Ultrasound Impact on Extraction Yield and Properties of Starch and Polyphenols from Canna indica L. Rhizomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Sample Preparation
2.2. Extraction Methods
2.2.1. Conventional Extraction (CE) of Starch
2.2.2. Ultrasound-Assisted Extraction (UAE) of Starch
2.3. Physicochemical Property Evaluation of Starch
2.3.1. Chemical Characterization
2.3.2. Swelling and Solubility
2.3.3. Oil and Water Absorption
2.4. Scanning Electron Microscopy
2.5. Response Surface Methodology (RSM) for Optimization
2.6. Statistics
2.7. Extraction of Polyphenols
2.7.1. Conventional Extraction
2.7.2. Ultrasound-Assisted Extraction of Polyphenols
2.8. Evaluation of Polyphenol Content
2.9. Antioxidant Activity Assay
3. Results and Discussion
3.1. Starch Extraction Process
3.2. Optimization of the Starch UAE Process
3.2.1. Analysis of Experimental Data
3.2.2. Model Fitting
3.2.3. Statistical Analysis
3.2.4. Effect of Process Variables on Starch Yield
3.2.5. Effect of Temperature
3.2.6. Effect of Time
3.2.7. Effect of Solid-to-Liquid Ratio
3.2.8. Optimization and Validation
3.3. Physicochemical Properties of Extracted Starch
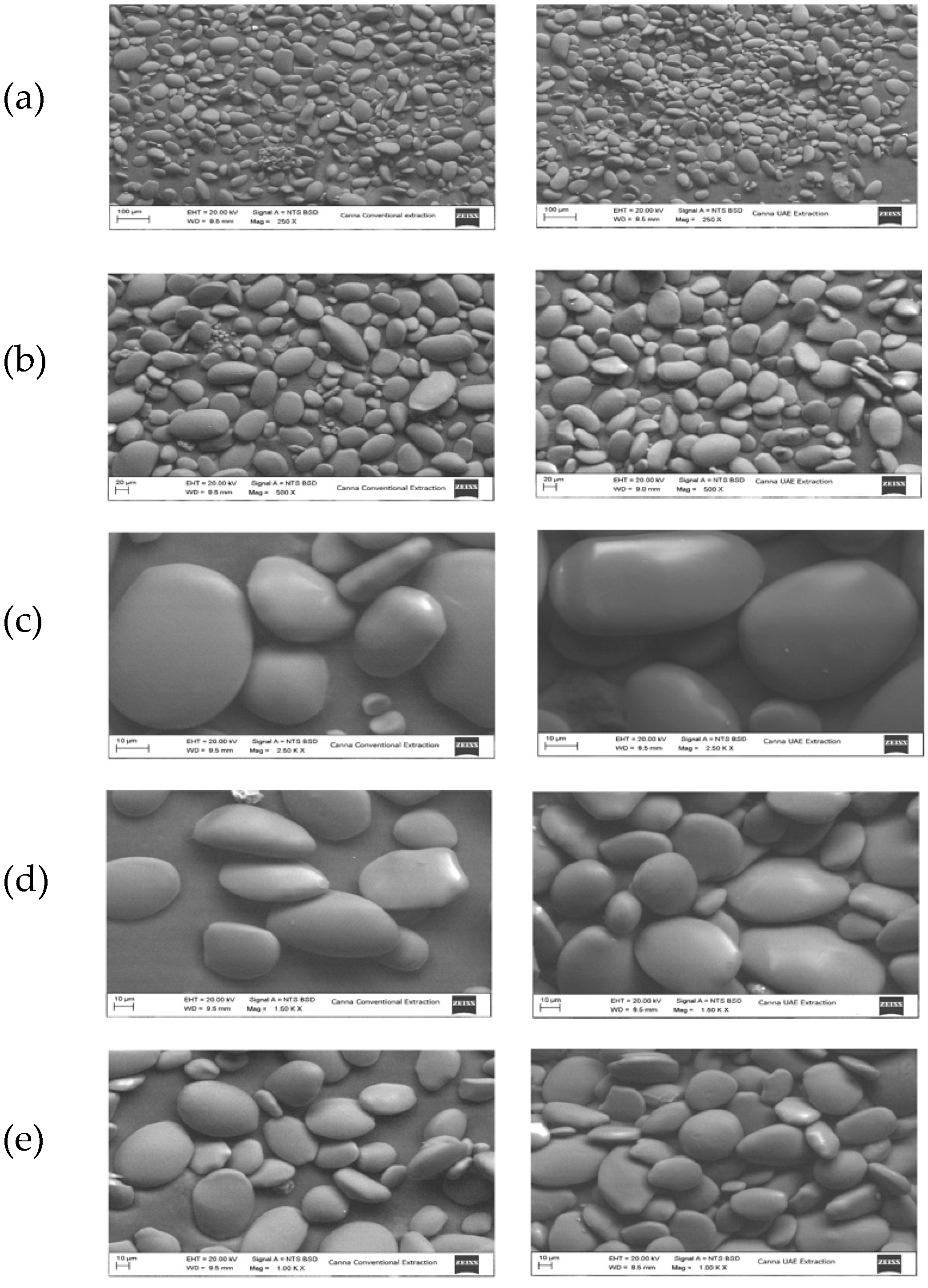
3.3.1. Micrographs of Sonicated Samples
3.3.2. Swelling and Solubility
3.3.3. Oil and Water Absorption
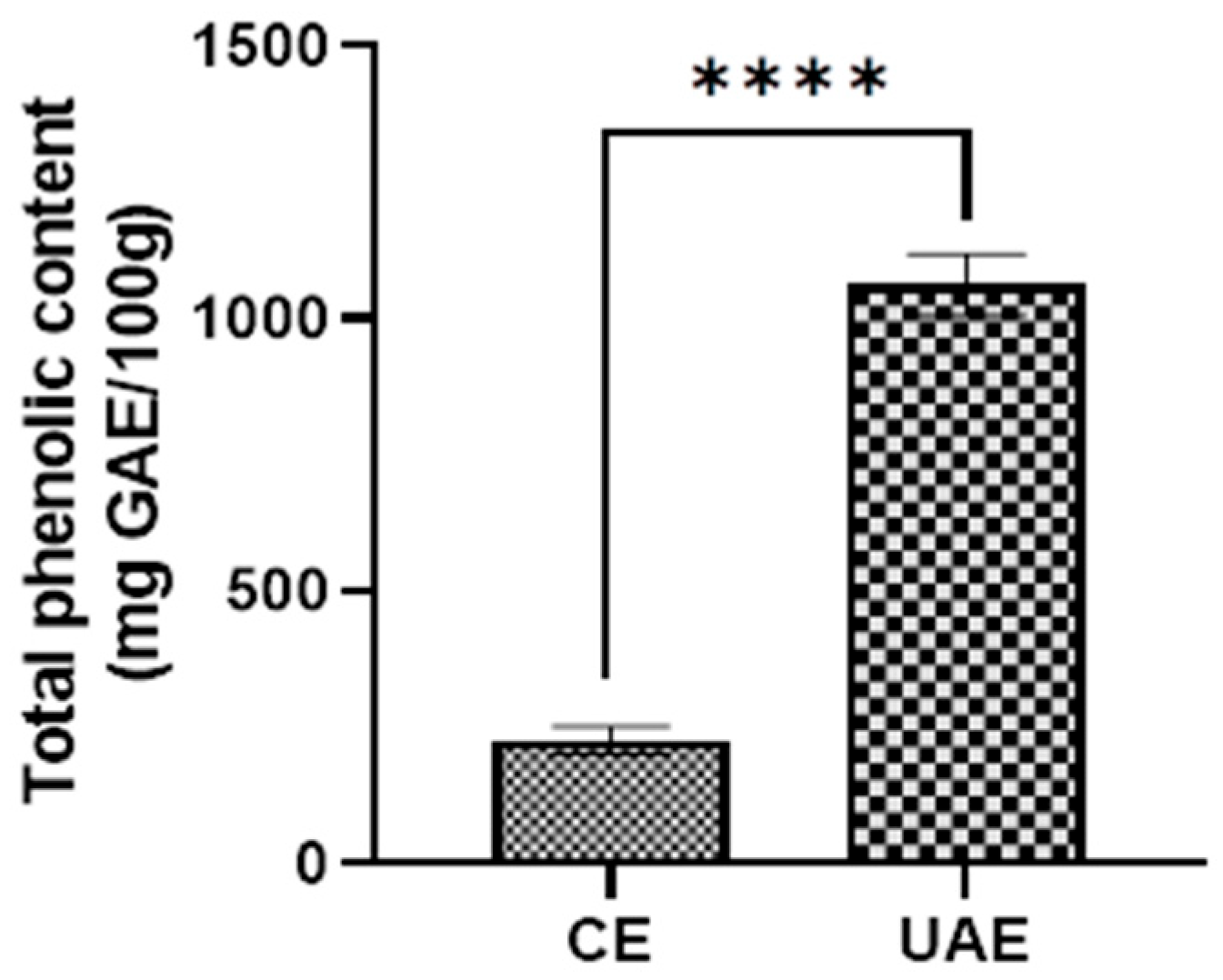
3.4. Effect of Ultrasound-Assisted Extraction on Total Polyphenol Content from Canna Rhizomes
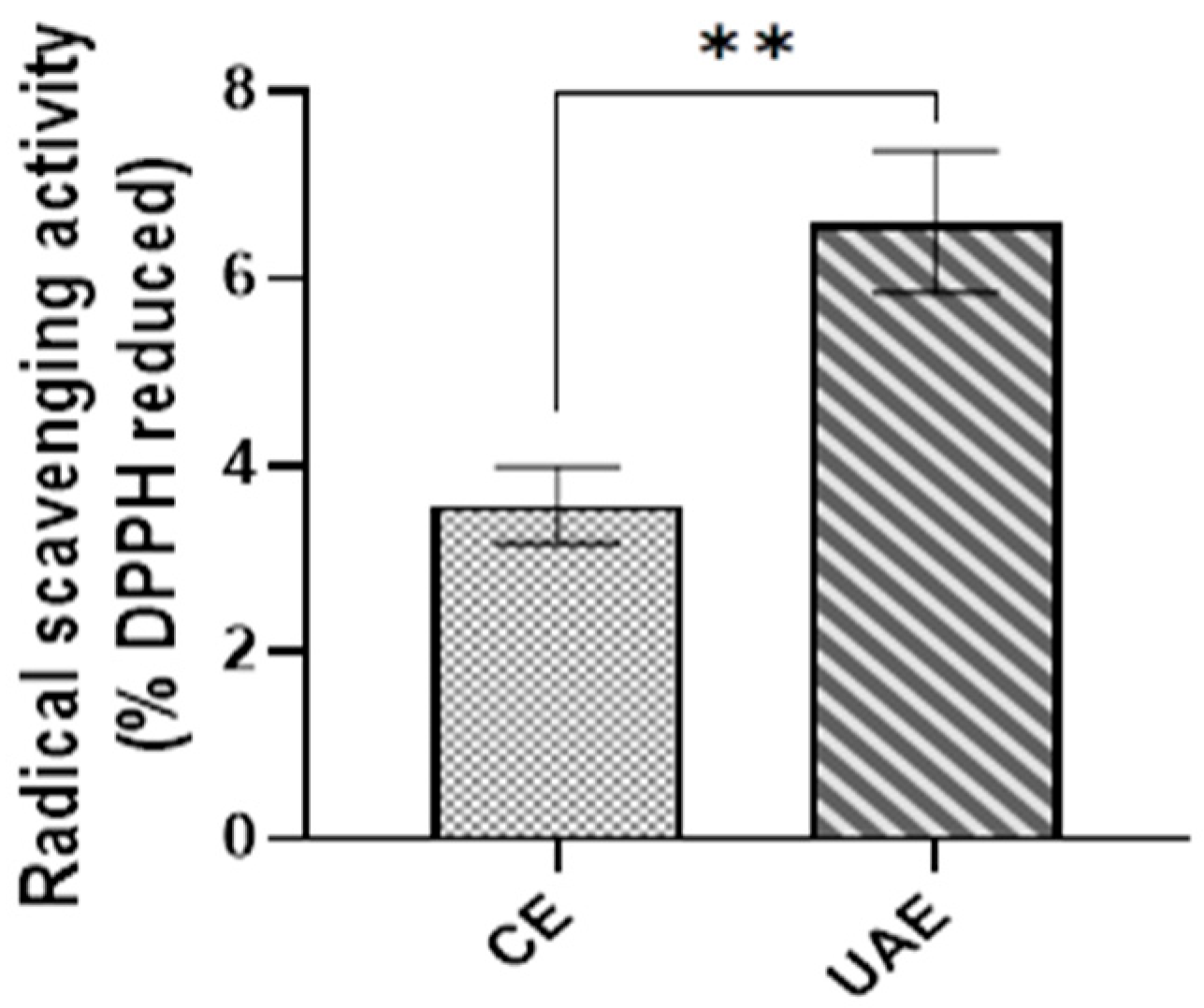
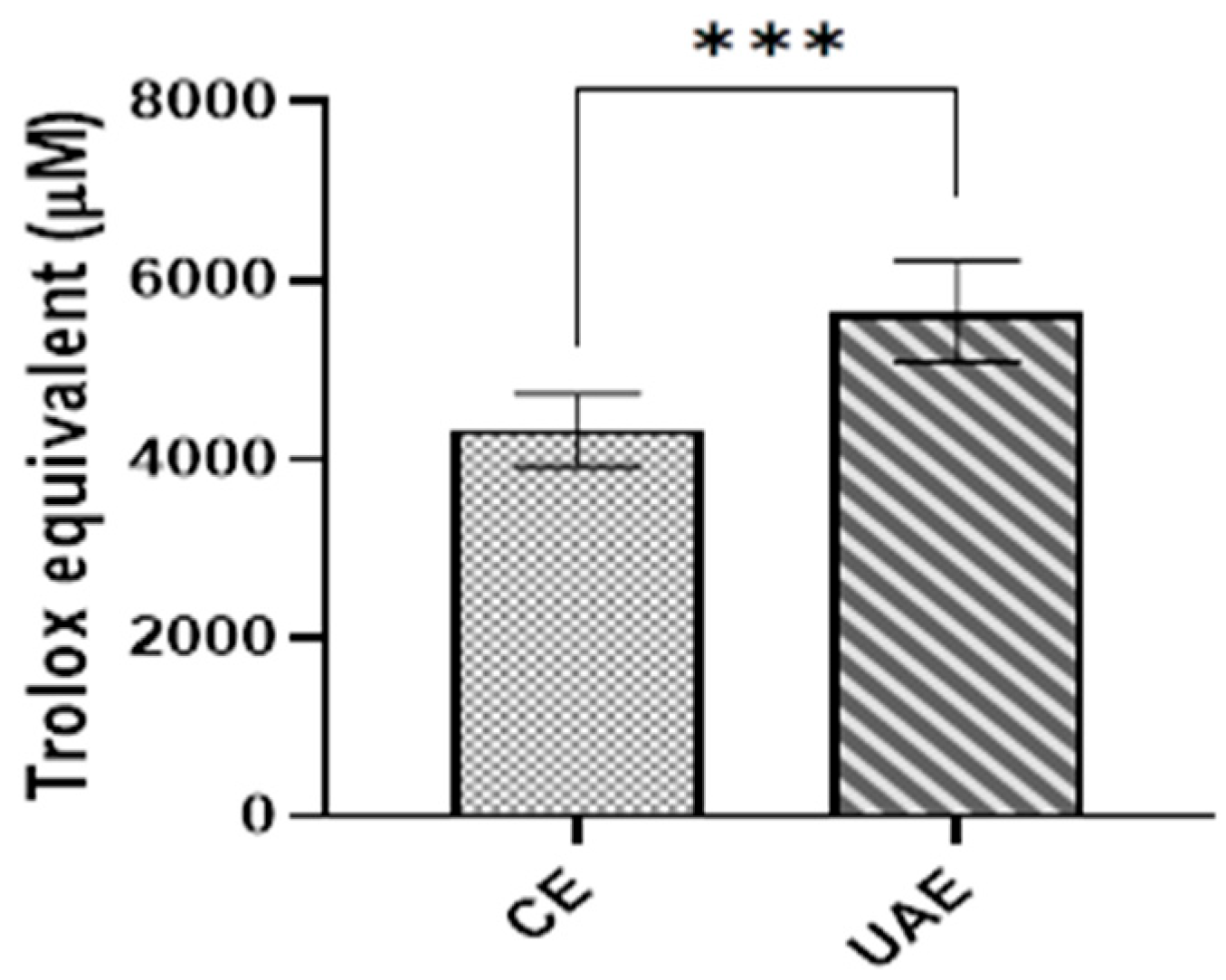
3.5. Effect of Extraction Methods on the Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soni, P.L.; Sharma, H.; Srivastava, H.C.; Gharia, M.M. Physicochemical Properties of Canna Edulis Starch—Comparison with Maize Starch. Starch-Stärke 1990, 42, 460–464. [Google Scholar] [CrossRef]
- Piyachomkwan, K.; Chotineeranat, S.; Kijkhunasatian, C.; Tonwitowat, R.; Prammanee, S.; Oates, C.G.; Sriroth, K. Edible canna (Canna edulis) as a complementary starch source to cassava for the starch industry. Ind. Crops Prod. 2002, 16, 11–21. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bewa, H.; Buy, Q.H.; Bouchet, B.; Szylit, O.; Sealy, L. On Ultrastructural and Nutritional Aspects of Some Tropical Tuber Starches. Starch-Stärke 1982, 34, 255–262. [Google Scholar] [CrossRef]
