High-Efficiency Uranium Adsorption from Real Salt-Lake Brine Using Amine-Functionalized Lignin Microspheres
Abstract
1. Introduction
2. Experiments
2.1. Experimental Instruments and Reagents
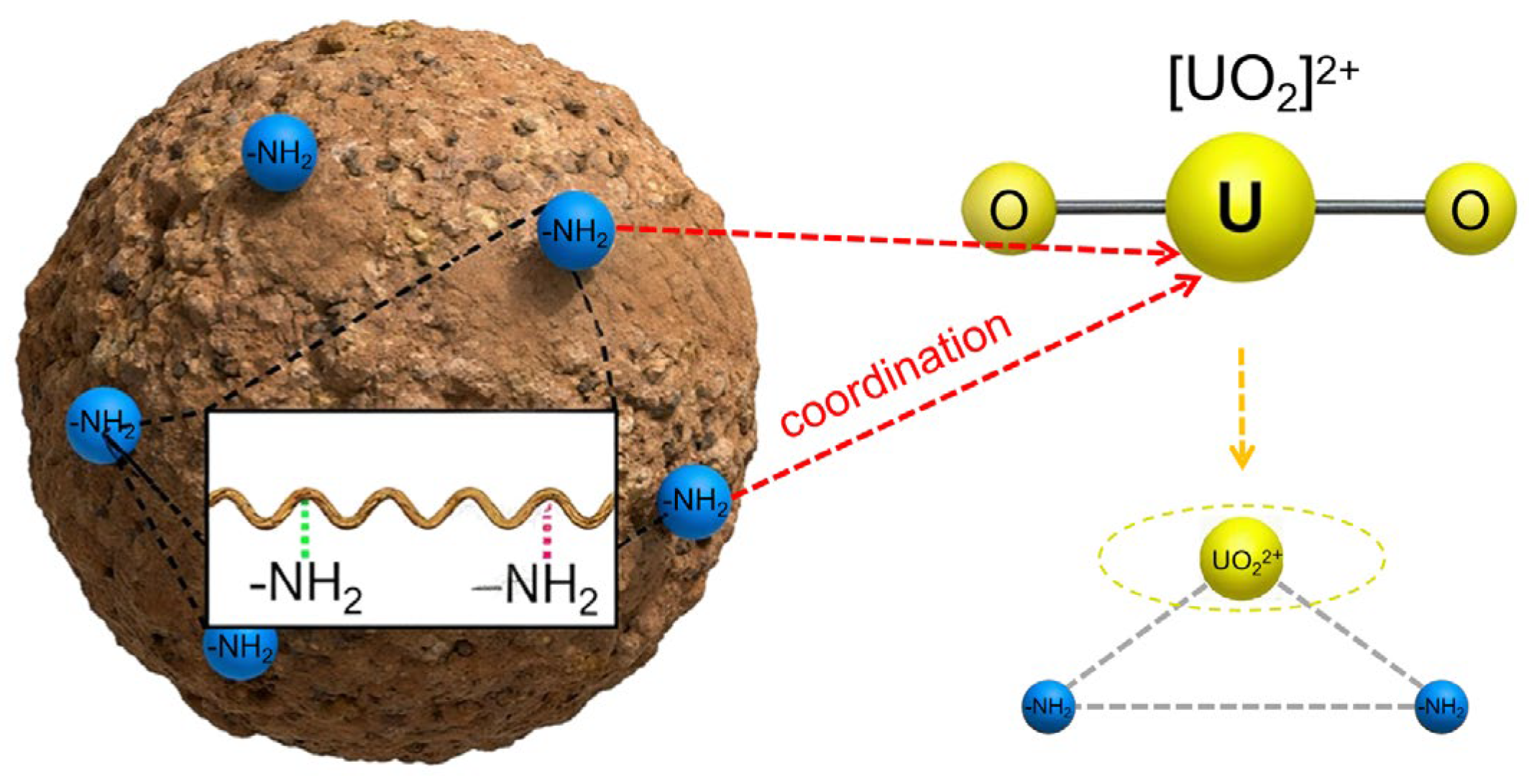
2.2. Preparation and Characterization of AL-PEI/GMS
2.3. Adsorption Experiments
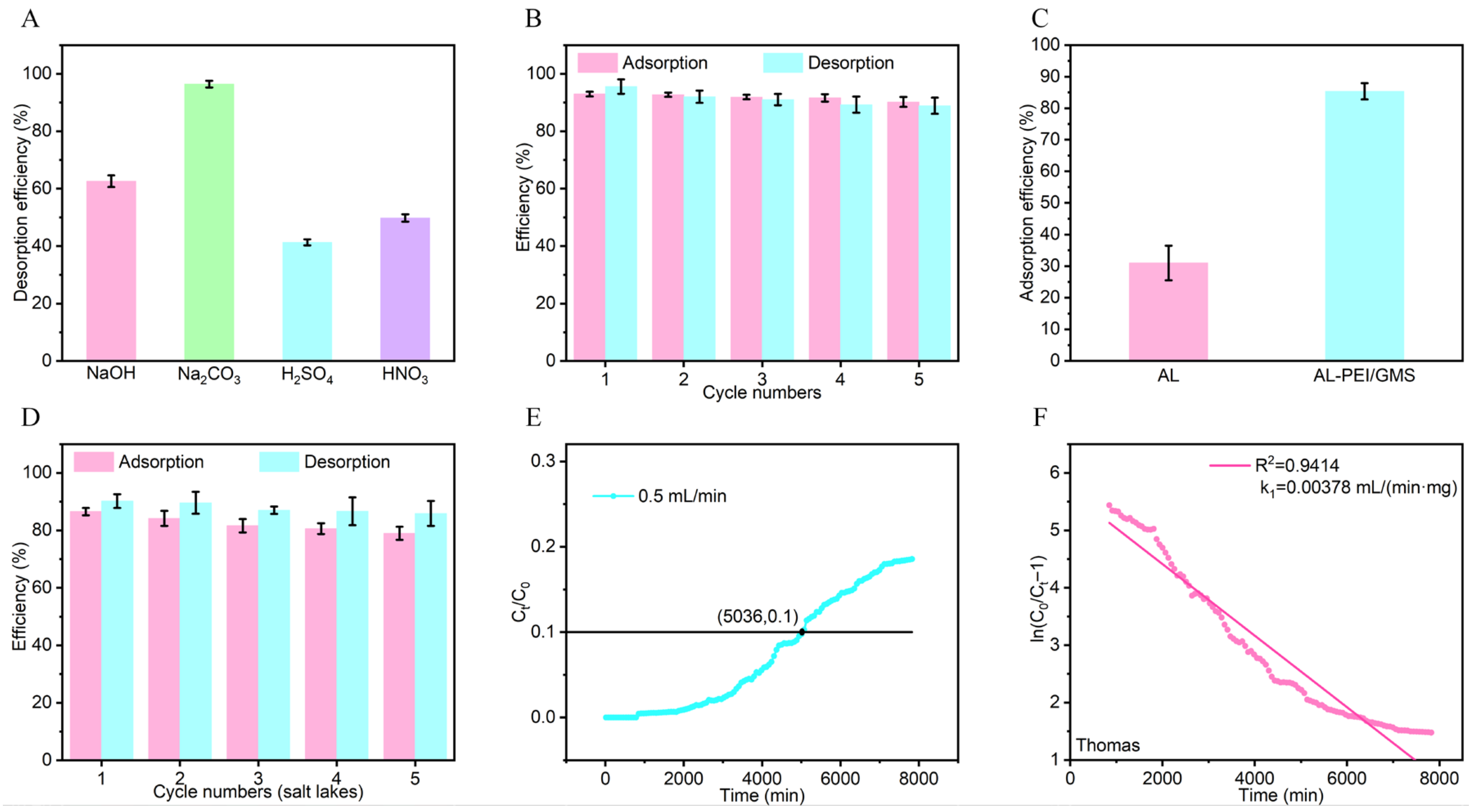
2.4. U(VI) Adsorption via Fix-Bed System
3. Results and Discussion
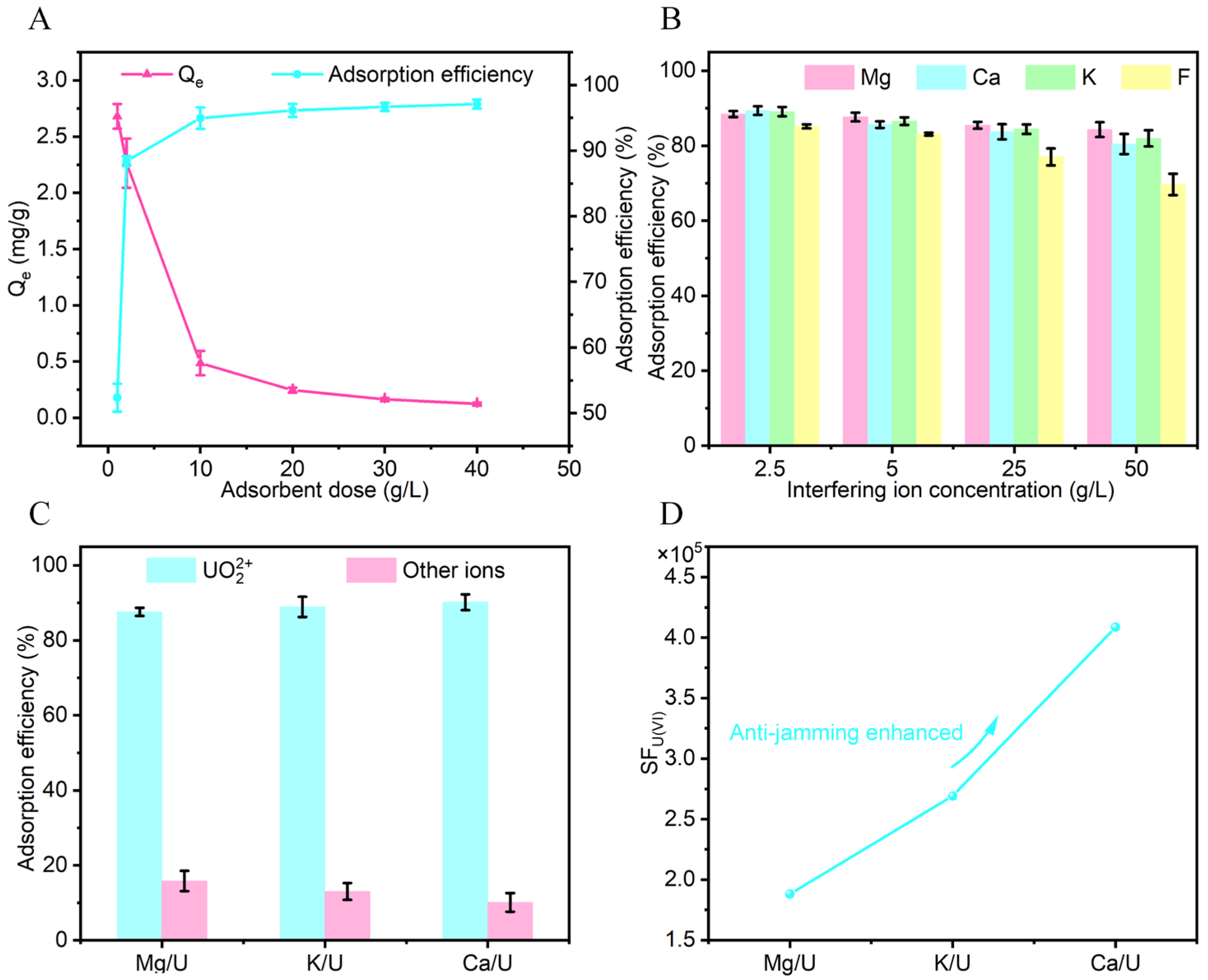
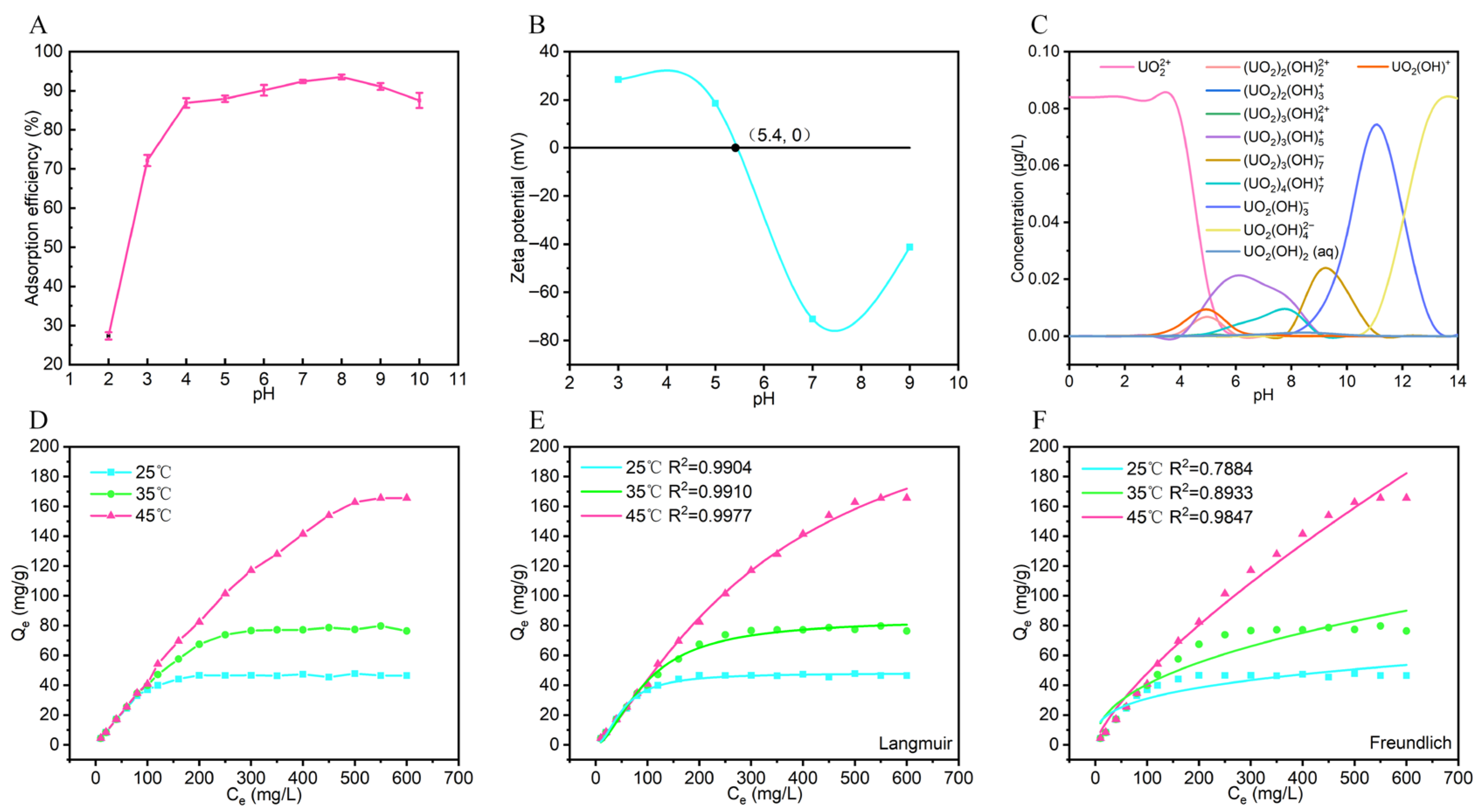
3.1. Exploration of U(VI) Adsorption Performance of AL-PEI/GMS
3.2. Industrial Application of AL-PEI/GMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghosh, S.K.; Ghosh, B.K. Fossil fuel consumption trend and global warming scenario: Energy overview. Glob. J. Eng. Sci. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Shuaibu, A.M.; Alhassan, U.H.; Shafi’I, A.M.; Kolere, M.S.; Sharma, R.; Abdurrashid, I. Biofuel: A sustainable and clean alternative to fossil fuel. GSC Adv. Res. Rev. 2024, 21, 204–222. [Google Scholar] [CrossRef]
- Arias, F.P.; Lampropoulos, G.; Sancho, A.Á.; Vergara, D. Progress, challenges, and sustainable perspectives in nuclear energy strategies. Appl. Sci. 2024, 14, 11864. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Li, M.; Liu, L.; Xu, P.; Xia, Y.; Tang, X. Cretaceous to paleogene polyphase faulting and paleostress inversions in the Taoshan-Zhuguangshan uranium metallogenic belt, south china block. J. Struct. Geol. 2025, 191, 105340. [Google Scholar] [CrossRef]
