1. Introduction
Histone proteins and their post-translational modifications (PTMs) play a central role in regulating chromatin structure and gene expression, making them key targets of study in structural biology [
1]. More precisely, modifications such as methylation, acylations, and phosphorylation modulate interactions between nucleosomes and associated proteins, thereby influencing cellular processes ranging from transcription to DNA repair [
2,
3]. To investigate the structural basis of the interaction with these modifications and their impact on chromatin architecture, synthetic histone peptides bearing specific PTMs are widely employed as molecular tools [
4,
5,
6,
7,
8].
The chemical synthesis of modified histone peptides and their purification are essential for producing homogeneous samples suitable for biochemical and structural studies. In particular, high-purity histone peptides are a prerequisite for successful crystallization and high-resolution structural analysis, such as X-ray crystallography or cryo-EM, where sample quality critically determines experimental outcomes [
9]. Obtaining synthetic histone peptides with post-translational modifications in high purity is crucial for advancing PTM-related research.
When the desired PTM containing building blocks are commercially available, the corresponding modification can be directly incorporated during solid-phase peptide synthesis (SPPS). However, in the absence of such modified building blocks, post-synthesis chemical modification strategies become inevitable. In frame of these post-synthesis approaches, optimization of both the incorporation conditions and the purification processes are of paramount importance. For instance, the post-synthesis incorporation of acylations such as an acetyl or a crotonyl group on the lysine side chain would potentially yield two results, one with the desired modification and one unreacted side-product. In these cases, the differences between the unmodified peptide and its PTM-bearing variants are minor. The two sequences differ by only 43 and 70 Daltons, respectively (
Figure 1). Furthermore, it is currently difficult to assess quantity by mass spectrometry thus, a heterogeneous sample including these two products would have an uncertain purity level. Even though the mass shift is minor, the differences can significantly influence downstream applications. That is why it would be ideal to obtain 100% yield during the modification and approve the absence of the unreacted side product by mass spectrometry, but this cannot be always achieved. The only remaining option to obtain high-purity product is to be able to elute those two species separately. Therefore, precise and efficient purification is essential to ensure the homogeneity of the final PTM-modified histone product. The study of PTM-bearing peptide purification has not been extensively elaborated in the literature.
Peptides are commonly purified using high-performance liquid chromatography (HPLC), a crucial step to obtain high-purity products for research and therapeutic applications. In HPLC, acetonitrile is widely used as an organic solvent due to its polarity character, low viscosity, and miscibility with water. It offers an effective elution capability with minimal UV absorbance above 190 nm, and low system backpressure. These characteristics make acetonitrile particularly well-suited for high-resolution separations and fractionations in biomolecular analysis, but it is toxic and harmful. Moreover, according to the International Council for Harmonisation (ICH) guidelines, acetonitrile (ACN) is designated as a Class 2 solvent that should be limited in pharmaceutical products because of its inherent toxicity [
10]. Therefore, in recent years, academia and industry have started searching for greener alternatives for both synthesis and purification of peptides [
11,
12,
13]. The physical properties of some green solvents that could be alternatives to the frequently used acetonitrile and methanol are summarized in
Table 1. However, not all green solvents are suitable for peptide purification, as some may not dissolve peptides well or offer proper separation or simply not compatible with the current purification instrumentations. Therefore, separation efficiency is influenced by the physicochemical properties of the solvent alongside those of the peptide, column, stationary phase, and the purification setup. So far, in HPLC studies, solvents like methanol, ethanol, and isopropanol, which are less harmful and mix well with water, have been tested as alternatives to acetonitrile [
14,
15,
16,
17,
18]. Employing these greener solvents not only minimizes the environmental footprint but also promotes safer working conditions in the laboratory.
