Phytochemical Profile, Antioxidant and Antiproliferative Activity of Randia spp. Fruit Extracts Obtained by Ultrasound-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Extraction Procedures and Traditional Wine Preparation Techniques
2.3. Phytochemical Profile
2.3.1. Qualitative Identification of Families
2.3.2. Total Phenolic Compounds Content
2.3.3. Total Flavonoid Content
2.4. Identification of Phenol Compounds by HPLC
2.5. Antioxidant Activity
2.6. Antiproliferative Activity and Cytotoxicity
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Profile
3.1.1. Qualitative Family Identification
3.1.2. Total Polyphenol and Flavonoid Contents
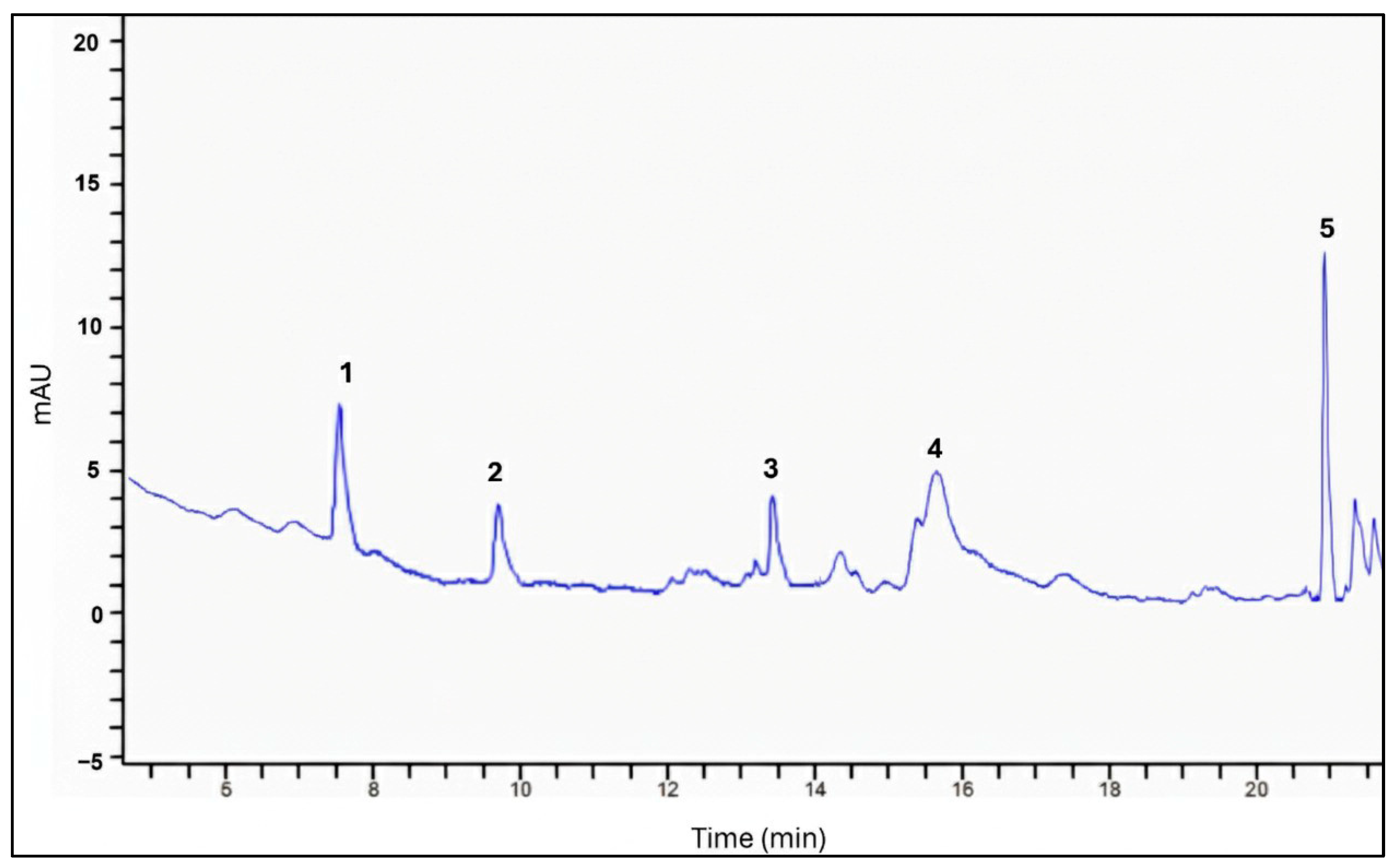
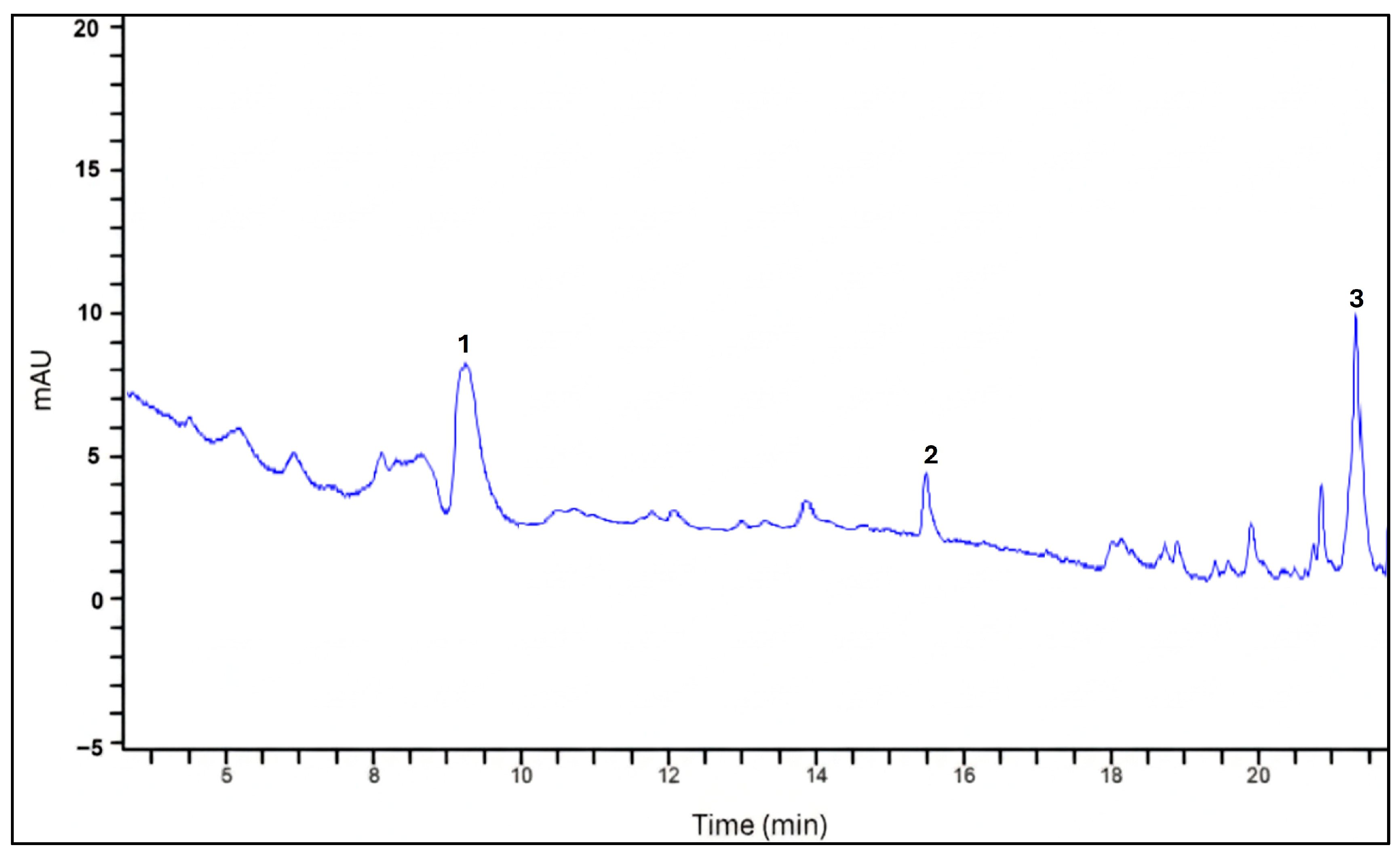
3.1.3. Identification of Phenolic Compounds by HPLC
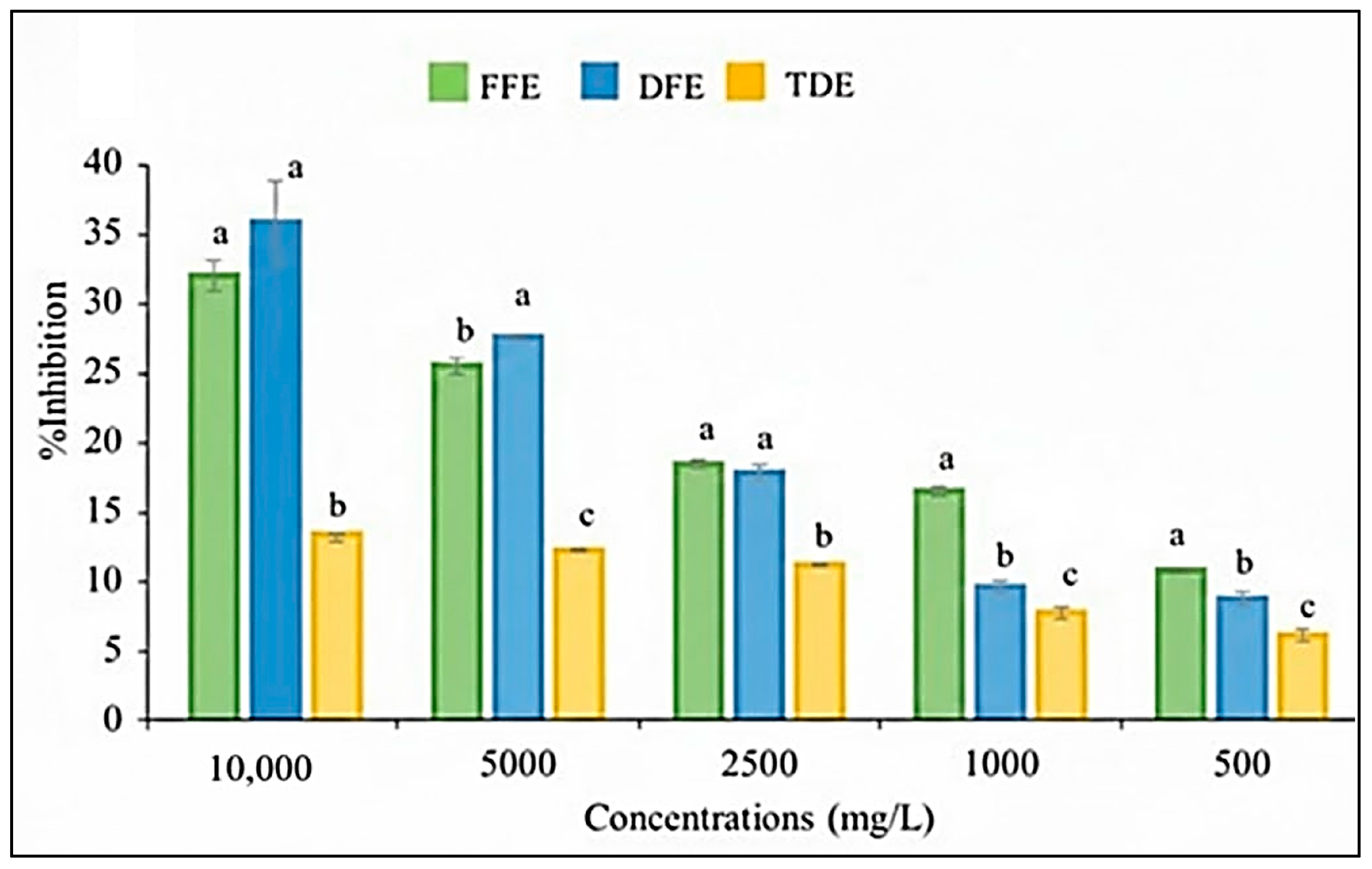
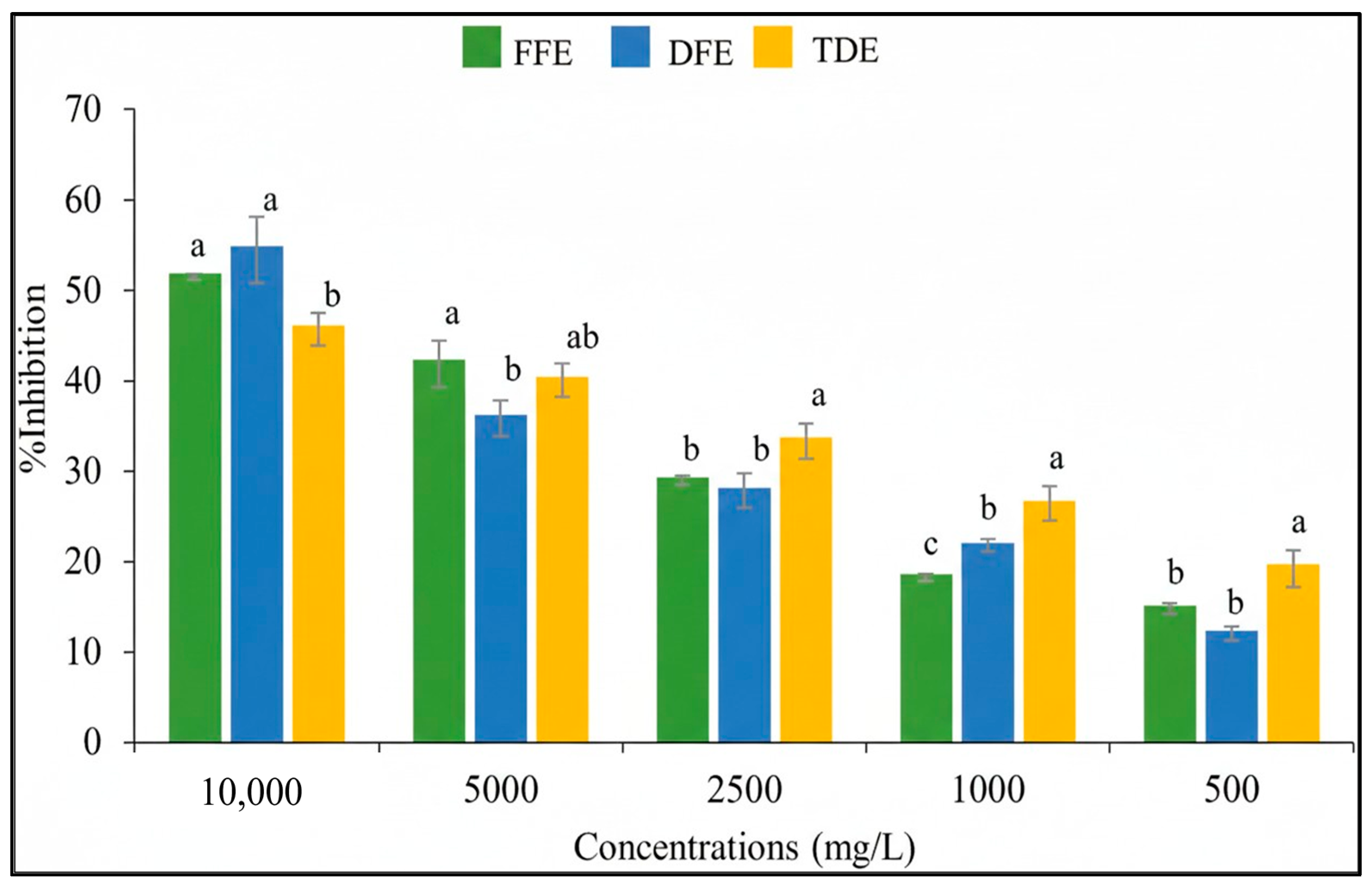
3.2. Antioxidant Activity
3.3. Antiproliferative Activity and Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gómez, X.; Sanon, S.; Zambrano, K.; Asquel, S.; Bassantes, M.; Morales, J.E.; Otáñez, G.; Pomaquero, C.; Villarroel, S.; Zurita, A.; et al. Key points for the development of antioxidant cocktails to prevent cellular stress and damage caused by reactive oxygen species (ROS) during manned space missions. NPJ Microgravity 2021, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Jiang, F.; Ding, Y.; Tian, Y.; Yang, R.; Quan, M.; Tong, Z.; Zhang, X.; Luo, D.; Chi, Z.; Liu, C. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomater. Adv. 2022, 133, 112637. [Google Scholar] [CrossRef]
