Phenolic Compounds in Plant-Based Milk Alternatives from the Greek Market
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Stock and Working Solutions
2.3. Instrumentation
2.4. Sample Preparation
2.5. Sampling
2.6. Statistical Analysis
3. Results
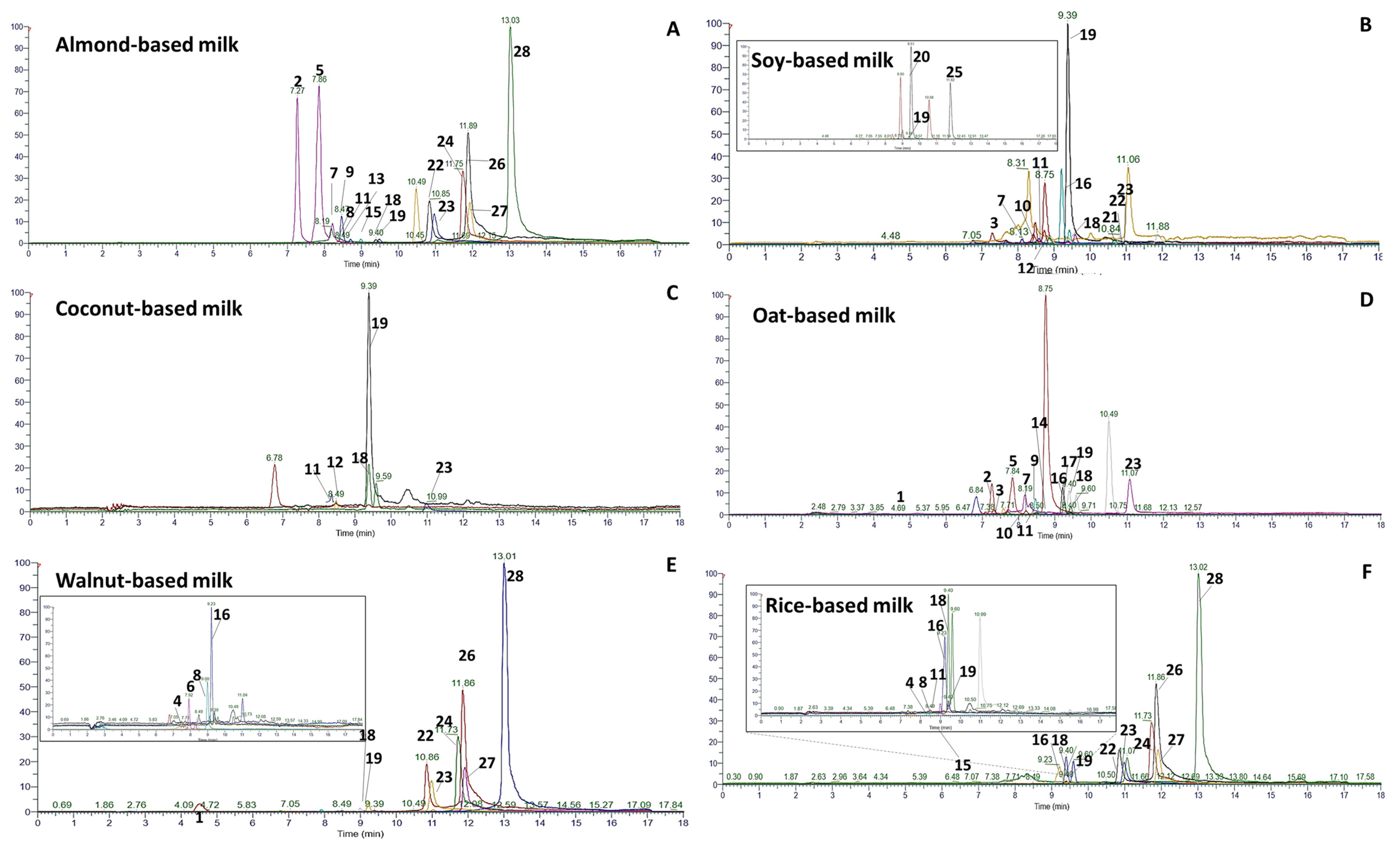
3.1. PBMAs’ Phenolic Content
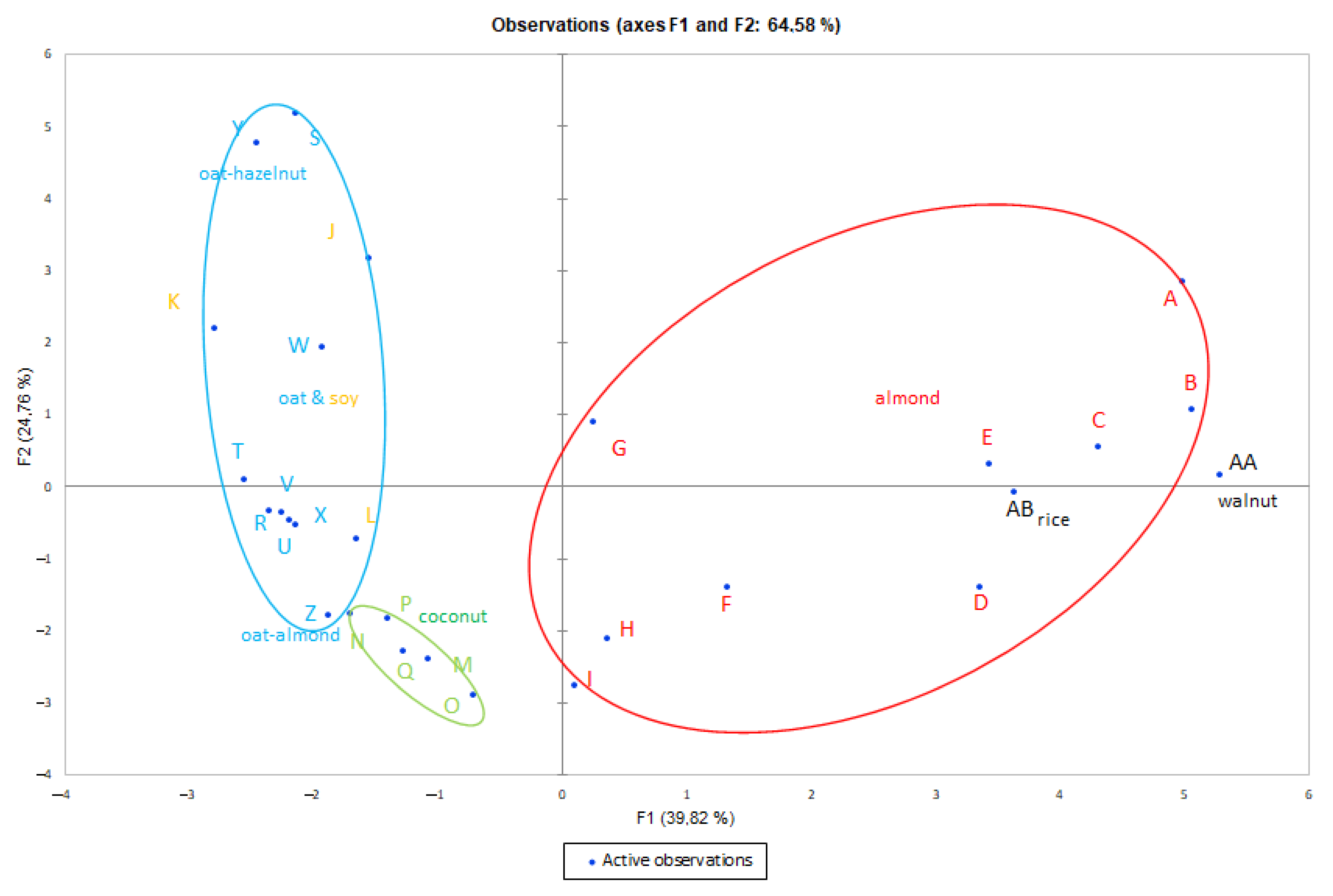
3.2. Principal Component Analysis (PCA)
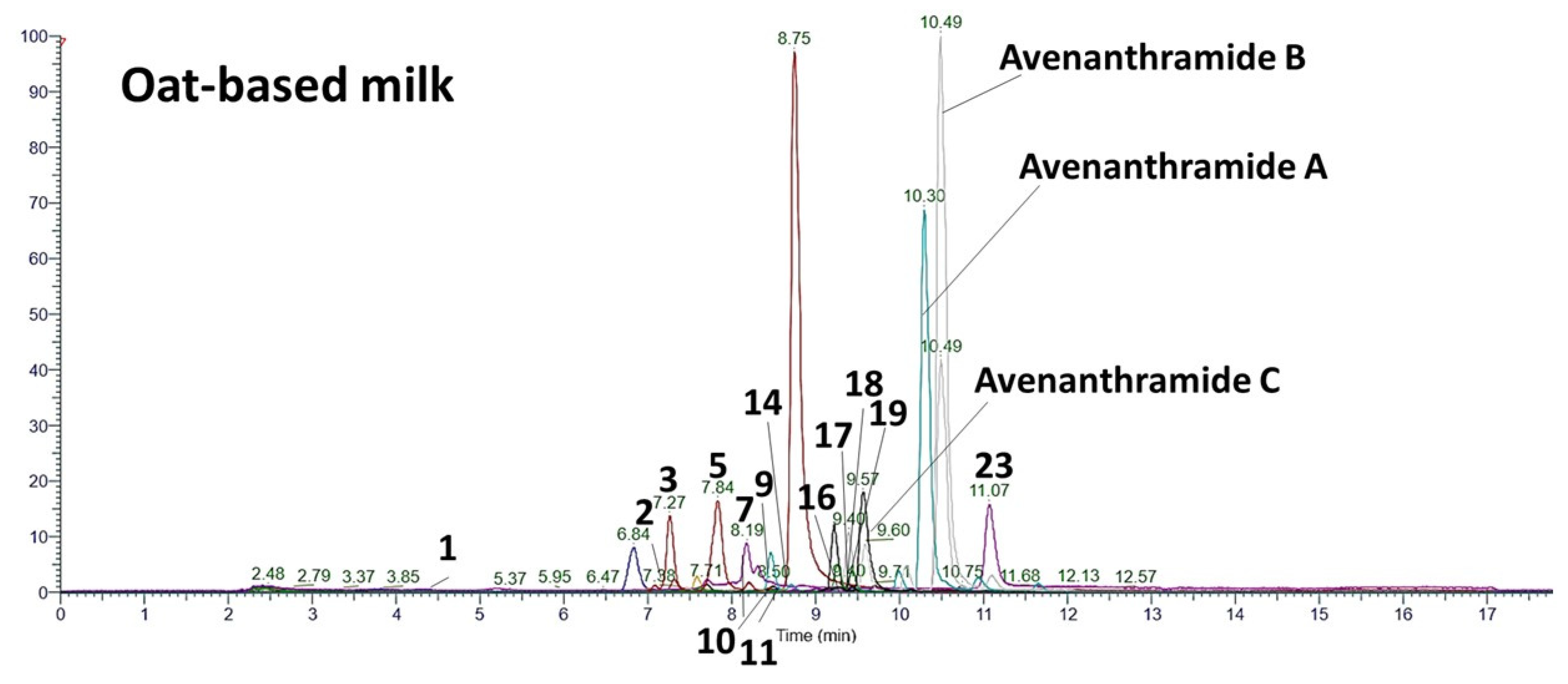
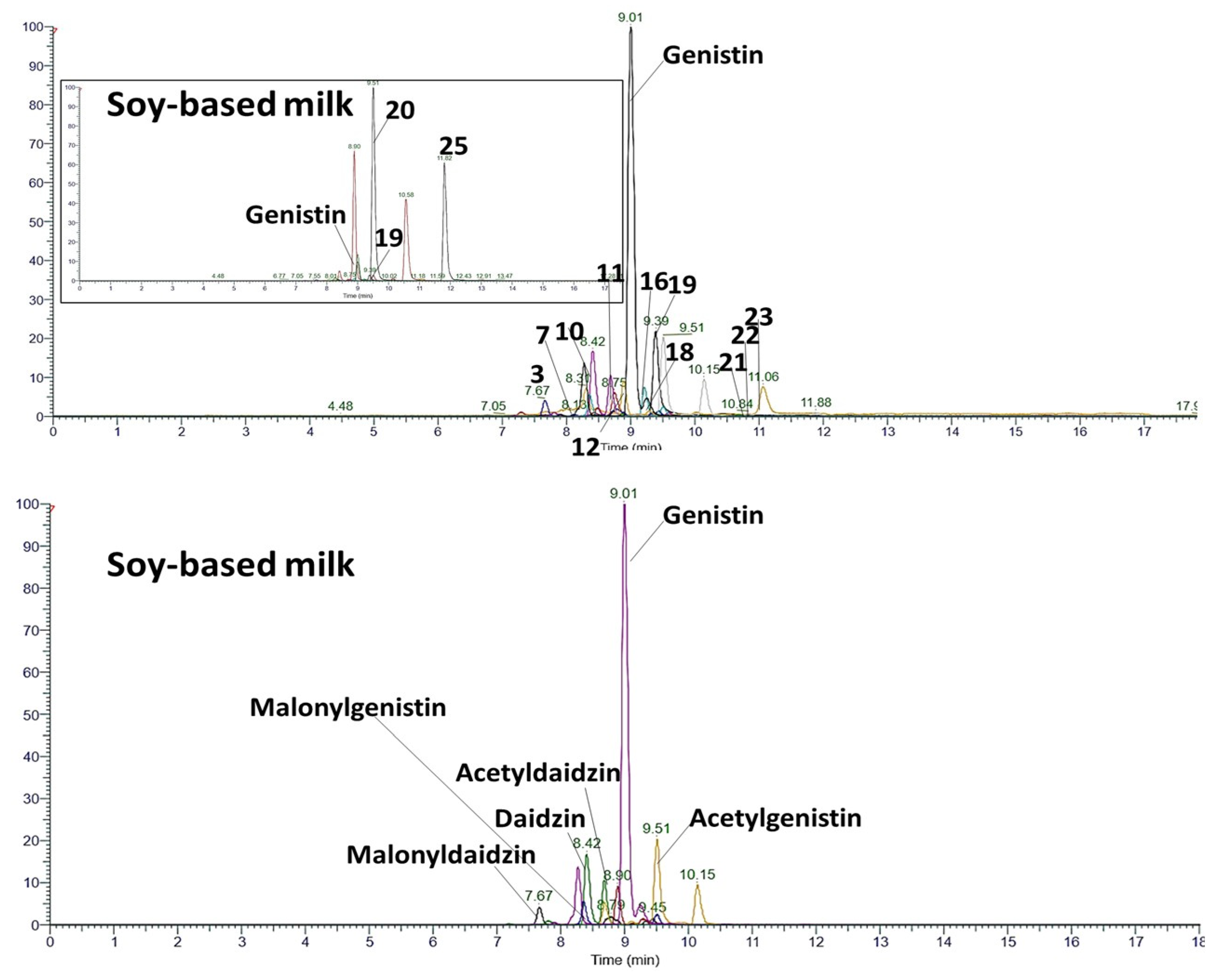
3.3. Suspect Screening of Phenolic Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fact. Mr Plant-Based Dairy Market Outlook (2024 to 2034). Available online: https://www.factmr.com/report/4963/plantbased-dairy-market (accessed on 5 August 2025).
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health Issues and Technological Aspects of Plant-Based Alternative Milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant Based Alternatives to Conventional Milk, Production, Potential and Health Concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- FDA Plant-Based Milk and Animal Food Alternatives. Available online: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/plant-based-milk-and-animal-food-alternatives (accessed on 15 July 2025).
- Grossmann, L.; Kinchla, A.J.; Nolden, A.; McClements, D.J. Standardized Methods for Testing the Quality Attributes of Plant-based Foods: Milk and Cream Alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2206–2233. [Google Scholar] [CrossRef]
- Bello, V.; Bodo, E.; Merlo, S. Speckle Pattern Acquisition and Statistical Processing for Analysis of Turbid Liquids. IEEE Trans. Instrum. Meas. 2023, 72, 7005004. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Aadil, R.M.; Rahaman, A.; Ahmed, Z.; Rehman, A.; Siddeeg, A.; Zeng, X.; Manzoor, A. Impact of Pulsed Electric Field on Rheological, Structural, and Physicochemical Properties of Almond Milk. J. Food Process Eng. 2019, 42, e13299. [Google Scholar] [CrossRef]
- Dhakal, S.; Giusti, M.M.; Balasubramaniam, V. Effect of High Pressure Processing on Dispersive and Aggregative Properties of Almond Milk. J. Sci. Food Agric. 2016, 96, 3821–3830. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Uniacke-Lowe, T.; O’Mahony, J.A.; Arendt, E.K. Physicochemical and Acid Gelation Properties of Commercial UHT-Treated Plant-Based Milk Substitutes and Lactose Free Bovine Milk. Food Chem. 2015, 168, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Kuchel, R.P.; Chandrawati, R. A Polydiacetylene-Based Colorimetric Sensor as an Active Use-By Date for Plant-Based Milk Alternatives. Macromol. Rapid Commun. 2020, 41, 2000172. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, S.; Wyrwa, J.; Ciepiela, F.; Jakubowska, M. The Application of Picein Wax Carbon Composite Electrode for Plant-Based Milk Profiling. Chemosensors 2023, 11, 513. [Google Scholar] [CrossRef]
- Achouri, A.; Boye, J.; Zamani, Y. Identification of Volatile Compounds in Soymilk Using Solid-Phase Microextraction-Gas Chromatography. Food Chem. 2006, 99, 759–766. [Google Scholar] [CrossRef]
- Kasapidou, E.; Basdagianni, Z.; Papatzimos, G.; Papadopoulos, V.; Tsiftsi, E.; Neki, I.; Nigianni, P.-A.; Mitlianga, P. Chemical Composition, Antioxidant Profile and Physicochemical Properties of Commercial Non-Cocoa- and Cocoa-Flavoured Plant-Based Milk Alternatives. Eur. Food Res. Technol. 2023, 249, 3011–3026. [Google Scholar] [CrossRef]
- Moore, S.S.; Costa, A.; Pozza, M.; Vamerali, T.; Niero, G.; Censi, S.; De Marchi, M. How Animal Milk and Plant-Based Alternatives Diverge in Terms of Fatty Acid, Amino Acid, and Mineral Composition. npj Sci. Food 2023, 7, 50. [Google Scholar] [CrossRef]
