CoFeNi-Layered Double Hydroxide Combined Activation of PMS and Ozone for the Degradation of Rhodamine B in Water
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemicals
2.2. Catalyst Synthesis
2.3. Characterization Methods
2.4. Experimental Procedure
2.5. Analysis and Calculation Methods
3. Results and Discussion
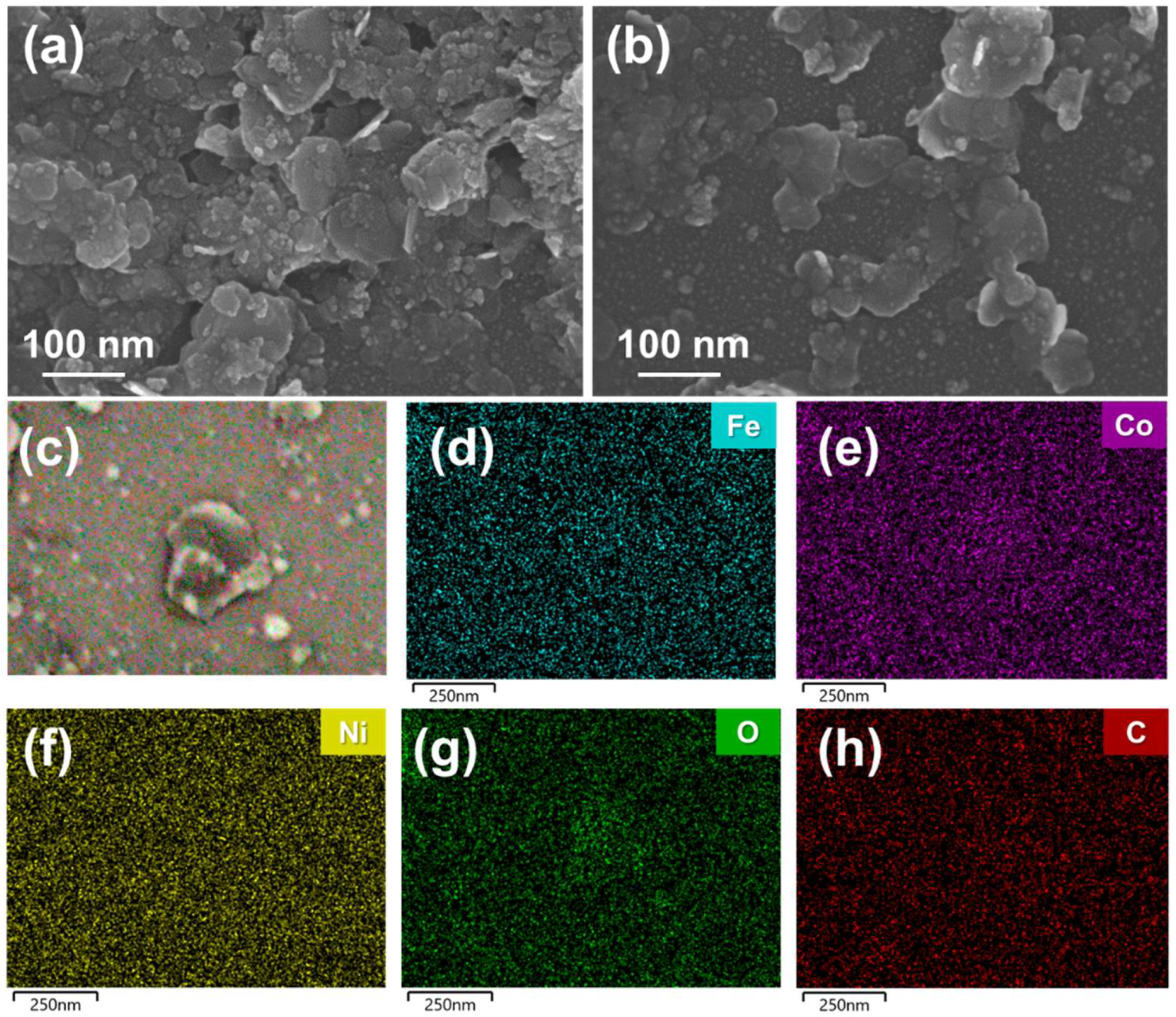
3.1. Characterization
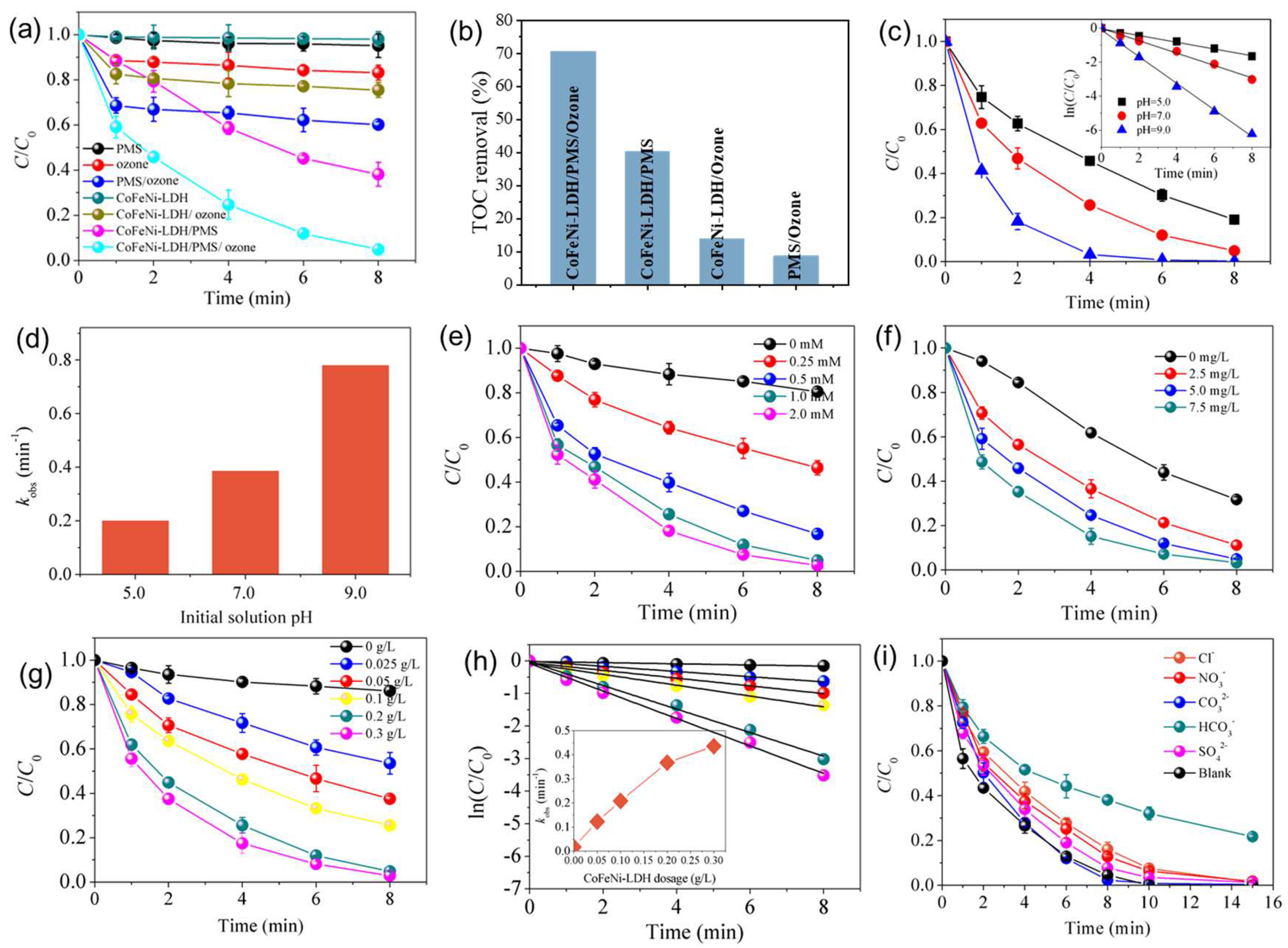
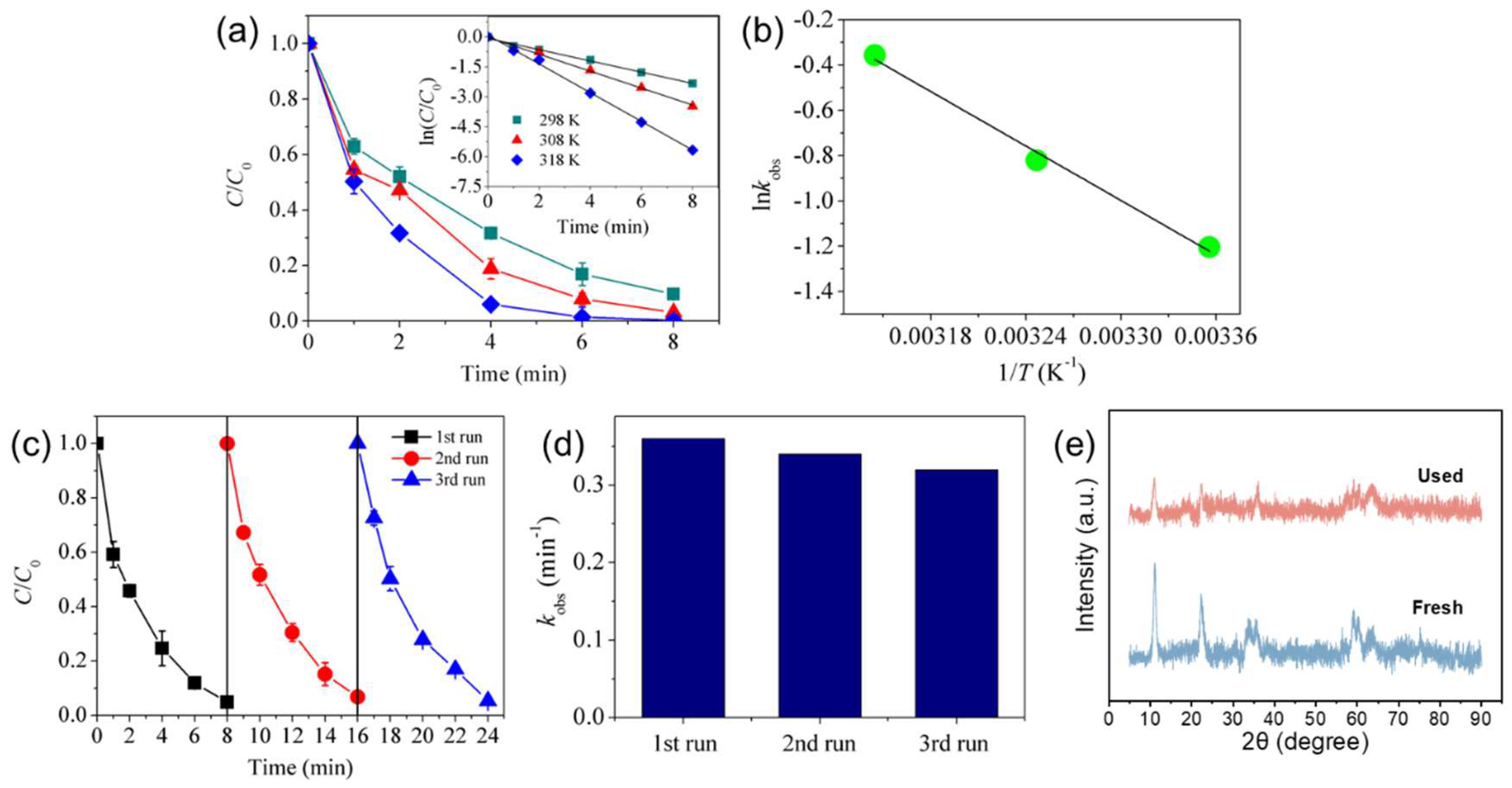
3.2. Catalytic Performance Tests
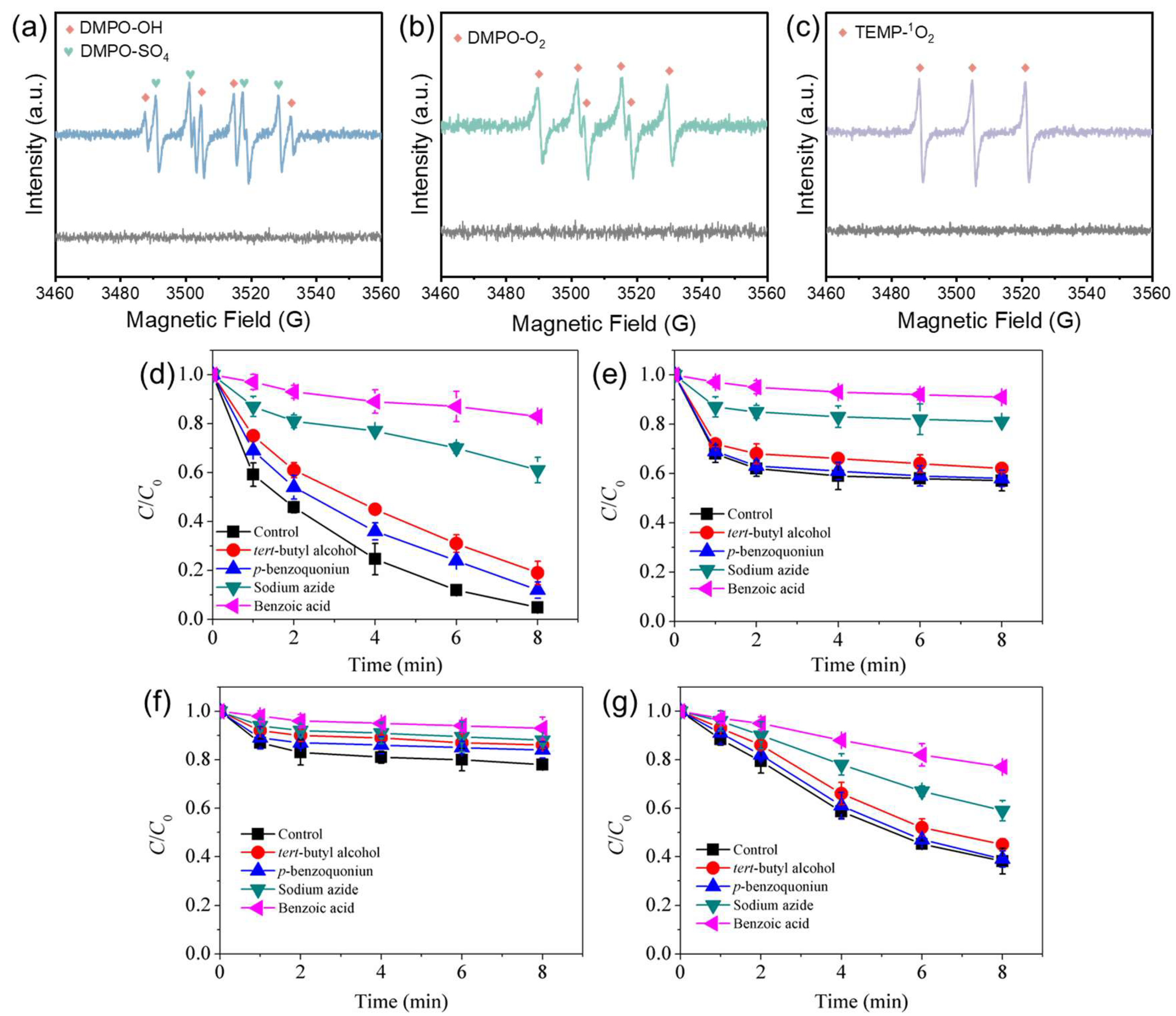
3.3. Reactive Species Identification
3.4. DFT Calculation
3.5. Possible Catalytic Mechanisms
3.6. Possible Degradation Pathway of RhB
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Sun, S.; Wang, Y.; Zhao, L.; Huang, F.; Li, S. Design of interfacial dual Schottky junctions to modulate charge transfer for enhanced piezo-assisted photocatalytic degradation RhB performances. J. Mater. Chem. A 2024, 12, 12146–12154. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Deng, L.; Zeng, H.; Shi, Z. Heterogeneous activation of hydrogen peroxide by cysteine intercalated layered double hydroxide for degradation of organic pollutants: Performance and mechanism. J. Colloid Interface Sci. 2019, 543, 183–191. [Google Scholar] [CrossRef]
- Jorge, A.M.S.; Athira, K.K.; Alves, M.B.; Gardas, R.L.; Pereira, J.F.B. Textile dyes effluents: A current scenario and the use of aqueous biphasic systems for the recovery of dyes. J. Water Process Eng. 2023, 55, 104125. [Google Scholar] [CrossRef]
- Ren, X.; Chu, Y.; Yuan, S.; Zheng, Y.; Zeng, Z.; Xia, C.; Zhao, L.; Wu, Y.; He, Y. Enhanced piezocatalytic RhB degradation with ZnSnO3 Nanocube-modified Bi4Ti3O12 composite catalyst by harnessing ultrasonic energy. J. Environ. Manag. 2024, 370, 122776. [Google Scholar] [CrossRef]
- Zeng, H.; Che, Y.; Yang, B.; Deng, J.; Zhang, C.; Wang, J.; Zhang, H. Differential catalytic mechanism induced by selective adsorption of pollutants in metal clusters decorated single atom catalyst mediated heterogeneous Fenton-like reaction. J. Hazard. Mater. 2025, 491, 138029. [Google Scholar] [CrossRef] [PubMed]