- Manikandan, R.; Anjali, R.; Beulaja, M.; Prabhu, N.M.; Koodalingam, A.; Saiprasad, G.; Chitra, P.; Arumugam, M. Synthesis, characterization, anti-proliferative and wound healing activities of silver nanoparticles synthesized from Caulerpa scalpelliformis. Process Biochem. 2019, 79, 135–141. [Google Scholar] [CrossRef]
- Said Nasser Al-Owamri, F.; Saleh Abdullah Al Sibay, L.; Hemadri Reddy, S.; Althaf Hussain, S.; Gangireddygari, V.S.R. Phytochemical, Antioxidant, hair growth and wound healing property of Juniperus excelsa, Olea oleaster and Olea europaea. J. King Saud Univ. Sci. 2023, 35, 102446. [Google Scholar] [CrossRef]
- Hang, C.; Sun, H.; Zhang, A.; Yan, G.; Lu, S.; Wang, X. Recent Developments in the Field of Antioxidant Activity on Natural Products. Aмypcкий Meдицинcкий Жypнaл 2015, 2, 44–48. [Google Scholar]
- Cano-Campos, M.C.; Díaz-Camacho, S.P.; Uribe-Beltran, M.J.; López-Angulo, G.; Montes-Avila, J.; Paredes-López, O.; Delgado-Vargas, F. Bio-guided fractionation of the antimutagenic activity of methanolic extract from the fruit of Randia echinocarpa (Sessé et Mociño) against 1-nitropyrene. Food Res. Int. 2011, 44, 3087–3093. [Google Scholar] [CrossRef]
- Stevens, W.D.; Ulloa, C.; Pool, A.; Montiel, O.M. Flora de Nicaragua, 1st ed.; Missouri Botanical Garden Press: St. Louis, Russia, 2001; p. 943. [Google Scholar]
- Gallardo-Casas, C.A.; Guevara-Balcázar, G.; Morales-Ramos, E.; Tadeo-Jiménez, Y.; Gutiérrez-Flores, O.; Jiménez-Sánchez, N.; Valadez-Omaña, M.T.; Valenzuela-Vargas, M.T.; Castillo-Hernández, M.C. Ethnobotanic study of Randia aculeata (Rubiaceae) in Jamapa, Veracruz, Mexico, and its anti-snake venom effects on mouse tissue. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 287–294. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Alvarado-Olivarez, M.; Luna-Solano, G.; Guerrero-Analco, J.A.; Jiménez-Fernández, M. Phenolic profile and antioxidative properties of pulp and seeds of Randia monantha Benth. Ind. Crops Prod. 2018, 124, 53–58. [Google Scholar] [CrossRef]
- Ojeda-Ayala, M.; Gaxiola-Camacho, S.M.; Delgado-Vargas, F. Phytochemical composition and biological activities of the plants of the genus Randia. Bot. Sci. 2024, 100, 779–796. [Google Scholar] [CrossRef]
- Mërtiri, I.; Coman, G.; Cotârlet, M.; Turturică, M.; Balan, N.; Râpeanu, G.; Stanciuc, N.; Mihalcea, L. Phytochemical Profile Screening and Selected Bioactivity of Myrtus communis Berries Extracts Obtained from Ultrasound-Assisted and Supercritical Fluid Extraction. Separations 2025, 12, 8. [Google Scholar] [CrossRef]
- Capataz-Tafur, J.; Orozco-Sánchez, F.; Vergara-Ruiz, R.; Hoyos-Sánchez, R. Efecto antialimentario de los extractos de suspensiones celulares de Azadirachta indica sobre Spodoptera frugiperda JE Smith en condiciones de laboratorio. Rev. Fac. Nac. Agron. Medellín 2007, 60, 3703–3715. [Google Scholar]
- Bulugahapitiya, V.P.; Rathnaweera, T.N.; Manawadu, H.C. Phytochemical composition and antioxidant properties of Dialium ovoideum thwaites (Gal Siyambala) leaves. Int. J. Min. Fruits, Med. and Arom. Plants 2020, 6, 13–19. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Méndez-Lagunas, L.L.; Cruz-Gracida, M.; Barriada-Bernal, L.G.; Rodríguez-Méndez, L.I. Profile of phenolic acids, antioxidant activity and total phenolic compounds during blue corn tortilla processing and its bioaccessibility. J. Food Sci. Technol. 