- Grainger, E.M.; Jiang, K.; Webb, M.Z.; Kennedy, A.J.; Chitchumroonchokchai, C.; Riedl, K.M.; Manubolu, M.; Clinton, S.K. Bioactive (Poly)Phenol Concentrations in Plant-Based Milk Alternatives in the US Market. J. Agric. Food Chem. 2024, 72, 18638–18648. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.L.; Serrano-Carretero, A.; García-Herrera, P.; Cámara-Hurtado, M.; Sánchez-Mata, M.C. Plant-Based Beverages as Milk Alternatives? Nutritional and Functional Approach through Food Labelling. Food Res. Int. 2023, 173, 113244. [Google Scholar] [CrossRef]
- Craig, W.J.; Fresán, U. International Analysis of the Nutritional Content and a Review of Health Benefits of Non-Dairy Plant-Based Beverages. Nutrients 2021, 13, 842. [Google Scholar] [CrossRef]
- Antunes, I.C.; Bexiga, R.; Pinto, C.; Roseiro, L.C.; Quaresma, M.A.G. Cow’s Milk in Human Nutrition and the Emergence of Plant-Based Milk Alternatives. Foods 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Yeasmen, N.; Dubé, L.; Orsat, V. A Review on Current Scenario and Key Challenges of Plant-Based Functional Beverages. Food Biosci. 2024, 60, 104320. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-Based Milk Substitutes: Bioactive Compounds, Conventional and Novel Processes, Bioavailability Studies, and Health Effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Marafon, K.; Prestes, A.A.; Carvalho, A.C.; De Souza, C.K.; Prudencio, E.S. Bioactive Compounds’ Importance in Plant-Based Beverages: A Review. Curr. Opin. Food Sci. 2025, 63, 101304. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Isoflavones, Flavan-3-Ols, Phenolic Acids, Total Phenolic Profiles, and Antioxidant Capacities of Soy Milk As Affected by Ultrahigh-Temperature and Traditional Processing Methods. J. Agric. Food Chem. 2009, 57, 4706–4717. [Google Scholar] [CrossRef]
- Yu, X.; Meenu, M.; Xu, B.; Yu, H. Impact of Processing Technologies on Isoflavones, Phenolic Acids, and Antioxidant Capacities of Soymilk Prepared from 15 Soybean Varieties. Food Chem. 2021, 345, 128612. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk Phenolic Compounds, Isoflavones and Antioxidant Activity as Affected by in Vitro Gastrointestinal Digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Messina, M.; Barnes, S.; Setchell, K.D. Perspective: Isoflavones—Intriguing Molecules but Much Remains to Be Learned about These Soybean Constituents. Adv. Nutr. 2025, 16, 100418. [Google Scholar] [CrossRef] [PubMed]
- Gerogianni, V.-E.; Panagopoulou, E.A.; Vasilakopoulou, P.B.; Karathanos, V.T.; Chiou, A. Free and Bound Polar Phenols in Corinthian Currants (Vitis vinifera L., Var. Apyrena). J. Food Compos. Anal. 2024, 125, 105789. [Google Scholar] [CrossRef]
- Gerogianni, V.-E.; Mantzourani, C.; Karathanos, V.T.; Kokotou, M.G.; Chiou, A. Suspect Phenolic Compound Profiling in Corinthian Currants (Vitis vinifera L., Var. Apyrena). Food Chem. 2025, 491, 145319. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Mantzourani, C.; Kokotos, G. Development of a Liquid Chromatography–High Resolution Mass Spectrometry Method for the Determination of Free Fatty Acids in Milk. Molecules 2020, 25, 1548. [Google Scholar] [CrossRef]
- Mantzourani, C.; Batsika, C.S.; Kokotou, M.G.; Kokotos, G. Free Fatty Acid Profiling of Greek Yogurt by Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS) Analysis. Food Res. Int. 2022, 160, 111751. [Google Scholar] [CrossRef]