- Ming, K.; Chen, F.; Zhu, L.; Xia, S.; Yang, L.; Shi, Z.; Deng, L.; Zhang, H. Perborate accelerated copper-immobilized carbon nanofibers activating peroxymonosulfate process for sulfadiazine degradation: Performance and mechanisms understanding. Sep. Purif. Technol. 2023, 324, 124587. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C.; Zeng, H.; Shi, Z.; Wu, H.; Deng, L. Novel sulfur vacancies featured MIL-88A(Fe)@CuS rods activated peroxymonosulfate for coumarin degradation: Different reactive oxygen species generation routes under acidic and alkaline pH. Process Saf. Environ. Prot. 2022, 166, 11–22. [Google Scholar] [CrossRef]
- Shao, B.; Hu, C.; Zhao, H.; Xiao, C.; Du, E.; Cai, A.; Deng, J. Degradation of ambroxol by UV/chloramine process: Kinetics, degradation pathway, and control of the risk of highly toxic disinfection by-products. Environ. Pollut. 2024, 363, 125091. [Google Scholar] [CrossRef]
- Chang, J.; Xia, S.; Shi, Z.; Zeng, H.; Zhang, H.; Deng, L. In situ anchoring of bimetal (Cu, Fe) sulfides featured by sulfur vacancy and phosphorus doping within porous carbon nanocubes derived from Prussian blue analogs to activate peroxymonosulfate for the efficient degradation of organic pollutants. Chem. Eng. J. 2024, 498, 155252. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, B.; Zhang, J.; Zhu, H.; Deng, J.; Shi, Z.; Zhou, S.; Zhang, H.; Cai, A.; Deng, L. MnFe layered double hydroxides confined MnOx for peroxymonosulfate activation: A novel manner for the selective production of singlet oxygen. Environ. Pollut. 2024, 348, 123865. [Google Scholar] [CrossRef]
- Zeng, H.; Zhu, H.; Deng, J.; Shi, Z.; Zhang, H.; Li, X.; Deng, L. New insight into peroxymonosulfate activation by CoAl-LDH derived CoOOH: Oxygen vacancies rather than Co species redox pairs induced process. Chem. Eng. J. 2022, 442, 136251. [Google Scholar] [CrossRef]
- Wang, T.; Sun, C.; Cheng, Y.; Huo, Z.; Gu, J.; Ma, J. Efficient elimination of sulfamethoxazole by UV/peroxymonosulfate/sulfite process: Activation of peroxymonosulfate by sulfite via nucleophilic addition. Chem. Eng. J. 2025, 511, 161492. [Google Scholar] [CrossRef]
- Ranaweera, R.; Wu, X.; Ng, D.; Reineck, P.; Zhang, J.; Williams, M.; Fan, L.; Xie, Z. Comparative study of adsorption, thermally activated peroxymonosulfate and wet air oxidation for tetracycline removal and wastewater treatment. J. Water Process Eng. 2025, 72, 107559. [Google Scholar] [CrossRef]