- Cisneros, F.H.; Zevillanos, R.; Cisneros-Zevallos, L. Characterization of Starch from Two Ecotypes of Andean Achira Roots (Canna Edulis). J. Agric. Food Chem. 2009, 57, 7363–7368. [Google Scholar] [CrossRef] [PubMed]
- Aprianita, A.; Vasiljevic, T.; Bannikova, A.; Kasapis, S. Physicochemical Properties of Wheat-Canna and Wheat-Konjac Composite Flours. J. Food Sci. Technol. 2014, 51, 1784–1794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishra, T.; Goyal, A.K.; Middha, S.K.; Sen, A. Antioxidative properties of Canna edulis Ker-Gawl. Indian. J. Nat. Prod. Resour. 2011, 2, 315–321. [Google Scholar][Green Version]
- Hung, P.; Morita, N. Physicochemical Properties and Enzymatic Digestibility of Starch from Edible Canna (Canna Edulis) Grown in Vietnam. Carbohydr. Polym. 2005, 61, 314–321. [Google Scholar] [CrossRef]
- Builders, P.F.; Arhewoh, M.I. Pharmaceutical Applications of Native Starch in Conventional Drug Delivery. Starch-Stärke 2016, 68, 864–873. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.M.; Tapia-Blácido, D.R.; Menegalli, F.C. Physical-Chemical, Thermal, and Functional Properties of Achira (Canna indica L.) Flour and Starch from Different Geographical Origin. Starch-Stärke 2012, 64, 348–358. [Google Scholar] [CrossRef]
- Falade, K.O.; Okafor, C.A. Physicochemical Properties of Five Cocoyam (Colocasia Esculenta and Xanthosoma Sagittifolium) Starches. Food Hydrocoll. 2013, 30, 173–181. [Google Scholar] [CrossRef]
- Rahman, S.M.M.; Rakshit, S.K. Effect of Endogenous and Commercial Enzyme on Improving Extraction of Sweet Potato Starch. In Proceedings of the 2004 ASAE Annual Meeting, Ottawa, ON, Canada, 1–4 August 2004. [Google Scholar]
- Daiuto, É.; Cereda, M.; Sarmento, S.; Vilpoux, O. Effects of Extraction Methods on Yam (Dioscorea Alata) Starch Characteristics. Starch-Stärke 2005, 57, 153–160. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Ramakrishna, M.; Lonsane, B.K.; Krishnaiah, M.M. Enzymic Treatment of Cassava Flour Slurry for Enhanced Recovery of Starch. Food Biotechnol. 1993, 7, 1–10. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-E-Huma; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Chan, H.-T.T.; Bhat, R.; Karim, A.A. Effects of Sodium Dodecyl Sulphate and Sonication Treatment on Physicochemical Properties of Starch. Food Chem. 2010, 120, 703–709. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on Supramolecular Structural Changes of Ultrasonic Treated Potato Starch Granules. Food Hydrocoll. 2012, 29, 116–122. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of Ultrasound-Assisted Extraction of Phenolic Compounds from Wheat Bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Shen, N. Optimization of Succinic Acid Production from Cane Molasses by Actinobacillus Succinogenes GXAS137 Using Response Surface Methodology (RSM). Food Sci. Biotechnol. 2014, 23, 1911–1919. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Ying, Z.; Han, X.; Li, J. Ultrasound-Assisted Extraction of Polysaccharides from Mulberry Leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Le, V.V.M. Comparison of Enzyme-Assisted and Ultrasound-Assisted Extraction of Vitamin C and Phenolic Compounds from Acerola (Malpighia Emarginata DC.) Fruit. Int. J. Food Sci. Technol. 2012, 47, 1206–1214. [Google Scholar] [CrossRef]
- Hromádková, Z.; Ebringerová, A.; Valachovič, P. Comparison of Classical and Ultrasound-Assisted Extraction of Polysaccharides from Salvia Officinalis L. Ultrason. Sonochem. 1999, 5, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Areca Husk. J. Food Process. Preserv. 2014, 38, 90–96. [Google Scholar] [CrossRef]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of Ultrasound-Assisted Extraction (UAE) of Flavonoids Compounds (FC) from Hawthorn Seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Sit, N.; Misra, S.; Deka, S.C. Yield and Functional Properties of Taro Starch as Affected by Ultrasound. Food Bioprocess. Technol. 2014, 7, 1950–1958. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International. Assoc. Off. Anal. Chem. 1990, 15, 2. [Google Scholar]
- Holm, J.; Björck, I.; Drews, A.; Asp, N. A Rapid Method for the Analysis of Starch. Starch-Stärke 1986, 38, 224–226. [Google Scholar] [CrossRef]
- Qi, Y. Optimization of Starch Isolation from Red Sorghum Using Response Surface Methodology. LWT-Food Sci. Technol. 2017, 91, 242–248. [Google Scholar] [CrossRef]
