Abstract
Randia spp. is a medicinal plant traditionally used to treat various diseases. In this study, the phytochemical composition and the antioxidant, antiproliferative, and cytotoxic activities of hydroalcoholic extracts from fresh and dried Randia spp. fruits were evaluated. The phytochemical profile was determined through qualitative assays and high-performance liquid chromatography (HPLC). Antioxidant activity was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. The antiproliferative effect was tested against CaCo-2 cells (human colon adenocarcinoma), the cytotoxicity was evaluated using J774.2 murine macrophages, and the selectivity index (SI) was calculated. The fresh and dried fruit extracts contained 50.27 and 47.22 mg QE/g extract of total phenols (TPC) and 27.08 and 35.53 mg QE/g extract of total flavonoids (TFC), respectively. In the fresh fruit extracts, four phenolic acids (caffeic, hydroxybenzoic, ferulic, and coumaric) and one flavonoid (kaempferol) were identified, and the dried fruit extracts contained ferulic acid, vanillic acid, and kaempferol. Kaempferol was the predominant compound in both extracts (137.55 and 42.10 mg/g dry sample in fresh and dried fruits, respectively). Both extracts displayed antioxidant activity, with IC50 values of 18.29 mg/mL (DPPH) and 8.70 mg/mL (ABTS). Among the tested samples, the dried fruit extract demonstrated the highest antiproliferative activity. Furthermore, the extract showed moderate antiproliferative effects against CaCo-2 cells (IC50 25.44 ± 0.16 µg/mL) and low cytotoxicity toward J774.2 cells (CC50 > 100 µg/mL), resulting in an SI = 3.92. Overall, the antioxidant and antiproliferative activities can be attributed mainly to kaempferol, given its high abundance in both extracts. The favorable selectivity index suggests that hydroalcoholic extracts of Randia spp. are safe and effective, highlighting their potential as candidates for further preclinical and clinical evaluation.
1. Introduction
Under normal physiological conditions, the human body continuously generates free radicals, which play essential roles in regulating key cellular processes, including tumor cell apoptosis, immune cell activation, and cell differentiation [1]. The balance of these reactive species is tightly controlled by endogenous antioxidant enzymes that protect cells from excessive oxidative damage. However, when the production of free radicals exceeds the capacity of these defense systems, oxidative stress occurs, contributing to the onset and progression of numerous chronic conditions, such as inflammation, cardiovascular disorders, neurodegenerative conditions, and cancer [2].
Inflammation, one of the critical stages of the wound-healing process and a vital component of the innate immune response to tissue injury, can become harmful when it is dysregulated or chronic [3]. Additionally, despite advances in modern medicine, cancer remains among the leading causes of mortality worldwide, highlighting the need for novel, safer, and more effective therapeutic strategies [4].
Since antiquity, medicinal plants have served as primary remedies for the treatment of diverse ailments. Even today, plant-derived compounds continue to be invaluable sources of bioactive molecules because of their safety, accessibility, and therapeutic efficacy [5]. In particular, increasing attention has been given to natural antioxidants capable of mitigating oxidative stress and modulating inflammatory responses. Compounds such as polyphenols, saponins, flavonoids, and vitamins have been extensively investigated for their pharmacological properties, including their antioxidant, anti-inflammatory, and anticancer activities [6].
In this context, the exploration of medicinal plants and their secondary metabolites provides an important avenue for the discovery of novel therapeutic agents. By elucidating their phytochemical composition and biological activities, it is possible to identify potential candidates for the prevention or complementary treatment of oxidative stress-related diseases, including cancer and chronic inflammation.
Randia is a neotropical genus comprising approximately 60 to 70 species distributed from the southern United States to South America. Mexico represents the center of greatest diversity, harboring 33 recognized species [7]. Commonly known as “crucetillo”, this genus is characterized by the presence of cross-shaped thorns along the stems of its trees [8]. Fruits are traditionally used in local medicine and are often prepared as alcoholic extracts mixed with brandy to counteract the effects of snake and insect envenomation (e.g., snakes, spiders, scorpions, toads, bees, wasps). Additionally, they are employed in the treatment of cancer, diabetes, inflammation, and pain [9].
Several species within the genus have demonstrated notable pharmacological properties. For example, Randia aculeata partially inhibited necrosis in skeletal and myocardial muscles following exposure to snake venom, thereby offering protection against venom-induced tissue damage. Furthermore, hydroalcoholic extracts of its fruits have been reported to exert analgesic activity at the visceral level [9,10]. These findings highlight the therapeutic potential of Randia species and support their relevance as a source of bioactive compounds for further pharmacological and biomedical research.
Despite its traditional importance, most species of the Randia genus remain poorly characterized from a phytochemical perspective. Existing qualitative studies are scarce and incomplete, with reports available for only eight species; in these species, flavonoids and tannins have been the most frequently detected compounds. Moreover, the isolation and identification of specific metabolites have been achieved in only six species [11]. Among them, Randia spp., distributed in the northern region of Oaxaca, Mexico, is particularly noteworthy because of its long-standing traditional use in the treatment of poisonous animal bites and in the management of chronic conditions such as diabetes and cancer. This ethnopharmacological relevance underscores the urgent need to advance research on its chemical composition and therapeutic potential.
The present study addresses this gap by conducting a detailed investigation of hydroalcoholic extracts obtained from fresh and dried fruits of Randia spp. Specifically, the phytochemical profile was characterized, and the antioxidant, antiproliferative, and cytotoxic activities were systematically evaluated. By integrating both chemical and biological assessments, this research helps expand the current knowledge of Randia spp. while also highlighting its potential as a promising source of bioactive compounds with applications in the pharmaceutical and functional food industries.
2. Materials and Methods
2.1. Sample Collection and Preparation
A total of 100 fruits of Randia spp. (5.7 kg) were collected in October 2023 from the city of Tuxtepec, Oaxaca, Mexico (18°01′21″ N, 96°12′10″ W; 35 m a.s.l.). The fruits were thoroughly washed to remove soil residues, and any samples showing signs of fungal contamination or physical damage (e.g., bruising) were discarded. The selected fruits were ground using a knife mill (model HC-2000Y, VEVOR, Shanghai, China) until a homogeneous paste was obtained. A portion of this paste was subjected to drying in a batch refractive window dryer at a constant temperature of 55 °C for 2.5 h. The dried material was subsequently reground in the same knife mill to obtain a fine powder with a particle size of 0.420 mm (mesh No. 40).