- Moreno Gracia, B.; Laya Reig, D.; Rubio-Cabetas, M.J.; Sanz García, M.Á. Study of Phenolic Compounds and Antioxidant Capacity of Spanish Almonds. Foods 2021, 10, 2334. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.S.; Karathanos, V.T. Nutritional Evaluation and Health Promoting Activities of Nuts and Seeds Cultivated in Greece. Int. J. Food Sci. Nutr. 2013, 64, 757–767. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D.; Lante, A. Insights and Perspectives on Plant-Based Beverages. Plants 2023, 12, 3345. [Google Scholar] [CrossRef]
- Arivalagan, M.; Roy, T.K.; Yasmeen, A.M.; Pavithra, K.C.; Jwala, P.N.; Shivasankara, K.S.; Manikantan, M.R.; Hebbar, K.B.; Kanade, S.R. Extraction of Phenolic Compounds with Antioxidant Potential from Coconut (Cocos nucifera L.) Testa and Identification of Phenolic Acids and Flavonoids Using UPLC Coupled with TQD-MS/MS. LWT 2018, 92, 116–126. [Google Scholar] [CrossRef]
- Toledo-Merma, P.R.; Arias-Santé, M.F.; Rincón-Cervera, M.Á.; Porras, O.; Bridi, R.; Rhein, S.; Sánchez-Contreras, M.; Hernandez-Pino, P.; Tobar, N.; Puente-Díaz, L.; et al. Phenolic Fractions from Walnut Milk Residue: Antioxidant Activity and Cytotoxic Potential. Plants 2024, 13, 3473. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, L.; Li, X.; Liu, J.; Li, H.; Gong, L.; Zhang, J.; Wang, J.; Sun, B. The Progress of Nomenclature, Structure, Metabolism, and Bioactivities of Oat Novel Phytochemical: Avenanthramides. J. Agric. Food Chem. 2022, 70, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lin, M.; Xiao, G.; Liu, H.; Wang, F.; Liu, D.; Ma, L.; Wang, Q.; Li, Z. Phenolic Amides (Avenanthramides) in Oats—An Update Review. Bioengineered 2024, 15, 2305029. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Suo, D.; Guan, X.; Wang, S.; Xiao, Z.; Li, Y.; Liu, X.; Fan, X. Effect of Germination on the Avenanthramide Content of Oats and Their in Vitro Antisensitivity Activities. Molecules 2022, 27, 6167. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, W.J.C.; Van Dinteren, S.; Gruppen, H.; Vincken, J.-P. Mass Spectrometric Characterisation of Avenanthramides and Enhancing Their Production by Germination of Oat (Avena sativa). Food Chem. 2019, 277, 682–690. [Google Scholar] [CrossRef]
- Evans, R.L.; Bryant, D.J.; Voliotis, A.; Hu, D.; Wu, H.; Syafira, S.A.; Oghama, O.E.; McFiggans, G.; Hamilton, J.F.; Rickard, A.R. A Semi-Quantitative Approach to Nontarget Compositional Analysis of Complex Samples. Anal. Chem. 2024, 96, 18349–18358. [Google Scholar] [CrossRef]
- Malm, L.; Palm, E.; Souihi, A.; Plassmann, M.; Liigand, J.; Kruve, A. Guide to Semi-Quantitative Non-Targeted Screening Using LC/ESI/HRMS. Molecules 2021, 26, 3524. [Google Scholar] [CrossRef]
- Pabich, M.; Marciniak, B.; Kontek, R. Phenolic Compound Composition and Biological Activities of Fractionated Soybean Pod Extract. Appl. Sci. 2021, 11, 10233. [Google Scholar] [CrossRef]
- Moretto, L.; Tonolo, F.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Comparative Analysis of the Antioxidant Capacity and Lipid and Protein Oxidation of Soy and Oats Beverages. Food Prod. Process. Nutr. 2021, 3, 1. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Zhang, H.-S.; Zhao, X.-S.; Xue, H.-H.; Xue, J.; Sun, Y.-H. Composition, Distribution, and Antioxidant Activity of Phenolic Compounds in 18 Soybean Cultivars. J. AOAC Int. 2018, 101, 520–528. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Z.; Zeng, M.; He, Z.; Tao, G.; Qin, F.; Chen, J. A Novel Isoflavone Profiling Method Based on UPLC-PDA-ESI-MS. Food Chem. 2017, 219, 40–47. [Google Scholar] [CrossRef]