- Yu, S.; Ma, Y.; Menager, L.; Sun, D.W. Physicochemical Properties of Starch and Flour from Different Rice Cultivars. Food Bioprocess. Technol. 2012, 5, 626–637. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Ultrasound-Treated Starch: SEM and TEM Imaging, and Functional Behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Priya, B.; Gurumoorthi, P. Box-Behnken Design Based Multi-Response Analysis and Optimization of Supercritical Carbon Dioxide Extraction of Bioactive Flavonoid Compounds from Tea (Camellia sinensis L.) Leaves. J. Food Sci. Technol. 2015, 52, 92–104. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Maran, J.P. Box-Behnken Design Based Statistical Modeling for Ultrasound-Assisted Extraction of Corn Silk Polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of Nutritional and Antioxidant Properties of the Tropical Fruits Banana, Litchi, Mango, Papaya, Passion Fruit and Pineapple Cultivated in R??Union French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. A Colorimetric Method for the Determination of Phenols (and Phenol Derivatives) in Urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Hatia, S. Evaluation of Antioxidant Properties of Major Dietary Polyphenols and Their Protective Effect on 3T3-L1 Preadipocytes and Red Blood Cells Exposed to Oxidative Stress. Free Radic. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, M.; Hamaker, B.R. Rapid Small-Scale Starch Isolation Using a Combination of Ultrasonic Sonication and Sucrose Density Separation. Starch-Starke 2011, 63, 333–339. [Google Scholar] [CrossRef]
- Park, S.H.; Bean, S.R.; Wilson, J.D.; Schober, T.J. Rapid Isolation of Sorghum and Other Cereal Starches Using Sonication. Cereal Chem. 2006, 83, 611–616. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.J. Rice Starch Isolation by Neutral Protease and High-Intensity Ultrasound. J. Cereal Sci. 2004, 39, 291–296. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, B.; Li, H.; Miao, S.; Zheng, B. Optimization of Ultrasound-Microwave Synergistic Extraction of Prebiotic Oligosaccharides from Sweet Potatoes (Ipomoea batatas L.). Innov. Food Sci. Emerg. Technol. 2019, 54, 51–63. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S. Response Surface Modeling and Optimization of Process Parameters for Aqueous Extraction of Pigments from Prickly Pear (Opuntia Ficus-Indica) Fruit. Dye. Pigment. 2012, 3, 465–472. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Ultrasound-Assisted Extraction of Pectin from Sisal Waste. Carbohydr. Polym. 2015, 115, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the Effects of Ultrasound on Vegetal Tissues during Solvent Extraction. Ultrason. Sonochem 2001, 2, 137–142. [Google Scholar] [CrossRef]