2.2. Extraction Procedures and Traditional Wine Preparation Techniques
Fresh fruit paste and dried fruit powder from Randia spp. were subjected to ultrasound-assisted extraction. The process was performed in an ultrasonic bath (Elmasonic P, D-78224, Singen/Htw., Singen, Germany) operating at a frequency of 80 kHz and 100% power for 30 min. A hydroalcoholic mixture of 70% ethanol in water was used as the extraction solvent at a plant material-to-solvent ratio of 1:10 (g/mL). After extraction, the solvent was filtered through Whatman N° 1 filter paper, and the filtrate was concentrated under reduced pressure using a Rotavapor® R-3 (Büchi Labortechnik, Flawil, Switzerland) at 40 °C and 60 rpm. It was then placed in an oven at 40 °C until a solid residue was obtained. The resulting extracts were stored at 4 °C until analysis [12,13].
The traditional beverage was formulated by combining 750 mL of sherry wine (La Lupe Mexican brand) with 250 mL of brandy, both of which were acquired from the local market. Eight fresh ground fruits (40 g) were subsequently added to the mixture to obtain the final preparation.
2.3. Phytochemical Profile
2.3.1. Qualitative Identification of Families
An aliquot of 50 mg of each extract was weighed and diluted in 2.5 mL of a 70% ethanol–water solution. The resulting mixture was clarified by filtration through a Pasteur pipette packed with diatomaceous earth and activated carbon because the extracts presented a dark coloration; thus, clarification was necessary to perform the colorimetric tests for qualitative identification. Phytochemical screening of the clarified extracts was carried out using standard qualitative tests: alkaloids were detected with Dragendorff’s reagent, and saponins were detected by their ability to produce stable foam, phenolic compounds with the Folin–Ciocalteu reagent, flavonoids by colorimetric reactions with NaOH and HCl, and sterols and triterpenes with the Liebermann–Burchard reagent [14]. The experiments were performed in triplicate.
2.3.2. Total Phenolic Compounds Content
The total polyphenol content (TPC) was determined following the procedure described by Singleton and Rossi [15], using gallic acid as the reference standard. The results are expressed as milligrams of gallic acid equivalents per gram of extract (mg GAE/g). For the assay, 600 µL of deionized water, 10 µL of sample solution (2.5 mg extract/mL H2O), and 50 µL of Folin–Ciocalteu reagent were added sequentially to Eppendorf tubes. After mixing, 150 µL of 20% (w/v) sodium carbonate solution was added, and the reaction mixtures were incubated at 27 °C for 2 h. The absorbance of each sample was then measured at 760 nm using a UV–Vis spectrophotometer (Cary 60 UV-Vis (Agilent Technologies, Santa Clara, CA, USA). The experiments were performed in triplicate.
2.3.3. Total Flavonoid Content
The total flavonoid content (TFC) was determined following the methodology described by Zhishen et al. [16]. Briefly, 1250 µL of deionized water was mixed with the sample solution (2.5 mg extract/mL H2O), followed by the addition of 75 µL of 5% NaNO2 solution. After incubation at room temperature for 6 min, 150 µL of 10% AlCl3 solution was added, and the mixture was allowed to stand for an additional 5 min. Subsequently, 500 µL of 1 M NaOH was added, and the final mixture was thoroughly mixed. The absorbance was recorded at 510 nm using a UV–Vis spectrophotometer. Quercetin was used as the reference standard, and the results are expressed as milligrams of quercetin equivalents per gram of extract (mg QE/g). The experiments were performed in triplicate.
2.4. Identification of Phenol Compounds by HPLC
Phenolic compounds were identified using a PerkinElmer Flexar high-performance liquid chromatography (HPLC) system (PerkinElmer Inc., Waltham, MA, USA) equipped with a quaternary pump and a UV detector, and operated with Chromera software (version 4.1.16396, PerkinElmer Inc., Waltham, MA, USA).
The procedure followed the method described by Méndez-Lagunas et al. [17]. Separation was achieved on a Zorbax Bonus-RP column (4.6 × 150 mm i.d.; Agilent Technologies) coupled to a UV detector (Perkin-Elmer Flexar, Burladingen, Baden-Wurtemberg, Germany). The mobile phase consisted of two solvents with different polarities: 0.085% orthophosphoric acid in water and acetonitrile. Phenolic acids, including ferulic, p-coumaric, syringic, caffeic, chlorogenic, 4-hydroxybenzoic, and vanillic acids, were identified by comparison with authentic standards. The concentration of the extracts was 10 mg/mL, and the injection volume was 1 μL. Quantification was performed using calibration curves prepared from the corresponding standards at a detection wavelength of 280 nm, and peak areas were used for calculations. The results are expressed as milligrams per 100 g of dry solids. The experiments were performed in triplicate.
2.5. Antioxidant Activity
The antioxidant activity of the extracts was evaluated on the basis of their ability to inhibit DPPH and ABTS radical activity. The percentage of DPPH inhibition was determined following the method of Brand-Williams et al. [18]. A stock solution of DPPH was prepared by dissolving 2.4 mg of the radical in methanol and adjusting the volume to 100 mL. For the assay, 975 μL of the DPPH solution was mixed with 25 μL of the extracts at different concentrations (10,000, 5000, 2500, 1000, and 500 mg/L) in Eppendorf tubes. The mixtures were incubated under dark conditions at room temperature for 30 min prior to absorbance measurements at 515 nm. The experiments were performed in triplicate.
The ABTS radical inhibition assay was performed according to the procedure described by Re et al. [19]. ABTS was prepared at a concentration of 7 mM in water, and the ABTS radical cation was generated by reacting the stock solution with 2.45 mM potassium persulfate (final concentration). The mixture was kept in the dark at room temperature for 12–16 h before use. The working ABTS solution was then diluted with ethanol to an absorbance of 0.70 ± 0.05 at 732 nm. The ABTS radical solution was added to the samples at a 1:10 (v/v) ratio of the extracts at different concentrations (10,000, 5000, 2500, 1000, 500 mg/L), and the results are expressed as percentage inhibition. The experiments were performed in triplicate.
