Abstract
The recycling of used lithium-ion batteries has become a growing concern. As a large number of rare metal elements are present in waste lithium-ion batteries, recycling them can significantly improve resource utilization and reduce the material cost of battery production. The process of recycling used lithium-ion batteries involves three main technology parts: pretreatment, material recovery, and cathode material recycling. Pretreatment includes discharge treatment, uniform crushing, and removing impurities. Material-recovery technology mainly involves traditional pyrometallurgical and hydrometallurgical technologies, as well as the developing biometallurgy technology. Analysis of existing data shows that pretreatment technology is crucial for the recycling of used lithium-ion batteries. Hydrometallurgical technology and pyro-hydrometallurgical technology are expected to be the most suitable industrialization technology paths in the future, with biometallurgical technology and direct recycling technology providing a low-pollution development direction. This article summarizes the different pretreatment techniques and valuable metal-recovery pathways. The advantages and disadvantages of each method were evaluated. The economic costs, environmental benefits, and degree of industrialization of each method were assessed. The possible development directions of various methods are summarized to provide reference for future research.
1. Introduction
The global new energy market has been proliferating in recent years. In 2022, over 7.8 million electric vehicles (EVs) were sold, marking a 68% year-on-year increase and constituting 10% of new vehicle sales [1]. Borgwardana’s 2023 performance report predicts EV sales will reach USD 2.3 to USD 2.8 billion in 2023. The company’s fourth-quarter 2023 reports highlight the significant market share of power batteries (as shown in Table 1) [2]. Properly managing waste lithium-ion batteries is essential for social development and ecological protection [3].

Table 1.
Fourth quarter 2023 organic net sales change (unaudited in millions) [2].
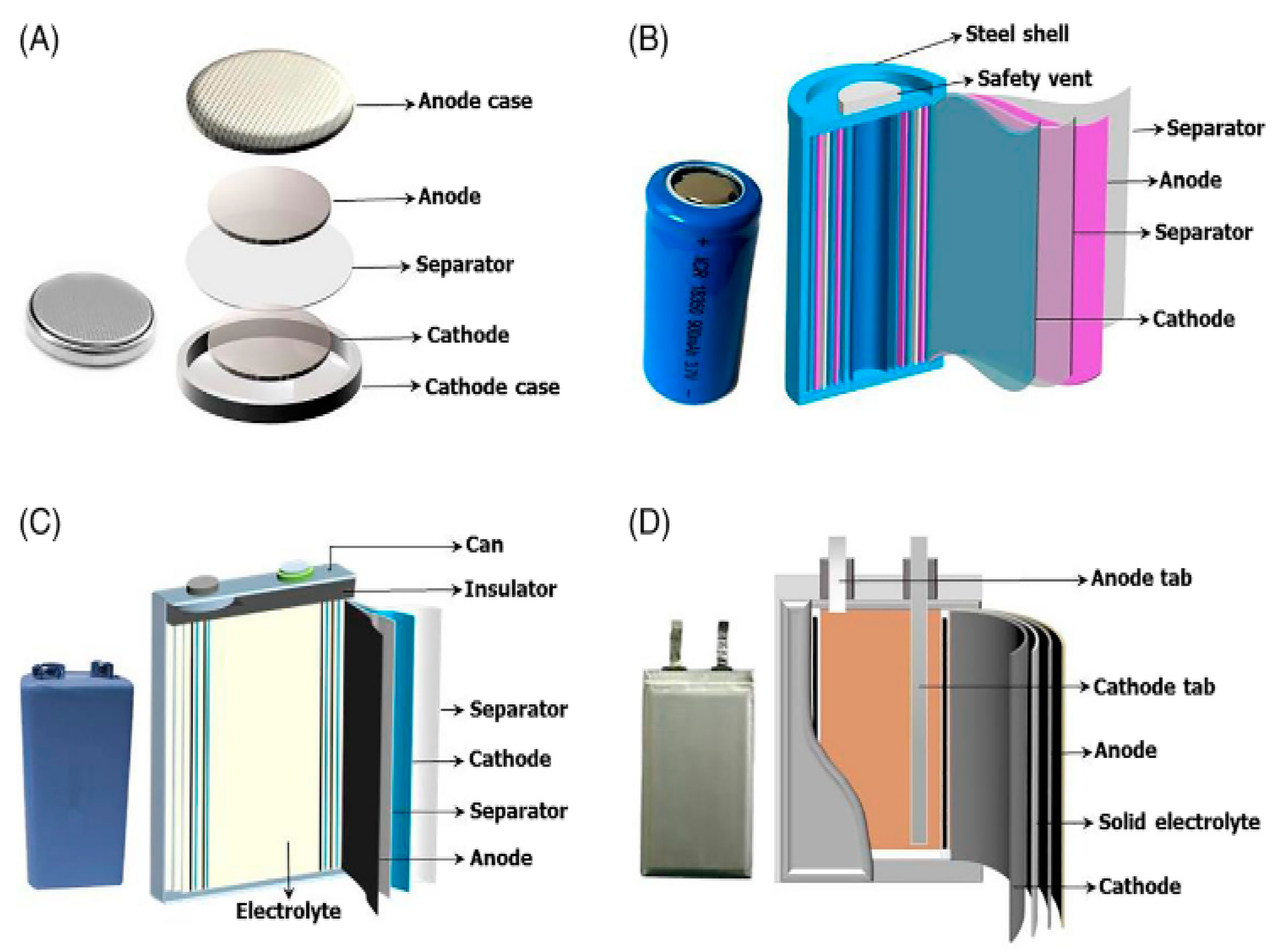
The power battery contains high-value metal materials such as Li, Ni, Co, and Mn, enriching its economic value. Generally, graphite is used as the anode material, and there are various choices of cathode materials, such as (LiFePO4) [4], (LiCoO2), (Li(NiCo-Mn)O2), (LiMn2O4), (LiV2O3), and (LiNiO2) [5]. The electrolyte usually consists of a vinyl carbonate solvent [6], dimethyl sulfoxide, propylene, and a salt solution. Even though the types of batteries differ, their internal structures are very similar, indicating that most batteries are rich in high-value metal materials (as shown in Figure 1) [7]. The cost of these cathode materials can typically account for 20% or more of the entire battery’s price, as the proportion of these metal materials in nature is very small, and the mining cost is relatively high. Therefore, there is a high motivation for recycling waste lithium-ion batteries at the economic level.
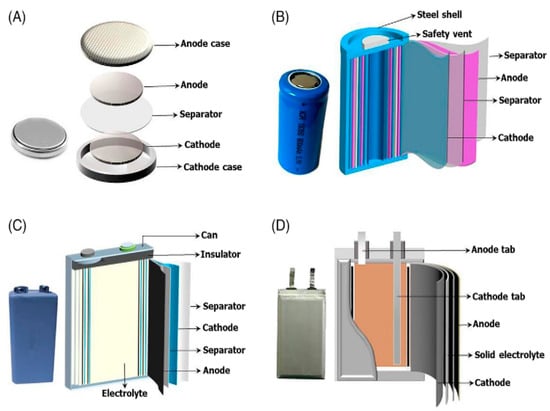
Figure 1.
Different kinds of battery structure [7]. (A) Small button battery, (B) small rechargeable battery, (C) power battery monomer, (D) another configuration of power battery unit. The structure of the above batteries can be classified into positive and negative electrode materials, shells, and electrolytic materials.
Secondly, due to the complex structure of the battery, as shown in Figure 1, spent lithium-ion batteries will cause great harm to the environment and human health. The enrichment of metal materials in the battery makes it difficult to degrade under natural conditions. Some substances in the electrolyte will produce harmful gases during the degradation process. For example, LiPF6 in water will react and generate HF, and P2O5 will be produced during incineration. Unhealthy substances can poison land resources, pollute groundwater resources and air, and ultimately lead to threats to human health [8]. Therefore, recycling used lithium-ion batteries is also necessary from an ecological and health perspective.
The first step in recycling batteries is to pre-treat used batteries, which includes discharge, crushing, and flotation. Currently, the more mature discharge schemes are the use of conductive salt solution [9], conductive metal powder, conductive graphite powder [10], and direct extrusion discharge. Among them, the salt solution discharge method is cost-effective. Crushing technology can be divided into dry crushing and wet crushing, as well as hammer crushing and shear crushing. Mechanical crushing is a prevalent and effective method in industrial production. Finally, flotation technology is used to remove some impurities from the material [11]. The second step is the material-recovery phase. In this article, we will introduce pyrometallurgical technology [12], hydrometallurgical technology [13], biometallurgical technology [14], and direct repair technology.
2. Pre-Processing Technology
According to a study by Yu Dawei’s group, it is important to note that the pre-processing technology of lithium-ion batteries is crucial for their recycling. After pre-processing lithium-ion batteries, we can effectively prevent environmental pollution caused by the liquid in the batteries and make better use of the precious metals found in spent lithium-ion batteries [15].
2.1. Discharge
When recycling spent lithium-ion batteries, it is important to note that the battery will not be fully discharged. Before mechanical treatment, the battery has to undergo a discharge treatment to reduce the risk of cell explosion in the subsequent process [16]. Typically, NaCl and FeSO4 solutions are used to soak the discharge of the recycled materials. This kind of technology effectively discharges the battery at a very low cost, but it also has a disadvantage—it can easily produce explosive gases like H2 [17].
Jiang’s team conducted a study on the impact of different salt solutions on battery discharge. They compared the metal ion residue and discharge time of 5% Na2S solution, NaCl, Na2SO4, and Na2CO3 discharge solutions. The results indicated that the Na2S solution had only 0.2 M Li, 0.3 M Ni, and 0.011 M cobalt plasma at the end of discharge. Additionally, the metal ion content in the precipitate was lower than that of other discharge solutions (see Table 2 and Table 3) [18]. However, the discharge process took longer. This method has minimal corrosive effects on the battery, resulting in low environmental pollution and good emission control, but the efficiency is relatively low.

Table 2.
Mass fraction of the major element ions in supernatant [18] (10−6).

Table 3.
Mass fraction of major elements in precipitates [18] (%).