In this study, we focus on the separation of histone H4 tail peptides bearing different site-specific lysine modifications. Specifically, we examine two peptide sequences that differ solely by the presence of a crotonyl group. To address this subtle difference, which corresponds to only a few additional atoms, we aim to optimize a HPLC method capable of efficiently resolving such closely related peptide species. The current literature lacks references on the purification of PTM-bearing histone peptides. Researching efficient methods for this purpose will inevitably accelerate PTM-related discoveries in molecular biology. More generally, the development of an HPLC method that can accurately distinguish modified peptides from their unmodified counterparts is particularly critical for the analysis of a multitude of peptides generated through post synthetic modifications. On the other hand, metabolomics and proteomics studies can benefit from this research for the isolation and identification of different PTM-bearing molecules present in the cell. Furthermore, in alignment with green chemistry principles, we have chosen to investigate the use of ethanol and isopropanol based on their UV cut-off and polarity (
Table 1) as an alternative mobile phase component to acetonitrile to ensure a reduced environmental impact while conducting basic research.
2. Materials and Methods
2.1. Reagents and Chemicals
All chemicals and reagents used were of analytical grade, unless otherwise specified. For peptide synthesis, Fmoc-amino acids, coupling reagents, Rink amide resin, DMF, DCM, piperidine, and TFA were obtained from Merck (Darmstadt, Germany). HPLC-grade solvents were purchased from Merck (Darmstadt, Germany).
2.2. Peptide Synthesis, Purification and Characterization
Histone H4 tail peptides (residues 1–18) were synthesized on a fully automated peptide synthesizer (Aapptec, Louisville, KY, USA) at 0.1 mmol scale using Fmoc chemistry on Rink amide resin. Site-specific lysine modifications were incorporated using commercially available Fmoc-Lys(Ac)-OH or Fmoc-Lys(Cro)-OH derivatives. Following synthesis, peptides were cleaved from the resin using a cleavage cocktail composed of TFA/TIS/H2O in a ratio of 95:2.5:2.5 for 1 h at room temperature. After cleavage, the crude peptides were precipitated in cold diethyl ether, collected by centrifugation, and dried under nitrogen flow. Crude products were then dissolved in water and purified on a reversed-phase C18 column (5 μm; 250 × 4.6 mm, Shim-pack GISS, Shimadzu, Kyoto, Japan) using a high-performance liquid chromatography system equipped with DAD detector (Shimadzu Nexera Prep, Kyoto, Japan). Gradient elution was performed using different combinations of two mobile phases, both including 0.05% TFA: solvent A was water in all cases and solvent B was either acetonitrile, ethanol, or isopropanol. Purified peptides were analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) using an Agilent 6420 Triple Quadrupole (Agilent Technologies, Santa Clara, CA, USA) and a Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Mathematical Formulas Used for the Analysis of Chromatograms
2.3.1. Theoretical Plates
The number of theoretical plates (
N) is calculated to assess column efficiency using Equation (1), where
tR is the retention time of the peptide (in minutes) and
W0.5 is the peak width at half-height (in minutes).
2.3.2. Height Equivalent to a Theoretical Plate (HETP)
The column efficiency is evaluated by calculating the Height Equivalent to a Theoretical Plate (HETP) using Equation (2), where
L is the length of the chromatographic column and
N is the number of theoretical plates.
2.3.3. Tailing Factor
Peak symmetry is assessed by calculating the tailing factor (
Tf) using Equation (3), where
W0.05 is the width of the peak at 5% of the peak height and
Q0.05 is the distance from the peak maximum to the front of the peak at 5% of the peak height.
2.3.4. Resolution
The resolution (
R) between two adjacent peaks was calculated with Equation (4) to evaluate the separation efficiency, where
tR and
tRP are the retention times of the first and second eluting peaks, and
W0.5 and
WP0.5 are the respective baseline peak widths of the two compounds.
2.3.5. The Capacity Factor k’
The capacity factor (
k′) was calculated to assess the retention of the peptides under the chromatographic conditions, using Equation (5) where
t is the retention time of the peptide and
t0 is the column void time.