- Xing, W.; Wang, A.; Yan, Q.; Chen, S. A study of China’s uranium resources security issues: Based on analysis of China’s nuclear power development trend. Ann. Nucl. Energy 2017, 110, 1156–1164. [Google Scholar] [CrossRef]
- Li, Q.; Sun, W.; Shang, B.; Zhang, N.; Zhao, L.; Deng, H.; Du, P.; Qiao, M.; Lu, J.; Wang, Z. Lignin/mesoporous hollow silica nanosphere thin film nanocomposite loose nanofiltration membrane for dye/salt separation. Desalination 2024, 575, 117332. [Google Scholar] [CrossRef]
- Qiang, T.; Wang, T.; Ruan, X.; Zhang, X.; Li, R.; Ren, L. Collagen-based porous aerogel with high adsorption, excellent antibacterial properties, and structural stability for specific uranium capture in seawater. Collagen Leather 2025, 7, 21. [Google Scholar] [CrossRef]
- Imran, M.; Zaman, K.; Nassani, A.A.; Dincă, G.; Khan, H.R.; Haffar, M. Does nuclear energy reduce carbon emissions despite using fuels and chemicals? Transition to clean energy and finance for green solutions. Geosci. Front. 2024, 15, 101608. [Google Scholar] [CrossRef]
- Han, J.; Jiang, H.; Xu, J.; Hussain, S.A.; Yuan, X.; Qin, X. Hydraulic connection affects uranium distribution in the Gas Hure salt lake, Qaidam Basin, China. Environ. Sci. Pollut. Res. Int. 2018, 25, 4881–4895. [Google Scholar] [CrossRef]
- Wang, C.; Jiao, H.; Wu, Y.; Na, P. Amidoxime-functionalized TiO2 nanotube arrays plate photocatalyst for efficient extracting and recovering uranium from salt lakes: Bench-scale experiments and theoretical calculation. Sep. Purif. Technol. 2024, 339, 126556. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, R.; Meng, Q.; Yang, Y.; Ma, X.; Ruan, X.; Yuan, Y.; Zhu, G. Constructing uranyl-specific nanofluidic channels for unipolar ionic transport to realize ultrafast uranium extraction. J. Am. Chem. Soc. 2021, 143, 14523–14529. [Google Scholar] [CrossRef]
