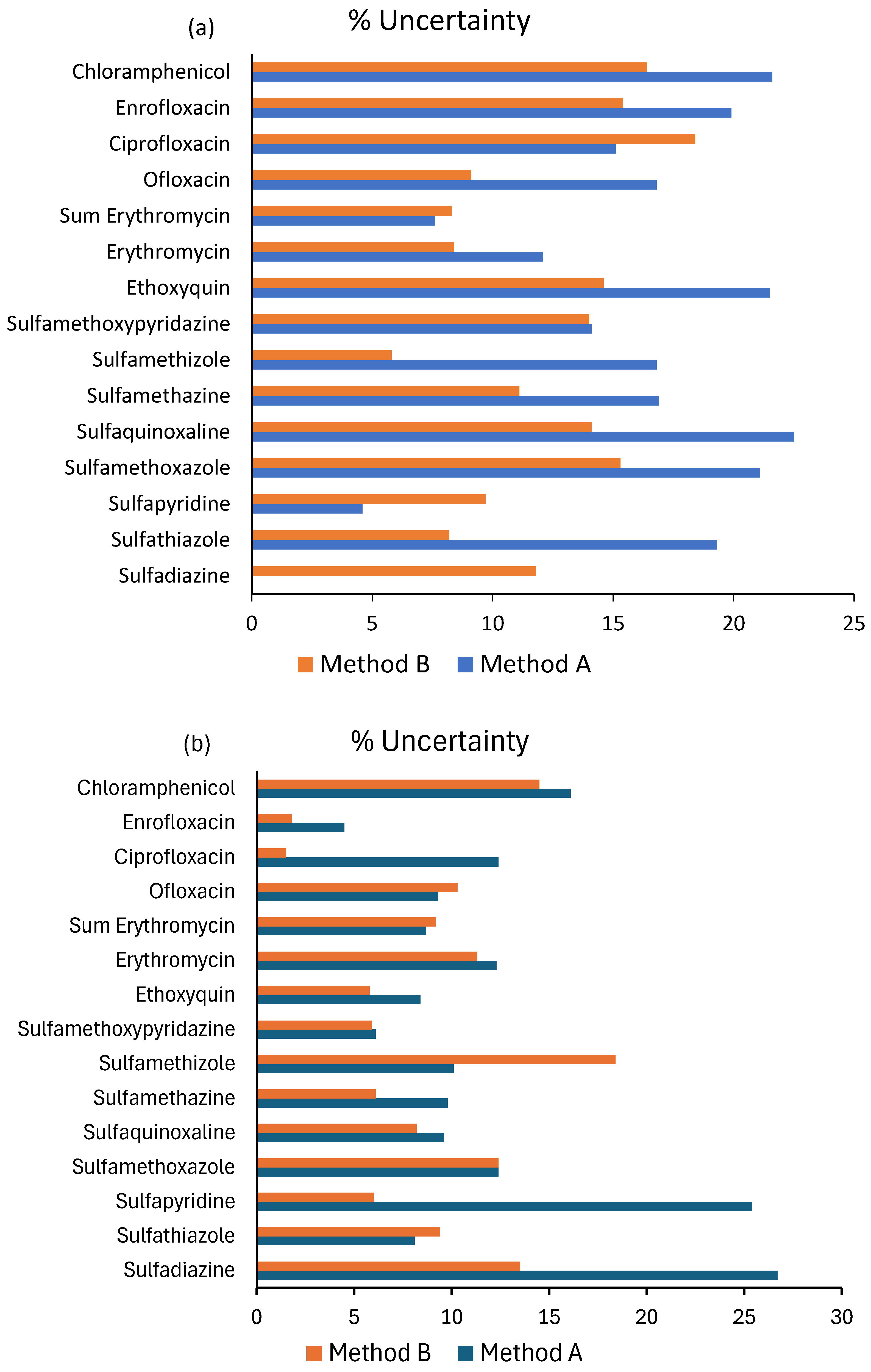
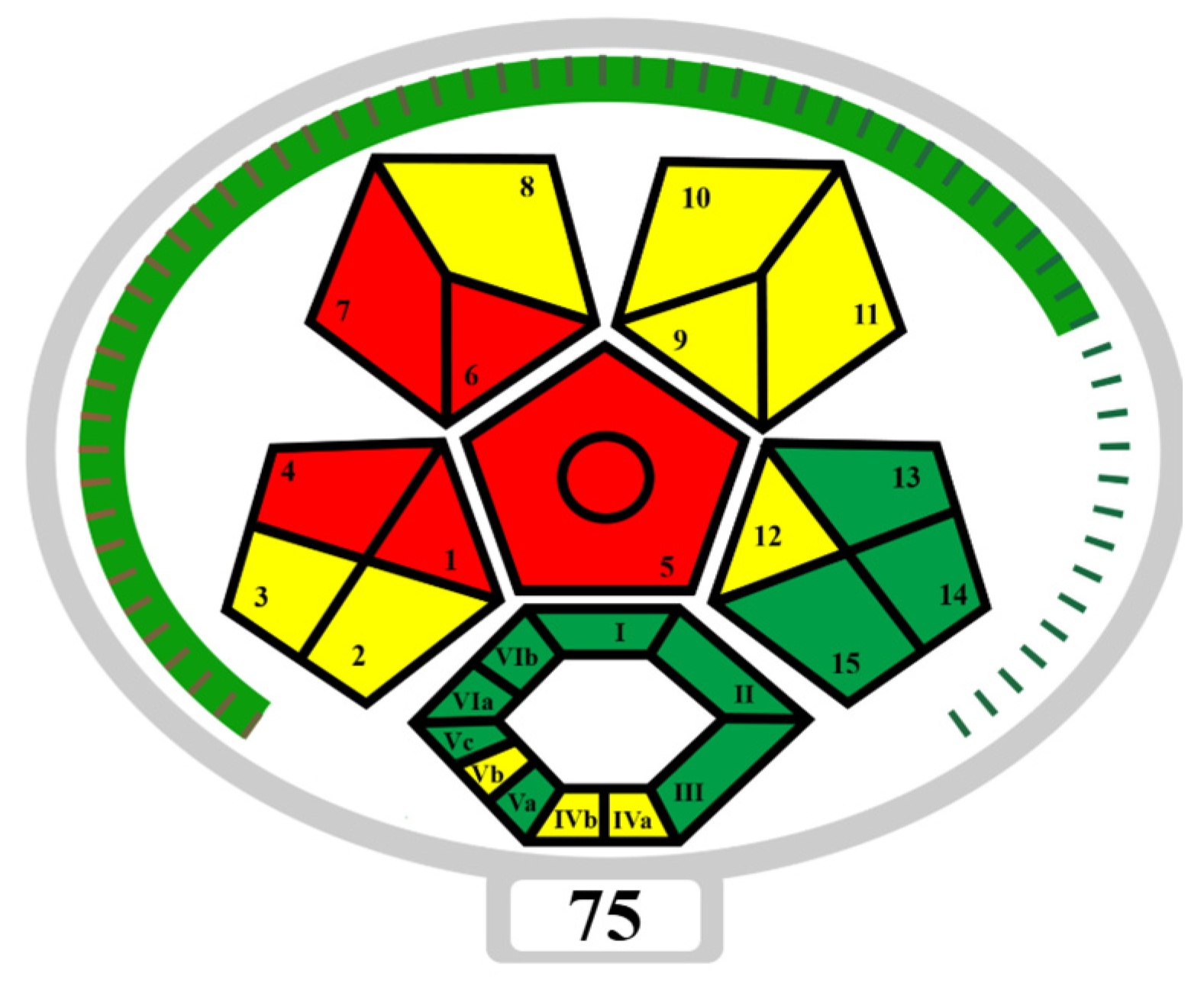
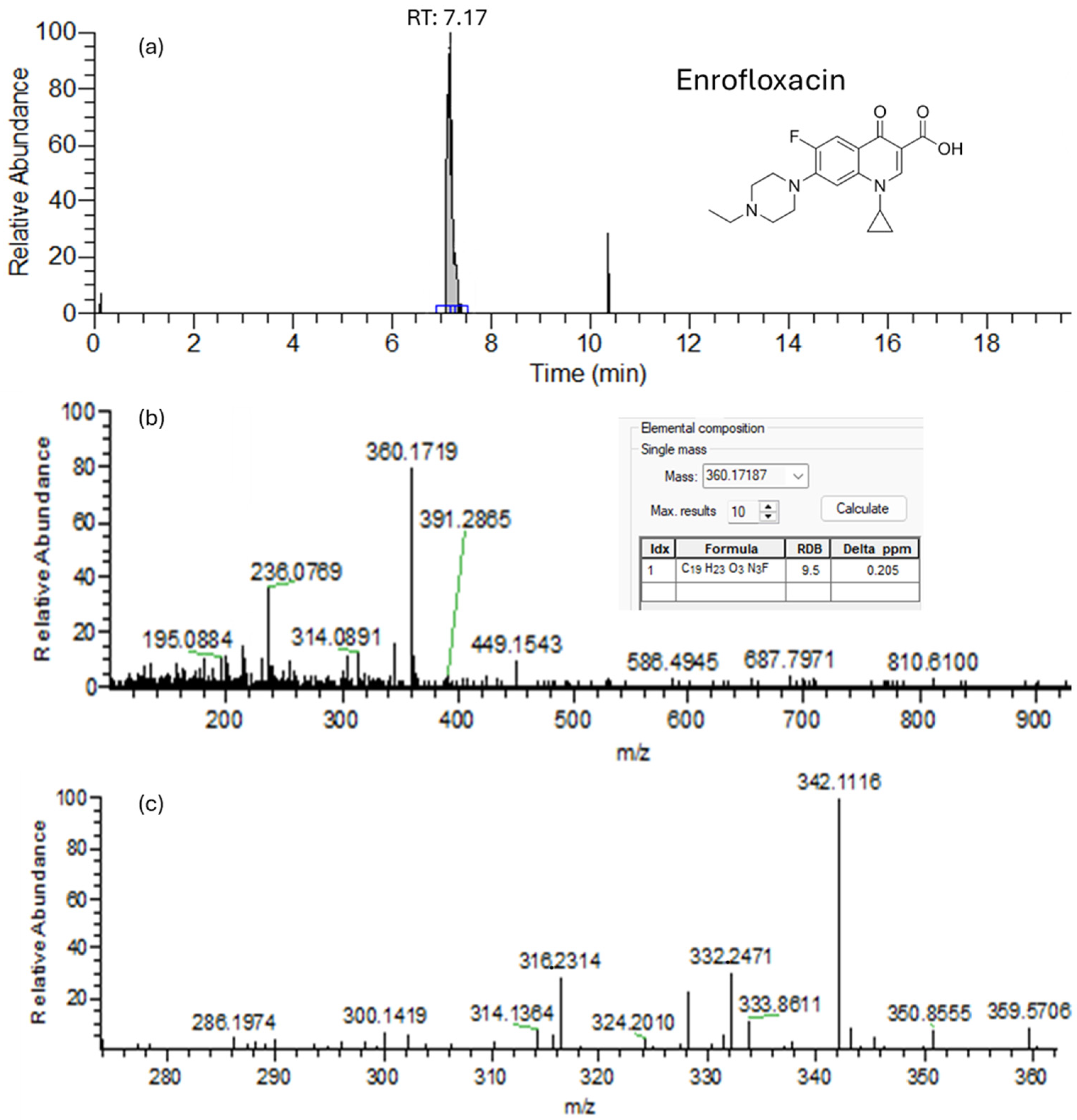

| Pharmaceuticals/ Additive | Retention Time (min) | Formula | Molecular Weight (g mol−1) | Precursor m/z | Mass Accuracy (Δppm) | RDB | Product Ions m/z |
|---|---|---|---|---|---|---|---|
| Sulfonamides | |||||||
| Sulfadiazine | 5.81 | C10H10N4O2S | 250.276 | 251.0597 | −0.539 | 6.5 | 156.0168 |
| Sulfathiazole | 6.29 | C9H9N3O2S2 | 255.310 | 256.0209 | −0.525 | 6.5 | 156.0411 |
| Sulfapyridine | 6.51 | C11H11N3O2S | 249.288 | 250.0645 | −0.414 | 7.5 | 184.0916/156.0060 |
| Sulfamethoxazole | 7.89 | C10H11N3O3S | 253.276 | 254.0594 | −0.780 | 6.5 | 156.0568/147.0573 |
| Sulfaquinoxaline | 8.20 | C14H12N4O2S | 300.336 | 301.0754 | −0.822 | 10.5 | 156.0391 |
| Sulfamethazine | 7.40 | C12H14N4O2S | 278.330 | 279.0910 | −0.743 | 7.5 | 186.0614/156.0796 |
| Sulfamethizole | 7.29 | C9H10N4O2S2 | 270.325 | 271.0318 | −0.367 | 6.5 | 156.0058/108.0688 |
| Sulfamethoxypyridazine | 7.53 | C11H12N4O3S | 280.302 | 281.0703 | −0.541 | 7.5 | 188.0027/156.0993/ 126.0733 |
| Macrolides | |||||||
| Erythromycin | 11.43 | C37H67NO13 | 733.937 | 716.4497 | −1.832 | 5.5 | 558.3030 |
| Erythromycin A | 11.18 | C37H69NO14 | 751.900 | 734.4685 | 0.344 | 4.5 | 716.4867/558.3203 |
| Quinoline-based additive | |||||||
| Ethoxyquin | 8.84 | C14H18NO | 217.310 | 218.1539 | −3.212 | 5.5 | 202.1215/176.0662 |
| Quinolones | |||||||