2020, 57, 4688–4696. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, L.D.; Moreno-Rodríguez, A.; Vázquez-Jiménez, L.K.; Delgado-Maldonado, T.; Juárez-Saldivar, A.; Ortiz-Pérez, E.; Rivera, G. Triose Phosphate Isomerase Structure-based virtual screening and in vitro biological activity of natural products as Leishmania Mexicana inhibitors. Pharmaceutics 2023, 15, 2046. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Mancera-Rodríguez, M.A.; Delgado-Buenrostro, N.L.; Moreno-Rodríguez, A.; Bautista-Martínez, J.L.; Díaz-Velásquez, C.E.; Martínez-Alarcón, S.A.; Torrens, H.; Godínez-Rodríguez, M.A.; Terrazas-Valdés, L.I.; et al. Novel thiosemicarbazones induce high toxicity in estrogen receptor-positive breast cancer cells (MCF7) and exacerbate cisplatin effectiveness in triple-negative breast (MDA-MB231) and lung adenocarcinoma (A549) cells. Investig. New Drugs 2019, 38, 558–573. [Google Scholar] [CrossRef]
- Chaparro-Hernández, I.; Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Sandoval-Torres, S.; Aquino-González, L.; Barriada-Bernal, G. Spray Drying of Stevia Rebaudiana Bertoni Aqueous Extract: Effect on Polyphenolic Compounds. Chem. Eng. Trans. 2023, 98, 33–38. [Google Scholar]
- Sepúlveda, C.T.; Zapata, J.E. Efecto de la Temperatura, el pH y el Contenido en Sólidos sobre los Compuestos Fenólicos y la Actividad Antioxidante del Extracto de Bixa orellana L. Inf. Technol. 2019, 30, 57–66. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Ozer, M.S.; Kirkanb, B.; Sarikurkcuc, C.; Cengizd, M.; Ceylane, O.; Atılgand, N.; Tepef, B. Onosma heterophyllum: Phenolic composition, enzyme inhibitory and antioxidant activities. Ind. Crops Prod. 2018, 111, 179–184. [Google Scholar] [CrossRef]