- Koundle, P.; Nirmalkar, N.; Momotko, M.; Boczkaj, G. Ozone nanobubble technology as a novel AOPs for pollutants degradation under high salinity conditions. Water Res. 2024, 263, 122148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, G.; Cao, Y.; Lu, X.; Guan, C.; Li, J.; Ma, J.; Jiang, J.; Yang, Y. Efficient degradation and deiodination of iopamidol by synergistic •OH and SO4•− oxidation in O3/PMS system. Chem. Eng. J. 2025, 521, 166362. [Google Scholar] [CrossRef]
- Deniere, E.; Van Hulle, S.; Van Langenhove, H.; Demeestere, K. Advanced oxidation of pharmaceuticals by the ozone-activated peroxymonosulfate process: The role of different oxidative species. J. Hazard. Mater. 2018, 360, 204–213. [Google Scholar] [CrossRef]
- Guo, W.; Li, C.; Zhao, J.; Ding, Y.; Yang, Q.; Guan, H. The treatment of petrochemical wastewater via ozone-persulfate coupled catalytic oxidation: Mechanism of removal of soluble organic matter. Environ. Sci. Pollut. Res. 2024, 31, 29400–29414. [Google Scholar] [CrossRef]
- How, Z.T.; Fang, Z.; Chelme-Ayala, P.; Ganiyu, S.O.; Zhang, X.; Xu, B.; Chen, C.; Gamal El-Din, M. Ozone-activated peroxymonosulfate (O3/PMS) process for the removal of model naphthenic acids compounds: Kinetics, reactivity, and contribution of oxidative species. J. Environ. Chem. Eng. 2023, 11, 109935. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Ma, J.; He, H. Effect of Interlayer Anions on NiFe Layered Double Hydroxides for Catalytic Ozone Decomposition. Environ. Sci. Technol. 2024, 58, 8597–8606. [Google Scholar] [CrossRef]
- Tang, D.; Zhong, D.; Zhou, Y.; Xu, Y.; Xiang, T.; Li, W.; Yang, Y.; Fan, C.; Chen, J. Catalytic Ozone Degradation of Dye Wastewater by CoAl-LDH@K-NSBC Modified with Magnetic CuFe2O4. Langmuir 2025, 41, 17295–17310. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, X.; Zhang, C. Sulfidation of Ni-Fe-LDH for enhanced Fenton-like catalysis: Experimental validation and theoretical calculation. Sep. Purif. Technol. 2024, 336, 126320. [Google Scholar] [CrossRef]