| Ofloxacin | 7.43 | C18H20FN3O4 | 361.368 | 362.1511 | −2.550 | 10.5 | 344.2135/318.2941 |
| Ciprofloxacin | 7.68 | C17H18FN3O3 | 331.346 | 332.1405 | −2.408 | 9.5 | 314.1526/288.2909 |
| Enrofloxacin | 7.73 | C19H22FN3O3 | 359.400 | 360.1718 | −2.793 | 9.5 | 342.2841/316.2802 |
| Amphenicols | |||||||
| Chloramphenicol | 7.75 | C11H13Cl2N2O5 | 323.132 | 321.0051 | 1.547 | 6.5 | 257.0257/249.1590 |
| Analytes | %Recoveries (n = 6) | Matrix Match Calibration Curve | LOD (ng g−1) | LOQ (ng g−1) | Intra-Day RSDr% (n = 6) | Inter-Day RSDR% (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | Linear Range (ng g−1) | R2 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | |||
| Sulfadiazine | - | 93 | 78 | 25–500 | 0.9970 | 10.0 | 25 | - | 9.6 | 3.9 | - | 11.9 | 5.8 |
| Sulfathiazole | 67 | 74 | 75 | 10–500 | 0.9996 | 2.2 | 7.3 | 2.9 | 4.4 | 3.9 | 4.9 | 5.2 | 5.3 |
| Sulfapyridine | 97 | 88 | 78 | 25–500 | 0.9970 | 3.0 | 10 | 4.9 | 10.6 | 2.3 | 3.1 | 13.5 | 10.0 |
| Sulfamethoxazole | 74 | 85 | 91 | 25–500 | 0.9970 | 3.0 | 10 | 2.6 | 12.9 | 6.2 | 10.3 | 9.4 | 4.1 |
| Sulfaquinoxaline | 66 | 78 | 81 | 10–500 | 0.9998 | 2.7 | 8.9 | 10.1 | 8.5 | 3.8 | 5.3 | 10.8 | 2.3 |
| Sulfamethazine | 71 | 85 | 93 | 10–500 | 0.9999 | 2.0 | 6.6 | 11.4 | 11.7 | 4.4 | 11.9 | 8.2 | 2.8 |
| Sulfamethizole | 62 | 73 | 74 | 10–500 | 0.9995 | 3.0 | 10 | 1.1 | 1.6 | 1.3 | 4.2 | 3.5 | 2.1 |
| Sulfamethoxypyridazine | 72 | 74 | 89 | 10–500 | 0.9995 | 3.0 | 10 | 3.1 | 4.9 | 4.9 | 4.3 | 5.2 | 5.5 |