- Wang, X.S.; Wu, Y.F.; Dai, S.L.; Chen, R.; Shao, Y. Ultrasound-Assisted Extraction of Geniposide from Gardenia Jasminoides. Ultrason. Sonochem 2012, 19, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and Opportunities for Ultrasound Assisted Extraction in the Food Industry—A Review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J. Comparative Study of Lignins Isolated by Alkali and Ultrasound-Assisted Alkali Extractions from Wheat Straw. Ultrason. Sonochem. 2002, 9, 85–93. [Google Scholar] [CrossRef]
- Chen, F. Optimization of Ultrasound-Assisted Extraction of Anthocyanins in Red Raspberries and Identification of Anthocyanins in Extract Using High-Performance Liquid Chromatography-Mass Spectrometry. Ultrason. Sonochem. 2007, 14, 767–778. [Google Scholar] [CrossRef]
- Puncha-arnon, S.; Pathipanawat, W.; Puttanlek, C.; Rungsardthong, V.; Uttapap, D. Effects of Relative Granule Size and Gelatinization Temperature on Paste and Gel Properties of Starch Blends. Food Res. Int. 2008, 41, 552–561. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Singh, N. Some Properties of Corn Starches II: Physicochemical, Gelatinization, Retrogradation, Pasting and Gel Textural Properties. Food Chem. 2007, 101, 1499–1507. [Google Scholar] [CrossRef]
- Jambrak, A.R. Ultrasound Effect on Physical Properties of Corn Starch. Carbohydr. Polym. 2010, 79, 91–100. [Google Scholar] [CrossRef]
- Halbrook, W.U.; Kurtzman, R.H., Jr. Water Uptake of Bean and Other Starches at High Temperatures and Pressures. Cereal Chem. 1975, 52, 156–161. [Google Scholar]
- Ekanayake, S.; Nair, B.M.; Asp, N.G.; Jansz, E.R. Effect of Processing of Sword Beans (Canavalia Gladiata) on Physicochemical Properties of Starch. Starch-Starke 2006, 58, 215–222. [Google Scholar] [CrossRef]
- Tester, R.F.; Morrison, W.R. Swelling and Gelatinization of Cereal Starches. II. Waxy Rice Starches. Cereal Chem. 1990, 67, 558–563. [Google Scholar]
- Wu, Y.; Du, X.; Ge, H.; Lv, Z. Preparation of Microporous Starch by Glucoamylase and Ultrasound. Starch-Starke 2011, 63, 217–225. [Google Scholar] [CrossRef]