The average inhibitory concentration (IC50) for antioxidant activity was calculated using the DPPH and ABTS assays from the percentage of inhibition for each sample concentration, and the concentration corresponding to 50% inhibition was determined using a regression curve.
2.6. Antiproliferative Activity and Cytotoxicity
The cytotoxicity assay was performed using the mouse macrophage cell line J774.2 (ATCC® TIB-67; Manassas, VA, USA). Cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM glutamine and maintained at 37 °C in a humidified atmosphere containing 5% CO2. A defined cell suspension (1 × 105 cells/mL) was incubated with fresh and dried fruit extracts of Randia spp. at concentrations ranging from 0.78 to 200 µg/mL for 48 h under the same conditions. Wells containing cells treated with 0.2% DMSO served as negative controls, while cisplatin was used as a positive control. Cell metabolic activity was assessed by the MTT assay, and cell viability (%) was calculated. The 50% cytotoxic concentration (CC50) was determined by probit analysis. All assays were performed in triplicate across three independent experiments [20].
Antiproliferative activity was evaluated using human colorectal adenocarcinoma Caco-2 cells (ATCC®HTB-37, Manassas, VA, USA). Cells were cultured in high-glucose DMEM supplemented with 20% FBS and 1% antibiotic–antimycotic solution and maintained at 37 °C in a humidified atmosphere with 5% CO2. The culture medium was replaced every 2–3 days, depending on cell confluence. For the assay, 15,000 cells per well were seeded into 96-well plates and incubated for 24 h. The cells were then treated with fresh and dried fruit extracts of Randia spp. at concentrations ranging from 0.78 to 200 µg/mL, washed with PBS, and incubated with fresh, treatment-free medium for an additional 22 h. Cisplatin was used as a positive control.
Cell viability was assessed using the MTT assay. After treatment, the culture supernatants were removed, the wells were washed with PBS, and 100 µL of serum-free medium supplemented with 0.5 mg/mL MTT was added. The plates were incubated at 37 °C for 2 h, after which the resulting formazan crystals were dissolved in isopropyl alcohol. The absorbance was measured at 540 nm using a microplate reader [21]. Experiments were performed in triplicate across three independent assays, and the results are expressed as the means ± standard error. The half-maximal inhibitory concentration (IC50) was determined by probit analysis.
The selectivity index (SI) of the fresh and dried fruit extracts was calculated as the ratio between CC50 and IC50 (SI = CC50/IC50) [20].
2.7. Statistical Analysis
All the results are presented as the means ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Tukey’s test to assess significant differences between means at the 95% confidence level (p < 0.05). Analyses were conducted using Statistica software, version 10.0 (StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Phytochemical Profile
3.1.1. Qualitative Family Identification
Colorimetric assays revealed the presence (+) or absence (−) of secondary metabolites, as summarized in Table 1. Extracts from fresh and dried fruits, as well as from traditional beverages, were positive for saponins, phenolic compounds, and flavonoids, compounds that are widely recognized for their potential contribution to antioxidant activity.

Table 1.
Identification of chemical families of extracts from the fresh and dried fruit of Randia spp.
The values represent the mean of three determinations ± standard deviation. Different letters in the same column indicate significantly different values (p < 5) according to Tukey’s test.
In the extracts of fresh and dried fruit, phenolic compounds and saponins were identified as the predominant metabolite families, whereas lower levels of flavonoids were detected than in traditional drinks. In contrast, alkaloids, steroids, and triterpenes were not detected in any of the evaluated extracts.
3.1.2. Total Polyphenol and Flavonoid Contents
The total polyphenol content of the fruit extracts is presented in Table 2, with values of 47.22 ± 0.96 mg GAE/g for the fresh fruit extract and 50.27 ± 0.14 mg GAE/g for the dried fruit extract. No significant differences were observed between the fresh and dried samples, indicating that drying using a refractive window did not notably affect the polyphenol content.

Table 2.
Total polyphenol and flavonoid content of fresh and dried fruit extracts of Randia spp.
Significant differences in total flavonoid content were observed among the extracts, with the dried fruit extract showing the highest value (35.53 ± 2.20 mg QE/g; Table 2). This increase is likely due to water removal during drying, which concentrates the compounds and disrupts cellular vacuoles, facilitating flavonoid extraction [22].
3.1.3. Identification of Phenolic Compounds by HPLC
The phenolic compounds of the Randia spp. fruit extracts, as determined by HPLC, are presented in Table 3. In the fresh fruit extract, four phenolic acids (caffeic, hydroxybenzoic, ferulic, and coumaric acids) and one flavonoid (kaempferol) were identified and quantified (Figure 1). In contrast, the dried fruit extract contained only two phenolic acids (ferulic and vanillic acids) along with kaempferol (Figure 2), suggesting that several phenolic compounds were degraded during the drying process because of thermal effects [23].

Table 3.
Phenolic profile of extracts from the fresh and dried fruit of Randia spp.
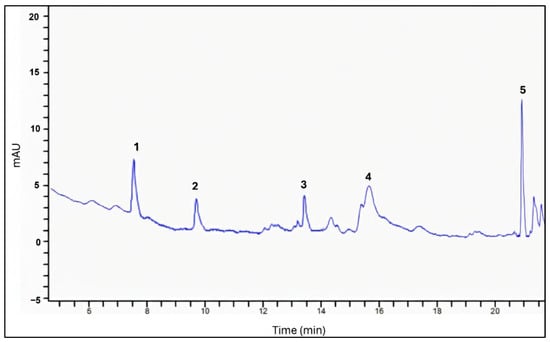
Figure 1.
Chromatogram of fresh fruit extract. The peaks correspond to hydroxybenzoic acid (1), caffeic acid (2), coumaric acid (3), ferulic acid (4), and kaempferol (5).