- Majdoubi, H.; Şimşek, S.; Billah, R.E.K.; Koçak, N.; Kaya, S.; Tamraoui, Y.; Katin, K.P.; Hannache, H.; Marzouki, R. Novel geopolymer composite based on oil shale and chitosan for enhanced uranium (VI) adsorption: Experimental and theoretical approaches. J. Mol. Liq. 2024, 395, 123951. [Google Scholar] [CrossRef]
- Hussein, G.M.; Muhammad, S.S.; Gomaa, N.A.; Shehata, M.R.; Hosny, W.M. A Study on the extraction of uranium (VI) from sulphate leach liquor using LIX63. J. Dispers. Sci. Technol. 2016, 38, 866–875. [Google Scholar] [CrossRef]
- Yang, L.; Qian, Y.; Zhang, Z.; Li, T.; Lin, X.; Fu, L.; Zhou, S.; Kong, X.; Jiang, L.; Wen, L. A marine bacteria-inspired electrochemical regulation for continuous uranium extraction from seawater and salt lake brine. Chem. Sci. 2024, 15, 4538–4546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Wang, J.; Liang, J.; Pan, D.; Li, P.; Fan, Q. Efficient recovery of uranium from saline lake brine through photocatalytic reduction. J. Mol. Liq. 2020, 308, 113007. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Y.; Ma, H.; Bai, Z.; Qiao, Z.; Ji, D.; Yan, C.; Yan, Y.; Wu, H. Study on uranium adsorption property of carbon nanotubes prepared by molten salt electrolysis. ACS Sustain. Chem. Eng. 2022, 10, 11990–11999. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Dong, C.; Sun, Y. Synthesis of magnetic biochar composites for enhanced uranium (VI) adsorption. Sci. Total Environ. 2018, 651, 1020–1028. [Google Scholar] [CrossRef]
