Abstract
Kudzu root (Puerariae lobatae Radix) is the tuberous root of Pueraria lobata, family Leguminosae. Kudzu root contains a variety of beneficial active ingredients such as puerarin, daidzin, daidzein, genistenin, 3′-hydroxy puerarin, β-sitosterol, stigmasterol, arachidic acid, and so on. Modern medical research shows that active ingredients in kudzu root are widely used clinically as raw materials for the treatment of hyperglycemia, non-alcoholic fatty liver disease, myocardial infarction, alcohol addiction, oxidative stress, inflammatory response, and retinal blockage due to their various pharmacological effects such as improving cardiovascular circulation, lowering blood lipids, lowering blood pressure, lowering blood sugar, being antipyretic, being estrogen-like, and relieving alcohol. China has rich resources of kudzu root, and active ingredients are usually extracted before it is made into a preparation, so whether the extraction and separation process is reasonable will directly affect the ease of preparation and the efficacy of the treatment. This paper reviews the process methods for the extraction and separation of active ingredients in kudzu root and its common pharmacological activities. The aim is to provide some references for readers to compare the advantages and disadvantages of various extraction and separation methods as well as understand the active ingredients and pharmacological activities of kudzu root.
1. Introduction
Kudzu (Pueraria lobata (Willd.) Ohwi), a perennial herbaceous vine of the Pueraria DC in the Leguminosae family, primarily grows in warm and moist slopes, ravines, and sunny dwarf bushes. It originated in China but is also found in Southeast Asia and Australia [1]. Kudzu exhibits a certain level of cold hardiness and drought resistance. Kudzu holds significant medicinal value. Its roots, stems, leaves, and flowers can all be utilized for medicinal purposes. The roots (Puerariae lobatae Radix), in particular, contain various active ingredients such as flavonoids, triterpenoids, coumarins, saponins, alkaloids, and polysaccharides [2], which have garnered attention from scholars worldwide. Modern medical research has demonstrated the excellent performance of kudzu root’s active ingredients in regulating blood circulation [3], reducing myocardial hypoxia [4], reducing alcohol dependence [5], lowering blood pressure, blood sugar, and blood lipids [6], preventing bone loss [7] and holding anti-tumor properties [8]. They have also been used in the treatment of retinal obstruction [9]. Consequently, how to effectively extract and separate these natural active ingredients from kudzu root has become an important area of research.
In recent years, there have been many review literature reports on kudzu root, mainly focusing on pharmacological activity, and there are relatively few summary reports on extraction and separation methods. Ma et al., Jiang et al., Wong et al., and Prasain et al. [10,11,12,13] summarized the positive effects of the active ingredients in kudzu root, such as puerarin, daidzein, etc., in the treatment of metabolic syndrome, cardiovascular disease, and type 2 diabetes, and explained its mechanism of action. He et al. [14] reviewed the protective effects of kudzu root flavonoids and triterpene saponins on the liver through different targeted therapy mechanisms, and summarized the therapeutic potential for different types of liver diseases. Wang et al. and Chauhan et al. [15,16] summarized the main biological functions and neuroprotective effects of puerarin in some neurological diseases, such as nerve cell damage, Alzheimer’s disease, etc. Liu et al. and Tomczyk et al. [17,18] reviewed the use of kudzu in reducing alcohol consumption. Tong et al. [19] summarized the extraction and purification methods of the active ingredients in kudzu root, but there were still some gaps in some extraction methods. This paper aims to review the extraction and separation methods of active ingredients in kudzu root by scholars both domestic and international. Additionally, it summarizes the common pharmacological applications of kudzu root.
2. The Main Components in Kudzu Root
After the study of kudzu root by many domestic and international scholars, numerous chemical components have been found, such as flavonoids, triterpenoid saponins, coumarins, alkaloids, polysaccharides, and essential amino acids and minerals [20]. Flavonoids are the main bioactive components of kudzu root, and their content is often used as an evaluation index for the quality identification of kudzu root; the representative compounds are puerarin, dadizin, daidzein, genistin, and formononetin. In addition, the main nutrient in kudzu root is starch, and the starch content in fresh kudzu root can range from 15% to 34.2% [21]. The kudzu root is shown in Figure 1 and the main chemical constituents in kudzu root are shown in Table 1 and Figure 2.

Figure 1.
Kudzu root (Puerariae lobatae Radix).

Table 1.
Main chemical constituents in kudzu root.
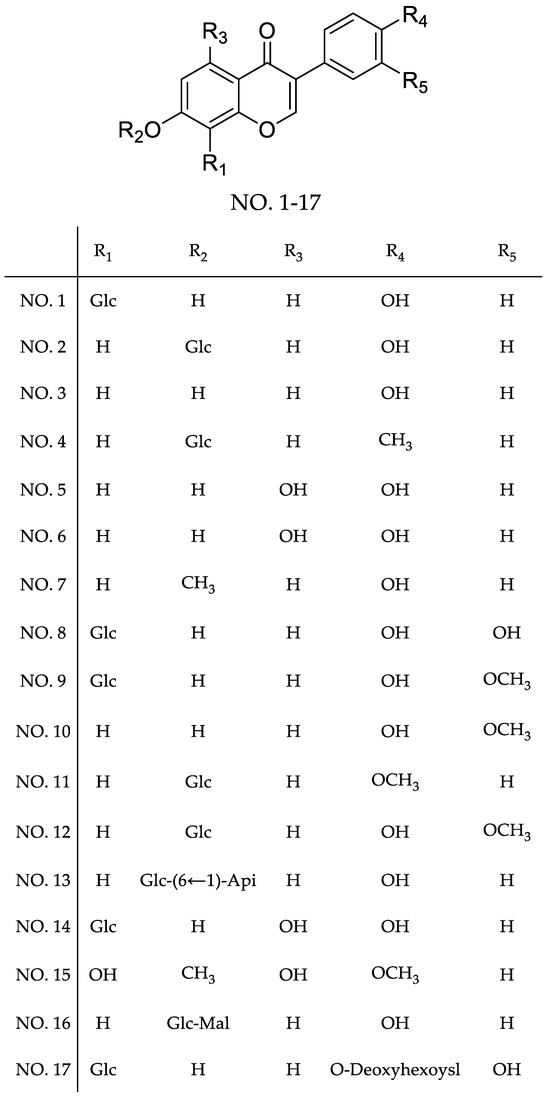
Figure 2.
The main chemical structures isolated from Puerariae lobatae Radix.
3. Extraction and Separation Methods of Flavonoids from Kudzu Root
China is rich in kudzu root resources, and flavonoids are the main compounds in the kudzu root. Their content can be used as a standard to judge whether a piece of kudzu root is good or bad. In addition to natural enrichment, the rationality of the extraction process is also a key factor affecting the full utilization of kudzu resources. At present, the common extraction and separation methods of kudzu root flavonoids include water decoction, solvent extraction, ultrasonic-assisted extraction, microwave-assisted extraction, pressurized solvent extraction, ionic liquid-assisted extraction, supercritical CO2 extraction, cyclodextrin-assisted extraction, molecularly imprinted solid-phase extraction, macroporous resin adsorption, high-performance liquid chromatography, and high-speed counter-current chromatography. The advantages and disadvantages of these methods are shown in Table 2.

Table 2.
Comparison of advantages and disadvantages of various extraction and separation methods.
3.1. Water Decoction Method
The water decoction method refers to the crushing of plant materials, adding distilled water with the corresponding liquid ratio, soaking for a certain period of time, and then boiling and filtering the mixture, because most of the active pigment ingredients in plants are soluble in water; this method is called the “water boiling method” [37]. Zhang et al. [38] conducted a study on the content of total flavonoids in kudzu root extracts using different decoction times. The study concluded that the maximum amount of total flavonoids from kudzu root was obtained when 10 times the amount of water compared to the original material was added. The optimal extraction process involved three water decoctions, with each decoction lasting for 3 h, 1 h, and 0.5 h, respectively. Li et al. [39] performed an analysis on the leaching rate of puerarin under various extraction times, temperatures, material–liquid ratios, and number of extractions. Through a one-way choice experiment, the optimal extraction conditions were determined using an orthogonal test. The results indicated that the extraction temperature should be set at 90 °C, the extraction time at 4 h, and the solid–liquid ratio at 1:45 (g/mL). Yang et al. [40] conducted experiments to investigate the optimal process parameters for flavonoids in kudzu root. The study also explored the purification conditions for the extracts, which were subsequently qualitatively identified by UV absorption spectroscopy. The experimental results revealed an optimal extraction time of 3 h, extraction temperature of 100 °C, and a material–liquid ratio of 1:10, through three extraction cycles. The extract of flavonoids from kudzu root was then purified using 3.75 g NaCl salting out, resulting in a yield of 6.45% and a content of 10.50%. The comparison of the purified product with the standard sample by UV absorption spectroscopy revealed the same UV absorption peak at 250 nm, indicating that the purified product mainly contained flavonoid compounds. Zhang et al. [41] determined the best extraction process of puerarin by comparing the extraction efficiency of water, methanol, and 95% ethanol with the content of total flavonoids in kudzu root as the index. Using water as the extraction solvent, the first time, four times the amount of water was added for 1.5 h, and the second time, two times the amount of water was added for 1.5 h. Combining the two extracts, the total flavonoids were obtained first with the content of 85.4%, and then refined by using D101 macroporous resin of seven times the amount of the crude extract and 12 times the amount of 70% ethanol in water as eluent. Through validation, it was found that puerarin was obtained with a yield of up to 90%. The advantage of this process is that compared to methanol and 95% ethanol, water is non-toxic, has a lower production cost and operational requirements, and there is no major difference in the yield. Therefore, considering factors such as cost and operation, water as an extractant is the most suitable for industrial production. Zhang et al. [42] successfully developed a kinetic model to determine the concentration changes of total flavonoids in the aqueous extract of kudzu root through water decoction at various extraction times and solvent multiplicities. The model is represented by the following equation:
where CB represents the mass concentration of the target fraction in the extract in g/L, t denotes the extraction time in min, and M signifies the solvent multiplication in mL/g. The model was validated and demonstrated a strong fit with minimal deviation.
3.2. Solvent Extraction Method
The solvent extraction (SE) method is a method to dissolve active ingredients from plant tissues by choosing solvents with high solubility for active ingredients or low solubility for ingredients that do not need to be dissolved according to the solubility properties of various ingredients in the solvent. When the solvent is added to the crushed plant material, the solvent gradually penetrates into the cell through the cell wall due to diffusion and permeation, and dissolves the soluble material, resulting in the concentration difference between inside and outside the cell, so that the concentrated solution inside the cell continuously diffuses outward, and the solvent continuously enters the plant tissue cell, and so on and so forth until the concentration of the solution inside and outside the cell reaches dynamic equilibrium. This saturated solution is filtered out, and new solutions are added several times to dissolve the desired components nearly completely or mostly. Common extraction solvents are methanol, 60–95% ethanol, alkaline alcohol, lime water, and acetone. In addition, in order to enable the active ingredients to be fully dissolved in the solvent, the plant material and the organic solvent are usually placed in a heated reflux device to react for a certain period of time, with a reflux temperature of 60–85 °C in general, and extracted 2–3 times [43]. Wu et al. [44] have successfully extracted puerarin from kudzu root by the ethanol hot reflux method and studied the effects of different extraction factors such as ethanol concentration, extraction temperature, solid–liquid ratio, and extraction times on the extraction rate of puerarin, as well as optimized the extraction conditions using orthogonal tests. The optimum extraction process was obtained as follows: the extraction temperature was 90 °C, the material–liquid ratio was 1:6, the ethanol concentration was 90%, and the extraction time was 2 h. The extraction rate of puerarin was 37.51 ± 0.64 mg/g under this condition, and the experimental results can provide the basis for the large-scale production of puerarin. Liu et al. [45] used a single-factor examination and orthogonal test design to investigate the content of puerarin. The selected process was to add nine times the amount of 70% ethanol and extract twice, the first time for 2 h and the second time for 1.5 h. This experimental condition was efficient in the extraction of puerarin and the results were stable. Han et al. [46] used an orthogonal test to optimize the extraction conditions of kudzu root herbs with the content of puerarin as the index of investigation. The results of the orthogonal test showed that the best extraction process of puerarin was to extract twice, each time with 10 times the amount of water, where the extraction times were 1.5 h and 1 h. Pan et al. [47] optimized the extraction process of flavonoids from kudzu root by six sets of one-way experiments and four sets of multifactorial orthogonal experiments. The experimental results showed that the best extraction of kudzu root flavonoids was carried out at 85 °C and pH 8 by using 70% ethanol at 10 times the amount of kudzu root for two times, each time for 3 h. The extraction rate was more than 9%, and the flavonoid content in the extract was more than 40%. Xu et al. [48] studied the extraction of puerarin from kudzu root using an n-butanol/water two-phase solvent system. An amount of 100 g of kudzu root was mixed with 1000 mL of the n-butanol/water phase solvent system with a volume ratio of 1:1, and then extracted at a temperature of 25 °C for 60 min. The experimental results showed that the partition coefficient of puerarin was influenced by the pH of extraction and the initial concentration of puerarin. The lower pH of the extraction was favorable to the extraction of puerarin from the aqueous phase to n-butanol, and conversely, the extraction from n-butanol to the aqueous phase. The first extraction in the experiment found that puerarin was unstable at a solution pH higher than 8, so the pH of the first solvent extraction was reduced, and the partition coefficient (K1) of puerarin did not change much when the extraction pH was lower than 4.0, so 4.0 was the best pH for the first extraction; and in the second extraction, because puerarin was free in the aqueous phase, in the very acidic medium, the free molecules occupied a dominant position. Increasing the pH meant that more free molecules could be converted to the organic phase. On the contrary, in very basic media, fewer free molecules were converted to the organic phase, and increasing the pH to 8.0 significantly increased the partition coefficient K2. In addition to the pH affecting the partition coefficient of puerarin, the initial concentration of puerarin also had an important effect on the partition coefficient: at low initial concentrations, changes in pH had a greater effect on the partition coefficient of puerarin, but as the initial concentration increased, the effect of pH on the partition coefficient gradually decreased. Two-solvent extraction can effectively separate puerarin from impurities by adjusting the extraction pH at low initial concentration.