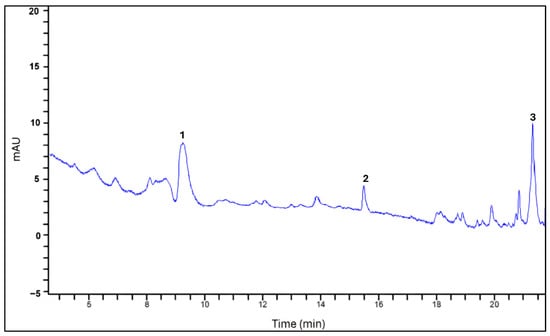
Figure 2.
Chromatogram of dried fruit extract. The peaks correspond to vanillic acid (1), ferulic acid (2), and kaempferol (3).
The detection of vanillic acid in the dried fruit extract is noteworthy, as it was not detected in the fresh fruit. This may be attributed to its formation as an intermediate from ferulic acid (present in fresh fruit) during vanillin biosynthesis, reflecting the structural diversity of phenolic compounds arising from oxidative coupling reactions [24]. No phenolic compounds were detected in the traditional beverage.
3.2. Antioxidant Activity
Several methods have been developed to assess the antioxidant activity of plant extracts, each providing a distinct perspective on their antioxidant potential. Consequently, the most comprehensive evaluation is achieved by combining two or more complementary methods [25]. In this study, the in vitro antioxidant activity of the extracts was evaluated using a DPPH radical scavenging assay and the ABTS method. The percentage inhibition of DPPH and ABTS radicals across concentrations ranging from 500 to 10,000 mg/L are shown in Figure 3 and Figure 4, respectively. The greatest inhibition was observed at the maximum concentration tested (10,000 mg/L), indicating concentration-dependent radical scavenging activity.
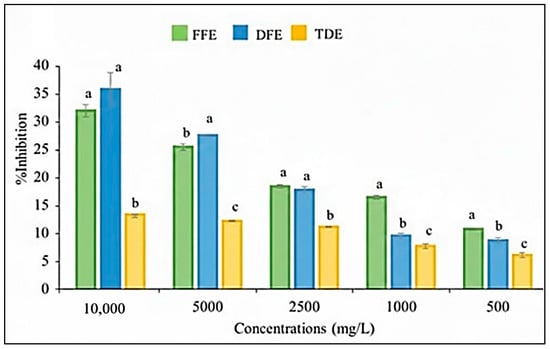
Figure 3.
Antioxidant activity (% inhibition) of the DPPH radical for fruit extracts and traditional fruit drink of Randia spp. at various concentrations. FFE = fresh fruit extract; DFE = dried fruit extract; TDE = traditional fruit drink extract. Different letters within the same concentration indicate significant differences (p < 0.05) according to Tukey’s test.
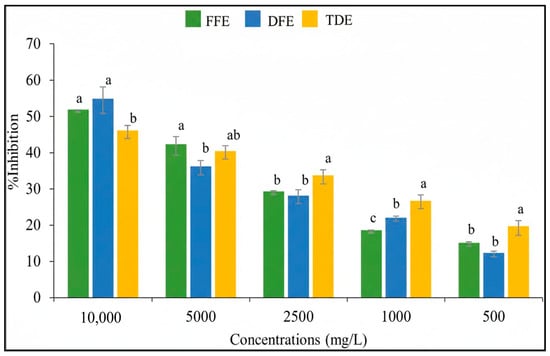
Figure 4.
Antioxidant activity (% inhibition) of the ABTS radical for fruit extracts and traditional fruit drink of Randia spp. at different concentrations. FFE = fresh fruit extract; DFE = dried fruit extract; TDE = traditional drink extract. Different letters within the same concentration indicate significant differences (p < 0.05) according to Tukey’s test.
No significant differences in antioxidant activity were observed between the fresh and dried fruit extracts with either method (Table 4), which correlates with the similar total polyphenol content observed in both extracts. These findings align with the observations of Martínez-Ceja et al. [26], who reported a strong relationship between phenolic compound concentration and antioxidant activity.

Table 4.
Antioxidant activity of fruit extracts of Randia spp.
The mean inhibitory concentration (IC50) was calculated to quantify the antioxidant capacity of the extracts. In the DPPH assay, the IC50 values were 18.29 ± 0.8 mg/mL for the fresh fruit extract and 14.22 ± 1.35 mg/mL for the dried fruit extract. For the ABTS assay, the IC50 values were 8.70 ± 0.14 mg/mL and 8.68 ± 0.62 mg/mL for the fresh and dried fruit extracts, respectively (Table 4). The IC50 values obtained for both the fresh and dried fruit extracts demonstrate a moderate radical-scavenging capacity against DPPH and ABTS radicals, thereby reflecting an intermediate antioxidant potential of the evaluated samples. No significant differences were detected between the IC50 values obtained for the two extracts using either method.
3.3. Antiproliferative Activity and Cytotoxicity
The assessment of cytotoxicity in medicinal plants has gained considerable importance, as it serves as an initial step in evaluating their safety and is also indicative of the biological activity of plant extracts or isolated compounds [27]. Table 5 presents the cytotoxic concentration (CC50) values of the extracts. Both the fresh and dried fruit extracts exhibited CC50 values greater than 100 µg/mL, indicating low cytotoxicity. In contrast, the CC50 value of the reference compound cisplatin, a chemotherapeutic agent, was 2.22 ± 0.21 µg/mL.

Table 5.
Cytotoxicity and antiproliferative activity of extracts from the fruit of Randia spp.
The antiproliferative activity of the extracts, evaluated using the Caco-2 cell line, is presented in Table 5. The dried fruit extract exhibited an aIC50 of 25.44 ± 0.16 µg/mL, which indicated that its activity was lower than that of the control (7.52 ± 0.41 µg/mL). With respect to the selectivity index, both the fresh and dried fruit extracts demonstrated greater selectivity than the traditional beverage and the control, with values of 1 and 3.92, respectively, whereas the control and beverage exhibited a selectivity index of 0.29.
4. Discussion
Most Randia species remain poorly characterized, with the earliest chemical studies dating back to the 1990 s and focusing primarily on Randia echinocarpa [28]. To date, qualitative phytochemical studies are still limited and incomplete for several species, with flavonoids and tannins being the most commonly reported metabolites. Cano-Campos and Ojeda-Ayala et al. [7,11] identified phenolic compounds, flavonoids, and saponins in Randia echinocarpa, Randia nitida, and Randia laevigata; these same classes of compounds were also detected in both fresh and dried fruit extracts in the present study. Such similarities may be related not only to the species but also to methodological aspects, including the extraction procedure and solvent system employed [29].