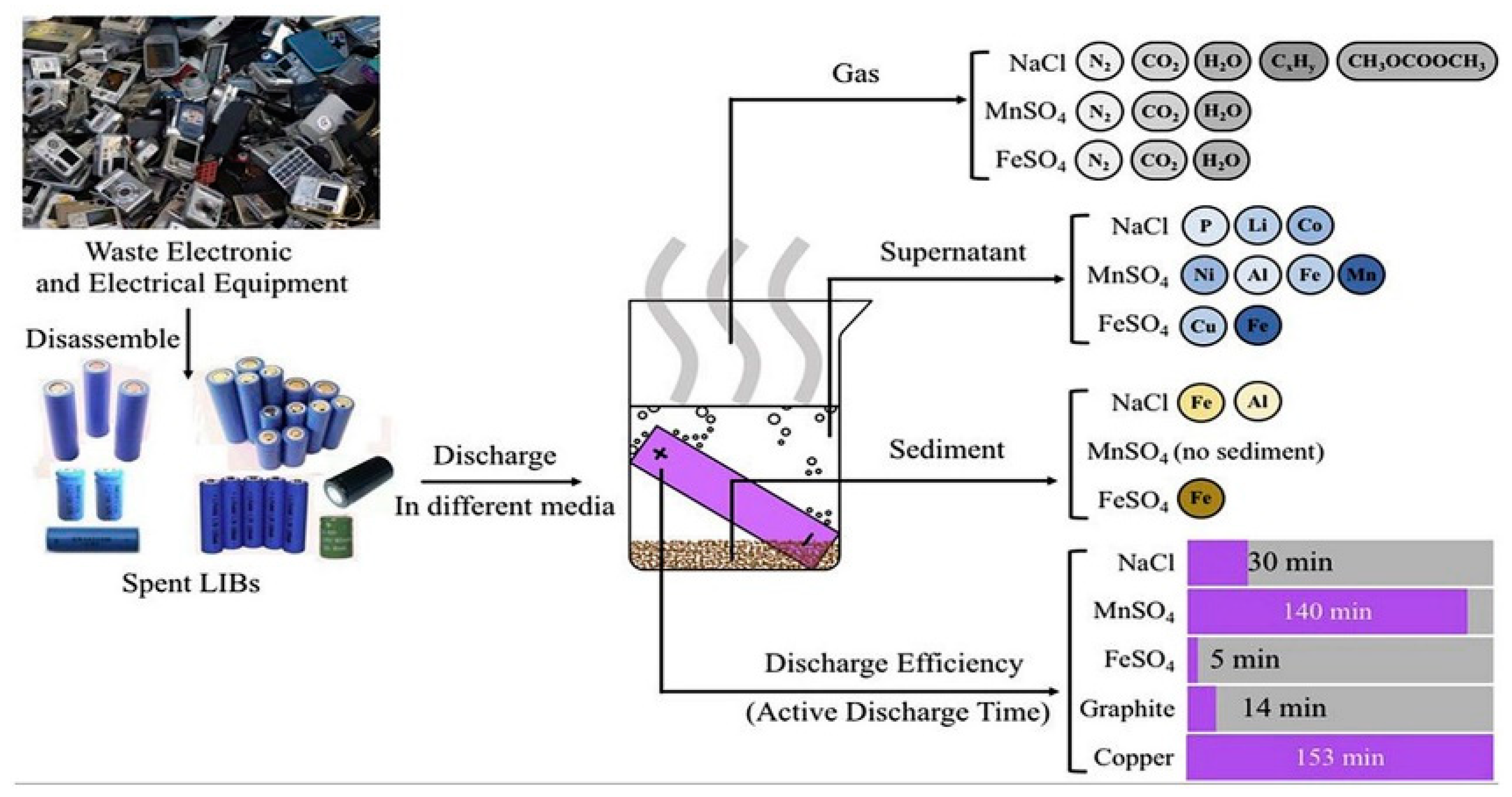
Yao’s team explored the effect of FeSO4 solution on battery discharge. FeSO4, NaCl, and MnSO4 solutions were used to conduct parallel experiments with the discharge rate and metal ion residue detection. A fast discharge model using FeSO4 solution reduces the residual voltage to 1.0 V at 125 min. A total discharge model using NaCl solution and FeSO4 solution reduces the residual voltage to 0.5 V or less in 183 min. At the same time, nitrogen, carbon dioxide, and water vapor were mainly excluded in experiments with FeSO4 solutions. Cu and Fe are mainly residuals in the solution. The results show that the FeSO4 discharge solution has less pollution to the environment and has high discharge efficiency (the process is shown in Figure 2) [19].
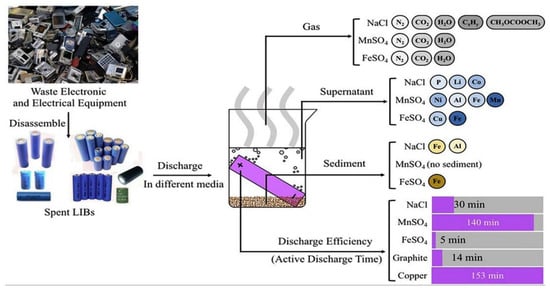
Figure 2.
Using FeSO4, NaCl, and MnSO4 solution to discharge spent battery. The gases produced in use of the three discharge solutions and the mixed discharge fluids, the ion content in the solution, the metal ion content in the precipitate, and the discharge time [19].
2.2. Crushing, Screening, and Sorting
Currently, crushing technologies mainly include hammer crushing, wet crushing, and shear crushing [20,21]. After crushing, the materials need to be sorted layer by layer to separate the plastic, separator, metal shell, and electrode powder. The electrode powder consists of graphite as well as valuable metal oxides, which require further processing.
Direct screening allows for the enrichment of particles of a specific size. Nan’s group used a mechanical disassembly method to effectively separate the metal casing while exposing the valuable materials inside the battery using a sieve [22]. Granata’s team obtained electrode powder, which accounts for 49% of the original mass of used lithium-ion batteries, after two crushing and mesh screening, while the rest of the metal casing remained on the sieve [20]. Maschler’s team grinding and sieving analysis of used lithium-ion batteries showed that the coarse particles (+200 μm) were mainly composed of ferrous metal shells, copper foil, and aluminum foil, while most of the substances containing Co and Li were concentrated in −100 μm. Later, more than 90% of the Co and Li were concentrated in −200 μM by fine crushing, magnetic separation, and fractionation [23].
Magnetic screening is an effective method for separating the metal shell and internal components of used lithium-ion batteries. Shin’s team utilized waste lithium-ion batteries and conducted two rounds of crushing and screening in addition to one round of magnetic separation to separate them from the plastic packaging. During the initial crushing vibration, the magnetic separator successfully separated the shell material in the battery, while during the second crushing vibration, the cathode material was completely separated from the aluminum foil [24]. Li’s team proposed a new method involving anaerobic roasting and wet magnetic separation to treat powders enriched with LiCoO2 and graphite after mechanical crushing. Through high-temperature burning, metal cobalt and lithium carbonate are directly obtained. Subsequently, the powder is treated with water using a magnetic stirrer, allowing the lithium carbonate to dissolve directly, the metal cobalt to be adsorbed on the magnetic stirrer, and the graphite to precipitate at the bottom of the water, thereby completing the separation of substances without the need for additional chemicals [25]. This method enables the eco-friendly and efficient recycling of used lithium-ion batteries.
The flotation process primarily utilizes hydrophilic cathode material, which sinks to the bottom of the water when wet. On the other hand, the anode material, graphite, is not hydrophilic and will adhere to air bubbles and float on the liquid’s surface. N-dodecane, kerosene, and similar substances are commonly used as collectors in flotation, while methyl isobutyl methanol (MIBC), octanol, pine oil, and others are used as foaming agents. Recent studies have shown that using the Fenton reagent in flotation can help reduce the impact of the organic film in batteries on the flotation process compared to direct flotation. Yu’s study found that the Fenton reagent increased the LiCoO2 grade from 55.63% to 90% for the same raw material [26]. Fenton’s reagent can enhance the mineral grade of the obtained substances by breaking down the organic film through the generation of hydroxyl radicals.
In general, there are three main methods of battery sorting technology. The results show that the direct sieving method is low-cost and effective for the separation of metal shell and electrode material. However, due to the mechanical structure, it is not possible to subdivide too small particles. The magnetic separation method is an efficient method of separating metal components from internal materials and can achieve very little pollution and waste through process optimization design, which is a green sorting method, but the equipment cost is high, and the economic cost of large-scale use needs to be considered. Finally, there is the flotation method, which makes good use of the hydrophilic and hydrophobic properties of different substances. However, it will be affected by the battery separator, which will lead to a decrease in efficiency. While Fenton’s reagent is a good solution to this problem, it does require the pH of the reaction. In addition, the handling of organic reagents is an issue that must be considered.
3. The Technology of the Material-Recycling Steps
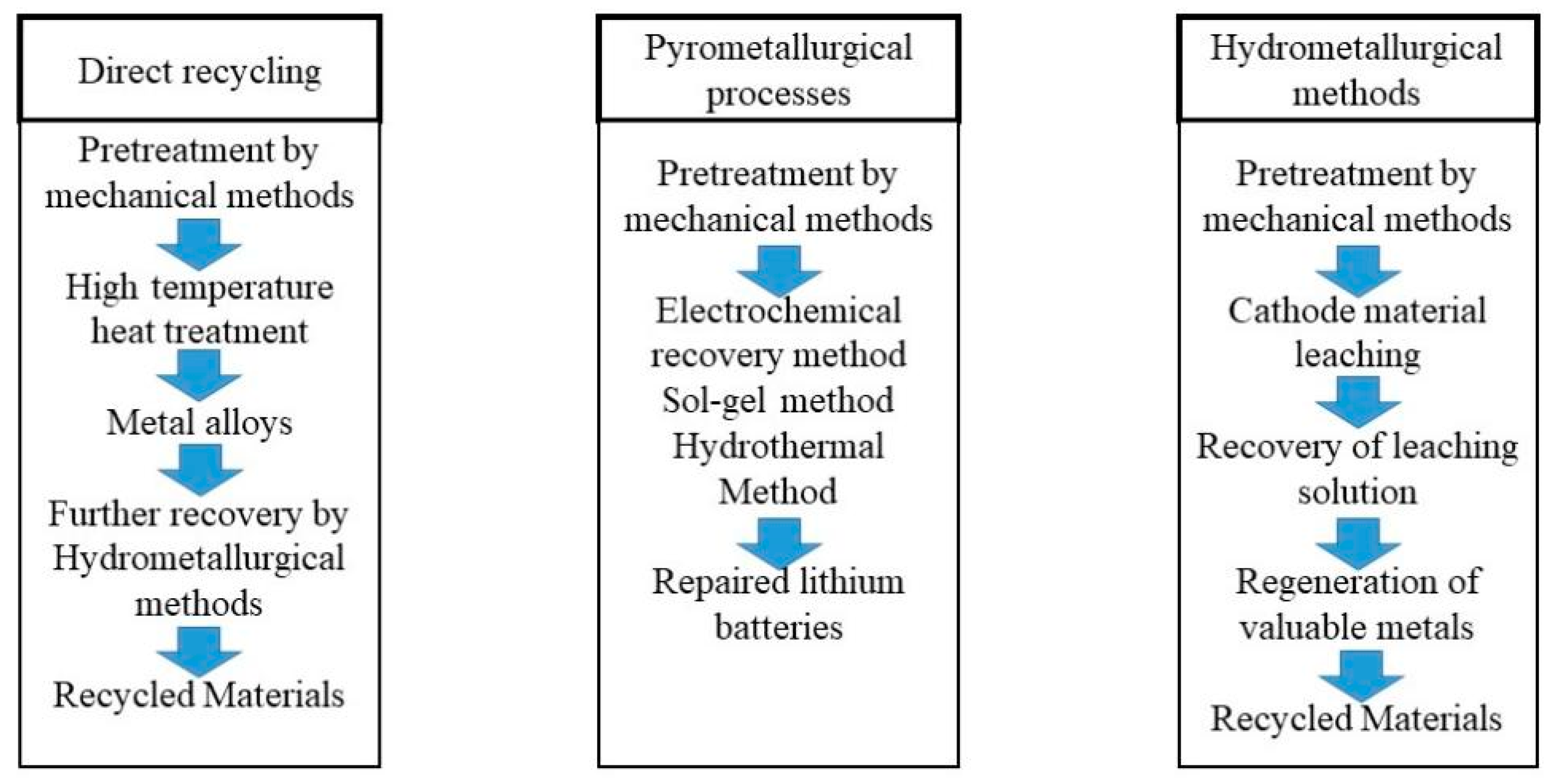
Now the spent battery technologies include a pyrometallurgical method, hydromel-metallurgical method, direct recovery method, and metallurgy method [27]. Figure 3 shows the path [28]. The first three techniques have been in development for a long time and they are more mature. The metallurgy one is currently more in the laboratory technology stage.
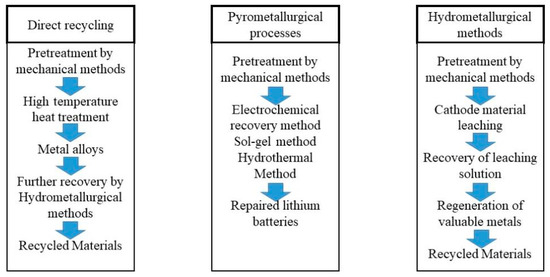
Figure 3.
Classic waste lithium-ion battery-recycling technology. The general process of direct repair, hydrometallurgy, and pyrometallurgy was introduced [28] (the content is from the quotation, and the picture is drawn by the author).