- Majzoobi, M.; Hedayati, S.; Farahnaky, A. Functional Properties of Microporous Wheat Starch Produced by α-Amylase and Sonication. Food Biosci. 2015, 11, 79–84. [Google Scholar] [CrossRef]
- Brusotti, G.; Ngueyem, T.A.; Biesuz, R.; Caccialanza, G. Optimum extraction process of polyphenols from Bridelia grandis stem bark using experimental design. J. Sep. Sci. 2010, 33, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M. An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles from Herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-Assisted Extraction of Soy Isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Adjé, F.; Lozano, Y.F.; Lozano, P.; Adima, A.; Chemat, F.; Gaydou, E.M. Optimization of Anthocyanin, Flavonol and Phenolic Acid Extractions from Delonix Regia Tree Flowers Using Ultrasound-Assisted Water Extraction. Ind. Crops Prod. 2010, 32, 439–444. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.; Meullemiestre, A.; Abert-vian, M. Ultrasonics Sonochemistry Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Food Sci. Technol. 2014, 11, 31–41. [Google Scholar]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-Assisted Extraction of Polyphenols (Flavanone Glycosides) from Orange (Citrus sinensis L.) Peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Chen, C. Ultrasound-Assisted Extraction from Defatted Oat (Avena sativa L.) Bran to Simultaneously Enhance Phenolic Compounds and β-Glucan Contents: Compositional and Kinetic Studies. J. Food Eng. 2018, 222, 1–10. [Google Scholar] [CrossRef]
- Dzah, C.S. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2019, 35, 100547. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH Assay Comparison to Assess Antioxidant Capacity of Tea Infusions: Relationship between Total Polyphenol and Individual Catechin Content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]


| Variables | Level (−1) | Level (0) | Level (1) |
|---|---|---|---|
| Temperature (°C) | 30 | 40 | 50 |
| Time (min) | 5 | 10 | 15 |
| Solid-to-liquid ratio (g/mL) | 1:1 | 1:2 | 1:3 |
| Experimental Design with Predicted and Experimental Values | |||||
|---|---|---|---|---|---|
| Factors (Coded and Actual Values) | Experimental Value | Predicted Value | |||
| Run | A: Temperature (°C) | B: Time (min) | C: Solid-to-Liquid (g/mL) | Starch Yield (%) | Starch Yield (%) |
| 1 | 40 (0) | 15 (1) | 1:1 (−1) | 7.2885 | 7.07 |