With the increasing development of modern solvent extraction techniques, one of the main challenges in the solvent extraction of flavonoids is to adopt economically feasible techniques for efficient and selective extraction of the target compounds using a minimum of non-toxic solvents. The emergence of natural deep eutectic solvents (NADESs) has attracted the interest of many researchers and scholars as green solvents consisting of a large number of hydrogen-bonding donors (HBDs) (usually carbohydrates or organic acids, such as oxalic acid, lactic acid, malic acid, citric acid, etc.) and hydrogen bond acceptors (HBAs) (usually amino acids or non-toxic quaternary ammonium salts) form a mixture. Compared with conventional organic solvents, NADESs have many advantages, such as good physicochemical properties (very low volatility, good thermal stability, non-flammability, and non-toxicity) and sustainable green properties (biodegradable and bio-renewable, with low or even negligible waste generated during the preparation process), which, due to these excellent properties, make NADESs very suitable for the manufacture of food, pharmaceuticals, and cosmetics [49]. NADESs have been widely used in the extraction of active ingredients from plants, such as polyphenols from green tea [50] and pectin from apple pomace [51]. Duru et al. [52] used quaternary ammonium choline chloride (ChCl) (HBA) and citric acid (HBD) as the raw materials, with a molar ratio of 1:2, to prepare a NADES, and took advantage of the ability of the two components to tightly bind to destroy the kudzu root’s cell wall, so as to extract the flavonoids. The NADES was prepared by mixing the two components in a molar ratio of 2:2. The effects on the extraction rate of flavonoids from kudzu root were investigated under different solid–liquid ratios and water contents of the NADES. The experimental results showed that the NADES successfully extracted and isolated three flavonoids, puerarin, daidzein, and genistein, from kudzu root, and the highest extraction rate of puerarin was 10.1 mg/g when the sample:NADES was 1:20 (m/v) and the concentration of NADES:water was 7:3 (at 80 °C for 2 h, ultrasonic power of 180 W). Saied et al. [53] prepared a NADES by mixing ChCl with HBD at a molar ratio of 1:1 and extracted flavonoids from kudzu root using ultrasonic extraction equipment at a temperature of 60 °C, a frequency of 37 KHZ, a power of 580 W, and an extraction time of 3 h. Systematic analysis using HPLC-DAD yielded five flavonoids: daidzein, genistein, puerarin, formononetin, and biochanin A. The experiment did not optimize the process parameters but simply extracted the active ingredients from kudzu root. The results showed that flavonoids can be effectively extracted from kudzu root using a NADES, which is a natural green solvent and is expected to be an alternative to conventional solvents. Huang et al. [54] prepared 25 different NADESs from kudzu root flavonoids by mixing three conventional HBAs, choline chloride, betaine, and L-proline, with representative HBDs, such as carbohydrates (glucose, maltose, sucrose), alcohols (xylitol, glycerol), organic acids (lactic acid, malic acid), and organic bases (urea, acetamide). Twenty-five different NADESs were mixed and prepared, and these were compared with water and methanol for the extraction of kudzu root flavonoids by determining the amount of puerarin, 3′-methoxy puerarin, and puerarin-6″-O-xyloside in kudzu root to determine the optimal extraction parameters. The experimental results showed that the extraction of these three active ingredients was significantly enhanced by most of the NADESs relative to the conventional solvents (methanol, water). In addition, by comparing the three conventional HBAs, it was found that NADESs with L-proline as HBA were more helpful in increasing the yield of the three components. After that, L-pro-Maa (L-proline-malic acid) was further selected as the extraction solvent, the four factors were optimized using a one-way experiment, and the optimal extraction process was determined as follows: a solid–liquid ratio of 25 mg/mL, an extraction temperature of 40 °C, an extraction time of 30 min, and a content of NADES of 60%. The three active ingredients of puerarin, 3′-methoxypuerarin, and puerarin-6″-O-xyloside from kudzu root were extracted using these conditions, and the yields obtained were 98.7 mg/g, 16.3 mg/g, 9.9 mg/g, respectively, which were 2.2, 2.9, and 3.4 times higher than the yields obtained with water as the extraction solvent.
3.3. Ultrasonic-Assisted Extraction Method
Ultrasonic-assisted extraction (UAE) refers to the use of ultrasound to produce strong vibration, a continuous stirring effect, and a high-speed strong cavitation effect in order to destroy the cells of plants, so that the solvent can penetrate into the cells faster and accelerate the precipitation of active ingredients [55]. Kwun et al. [56] examined the effects of extraction time, extraction temperature, ultrasonic power, ethanol concentration, and other factors on the extraction of flavonoids by using kudzu root waste left over from squeezing out kudzu root juice. The experimental results showed that an 80% aqueous solution of ethanol could be used to extract six flavonoids, daidzein, daidzin, genistein, genistin, glycitein, and puerarin, from the kudzu root waste with high efficiency extraction, and the flavonoids extraction rate increased with the increase in extraction time and extraction temperature. An amount of 7.26 g of flavonoids (per 100 g of kudzu root waste) can be obtained in 3 h at 80 °C with 40 KHz ultrasonic power combined with 80% ethanol water, which is a 2.5 times increase compared with the solvent extraction of 80% ethanol water alone. In this study, the total content of flavonoids in kudzu root dregs was 8%, and the final extraction obtained by using UAE was 7.33%, with an extraction rate of 91.6%. Zhu et al. [57] used an orthogonal test to investigate the effects of ultrasound power and extraction time on the flavonoids extract of kudzu root, and with the increase in ultrasound power, the flavonoids extraction rate showed a line increasing and then decreasing trend, and when the ultrasound power was in the range of 200–600 W, the two variables had a positive effect on the extraction rate. There was a positive correlation between the two variables, and the extraction rate reached the highest at 600 W. Thus, 600W was the optimal ultrasonic power for kudzu root flavonoids extraction. In terms of extraction time, the extraction rate of flavonoids was analyzed under the conditions of ultrasonic power of 400 W, a 60% concentration of ethanol, and a solid–liquid ratio of 1:20 for different extraction times, and the results showed that the extraction rate reached a maximum of 4.78% at 30 min, while the extraction rate of flavonoids decreased when it exceeded 30 min. Zeng et al. [58] used a pilot type ultrasonic extractor to extract puerarin and studied the effects of extraction time and ultrasonic frequency on extraction rate and compared with conventional reflux. The results showed that the extraction rate of 90% was basically the same between UAE and conventional SE under the same solid–liquid ratio (1:20) and solvent conditions (50% ethanol), but the extraction temperature could be reduced to 60 °C and the extraction time was just 30 min by using UAE when the ultrasonic power was 25 W. Lee et al. [59] used ultrasound technology to extract puerarin, daidzin, and daidzein from kudzu root. The experimental results showed that when the ethanol concentration was 95%, the solid–liquid ratio was 1:10, the extraction time was 5 min, and the extraction temperature was lower than 37 °C; the extraction of daidzin and daidzein reached the maximum at 40 MJ, and puerarin required 60 MJ to reach the maximum. However, when the solid–liquid ratio was 1:5, all three compounds reached the maximum at 60 MJ. Wong et al. [60] used ultrasonic power to extract and isolate 3′-hydroxypuerarin, puerarin, 6″-O-D-xylosylpuerarin, 3′-methoxypuerarin, daidzin, genistin, and daidzein from kudzu root and optimized the extraction process by the response surface optimization method, where the following best extraction parameters were mentioned: an extraction time of 16.02 min, an ethanol concentration of 41.41%, and a liquid–solid ratio of 44.35 mL/g when the ultrasonic power was 300 W and the temperature was 40 °C. The experimental validation of the extraction parameters was carried out by taking the gain effect of the extracts on cellular antioxidant capacity as an indicator, and it was found that the predicted value differed from the experimental value by 3.26%, which verified the validity of the optimization model.
3.4. Microwave-Assisted Extraction Method
The microwave-assisted extraction (MAE) method refers to the use of a microwave to destroy the cell wall and cell membrane of plant cells, so that the extracted material is separated from the body or tissue into the extraction solvent with a small dielectric constant and poor microwave absorption capacity, in order to achieve the purpose of extracting the active ingredients in the cells [61]. Sun et al. [62] took 10 g of Xiangxi wild kudzu as the raw material, added 40% ethanol solution, and destroyed its tissue cells by high-speed shearing, coupled with MAE to extract puerarin. The effects of shearing time, shearing speed, average microwave radiation power, and radiation time on the extraction rate of puerarin were investigated. The experimental results showed that the best extraction process was 6 min shear time, 6000 r/min shear speed, microwave radiation for 20 s, and microwave power of 467.27 W, where the content of puerarin was 2.05 mg/g. Zhou et al. [63] studied the process conditions of MAE of the total flavonoids of kudzu root by an orthogonal test, and the results showed that with 90% ethanol, the particle size of kudzu root for 40 mesh, a solid–liquid ratio of 1:15, microwave power of 700 W, a temperature of 78 °C, pre-soaking for 1–2 h, and microwave radiation for 40 s, the leaching rate of the total flavonoids of kudzu root reached more than 96%, which is not only a high yield but also fast and energy-saving compared with the traditional hot leaching method. Zhu et al. [57] investigated the effect of different microwave power on the extraction rate of kudzu root flavonoid compounds under 200 W, 400 W, 600 W, 800 W, and 1000 W microwave power conditions by combining MAE with UAE. The experimental results showed that with the increase of microwave power, the extraction rate of flavonoids showed a trend of increasing and then decreasing, and the extraction rate reached the maximum of 4.92% at the microwave power of 800 W (temperature of 55 °C, ultrasonic power of 400 W, 60% ethanol, solid–liquid ratio of 1:20, extraction time for 40 min). This study provides a useful basis for the combined processing technology for the application of ultrafine kudzu root powder as a functional food. Du et al. [64] determined the 14 major phenolic compounds, namely puerarin-4′-O-glucoside, puerarin-3′-methyoxy-4′-O-glucoside, daidzein-4′,7-O-glucoside, puerarin, mirificin, daidzin, 6″-O-xylosylpuerarin, 3′-methoxypuerarin, genistin, sophoraside A, ononin, daidzein, genistein, and formononetin, in kudzu root by combining ultra-high-performance liquid chromatography–diode array detector–time of flight mass spectrometry (UHPLC-DAD-TOF-MS) with microwave-assisted extraction (MAE), UAE, SE, and pressurized liquid extraction (PLE). The geometric mean values of the contents of the 14 target compounds extracted by the four methods were compared, and it was found that the extracted amounts obtained by the four extraction methods were similar in terms of phenolic compounds with low contents (<2 mg/g), such as puerarin-3′-methyoxy-4′-O-glucoside, genistein, and formononetin. However, for some major phenolic compounds (>6 mg/g) in kudzu root, such as puerarin, mirificin, daidzin, and daidzein, the peak areas obtained by MAE were similar to those of PLE and were significantly larger than those of SE and UE. The factors show that MAE is an excellent extraction method. In addition, the team used the response surface optimization method to optimize the conditions of particle size, extraction temperature, ethanol dosage, ethanol concentration, microwave power, and extraction time in MAE, and the optimization results obtained were as follows: for 0.3 g of kudzu root powder, with a particle size of 0.18–0.35 mm, an ethanol concentration of 65%, a dosage of 17 mL, an extraction temperature of 100 °C, an extraction time of 2 min, and the microwave power of 600 W, the extraction rate could be optimal. The optimized conditions were successfully used for the determination of the compounds in kudzu root samples of 20 different varieties.