3.1. Combination of Pyrometallurgical Technology and Hydrometallurgical Technology
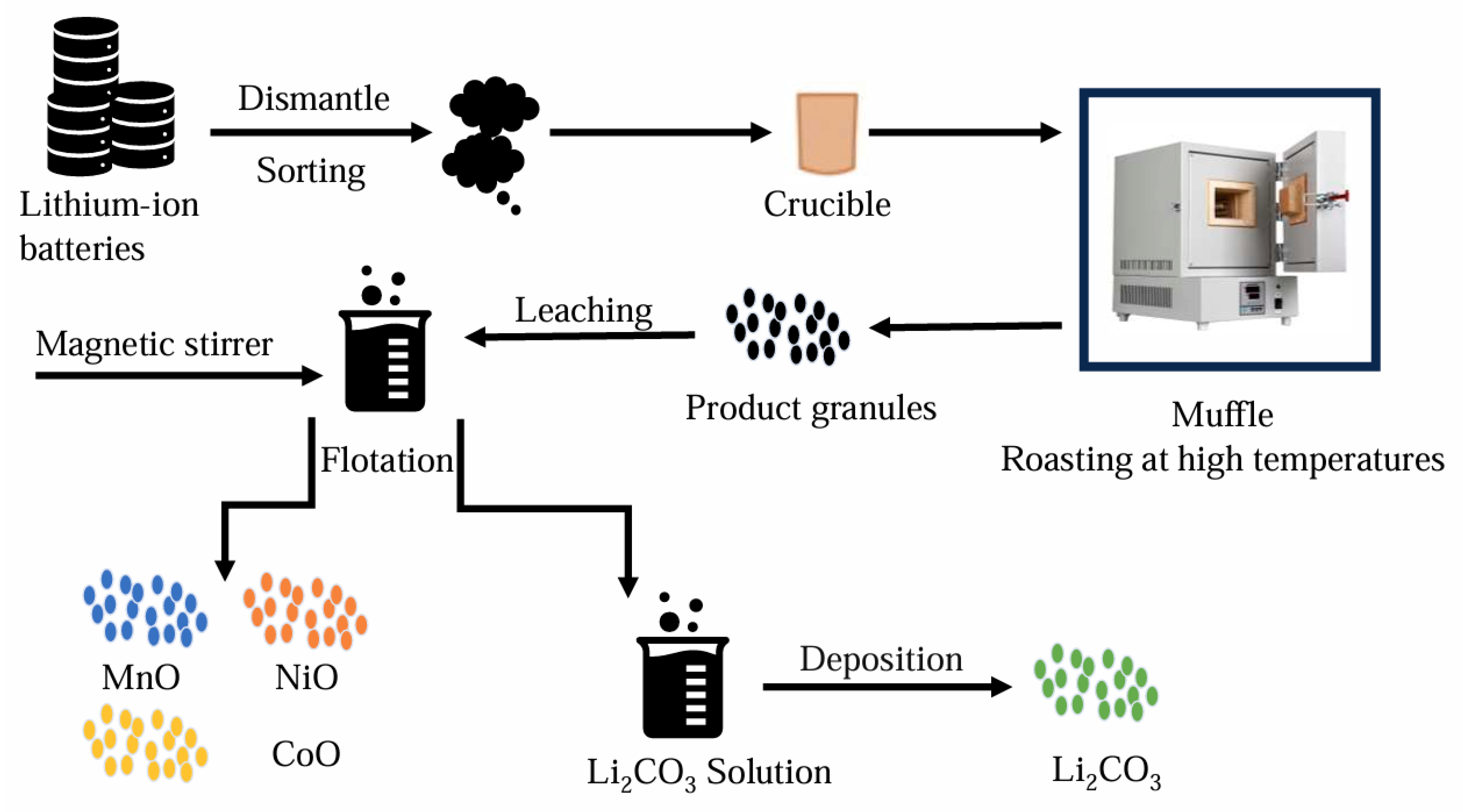
Pyrometallurgy is the process of using high-temperature heat to separate target metal materials from impurities through physical and chemical reactions. The use of pyrometallurgical technology is a classic treatment method for waste resources. High-temperature treatment can destroy the complex structure in the battery and melt the metals. The separation of the essential components in the cathode of a lithium-ion battery is achieved via a high-temperature method [29]. In general, manganese and lithium produce oxides in oxygen-rich environments. Other metals are alloyed at high temperatures, which makes it relatively easy to separate the relevant metal materials from waste lithium-ion battery materials [30]. However, from the perspective of environmental protection, pyrometallurgical technology has to produce a large number of harmful gases and sewage. And in the smelting process, there will be more loss of valuable metal materials. Therefore, from the perspective of the recycling and environmental benefits of the whole process, pyrometallurgical technology is facing great challenges [31,32]. Therefore, an increasing number of scholars have started studying the combination of pyrometallurgy and hydrometallurgy. They aim to utilize the characteristics of pyrometallurgy to eliminate complicated pretreatment steps and, at the same time, employ hydrometallurgy to achieve higher metal-recovery efficiency. (as shown in Figure 4).
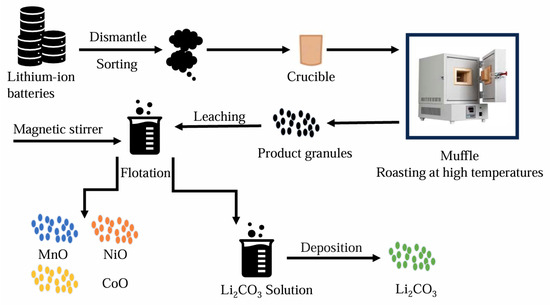
Figure 4.
Schematic diagram of the hydro-pyrometallurgical recycling process for spent lithium-ion batteries (drawn for the content of the article).
Dang’s team investigated the effect of Gibbs’s free energy and reaction equilibrium constants using calcium chloride to affect metallurgical reactions on the pyrometallurgical treatment of used batteries. They added CaCl2 at a ratio of 1.8:1 Cl:Li, and roasted at a temperature of not less than 500 °C. The results showed that LiCl produced in the chlorination reaction was a good chlorine donor, and the final optimal roasting conditions at 800 °C for 60 min, Cl/Li molar ratio of 1.8:1, and subsequent leaching conditions at 60 °C for 30 min, with a water/calcination mass ratio of 30:1, could produce a maximum lithium-recovery rate of 90.58% [33].
Gou Haipeng researched the use of pyrometallurgy to extract metals from waste batteries in a nitrogen atmosphere. Nitrogen was used as the gas atmosphere in the experiment, and activated carbon and alkali absorption devices were used to adsorb harmful gases. The results indicated that at 650 degrees Celsius, a black powder rich in graphite and metal can be obtained. The percentage of this powder is shown in Table 4 [34]. This method resolves the exhaust gases generated during the process and eliminates the risk of explosion. Afterward, the powder needs to undergo treatment using hydrometallurgical methods.

Table 4.
Proportion of different metals after purification [34].
Meng studied the effect of synergistic pyrometallurgy with organic waste on the pyro-wet treatment of spent lithium-ion batteries. In his experiments, he used waste plastics as carbon and hydrogen sources to produce reducing agents to achieve the reduction of valuable metals. The experimental results show that LiCoO2 is completely decomposed into Li2CO3, CoO, Co, and chloride for 90 min at 450 °C with a PVC/LCO mass ratio of 1:1. High recoveries of Li (92.50%) and Co (94.85%) can be achieved by water immersion. It is important to note that the Cl compounds released by the decomposition of PVC may be in metal chlorides. During the pyrolysis process, PVC can initially decompose to form carbon, gaseous hydrocarbons, and hydrochloric acid [35].
Liu studied the reactions of different spent lithium-ion batteries (LCO, LMO, NMC) and benzene-containing plastics (polycarbonate, polyethylene terephthalate, polybutylene terephthalate, polystyrene) in sealed reactors. According to the experimental results, the pyrolysis of the two together produces Li2CO3, metals, and metal oxides. The use of closed reactors can make the polycyclic aromatic hydrocarbons and lithium transition metal oxides undergo sufficient reduction reactions, and the lithium recoveries of LCO, LMO, and NMC can reach 98.3%, 99.9%, and 97.5% [36].
Recycling used lithium-ion batteries using a single pyrometallurgical technology is challenging due to environmental and economic reasons [37]. Currently, efforts are focused on combining pyrometallurgy with hydrometallurgy to create a more efficient recovery process that eliminates complex pretreatment steps. However, this approach requires significant equipment investment and still poses a gas pollution issue. Collaborative pyrolysis technology offers a promising solution by efficiently utilizing plastic waste and used batteries, but optimizing the process parameters, conditions, and equipment selection is crucial. When using household waste as a source of carbon and hydrogen, the presence of inorganic substances like minerals can significantly impact the recovery of valuable metals in cathode materials through pyrolysis. Additionally, addressing the emission of polluting gases during pyrometallurgy remains an important challenge to address [38].
3.2. The Inorganic Acid Hydrometallurgy Technology
The hydrometallurgical method is a method in which the useful metals contained in the raw materials are transferred to the liquid phase through chemical reactions after contact with aqueous solutions or other liquids, and then the various useful metals contained in the liquid phase are separated and enriched and finally recovered in the form of metals or other compounds. It mainly includes unit operation processes such as leaching, liquid-solid separation, solution purification, metal extraction in solution, and wastewater treatment. Compared to the limitation of the pyrometallurgy tech, the hydrometallurgy tech is used more widely than the former, and the recycling effect is better too [39].
Now, hydrometallurgy technology can process popular lithium iron phosphate batteries, ternary lithium batteries, nickel-metal hydride batteries, and so on [40,41]. The hydrometallurgy technology can be widely used, and there is in-depth study in this area. Based on the chemical properties of the leaching solution, the hydrometallurgical method can be roughly divided into two groups: the inorganic acid leaching method and the organic acid leaching method [42]. The concentration, temperature, solid–liquid ratio, and pH value of the reagents used in hydrometallurgy are all decisive factors.
Inorganic acid leaching is a well-established technique for extracting high-purity valuable metals from spent lithium-ion battery materials. This technique primarily utilizes solutions such as HCl, HNO3, and H2SO4. The final effect of these three leaching solutions varies due to their chemical properties. When using sulfuric acid and nitric acid as extractors, hydrogen peroxide needs to be added as an auxiliary infusion agent to achieve a better leaching effect [43]. Wang’s group uses sulfuric acid as an extractor to process spent lithium-ion batteries. Let Li, Co, Ni, Mn, and other valuable metal elements be leached. At the same time, add the KMnO4 to the leachate to selectively react with Mn to generate MnO2 and manganese hydroxide. Then Ni was extracted and separated using butanediketoxime, followed by selective precipitation of Co by sodium hydroxide (pH = 11). Finally, the Na2Co3 was used to form the Li2CO3 precipitation of Li in solution. The results of the test showed that it can achieve extraction purities of Li, Mn, Co, and Ni of 96.97%, 98.23%, 96.94%, and 97.43%, respectively [44].
Barik’s team investigated the effect of HCl as the leaching agent and HClO as the impurity remover on the extraction of used lithium-ion battery materials. They used a multi-dimensional, single-variable experiment to explore the optimal extraction conditions, and the results showed that the temperature was 50 °C, the concentration was 1.75 mol/L HCl, and the condition was within 90 min. The leaching efficiency of O2− and Mn2+ was higher than 99%. Also, 90% total Co recovery and 95% total Mn recovery can be achieved when the treatment scale is scaled [45].
Dorella’s group also used H2SO4 and H2O2 as the leaching agent to leach the cathode material of lithium cobalt oxide battery. Then they used NH4OH to remove Al from the leachate. Finally, Cyanex272 was used for extraction to separate the cobalt from the leachate. A study shows when the H2SO4 concentration is 6%, the H2O2 concentration is 1%, the solid–liquid ratio is 1:30, and the leaching rates of Co and Li can reach 80% and 95%, respectively [46].