With respect to total polyphenol content (TPC), previous reports on the dried pulp of Randia monantha Bent reported values of 413 ± 0.61, 125 ± 0.63, and 57 ± 0.70 mg GAE/g for aqueous, methanolic, and ethanolic extracts, respectively [10]. For the dried seeds of the same species, TPC values of 276 ± 0.36, 146 ± 1.76, and 268 ± 0.27 mg GAE/g were reported for aqueous, methanolic, and ethanolic extracts, respectively [10]. Martínez-Ceja et al. [26] reported a TPC of 30.65 ± 0.00 mg GAE/g in methanolic extracts of Randia aculeata leaves. In comparison, the values obtained in the present work were lower than those reported for the seeds and dried pulp of R. monantha but higher than those reported for the methanolic extract of R. aculeata leaves.
Notably, in contrast to most previous studies in which pulp and seeds were analyzed separately, the whole fruit was evaluated in the present study. This difference likely explains the variation in TPC values, as polyphenolic compounds are differentially distributed across plant tissues [30]. Such distributional differences highlight the importance of considering the plant part analyzed and the extraction strategy employed when comparing phytochemical profiles across Randia species.
The phytochemical composition observed in this study is consistent with the biological activities obtained. The comparable antioxidant activities of fresh and dried fruit extracts, measured by DPPH and ABTS assays, correlate with the similar polyphenol contents observed between the two treatments. Moreover, the presence of bioactive compounds such as phenolic acids and flavonoids may explain the low cytotoxicity and selective antiproliferative effects detected in the Caco-2 cell line. These findings reinforce the idea that phenolic composition not only varies among Randia species and plant parts but also plays a critical role in defining their functional and therapeutic potential.
Flavonoids are a major subclass of polyphenolic compounds widely distributed in plants and are known for their diverse biological activities. Qualitative phytochemical studies in Randia species have consistently reported the presence of flavonoids, with seeds being particularly rich in these metabolites [11]. In the present study, flavonoids were quantitatively assessed, and the flavonoid content of the dried fruit extract was greater than that of the fresh fruit extract. This increase may be attributed to the mechanical processes involved in powder production (grinding and sieving), which likely promoted the rupture of cellular vacuoles and cell walls, thereby increasing the release and extraction efficiency of flavonoids [22]. These findings highlight the influence of postharvest processing on the phytochemical yield of plant materials.
Despite the growing interest in Randia species, few studies have systematically identified and quantified individual phenolic compounds in Randia fruits. Notably, Juárez-Trujillo et al. [10] conducted the first comprehensive profiling of phenolic compounds in the pulp and seed extracts of Randia monantha using aqueous, methanolic, and ethanolic solvents. They identified 10 compounds in the pulp and 13 in the seeds, most of which were classified as phenolic acids. Compared with the pulp, the seed extracts consistently exhibited greater diversity and concentrations of individual phenolics.
In their study, chlorogenic acid was the predominant compound in the aqueous seed extract (81.11 ± 1.94 μg/g), followed by rutin (51.61 ± 3.12 μg/g), 4-coumaric acid (30.29 ± 0.06 μg/g), and caffeic acid (21.95 ± 0.25 μg/g). The other compounds detected at relatively low concentrations across all the seed extracts included ferulic acid, kaempferol, vanillic acid, quercetin, (–)-epicatechin, 4-hydroxybenzoic acid, vanillin, 2,4-dimethoxy-6-methylbenzoic acid, and scopoletin. In contrast, the pulp extracts contained lower concentrations of phenolics overall. Chlorogenic acid was also the major compound in the pulp, with concentrations of 39.81, 20.74, and 13.01 μg/g in the ethanolic, aqueous, and methanolic extracts, respectively. Vanillic acid was consistently present in all pulp extracts, with the highest concentration in the aqueous extract (9.83 ± 0.28 μg/g), followed by the methanolic (4.87 ± 0.48 μg/g) and ethanolic (2.17 ± 0.22 μg/g) extracts. Caffeic acid was also detected at low concentrations in the ethanolic pulp extract (3.11 ± 0.09 μg/g).
Several of these phenolic acids—including chlorogenic acid, vanillic acid, and caffeic acid—were also detected and quantified in the fresh and dried fruit extracts of Randia spp. analyzed in the present work. The overlap in compound profiles between our results and those of previous studies supports the reproducibility of the phytochemical composition across different Randia species and extraction methods. However, differences in compound abundance are likely influenced by several factors, including the plant organ analyzed (whole fruit versus separated pulp or seed), the solvent polarity, and the extraction conditions employed.
These findings contribute to the limited but growing body of knowledge on the phytochemistry of Randia fruits. By combining qualitative and quantitative data, the present study underscores the relevance of both processing techniques and analytical methods in shaping the phytochemical profile, particularly with respect to flavonoid and phenolic acid contents. Such insights are essential for understanding the bioactive potential of Randia species and for guiding future research aimed at their pharmacological and nutraceutical applications.
A comparison of the results obtained in this study with those of previous reports highlights important differences in the phenolic profiles of Randia spp. [10]. The main compound identified in both the fresh and dried fruit extracts was kaempferol, whereas Juarez Trujillo et al. [10] reported low concentrations of kaempferol in the seed extracts of Randia monantha Benth (1.14 ± 0.01), and kaempferol was not detected in the pulp. A key distinction is that the whole fruit (pulp and seeds) was evaluated in the present study, which likely accounts for part of the observed variation.
The chemical composition of plant extracts is strongly influenced by the solubility of metabolites in the solvent system employed. In this study, 70% ethanol was selected, as it is well recognized for its efficiency in extracting flavonoids and phenolic acids, although its selectivity toward different classes of polyphenols can vary. This factor, together with the use of whole fruit, may explain the differences between the extracts analyzed here and those previously reported [30,31]. In addition to solvent choice, phytochemical composition can also be affected by other variables, including the extraction method, analytical methods, environmental conditions, and geographical location of plant growth [12].