3.5. Pressurized Solvent Extraction Method
Pressurized solvent extraction (PSE) technology is an automated method for extracting solid or semi-solid samples using organic solvents at higher temperature and pressure. In recent years, it has been widely used in the fields of food, medicine, etc. [65]. Lee et al. [59] found that when 1 g of powdered kudzu root was mixed with 10 mL of 95% ethanol, the pressure was set at 1400 psi, and the temperature of extraction was set at 60, 80, and 100 °C, respectively. Under the extraction for 10 min, it was found that the extraction of daidzein, puerarin, and daidzin increased when the temperature was 100 °C compared to 60 °C, which increased 2.6–3.7 times, 1.8–2.4 times, and 1.4–1.8 times, respectively. Compared with the conventional SE, PSE is a more excellent extraction method. Du et al. [64] mixed kudzu root powder with diatomaceous earth at the ratio of 1:2, put it into a pressurized extractor, added 60% of methanol solution, controlled the extraction temperature at 140 °C, with a pressure of 1500 psi, and extracted it statically for 10 min, and then filtered the obtained extract and injected it into ultra-high-performance liquid chromatography for analysis, successfully extracting and isolating 14 phenolic compounds from kudzu root, including puerarin, dadizin, daidzein, genistein, and formononetin.
3.6. Ionic Liquid-Assisted Extraction Method
Ionic liquids (ILs) are inorganic or organic salts that are thermally and chemically stable at room temperature, in addition to being renewable, miscible in water and organic solvents, and easy to extract a variety of organic compounds, which make them ideal solvents for green chemistry, and have been successfully used for the extraction of active ingredients from medicinal plants [66,67]. Mocan et al. [68] selected 1-butyl-3-methylimidazolium bromide as the IL to optimize the extraction of kudzu root flavonoids. They added 10% (w/v) of 1-butyl-3-methylimidazolium bromide to the extraction solvents (water or 65% ethanol) for conventional SE, UAE, and MAE, and determined the amount of the four most representative flavonoids in the kudzu root extract (puerarin, dadizin, daidzein, genistein) by comparing the amount of the optimal extraction process parameters. The results demonstrated that the inclusion of 1-butyl-3-methylimidazolium bromide enhanced the extraction efficiency of puerarin and daidzin in traditional solvents using water as the extraction solvent. Additionally, the extraction efficiency of daidzein was improved in traditional solvent extraction and UAE by incorporating 65% ethanol as the extraction solvent. Taking the above factors into account, the optimal extraction conditions for puerarin, dadizin, and genistein were as follows: water as solvent, a solid–liquid ratio of 1:5 (m/v), and an extraction time of 30 min, whereas the optimal conditions for daidzin were as follows: 65% (±5 or 10) ethanol as solvent, and an extraction time of 30 min. Zhang et al. [69] obtained the optimal results by choosing [bmim]Br as a solvent, which is a kind of room temperature ionic liquid (RTIL), and which is expected to be a green solvent instead of traditional volatile organic solvents. By combining MAE, the effects of different extraction times, extraction temperatures, microwave power and [bmim]Br concentrations on the extraction rate of total flavonoids from kudzu root were analyzed. A prediction model was established by the response surface optimization method to simulate and optimize the extraction process, and the multivariate quadratic regression equation was obtained by using a central combinatorial design. The experimental results showed that the extraction rate of total flavonoids of kudzu root reached the optimum of up to 10.09% when the weight of the kudzu root sample was 0.5 g, the extraction temperature was 70 °C, the extraction time was 8 min, the microwave power was 400 W, and the concentration of [bmim]Br was 1.2 mol/L, which was very mild compared with the value of the prediction model. Compared with conventional organic solvents, ILs combined with MAE could lead to a shorter extraction time and a higher extraction rate could be obtained. Wang et al. [70] investigated different cations liquids ([EMim]HSO4, [BMim]HSO4, [HMim]HSO4, [OMim]HSO4) and anionic liquids ([Omim]HSO4, [Omim]TsO, [Omim]Cl, [Omim]BF4, [OMim]Br), and finally chose [Omim]HSO4 as the target ionic liquid to be added into the kudzu root extract of MAE. The response surface optimization method was used to investigate the effects of different dosages of [Omim]HSO4, different concentrations, microwave power, microwave times, and other process parameters on the extraction rate of the flavonoids, and the optimal conditions of the process were determined as follows: for 5 mL, 0.45 mg/mL aqueous solution of kudzu root crude extracts, 0.82 mol/L, 1.42 mL of [Omim]HSO4, a microwave time of 7 min, and microwave power of 400 W. Under these conditions, the average extraction rate of kudzu root and daidzein was 57.93 ± 3.08%, which makes IL–MAE a fast, simple, and efficient extraction technique compared with single MAE.
3.7. Supercritical CO2 Extraction Method
Supercritical fluid is a fluid with a temperature and pressure higher than the critical value, and supercritical fluid extraction refers to the contact between a supercritical fluid and a substance to be extracted in a supercritical state, so that components with a certain polarity, boiling point, and molecular weight can be extracted sequentially and selectively. This technology has been widely used in food and medical fields in recent years [71]. The extraction solvents commonly used in supercritical techniques are carbon dioxide (CO2), ammonia (NH3), diethyl ether (C2H5OC2H5), methane (CH4), ethane (C2H4), ethanol (CH3CH2OH), water (H2O), methanol (CH3OH), ethylene (CH2CH2), etc. Wang et al. [72] extracted puerarin from 40 g of kudzu root using supercritical CO2 extraction. The effects of pressure, temperature, time, and co-solvent amount on the yield of puerarin were investigated. The experimental results showed that all four factors had a significant effect on the yield of puerarin. In terms of pressure, the researchers explored the yield of puerarin under pressure by setting the experimental temperature at 50 °C, extraction time at 90 min, and co-solvent dosage at 150 mL. It was found that the maximum yield of puerarin, 5.8 ± 0.3 mg/g, was achieved at a pressure value of 20 MPa, and the yield decreased above and below this pressure value. However, the optimum pressure value for the puerarin yields may change when different temperatures, times, or amounts of co-solvent are used. In terms of extraction temperature, the puerarin yields were investigated at extraction temperatures of 40 °C, 45 °C, 50 °C, 55 °C, and 60 °C, pressure values of 20 MPa, a time of 90 min, and a co-solvent dosage of 150 mL. It was found that the highest yield of puerarin, 3.1 ± 0.2 mg/g, was obtained at an extraction temperature of 50 °C. Temperature is one of the more important factors affecting the yield, and an increase in temperature is feasible until 50 °C, because it accelerates the mass transfer and increases the extraction rate. The yield of puerarin decreases when the temperature exceeds 50 °C, because the increase in temperature causes a corresponding increase in the vapor pressure and volatilization of solutes. In terms of time, the yield of puerarin increased with the increase in extraction time, and there was a positive correlation between the two. For the co-solvent, the ethanol [73] was chosen in this experiment to increase the polarity of CO2 and thus improve the extraction rate of supercritical CO2. As the amount of co-solvent increased, the yield of puerarin increased accordingly, and this effect was the same as that of the extraction time. Yang et al. [74] used supercritical CO2 fluid instead of conventional ethanol solution to extract 3′-hydroxy puerarin, puerarin, 3′-methoxy puerain, daidzin, ononin, and daidzein from kudzu root, with supercritical CO2 as the extracting solution and ethanol–methanol as the co-solvent, where the flow rate of both of them was 24 L/h and 2 L/h, respectively, and they investigated the effects of the change of temperature, pressure, and the concentration of ethanol in the co-solvent on the extraction rate. The optimum extraction parameters of kudzu root flavonoids were obtained by the response surface optimization method: taking the content of daidzein as an index, the extraction rate of daidzein reached the maximum value of about 30 μg/g when the temperature was 51.5 °C, the pressure was 31.8 MPa, and the concentration of ethanol in the co-solvent was 36.7%. Supercritical CO2 was a green, efficient, and simple method that can be applied to the pharmaceutical industry and provide a reference for the large-scale extraction of the natural active ingredients from medicinal plants.
3.8. Cyclodextrin-Assisted Extraction Method
Cyclodextrin (CD) is a cyclic oligosaccharide consisting of α-(1,4)-linked glucose units, which is widely used in the chemical synthesis of natural products, drug carriers, the food industry, and other fields due to its unique cyclic structure. The main principle of cyclodextrin-assisted extraction (CAE) of natural products is to separate natural products from their components with higher affinity (e.g., non-biologically active components, pigments, etc.) through its hydrophilic effect and hydrophilicity, thus improving the extraction efficiency of natural products [75]. Feng et al. [76] compared the effect of the addition of conventional cyclodextrins: β-cyclodextrin (β-CD), γ-cyclodextrin (γ-CD); water-soluble cyclodextrin derivatives: hydroxypropyl-β-cyclodextrin (HP-β-CD), hydroxypropyl-γ-cyclodextrin (HP-γ-CD); and ionic cyclodextrin derivatives: carboxymethyl-β-cyclodextrin (CM-β-CD), sulphonatobutyl ether-β-cyclodextrin (SBE-β-CD) for five flavonoids extracted from kudzu root, namely puerarin, daidzein, daidzin, genistein, and genistein. This was performed by mixing 5 g of kudzu root with 0.25 g of individual CDs in 125 mL of water, first soaked for 1 h and then decocted for 2 h, and compared with the extract without any added CDs. The results of HPLC analyses showed that the peak areas of these five flavonoids were significantly increased by the addition of CDs, indicating that CDs could improve the extraction capacity of total flavonoids from kudzu root. In addition, the results of statistical analyses showed that different types of CDs had different solubilizing effects, in the following order: ionic cyclodextrin derivatives > water-soluble cyclodextrin derivatives > conventional cyclodextrins, in the case of this experiment. Among the ionic cyclodextrin derivatives, SBE-β-CD had the best solubilizing effect on the flavonoids in kudzu root, which has a promising application. He et al. [77] also used an epichlorohydrin polymerized β-CD ligand with allyl bromide coupled with a substituted Sepharose HP base substrate to further isolate puerarin from kudzu root extract by adsorption chromatography. In the experiment, 20% ethanol was used as the solvent, the optimal mobile phase was 10% acetic acid, the linear flow rate was 1 cm/min, and the optimum loading capacity was around 1.2 mg crude extract/mL packed gel. The authors found that the substituted base substrate can be used as a ligand for the separation and purification of puerarin, and the purity of the obtained puerarin was tested to be 98% with a yield of about 62%. Lv et al. [78] prepared a novel monomeric and polymeric β-CD-functionalized poly: poly (isocyanatoethyl methacrylate-co-methyl methacrylate-co-ethylene dimethacrylate) (poly(IEM-co-MMA-co-EDMA)) and poly (glycidyl methacrylate-co-EDMA) (poly(GMA-co-EDMA)) were used for the rapid isolation and purification of puerarin from kudzu root. The experimental results showed that the introduction of polymeric β-CD significantly improved the selectivity of monoliths for puerarin compared to mono-β-CD. The β-CD-functionalized poly (IEM-co-MMA-co-EDMA) monolith was used for the separation and purification of puerarin from the crude extract of kudzu root with 20 μL 0.6 mg/mL as the injection volume, 4:3:93 methanol/acetic acid/water as the mobile phase, 361 cm/h as the flow rate, 254 nm as the detection wavelength, and an extraction rate of 89% and purity of 96% were mentioned. The single-step separation could be accomplished in only 15 min. Using ethylenediamine-β-CD-functionalized poly (GMA-co-EDMA) monolith separation and purification, kudzu root was mentioned with an extraction rate of 79% and a purity of 86% using 12.5% acetic acid aqueous solution as the mobile phase. The separation efficiency is lower than that of the former. This is the first time that a study on β-CD-functionalized monoliths separation and purification of active ingredients from natural products was reported, which has very important guiding significance.