- Multari, S.; Pihlava, J.-M.; Ollennu-Chuasam, P.; Hietaniemi, V.; Yang, B.; Suomela, J.-P. Identification and Quantification of Avenanthramides and Free and Bound Phenolic Acids in Eight Cultivars of Husked Oat (Avena sativa L.) from Finland. J. Agric. Food Chem. 2018, 66, 2900–2908. [Google Scholar] [CrossRef]
- Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Da Silva, L.R.; Velasco, J.I.; Fakhouri, F.M. Use of Rice on the Development of Plant-Based Milk with Antioxidant Properties: From Raw Material to Residue. LWT 2023, 173, 114271. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.C.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Rev. Int. 2023, 39, 2320–2351. [Google Scholar] [CrossRef]
- McCarron, R.; Methven, L.; Grahl, S.; Elliott, R.; Lignou, S. Oat-Based Milk Alternatives: The Influence of Physical and Chemical Properties on the Sensory Profile. Front. Nutr. 2024, 11, 1345371. [Google Scholar] [CrossRef]
- McCarron, R.; Methven, L.; Ghawi, S.K.; Grahl, S.; Elliott, R.; Lignou, S. The Effects of Processing Steps on Avenanthramides, Avenacosides and β-Glucan Content during the Production of Oat-Based Milk Alternatives. Food Chem. Adv. 2025, 6, 100907. [Google Scholar] [CrossRef]
- Rocchetti, G.; Ghilardelli, F.; Mosconi, M.; Masoero, F.; Gallo, A. Occurrence of Polyphenols, Isoflavonoids, and Their Metabolites in Milk Samples from Different Cow Feeding Regimens. Dairy 2022, 3, 314–325. [Google Scholar] [CrossRef]
- Besle, J.M.; Viala, D.; Martin, B.; Pradel, P.; Meunier, B.; Berdagué, J.L.; Fraisse, D.; Lamaison, J.L.; Coulon, J.B. Ultraviolet-Absorbing Compounds in Milk Are Related to Forage Polyphenols. J. Dairy Sci. 2010, 93, 2846–2856. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Oliva, E.; Bennato, F.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Sergi, M.; Martino, G. Effect of Olive Leaves Feeding on Phenolic Composition and Lipolytic Volatile Profile in Goat Milk. J. Dairy Sci. 2021, 104, 8835–8845. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Oliva, E.; Franceschini, N.; Grotta, L.; Sergi, M.; Martino, G. Characterization of Phenolic Profile in Milk Obtained by Ewes Fed Grape Pomace: Reflection on Antioxidant and Anti-Inflammatory Status. Biomolecules 2023, 13, 1026. [Google Scholar] [CrossRef]
- Kalantar, M. The Importance of Flavonoids in Ruminant Nutrition. Arch. Anim. Husb. Dairy Sci. 2018, 504, 2. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Significance and Applications of Phenolic Compounds in the Production and Quality of Milk and Dairy Products: A Review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total Phenolic Compounds in Milk from Different Species. Design of an Extraction Technique for Quantification Using the Folin–Ciocalteu Method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef] [PubMed]
| Code | Brand | Ingredients Declared by the Producers | Nutritional Information Declared by the Producers per 100 mL |
|---|---|---|---|
| Almond milk | |||
| A | Almond brand 1 | Water, almond (3%), calcium, sea salt, emulsifier (sunflower lecithin), stabilizer (gellan gum), vitamins (B2, B12, D, E). | Fat: 1.7 g. Saturated fatty acids: 0.1 g. Carbohydrates: 3.0 g. Sugars: 2.0 g. Fibers: 0.4 g. Proteins: 0.7 g. Salt: 0.10 g. |
| B | Almond brand 2 | Water, almond (3%), dietary fiber, stabilizers (gellan gum, locust bean gum), emulsifier (sunflower lecithin), sea salt, flavorings, calcium phosphate, sodium bicarbonate, vitamins (B2, B12, D2, E). | Fat: 1.7 g. Saturated fatty acids: 0.1 g. Carbohydrates: 0.5 g. Sugars: 0.5 g. Fibers: 1.8 g. Proteins: 0.7 g. Salt: 0.10 g. |