All compounds identified in the fresh and dried fruit extracts of Randia spp. contain conjugated double bonds in their chemical structures, which enable electronic delocalization. This structural feature is closely associated with the antioxidant and anti-inflammatory properties of polyphenols described in various plants [32] and may therefore contribute to the biological activities observed in the present study.
In contrast, no compounds were detected in the traditional beverage extract. The absence of caffeic and hydroxybenzoic acids in the dried fruit extracts may be attributed to degradation during the drying process. Thermal treatment, exposure to oxygen, or enzymatic activity can lead to the partial or complete breakdown of certain phenolic compounds. This phenomenon has been reported in similar studies in which drying or heat processing reduced the concentration of heat-sensitive phenolics.
Vanillic acid, identified in the dried fruit extract of Randia spp., is a phenolic compound previously reported to possess anti-snakebite properties [33]. Phenolic compounds are also recognized for their anti-inflammatory potential in various pathologies, which may explain their traditional use in treating animal bites. Another key compound detected in the extracts was kaempferol, a flavonoid widely known for its antioxidant, anti-inflammatory, and neuroprotective activities. Kaempferol contributes to cellular protection by neutralizing reactive oxygen species and reducing oxidative stress [34]. The high content of this compound in both fresh and dried fruit extracts highlights Randia spp. as a rich source of bioactive secondary metabolites with potential applications in the pharmaceutical and food industries [30].
Furthermore, the strong correlation between total polyphenol content and antioxidant activity, which was consistently demonstrated in this and other studies [35,36], reinforces the role of phenolic compounds as major contributors to the biological activity of Randia spp. extracts.
Although studies have reported the antioxidant activity of the pulp, seeds, and leaves of Randia species [18], no previous evaluations have been conducted specifically on whole fruits. In the present study, antioxidant activity was assessed by determining the percentage of inhibition of DPPH and ABTS radicals, as well as the IC50 values for both assays. The activity observed in the fresh and dried fruit extracts can be largely attributed to kaempferol, which, as noted above, exhibits antioxidant, anti-inflammatory, and neuroprotective properties by effectively neutralizing reactive oxygen species and protecting cells against oxidative stress [34].
Notably, compared with the DPPH assay, the ABTS assay resulted in higher inhibition percentages. This difference is consistent with the broader applicability of the ABTS method, which is capable of evaluating both hydrophilic and lipophilic antioxidant systems, whereas the DPPH assay is more restricted to hydrophobic environments [37].
The evaluation of cytotoxicity has become increasingly important as an initial step in assessing the safety of medicinal plants since it provides valuable insights into the biological activity of plant extracts and isolated compounds [27]. In this context, previous studies on Randia ferox leaf extracts demonstrated that peripheral blood mononuclear cells treated for 24 h maintained normal cell viability. Moreover, all tested concentrations reduced intracellular reactive oxygen species (ROS) levels without affecting nitric oxide (NO) production, and most did not alter double-stranded DNA (dsDNA) release [38].
In the present study, a ROS analysis was not performed, such as a hydrogen peroxide uptake reducing power assay; however, according to the literature, the antioxidant activity of phenolic compounds can prevent cellular damage in normal cells and cancer cells and reduce ROS levels by altering protumorigenic signaling and, consequently, cell proliferation and tumor progression [39]. However, the cytotoxicity of hydroalcoholic extracts from fresh and dried fruit, as well as from the traditional beverage prepared from Randia spp., was evaluated in the J774.2 cell line. The extracts exhibited low cytotoxicity compared with that of cisplatin, suggesting a favorable safety profile. These findings highlight the potential of Randia spp. fruit as a safe source of bioactive compounds for therapeutic applications, and to support its traditional use in the treatment of various diseases and symptoms.
Species of the genus Randia have been shown to have diverse biological activities; however, only their antioxidant, anti-inflammatory, and antimicrobial properties have been experimentally validated [11]. In the present study, hydroalcoholic extracts of dried Randia spp. fruit exhibited an IC50 of 25.44 ± 0.016 µg/mL against a colon adenocarcinoma cell line (CaCo-2) according to the results of the MTT assay. These findings suggest a potential antiproliferative effect, which may be primarily attributed to kaempferol, a flavonoid identified in the extracts. Previous studies have demonstrated that flavonoids possess anticancer activity but generally exhibit lower toxicity than do conventional chemotherapeutic agents [40,41]. Previous studies have shown that kaempferol is a potent promoter of apoptosis and regulates a variety of signaling pathways within cells. Furthermore, kaempferol inhibits cancer cell proliferation by interrupting the cell cycle at checkpoints [42]. Kaempferol promotes apoptosis by regulating caspases, Bik, PARP, and Bcl-xL and inhibits cancer cell proliferation by reducing the expression of Ki67 and LGR5 [43]. However, in this work, the complete extract was used; that is, no fractions were made nor was a particular compound isolated. Thus, it could be not only kaempferol but also the synergy of other phenolic compounds (flavonoids, phenolic acids) that exhibited antiproliferative effects.
Furthermore, comparison of the selectivity index (SI) of the fresh and dried Randia spp. fruit extracts with that of cisplatin, a standard drug used in cancer therapy, revealed that the extracts displayed higher SI values. This is a desirable characteristic, as a higher SI indicates greater safety and efficacy. Ideally, a compound should demonstrate minimal cytotoxicity at high concentrations while maintaining biological activity at low concentrations, thus yielding a high SI value [44]. These results highlight the potential of Randia spp. fruit extracts as promising candidates for the development of safer, plant-derived anticancer agents.
5. Conclusions
In this study, the phytochemical composition, antioxidant and antiproliferative activities, and cytotoxicity of hydroalcoholic extracts from fresh and dried Randia spp. fruits were evaluated. Qualitative analysis confirmed the presence of saponins, phenols, and flavonoids. The dried fruit extract resulted in higher levels of total polyphenols and flavonoids, likely because of concentration effects during drying when a refractive window was used. HPLC analysis revealed that the fresh fruit extract contained a greater diversity of phenolic compounds, while kaempferol was the predominant compound in both extracts, contributing significantly to their antioxidant and antiproliferative activities. Both extracts demonstrated low cytotoxicity and high selectivity indices, supporting their potential safety and therapeutic value in the prevention or management of chronic degenerative diseases.