| Erythromycin | 77 | 81 | 99 | 10–500 | 0.9999 | 2.4 | 7.9 | 3.1 | 2.5 | 1.6 | 6.1 | 2.0 | 3.0 |
| Sum Erythromycin | 86 | 87 | 90 | 10–500 | 0.9985 | 3.0 | 10 | 8.6 | 6.8 | 2.7 | 9.4 | 7.3 | 2.1 |
| Ethoxyquin | 70 | 74 | 97 | 10–500 | 0.9971 | 0.7 | 2.3 | 5.8 | 1.2 | 0.8 | 6.9 | 3.4 | 2.5 |
| Ofloxacin | 71 | 81 | 100 | 5–500 | 0.9985 | 0.2 | 0.7 | 1.2 | 3.0 | 3.1 | 5.0 | 1.8 | 4.3 |
| Ciprofloxacin | 82 | 88 | 102 | 5–500 | 0.9996 | 0.4 | 1.3 | 1.2 | 0.34 | 0.55 | 5.3 | 1.1 | 1.5 |
| Enrofloxacin | 77 | 85 | 95 | 5–500 | 0.9993 | 0.2 | 0.7 | 1.6 | 3.9 | 0.6 | 6.3 | 9.1 | 7.9 |
| Chloramphenicol | 79 | 80 | 87 | 5–500 | 0.9939 | 0.3 | 1.1 | 5.3 | 3.0 | 2.2 | 7.8 | 8.5 | 4.8 |
| Analytes | %Recoveries (n = 6) | Matrix Match Calibration Curve | LOD (ng g−1) | LOQ (ng g−1) | Intra-Day RSDr% (n = 6) | Inter-Day RSDR% (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | Linear Range (ng g−1) | R2 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | |||
| Sulfadiazine | 95 | 93 | 104 | 50–500 | 0.9951 | 3.6 | 11.9 | 3.1 | 1.1 | 0.8 | 6.2 | 1.2 | 2.3 |
| Sulfathiazole | 77 | 95 | 101 | 50–500 | 0.9926 | 3.8 | 12.5 | 1.3 | 0.4 | 0.6 | 1.1 | 1.0 | 0.8 |
| Sulfapyridine | 80 | 99 | 100 | 25–500 | 0.9979 | 3.6 | 11.9 | 2.4 | 0.8 | 0.4 | 4.4 | 0.9 | 0.5 |
| Sulfamethoxazole | 85 | 89 | 91 | 25–500 | 0.9902 | 3.9 | 12.9 | 1.7 | 0.9 | 6.6 | 2.2 | 5.3 | 8.2 |
| Sulfaquinoxaline | 88 | 93 | 98 | 25–500 | 0.9994 | 3.0 | 10 | 3.1 | 1.7 | 2.3 | 1.4 | 1.5 | 4.2 |