3. Results
We synthesized two different peptides derived from the same histone H4 sequence (
Table 2). One peptide contained a single post-translational modification (PTM), an acetylated lysine, while the other carried two distinct PTMs: one acetylated lysine and one crotonylated lysine.
Following synthesis, the peptides were subjected to purification by high-performance liquid chromatography (HPLC). The conventional HPLC peptide purification protocol uses elution on a C18 column with an increasing gradient of acetonitrile in water. Several parameters must be considered while choosing a C18 column, including particle and pore size, carbon loading, and column dimensions. Due to pressure limits, we could choose 5 μm particle size at best. Additionally, we decided to use a 200 Å pore size based on the peptides’ molecular weights. Finally, we took a 250 mm long column to ensure the best resolution possible. HPLC profile of crude peptides are given in
Supplementary Figures S1 and S2 for H4K5ac and H4K5acK8cro, respectively.
The molecular weight of the synthesized peptides were verified by LC-MS/MS, and their spectra are provided in the
Supplementary Files (Figures S3–S10). The calculated monoisotopic
m/
z values for the fragment ions of peptides H4K5ac and H4K5acK8cro are listed in
Table 3. The detection of molecular ion peaks with +2, +3, and +4 charge states in the MS spectra of the peptide H4K5ac confirmed its proper synthesis. Likewise, the synthesis of the peptide H4K5acK8cro was confirmed with the presence of +2, +3, +4 charge states in its MS spectra.