It is suggested to continue characterizing the chemical compounds of the fruit using highly specific equipment such as ultrahigh-resolution liquid chromatographs coupled with a quadrupole time-of-flight mass spectrometer (UPLC-MS-q-TOF). In addition, extract fractionation techniques should be applied for chemical and biological characterization and decreasing polarity extraction methods should be implemented to expand phytochemical screening.
Author Contributions
Conceptualization, V.M.F.-L.; methodology, V.M.F.-L., L.L.M.-L., A.M.-R. and I.G.-M.; software, E.H.-L.; validation, A.M.-R. and L.L.M.-L.; formal analysis, C.E.M.-S., E.H.-L. and I.G.-M.; investigation, V.M.F.-L., C.E.M.-S. and I.G.-M.; resources, C.E.M.-S. and A.M.-R.; data curation, methodology, writing—original draft preparation, V.M.F.-L. and I.G.-M.; writing—review and editing, C.E.M.-S., A.M.-R., L.A.-F. and I.G.-M.; visualization, E.H.-L.; supervision, L.L.M.-L. and L.A.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretariat of Science, Technology, Humanities and Innovation of Mexico (SECIHTI) through grant 832851 and the National Technological Institute of Mexico Campus Tuxtepec for infrastructure support.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gómez, X.; Sanon, S.; Zambrano, K.; Asquel, S.; Bassantes, M.; Morales, J.E.; Otáñez, G.; Pomaquero, C.; Villarroel, S.; Zurita, A.; et al. Key points for the development of antioxidant cocktails to prevent cellular stress and damage caused by reactive oxygen species (ROS) during manned space missions. NPJ Microgravity 2021, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Jiang, F.; Ding, Y.; Tian, Y.; Yang, R.; Quan, M.; Tong, Z.; Zhang, X.; Luo, D.; Chi, Z.; Liu, C. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomater. Adv. 2022, 133, 112637. [Google Scholar] [CrossRef]
- Manikandan, R.; Anjali, R.; Beulaja, M.; Prabhu, N.M.; Koodalingam, A.; Saiprasad, G.; Chitra, P.; Arumugam, M. Synthesis, characterization, anti-proliferative and wound healing activities of silver nanoparticles synthesized from Caulerpa scalpelliformis. Process Biochem. 2019, 79, 135–141. [Google Scholar] [CrossRef]
- Said Nasser Al-Owamri, F.; Saleh Abdullah Al Sibay, L.; Hemadri Reddy, S.; Althaf Hussain, S.; Gangireddygari, V.S.R. Phytochemical, Antioxidant, hair growth and wound healing property of Juniperus excelsa, Olea oleaster and Olea europaea. J. King Saud Univ. Sci. 2023, 35, 102446. [Google Scholar] [CrossRef]
- Hang, C.; Sun, H.; Zhang, A.; Yan, G.; Lu, S.; Wang, X. Recent Developments in the Field of Antioxidant Activity on Natural Products. Aмypcкий Meдицинcкий Жypнaл 2015, 2, 44–48. [Google Scholar]
- Cano-Campos, M.C.; Díaz-Camacho, S.P.; Uribe-Beltran, M.J.; López-Angulo, G.; Montes-Avila, J.; Paredes-López, O.; Delgado-Vargas, F. Bio-guided fractionation of the antimutagenic activity of methanolic extract from the fruit of Randia echinocarpa (Sessé et Mociño) against 1-nitropyrene. Food Res. Int. 2011, 44, 3087–3093. [Google Scholar] [CrossRef]
- Stevens, W.D.; Ulloa, C.; Pool, A.; Montiel, O.M. Flora de Nicaragua, 1st ed.; Missouri Botanical Garden Press: St. Louis, Russia, 2001; p. 943. [Google Scholar]
- Gallardo-Casas, C.A.; Guevara-Balcázar, G.; Morales-Ramos, E.; Tadeo-Jiménez, Y.; Gutiérrez-Flores, O.; Jiménez-Sánchez, N.; Valadez-Omaña, M.T.; Valenzuela-Vargas, M.T.; Castillo-Hernández, M.C. Ethnobotanic study of Randia aculeata (Rubiaceae) in Jamapa, Veracruz, Mexico, and its anti-snake venom effects on mouse tissue. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 287–294. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Alvarado-Olivarez, M.; Luna-Solano, G.; Guerrero-Analco, J.A.; Jiménez-Fernández, M. Phenolic profile and antioxidative properties of pulp and seeds of Randia monantha Benth. Ind. Crops Prod. 2018, 124, 53–58. [Google Scholar] [CrossRef]
- Ojeda-Ayala, M.; Gaxiola-Camacho, S.M.; Delgado-Vargas, F. Phytochemical composition and biological activities of the plants of the genus Randia. Bot. Sci. 2024, 100, 779–796. [Google Scholar] [CrossRef]
- Mërtiri, I.; Coman, G.; Cotârlet, M.; Turturică, M.; Balan, N.; Râpeanu, G.; Stanciuc, N.; Mihalcea, L. Phytochemical Profile Screening and Selected Bioactivity of Myrtus communis Berries Extracts Obtained from Ultrasound-Assisted and Supercritical Fluid Extraction. Separations 2025, 12, 8. [Google Scholar] [CrossRef]
- Capataz-Tafur, J.; Orozco-Sánchez, F.; Vergara-Ruiz, R.; Hoyos-Sánchez, R. Efecto antialimentario de los extractos de suspensiones celulares de Azadirachta indica sobre Spodoptera frugiperda JE Smith en condiciones de laboratorio. Rev. Fac. Nac. Agron. Medellín 2007, 60, 3703–3715. [Google Scholar]