The hydrometallurgical treatment of waste lithium-ion batteries using inorganic acids is an effective, stable, and widely applicable method. The extractant is cost-effective and has high extraction efficiency. However, a common disadvantage is the strong corrosive effect on the equipment. Additionally, the reuse performance of the extractant is poor. From an economic point of view, inorganic acid extraction is a technology with a small upfront investment, but the equipment maintenance cost is high in the later stage. Industrialization is relatively easy and suitable for large-scale production. From an environmental point of view, the inorganic acid extraction method produces a large amount of wastewater and complex waste gases, which needs to be purified before it can be used. Table 5 provides a summary of the above technical paths.

Table 5.
Leaching effects of inorganic acids.
3.3. The Organic Acid Leaching Method
The inorganic acid leaching method offers several technical advantages such as good scale efficiency, a simple technical process, and moderate cost. However, it generates a large amount of harmful gases and leads to strong corrosion of the recycling equipment, significantly reducing its service life [47]. On the other hand, the organic acid leaching method has emerged as a major research focus in hydrometallurgy due to its low pollution and low corrosive properties [48,49].
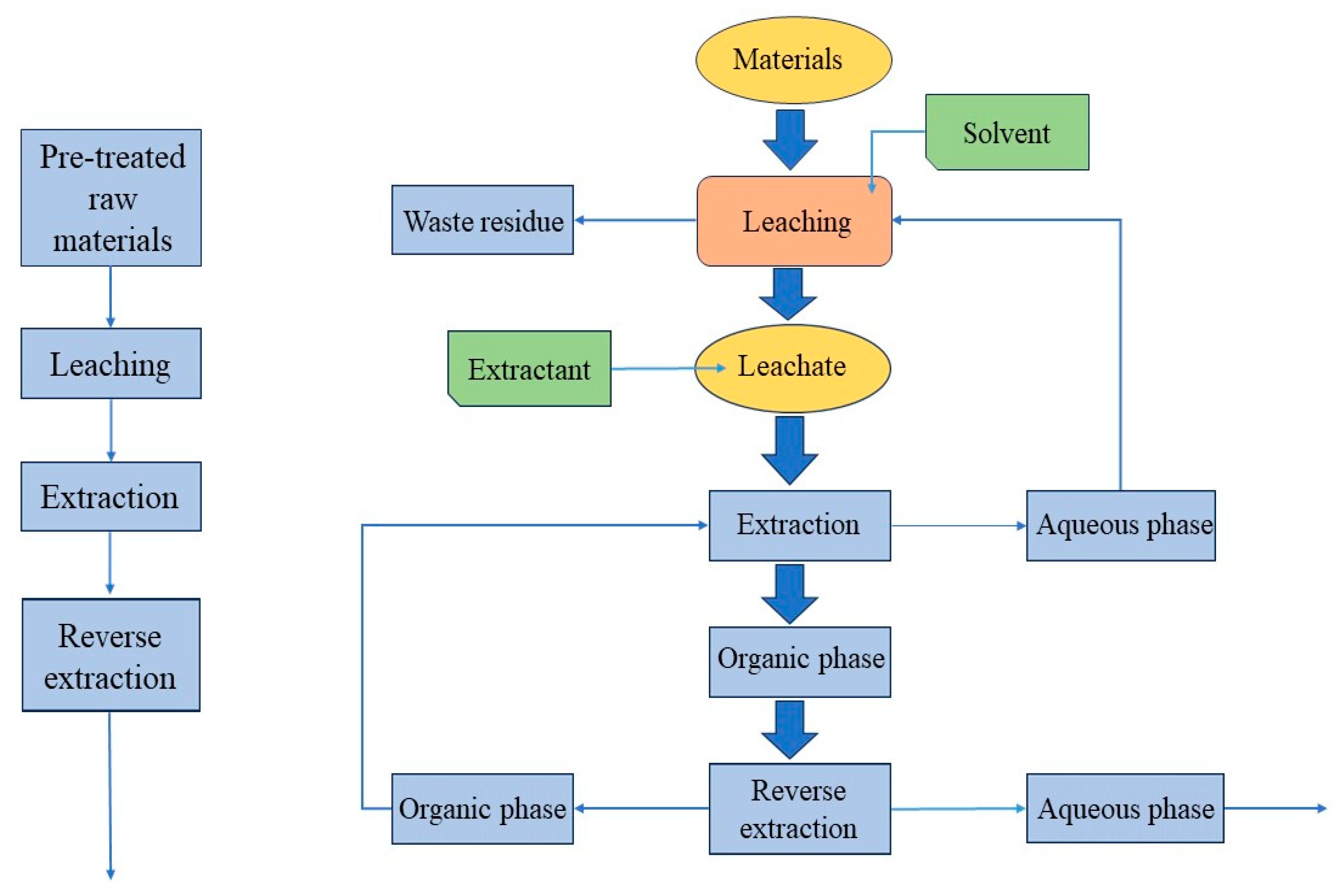
Organic acids can often be chelated with metal lithium ions due to their chemical structure and physical and chemical properties. The chelation reaction between organic acids and metal ions is usually multi-point, involving a variety of molecular forces. A strong chelating structure can be formed. So organic acid extraction can achieve the leaching effect of strong inorganic acids in a low acidity state. At the same time, many organic acids have their own reducing ability such as oxalic acid, citric acid, and ascorbic acid. As a leaching agent can promote the metal ions from a high-valence state to a low-valence state, researchers are trying to replace inorganic acids with organic acids to become the leaching agent of spent lithium-ion batteries [50]. The hydrometallurgical process of organic acids is mainly divided into several important steps: pretreatment, extraction, separation, and reverse extraction (as shown in Figure 5).
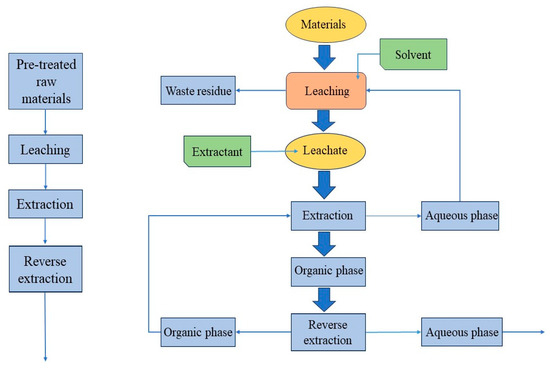
Figure 5.
Organic acid hydrometallurgical treatment process of waste lithium-ion batteries [51] (drawn for content).
Meng’s team studied the extraction of valuable metals from used lithium-ion batteries using malic acid as the extractant, and they used 1.5 M malic acid, 8 V voltage, 300 r/min, 60 °C, and extraction for 30 min. The results showed that the extraction rates of Li, Co, Ni, and Mn were 100%, 99.87%, 99.58%, and 99.82%, respectively [52]. Pant and Dolker proposed to use citrus juice (CJ) components to help dispose of spent lithium-ion batteries in their study. CJ is rich in citric acid malic acid and other organic acids. They can be used well as a complexing agent for ascorbic acid and citrus flavonoids for the reduction of a variety of metals. In the experiment, the positive electrode leachate was obtained by this method and then the pH value of Na2CO3 was adjusted to achieve selective precipitation. It is calculated that the method can achieve Li, Mn, Ni, and Co leaching rates of 100%, 99%, 98%, and 94% [53]. Sun’s group conducts leaching treatment with LiCoO2 by combining maleic acid and SnCl2. It was found that the leaching rates of Li and Co reached 98.67% and 97.5%, respectively, under the conditions of 1 mol/L maleic acid and 0.3 mol/L SnCl2 [54].
In summary, organic acids can be used as extractants to obtain better extraction results under milder conditions. This can greatly reduce the degree of corrosion in the operating equipment. However, compared with the low price of inorganic acids, the price of organic acids is higher, and the cost of use is relatively large. Yang studied the extraction effect of a mixture of acetic acid and ascorbic acid. They experimented with different concentration ratios and experimental conditions. Finally, it was found that 1 M acetic acid was mixed with 0.1 M ascorbic acid 1:1, working voltage of 4 V, reaction temperature of 25 °C, and reaction time of 70 min. The leaching rates of Ni, Co, Mn, and Li reached 99.8%, 99.8%, 99.8%, and 99.9%, respectively. At the same time, Yang proved that the surface chemical reaction of the metal determines the speed of the reaction [55]. This process also shows that chelation reactions are very important in organic acid leaching. This is also the difference between organic acid leaching and inorganic acid leaching.
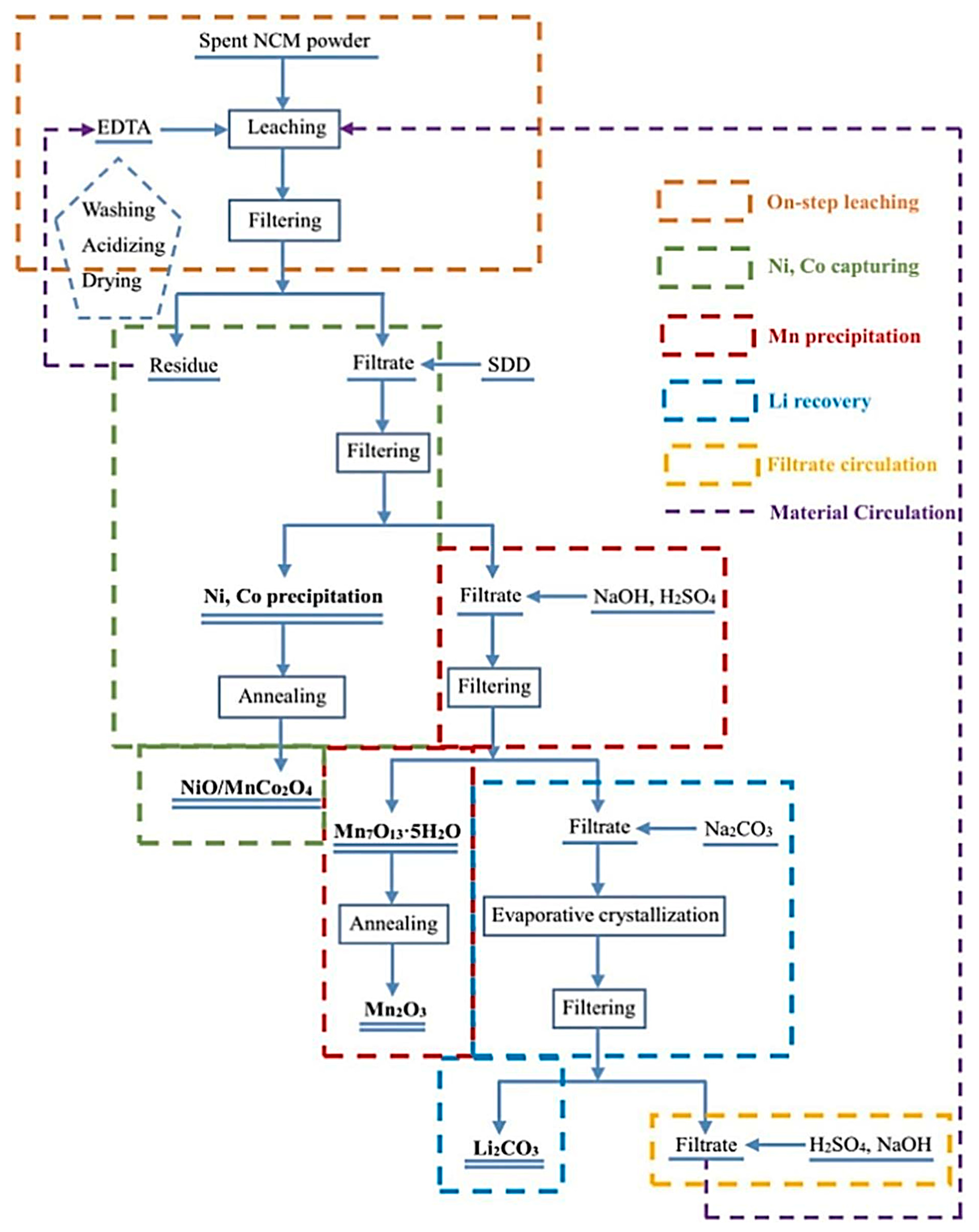
Su proposed the use of EDTA as a leaching-reducing agent to treat waste lithium-ion battery materials. Under the condition of 0.5 M EDTA, a solid–liquid ratio of 30:1 mL/g, 353 K, 2 h can achieve a metal ion leaching rate of 99.9%. In the experiment, the reaction thermodynamic mechanism proved that there is a synergistic leaching effect of Fe and Al. EDTA can also be recycled after separating the various ions by precipitation (as shown in Figure 6) [56].