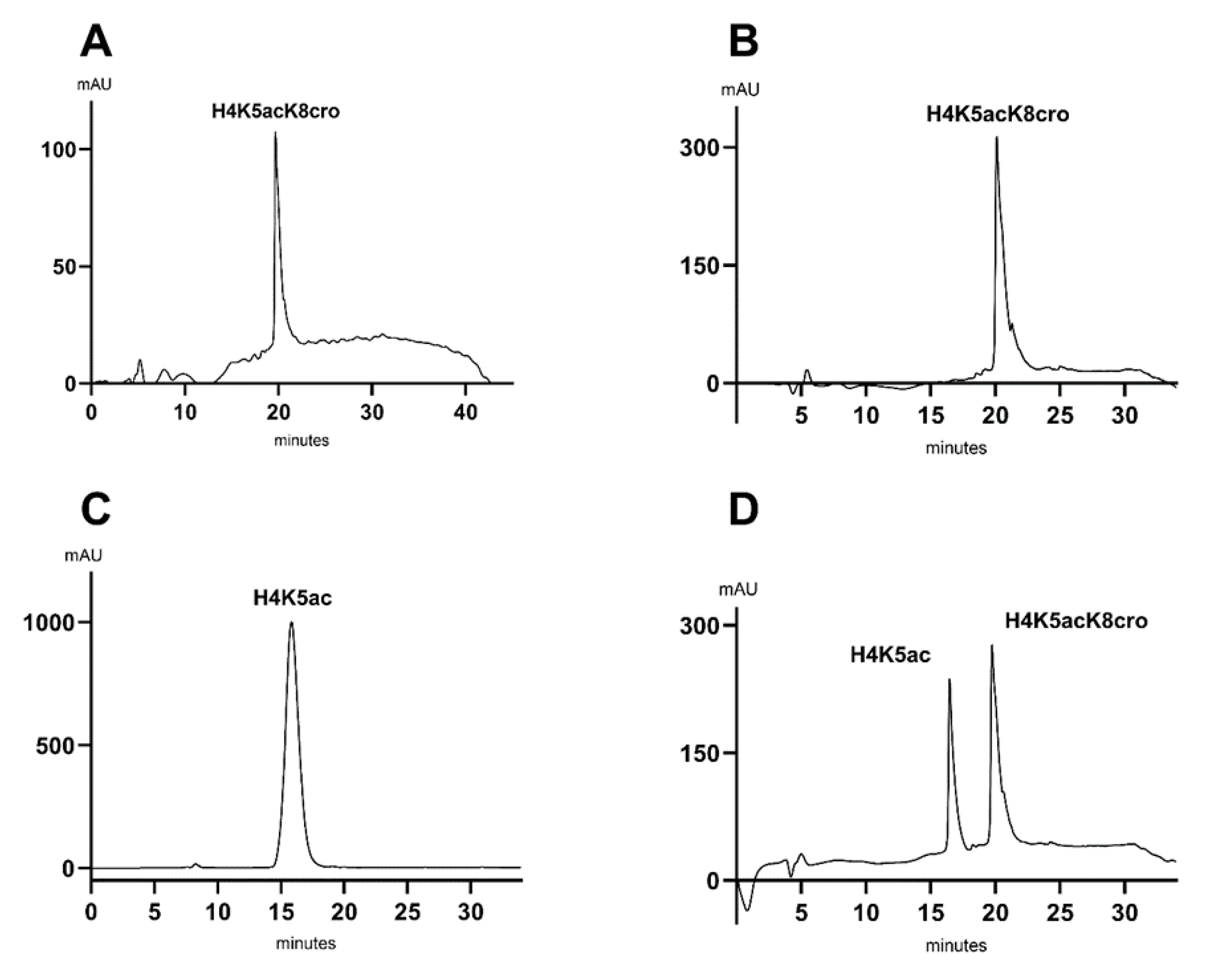
Once we obtained our two peptides of interest, we first wanted to validate the maximum concentration of acetonitrile that would elute the peptides. We first tested a run with the 5 to 80% in 30 min protocol that translated to an increment of 2.5% acetonitrile per minute. Finding the maximum concentration that elutes the peptides helps in shortening the HPLC method to gain time for the following runs but also limits solvent consumption. In that sense, we individually ran peptide H4K5acK8cro (
Figure 2A), which was more hydrophobic and should elute later than peptide H4K5ac. That result indicated that peptide H4Kac5K8cro already eluted by minute 20. This led us to shorten the method by finishing it at 52% acetonitrile instead of 80%. To validate the percentage of elution, both peptides were run individually with the new 5 to 52% in 19 min. Peptide H4K5acK8cro eluted at minute 20 (
Figure 2B) and H4K5ac eluted at minute 15 (
Figure 2C). Since the elution times of these two peptides differed significantly with this gradient, the method was deemed suitable for subsequent tests aimed at separating the two molecules. To validate the method, we prepared a mixture of the two peptides and co-injected them. As expected, the two peptides eluted separately (
Figure 2D). We evaluated the separation efficiency with the respective number of theoretical plates and resolution parameters summarized in
Table 4.