- Bulugahapitiya, V.P.; Rathnaweera, T.N.; Manawadu, H.C. Phytochemical composition and antioxidant properties of Dialium ovoideum thwaites (Gal Siyambala) leaves. Int. J. Min. Fruits, Med. and Arom. Plants 2020, 6, 13–19. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Méndez-Lagunas, L.L.; Cruz-Gracida, M.; Barriada-Bernal, L.G.; Rodríguez-Méndez, L.I. Profile of phenolic acids, antioxidant activity and total phenolic compounds during blue corn tortilla processing and its bioaccessibility. J. Food Sci. Technol. 2020, 57, 4688–4696. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, L.D.; Moreno-Rodríguez, A.; Vázquez-Jiménez, L.K.; Delgado-Maldonado, T.; Juárez-Saldivar, A.; Ortiz-Pérez, E.; Rivera, G. Triose Phosphate Isomerase Structure-based virtual screening and in vitro biological activity of natural products as Leishmania Mexicana inhibitors. Pharmaceutics 2023, 15, 2046. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Mancera-Rodríguez, M.A.; Delgado-Buenrostro, N.L.; Moreno-Rodríguez, A.; Bautista-Martínez, J.L.; Díaz-Velásquez, C.E.; Martínez-Alarcón, S.A.; Torrens, H.; Godínez-Rodríguez, M.A.; Terrazas-Valdés, L.I.; et al. Novel thiosemicarbazones induce high toxicity in estrogen receptor-positive breast cancer cells (MCF7) and exacerbate cisplatin effectiveness in triple-negative breast (MDA-MB231) and lung adenocarcinoma (A549) cells. Investig. New Drugs 2019, 38, 558–573. [Google Scholar] [CrossRef]
- Chaparro-Hernández, I.; Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Sandoval-Torres, S.; Aquino-González, L.; Barriada-Bernal, G. Spray Drying of Stevia Rebaudiana Bertoni Aqueous Extract: Effect on Polyphenolic Compounds. Chem. Eng. Trans. 2023, 98, 33–38. [Google Scholar]
- Sepúlveda, C.T.; Zapata, J.E. Efecto de la Temperatura, el pH y el Contenido en Sólidos sobre los Compuestos Fenólicos y la Actividad Antioxidante del Extracto de Bixa orellana L. Inf. Technol. 2019, 30, 57–66. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Ozer, M.S.; Kirkanb, B.; Sarikurkcuc, C.; Cengizd, M.; Ceylane, O.; Atılgand, N.; Tepef, B. Onosma heterophyllum: Phenolic composition, enzyme inhibitory and antioxidant activities. Ind. Crops Prod. 2018, 111, 179–184. [Google Scholar] [CrossRef]
- Martínez-Ceja, A.; Romero-Estrada, A.; Columba-Palomares, M.C.; Hurtado-Díaz, I.; Alvarez, L.; Teta-Talixtacta, R.; Bernabé-Antonio, A. Anti-inflammatory, antibacterial and antioxidant activity of leaf and cell cultures extracts of Randia aculeata L. and its chemical components by GC-MS. S. Afr. J. Bot. 2022, 144, 206–218. [Google Scholar] [CrossRef]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Cytotoxicity of African medicinal plants against normal animal and human cells. In Toxicological Survey of African Medicinal Plants, 1st ed.; Kuete, V., Ed.; Elsevier: London, UK, 2014; pp. 181–233. [Google Scholar]
- Bye, R.; Linares, E.; Mata, R.; Albor, C.; Casteñeda, P.C.; Delgado, G. Ethnobotanical and phytochemical investigation of Randia echinocarpa (Rubiaceae). An. Inst. Biol. Ser. Bot. 1991, 62, 87–106. [Google Scholar]
- Charoensin, S. Antioxidant and anticancer activities of Moringa oleifera leaves. J. Med. Plants Res. 2014, 8, 318–325. [Google Scholar] [CrossRef]
- Sahlabgi, A.; Lupuliasa, D.; Stoicescu, I.; Vlaia, L.L.; Licu, M.; Popescu, A.; Mititelu, M. Determination of the Phytochemical Profile and Antioxidant Activity of Some Alcoholic Extracts of Levisticum officinale with Pharmaceutical and Cosmetic Applications. Separations 2025, 12, 79. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Oracz, J. Effect of addition of green coffee extract and nanoencapsulated chlorogenic acids on aroma of different food products. LWT 2016, 73, 197–204. [Google Scholar] [CrossRef]
- Zhuang, X.; Shi, W.; Shen, T.; Cheng, X.; Wan, Q.; Fan, M.; Hu, D. Research Updates and Advances on Flavonoids Derived from Dandelion and Their Antioxidant Activities. Antioxidants 2024, 13, 1449. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Tapia-Hernández, F.E.; Alvarado-Olivarez, M.; Beristain-Guevara, C.I.; Pascual-Pineda, L.A.; Jiménez-Fernández, M. Antibacterial activity and acute toxicity study of standardized aqueous extract of Randia monantha Benth fruit. Biotecnia 2022, 24, 38–45. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Pappis, L.; Prates-Ramos, A.; Fontana, T.; Geraldo-Sangoi, G.; Castro-Dornelles, R.; Bolssoni-Dolwitsch, C.; Rorato-Sagrillo, M.; Cadoná, F.C.; Kolinski-Machado, A.; Bauermann, L.D.F. Randia ferox (Cham & Schltdl) DC: Phytochemical composition, in vitro cyto-and genotoxicity analyses. Nat. Prod. Res. 2021, 36, 4170–4176. [Google Scholar]
- Do Carmo, M.A.V.; Granato, D.; Azevedo, L. Antioxidant/pro-oxidant and antiproliferative activities of phenolic-rich foods and extracts: A cell-based point of view. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2021; Volume 98, pp. 253–280. [Google Scholar]
- Saklani, A.; Kutty, S.K. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 1, 721402. [Google Scholar] [CrossRef] [PubMed]
- Archoo, S.; Naikoo, S.H.; Tasduq, S.A. Role of herbal products as therapeutic agents against ultraviolet radiation-induced skin disorders. In Herbal Medicines; Academic Press: Cambridge, MA, USA, 2022; pp. 345–360. [Google Scholar]
- Kaur, S.; Mendonca, P.; Soliman, K.F. The anticancer effects and therapeutic potential of kaempferol in triple-negative breast cancer. Nutrients 2024, 16, 2392. [Google Scholar] [CrossRef] [PubMed]
- Subramani, C.; Sharma, G.; Chaira, T.; Barman, T.K. High content screening strategies for large-scale compound libraries with a focus on high-containment viruses. Antivir. Res. 2024, 221, 105764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).