| C | Almond brand 3 | Water, almond (2.3%), calcium (calcium carbonate), sea salt, stabilizers (guar gum, gellan gum), emulsifier (lecithins), natural flavorings, vitamins (B12, D2, E). | Fat: 1.2 g. Saturated fatty acids: 0.1 g. Carbohydrates: 2.6 g. Sugars: 2.3 g. Fibers: 0.3 g. Proteins: 0.5 g. Salt: 0.15 g. |
| D | Almond brand 4 | Water, almond (2.5%), sugar, fructose, acidity regulator (potassium phosphates), calcium carbonate, natural flavors, stabilizers (gellan gum, guar gum), sea salt. | Fat: 1.2 g. Saturated fatty acids: 0.1 g. Carbohydrates: 2.6 g. Sugars: 2.5 g. Fibers: 0.3 g. Proteins: 0.5 g. Salt: 0.08 g. |
| E | Almond brand 5 | Water, almond (2.3%), pea protein, calcium salts of orthophosphoric acid, sea salt, acidity regulator (potassium phosphate), stabilizer (gellan gum), emulsifier (sunflower lecithin), natural flavorings, vitamins (B2, B12, D). | Fat: 1.6 g. Saturated fatty acids: 0.1 g. Carbohydrates: 0.0 g. Sugars: 0.0 g. Fibers: 0.2 g. Proteins: 1.7 g. Salt: 0.16 g. |
| F | Almond brand 6 | Water, almond (2.3%), neutral calcium phosphate, sea salt, stabilizers (locust bean gum, gellan gum), emulsifier (sunflower lecithins), vitamins (B2, B12, E, D2). | Fat: 1.2 g. Saturated fatty acids: 0.1 g. Carbohydrates: 0.0 g. Sugars: 0.0 g. Fibers: 0.3 g. Proteins: 0.5 g. Salt: - g. |
| G | Almond brand 7 | Water, almond (3%), calcium, sea salt, emulsifier: sunflower lecithin, stabilizer: gellan gum, vitamins (B2, B12, D, E). | Fat: 1.7 g. Saturated fatty acids: 0.1 g. Carbohydrates: 4.4 g. Sugars: 3.6 g. Fibers: 0.4 g. Proteins: 0.7 g. Salt: 0.10 g. |
| H | Almond brand 8 | Water, almond * (6%), tapioca starch *, natural almond flavor *. * Organic ingredient. | Fat: 1.9 g. Saturated fatty acids: 0.3 g. Carbohydrates: 3.2 g. Sugars: <0.3 g. Fibers: - g. Proteins: 1.0 g. Salt: 0.14 g. |
| I | Almond brand 9 | Water, almonds* (2.8%), rice starch *, natural almond flavor, sea salt, stabilizers (guar gum *, xanthan gum). * Organic ingredient. | Fat: 1.7 g. Saturated fatty acids: 0.2 g. Carbohydrates: - g. Sugars: <0.5 g. Fibers: - g. Proteins: 1.6 g. Salt: 0.05 g |
| Soy milk | |||
| J | Soy brand 1 | Soy base [water, peeled soy beans (8.7%)], acidity regulators (potassium phosphates), calcium carbonate, flavors, sea salt, stabilizer (gellan gum), vitamins (B2, B12, D2). | Fat: 1.8 g. Saturated fatty acids: 0.3 g. Carbohydrates: 0.0 g. Sugars: 0.0 g. Fibers: 0.6 g. Proteins: 3.3 g. Salt: - g. |
| K | Soy brand 2 | Soy base, water, peeled soy beans (8%), sugar, acidity regulators (potassium phosphates), calcium carbonate, sea salt, stabilizer (gellan gum), vitamins (B2, B12, D2). | Fat: 1.8 g. Saturated fatty acids: 0.3 g. Carbohydrates: 2.5 g. Sugars: 2.5 g. Fibers: 0.5 g. Proteins: 3.0 g. Salt: 0.09 g. |
| L | Soy brand 3 | Tonyu preparation * (water, dehulled non-GMO soy beans * 8%). * Organic farming. | Fat: 2.6 g. Saturated fatty acids: 0.6 g. Carbohydrates: 0.7 g. Sugars: <0.5 g. Fibers: 0.5 g. Proteins: 3.9 g. Salt: 0.03 g. |
| Coconut milk | |||
| M | Coconut brand 1 | Water, coconut (7%), rice (3%), calcium, sea salt, natural flavor, stabilizer (gellan gum), vitamins (B2, B12, D2, E). | Fat: 1.5 g. Saturated fatty acids: 1.0 g. Carbohydrates: 5.0 g. Sugars: 2.7 g. Fibers: 0.2 g. Proteins: 0.2 g. Salt: 0.10 g. |
| N | Coconut brand 2 | Water, coconut milk (5.3%) (coconut cream, water), rice (3.3%), neutral calcium phosphate, stabilizers (guar gum, gellan gum, xanthan gum), sea salt, flavors, vitamins (B12, D2). | Fat: 1.2 g. Saturated fatty acids: 1.1 g. Carbohydrates: 0.0 g. Sugars: 0.0 g. Fibers: 0.0 g. Proteins: 0.1 g. Salt: 0.3 g. |