3.9. Molecularly Imprinted Solid-Phase Extraction Method
Molecularly imprinted solid-phase extraction (MISPE) is a solid-phase extraction (SPE) method based on molecularly imprinted technology (MIT). MIT is a method for the selective identification and extraction of target molecules by specific recognition of the target molecules using template molecules that form a specific cavity structure in a polymer matrix. The principle of MISPE is to use molecularly imprinted polymers with affinity to be immobilized on a solid-phase carrier to achieve the extraction and enrichment of target molecules through the specific interaction of the molecularly imprinted polymer with the target molecules. MISPE has been widely used in the fields of environmental monitoring, food safety monitoring, drug analysis, bioanalysis, and so on [79]. Chen et al. [80] have developed a new efficient and selective method for the enrichment of puerarin using molecularly imprinted polymers (MIPs) as a solid-phase extraction material, which allows the selective enrichment and high purification of the analytes. Puerarin was used as a template, monomer acrylamide (AA) was used as a functional monomer, and ethylene glycol dimethacrylate (EGDMA) was used a crosslinker. The optimal molar ratio for the preparation of the MIPs was puerarin:AA:EDGMA = 1:4:20. The addition of 0.2 mg/mL, 10 mL of kudzu root extract to the column followed by elution with different volume ratios of toluene/methanol revealed that 10:2 (v/v) was the suitable washing solvent to reduce the non-specific interactions. Moreover, 10 mL of methanol/acetic acid (9:1, v/v) was the optimal eluent to elute puerarin. Under these conditions, the yield of puerarin extracted from kudzu root at one time was up to 86%, and the purity of puerarin obtained by high-performance liquid chromatography analysis was greater than 86%. MISPE provides the possibility of efficient and selective separation and purification of puerarin from kudzu root. Luo et al. [81] used quantum mechanical methods to screen three functional monomers that may interact with puerarin (PR): acrylamide (AA), methacrylic acid (MAA), and 4-vinylpyridine (4-VP), and they found that AA was more likely to form strong complexes with PR by comparing the hydrogen-bonding strengths of the three, as well as by molecular dynamics simulations. At the same time, the template was coordinated with the three monomers in a molar ratio of 1:4 to form a complex to coat the SiO2 surface, and it was found that the binding energy of the synthesized SiO2@PR-MIP particles was the largest, so AA was the best functional monomer; the possible molar ratios for the interaction between PR and AA were also investigated, and 1:4 (PR/AA) was determined as the best molar ratio. In addition, equal amounts of SiO2@PR-MIP, SiO2@NIP (non-imprinted particles without added PR template), and commercial resins were taken to investigate the binding ability of the three to puerarin and other similar compounds in the crude extract of kudzu root. The experimental results showed that SiO2@PR-MIP microparticles could selectively enrich the flavonoids in the crude extract of kudzu root, and successfully recovered 90% of the three flavonoids (puerarin, dadizin, genistin) from the original herb; for the non-imprinted adsorbent SiO2@NIP and the commercial resin, the final amount of flavonoids adsorbed was significantly lower than that obtained by SiO2@PR-MIP. The above results indicate that SiO2@PR-MIP is a more desirable adsorbent, which is capable of specific recognition and selective enrichment of PR and similar compounds, in addition to good renewability and reuse for at least five times without significant loss of the adsorbed amount, which is expected to open up a new research direction for the selective enrichment of active ingredients in medicinal plants.
3.10. Macroporous Resin Adsorption Method
The adsorption action of the macroporous resin relies on the Van Der Waals force between it and the adsorbed molecules (adsorbent) and physically adsorbs through its huge specific surface, and then, based on the adsorption force and molecular weight size of the adsorbent, it achieves the purpose of isolation and purification through the elution of certain solvents. Meng [82] studied the process of purification of puerarin by the macroporous resin AB-8, and the conditions of ethanol concentration, adsorption flow rate, desorption flow rate, and sample concentration were optimized by an orthogonal test, where the following optimal purification parameters were obtained: ethanol concentration of 80%, adsorption flow rate of 9 BV/h, desorption flow rate of 4 BV/h, and sample solution concentration of 0.8 g/mL. The factors affecting the purification of the macroporous resin were in the order of resolution flow rate > adsorption flow rate > concentration of sample solution > concentration of ethanol. Chi et al. [83] investigated the adsorption properties of puerarin on S-8 resin and analyzed the effect of different concentrations of desorption solvents on the desorption of puerarin. The experimental results showed that the adsorption process of puerarin was in accordance with the Langmuir and Freundlich equations, the rate constant k increased with the increase in the initial concentration of puerarin, the adsorption equilibrium state was reached after 60 min, and the maximum adsorption rate could reach 95%. In addition, puerarin adsorbed on S-8 resin could be desorbed by different concentrations of ethanol–water solution, and with the increase in the volume of the desorption solvent, the desorption amount of puerarin firstly increased rapidly and slowly decreased, the desorption curves of each concentration had a maximum value, the 80% ethanol solution was chosen to be eluted by comparison, and the eluent was collected and concentrated, then compared with the standard of puerarin and the crude extracts of puerarin by HPLC analysis. The peak of puerarin appeared at the retention time of 10 min, and the impurity peak was significantly reduced compared with the crude extract without S-8 resin purification, which indicated that the S-8 resin was the ideal medium for the purification of puerarin, 80% ethanol was the optimal desorption solvent, and the final content of puerarin isolated was as high as 43.75%. Guo et al. [84] examined the adsorption of puerarin by six resins (D101, S-8, H103, X-5, HPD600, and AB-86) at a temperature of 25 °C and an adsorption duration of 3 h. From the adsorption isotherms, it was found that the adsorption amounts of all six resins increased with the increase in the equilibrium concentration of puerarin, and the adsorption amount of H103 was significantly higher than that of the other resins. Further analysis of H103 showed that it had the largest specific surface area and KL, indicating that this resin had the strongest adsorption affinity for puerarin and had the best adsorption effect. In addition, in this study, a process was developed for the large-scale isolation and purification of puerarin in kudzu root by selecting H103 as the purification resin, using 10% ethanol as the extraction solvent and adsorption solvent, and 75% ethanol as the desorption solvent, with a purification product yield of 11.65 g/100 g. The purified product of the resin was then hydrolyzed by mixing it with HCl to remove impurities. The contents of puerarin, daidzin, and daidzein were analyzed by HPLC and compared with the crude extract without resin purification. The results showed that the product yield decreased from 34.17% to 11.65%, and the content of puerarin increased from 14.25% to 41.78% by H103 resin purification, which was nearly three times higher. In conclusion, a strategy for the large-scale isolation and purification of puerarin can be established by resin and acid hydrolysis.
3.11. High-Performance Liquid Chromatography
High-performance liquid chromatography (HPLC) is an important branch of chromatography, which uses liquid as the mobile phase, and adopts a high-pressure infusion system to pump mobile phases such as single solvents with different polarities or mixed solvents and buffers with different ratios into a column equipped with a stationary phase, and then after the components are separated inside the column, they enter the detector for detection, so as to realize the analysis of the specimen. Niu et al. [85] achieved the separation and identification of 22 flavonoids, such as puerarin, ononin, daidzein, etc., in kudzu root by combining post-column derivatization (PCD) with high-performance liquid chromatography–diode array detector–sequential ion-trap time-of-flight mass spectrometry (HPLC-DAD-IT-TOF-MSn). The separation was performed on a YMCTMC18 (150 mm × 4.6 mm; 5 μm) column with a binary gradient of 0.02% formic acid acetonitrile solution (solvent A) and 0.02% formic acid aqueous solution (solvent B), the linear gradient of solvent B was controlled to be 90–75% from 0–30 min, and the linear gradient of solvent B was 75–10% from 30–40 min, with the injection volume of 1 μL and the flow rate of 0.8 mL/min, the column temperature at 40 °C, and the UV spectral data recorded in the range of 190–400 nm. The experimental results showed that 22 flavonoids could be separated within 35 min, and the purity of the obtained compounds was above 95%. This is the first on-line method for the identification of kudzu root flavonoids, which can quickly and comprehensively evaluate the quality of the sample, and which provides an efficient method for the analysis and quality control of complex components in medicinal plants. Feng et al. [76] chose an Elite SinoChrom ODS-BP column (250 mm × 4.6 mm, 5 µm) as the chromatographic column, and the separation was carried out with formic acid/water (1 mL/L) (solvent A) and methanol (solvent B) as the mobile phases to establish 5–95% B, 0–40 min, 95% B, 40 min, injection volume of 10 μL, flow rate of 1 mL/min, and detection wavelengths of 254 nm (for puerarin, daidzein, daidzin and genistin) and 360 nm (for genistein). The samples were analyzed for 8 min, and the peak areas of the resulting UV curves were recorded to determine the extraction efficiencies of the six cyclodextrins’ kudzu root flavonoids. Du et al. [64] used an ultra-HPLC-DAD-TOF-MS system and selected Zorbax SB C18 (50 mm × 4.6 mm, 1.8 μm) as the chromatographic column, with the mobile phases of 0.1% formic acid (solvent A) and methanol (solvent B), and the gradient elution profiles of 0–3 min, 20–30% B; 3–4 min, 30–32% B; and 4–8 min, 32–57% B, at a flow rate of 2.0 mL/min, a column temperature of 48 °C, and with UV spectra collected from 190–400 nm to qualitatively and quantitatively analyze 14 major phenolic compounds including puerarin, daidzin, daidzein, genistein, etc., in kudzu root. The results showed that this technique can successfully determine the contents of the compounds contained in kudzu root samples. It is a rapid method for separation and purification. Wong et al. [60] analyzed the ethanolic extract of kudzu root under UHPLC, choosing a BEH C18 (1.7 μm, 2.1 mm × 150 mm) column with 0.3% acetic acid–water (solvent A) and 0.3% acetic acid–acetonitrile (solvent B) as the mobile phases, and gradient elution profiles of 10–25% B, 0–7 min; 25–28% B, 7–8min; 28–32% B, 8–12 min; 32–40% B, 12–13 min; 40% B, 13–15 min; 40–10% B, 15–16 min; and 10% B, 16–20 min, with a column temperature of 25 °C, a flow rate of 0.2 mL/min, and an injection volume of 0.5 μL. Puerarin, 3′-hydroxypuerarin, 6″-O-D-xylosylpuerarin, and 3′-methoxypuerarin with cytoprotective effects were successfully isolated and identified from the extracts. Xu [86] determined the content of puerarin in 30% ethanol extract of kudzu root by UHPLC-MS on a ChromCore C18 column (2.1 mm × 100 mm, 1.8 μm) with an injection volume of 5 μL at a flow rate of 0.3 mL/min, performing isocratic elution for 5 min with a mobile phase system consisting of 25% acetonitrile–water. The content of puerarin (99%) was determined by the standard curve method, which provides an efficient and rapid method for the quantitative analysis of active ingredients in traditional Chinese medicine.
3.12. High-Speed Counter-Current Chromatography
High-speed counter-current chromatography (HSCCC) is a continuous and highly efficient liquid–liquid partition chromatographic separation technique developed in the 1980s, in which a special unidirectional hydrodynamic equilibrium is established by the two-phase solvent system in the high-speed rotating helical tube, so as to achieve the separation of substances by the difference of partition coefficients in the two phases. This technology is widely used in the fields of herbal composition separation, biochemistry, organic synthesis, healthy food, environmental analysis, etc. Cao et al. [87] used HSCCC for the semi-preparative separation and purification of puerarin and related flavonoids from crude extracts of kudzu root. The solvent system of ethyl acetate–n-butanol–water with a volume ratio of 2:1:3 was selected, where the injection volume was 8 mg/mL, the flow rate was 2.0 mL/min, the speed was 800 rpm, and the detection wavelength was 254 nm. Using the above separation conditions, five flavonoids, including puerarin, 3′-hydroxy puerarin, 3′-methtoxy puerarin, daidzin, and puerarinxyloside, were successfully separated and purified from 80 mg of crude extract. This showed that HSCCC is a powerful tool for the purification of the crude product. Sun et al. [88] purified the crude extract of kudzu root by HSCCC using an acetate–n-butanol–water (2:1:3) biphasic solvent system at 800 r/min, with a flow rate of 2.0 mL/min, and a sample volume of 10 mg. The chromatograms were analyzed to obtain the purity of puerarin, retention time, separation, and stationary phase retention, which were less than 90%, 27.502 min, 47%, and less than 0.5%, respectively, which indicates an effective method for the separation and purification of puerarin. Shi et al. [89] extracted the crude extracts of kudzu flower with petroleum ether, ethyl acetate, and n-butanol, respectively, and separated and purified the ethyl acetate site with HSCCC, where the selected solvent system was petroleum ether–ethyl acetate–methanol–water, with a flow rate of 1.2 mL/min, a separation speed of 850 rpm, a detection wavelength of 254 nm, a separation temperature of 25 °C, and where the separated compounds were recrystallized with methanol, then analyzed by HPLC, and structurally identified by UV, MS, and 1D and 2D NMR, where 18 antioxidant active compounds, including puerarin, daidzin, daidzein, glycitin, puerarin-4′-O-β-D-glucopyranoside, irisolidone, etc., were obtained. This shows that HSCCC is a very effective method for the rapid isolation of the components. With the development of separation technology in recent years, the “high g-value” technique has been successfully applied to HSCCC, resulting in a much shorter separation time, and this new type of instrument, which can analyze samples efficiently, is known as high-performance counter-current chromatography (HPCCC). Its advantage lies in the fact that the elution rate and injection volume can be increased linearly according to the change in instrument volume, separation on the order of hundred grams can be achieved with the same separation time, and the separation of the products remains unchanged compared to the general HSCCC. Li et al. [90] obtained the crude extract by ethanol solvent extraction of kudzu root powder, then the extract was extracted with petroleum ether and ethyl acetate, and then the ethyl acetate was taken for HPCCC analysis. The conditions of analysis were a temperature of 30 °C, a wavelength of 250 nm, and a solvent system of hexane–ethyl acetate–n-butanol–ethanol–water = 0.5:2:1:0.5:3.5 (v/v/v/v/v/v). The purities of puerarin, daidzin, daidzein, 3‘-hydroxy puerarin, and 3′-methoxy puerarin obtained were 98.77%, 96.53%, 97.59%, 90.21%, and 98.36%, respectively. The above results showed that HPCCC can rapidly and efficiently separate the five major flavonoids in kudzu root with high purity.