- Zeng, H.; Deng, L.; Zhang, H.; Zhou, C.; Shi, Z. Development of oxygen vacancies enriched CoAl hydroxide@hydroxysulfide hollow flowers for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for sulfamethoxazole degradation. J. Hazard. Mater. 2020, 400, 123297. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liang, M.; Liu, C.; Xu, Z.; Yu, Y.; Xu, J.; You, S.; Wang, D.; Rad, S. Peroxymonosulfate activation by CuMn-LDH for the degradation of bisphenol A: Effect, mechanism, and pathway. Ecotoxicol. Environ. Saf. 2024, 270, 115929. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, W.; Deng, L.; Luo, J.; Zhou, S.; Liu, X.; Pei, Y.; Shi, Z.; Crittenden, J. Degradation of dyes by peroxymonosulfate activated by ternary CoFeNi-layered double hydroxide: Catalytic performance, mechanism and kinetic modeling. J. Colloid Interface Sci. 2018, 515, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, Y.; Liu, W.; Hou, Y.; Wang, C.; Shang, J.; Cheng, X. Degradation of Rhodamine B by MnFe-LDH/PMS/O3 three-phase catalytic system: Performance, mechanism and ecotoxicity studies. Front. Environ. Sci. Eng. 2023, 18, 9. [Google Scholar] [CrossRef]
- Deng, J.; Xiao, L.; Yuan, S.; Wang, W.; Zhan, X.; Hu, Z.-H. Activation of peroxymonosulfate by CoFeNi layered double hydroxide/graphene oxide (LDH/GO) for the degradation of gatifloxacin. Sep. Purif. Technol. 2021, 255, 117685. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Jensen, F. Activation energies and the arrhenius equation. Qual. Reliab. Eng. Int. 1985, 1, 13–17. [Google Scholar] [CrossRef]
- Petersen, A.C.; Crockett, L.; Richards, M.; Boxer, A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc. 1988, 17, 117–133. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Tang, H.; Shahi, C. Origin of the size-dependence of the equilibrium van der Waals binding between nanostructures. J. Chem. Phys. 2018, 148, 074110. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O.; Gazi, M. Magnetic LDH-based CoO–NiFe2O4 catalyst with enhanced performance and recyclability for efficient decolorization of azo dye via Fenton-like reactions. Appl. Catal. B Environ. 2019, 243, 243–252. [Google Scholar] [CrossRef]
- Xiao, Y.; Lu, J.; Cheng, S.; Wang, Z.; Shi, A.; Shen, J.; Jiang, Z. Performance and influencing mechanisms of magnetically separable Ni-M layered double hydroxides (M = Fe, Al) for catalytic ozonation. J. Ind. Eng. Chem. 2024, 130, 178–190. [Google Scholar] [CrossRef]