- Martínez-Ceja, A.; Romero-Estrada, A.; Columba-Palomares, M.C.; Hurtado-Díaz, I.; Alvarez, L.; Teta-Talixtacta, R.; Bernabé-Antonio, A. Anti-inflammatory, antibacterial and antioxidant activity of leaf and cell cultures extracts of Randia aculeata L. and its chemical components by GC-MS. S. Afr. J. Bot. 2022, 144, 206–218. [Google Scholar] [CrossRef]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Cytotoxicity of African medicinal plants against normal animal and human cells. In Toxicological Survey of African Medicinal Plants, 1st ed.; Kuete, V., Ed.; Elsevier: London, UK, 2014; pp. 181–233. [Google Scholar]
- Bye, R.; Linares, E.; Mata, R.; Albor, C.; Casteñeda, P.C.; Delgado, G. Ethnobotanical and phytochemical investigation of Randia echinocarpa (Rubiaceae). An. Inst. Biol. Ser. Bot. 1991, 62, 87–106. [Google Scholar]
- Charoensin, S. Antioxidant and anticancer activities of Moringa oleifera leaves. J. Med. Plants Res. 2014, 8, 318–325. [Google Scholar] [CrossRef]
- Sahlabgi, A.; Lupuliasa, D.; Stoicescu, I.; Vlaia, L.L.; Licu, M.; Popescu, A.; Mititelu, M. Determination of the Phytochemical Profile and Antioxidant Activity of Some Alcoholic Extracts of Levisticum officinale with Pharmaceutical and Cosmetic Applications. Separations 2025, 12, 79. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Oracz, J. Effect of addition of green coffee extract and nanoencapsulated chlorogenic acids on aroma of different food products. LWT 2016, 73, 197–204. [Google Scholar] [CrossRef]