| Sulfamethazine | 80 | 83 | 96 | 10–500 | 0.9906 | 2.0 | 6.6 | 0.4 | 0.9 | 4.7 | 4.8 | 2.0 | 4.6 |
| Sulfamethizole | 93 | 85 | 87 | 50–500 | 0.9987 | 3.8 | 12.5 | 3.4 | 0.2 | 0.6 | 3.6 | 0.5 | 0.6 |
| Sulfamethoxypyridazine | 85 | 93 | 97 | 25–500 | 0.9974 | 3.9 | 12.9 | 0.7 | 1.8 | 1.1 | 6.5 | 1.2 | 1.7 |
| Erythromycin | 71 | 82 | 88 | 5–500 | 0.9954 | 0.4 | 1.3 | 3.1 | 2.5 | 2.1 | 4.9 | 4.5 | 2.8 |
| Sum Erythromycin | 98 | 110 | 105 | 10–500 | 0.9999 | 1.6 | 5.3 | 2.6 | 1.5 | 1.1 | 5.3 | 1.7 | 1.9 |
| Ethoxyquin | 70 | 87 | 96 | 10–500 | 0.9934 | 0.6 | 1.9 | 4.7 | 2.1 | 1.1 | 9.9 | 6.1 | 4.1 |
| Ofloxacin | 69 | 82 | 88 | 5–500 | 0.9997 | 0.2 | 0.7 | 5.2 | 3.4 | 4.6 | 5.9 | 4.4 | 6.7 |
| Ciprofloxacin | 70 | 85 | 96 | 5–500 | 0.9986 | 0.4 | 1.3 | 12.4 | 4.9 | 6.7 | 13.0 | 3.9 | 7.4 |
| Enrofloxacin | 84 | 94 | 108 | 5–500 | 0.9999 | 0.2 | 0.7 | 7.6 | 2.3 | 9.3 | 11.3 | 13.6 | 8.9 |
| Chloramphenicol | 86 | 88 | 93 | 5–500 | 0.9942 | 0.2 | 0.7 | 3.1 | 1.1 | 1.0 | 6.2 | 1.2 | 2.3 |
| Analytes | %Recoveries (n = 6) | Matrix Match Calibration Curve | LOD (ng g−1) | LOQ (ng g−1) | Intra-Day RSDr% (n = 6) | Inter-Day RSDR% (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | Linear Range (ng g−1) | R2 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | |||
| Sulfadiazine | 68 | 85 | 87 | 25–500 | 0.9894 | 3.4 | 11.1 | 12.9 | 11.9 | 7.7 | 19.7 | 7.6 | 5.8 |
| Sulfathiazole | 60 | 60 | 92 | 10–500 | 0.9918 | 3.0 | 10 | 6.2 | 4.2 | 1.3 | 7.3 | 6.4 | 5 |