- Abd, M.O.; Manaa, E.S.A.; Youssef, M.A.M.; Kouraim, M.N.; Dhmees, A.S.; Eldesouky, E.M. Uranium removal from aqueous medium using Co0.5Mn0.5Fe2O4 nanoparticles. J. Radioanal Nucl. Chem. 2021, 327, 745–753. [Google Scholar]
- Li, M.; Qing, B.; Luo, H.; Gao, W.; Shou, Q.; Wu, S.; Yao, H.; Liang, X.; Liu, H. Recyclable covalent organic frameworks/cellulose aerogels for efficient uranium adsorption. Int. J. Biol. Macromol. 2024, 282, 137156. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Liu, F.; Hu, B. Effective separation and immobilization of uranium (VI) from wastewater using amino and carboxyl groups functionalized covalent organic frameworks. Sep. Purif. Technol. 2024, 338, 126501. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Sun, R. Fractionational and structural characterization of lignin and its modification as biosorbents for efficient removal of chromium from wastewater: A review. J. Leather Sci. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Shi, M.; Wang, S.; Xi, Y.; Yang, W.; Lin, X.; Qiu, X.; Zhang, F.; Kong, F. Preparation of chemically modified lignin and its application in advanced functional materials. Resour. Chem. Mater. 2025, 4, 100104. [Google Scholar] [CrossRef]
- Selçuk, Ş.; Savaş, K.; Nida, J.S. Adsorption properties of amine modified lignin-hydrogel composite for uranyl ions: Theoretical and experimental insights. J. Indian Chem. Soc. 2023, 100, 100924. [Google Scholar] [CrossRef]