- Zhuang, X.; Shi, W.; Shen, T.; Cheng, X.; Wan, Q.; Fan, M.; Hu, D. Research Updates and Advances on Flavonoids Derived from Dandelion and Their Antioxidant Activities. Antioxidants 2024, 13, 1449. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Tapia-Hernández, F.E.; Alvarado-Olivarez, M.; Beristain-Guevara, C.I.; Pascual-Pineda, L.A.; Jiménez-Fernández, M. Antibacterial activity and acute toxicity study of standardized aqueous extract of Randia monantha Benth fruit. Biotecnia 2022, 24, 38–45. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Pappis, L.; Prates-Ramos, A.; Fontana, T.; Geraldo-Sangoi, G.; Castro-Dornelles, R.; Bolssoni-Dolwitsch, C.; Rorato-Sagrillo, M.; Cadoná, F.C.; Kolinski-Machado, A.; Bauermann, L.D.F. Randia ferox (Cham & Schltdl) DC: Phytochemical composition, in vitro cyto-and genotoxicity analyses. Nat. Prod. Res. 2021, 36, 4170–4176. [Google Scholar]
- Do Carmo, M.A.V.; Granato, D.; Azevedo, L. Antioxidant/pro-oxidant and antiproliferative activities of phenolic-rich foods and extracts: A cell-based point of view. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2021; Volume 98, pp. 253–280. [Google Scholar]
- Saklani, A.; Kutty, S.K. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 1, 721402. [Google Scholar] [CrossRef] [PubMed]
- Archoo, S.; Naikoo, S.H.; Tasduq, S.A. Role of herbal products as therapeutic agents against ultraviolet radiation-induced skin disorders. In Herbal Medicines; Academic Press: Cambridge, MA, USA, 2022; pp. 345–360. [Google Scholar]
- Kaur, S.; Mendonca, P.; Soliman, K.F. The anticancer effects and therapeutic potential of kaempferol in triple-negative breast cancer. Nutrients 2024, 16, 2392. [Google Scholar] [CrossRef] [PubMed]