- Dung, N.T.; Ha, D.T.H.; Thao, V.D.; Thao, N.P.; Lam, T.D.; Lan, P.T.; Trang, T.T.; Ngan, L.V.; Nhi, B.D.; Thuy, N.T.; et al. Effective activation of peroxymonosulfate by CoCr-LDH for removing organic contaminants in water: From lab-scale to practical applications. Environ. Sci. Pollut. Res. 2024, 31, 26773–26789. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Fui, H.; Gao, S.; Ma, X.; Huang, Y. Facile fabrication of CoAl-LDH nanosheets for efficient rhodamine B degradation via peroxymonosulfate activation. RSC Adv. 2023, 13, 29695–29705. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Jin, Q. Enhancement on peroxymonosulfate activation by Fe-Al layered double hydroxide loaded on super activated carbon for the degradation of organic pollutants. J. Water Process Eng. 2024, 68, 106486. [Google Scholar] [CrossRef]
- Li, T.; Du, X.; Deng, J.; Qi, K.; Zhang, J.; Gao, L.; Yue, X. Efficient degradation of Rhodamine B by magnetically recoverable Fe3O4-modified ternary CoFeCu-layered double hydroxides via activating peroxymonosulfate. J. Environ. Sci. 2021, 108, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Chengqian, F.; Wanbing, L.; Yimin, D.; Zhiheng, W.; Yaqi, L.; Ling, C.; Bo, L.; Siwen, Y.; Junlong, W.; Xianglong, D.; et al. Synthesis of a novel hierarchical pillared Sep@Fe3O4/ZnAl-LDH composite for effective anionic dyes removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 130921. [Google Scholar] [CrossRef]
- Miao, W.; Liu, Y.; Wang, D.; Du, N.; Ye, Z.; Hou, Y.; Mao, S.; Ostrikov, K. The role of Fe-Nx single-atom catalytic sites in peroxymonosulfate activation: Formation of surface-activated complex and non-radical pathways. Chem. Eng. J. 2021, 423, 130250. [Google Scholar] [CrossRef]
- Jo, Y.; Kim, C.; Moon, G.-H.; Lee, J.; An, T.; Choi, W. Activation of peroxymonosulfate on visible light irradiated TiO2 via a charge transfer complex path. Chem. Eng. J. 2018, 346, 249–257. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, H.; Deng, L.; Shi, Z. Peroxymonosulfate-assisted photocatalytic degradation of sulfadiazine using self-assembled multi-layered CoAl-LDH/g-C3N4 heterostructures: Performance, mechanism and eco-toxicity evaluation. J. Water Process Eng. 2020, 33, 101084. [Google Scholar] [CrossRef]