- Guo, L.; Peng, L.; Li, J.; Zhang, W.; Shi, B. Ultrafast uranium recovery from high-salinity nuclear wastewater using amine-functionalized lignin microspheres. J. Clean. Prod. 2023, 388, 136006. [Google Scholar] [CrossRef]
- Negm, S.; Mahmoud, O.; Wael, M.; Mostafa, M.; Samia, M.; Mahmoud, A.; Khaled, A.; Mohamed, N.; Mohamed, F. Appreciatively efficient sorption achievement to U(VI) from the El sela area by ZrO2/Chitosan. Separations 2022, 9, 311. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, C.; Shu, Q. Study on the adsorption characteristics of spirulina dry powder biomass for rare earth element praseodymium (III): Adsorption isotherms, kinetics, and thermodynamics analysis. Separations 2025, 12, 195. [Google Scholar] [CrossRef]
- Michael, B.; Nicolas, S.; Georges, W. Density functional study of aqueous uranyl (VI) fluoride complexes. Chem. Phys. Lett. 2008, 467, 287–293. [Google Scholar]
- Lv, Y.; Han, S.; Wen, W.; Bai, X.; Sun, Q.; Chen, L.; Zhang, H.; Mu, F.; Luo, M. Preparation of EDTA-2Na-Fe3O4-activated carbon composite and its adsorption performance for typical heavy metals. Separations 2025, 12, 205. [Google Scholar] [CrossRef]
- Chang, Z.; He, B.; Gong, X.; Qi, X.; Liu, K. Cr-based metal–organic frameworks (MOFs) with high adsorption selectivity and recyclability for Au (III): Adsorption behavior and mechanism study. Sep. Purif. Technol. 2023, 325, 124612. [Google Scholar]
- Nisha, M.; Manpreet, K.; Vasundhara, S. Adsorption studies on hydrophobic disperse dye using cellulose derived mesoporous activated carbon. Mater. Today Proc. 2022, 62, 7595–7599. [Google Scholar]