| O | Coconut brand 3 | Water, coconut milk (7%) (coconut cream, water), coconut water (2.6%), neutral calcium phosphate, natural coconut flavor, stabilizers (guar gum, xanthan gum, gellan gum), sea salt, vitamins (B12, D2). | Fat: 0.8 g. Saturated fatty acids: 0.8 g. Carbohydrates: 2.7 g. Sugars: 1.9 g. Fibers: 0.1 g. Proteins: 0.1 g. Salt: 0.12 g. |
| P | Coconut brand 4 | Water, coconut milk * (8.5%), tapioca starch *, natural coconut flavor *, sea salt *, stabilizer (gellan gum). * Product of organic farming. | Fat: 2.7 g. Saturated fatty acids: 2.5 g. Carbohydrates: 2.0 g. Sugars: <0.3 g. Fibers: - g. Proteins: 0.2 g. Salt: 0.1 g. |
| Q | Coconut brand 5 | Water, coconut * (59.6%), guar gum *. * Product of organic farming. | Fat: 1.6 g. Saturated fatty acids: 1.4 g. Carbohydrates: 2.4 g. Sugars: 2.1 g. Fibers: <0.5 g. Proteins: <0.5 g. Salt: 0.09 g. |
| Oat milk | |||
| R | Oat brand 1 | Oat base [water, oat (EU/non-EU) (8.7%)], soluble corn fiber, sunflower oil, calcium carbonate, sea salt, stabilizer (gellan gum), vitamins (B2, B12, D2). | Fat: 1.5 g. Saturated fatty acids: 0.2 g. Carbohydrates: 5.8 g. Sugars: 0.0 g. Fibers: 1.2 g. Proteins: 0.2 g. Salt: 0.10 g. |
| S | Oat brand 2 | Water, oats (12%), dietary fiber, sunflower oil, calcium phosphate, sea salt, sodium bicarbonate, stabilizer (gellan gum), emulsifier (sunflower lecithin), vitamins (B2, B12, D2, E). | Fat: 1.5 g. Saturated fatty acids: 0.3 g. Carbohydrates: 8.0 g. Sugars: 4.9 g. Fibers: 1.7 g. Proteins: 1.2 g. Salt: 0.11 g. |
| T | Oat brand 3 | Water, oats (10%), pea protein, sunflower oil, calcium salts of orthophosphoric acid, sea salt, acidity regulator (potassium phosphate), stabilizer (gellan gum), natural flavors, vitamins (B2, B12, D). | Fat: 1.5 g. Saturated fatty acids: 0.2 g. Carbohydrates: 6.2 g. Sugars: 3.5 g. Fibers: 0.5 g. Proteins: 1.7 g. Salt: 0.18 g. |
| U | Oat brand 4 | Water, oats * (16%), sunflower oil *. * Product of organic farming. | Fat: 1.2 g. Saturated fatty acids: 0.2 g. Carbohydrates: 9.6 g. Sugars: 5.7 g. Fibers: - g. Proteins: 1.1 g. Salt: 0.12 g. |
| V | Oat brand 5 | Oat base, water, oat (8.7%), soluble corn fiber, sunflower oil, calcium carbonate, sea salt, stabilizer (gellan gum), vitamins (B2, B12, D2). | Fat: 1.5 g. Saturated fatty acids: 0.2 g. Carbohydrates: 6.6 g. Sugars: 3.3 g. Fibers: 1.4 g. Proteins: 0.8 g. Salt: 0.08 g. |
| W | Oat brand 6 | Water, oats (8%), sunflower seeds (1%), pumpkin seeds (0.5%), sesame (0.5%), calcium, sea salt, emulsifier (sunflower lecithin), stabilizer (gellan gum), vitamins (B12, D, E). | Fat: 0.8 g. Saturated fatty acids: 0.1 g. Carbohydrates: 2.7 g. Sugars: 1.6 g. Fibers: 0.2 g. Proteins: 0.3 g. Salt: 0.10 g. |
| X | Oat brand 7 | Water, oats * (11%), cold-pressed sunflower oil *, sea salt. * Organic farming. | Fat: 0.9 g. Saturated fatty acids: 0.2 g. Carbohydrates: 4.5 g. Sugars: <0.5 g. Fibers: - g. Proteins: 0.6 g. Salt: 0.09 g. |
| Oat and hazelnut or almond milk | |||
| Y | Oat and hazelnut | Water, oats (8%), hazelnuts (2.5%), dietary fiber, calcium phosphate, sea salt, potassium phosphate, stabilizer (gellan gum), emulsifier (sunflower lecithin), flavorings, vitamins (B2, B12, D2, E). | Fat: 2.2 g. Saturated fatty acids: 0.2 g. Carbohydrates: 5.6 g. Sugars: 3.5 g. Fibers: 1.7 g. Proteins: 1.2 g. Salt: 0.11 g. |
| Z | Oat and almond | Oat base (water, oat (3.2%)), almond (1.7%), vegetable fiber from chicory roots, neutral calcium phosphate, stabilizers (locust bean gum, gellan gum), sea salt, natural flavors, vitamins (B2, B12, E, D2). | Fat: 1.0 g. Saturated fatty acids: 1.0 g. Carbohydrates: 2.4 g. Sugars: 2.4 g. Fibers: 1.5 g. Proteins: 0.3 g. Salt: 0.11 g. |