- Zeng, H.; Deng, L.; Yang, L.; Wu, H.; Zhang, H.; Zhou, C.; Liu, B.; Shi, Z. Novel Prussian blue analogues@MXene nanocomposite as heterogeneous activator of peroxymonosulfate for the degradation of coumarin: The nonnegligible role of Lewis-acid sites on MXene. Chem. Eng. J. 2021, 416, 128071. [Google Scholar] [CrossRef]
- Akbari, S.; Ghanbari, F.; Moradi, M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: Applying low current density for oxidation mechanism. Chem. Eng. J. 2016, 294, 298–307. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, Q.; Ding, J.; Ou, M.; Lv, Z.; Song, F. Low-Temperature NOX (x = 1, 2) Removal with •OH Radicals from Catalytic Ozonation over α-FeOOH. Ozone Sci. Eng. 2016, 38, 382–394. [Google Scholar] [CrossRef]
- Jackson, P.E.; Weigert, C.; Pohl, C.A.; Saini, C. Determination of inorganic anions in environmental waters with a hydroxide-selective column. J. Chromatogr. A 2000, 884, 175–184. [Google Scholar] [CrossRef]
- Yang, X.; Wu, P.; Chu, W.; Wei, G. Peroxymonosulfate/LaCoO3 system for tetracycline degradation: Performance and effects of co-existing inorganic anions and natural organic matter. J. Water Process Eng. 2021, 43, 102231. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Wang, Y.; Fu, J.; Hou, Y.; Yang, B.; Lei, L.; Al-Anazi, A.; Jiang, G.; Li, Z. New insights into activation mechanisms of peroxymonosulfate by halide ions for micropollutant abatement: The generation routes and contributions of reactive radicals and hypohalous acid. Chem. Eng. Sci. 2025, 305, 121178. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Zhan, J.; Feng, A.; Zhou, C.; Chen, J. Disparate effects of four anions on the non-radical oxidation process of sulfamethoxazole by peroxymonosulfate: Kinetics, mechanism and toxicity variation. J. Environ. Chem. Eng. 2022, 10, 107572. [Google Scholar] [CrossRef]
- Yang, P.; Long, Y.; Huang, W.; Liu, D. Single-atom copper embedded in two-dimensional MXene toward peroxymonosulfate activation to generate singlet oxygen with nearly 100% selectivity for enhanced Fenton-like reactions. Appl. Catal. B Environ. 2023, 324, 122245. [Google Scholar] [CrossRef]
- Chen, Q.; Ji, F.; Liu, T.; Yan, P.; Guan, W.; Xu, X. Synergistic effect of bifunctional Co–TiO2 catalyst on degradation of Rhodamine B: Fenton-photo hybrid process. Chem. Eng. J. 2013, 229, 57–65. [Google Scholar] [CrossRef]