4. The Pharmacological Activities of Kudzu Root
The natural active ingredients extracted from kudzu root can be used to treat a wide range of diseases and are a potential source for research into new medicines. The following is a description of some scholars who have experimentally studied the pharmacological activities of kudzu root.
4.1. Regulates Vasodilation
In 2019, cardiovascular diseases were responsible for 19.7 million deaths globally and occur mainly in low- and middle-income countries [91]. In Haiti, cardiovascular diseases account for 36% of all deaths among adults over the age of 20 in the country, far exceeding the mortality rate from infectious diseases such as HIV [92]. Kudzu root has a protective effect on blood vessels, preventing the occurrence of cardiovascular diseases such as infarction, angina pectoris, cerebral ischemia, and hypertension. Chen et al. [93] executed C57BL/6 mice by carbon dioxide inhalation and removed the common carotid arteries, after which the carotid arteries of intact endothelium were pre-treated with the NO synthase inhibitor L-NAME, and acetylcholine (Ach) induced endothelium-dependent contractions (EDCs) in mouse arteries. Prior to the addition of cumulative concentrations of acetylcholine, the arteries were pretreated with puerarin, the COX inhibitors, and S18886. HPLC-MS was used to determine the concentrations of four prostaglandins (PGs): thromboxane A2 (TXA2), prostaglandin D2 (PGD2), prostaglandin I2 (PGI2), prostaglandin E2 (PGE2), and prostaglandin F2α (PGF2α). Acute 40 min puerarin treatment was found to reduce EDCs in a concentration-dependent manner and failed to inhibit the production of PGs in intact endothelial arteries, suggesting that puerarin is able to inhibit EDCs induced via Ach independently of the inhibition of TP receptor- or COX2-derived PGs. Zhou et al. [94] pretreated first-order mouse mesenteric resistance arteries with intact or incomplete endothelium using 1 μM phenylephrine (Phe) to constrict them and then exposed them to cumulative doses of puerarin (0.01, 0.1, 1, 10, 20, 50, 100, and 200 μM), and they found that mesenteric arteries with intact endothelium in mice treated with puerarin relaxed and that the degree of relaxation was correlated with the dose of puerarin. Subsequently, to investigate the role of transient receptor potential vanilloid 4 (TRPV4) channels in the modulation of vasorelaxation by puerarin, mesenteric arteries of TRPV4−/− mice were selected. The results showed that puerarin was unable to regulate vasorelaxation due to TRPV4 deficiency, suggesting that puerarin may regulate vasorelaxation through TRPV4 channels. In-depth studies revealed that the ability of puerarin to regulate vasodilation was significantly inhibited in the presence of an IKCa inhibitor (TRAM-34, 1 μM) and an SKCa inhibitor (apamin, 100 nM), suggesting that puerarin regulates mesenteric endothelial vasodilatation through the TRPV4-IKCa/SKCa pathway. Lam et al. [95] killed rats with CO2 to separate the basilar artery from the brain and washed with Krebs–Henseleit solution, after which U46619 (100 nM) was added to induce vasoconstriction. Subsequently, mixed extracts of danshen and kudzu root were added at 5 min intervals to obtain concentration–mixture response curves. HPLC analysis showed that danshensu, protocatechuic aldehyde, puerarin, daidzein 8-C-apiosyl-glucoside, daidzin, salvianolic acid B, daidzein, cryptotanshinone, tanshinone I, and tanshinone IIA were detected in the mixture. Endothelium-dependent mechanisms were investigated by mechanical removal of the endothelium. The extracts were found to produce concentration-dependent relaxation of arterial rings with the mechanical removal of the endothelium, and the BKCa channel inhibitor iberiotoxin (100 nM), the KV channel inhibitor 4-aminopyridine (1 mM), and the KIR channel inhibitor barium chloride (100 μM) had no effect on the ability of the extracts to modulate vasodilation. This suggests that the vasolytic effect of the mixed extracts of danshen and kudzu root on rat basilar artery vasorelaxants is independent of endothelial-derived mediators and can be used as a useful brain-protective agent in some occlusive cerebrovascular patients.
4.2. Treatment of Inflammatory Diseases
Atopic dermatitis (AD) is one of the common inflammatory disorders of the immune system and is a highly pruritic, chronic inflammatory dermatosis with the main symptoms of dry skin, skin irritation, skin thickening, and severe induced pruritic eczema lesions [96]. The incidence of the disease is increasing year by year worldwide [97]. Lee et al. [98] sub-randomized BALB/c mice into six groups, one of which was a normal group, and each of the other groups was induced with 2,4-dinitrochlorobenzene (DNCB) for three days. After that, three groups of mice were selected to be administered with an oral dosage of 10 mg/kg, 30 mg/kg, and 50 mg/kg doses of puerarin, and it was found that the skin thickness of mice decreased after oral puerarin treatment, and that the degree of decrease was positively correlated with the dose. In addition, the effect of puerarin on the levels of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-4, IL-6, and IL-31 in mice was also investigated, and it was found that the levels of pro-inflammatory cytokines were significantly reduced after treatment with puerarin. An in-depth study revealed that the secretion of pro-inflammatory cytokines is caused by the activation of the MAPKs (p38, ERK, and JNK) signaling pathway. Puerarin appears to have a therapeutic effect on AD by reducing cytokine and chemokine expression in DNCB-induced AD mice through the inhibition of the STAT-1 and NK-κB pathways, as well as the activation of the MAPKs and Akt signaling pathways. In addition, puerarin can be used to treat acute liver injury (ALI), which is a disease in which drug overdose or severe infections lead to liver damage, by inhibiting the inflammatory response [99]. Yang et al. [100] induced ALI in C57BL/6 mice by LPS/D-Gal, and after that, puerarin was orally administered at different concentrations (25 mg/kg, 50 mg/kg, 100 mg/kg), and observed the liver tissue changes in mice. It was found that ALI in mice treated with oral puerarin was significantly relieved, with a reduction in hepatic lobular structure and inflammatory cell infiltration. To determine the degree of remission, IL-1β, TNF-α, and IL-6 levels were monitored, and it was found that the expression levels of IL-1β, TNF-α, and IL-6 were increased in untreated puerarin mice, whereas the expression levels of all three pro-inflammatory cytokines were significantly reduced after treatment with kudzu root, which suggests that kudzu root has a significant anti-inflammatory and preventive effect on LPS/D-Gal-induced ALI. ZEB2 has a significant effect on pro-inflammatory cytokine production and is essential for the production of pro-inflammatory cytokines and epithelial-to-mesenchymal transition (EMT), and puerarin may activate the expression level of ZEB2 by inhibiting the NF-κB signaling pathway, thus inhibiting the production of IL-1β, TNF-α, and IL-6 and thus achieving the therapeutic purpose of inflammation. Noh et al. [101] evaluated the effects of puerarin on obesity and metabolic complications and investigated the underlying immunological mechanisms. Obesity causes adipocyte hypertrophy and increases the levels of pro-inflammatory cytokines in adipose tissue. Chronic tissue inflammation begins with the hyperinfiltration of adipose tissue macrophages (ATMs), which leads to multiple metabolic complications such as dyslipidemia and insulin resistance [102]. A total of 20 male C57BL/6 mice were divided into four groups: normal diet group (NC), high-fat diet group (HFD), HFD + puerarin 200 mg/kg group (PUE), and HFD + atorvastatin 10 mg/kg group (ATO), and body weight, fat weight, and inflammatory cytokine expression levels were compared. It was found that body weight and fat weight increased significantly in the HFD group and fat weight decreased but body weight remained unchanged in the PUE group compared to the NC group. In addition, in terms of inflammatory gene expression, the PUE group significantly reduced the expression of F4/80 and TNF-α. These findings suggest that puerarin reduces adipose tissue inflammation by lowering lipids and attenuating ATM aggregation through the inhibition of the TNF-α/NF-κB pathway, decreasing differentiation to M1 ATM and suppressing the expression levels of F4/80 and TNF-α. This is the first observation of an anti-inflammatory effect on ATM.
4.3. Antioxidant Effect
Kudzu root, as a natural medicinal plant, has extracts that are not only low in cytotoxicity but also have excellent antioxidant activity. Oxidative stress is a state of imbalance between oxidative and antioxidant effects in the body, favoring oxidation, leading to an inflammatory infiltration of neutrophils, an increase in the secretion of proteolytic enzymes, and the production of large amounts of oxidative intermediates. It is a negative effect produced in the body by free radicals and is considered one of the main causes of skin ageing and skin diseases [103]. Hydroquinone, tretinoin, azelaic acid, and ascorbic acid are widely used in cosmetic products due to their ability to prevent skin aging. Although these compounds are very effective, they can also cause certain side effects such as dermatitis and erythema [104]. Bacanli et al. [105] examined the antioxidant capacity of puerarin, galangin, and ursolic acid by using Trolox equivalent antioxidant capacity (TEAC), and the experimental results showed that puerarin, galangin, and ursolic acid had antioxidant activity in the concentration ranges of 2–2000 μM, 1–2000 μM, and 100–2000 μM, respectively. Compared with the reference antioxidant Trolox, the antioxidant capacity of the three was weak. Yu et al. [106] investigated the scavenging activity of total kudzu root flavonoids against four different reactive oxygen species (ROS), including superoxide anion (O2.−), hydroxyl radical (OH.), lipid-derived radical (R.), and single-linear oxygen species (1O2), by using chemiluminescence, and they found that the radicals of total kudzu root flavonoids were able to scavenge all ROS radicals, and that they were a strong antioxidant. Subsequently, by comparing the EC50 values of total kudzu root flavonoids, puerarin, and tea polyphenols, it was found that the free radical scavenging ability of total kudzu root flavonoids was significantly higher than that of the main constituent, puerarin, and similar to the EC50 value of tea polyphenols. This result also suggests that the flavonoids in kudzu root may have a synergistic effect in free radical scavenging, or that other components with stronger free radical scavenging activity exist. Li et al. [107] selectively catalyzed puerarin by the selective catalysis of aspergillus oryzae to puerarin monoester, which has higher fat solubility and is better absorbed by the human body. A UV-visible spectrophotometer was used to determine the absorbance of DPPH and ABTS radicals after the addition of puerarin and puerarin monoester at 517 nm and 734 nm to judge the scavenging ability of the two compounds on the two radicals. The experimental results showed that puerarin has high antioxidant activity due to the presence of phenolic hydroxyl groups, and compared with puerarin, puerarin monoesters have a lower scavenging ability of free radicals, i.e., lower antioxidant capacity. Puerarin can be effectively modified by new biocatalytic methods, which can improve the lipophilicity and bioactivity and greatly increase the value of kudzu root utilization. Ye et al. [108] were able to convert puerarin into 3′-hydroxypuearin (3′-OHP) by screening a strain named Trichoderma harzianum NJ01 from soil, and its solubility was greatly increased. The team also compared puerarin, 3′-OHP, and the traditional antioxidant α-tocopherol in terms of their ability to scavenge DPPH free radicals to determine the antioxidant activity of the three. The results showed that the biotransformed 3′-OHP had a 20 times higher antioxidant capacity than puerarin and three times the capacity of the traditional antioxidant α-tocopherol, and that the scavenging capacity increased with increasing concentration. The content of 3′-OHP in natural kudzu root is minimal, while the 3′-OHP produced through the biotransformation of puerarin is more economically valuable. This has significant implications for the advancement of medicinal chemistry.