| Sulfapyridine | 59 | 81 | 112 | 10–500 | 0.9896 | 2.2 | 7.1 | 11.2 | 10.3 | 6.5 | 18.6 | 12.2 | 7 |
| Sulfamethoxazole | 64 | 82 | 93 | 10–500 | 0.9895 | 2.8 | 9.1 | 14.9 | 5.4 | 0.91 | 9.8 | 3.1 | 4.2 |
| Sulfaquinoxaline | 68 | 79 | 84 | 25–500 | 0.9906 | 3.8 | 12.5 | 5.2 | 5.7 | 4.1 | 8.1 | 6.3 | 4.8 |
| Sulfamethazine | 64 | 91 | 107 | 10–500 | 0.9956 | 1.4 | 4.8 | 4 | 3.6 | 0.5 | 3.8 | 1.4 | 0.7 |
| Sulfamethizole | 63 | 81 | 94 | 25–500 | 0.9915 | 3.8 | 12.5 | 9.8 | 5 | 1.1 | 11.8 | 7.6 | 2.3 |
| Sulfamethoxypyridazine | 66 | 88 | 93 | 10–500 | 0.9955 | 2.2 | 7.1 | 8 | 4.9 | 3.5 | 6 | 3.1 | 2.7 |
| Erythromycin | 70 | 82 | 94 | 25–500 | 0.9993 | 3.4 | 11.1 | 6.1 | 1.7 | 0.6 | 7 | 2.7 | 0.1 |
| Sum Erythromycin | 71 | 82 | 94 | 25–500 | 0.9928 | 3.8 | 12.5 | 5.8 | 3.5 | 1.8 | 7.8 | 6.5 | 4.3 |
| Ethoxyquin | 72 | 84 | 106 | 25–500 | 0.9983 | 3.8 | 12.5 | 4.2 | 3.2 | 1.1 | 6.4 | 0.6 | 2.2 |
| Ofloxacin | 63 | 74 | 82 | 10–500 | 0.9935 | 2.0 | 6.7 | 12.3 | 11.8 | 3.3 | 13.3 | 11.9 | 7.8 |
| Ciprofloxacin | 66 | 79 | 110 | 10–500 | 0.9948 | 0.98 | 3.2 | 4.8 | 2 | 1.2 | 2.8 | 3.6 | 3.7 |
| Enrofloxacin | 73 | 85 | 99 | 10–500 | 0.9953 | 1.2 | 3.8 | 9.6 | 2.7 | 1.8 | 7.6 | 2.7 | 0.6 |
| Chloramphenicol | 66 | 81 | 96 | 10–500 | 0.9962 | 1.4 | 4.8 | 8.1 | 7.9 | 3.3 | 8.8 | 6.5 | 6.8 |
| Analytes | %Recoveries (n = 6) | Matrix Match Calibration Curve | LOD (ng g−1) | LOQ (ng g−1) | Intra-Day RSDr% (n = 6) | Inter-Day RSDR% (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | Linear Range (ng g−1) | R2 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | 15 ng g−1 | 40 ng g−1 | 90 ng g−1 | |||