- Subramani, C.; Sharma, G.; Chaira, T.; Barman, T.K. High content screening strategies for large-scale compound libraries with a focus on high-containment viruses. Antivir. Res. 2024, 221, 105764. [Google Scholar] [CrossRef]
| Family | Fresh Fruit | Dried Fruit | Traditional Drink |
|---|---|---|---|
| Alkaloids | - | - | - |
| Saponins | ++ | ++ | + |
| Sterols and triterpenes | - | - | - |
| Phenolic compounds | ++ | ++ | + |
| Flavonoids | + | + | ++ |
| Extract | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) |
|---|---|---|
| Fresh | 47.22 ± 0.96 A | 27.08 ± 1.36 B |
| Dry | 50.27 ± 0.14 A | 35.53 ± 2.20 A |
| Traditional drink | 38.583 ± 2.677 B | 18.660 ± 1.696 C |
| Compound (mg/gdb) | Fresh | Dry | Traditional Drink |
|---|---|---|---|
| Caffeic acid | 1.05 ± 0.01 | nd | nd |
| Hydroxybenzoic acid | 5.34 ± 0.1 | nd | nd |
| Ferulic acid | 2.30 ± 0.03 A | 1.94 ± 0.05 B | nd |
| Cumaric acid | 1.54 ± 0.05 | nd | nd |
| Kaempferol | 137.55 ± 0.16 A | 42.10 ± 0.20 B | nd |
| Vanillic acid | nd | 7.51 ± 0.33 | nd |
| Extract | DPPH (%) | IC50 (mg/mL) | ABTS (%) | IC50 (mg/mL) |
|---|---|---|---|---|
| Fresh | 31.8 ± 1.85 A | 18.29 ± 0.8 A | 51.54 ± 2.12 A | 8.70 ± 0.14 A |
| Dried | 35.7 ± 1.92 A | 14.22 ± 1.35 A | 54.54 ± 1.87 A | 8.68 ± 0.62 A |
| Traditional drink | 14.6 ± 1.92 B | 61.933 ± 1.042 B | 45.54 ± 1.92 B | 61.933 ± 1.042 B |
| Extract | Cytotoxicity a CC50 (µg/mL) | Antiproliferative Activity b IC50 (µg/mL) | SI c |
|---|---|---|---|
| Fresh fruit | >100 | >100 | 1 |
| Dried fruit | >100 | 25.44 ± 0.16 | 3.92 |