4.4. Treatment of Eye Diseases
The flavonoids component extracted from kudzu root has been shown to be effective in the treatment of a wide range of ophthalmic diseases. Hao et al. [109] induced RVO in 10 SD rats by the laser thrombosis method to reduce venous blood flow to 50% of the normal value, after which the RVO model rats were randomly divided into four groups, one of which was the model group, and the remaining three were given different doses of puerarin (20, 40, and 80 mg/kg), analyzed for vascular endothelial growth factor (VEGF), IL-1β, and NO levels, and then underwent histopathological examination. The results of the experiments revealed that the retinal nerves of RVO rats were significantly edematous and thickened, with swollen and disorganized cells in the inner and outer nuclear layers, and a large number of vacuoles could be seen when compared with the normal rat retina. At the end of the experiment, the mean venous blood flow in the model group was reduced by 73.5% compared with the normal value, which was increased by 9.3%, 33.1%, and 41.5% after treatment with puerarin (20, 40, and 80 mg/kg, respectively). Symptoms such as thick edema in the neural retinal layer and swelling inner and outer nuclear layers of the rats were significantly relieved. In addition, puerarin reduced the levels of VEGF and IL-1β and increased the levels of NO in the vitreous fluid compared with the model group. In addition, puerarin reduced the levels of VEGF and IL-1β and increased the levels of NO in the vitreous fluid compared with the model group. In conclusion, puerarin may be a treatment for RVO model rats through inhibiting neovascularization, being anti-inflammatory, and increasing NO-mediated pathways. Guan et al. [110] induced retinal ischemia/reperfusion (I/R) injury in the left eyes of male SD rats, after which the I/R-injured rats were randomly divided into four groups: three groups of rats were gavaged with puerarin at the doses of 25 mg/kg, 50 mg/kg, and 100 mg/kg at 1 h, 24 h, and 48 h, respectively, and the other group was designated as the model group. The latter group was compared with the normal group. It was found that, compared with the normal group, the model group of rats had a weakened light reflex, longer pupil constriction diameters and larger constriction areas, and a decreased thickness of the ganglion cell layer (GCL). After treatment with puerarin, the impaired light reflexes of the rats were significantly reduced and the decrease in GCL thickness was restored. In addition, puerarin was found to inhibit the activation of TLR4/NLRP3 inflammasomes caused by I/R injury and to reduce the damage to retinal ganglion cells (RGCs), suggesting that puerarin could be used as an adjunctive drug for the treatment of inflammatory responses to retinal I/R injury. Song et al. [111] investigated the in vivo effects and possible mechanisms of puerarin on retinal damage by inducing iron overload in the retina of mice through repeated injections of iron dextrose followed by oral administration of puerarin. It was found that iron content in serum and retinal homogenate was significantly elevated, and the average thickness of the outer nuclear layer of the retina was thinner and iron deposition was more pronounced in iron-overloaded mice than in normal healthy mice. These symptoms were significantly improved after treatment with puerarin. The potential mechanism of puerarin on iron overload retinal injury was investigated and it was found that puerarin helped to reduce the excess iron in the body to alleviate retinal injury by inhibiting the expression of TfR, Ft, DMT-1, and Cav1.2. In addition, puerarin enhanced CAT, SOD, and GSH-Px activities, decreased MDA content, enhanced antioxidant capacity in iron-overloaded mice, inhibited MAPK, STAT3 activation, and apoptotic pathways, and maintained retinal cell activity under iron overload. In conclusion, puerarin may have some therapeutic effects on retinal damage in iron-overloaded mice, and the specific mechanism of action remains to be further explored.
4.5. Treatment of Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) is a common clinicopathological syndrome characterized by excessive deposition of fat in the liver, with the exception of alcohol and other well-defined hepatic injury factors. Excessive fat deposition leads to reduced levels of autophagy, which is detrimental to hepatic lipolysis and produces inflammation [112]. Sun et al. [113] induced NAFLD in mice by HFD feeding for 16 weeks and observed the effect on HFD-induced lipid deposition by feeding different doses of kudzu root flavonoids (50, 100, and 200 mg/kg/d) for 4 weeks. It was found that the lipid deposition was decreased and inflammatory cell infiltration of liver histiocytes was significantly reduced after treatment with kudzu root flavonoids, and the best effect was achieved with 200 mg/kg/d. Puerarin, daidzin, afzelin, ononin, puerarin xyloside, and other ingredients were isolated from kudzu root flavonoids by UHPLC-QTOF-MS analysis, suggesting that the active ingredient may be one or more of them. In summary, kudzu root flavonoids activated autophagy by inhibiting the phosphorylation of the PI3K/Akt/mTOR signaling pathway, reduced the expression levels of IL-1β, IL-18, IL-33, and IL-6 mRNA in mouse liver cells, reduced lipid deposition and lowered inflammation, and thus achieved the therapeutic effect of NAFLD. Wang et al. [114] compared the therapeutic effects of puerarin and curcumin in a methionine–choline-deficient (MCD) diet-induced NAFLD C57BL/6 mouse model. By dividing the mice into normal control, model control, curcumin-treated, and puerarin-treated groups, the mice in the normal control group were on a methionine–choline-sufficient (MCS) diet, and the other groups were MCD-fed for a fortnight, after which the histopathological evaluation of the liver and the detection of serum TG, TC, HDL, LDL, TNF-α, IL-6, and liver levels of PPARγ and NF-κB were performed. The results showed that treatment with puerarin and curcumin attenuated lipid deposition caused by the MCD diet, reduced liver damage, and significantly reduced serum levels of TG and TC in the puerarin treatment compared to the normal control group, and reduced serum levels of LDL only in the puerarin group compared to the model control group. In terms of TNF-α and IL-6 expression levels, both curcumin and puerarin inhibited TNF-α secretion compared to the model control group, but puerarin had no inhibitory effect on IL-6 secretion. It was found that puerarin and curcumin treat NAFLD through different mechanisms, puerarin may regulate lipid metabolism through the PPARγ pathway, while curcumin may inhibit inflammatory responses through the PPARγ/NF-κB pathway, and this study provides an effective strategy for the development of drugs for the treatment of NAFLD. Zhou et al. [115] established a high-fat–high-fructose (HFFD)-induced NAFLD rat model and divided the rats into five groups: normal group (NG); model group: HFFD-fed (MG); low-dose puerarin group: HFFD + 0.11% puerarin (PL); high-dose puerarin group: HFFD + 0.22% puerarin (PH); and fenofibrate group: HFFD + fenofibrate (FG), and the effect of puerarin in treating NAFLD was comprehensively evaluated by serum and liver lipid indexes, antioxidant markers, and inflammatory factors. It was found that severe oxidative stress, inflammation, and liver injury occurred in the MG group compared to the NG group. After treatment with puerarin, TG, TC, and FFA levels in the serum and liver decreased, VLDL levels increased, and lipid levels significantly improved compared with the MG group, and the improvement effect of low-dose puerarin was better than that of high-dose puerarin. Regarding the changes in the content of antioxidant markers, the content of SOD, CAT, and GSH-Px in rats and the serum in the puerarin group was significantly higher than that in MG group. In addition, low-dose puerarin significantly reduced the levels of IL-1b, IL-6, and TNF-α in the serum and liver of HFFD rats, and the inflammatory response was significantly reduced. In conclusion, puerarin ameliorated NAFLD by modulating hepatic lipid accumulation, liver function, oxidative stress, and inflammatory response.
4.6. Lowering Blood Sugar
Diabetes mellitus is a common disease and is known as one of the three major killers of human health along with hypertension and cardiovascular disease. In recent years, as people’s living standards continue to improve, the prevalence of diabetes mellitus has been increasing, with a global average growth rate of 51% [116]. Flavonoid compounds extracted from kudzu root have good ameliorative and therapeutic effects on hyperglycemia, insulin resistance, and hyperlipidemia. It has been suggested that kudzu root extracts may increase glucose handling through mechanisms such as increased receptor insulin levels, insulin expression [117], and activation of the antidiabetic TGFBeta 1/Smad2 pathway [118]. Carlson et al. [119] stimulated CD-1 mice with glucose orally by causing their blood glucose elevation and then by acute or chronic supplementation of kudzu root extract on blood glucose levels in mice. The results showed that both modalities resulted in a decrease in blood glucose levels and an improvement in glucose tolerance in mice chronically administered with kudzu root extract, presumably mediated by enhanced insulin sensitivity (chronic) and the inhibition of sodium-dependent glucose transport. As for which specific flavonoid compounds in kudzu root work, there is no specific analysis in the article. It is speculated that a variety of flavonoids in kudzu root may synergize or antagonize each other to show activity. Chen et al. [120] used streptozotocin (STZ) to induce type I diabetes mellitus in rats to analyze the mechanism of action of puerarin in lowering blood glucose in rats. After the rats were injected intravenously with puerarin at a concentration of 15 mg/kg, the plasma glucose concentration decreased from 25.7 ± 2.8 mmol/L to 17.1 ± 3.1 mmol/L, and the hypoglycemic activity of kudzu root was about 33.8 ± 3.7 mmol/L. An in-depth study on the hypoglycemic mechanism of kudzu root revealed that due to its ability to activate the α1-adrenergic receptor and promote the β1-adrenoceptor, this leads to the reduction of blood glucose in STZ-induced rats. Duru et al. [121] investigated the anti-glycemic effect of kudzu root flavonoids on tetraoxydipyridine-induced diabetic rats in terms of glycemic control, hematological indices, and the antidiabetic properties of the extract evaluated on key parameters such as liver enzyme activity and pancreatic β-cell regeneration. The HPLC analysis showed that the obtained kudzu root extract contains puerarin, daidzein, and genistein. The results showed that the extract reduced hyperglycemia and cellular HbA1c levels, improved glucose tolerance, promoted pancreatic β-cell regeneration, and also prevented diabetes-related alterations in renal tissue structure and function when given at doses above a certain value (200 mg/kg).
4.7. Reducing Alcohol Dependence
Excessive drinking is a pervasive problem in society that can cause early deaths, injuries, violence, crime, property damage, disease, and other harms [122]. In the United States, more than 95,000 deaths are caused by excessive alcohol consumption each year [123]. The active ingredients extracted from kudzu root have been used as anti-alcoholic agents for more than 1000 years [124], and in recent years have been used by many scholars for the treatment of alcohol dependence. Lin et al. [125] administered daidzin, daidzein, and puerarin orally to P rats for 7 days at a dose of 100 mg/kg/day, and observed the rats’ alcohol, water, and food consumption. It was found that oral administration of daidzein, daidzin, and puerarin resulted in a 75%, 50%, and 40% decrease in alcohol intake, respectively, and food consumption remained unchanged after oral administration, indicating that all three compounds can inhibit voluntary alcohol consumption without affecting appetite. In addition, the decrease in alcohol consumption was compensated by an increase in water intake. In summary, it can be seen that flavonoid compounds extracted from kudzu root are effective in reducing alcohol dependence, and that this effect is specific and may be due to the inhibition or stimulation of neurotransmitter/modulatory systems in the central nervous system in the reward pathway in response to ethanol. Penetar et al. [126] used a double-blind, crossover design experiment on 10 volunteers to take puerarin for one week prior to an experiment of drinking alcohol that lasted 1.5 h and recorded the amount of alcohol consumed. The results of the experiment showed that the amount of alcohol consumed decreased significantly after the puerarin treatment, and the average duration of one drink increased significantly compared to the previous one. This study demonstrates that the flavonoids in kudzu root can change human drinking habits and may be an aid in the treatment of excessive alcohol consumption. In addition, Penetar et al. [127] built on the results of a previous study to explore whether taking a single dose of kudzu root extract shortly before drinking alcohol would reduce alcohol intake. The extract contains puerarin, 3′-methoxypuerarin, daidzin, and daidzein. Thirty-two volunteers aged between 21 and 40 years were selected and randomly assigned to either a placebo or kudzu root extract group. The experiment was divided into two phases, starting with a drinking without dosing phase, followed by a seven-day interval in which either kudzu root extract or placebo was administered orally in a double-blind manner 2.5 h before drinking. The results of the experiment found that compared to the placebo group, the average beer consumption in the kudzu root extract group decreased from 3.0 ± 1.7 beers to 1.9 ± 1.3 beers, and the time to have one beer was relatively slower, which proved that kudzu root extract was effective in reducing alcohol consumption; furthermore, there were no harmful side effects of taking kudzu root extract to drink alcohol, suggesting that a moderate single dose of kudzu root extract could reduce alcoholism, exerts beneficial effects within a few hours of taking it, and may be an adjunct in the treatment of alcohol addiction.
5. Conclusions
In this paper, the main chemical constituents in kudzu root, mainly flavonoids such as puerarin, dadizin, and daidzein, were introduced from the perspective of kudzu root, and some methods of extraction and isolation of these compounds were summarized. These methods have been experimented with by both domestic and international scholars and have achieved remarkable results. The extraction and separation methods listed in the paper have their own advantages and disadvantages, and readers are required to select the best program suitable for themselves according to the local conditions. In addition, some common pharmacological activities of kudzu root are discussed, which can be used as pharmaceutical ingredients to treat some common diseases. The medicinal value of kudzu root is certainly not limited to this—as a natural Asian herb, it has been the focus of attention of various scholars. In conclusion, more time is needed for researchers to optimize the extraction process of kudzu root active ingredients and to explore the new medicinal values of kudzu root for the human body.