Figure 6.
Schematic diagram of the extraction of used lithium-ion batteries using EDTA as an extractant [56]. The diagram illustrates the recycling process of organic extracts.
In conclusion, the above two methods show that the mechanism of organic acid extraction is not only the solubility of ions in different solutions, but also the complexation effect of organic solvents can enhance the extraction effect, and the recycling performance of the organic extraction system is better, which can reduce the increase in the cost of extractants.
On top of the non-selective leaching methods described above, selective extraction methods have also been proposed in some studies. Selective extraction of partial ions is achieved by different metal salts having different solubilities in the same extractant. Rouquette used the different solubility of different transition metal salts in oxalic acid as the main driving force for selective extraction. Under the conditions of 60 °C, 60 min, and 0.6 M oxalic acid, the leaching rate of lithium reached 98.8%. The leaching rate of cobalt and nickel is only 1.5%. Efficient recovery of lithium is achieved [57].
Wang Kun used a completely different extraction strategy in his study. The simulated complex metal ion solution was extracted using an extraction system consisting of 0.4 mol/L 2-thenoyltrifluoroacetone (HTTA), 0.4 mol/L trioctylphosphine oxide (TOPO), and ionic liquid (IL) 1-butyl-3-methylimidazolium bis (trifluo-romethyl sulfonyl) imide. Under the conditions of O/A = 1/1, 25 °C, 20 min, and pH = 3.65, the extraction rates of Co, Mn, and Ni reached 99.54%, 99.85%, and 99.68%, while the extraction rate of Li was only 2.84%. This achieves the effect of selective extraction [58]. The above two solutions can achieve high-purity extraction of Li to prepare for the subsequent efficient recovery of metal materials and the production of high-quality recycled cathode materials. Table 6 will show some representative techniques.

Table 6.
Leaching effects of organic acids .
In summary, the organic acid leaching method can achieve the extraction effect of inorganic acids under milder extraction conditions under more extreme pH conditions, which can reduce the corrosion rate of the equipment. The upfront investment cost of organic acid extraction is relatively high, and the price of reagents is also high. However, it can effectively solve the problem of poor circulation of inorganic acids and serious corrosion of equipment. Similarly, the exhaust gases obtained by the organic acid extraction method are complex and can be used in other industrial processes after purification. With the reasonable circulation design, the wastewater generated by the organic acid extraction method can be greatly reduced and the environmental pollution can be reduced [62]. There is still great room for the development of the organic acid leaching method in the future. We need to focus on finding an organic acid leaching agent with high unit leaching efficiency and relatively low cost.
3.4. The Direct Recycling Technology
The charging and discharging of lithium-ion batteries is essentially the cyclic process of directional movement and removal of Li+ inside the battery. As the charging and discharging process progresses, the crystal structure of the cathode material of lithium-ion batteries continues to expand and contract. As a result, the battery loosens the cathode material structure, which is reflected in the macroscopic decline of the capacity of the battery [63].
The direct recycling method repairs the cathode material of lithium-ion batteries that have been retired because of battery capacity decline through some specific physical and chemical reactions. Now the main technical paths include atmospheric pressure lithiation of low eutectic solvents, hydrothermal lithiation, electrochemical method, solid phase method, and so on [64].
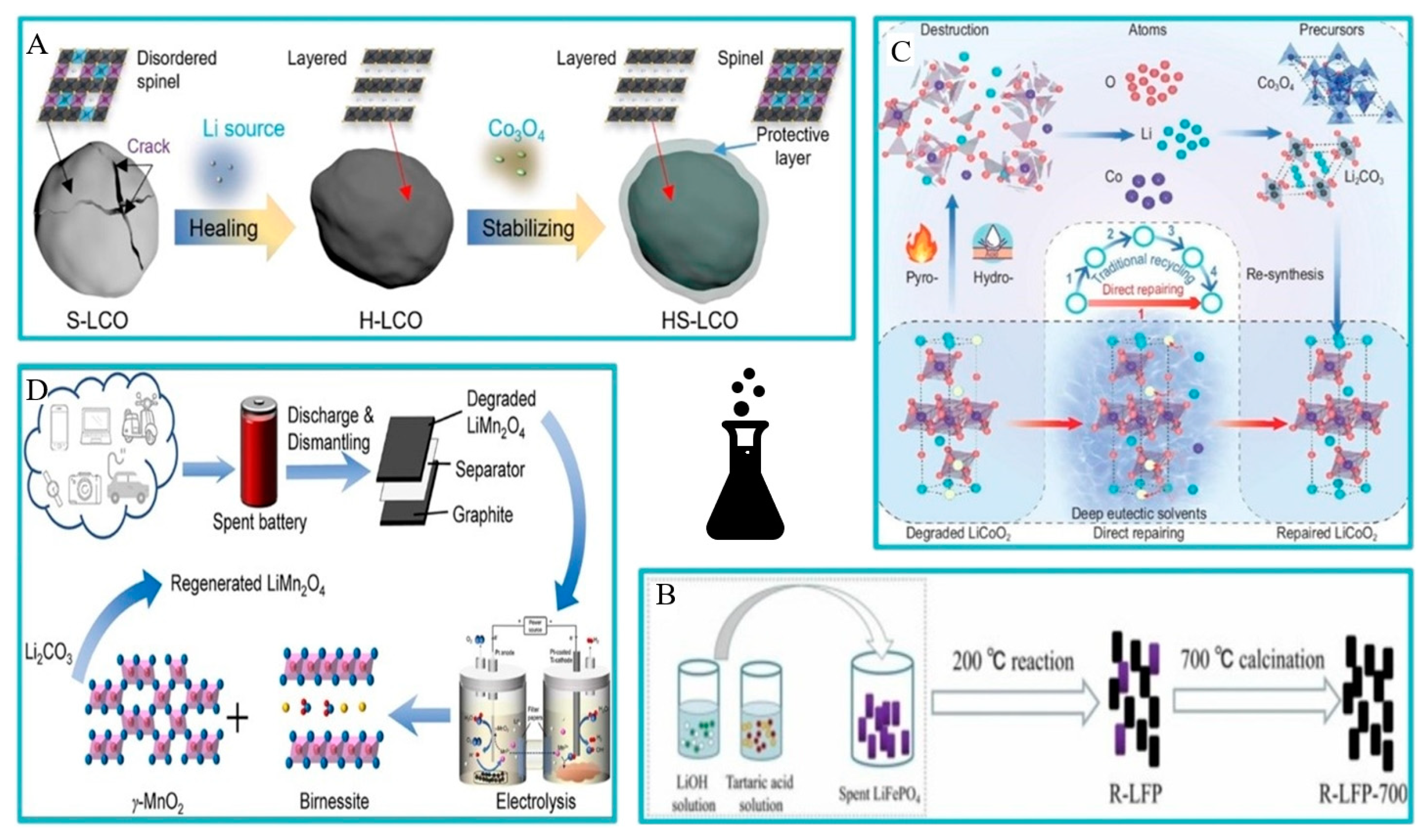
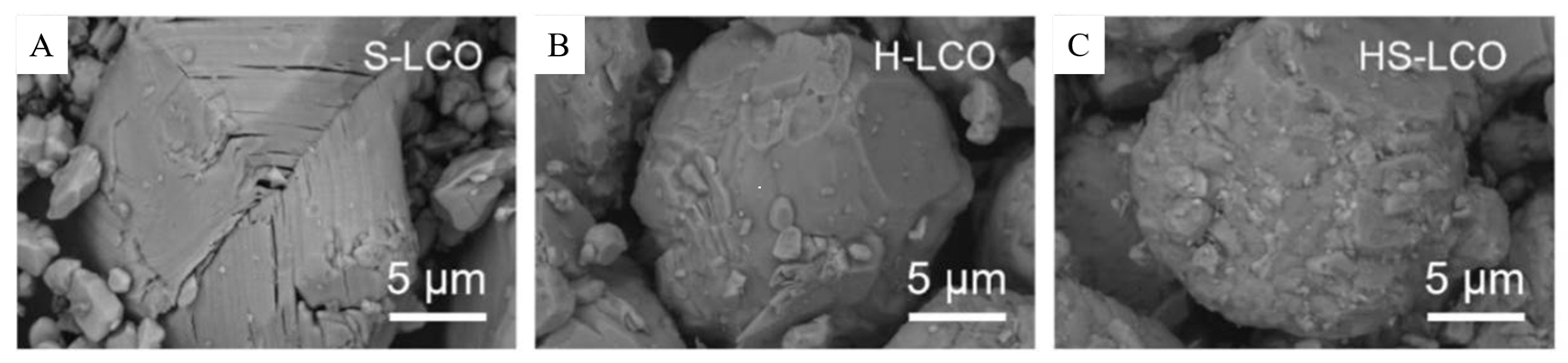
In Ji’s solid-state method research, dimethyl carbonate was used to clean disassembled mobile phone batteries. The batteries were then calcined into spent LiCoO2 (S-LCO) powder in the air. Annealing with a 1.03 ratio of lithium carbonate to Li/Co mixture at 850 °C for 10 h was carried out. The experimental results suggest that this process smoothens the surface of LCO and forms H-LCO, which helps repair the damaged structure. The resulting product is mixed with Co3O4 at 300 °C and further annealed to create a spinel protective layer, forming the regenerated LCO (HS-LCO) (this is shown in Figure 7B). HS-LCO demonstrates a high specific capacity retention rate of 85.9% after 100 cycles (as shown in Figure 8) [65].
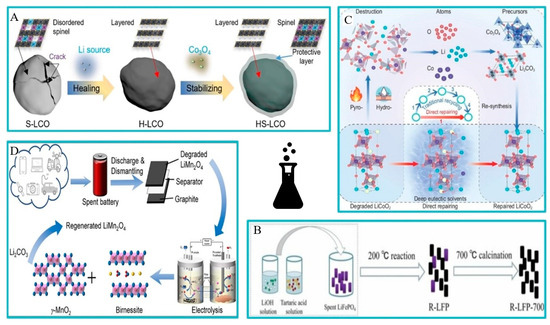
Figure 7.
Schematic diagram of the different direct recycling processes. (A) Schematic diagram of the solid-phase method [65]. (B) Schematic diagram of hydrothermal lithification [66]. (C) Schematic diagram of the physical and chemical method of low eutectic solvent under atmospheric pressure [67]. (D) Schematic diagram of the electrochemical method [68].

Figure 8.
Morphology and structural characterizations of S-LCO, H-LCO, and HS-LCO. SEM images of (A) S-LCO, (B) H-LCO, and (C) HS-LCO. It was proved that this method can repair the damage of the material [65].