| Walnut milk | |||
| AA | Walnut | Water, cane sugar, walnut paste (2%), calcium phosphate, stabilizers (locust bean gum, gellan gum), salt, emulsifier (mono- and diglycerides of fatty acids), flavoring, vitamins (B2, B12, D2). | Fat: 1.3 g. Saturated fatty acids: 0.2 g. Carbohydrates: 2.9 g. Sugars: 2.8 g. Fibers: 0.1 g. Proteins: 0.3 g. Salt: 0.10 g. |
| Rice milk | |||
| AB | Rice | Water, rice * (13.7%), cold-pressed sunflower oil *, sea salt. * Organic farming. | Fat: 0.7 g. Saturated fatty acids: 0.1 g. Carbohydrates: 8.8 g. Sugars: <0.5 g. Fibers: - g. Proteins: <0.5 g. Salt: 0.04 g. |
| Sample | Almond (n = 9) | Soy (n = 3) | Coconut (n = 5) | Oat (n = 9) | Walnut (n = 1) | Rice (n = 1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (μg/mL) | % | Mean ± SD (μg/mL) | % | Mean ± SD (μg/mL) | % | Mean ± SD (μg/mL) | % | (μg/mL) | % | (μg/mL) | % | |
| Phenolic aldehyde | ||||||||||||
| Vanillin | 0.14 ± 0.12 | 5.62 | 36.85 ± 52.9 | 94.72 | 1.23 ± 1.98 | 72.35 | 1.21 ± 2.02 | 13.20 | 0.08 ± 0.00 | 3.48 | 0.11 ± 0.00 | 9.17 |
| Benzoic acid derivatives | ||||||||||||
| Gallic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.16 ± 0.04 | 6.96 | <LOQ | <LOQ |
| Protocatechuic acid | <LOQ | <LOQ | 0.01 ± 0.01 | 0.03 | <LOQ | <LOQ | 0.02 ± 0.03 | 0.22 | <LOQ | <LOQ | <LOQ | <LOQ |
| Syringic acid | <LOQ | <LOQ | 0.10 ± 0.15 | 0.26 | <LOQ | <LOQ | 0.56 ± 1.13 | 6.05 | <LOQ | <LOQ | <LOQ | <LOQ |
| Vanillic acid | 0.08 ± 0.11 | 3.37 | 0.22 ± 0.14 | 0.56 | 0.03 ± 0.02 | 2.00 | 0.30 ± 0.54 | 3.22 | 0.01 ± 0.00 | 0.43 | 0.02 ± 0.00 | 1.67 |
| Homovanillic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.06 ± 0.18 | 0.66 | <LOQ | <LOQ | <LOQ | <LOQ |
| p-Hydroxybenzoic acid | 0.01 ± 0.00 | 0.40 | 0.01 ± 0.00 | 0.03 | 0.01 ± 0.00 | 0.59 | 0.01 ± 0.00 | 0.11 | 0.01 ± 0.00 | 0.43 | 0.01 ± 0.00 | 0.83 |
| Cinnamic acid derivatives | ||||||||||||
| Caffeic acid | 0.02 ± 0.04 | 0.94 | <LOQ | <LOQ | <LOQ | <LOQ | 0.08 ± 0.11 | 0.82 | <LOQ | <LOQ | <LOQ | <LOQ |
| Ferulic acid | 0.18 ± 0.01 | 7.11 | 0.22 ± 0.02 | 0.57 | 0.19 ± 0.03 | 10.94 | 1.22 ± 1.53 | 13.29 | 0.18 ± 0.00 | 7.83 | 0.36 ± 0.08 | 30.00 |
| p-Coumaric acid | <LOQ | <LOQ | 0.06 ± 0.02 | 0.15 | <LOQ | <LOQ | 0.08 ± 0.11 | 0.86 | 0.01 ± 0.00 | 0.43 | 0.01 ± 0.00 | 0.83 |
| Neochlorogenic acid | 0.71 ± 1.26 | 28.70 | <LOQ | <LOQ | <LOQ | <LOQ | 2.08 ± 4.72 | 22.63 | <LOQ | <LOQ | <LOQ | <LOQ |
| trans-Chlorogenic acid | 0.78 ± 1.38 | 31.40 | <LOQ | <LOQ | <LOQ | <LOQ | 2.68 ± 6.10 | 29.11 | <LOQ | <LOQ | <LOQ | <LOQ |
| Sinapic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.38 ± 0.89 | 4.18 | <LOQ | <LOQ | <LOQ | <LOQ |
| Caftaric acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.02 ± 0.01 | 0.87 | 0.01 ± 0.00 | 0.83 |
| Flavan-3-ols | ||||||||||||
| Catechin | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.01 ± 0.00 | 0.43 | <LOQ | <LOQ |
| Epicatechin gallate | 0.01 ± 0.02 | 0.49 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.04 ± 0.02 | 1.74 | 0.01 ± 0.00 | 0.83 |
| Epigallocatechin gallate | 0.03 ± 0.03 | 1.21 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.09 ± 0.04 | 3.91 | 0.04 ± 0.01 | 3.33 |
| Flavones | ||||||||||||
| Luteolin | 0.02 ± 0.04 | 0.90 | 0.01 ± 0.01 | 0.02 | <LOQ | <LOQ | <LOQ | <LOQ | 0.12 ± 0.06 | 5.22 | 0.03 ± 0.01 | 2.50 |
| Apigenin | 0.01 ± 0.01 | 0.13 | 0.01 ± 0.01 | 0.02 | <LOQ | <LOQ | <LOQ | <LOQ | 0.02 ± 0.01 | 0.87 | <LOQ | <LOQ |
| Chrysin | 0.02 ± 0.03 | 0.85 | 0.01 ± 0.01 | 0.01 | <LOQ | <LOQ | <LOQ | <LOQ | 0.12 ± 0.05 | 5.22 | 0.04 ± 0.01 | 3.33 |