| 2 | 30 (−1) | 10 (0) | 1:3 (1) | 18.1802 | 17.97 |
| 3 | 40 (0) | 5 (−1) | 1:3 (1) | 16.0479 | 16.26 |
| 4 | 30 (−1) | 10 (0) | 1:1 (−1) | 8.4524 | 8.71 |
| 5 | 50 (1) | 10 (0) | 1:1 (−1) | 8.6085 | 8.82 |
| 6 | 50 (1) | 5 (−1) | 1:2 (0) | 9.99 | 10.03 |
| 7 | 40 (0) | 10 (0) | 1:2 (0) | 15.5108 | 15.47 |
| 8 | 40 (0) | 10 (0) | 1:2 (0) | 15.3737 | 15.47 |
| 9 | 50 (1) | 15 (1) | 1:2 (0) | 13.2622 | 13.26 |
| 10 | 50 (1) | 10 (0) | 1:3 (1) | 19.5256 | 19.27 |
| 11 | 30 (−1) | 5 (−1) | 1:2 (0) | 11.8913 | 11.89 |
| 12 | 40 (0) | 10 (0) | 1:2 (0) | 15.0737 | 15.47 |
| 13 | 40 (0) | 15 (1) | 1:3 (1) | 17.2132 | 17.47 |
| 14 | 40 (0) | 10 (0) | 1:2 (0) | 16.0211 | 15.47 |
| 15 | 30 (−1) | 15 (1) | 1:2 (0) | 10.0239 | 9.98 |
| 16 | 40 (0) | 5 (−1) | 1:1 (−1) | 7.2052 | 6.95 |
| 17 | 40 (0) | 10 (0) | 1:2 (0) | 15.3785 | 15.47 |
| Model Summary Statistics | ||||||
|---|---|---|---|---|---|---|
| Source | Sequential Sum of Squares (p-Value) | R2 | Adjusted R2 | Predicted R2 | Press | |
| Linear | 0.0002 | 0.7783 | 0.7271 | 0.6332 | 92.3858 | |
| 2FI | 0.6923 | 0.8071 | 0.6913 | 0.4134 | 147.7631 | |
| Quadratic | <0.0001 | 0.9963 | 0.9916 | 0.9686 | 7.9030 | Suggested |
| Cubic | 0.4051 | 0.9981 | 0.9924 | Aliased | ||
| ANOVA | |||||||
|---|---|---|---|---|---|---|---|
| Source | Coefficient Estimate | Sum of Squares | DF | Mean Square | F-Value | p-Value | |
| Model | 15.47 | 250.97 | 9 | 27.89 | 210.55 | <0.0001 | significant |
| A-Temperature | 0.35 | 1.01 | 1 | 1.01 | 7.60 | 0.0282 | |
| B-Time | 0.33 | 0.88 | 1 | 0.88 | 6.64 | 0.0366 | |
| C-Solid-to-Liquid Ratio | 4.93 | 194.17 | 1 | 194.17 | 1466.00 | <0.0001 | |
| AB | 1.28 | 6.60 | 1 | 6.60 | 49.86 | 0.0002 | |
| AC | 0.30 | 0.35 | 1 | 0.35 | 2.67 | 0.1463 | |
| BC | 0.27 | 0.29 | 1 | 0.29 | 2.21 | 0.1807 | |
| A2 | −1.21 | 6.20 | 1 | 6.20 | 46.80 | 0.0002 | |
| B2 | −2.97 | 37.05 | 1 | 37.05 | 279.73 | <0.0001 | |
| C2 | −0.57 | 1.35 | 1 | 1.35 | 10.20 | 0.0152 | |
| Residual | 0.93 | 7 | 0.13 | ||||
| Lack of Fit | 0.45 | 3 | 0.15 | 1.24 | 0.4051 | not significant | |
| Pure Error | 0.48 | 4 | 0.12 | ||||
| Cor Total | 251.90 | 16 | |||||
| Mean | 13.24 | ||||||
| Coefficient of Variance (CV) % | 2.75 | ||||||
| Determination Coefficient (R2) | 0.9963 | ||||||
| Correlation Coefficient (R) | 0.9981 | ||||||