- Su, M.; Zhu, J.; Wu, R.; Pan, J.; Yang, J.; Zhao, J.; Chen, D.; Liao, C.; Shih, K.; Ma, S. Synthesis of Zeolitic Imidazolate Framework-8 from Waste Electrodes via Ball Milling for Efficient Uranium Removal. Separations 2025, 12, 40. [Google Scholar] [CrossRef]
- Li, J.; Long, W.; Peng, L.; Guo, L.; Zhang, W. An investigation into the stability source of collagen fiber modified using Cr (III): An adsorption isotherm study. Molecules 2024, 29, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, J.; Yang, S.; Guo, X.; Jia, Y.; Chen, N.; Zhou, J.; Zhang, S.; Wang, S.; Li, J.; et al. Extended X-ray absorption fine structure and density functional theory studies on the complexation mechanism of amidoximate ligand to uranyl carbonate. Ind. Eng. Chem. Res. 2016, 55, 4224–4230. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Chen, Y.; Ye, Q.; Xian, L.; Qian, J. Amino-functionalized yolk-shell magnetic silica nanoparticles for the selective removal of heavy metal ions. Microporous. Mesoporous. Mater. 2025, 387, 113535. [Google Scholar] [CrossRef]
- Abida, K.; Nawaz, B.H.; Munawar, I.; Aisha, A. Batch versus column modes for the adsorption of radioactive metal onto rice husk waste: Conditions optimization through response surface methodology. Water Sci. Technol. 2017, 76, 1035–1043. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Zeng, S.; Li, Z.; Zhang, Q.; He, X.; Liu, Y.; Zhao, X.; Wang, Y.; Yuan, D. Synthesis of amidoxime functionalized core-shell magnetic composites for the highly efficient removal of uranium from simulated and real wastewater. Sep. Purif. Technol. 2025, 357, 130119. [Google Scholar] [CrossRef]