Having demonstrated that these two peptides eluted separately with acetonitrile on our C18 column, we next explored the use of alternative solvents more aligned with green chemistry principles. In this context, ethanol was selected as the first candidate. Although ethanol is considered a greener solvent, its higher viscosity compared to acetonitrile (
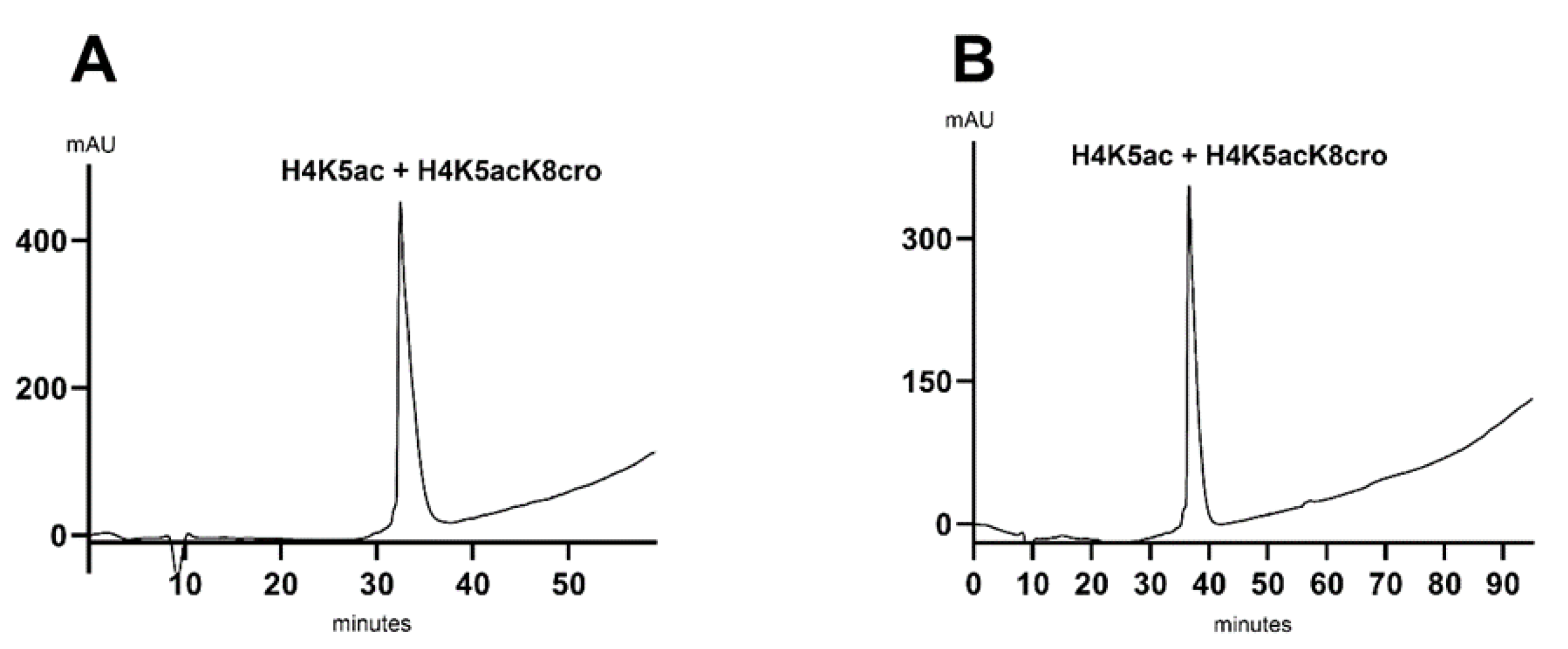
Table 1) results in increased backpressure on the column. One common strategy to mitigate this issue is to increase the mobile phase temperature using a column oven. However, as our HPLC system was not equipped with a column oven, we instead reduced the flow rate to avoid exceeding the 200 bar pressure limit of the column. Upon co-injection of the peptide mixture and analysis using a 5% to 52% gradient method, only a single peak was observed at 30 min, indicating co-elution of the peptides (
Figure 3A). To assess whether ethanol could achieve separation of the peptides using the existing column, we opted to reduce the gradient to 1% per minute (
Figure 3B), as opposed to the 2.5% per minute previously applied with acetonitrile, prior to evaluating alternative columns. Despite the modified gradient, both peptides still co-eluted as a single peak.