| Sulfadiazine | 73 | 104 | 115 | 25–500 | 0.9901 | 3.8 | 12.5 | 19.6 | 5.3 | 4.1 | 13.4 | 9.1 | 8.3 |
| Sulfathiazole | 70 | 78 | 84 | 25–500 | 0.9950 | 3.4 | 11.1 | 8.9 | 6.3 | 1.5 | 9.8 | 4.2 | 1.2 |
| Sulfapyridine | 72 | 80 | 97 | 25–500 | 0.9899 | 3.3 | 10 | 8.5 | 3.1 | 0.6 | 11.9 | 10.9 | 2 |
| Sulfamethoxazole | 76 | 88 | 119 | 10–500 | 0.9995 | 2.2 | 7.1 | 5.2 | 3.1 | 1.6 | 9.9 | 6.4 | 2.1 |
| Sulfaquinoxaline | 70 | 92 | 106 | 25–500 | 0.9928 | 3.3 | 10 | 11.5 | 9.6 | 0.9 | 15.8 | 12.4 | 2.7 |
| Sulfamethazine | 76 | 90 | 93 | 10–500 | 0.9996 | 1.9 | 6.3 | 7.5 | 6.8 | 0.9 | 8.6 | 7.6 | 2.8 |
| Sulfamethizole | 71 | 86 | 100 | 25–500 | 0.9987 | 3.3 | 10 | 4.7 | 2.5 | 2.2 | 5.4 | 3.8 | 2.7 |
| Sulfamethoxypyridazine | 68 | 95 | 99 | 10–500 | 0.9986 | 1.5 | 5 | 9.5 | 5.4 | 7.2 | 9.6 | 8.4 | 7 |
| Erythromycin | 71 | 81 | 105 | 25–500 | 0.9971 | 3.3 | 10 | 13.6 | 3.9 | 2.1 | 18 | 8.3 | 6.3 |
| Sum Erythromycin | 75 | 74 | 90 | 25–500 | 0.9957 | 3.8 | 12.5 | 12.9 | 5.1 | 4.9 | 12.5 | 7.1 | 5.1 |
| Ethoxyquin | 70 | 86 | 98 | 10–500 | 0.9909 | 2.5 | 8.3 | 9.8 | 8.7 | 1.5 | 13.5 | 12.2 | 7 |
| Ofloxacin | 74 | 97 | 107 | 10–500 | 0.9960 | 2 | 6.6 | 3.9 | 2.2 | 0.4 | 8.8 | 7.3 | 5.2 |
| Ciprofloxacin | 78 | 95 | 110 | 10–500 | 0.9825 | 1.7 | 5.5 | 1 | 0.8 | 0.6 | 7.7 | 2.8 | 0.3 |