| Flavonols | ||||||||||||
| Quercetin | 0.40 ± 0.25 | 16.06 | 0.28 ± 0.04 | 0.71 | 0.24 ± 0.00 | 14.12 | 0.26 ± 0.05 | 2.84 | 1.05 ± 0.38 | 45.65 | 0.46 ± 0.06 | 38.33 |
| Isorhamnetin | 0.04 ± 0.07 | 1.53 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.22 ± 0.09 | 9.57 | 0.06 ± 0.02 | 5.00 |
| Kaempferol | 0.03 ± 0.05 | 1.26 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.16 ± 0.06 | 6.96 | 0.05 ± 0.01 | 4.17 |
| Flavonol glucoside | ||||||||||||
| Rutin | <LOQ | <LOQ | 0.12 ± 0.10 | 0.32 | 0.01 ± 0.02 | 0.71 | 0.01 ± 0.00 | 0.01 | <LOQ | <LOQ | <LOQ | <LOQ |
| Isoflavones | ||||||||||||
| Genistein | <LOQ | <LOQ | 0.41 ± 0.29 | 1.05 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Glycitein | <LOQ | <LOQ | 0.05 ± 0.03 | 0.11 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Daidzein | <LOQ | <LOQ | 0.52 ± 0.38 | 1.34 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Stilbenoids | ||||||||||||
| Piceid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.29 ± 0.64 | 3.18 | <LOQ | <LOQ | <LOQ | <LOQ |
| Compound Name | Molecular Formula | Theoretical Mass | Measured Mass | Mass Error | MS Rank | Rt | Ion Fragments | Content | ||
|---|---|---|---|---|---|---|---|---|---|---|
| [M-H]− | [M-H]− | (ppm) | (min) | μg/mL ± SD | ||||||
| Avenanthramide A | C16H13NO5 | 298.0721 | 298.0723 | −0.67 | 97.9 | 10.30 | 254.08 | 163.06 | 161.04 | 25.22 ± 36.88 |
| Avenanthramide B | C17H15NO6 | 328.0827 | 328.0829 | −0.61 | 97.1 | 10.50 | 284.09 | 193.05 | 191.06 | 38.42 ± 57.97 |
| Avenanthramide C | C16H13O6 | 314.0670 | 314.0675 | −1.59 | 96.6 | 9.58 | 270.08 | 179.03 | 177.04 | 0.93 ± 1.28 |
| Compound Name | Molecular Formula | Theoretical Mass | Measured Mass | Mass Error | MS Rank | Rt | Ion Fragments | Content | |
|---|---|---|---|---|---|---|---|---|---|
| [M-H]− | [M-H]− | (ppm) | (min) | μg/mL ± SD | |||||
| Isoflavonoids | |||||||||
| Malonyldaidzin | C24H22O12 | 501.1038 | 501.1024 | 2.79 | 94.5 | 7.67 | 415.10 | 253.05 | 0.01 ± 0.00 |
| Acetyldaidzin | C23H22O10 | 457.1140 | 457.1147 | −1.53 | 94.8 | 8.88 | 415.10 | 253.05 | 0.01 ± 0.00 |
| Daidzin | C21H20O9 | 415.1035 | 415.1039 | −0.96 | 93.7 | 8.41 | 253.05 | 0.02 ± 0.00 | |
| Malonylgenistin | C24H22O13 | 517.0988 | 517.0977 | 2.13 | 95.3 | 8.36 | 431.10 | 269.04 | 0.01 ± 0.00 |
| Acetylgenistin | C23H22O11 | 473.1089 | 473.1092 | −0.63 | 97.8 | 9.51 | 431.10 | 269.04 | 0.01 ± 0.00 |
| Genistin | C21H20O10 | 431.0984 | 431.0987 | −0.70 | 98.8 | 9.01 | 269.04 | 0.12 ± 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerogianni, V.-E.; Mantzourani, C.; Theodoropoulou, M.A.; Chiou, A.; Kokotou, M.G. Phenolic Compounds in Plant-Based Milk Alternatives from the Greek Market. Separations 2025, 12, 282. https://doi.org/10.3390/separations12100282
Gerogianni V-E, Mantzourani C, Theodoropoulou MA, Chiou A, Kokotou MG. Phenolic Compounds in Plant-Based Milk Alternatives from the Greek Market. Separations. 2025; 12(10):282. https://doi.org/10.3390/separations12100282
Chicago/Turabian StyleGerogianni, Velisaria-Eleni, Christiana Mantzourani, Maria A. Theodoropoulou, Antonia Chiou, and Maroula G. Kokotou. 2025. "Phenolic Compounds in Plant-Based Milk Alternatives from the Greek Market" Separations 12, no. 10: 282. https://doi.org/10.3390/separations12100282
APA StyleGerogianni, V.-E., Mantzourani, C., Theodoropoulou, M. A., Chiou, A., & Kokotou, M. G. (2025). Phenolic Compounds in Plant-Based Milk Alternatives from the Greek Market. Separations, 12(10), 282. https://doi.org/10.3390/separations12100282