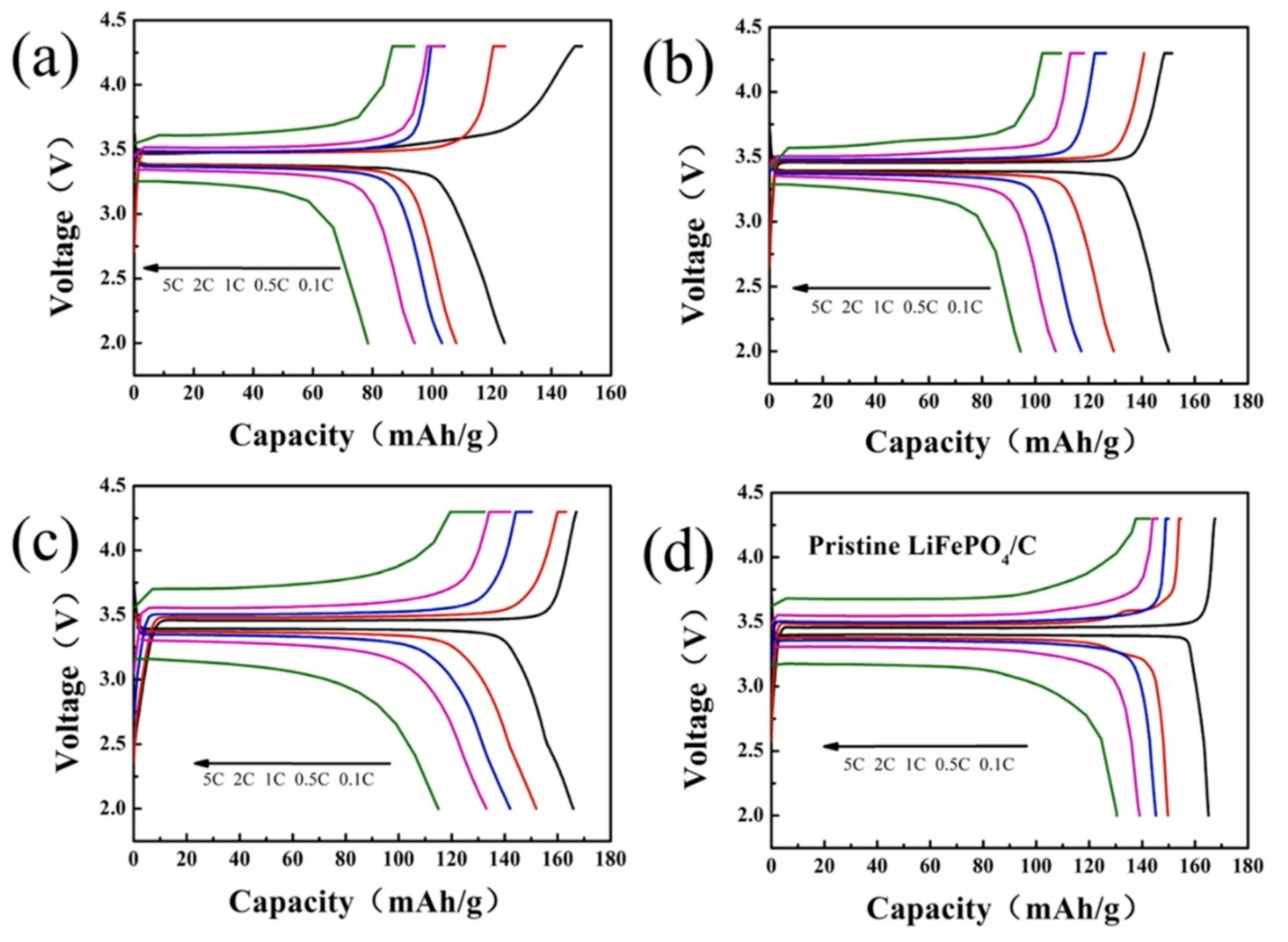
Chen et al. used LiOH as a lithium source and tartaric acid as a reducing agent in their study. After hydrothermal treatment, the regenerated LFP was annealed in Ar gas at 700 °C for 2 h (as shown in Figure 7C). The characterization image shows that the lattice distortion and fringes disappeared after processing. Due to the combination of hydrothermal treatment and annealing, the discharge capacity of the regenerative battery material at 1 °C is 145.92 mAh·g. Experiments show that the discharge performance of the repaired material is greatly increased (as shown in Figure 9) [66].
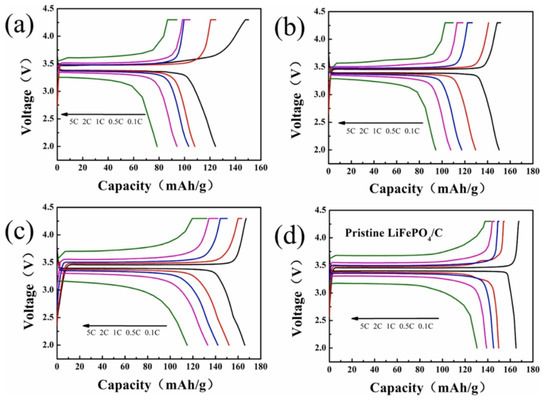
Figure 9.
The first charge and discharge curves of the sample at different rates: (a) spent LFP, (b) regenerated LFP, (c) regenerated LFP 700, (d) pristine LiFePO4/C [66].
Wang’s team conducted experiments using eutectic solvent culture. Direct remediation of degraded LiCoO2 was performed using LiCl–CH4N2O deep eutectic solvent (DES) (as shown in Figure 7A). The results suggest that DES is not used to dissolve LiCoO2, but is used directly as a carrier for selective supplementation of lithium and cobalt. Lithium supplementation restored LiCoO2 to a capacity of 130 mAh·g (0.1 C rate) at different states of charge, while cobalt supplementation increased capacity retention by 90% after 100 cycles [67]. Zhou’s team researched methods to recover unused lithium and manganese from LiMn2O4 using a pH gradient created in neutral water. The study found that lithium and manganese ions can be extracted from LiMn2O4 at low pH in the anode chamber, while manganese oxide can be precipitated at high pH in the cathode chamber. Additionally, hydrogen and oxygen are produced as useful byproducts. Lithium can be recovered in the form of lithium carbonate, and the LiMn2O4 can be re-synthesized, completing the process loop (As shown in Figure 7D). The study also carefully examined the specific roles of various factors in the leaching of LiMn2O4 and the resulting phase changes [68]. Table 7 summarizes the results of technical research in different directions.

Table 7.
Summary of different direct recycling techniques.
In short, direct recycling is an emerging technology with the potential to significantly reduce the use of chemical reagents and be environmentally friendly. Additionally, it exhibits strong selectivity for specific metal ions and has demonstrated promising results in laboratory settings. However, as of now, this technology is only being investigated on a small scale in laboratories and has not been applied on an industrial level. Processing the cathode material is currently not feasible.
3.5. The Biometallurgy Method
Biometallurgical technology refers to the method of using microorganisms as the main body to dissolve metal ions into the leaching solution or enrich them in microorganisms by using microbial catalytic oxidation. It mainly includes four main steps: bioleaching, bioabsorption, bioselection and enrichment, and waste biological treatment [76].
Biometallurgy technology has been developed alongside bioengineering. However, biometallurgy technology has been significantly hindered by its low recovery efficiency and selectivity. To overcome this technical barrier, researchers have shifted their focus to developing new strains [77]. Gumulya illustrates that microorganisms used in metallurgical technology have good acidophilic, autotrophic, and heavy metal resistance. This allows them to obtain energy from metal oxide at lower pH and in a relatively high concentration of heavy metal ions. Through genetic modification technology, these sexual transformations can be made more significant, and finally suitable for industrial production [78].
Roy’s team studied the extraction effect of A. ferroxidase under specified conditions. They find the optimal extraction conditions by controlling the inoculum amount, pH, liquid ratio, temperature, and rotational speed. The experimental results showed that when the pH value was equal to 2, 10% of the bacterial solution was added, and the inoculation amount of 100 g/L of the strain could obtain a good effect of 94% Co extraction rate [79].
Ghassa’s team used a consortium of moderately thermophilic bacteria as the extraction microorganism. At pH 1.8, 10% inoculation at 45 °C, and 130 rpm, the extraction of 99.9% Co, 99.7% Ni, and 84% Li was achieved [80]. Bahaloo-Horeh’s team used Aspergillus niger at 26.478 (g L−1) sucrose concentration, 3.45% (V V−1) inoculum amount, and pH 5.44. They achieved an extraction rate of 100% of Cu, 100% of Li, and 77% of Mn. They also tried other extraction conditions and finally achieved satisfactory results (as shown in Table 8) [81].

Table 8.
Leaching effects of bioleaching.
Biometallurgy technology has shown promise in achieving improved selectivity and higher extraction rates, while also causing less pollution in laboratory settings. However, the impact of heavy metal poisoning on microorganisms, the high cost of cultivating microorganisms, and the challenges associated with recycling make the process economically costly. Moreover, the extraction efficiency tends to decrease when dealing with high concentrations of metal ions.
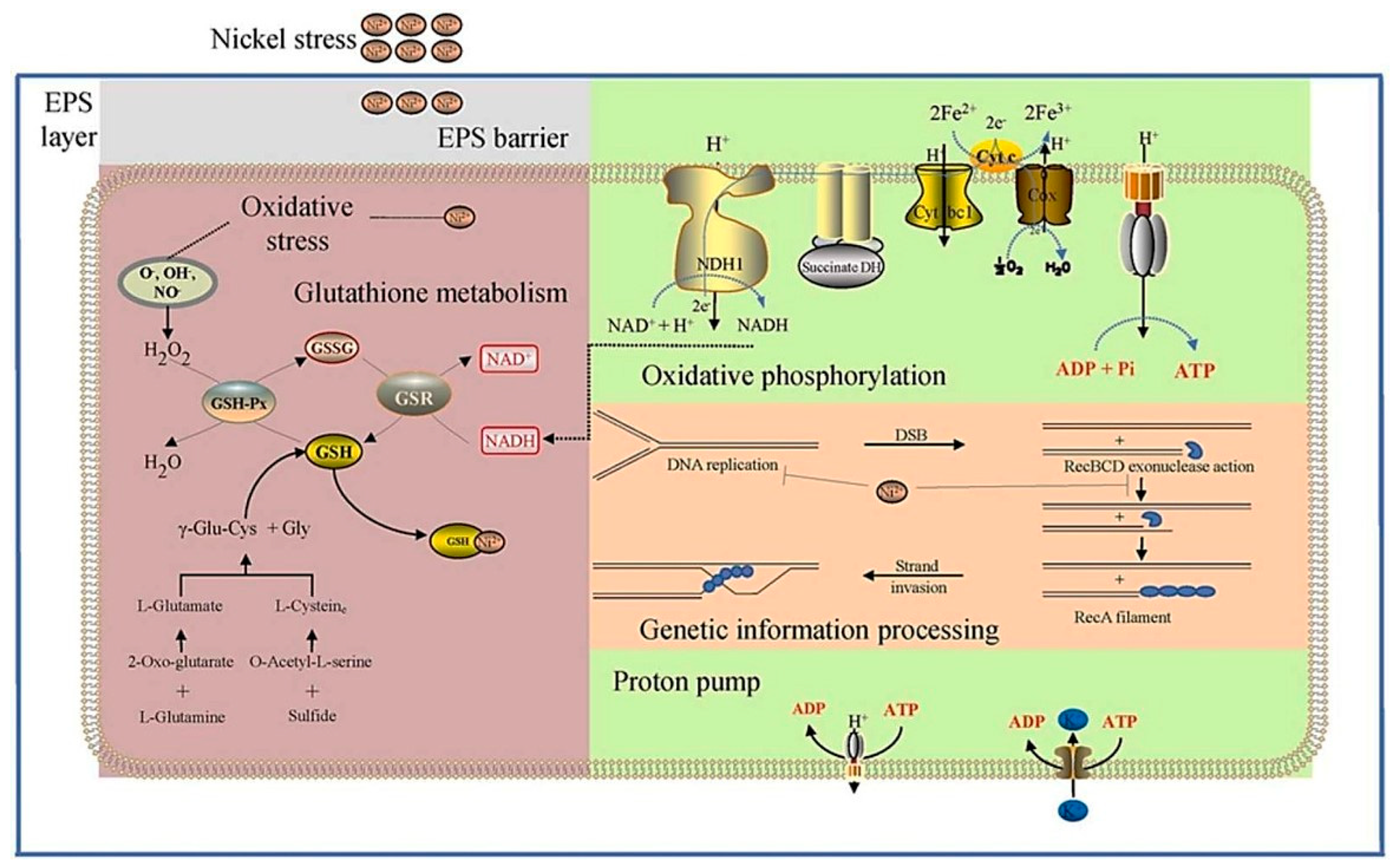
Chen’s team pointed out in a study that Ni2+ produces nickel ion stress when processing spent lithium-ion battery materials. This threatens the health of microorganisms and results in a decrease in the treatment effect. By studying the mechanism of sulfadoxine on nickel ion stress, they looked for the improvement of biological resistance to nickel ion stress by increasing the activity of ATP-reducing coenzymes. At the same time, RNA-sep genes identify what we know differentially expressed by nickel ion stress. That provided ideas for understanding metallurgy technology (as shown in Figure 10) [83].