- Channab, B.-E.; El Ouardi, M.; Marrane, S.E.; Layachi, O.A.; El Idrissi, A.; Farsad, S.; Mazkad, D.; BaQais, A.; Lasri, M.; Ait Ahsaine, H. Alginate@ZnCO2O4 for efficient peroxymonosulfate activation towards effective rhodamine B degradation: Optimization using response surface methodology. RSC Adv. 2023, 13, 20150–20163. [Google Scholar] [CrossRef]
- Kwan, W.P.; Voelker, B.M. Decomposition of Hydrogen Peroxide and Organic Compounds in the Presence of Dissolved Iron and Ferrihydrite. Environ. Sci. Technol. 2002, 36, 1467–1476. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, P.; Ji, Y.; Lu, J. The Role of Natural Organic Matter in the Degradation of Phenolic Pollutants by Sulfate Radical Oxidation: Radical Scavenging vs Reduction. Environ. Sci. Technol. 2025, 59, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, L.; Chen, J.; Zhang, Y.; Liu, M.; Han, Y.; Chen, Y.; Zeng, H.; Shi, Z. How MoS2 assisted sulfur vacancies featured Cu2S in hollow Cu2S@MoS2 nanoboxes to activate H2O2 for efficient sulfadiazine degradation? Chem. Eng. J. 2022, 446, 137364. [Google Scholar] [CrossRef]
- Zeng, H.; Ling, X.; Deng, J.; Deng, L.; Zhang, H.; Shi, T.; Zhou, S.; Shi, Z. Activation of peroxymonosulfate via peroxyborate: Electrophilic substitution induced strong electrophile generation process. Chem. Eng. J. 2023, 451, 138925. [Google Scholar] [CrossRef]
- Li, Z.; Huang, D.; Wang, Y.; Yan, J.; Liu, Y.; Zhao, H.; Lan, X.; Huang, Y.; Astruc, D.; Liu, X. Sustainability-Inspired Upcycling of Organophosphorus Pollutants into Phosphatic Fertilizer in a Continuous-Flow Reactor. Angew. Chem. Int. Ed. 2025, 64, e202502408. [Google Scholar]
- Sun, Z.; Zhao, M.; Zhou, X. Reliability analysis of NaN3 as quencher of 1O2 in heterogeneous persulfate catalytic oxidation system. Catal. Commun. 2023, 183, 106774. [Google Scholar] [CrossRef]
- Zeng, H.; Shi, W.; Yang, B.; Deng, J.; Wang, J.; Zhang, H. Co4(PW9O34)2 Polyoxmetalate Cluster Intercalated in Layered Double Hydroxides as Catalyst for the Oxidation of p-Arsanilic Acid and Subsequent Immobilization of Arsenic-Containing Byproducts. ACS Appl. Nano Mater. 2024, 7, 23008–23017. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, L.; Deng, L.; Zhang, H.; Zeng, H.; Shi, Z. Efficient activation of peroxymonosulfate on CuS@MIL-101(Fe) spheres featured with abundant sulfur vacancies for coumarin degradation: Performance and mechanisms. Sep. Purif. Technol. 2021, 276, 119404. [Google Scholar] [CrossRef]