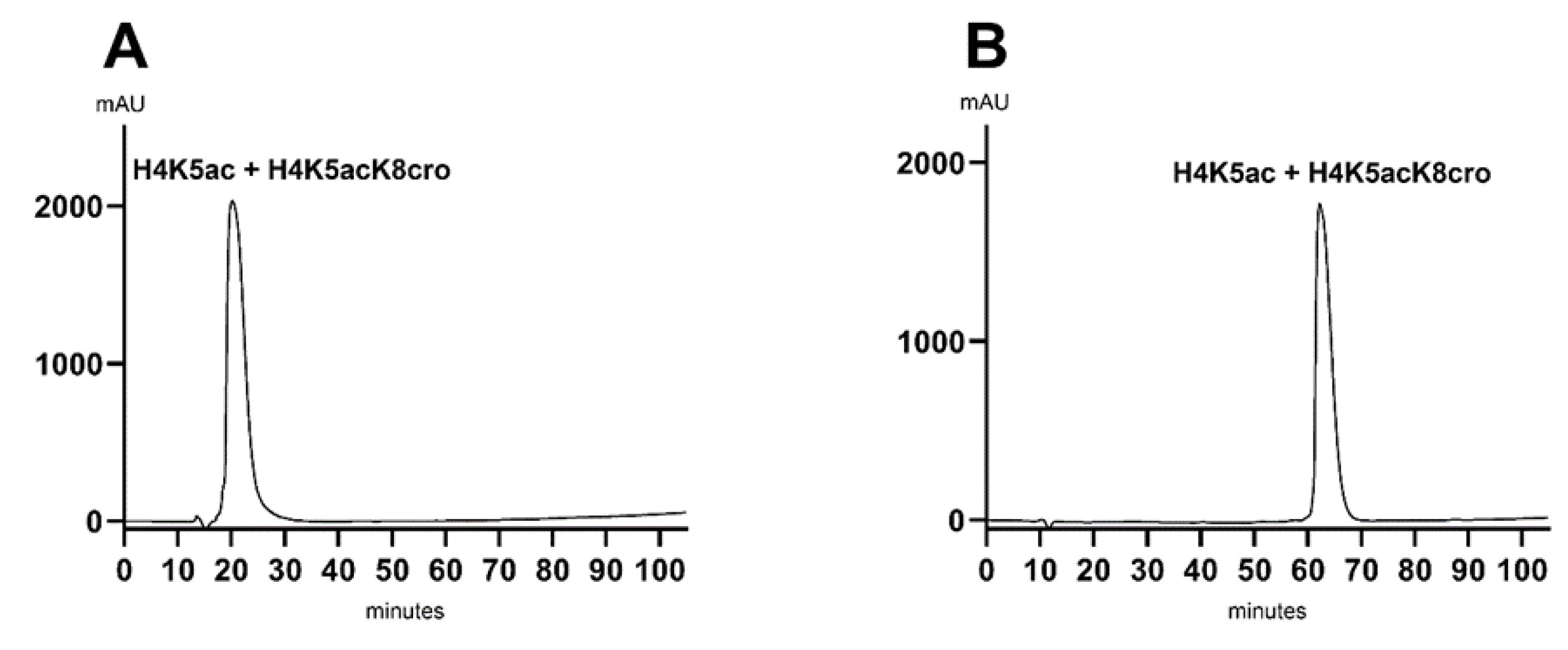
Before pursuing further optimization with ethanol, we decided to also evaluate isopropanol as a substitute for acetonitrile, given that its polarity is more similar to that of acetonitrile. However, like ethanol, isopropanol presents challenges due to its higher viscosity. Consequently, it was necessary to also reduce the flow rate when using this solvent. Using the 5% to 52% gradient method, we observed that the peptides did not bind to the column, as a single peak appeared in the unbound fraction (
Figure 4A). To promote peptide adsorption, we modified the method to start the gradient from 0% instead of 5%. As anticipated, initiating the gradient at 0% allowed the peptides to bind to the column, as evidenced by their elution occurring after the unbound fraction. However, despite improved retention, the peptides continued to co-elute as a single peak (
Figure 4B).