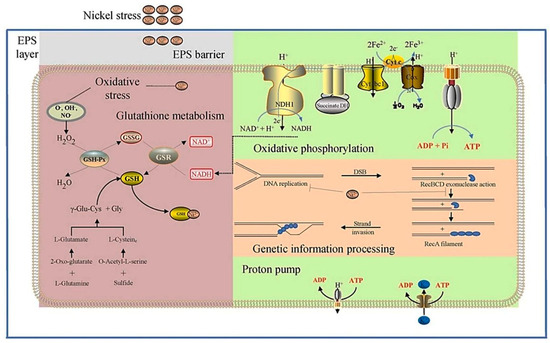
Figure 10.
Schematic diagram of the principle of the reduction of extraction efficiency due to the internal influence of nickel ions on microorganisms. There were differences in the expression of genes responsible for intracellular energy utilization, intracellular antioxidant capacity and DNA damage repair under nickel stress analysis [83].
All in all, the factors influencing biometallurgy technology’s effectiveness are primarily two-fold. First, there is the microbial species, and second, there is the influence of the environment on the microorganisms themselves [84]. Biometallurgy is undoubtedly a green technology. The waste gas produced mainly comes from microbial catalytic oxidation reactions, and the pH value and other conditions of wastewater are also relatively mild. However, at present, its cost is high, and the efficiency does not have much advantage over traditional technologies.
3.6. Summary of Technologies for the Material-Recycling Phase
In general, the primary methods for recycling mature materials are pyrometallurgy and hydrometallurgy. Direct repair technology and biometallurgy require significant development time to become industrialized. Pyrometallurgy technology is relatively easy to achieve. However, it consumes a lot of energy and the quality and recovery efficiency of material recovery are not high. Therefore, it is necessary to combine it with certain hydrometallurgical technologies to achieve a high recovery rate [85].
Currently, hydrometallurgy technology is expected to become a more widely used recycling method in the future. It offers better recovery efficiency, lower energy consumption, and causes slightly less direct pollution to the environment compared to pyrometallurgy. However, the use of organic acids for hydrometallurgy is still in the developmental stage [86]. Additionally, the hydrometallurgical technology using inorganic acids results in high losses on equipment [87]. Proper treatment of a large volume of toxic and harmful waste gas and waste liquid generated in the recycling process is a significant issue that needs to be addressed. At present, some scholars are studying the use of waste gas mineralization technology, which can also be applied to the recovery process to reduce CO2 and other gas emissions [88].
At present, biometallurgy and direct repair technology can effectively treat waste lithium-ion batteries under specific laboratory conditions. However, biometallurgy technology faces two major challenges: cost and the difficulty of finding suitable tolerance microorganisms [89]. Direct repair technology is not efficient, and the cost is high. Economic and environmental indicators are challenging to judge with data. A summary in Table 9 will provide an overview of the current status of relevant technologies based on “high”, “medium”, and “low” judgments from multiple dimensions.

Table 9.
Summary of comprehensive evaluation and development of various recycling technologies.
In the short term, the recycling of waste lithium-ion batteries should focus on developing wet-firing mixing and hydrometallurgical technologies that use organic acids. In the long term, biometallurgy technology could offer a more environmentally friendly approach to recycling lithium-ion batteries.
4. Conclusions
It is essential to note that, in addition to recycling electrode materials and precursors from spent lithium-ion batteries, significant profits can also be gained from the pretreatment process. The metals, such as aluminum, copper, and iron, recovered during the pretreatment phase hold great value [90]. Pyrometallurgical technology can process large quantities of materials while also being very simple but with high energy consumption and low recovery of valuable metals. The advantages of hydrometallurgical technology are that the recovery efficiency of valuable metals is high, the application scenarios are very wide, and the application is also very flexible. Among them, organic acid extraction is becoming a green and effective process method [91]. The main disadvantage of hydrometallurgical technology is that it has a long process flow, which leads to a slightly more complex process flow. Metals from spent lithium-ion batteries are recycled, mainly focusing on pyrometallurgical or hydrometallurgical methods or a combination of the two [92]. Biometallurgical technology belongs among the current cutting-edge technology, it has the advantages of being green and effective, and the bioleaching process is inevitably accompanied by an increase in Ni2+ accumulation in the later stage because the battery is rich in Ni. Therefore, how to reduce the impact of this problem on microorganisms will be a major concern in the future. At the same time, most of the results are currently obtained in the laboratory; achieving industrialization requires more exploration.
Author Contributions
L.L.: primary writing, revising, literature searching. Y.L.: secondary writing, literature searching. G.Z.: supervised writing and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sichuan Science and Technology Program 2024YFHZ0105 and 2024ZHCG0125, Chengdu Science and Technology Project 2022-YF05-00358-SN.
Institutional Review Board Statement
Not applicable, this manuscript does not contain any section on human animals.
Informed Consent Statement
Permission to publish this manuscript has been obtained from the other authors.
Data Availability Statement
The data cited in this manuscript are available in public repositories of publicly DOI published datasets.
Acknowledgments
Sichuan Science and Technology Program 2024YFHZ0105. Chengdu Science and Technology Project 2022-YF05-00358-SN.
Conflicts of Interest
There are no competing interests in this manuscript.
Abbreviations
| SEM | Scanning electron microscopy |
| XRD | X-ray Diffraction |
References
- Zhou, Z. Strong growth in global electric vehicle sales. People’s Daily, 1 March 2023. [Google Scholar] [CrossRef]
- BorgWarner. BorgWarner Reports First Quarter 2023 Results, Expects 2023 eProduct Sales of $2.3 Billion to $2.6 Billion. BorgWarner Reports, 4 May 2023.
- Dehghani-Sanij, A.; Tharumalingam, E.; Dusseault, M. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, M.; Miao, B.; Liu, R. Volumetric capacity enhancement in LiFePO4 cathodes by hot isostatic pressing. Scr. Mater. 2021, 194, 113638. [Google Scholar] [CrossRef]
- Su, Y. Comparative Analysis of Lithium Iron Phosphate Battery and Ternary Lithium Battery. In Proceedings of the 2021 International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2021), Online, 11–16 November 2021; pp. 425–431. [Google Scholar] [CrossRef]
- Saeed, A.M.N.; Hezam, A.; Al-Gunaid, M.Q.A.; Somesh, T.E.; Siddaramaiah. Effect of ethylene carbonate on properties of PVP-CsAlO;-LiClO;solid polymer electrolytes. Polym. Technol. Mater. 2021, 60, 132–146. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirici, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Stich, M.; Göttlinger, M.; Kurniawan, M. Hydrolysis of LiPF6 in Carbonate-Based Electrolytes for Lithium-Ion Batteries and Aqueous Media. J. Phys. Chem. 2018, 122, 8836–8842. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries. Waste Manag. 2016, 52, 221–227. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Ma, X.; Ge, P.; Wang, L.; Sun, W.; Bu, Y.; Sun, M.; Yang, Y. The Recycling of Spent Lithium-Ion Batteries: Crucial Flotation for the Separation of Cathode and Anode Materials. Molecules 2023, 28, 4081. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, A.; Alessandri, I.; Cornelio, A.; Frontera, P.; Malara, A.; Mousa, E.; Ye, G.; Valentim, B.; Bontempi, E. A microwave-enhanced method able to substitute traditional pyrometallurgy for the future of metals supply from spent lithium-ion batteries. Resour. Conserv. Recycl. 2023, 194, 106989. [Google Scholar] [CrossRef]
- An, B.; Lee, T.; Khan, T.T.; Seo, H.; Hwang, H.J.; Jun, Y. Optical and quantitative detection of cobalt ion using graphitic carbon nitride-based chemosensor for hydrometallurgy of waste lithium-ion batteries. Chemosphere 2023, 315, 137789. [Google Scholar] [CrossRef]
- Kanesalingam, B.; Fernando, W.A.M.; Panda, S.; Jayawardena, C.; Attygalle, D.; Amarasinghe, D.A.S. Harnessing the Capabilities of Microorganisms for the Valorisation of Coal Fly Ash Waste through Biometallurgy. Minerals 2023, 13, 724. [Google Scholar] [CrossRef]
- Yu, D. Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review. Miner. Eng. 2021, 173, 107–218. [Google Scholar] [CrossRef]
- Gao, T.; Dai, T.; Fan, N.; Han, Z.; Gao, X. Comprehensive review and comparison on pretreatment of spent lithium-ion battery. J. Environ. Manag. 2024, 363, 121314. [Google Scholar] [CrossRef] [PubMed]
- Seoa, K.; Jaeyeon, B. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, W.; Zhang, G. Research on green discharge technology for pretreatment of waste lithium-ion batteries. J. Cent. South Univ. 2023, 54, 684–693. [Google Scholar] [CrossRef]
- Yao, L.P.; Zeng, Q.; Qi, T.; Li, J. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries. J. Clean. Prod. 2020, 245, 118820. [Google Scholar] [CrossRef]
- Granata, G.; Pagnanelli, F.; Moscardini, E.; Takacova, Z.; Havlik, T.; Toro, L. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: Accomplishment of European Guidelines by optimizing mechanical pre-treatment and solvent extraction operations. J. Power Sources 2012, 212, 205–211. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Ge, L.; Fu, R.; Zhang, X.; Huang, Y. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J. Power Sources 2013, 240, 766–771. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Yang, M.; Cui, M.; Hou, X. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 2006, 84, 75–80. [Google Scholar] [CrossRef]
- Meshram, P.; Somani, H.; Pandey, B.D.; Mankhand, T.R.; Deveci, H.; Abhilash. Two stage leaching process for selective metal extraction from spent nickel metal hydride batteries. J. Clean. Prod. 2017, 157, 322–332. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, Y.; Li, H.; Xie, W.; Zhang, T. Effect of the secondary product of semi-solid phase Fenton on the flotability of electrode material from spent lithium-ion battery. Powder Technol. 2017, 315, 139–146. [Google Scholar] [CrossRef]
- Kaya, M. State-of-the-art lithium-ion battery recycling technologies. Circ. Econ. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Xu, J. Research progress of cathode materials for recycled waste lithium batteries by hydrometallurgy. CIESC J. 2022, 73, 85–96. [Google Scholar] [CrossRef]
- Sommerfeld, M. A Combined Pyro- and Hydrometallurgical Approach to Recycle Pyrolyzed Lithium-Ion Battery Black Mass Part 1: Production of Lithium Concentrates in an Electric Arc Furnace. Metals 2020, 10, 1069. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Holzer, A.; Ponak, C.; Raupenstrauch, H. Pyrometallurgical Lithium-Ion-Battery Recycling: Approach to Limiting Lithium Slagging with the InduRed Reactor Concept. Processes 2021, 9, 84. [Google Scholar] [CrossRef]