| Enrofloxacin | 69 | 93 | 97 | 10–500 | 0.9963 | 1.6 | 5.3 | 9.2 | 8.7 | 1.5 | 15.5 | 10.7 | 4 |
| Chloramphenicol | 71 | 88 | 96 | 10–500 | 0.9978 | 2.2 | 7.1 | 8.2 | 6.7 | 3.2 | 11.8 | 7.3 | 3.5 |
| Analytes | CCα (ng g−1) | CCβ (ng g−1) | CCα (ng g−1) | CCβ (ng g−1) |
|---|---|---|---|---|
| Method A | Method B | |||
| Sulfadiazine | 100.7 | 101.5 | 100.7 | 101.5 |
| Sulfathiazole | 100.6 | 101.3 | 100.6 | 101.3 |
| Sulfapyridine | 100.8 | 101.6 | 100.6 | 101.2 |
| Sulfamethoxazole | 102.2 | 104.4 | 104.8 | 109.6 |
| Sulfaquinoxaline | 103.1 | 106.2 | 105.5 | 110.9 |
| Sulfamethazine | 101.5 | 103.1 | 103.5 | 106.9 |
| Sulfamethizole | 100.1 | 100.2 | 100.7 | 101.5 |
| Sulfamethoxypyridazine | 100.9 | 101.8 | 100.5 | 101.1 |
| Erythromycin | 101.5 | 103.1 | 102.5 | 104.9 |
| Sum Erythromycin | 103.9 | 107.2 | 102.3 | 106.6 |
| Ethoxyquin | 0.10 | 0.20 | 0.11 | 0.19 |
| Ofloxacin | 101.3 | 102.5 | 105.1 | 109.6 |
| Ciprofloxacin | 100.5 | 101.0 | 104.6 | 109.2 |
| Enrofloxacin | 101.7 | 103.3 | 100.7 | 102.4 |
| Chloramphenicol | 0.30 | 0.52 | 0.09 | 0.11 |
| Reagents | Penalty Points | Reagents | Penalty Points |
|---|---|---|---|
| ACN (7.5 mL) | 4 | ACN (9 mL) | 4 |
| MeOH (2.5 mL) | 6 | Formic acid (1 mL) | 8 |
| Extraction Waste | 3 | Extraction Waste | 1 |
| Instruments | Instruments | ||
| Centrifugation | 1 | Centrifugation | 1 |
| Sonication | 0 | Sonication | 0 |
| LC-MS | 2 | LC-MS | 2 |
| LC-MS Waste | 3 | LC-MS Waste | 3 |
| Total Penalty Points | 19 | Total Penalty Points | 19 |