| Adjusted Determination Coefficient (Adj-R2) | 0.9916 | ||||||
| Predicted Determination Coefficient (Pred-R2) | 0.9686 | ||||||
| Factors | Optimum Conditions | Predicted Values of Starch Yield (%) | Experimental Values of Starch Yield (%) |
|---|---|---|---|
| Temperature | 40 °C | 19.7417 | 19.871 |
| Time | 10 min | ||
| Solid-to-Liquid Ratio | 1:30 |
| Canna Starch | ||||
|---|---|---|---|---|
| Physicochemical Properties | CE | UAE | ||
| Mean | SD | Mean | SD | |
| Water Absorption Index (%) | 168.7000 | 2.3774 | 174.9400 | 3.06 |
| Oil Absorption Index (%) | 219.7496 | 7.2849 | 217.5746 | 14.13 |
| Water Solubility Index (55° C) | 201.8700 | 1.2768 | 212.1186 | 1.26 |
| Water Solubility Index (85 °C) | 1339.1000 | 39.2340 | 1345.9000 | 2.78 |
| Swelling Power (%) (55 °C) | 2.1153 | 0.1174 | 2.1282 | 0.01 |
| Swelling Power (%) (85 °C) | 13.0990 | 0.2867 | 13.2850 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, V.N.; Silvestre, C.; Antih, J.; Jeganathan, P.M.; Portet, K.; Vesta, G.; Kodja, H.; Petit, T.; Souidi, K.; Bichon, F.; et al. Ultrasound Impact on Extraction Yield and Properties of Starch and Polyphenols from Canna indica L. Rhizomes. Separations 2025, 12, 307. https://doi.org/10.3390/separations12110307
Chandrasekaran VN, Silvestre C, Antih J, Jeganathan PM, Portet K, Vesta G, Kodja H, Petit T, Souidi K, Bichon F, et al. Ultrasound Impact on Extraction Yield and Properties of Starch and Polyphenols from Canna indica L. Rhizomes. Separations. 2025; 12(11):307. https://doi.org/10.3390/separations12110307
Chicago/Turabian StyleChandrasekaran, Vigna Nivetha, Charlotte Silvestre, Julien Antih, Prakash Maran Jeganathan, Karine Portet, Gaelle Vesta, Hippolyte Kodja, Thomas Petit, Kaies Souidi, Florence Bichon, and et al. 2025. "Ultrasound Impact on Extraction Yield and Properties of Starch and Polyphenols from Canna indica L. Rhizomes" Separations 12, no. 11: 307. https://doi.org/10.3390/separations12110307
APA StyleChandrasekaran, V. N., Silvestre, C., Antih, J., Jeganathan, P. M., Portet, K., Vesta, G., Kodja, H., Petit, T., Souidi, K., Bichon, F., & Poucheret, P. (2025). Ultrasound Impact on Extraction Yield and Properties of Starch and Polyphenols from Canna indica L. Rhizomes. Separations, 12(11), 307. https://doi.org/10.3390/separations12110307