- Georgi-Maschler, T. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Brian, M. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Dang, H.; Li, N.; Chang, Z.; Wang, B.; Zhan, Y.; Wu, X.; Liu, W.; Ali, S.; Li, H.; Guo, J.; et al. Lithium leaching via calci-um chloride roasting from simulated pyrometallurgical slag of spent lithium ion battery. Sep. Purif. Technol. 2020, 233, 116025. [Google Scholar] [CrossRef]
- Gou, H. Research on the process of treating waste ternary lithium-ion batteries by fire. China Nonferrous Metall. 2019, 48, 79–83. [Google Scholar] [CrossRef]
- Meng, Z.; Huang, W.; Gao, L.; Dai, J.; Lu, X.; Liu, J.; Huang, H.; Shih, K.; Tang, Y. Green and Energy-Saving Recycling of LiCoO2 by Synergetic Pyrolysis with Polyvinyl Chloride Plastics. ACS Sustain. Chem. Eng. 2022, 10, 12329–12341. [Google Scholar] [CrossRef]
- Qiu, B.; Liu, M.; Qu, X.; Zhang, B.; Xie, H.; Wang, D.; Lee, L.Y.S.; Yin, H. Recycling Spent Lithium-Ion Batteries Using Waste Benzene-Containing Plastics: Synergetic Thermal Reduction and Benzene Decomposition. Environ. Sci. Technol. 2023, 57, 7599–7611. [Google Scholar] [CrossRef] [PubMed]
- Yonglin, Y.; Meiying, Z.; Zhuo, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Pan, C.; Shen, Y. Pyrometallurgical recycling of spent lithium-ion batteries from conventional roasting to synergistic pyrolysis with organic wastes. J. Energy Chem. 2023, 85, 547–561. [Google Scholar] [CrossRef]
- Petranikova, M. Hydrometallurgical processes for recovery of valuable and critical metals from spent car NiMH batteries optimized in a pilot plant scale. Hydrometallurgy 2017, 171, 128–141. [Google Scholar] [CrossRef]
- Li, B. Deep eutectic solv ent for spent lithium-ion battery recycling: Comparison with inorganic acid leaching. Phys. Chem. Chem. Phys. 2022, 24, 19029–19051. [Google Scholar] [CrossRef] [PubMed]
- Dolotko, O.; Gehrke, N.; Knapp, M.; Ehrenberg, H. Mechanochemically induced hydrometallurgical method for recycling d-elements from Li-ion battery cathodes. J. Alloy. Compd. 2024, 976, 172884. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Lee, M.S. Separation ofCo(II), Ni(II), Mn(II) and Li(I) from synthetic sulfuric acid leaching solution of spent lithium ion batteries by solvent extraction. J. Chem. Technol. Biotechnol. 2020, 96, 1205–1217. [Google Scholar] [CrossRef]
- Xu, Z. Research progress on wet recycling of valuable metals of waste lithium-ion batteries. J. China Univ. Min. Technol. 2022, 51, 454–465. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Y.; Wu, S. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Wenhao, Y.; Yi, G.; Zhen, S. A review on comprehensive recycling of spent power lithium-ion battery in China. eTransportation 2022, 11, 100155. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, X.; Shen, Q.; Zhao, Q.; Li, Y.; Wu, T. A novel approach for ultrasonic assisted organic acid leaching of waste lithium-containing aluminum electrolyte and recovery of lithium. Chem. Eng. Process. Process Intensif. 2023, 192, 109508. [Google Scholar] [CrossRef]
- Qing, X.; Zhang, X.; Li, R.; Chen, F. Recovery of valuable metals from spent lithium ion batteries with organic acid leaching. In Proceedings of the 18th National Conference on Solid State Ionology and International Forum on Electrochemical Energy Storage Technology, Guilin, China, 3 November 2016; p. 314. [Google Scholar]
- Yang, J. Research progress on wet recycling of waste lithium-ion batteries. J. Cent. South Univ. Nat. Sci. Ed. 2020, 51, 3261–3278. [Google Scholar]
- Jumari, A.; Yudha, C.S.; Nizam, M.; Dyartanti, E.R.; Suranto; Purwanto, A. An environmentally friendly hydrometallurgy process for the recovery and reuse of metals from spent lithium-ion batteries, using organic acid. Open Eng. 2022, 12, 485–494. [Google Scholar] [CrossRef]
- Meng, Q. A novel process for leaching of metals from LiNi1/3Co1/3Mn1/3O2 material of spent lithium ion batteries: Process optimization and kinetics aspects. J. Ind. Eng. Chem. 2018, 61, 133–141. [Google Scholar] [CrossRef]
- Deepak, P.; Tenzin, D. Green and facile method for the recovery of spent Lithium Nickel Manganese Cobalt Oxide (NMC) based Lithium ion batteries. Waste Manag. 2017, 60, 689–695. [Google Scholar] [CrossRef]
- Sun, L. Hydrometallurgical recycling of valuable metals from spent lithium-ion batteries by reductive leaching with stannous chloride. Int. J. Miner. Metall. Mater. 2021, 28, 991–1000. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, C.; Li, J.; Meng, B.; Zhong, K.; Huang, W.; Yu, J.; Fang, Z. Electric field-assisted leaching of valuable metals from spent lithium-ion batteries in a mixture of acetic acid and ascorbic acid. Hydrometallurgy 2023, 221, 106152. [Google Scholar] [CrossRef]
- Su, F.; Meng, Q.; Zhou, X.; Liu, X.; Yang, J.; Tang, J.; Yang, W.; Cao, P.; Li, Z.; Wang, H.; et al. One-step leaching mechanism for total elemental recovery from spent lithium-ion batteries utilizing ethylene diamine tetraacetic acid. J. Environ. Chem. Eng. 2023, 11, 110275. [Google Scholar] [CrossRef]
- Rouquette, L.M.; Petranikova, M.; Vieceli, N. Complete and selective recovery of lithium from EV lithium-ion batteries: Modeling and optimization using oxalic acid as a leaching agent. Sep. Purif. Technol. 2023, 320, 124143. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, G.; Luo, M.; Li, J. Separation of metals from acetic acid leaching solution of spent lithium-ion batteries by ionic liquid system. Chem. Eng. J. 2023, 472, 145006. [Google Scholar] [CrossRef]
- Lerchbammer, R.; Gerold, E.; Antrekowitsch, H. Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material. Metals 2023, 13, 1330. [Google Scholar] [CrossRef]
- Sahu, S.; Devi, N. Two-step leaching of spent lithium-ion batteries and effective regeneration of critical metals and graphitic carbon employing hexuronic acid. RSC Adv. 2023, 13, 7193–7205. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Devi, N. Effective leaching of spent lithium-ion batteries using DL-lactic acid as lixiviant and selective separation of metals through precipitation and solvent extraction. Environ. Sci. Pollut. Res. Int. 2023, 30, 90152–90167. [Google Scholar] [CrossRef] [PubMed]
- Meshram, P.; Mishra, A.; Abhilash; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Tang, D. Research status and prospect of direct recycling of cathode materials for retired lithium-ion batteries. Inorg. Salt Ind. 2023, 55, 15–25. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, D.; Pan, Y.; Liu, Z.; Zhu, Z.; Qi, X.; Ma, M.; Jiang, R.; Yang, F.; Shi, K.; et al. Directly-regenerated LiCoO2 with a superb cycling stability at 4.6 V. Energy Storage Mater. 2023, 60, 102801. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Cao, S.; Hu, H.; Chen, G.; Guo, X.; Wang, X. Direct regeneration and performance of spent LiFePO4 via a green efficient hydrothermal technique. J. Alloys Compd. 2022, 924, 166487. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Sheng, J.; Liang, Z.; Ma, J.; Chen, Y.; Zhou, G.; Cheng, H.M. Direct and green repairing of degraded LiCoO2 for reuse in lithium-ion batteries. Natl. Sci. Rev. 2022, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bing, J.; Ni, J.; Wang, X.; Guan, X. Recycling the waste LiMn2O4 of spent Li-ion batteries by pH gradient in neutral water electrolyser. Mater. Today Sustain. 2022, 20, 100205. [Google Scholar] [CrossRef]
- Nie, H.; Xu, L.; Song, D.; Song, J.; Shi, X.; Wang, X.; Zhang, L.; Yuan, Z. LiCoO2: Recycling from spent batteries and regeneration with solid state synthesis. Green Chem. 2015, 17, 1276–1280. [Google Scholar] [CrossRef]
- Zhang, N.; Deng, W.; Xu, Z.; Wang, X. Upcycling of spent LiCoO2 cathodes via nickel- and manganese-doping. Carbon Energy 2022, 9, 104789. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Chen, Z. Effective regeneration of LiCoO2 from spent lithium-ion batteries: A direct approach towards high-performance active particles. Green Chem. 2018, 20, 851–862. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Yang, H.; Wang, D. One-pot compositional and structural regeneration of degraded LiCoO2 for directly reusing it as a high-performance lithium-ion battery cathode. Green Chem. 2020, 22, 6489–6496. [Google Scholar] [CrossRef]
- Li, J.; Zhong, S.; Xiong, D.; Chen, H. Synthesis and electrochemical performances of LiCoO2 recycled from the incisors bound of Li-ion batteries. Rare Met. 2009, 28, 328–332. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Sun, F.; Wu, F.; Liu, J. Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process. Hydrometallurgy 2011, 108, 220–225. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Meng, Q.; Dong, P.; Yang, X.; Liu, P.; Li, Q.; Fei, Z. Recycling of spent LiCoO2 materials by electrolytic leaching of cathode electrode plate. J. Environ. Chem. Eng. 2021, 9, 104789. [Google Scholar] [CrossRef]
- Jegan, J.R. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Yu, Z. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef] [PubMed]
- Gumulya, Y.; Boxall, N.J.; Khaleque, H.N.; Santala, V.; Carlson, R.P.; Kaksonen, A.H. In a quest for engineering acidophiles for biomining applications: Challenges and opportunities. Genes 2018, 9, 116. [Google Scholar] [CrossRef]
- Jegan Roy, J.; Srinivasan, M.; Cao, B. Bioleaching as an Eco-Friendly Approach for Metal Recovery from Spent NMC-Based Lithium-Ion Batteries at a High Pulp Density. ACS Sustain. Chem. Eng. 2021, 197, 3060–3069. [Google Scholar] [CrossRef]
- Ghassa, S.; Farzanegan, A.; Gharabaghi, M.; Abdollahi, H. Novel bioleaching of waste lithium ion batteries by mixed moderate thermophilic microorganisms, using iron scrap as energy source and reducing agent. Hydrometallurgy 2020, 197, 105465. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Chen, G.; Shi, H. Multi-scale analysis of nickel ion tolerance mechanism for thermophilic Sulfobacillus thermosulfidooxidans in bioleaching. J. Hazard. Mater. 2022, 443, 130245. [Google Scholar] [CrossRef]
- Zhuang, W.-Q.; Fitts, J.P.; Ajo-Franklin, C.M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Kim, S.; Yun, Y. Simple, green organic acid-based hydrometallurgy for waste-to-energy storage devices: Recovery of NiMnCoC2O4 as an electrode material for pseudocapacitor from spent LiNiMnCoO2 batteries. J. Hazard. Mater. 2022, 424, 127481. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Wu, Z.; Liang, Y.; Wang, W.; Li, Y.; Tian, X.; Feng, L.; Sui, Z.; Chen, Q. Sulfonic acid functionalized covalent organic frameworks for lithium-sulfur battery separator and oxygen evolution electrocatalyst. J. Colloid Interface Sci. 2023, 645, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, N.; Wen, L. Recent progress on the recycling technology of Li-ion batteries. J. Energy Chem. 2021, 55, 391–419. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Wang, K.; Li, G.; Zhang, G. Review of carbon dioxide mineralization of magnesium-containing materials. Carbon Neutralization 2023, 2, 574–584. [Google Scholar] [CrossRef]
- Biotech Week. Technology Recent Research from Ghent University Highlight Findings in Biotechnology (Recovery of critical metals using biometallurgy). ProQuest, 29 July 2015.
- Yang, Y.; Emenike, G. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).