- Mi, X.; Ma, R.; Pu, X.; Fu, X.; Geng, M.; Qian, J. FeNi-layered double hydroxide (LDH)@biochar composite for, activation of peroxymonosulfate (PMS) towards enhanced degradation of doxycycline (DOX): Characterizations of the catalysts, catalytic performances, degradation pathways and mechanisms. J. Clean. Prod. 2022, 378, 134514. [Google Scholar] [CrossRef]

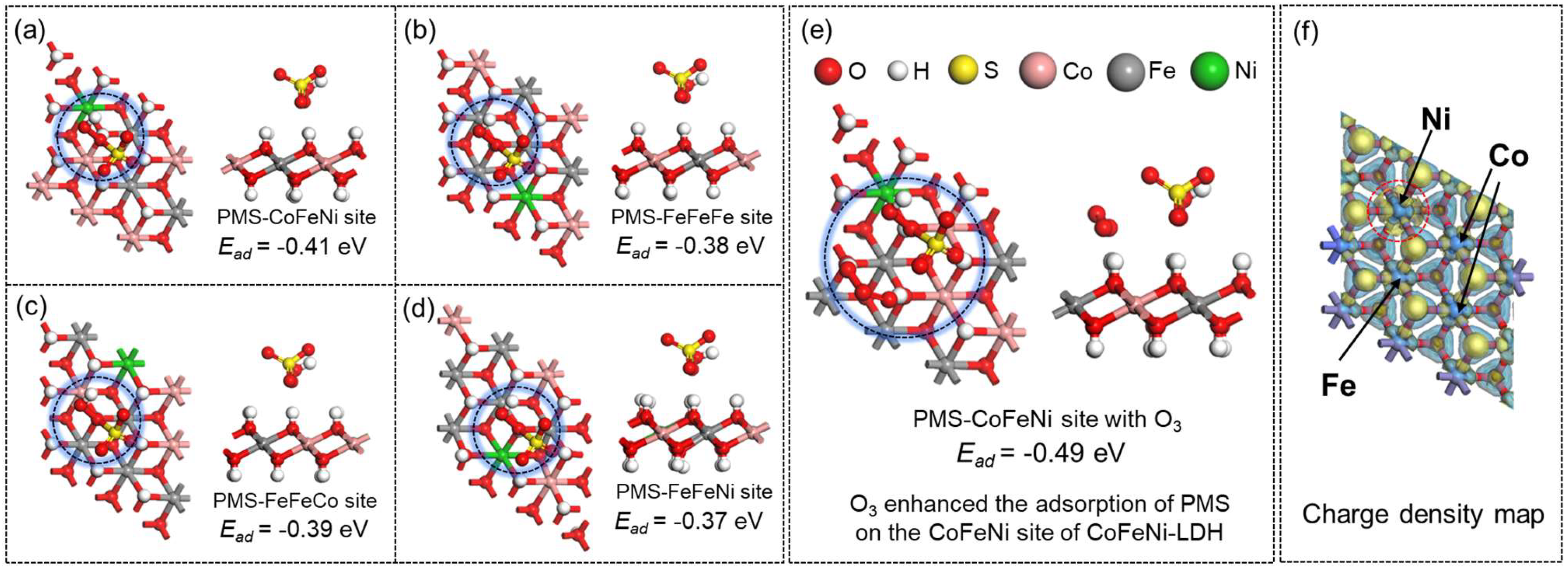

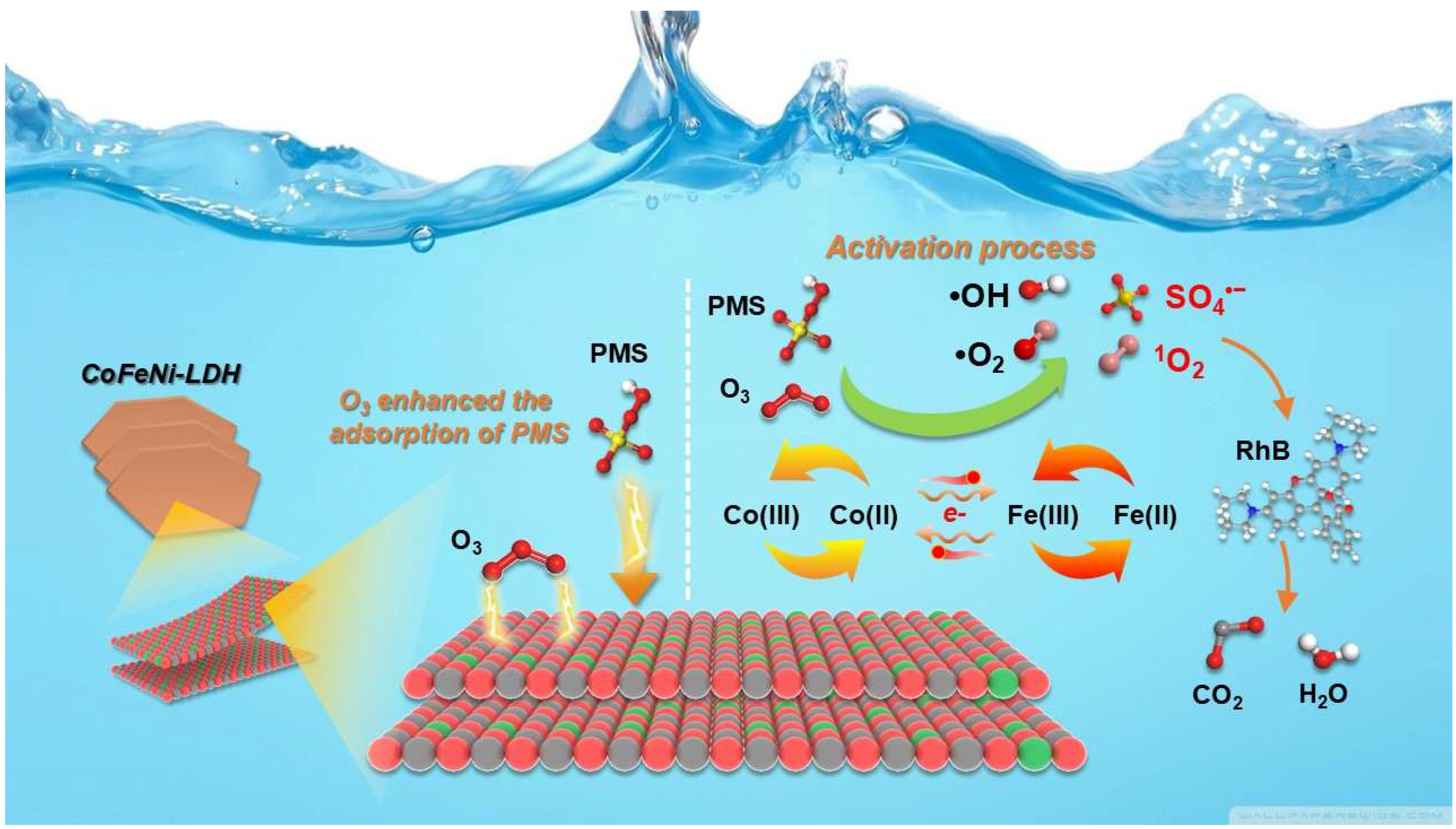
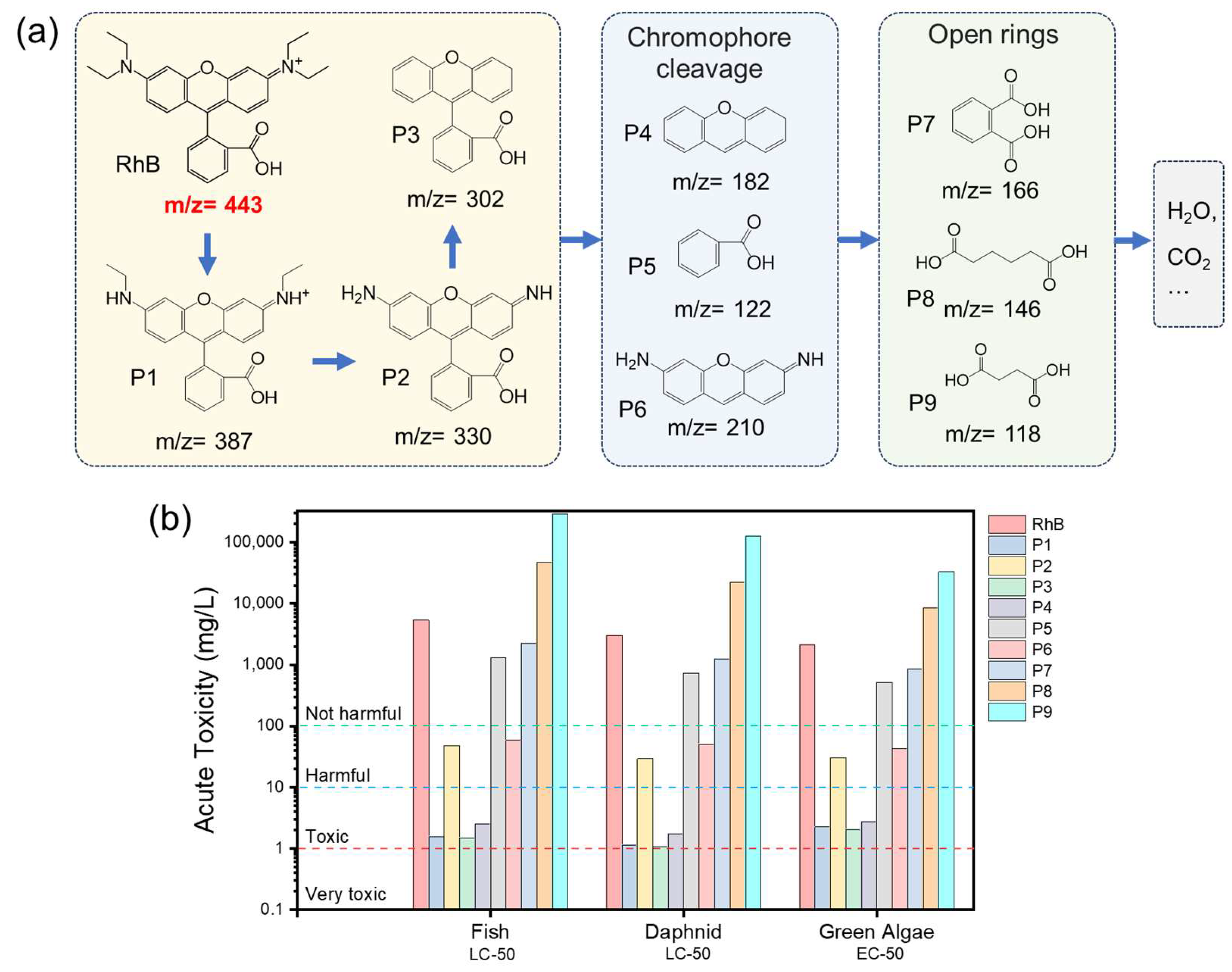
| Dosage (g/L) | PMS (mM) | Ozone | RhB (mg/L) | kobs (min−1) | Ref. | |
|---|---|---|---|---|---|---|
| ZnFe-LDH | 0.5 | - | 65 mg/h | 50 | 0.058 | [32] |
| MgFe-LDH | 0.5 | - | 65 mg/h | 50 | 0.054 | [32] |
| MgAl-LDH | 0.5 | - | 65 mg/h | 50 | 0.053 | [32] |
| NiFe–-LDH | 0.5 | - | 65 mg/h | 50 | 0.065 | [32] |
| NiAlLDH | 0.5 | - | 65 mg/h | 50 | 0.069 | [32] |
| CoCr-LDH | 0.02 | 2 | - | 50 | 0.079 | [33] |
| FeCo-LDH | 0.2 | 1 | - | 50 | 0.011 | [34] |
| CoAl-LDH | 0.1 | 0.3 | - | 80 | 0.1687 | [35] |
| FeAl-LDH | 0.1 | 0.66 | - | 10 | 0.41 | [36] |
| CoFeCu-LDH | 0.2 | 1 | - | 50 | 0.33 | [37] |
| MnFe-LDH | 0.3 | 1.3 | 0.1 L/min | 20 | 0.34 | [25] |
| CoFeNi-LDH | 0.2 | 1 | 5 mg/L | 20 | 0.42 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Song, L.; Miao, J. CoFeNi-Layered Double Hydroxide Combined Activation of PMS and Ozone for the Degradation of Rhodamine B in Water. Separations 2025, 12, 276. https://doi.org/10.3390/separations12100276
Zhu X, Song L, Miao J. CoFeNi-Layered Double Hydroxide Combined Activation of PMS and Ozone for the Degradation of Rhodamine B in Water. Separations. 2025; 12(10):276. https://doi.org/10.3390/separations12100276
Chicago/Turabian StyleZhu, Xiaohan, Liang Song, and Jia Miao. 2025. "CoFeNi-Layered Double Hydroxide Combined Activation of PMS and Ozone for the Degradation of Rhodamine B in Water" Separations 12, no. 10: 276. https://doi.org/10.3390/separations12100276
APA StyleZhu, X., Song, L., & Miao, J. (2025). CoFeNi-Layered Double Hydroxide Combined Activation of PMS and Ozone for the Degradation of Rhodamine B in Water. Separations, 12(10), 276. https://doi.org/10.3390/separations12100276