4. Discussion
Using an increasing gradient of acetonitrile, we successfully achieved the separation of two peptide molecules differing by only 70 Da. Having such a precise purification method is crucial for accurately assessing purity levels when incorporating post-translational modifications (PTMs) after peptide synthesis. To align with green chemistry principles, we first developed a shorter method, recognizing that minimizing solvent consumption is as important as selecting environmentally friendly solvents. Furthermore, we explored ethanol and isopropanol as alternative solvents to acetonitrile. We limited our study to these two solvents because their UV cut-off permitted peptide monitoring by UV at 214nm. Indeed, the solvent’s UV cut-off value must be below the UV absorption maxima of the studied molecule. At the same time, the solvent’s viscosity must be low to avoid generating high column backpressure. For example, glycerol has a UV cut-off below 214 nm but its high viscosity (
Table 1) makes it almost impossible to use in HPLC studies. Alternatively, a mass detector could be used instead of a UV detector; however, this setup is less common in laboratories and thus difficult to access. Even so, there are applications in the literature, for example, some research has been conducted with acetone, demonstrating that it can successfully replace acetonitrile in RP-HPLC when mass spectrometry detectors are used for peptide analysis [
19,
20,
21]. However, as stated above, UV detectors are still the most commonly used in pharmaceutical analysis, meaning acetone is not a favored green solvent to substitute acetonitrile in RP-HPLC. Another green solvent, dimethyl carbonate (DMC), is considered a promising alternative for industrial use. Recently, a study by Bozza et al. [
22] showed that DMC could be used in the mobile phase for preparative chromatography to purify a therapeutic peptide (Icatibant). However, its use in liquid chromatography is still very limited [
23], which might be due to the lack of commercially available HPLC-grade DMC. In frame with the objective of our study, it might be promising to also test DMC in the future, when HPLC-grade DMC will become available.
For the reasons mentioned above, our study was limited to only three solvents. Among them, acetonitrile remained the most effective, as it was the only one that enabled the separation of peptides differing by just 70 Da in molecular weight using our C18 column (5 μm, 4.6 × 250 mm). At present, we were unable to achieve baseline separation of these peptides using ethanol or isopropanol. A plausible reason could be the molecular interactions between the mobile phase, the peptide and the stationary phase. Ethanol and isopropanol are polar protic solvents, whereas acetonitrile misses hydrogen atoms, making it aprotic but still polar. Thus, compared to acetonitrile, the greener solvents evaluated in this study might have stronger interactions with the peptides due to hydrogen bonds formed with the solvent’s alcohol groups. Additionally, compared to acetonitrile, ethanol and isopropanol should have fewer interactions with the C18 column. The protic solvents’ higher affinity towards the peptides relative to the stationary phase could be eluting the peptides more rapidly from the C18 stationary phase; thus, it would decrease the elution sensitivity for closely similar moieties. Similarly, the protic solvents’ weaker affinity towards the C18 hydrophobic stationary phase may lead to heterogeneous interactions between the solvent, column, and peptides. Achieving a more homogeneous distribution between the mobile and stationary phases could improve the separation of similar peptides. As the two peptides in this study differ by only a single charge on the lysine residue, it might be difficult to separate them based on hydrogen interactions. The addition of acylation on lysine residues removes their charge. It would be more suitable to have significant charge differences between the peptides to separate them when working with protic solvents. Furthermore, a hydrophilic stationary phase might give more promising results for separations based on hydrogen interactions. This kind of alternative stationary phase screening should also be tested in the future for the investigation of ethanol and isopropanol in the purification of PTM-bearing peptides. In conclusion, our findings should not be interpreted as a definitive limitation of these solvents for the purification of PTM-bearing peptides. Other post-translational modifications, which were not examined in this study, may respond differently to these solvents. Moreover, the absence of a column oven in our setup hindered full optimization, as elevated temperatures could reduce solvent viscosity and improve chromatographic performance. However, with industrial applications in mind, a green solvent capable of separating these two peptides without heating would be more suitable, since column ovens are rarely used at industrial scale.
In summary, an RP-HPLC method capable of separating PTM-bearing peptides was found. Additionally, for future greener approaches, testing different HPLC columns, both hydrophobic but also hydrophilic, alongside broader solvent screening is necessary.