Author Contributions
Writing—original draft preparation, E.G.; supervision and validation, W.W.; writing—review and editing, Y.H.; writing—review and editing, Z.L.; writing—editing, B.C.; writing—review and editing, S.X.; supervision, D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFD2200604) and the Scientific Research Funds of Huaqiao University.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yi, H.; Yang, H. Phytochemicial and Extracting Technology Research Progress of Pueraria lobata. Chin. J. Exp. Tradit. Med. Formulae. 2006, 60–63. [Google Scholar]
- Fan, M.Y.; Liu, R.H.; Shao, F.; Meng, X.W.; Zhu, W.F.; Zhang, P.Z.; Zhang, Y.M. Chemical Constituents of Pueraria lobata Roots. J. Chin. Med. Mater. 2022, 45, 1877–1885. [Google Scholar]
- Deng, Y.; Lei, T.W.; Li, H.M.; Mo, X.C.; Wang, Z.T.; Ou, H.L. ERK5/KLF2 activation is involved in the reducing effects of puerarin on monocyte adhesion to endothelial cells and atherosclerotic lesion in apolipoprotein E-deficient mice. BBA-Mol. Basis. Dis. 2018, 1864, 2590–2599. [Google Scholar] [CrossRef]
- Tang, L.; Liu, D.; Yi, X.Q.; Xu, T.T.; Liu, Y.; Luo, Y.C.; Yin, D.; He, M. The protective effects of puerarin in cardiomyocytes from anoxia/reoxygenation injury are mediated by PKCε. Cell Biochem. Funct. 2014, 32, 378–386. [Google Scholar] [CrossRef]
- Bracken, B.K.; Penetar, D.M.; Maclean, R.R.; Lukas, S.E. Kudzu Root Extract Does Not Perturb the Sleep/Wake Cycle of Moderate Drinkers. J. Altern. Complement. Med. 2011, 17, 961–966. [Google Scholar] [CrossRef]
- Shi, W.L.; Yuan, R.; Chen, X.; Xin, Q.; Wang, Y.; Shang, X.H.; Cong, W.H.; Chen, K.J. Puerarin Reduces Blood Pressure in Spontaneously Hypertensive Rats by Targeting eNOS. Am. J. Chin. Med. 2019, 47, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Zheng, S.; Ding, Y.J.; Dai, Y.Q.; Zhou, Y.; Xiang, R.F.; Bay-Jensen, A.C.; Karsdal, M.A.; Qvist, P.; Zheng, Q.L. Preventive effects of kudzu root on bone loss and cartilage degradation in ovariectomized rat. Am. J. Transl. Res. 2017, 9, 3517–3527. [Google Scholar] [PubMed]
- Jia, L.H.; Hu, Y.L.; Yang, G.H.; Li, P.L. Puerarin suppresses cell growth and migration in HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR signaling pathway. Exp. Ther. Med. 2019, 18, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Mei, Z.G.; Fu, Y.; Yang, S.B.; Zhang, S.Z.; Huang, W.F.; Xiong, L.; Zhou, H.J.; Tao, W.; Feng, Z.T. Puerarin protects rat brain against ischemia/reperfusion injury by suppressing autophagy via the AMPK-mTOR-ULK1 signaling pathway. Neural Regener. Res. 2018, 13, 989–998. [Google Scholar] [CrossRef]
- Ma, X.J.; Ma, J.R.; Leng, T.; Yuan, Z.Z.; Hu, T.T.; Liu, Q.Y.; Shen, T. Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Renal Fail. 2023, 45, 2146512. [Google Scholar] [CrossRef]
- Jiang, Z.L.; Cui, X.N.; Qu, P.R.; Shang, C.; Xiang, M.; Wang, J. Roles and mechanisms of puerarin on cardiovascular disease: A review. Biomed. Pharmacother. 2022, 147, 112655. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011, 134, 584–607. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Barnes, S.; Wyss, J.M. Kudzu isoflavone C-glycosides: Analysis, biological activities, and metabolism. Food Front. 2021, 2, 383–389. [Google Scholar] [CrossRef]
- He, H.; Peng, S.W.; Song, X.; Jia, R.Y.; Zou, Y.F.; Li, L.X.; Yin, Z.Q. Protective effect of isoflavones and triterpenoid saponins from pueraria lobata on liver diseases: A review. Food Sci. Nutr. 2022, 10, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, Z.N.; Zhang, S.J.; Sun, Y.; Zheng, F.J.; Li, Y.H. Protective effects and mechanism of puerarin targeting PI3K/Akt signal pathway on neurological diseases. Front. Pharmacol. 2022, 13, 1022053. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Wadhwa, K.; Mishra, R.; Gupta, S.; Ahmad, F.; Kamal, M.; Iqbal, D.; Alsaweed, M.; Nuli, M.V.; Abomughaid, M.M.; et al. Investigating the Potential Therapeutic Mechanisms of Puerarin in Neurological Diseases. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Y.C.; Lee, D.Y.W. Applications of Pueraria lobata in treating diabetics and reducing alcohol drinking. Chin. Herb. Med. 2019, 11, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Zovko-Koncic, M.; Chrostek, L. Phytotherapy of Alcoholism. Nat. Prod. Commun. 2012, 7, 273–280. [Google Scholar] [CrossRef]
- Xuan, T.; Liu, Y.H.; Liu, R.; Liu, S.; Han, J.Q.; Bai, X.Y.; Wu, J.; Fan, R.H. Advances in Extraction, Purification, and Analysis Techniques of the Main Components of Kudzu Root: A Comprehensive Review. Molecules 2023, 28, 6577. [Google Scholar] [CrossRef]
- Shen, Z.W.; Rong, H.D.; Wei, X.S.; Bing, D.; Pan, L. Progress in Research on Composition, Structure, Functions and Mechanism of Action of Radix puerariae Isoflavones. Food Sci. 2023, 44, 1877–1885. [Google Scholar]
- Fei, J.P. Qualities Analysis and Functional Evaluation of Pueraria Fermentedwine. Master’s Thesis, Northwest A&F University, Shanxi, China, 2018. [Google Scholar]
- Fei, C.J.; Gang, Z.G.; Ping, L.; Ping, K.L. Chemical constituents of Radix Puerariae from Anhui Province. Chin. J. Med. Chem. 2007, 1, 47–49. [Google Scholar]
- Jun-Ei, K.; Un-Ichi, F.; Junko, B.; Takashi, T.; Masaki, Y.; Toshihiro, N. Studies on the Constituents of Pueraria lobata. III. Isoflavonoids and Related Compounds in the Roots and the Voluble Stems. Chem. Pharm. Bull. 1987, 35, 4846–4850. [Google Scholar]
- Rong, H.; Stevens, J.F.; Deinzer, M.L.; Cooman, L.D.; Keukeleire, D.D. Identification of isoflavones in the roots of Pueraria lobata. Planta Med. 1998, 64, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Zhang, Q.W.; Wang, Y.T. Chemical constituents from roots of Pueraria lobata. China J. Chin. Mater. Medica 2010, 35, 3156–3160. [Google Scholar]
- Liu, C.J.; Zhao, Z.H.; Wang, D.Y. Studies on the chemical composition of kudzu root from the USA. China J. Chin. Mater. Medica 1992, 2, 101–102. [Google Scholar]
- Hirakura, K.; Morita, M.; Nakajima, K.; Sugama, K.; Takagi, K.; Niitsu, K.; Ikeya, Y.; Maruno, M.; Okada, M. Phenolic glucosides from the root of Pueraria lobata. Phytochemistry 1997, 46, 921–928. [Google Scholar] [CrossRef]
- Sang, Y.S.; Shi, H.M.; Min, Z.D. Studies on chemical constituents from root of Pueraria omeiensis. Chin. Tradit. Herb. Drugs 2002, 33, 11–13. [Google Scholar]
- Zhang, D.W.; Dai, S.J.; Li, G.H.; Dai, J.G. Chemical constituents in cane of Pueraria lobata. Chin. Tradit. Herb. Drugs 2011, 42, 649–651. [Google Scholar]
- Junei, K.; Izumi, M.; Kotaro, M.; Kiyoshi, K.; Toshiaki, T.; Masaki, Y.; Toshihiro, N. Oleanene-sapogenols from puerariae radix. Chem. Pharm. Bull. 1985, 33, 1293–1296. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.-H.; Tan, Y.; Kano, Y.; Yuan, D. New triterpenoid saponins from the flowers of Pueraria thomsonii. J. Asian. Nat. Prod. Res. 2013, 15, 1065–1072. [Google Scholar] [CrossRef]
- Tomonori, A.; Junnei, K.; Toshihiro, N.; Ryuichi, I. Oleanene-Type Triterpene Glycosides from Puerariae Radix. IV. Six New Saponins from Pueraria lobata. Chem. Pharm. Bull. 1997, 45, 362–366. [Google Scholar] [CrossRef]
- Ohshima, Y.; Okuyama, T.; Takahashi, K.; Takizawa, T.; Shibata, S. Isolation and high performance liquid chromatography (HPLC) of isoflavonoids from the Pueraria root. Planta Med. 1988, 54, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Study on the Chemical Constituents of the Root of Pueraria lobata. Master’s Thesis, Shenyang Pharmaceutical University, Liaoning, China, 2011. [Google Scholar]
- Chen, M.H.; Zhang, S.J. Studies on the chemical composition of kudzu root. China J. Chin. Mater. Medica. 1985, 6, 36–38. [Google Scholar]
- Fan, C.H.; Chang, L. Research Progress on Chemical Constituents, Biological Activities and Processing and Utilization of Puerariae lobatae Radix. China Fruit Veg. 2022, 42, 36–40. [Google Scholar]
- Liu, M.; Li, Z.; Li, X.Y.; Tan, W.Y.; Wang, Q.; Liu, T. Study on kinetic model of extracting polysaccharide from Polygonatum odoratum. J. Guangdong Pharm. Univ. 2015, 31, 161–166. [Google Scholar]
- Zhang, A.C.; Li, Z.G.; Yu, L.Z. Effect of decoction time on the water-soluble components of kudzu root. J. Chin. Med. Mater. 1997, 2, 93–94. [Google Scholar]
- Li, Y.J.; Juan, S.Z.; Xian, T.H.; Min, L.G. Study on extraction technology of soluble ohwi pigment from ohwi. Food Ferment. Ind. 2003, 6, 51–54. [Google Scholar]
- Yang, S.J.; Zhang, H.X.; Zhou, Y.J. Study on the optimum extraction and purification technology of isoflavonoid of pueraria lobata. Food Sci. 2004, 8, 124–127. [Google Scholar]
- Zhang, X.G.; Wang, D.M. Research on the extraction process of puerarin. J. Chin. Med. Mater. 2004, 9, 680–682. [Google Scholar]
- Zhang, J.; Chen, Y.; Cao, S.L.; Yin, X.B.; Qi, J.J.; Zhang, H.; Dang, X.F.; Ni, J. Study on applicability of kinetic model for water extracts from Puerariae Radix. China J. Chin. Mater. Medica 2013, 38, 2788–2792. [Google Scholar]
- Li, J.P.; Jia, L.N.; Nie, T.T. Study on extraction of the effective composition of silybum marianum by organic solvent-internal reflux method. Liaoning Chem. Ind. 2004, 33, 497–499. [Google Scholar]
- Wu, Y.F.; Bai, J.H.; Zhao, E.L. Optimization of microwave-assisted extraction technology of puerarin from radix puerariae by orthogonal experiment. Hubei Agric. Sci. 2012, 51, 989–991. [Google Scholar]
- Liu, M.Y.; Dong, C.; Cao, X.Q.; Qin, L.; Xu, H.H.; Wang, Y.F.; Li, X.Y. Study on extraction process of radix puerariae. China Pharm. 2008, 3, 24–25. [Google Scholar]
- Han, J.; Cao, W.; Yin, H.; Yan, H.S.; Wang, C.L. Study on extraction of puerarin from Radix puerariae by orthogonal test. Chin. J. Hosp. Pharm. 2007, 3, 332–333. [Google Scholar]
- Pan, J.; Chen, Q.; Wang, G.X.; Zhang, J.; Xie, H.M. Optimum extracting technology of flavonoids in Radix Puerariae. Trans. Chin. Soc. Agric. Eng. 1998, 4, 236–239. [Google Scholar]
- Xu, H.N.; He, C.H. Separation and purification of puerarin with solvent extraction. Sep. Purif. Technol. 2007, 56, 397–400. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Kloskowskic, A.; Namiesnik, J. Perspectives on the replacement of harmful organic solvents in analytical methodologies: A framework toward the implementation of a generation of eco-friendly alternatives. Green Chem. 2015, 17, 3687–3705. [Google Scholar] [CrossRef]
- Cui, Z.F.; Djocki, A.V.E.; Yao, J.H.; Wu, Q.W.; Zhang, D.Q.; Nan, S.W.; Gao, J.; Li, C.L. COSMO-SAC-supported evaluation of natural deep eutectic solvents for the extraction of tea polyphenols and process optimization. J. Mol. Liq. 2021, 328, 115406. [Google Scholar] [CrossRef]
- Chen, M.; Lahaye, M. Natural deep eutectic solvents pretreatment as an aid for pectin extraction from apple pomace. Food Hydrocolloid. 2021, 115, 106601. [Google Scholar] [CrossRef]
- Duru, K.C.; Slesarev, G.P.; Aboushanab, S.A.; Kovalev, I.S.; Zeidler, D.M.; Kovaleva, E.G.; Bhat, R. An eco-friendly approach to enhance the extraction and recovery efficiency of isoflavones from kudzu roots and soy molasses wastes using ultrasound-assisted extraction with natural deep eutectic solvents (NADES). Ind. Crop. Prod. 2022, 182, 114886. [Google Scholar] [CrossRef]