| Traditional drink | 29.99 ± 8.45 | 100.50 ± 6.54 | 0.29 |
| Control (cisplatin) | 2.22 ± 0.21 | 7.52 ± 0.41 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sánchez, C.E.; Herman-Lara, E.; Fernández-López, V.M.; Méndez-Lagunas, L.L.; Moreno-Rodríguez, A.; Argueta-Figueroa, L.; Gallegos-Marín, I. Phytochemical Profile, Antioxidant and Antiproliferative Activity of Randia spp. Fruit Extracts Obtained by Ultrasound-Assisted Extraction. Separations 2025, 12, 292. https://doi.org/10.3390/separations12110292
Martínez-Sánchez CE, Herman-Lara E, Fernández-López VM, Méndez-Lagunas LL, Moreno-Rodríguez A, Argueta-Figueroa L, Gallegos-Marín I. Phytochemical Profile, Antioxidant and Antiproliferative Activity of Randia spp. Fruit Extracts Obtained by Ultrasound-Assisted Extraction. Separations. 2025; 12(11):292. https://doi.org/10.3390/separations12110292
Chicago/Turabian StyleMartínez-Sánchez, Cecilia E., Erasmo Herman-Lara, Víctor M. Fernández-López, Lilia L. Méndez-Lagunas, Adriana Moreno-Rodríguez, Liliana Argueta-Figueroa, and Ivet Gallegos-Marín. 2025. "Phytochemical Profile, Antioxidant and Antiproliferative Activity of Randia spp. Fruit Extracts Obtained by Ultrasound-Assisted Extraction" Separations 12, no. 11: 292. https://doi.org/10.3390/separations12110292
APA StyleMartínez-Sánchez, C. E., Herman-Lara, E., Fernández-López, V. M., Méndez-Lagunas, L. L., Moreno-Rodríguez, A., Argueta-Figueroa, L., & Gallegos-Marín, I. (2025). Phytochemical Profile, Antioxidant and Antiproliferative Activity of Randia spp. Fruit Extracts Obtained by Ultrasound-Assisted Extraction. Separations, 12(11), 292. https://doi.org/10.3390/separations12110292