| Major Ions | Concentration (μg/L) |
|---|---|
| Mg2+ | 6.41 × 107 |
| Ca2+ | 1.69 × 106 |
| K+ | 1.67 × 106 |
| UO22+ | 165 |
| Adsorbent | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| q1/(mg·g−1) | k1/min−1 | R2 | q2/(mg·g−1) | k2/g·mg−1·min−1 | R2 | |
| AL-PEI/GMS | 1.59 | 0.0539 | 0.8781 | 2.71 | 0.0295 | 0.9914 |
| AL | 0.67 | 0.0552 | 0.8876 | 1.21 | 0.0717 | 0.9894 |
| Adsorbent | k1/(mg·g−1·min−2) | k2/(mg·g−1·min−2) | k3/(mg·g−1·min−2) |
|---|---|---|---|
| AL-PEI/GMS | 0.2958 | 0.0867 | 0.0013 |
| AL | 0.1458 | 0.0543 | 0.0033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, L.; Peng, L.; Zhang, W.; Shi, B. High-Efficiency Uranium Adsorption from Real Salt-Lake Brine Using Amine-Functionalized Lignin Microspheres. Separations 2025, 12, 300. https://doi.org/10.3390/separations12110300
Wang X, Guo L, Peng L, Zhang W, Shi B. High-Efficiency Uranium Adsorption from Real Salt-Lake Brine Using Amine-Functionalized Lignin Microspheres. Separations. 2025; 12(11):300. https://doi.org/10.3390/separations12110300
Chicago/Turabian StyleWang, Xiaodong, Lijun Guo, Liangqiong Peng, Wenhua Zhang, and Bi Shi. 2025. "High-Efficiency Uranium Adsorption from Real Salt-Lake Brine Using Amine-Functionalized Lignin Microspheres" Separations 12, no. 11: 300. https://doi.org/10.3390/separations12110300
APA StyleWang, X., Guo, L., Peng, L., Zhang, W., & Shi, B. (2025). High-Efficiency Uranium Adsorption from Real Salt-Lake Brine Using Amine-Functionalized Lignin Microspheres. Separations, 12(11), 300. https://doi.org/10.3390/separations12110300