| Analytical Eco-Scale score (Method A) | 81 | Analytical Eco-Scale score (Method B) | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miserli, K.; Boti, V.; Hela, D.; Albanis, T.; Konstantinou, I. Comparison and Validation of QuEChERS Extraction Methods Coupled with UHPLC/Orbitrap HR-MS for the Determination of Antibiotics and Related Compounds in Fish and Fish Feed. Separations 2025, 12, 321. https://doi.org/10.3390/separations12110321
Miserli K, Boti V, Hela D, Albanis T, Konstantinou I. Comparison and Validation of QuEChERS Extraction Methods Coupled with UHPLC/Orbitrap HR-MS for the Determination of Antibiotics and Related Compounds in Fish and Fish Feed. Separations. 2025; 12(11):321. https://doi.org/10.3390/separations12110321
Chicago/Turabian StyleMiserli, Kleopatra, Vasiliki Boti, Dimitra Hela, Triantafyllos Albanis, and Ioannis Konstantinou. 2025. "Comparison and Validation of QuEChERS Extraction Methods Coupled with UHPLC/Orbitrap HR-MS for the Determination of Antibiotics and Related Compounds in Fish and Fish Feed" Separations 12, no. 11: 321. https://doi.org/10.3390/separations12110321
APA StyleMiserli, K., Boti, V., Hela, D., Albanis, T., & Konstantinou, I. (2025). Comparison and Validation of QuEChERS Extraction Methods Coupled with UHPLC/Orbitrap HR-MS for the Determination of Antibiotics and Related Compounds in Fish and Fish Feed. Separations, 12(11), 321. https://doi.org/10.3390/separations12110321