- Aboushanab, S.A.; Shevyrin, V.A.; Slesarev, G.P.; Melekhin, V.V.; Shcheglova, A.V.; Makeev, O.G.; Kovaleva, E.G.; Kim, K.H. Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS. Plants 2022, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, J.; Zhao, Y.; Yu, L.; He, Y.; Wan, H.; Li, C. Screening, Optimization, and Bioavailability Research of Natural Deep Eutectic Solvent Extracts from Radix pueraria. Molecules 2021, 26, 729. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.B. Purification and Anti-Oxidation Activity of Flavonoids from Hippophae rhamnoides Linn Leaves. Master’s Thesis, Huaqiao University, Fujian, China, 2016. [Google Scholar]
- Kwun, K.H.; Kim, G.J.; Shin, H.J. Ultrasonication assistance increases the efficiency of isoflavones extraction from kudzu (Pueraria lobata Ohwi) roots waste. Biotechnol. Bioprocess Eng. 2009, 14, 345–348. [Google Scholar] [CrossRef]
- Zhu, H.; Xing, Y.X.; Akan, O.D.; Yang, T. Ultrafine comminution-assisted ultrasonic-microwave synergistic extraction of Pueraria mirifica (Kudzu flower and root) flavonoids. Heliyon 2023, 9, e21137. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.H.; Li, J.H.; Liu, C.H. Study on extracting technology of Puerarin by large-scale ultrasonic extraction. China Pract. Med. 2007, 2, 1–2. [Google Scholar]
- Lee, M.H.; Lin, C.C. Comparison of techniques for extraction of isoflavones from the root of Radix Puerariae: Ultrasonic and pressurized solvent extractions. Food Chem. 2007, 105, 223–228. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.P. The Process of Microwave-Assisted Extraction of Potato Polyphenols. Food Ind. 2022, 43, 31–34. [Google Scholar]
- Sun, S.G.; Ma, C.J.; Huang, Q.; Gu, R.Y.; Luo, Z.Q. Combined High Speed Shear and Microwave Treatment for Extraction of Puerarin from Radix puerariae (Kudzu Root). Food Sci. 2009, 30, 50–53. [Google Scholar]
- Zhou, W.B. Study on the Total Isoflavones in Pueraria Root by Microwave-assisted Extraction. Food Sci. 2004, 25, 100–102. [Google Scholar]
- Du, G.; Zhao, H.Y.; Zhang, Q.W.; Li, G.H.; Yang, F.Q.; Wang, Y.; Li, Y.C.; Wang, Y.T. A rapid method for simultaneous determination of 14 phenolic compounds in Radix Puerariae using microwave-assisted extraction and ultra high performance liquid chromatography coupled with diode array detection and time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical Water Technology for Extraction of Phenolic Compounds from Chlorella sp. Microalgae and Assessment on Its Antioxidant Activity. Molecules 2017, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.K.; Bi, W.T.; Tian, M.L.; Row, K.H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2012, 904, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Mao, X.H.; Li, G.K. Application of ionic liquids in the microwave-assisted extraction of trans-resveratrol from Rhizma Polygoni Cuspidati. J. Chromatogr. A 2007, 1140, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Carradori, S.; Locatelli, M.; Secci, D.; Cesa, S.; Mollica, A.; Riga, S.; Angeli, A.; Supuran, C.T.; Celia, C.; et al. Bioactive isoflavones from Pueraria lobata root and starch: Different extraction techniques and carbonic anhydrase inhibition. Food Chem. Toxicol. 2018, 112, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Li, Y.; Chi, R. Optimization of ionic liquid-based microwave-assisted extraction of isoflavones from Radix puerariae by response surface methodology. Sep. Purif. Technol. 2014, 129, 71–79. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Peng, N.; Zhou, J.; Yong, X.; Yuan, H.; Zheng, T. Optimization of ionic liquids-based microwave-assisted hydrolysis of puerarin and daidzein derivatives from Radix Puerariae Lobatae extract. Food Chem. 2018, 256, 149–155. [Google Scholar] [CrossRef]
- Ramezani, N.; Raji, F.; Rezakazemi, M.; Younas, M. Juglone extraction from walnut (Juglans regia L.) green husk by supercritical CO2: Process optimization using Taguchi method. J. Environ. Chem. Eng. 2020, 8, 103776. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Du, X. Investigation of supercritical fluid extraction of puerarin from Pueraria lobata. J. Food Process. Eng. 2009, 32, 682–691. [Google Scholar] [CrossRef]
- Choi, Y.H.; Chin, Y.W.; Kim, J.; Jeon, S.H.; Yoo, K.P. Strategies for supercritical fluid extraction of hyoscyamine and scopolamine salts using basified modifiers. J. Chromatogr. A 1999, 863, 47–55. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Z.X.; Wang, G.J.; Ruan, X.; Wu, Q.F.; Luo, C.C.; Wu, Z.G.; Wei, F.; Zhao, Y.X.; Wang, Q. Supercritical extraction and antioxidant activity of major ingredients in Puerariae lobatae root, Pinus massoniana needle, Citrus reticulata peel and their mixture. J. CO₂ Util. 2021, 48, 101518. [Google Scholar] [CrossRef]
- Bai, C.C.; Tian, B.R.; Zhao, T.; Huang, Q.; Wang, Z.Z. Cyclodextrin-Catalyzed Organic Synthesis: Reactions, Mechanisms, and Applications. Molecules 2017, 22, 1475. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Liu, F.; Sun, L.; Huo, H.N.; Ren, X.L.; Wang, M. Associated-Extraction Efficiency of Six Cyclodextrins on Various Flavonoids in Puerariae Lobatae Radix. Molecules 2019, 24, 93. [Google Scholar] [CrossRef]
- He, X.L.; Tan, T.W.; Xu, B.Z.; Janson, J.C. Separation and purification of puerarin using β-cyclodextrin-coupled agarose gel media. J. Chromatogr. A 2004, 1022, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Hughes, T.C.; Hao, X.; Mei, D.; Tan, T. Preparation of monomeric and polymeric β-cyclodextrin functionalized monoliths for rapid isolation and purification of puerarin from Radix puerariae. J. Sep. Sci. 2011, 34, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Wang, Z.N.; Zhou, T.; Song, X.Q.; Liu, Q.Y.; Zhang, Y.M.; He, L.M. Determination of cyproheptadine in feeds using molecularly imprinted solid-phase extraction coupled with HPLC. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2015, 990, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.N.; Jia, X.J.; Lu, Q.; Peng, Y.; Du, S.H.; Chen, Q. Highly efficient and selective enrichment of puerarin from Radix puerariae by molecularly imprinted solid-phase extraction. Sep. Purif. Technol. 2010, 71, 324–330. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, L.Y.; Chen, D.J.; Wang, P.; Zhao, J.W.; Peng, Y.; Du, S.H.; Zhang, Z.P. Molecularly imprinted layer-coated monodisperse spherical silica microparticles toward affinity-enrichment of isoflavonoid glycosides from Radix puerariae. Analyst 2012, 137, 2891–2902. [Google Scholar] [CrossRef]
- Meng, L. Purification of puerarin by macroporous resin adsorption and its antioxidant activity. Tianjin Agric. Sci. 2016, 22, 16–18. [Google Scholar]
- Chi, R.A.; Zhou, F.; Huang, K.; Zhang, Y.F. Adsorption behaviors of puerarin on S-8 macroporous resin. Chin. J. Nat. Med. 2011, 9, 120–125. [Google Scholar]
- Guo, H.D.; Zhang, Q.F.; Chen, J.G.; Shang, G.; Cheng, X.; Guo, Y.X. Large scale purification of puerarin from Puerariae lobatae Radix through resins adsorption and acid hydrolysis. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2015, 980, 8–15. [Google Scholar] [CrossRef]
- Niu, Y.; Li, H.; Dong, J.; Wang, H.; Hashi, Y.K.; Chen, S.Z. Identification of isoflavonoids in Radix puerariae for quality control using on-line high performance liquid chromatography-diode array detector-electrospray ionization-mass spectrometry coupled with post-column derivatization. Food Res. Int. 2012, 48, 528–537. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Talpur, Z.H.; Yang, W.Y.; Xiong, Y.R.; Wu, T.; Zhang, Y.Q.; Shen, X.Y.; Du, Y.P. Dual-spectrum online monitoring of puerarin and total flavonoids contents during the extraction process of Pueraria lobata. Talanta 2022, 248, 123608. [Google Scholar] [CrossRef]
- Cao, X.L.; Tian, Y.; Zhang, T.Y.; Li, X.; Ito, Y. Separation and purification of isoflavones from Pueraria lobata by high-speed counter-current chromatography. J. Chromatogr. A 1999, 855, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.L.; Yang, Y.F.; Hu, X.; Xie, X.X.; Meucci, J.; Fang, M.; Zhao, L.J. Purification of Puerarin from Pueraria lobata by FCPC versus HSCCC Using Small-volume Columns. Chin. Herb. Med. 2014, 6, 140–144. [Google Scholar] [CrossRef]
- Shi, S.Y.; Ma, Y.J.; Zhang, Y.P.; Liu, L.L.; Liu, Q.; Peng, M.J.; Xiong, X. Systematic separation and purification of 18 antioxidants from Pueraria lobata flower using HSCCC target-guided by DPPH-HPLC experiment. Sep. Purif. Technol. 2012, 89, 225–233. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Zhao, A.Q.; Han, B.H.; Fan, Y.J.; Liu, C.M.; Liu, J.J. Preparative separation of isoflavones in plant extract of Pueraria lobata by high performance counter-current chromatography. Anal. Methods 2015, 7, 1321–1327. [Google Scholar] [CrossRef]
- Vos, T. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1562. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.D.; Sufra, R.; St Sauveur, R.; Jean-Pierre, M.C.; Apollon, A.; Malebranche, R.; Theard, M.; Pierre, G.; Devieux, J.; Lau, J.; et al. Spectrum of prevalent cardiovascular diseases in urban Port-au-Prince, Haiti: A population-based cross-sectional study. Lancet Reg. Health 2024, 33, 100729. [Google Scholar] [CrossRef]
- Chen, M.; Xiang, L.; Wu, G.L.; Liao, Y.D.; Cai, Y.F. Puerarin Inhibits Endothelium-Dependent Contractions in Mouse Carotid Arteries. Med. Sci. Monit. 2020, 26, 923163. [Google Scholar] [CrossRef]
- Zhou, T.T.; Wang, Z.W.; Guo, M.T.; Zhang, K.; Geng, L.; Mao, A.Q.; Yang, Y.J.; Yu, F. Puerarin induces mouse mesenteric vasodilation and ameliorates hypertension involving endothelial TRPV4 channels. Food Funct. 2020, 11, 10137–10148. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.F.Y.; Deng, S.Y.; Ng, E.S.K.; Yeung, J.H.K.; Kwan, Y.W.; Lau, C.B.S.; Koon, J.C.M.; Zhou, L.; Zuo, Z.; Leung, P.C.; et al. Mechanisms of the relaxant effect of a Danshen and Gegen formulation on rat isolated cerebral basilar artery. J. Ethnopharmacol. 2010, 132, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Sidbury, R.; Davis, D.M.; Cohen, D.E.; Cordoro, K.M.; Berger, T.G.; Bergman, J.N.; Chamlin, S.L.; Cooper, K.D.; Feldman, S.R.; Hanifin, J.M.; et al. Guidelines of care for the management of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 71, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, X.; Hou, C.; Yu, S.; Zhang, Y.; Yu, Z.; Kong, L.; Liu, C.; Feng, L.; Wang, D.; et al. Cold Plasma Irradiation Attenuates Atopic Dermatitis via Enhancing HIF-1α-Induced MANF Transcription Expression. Front. Immunol. 2022, 13, 941219. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, Y.D.; Lee, Y.M.; Kim, D.K. The suppressive effect of puerarin on atopic dermatitis-like skin lesions through regulation of inflammatory mediators in vitro and in vivo. Biochem. Bioph. Res. Commun. 2018, 498, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lofthus, D.M.; Stevens, S.R.; Armstrong, P.W.; Granger, C.B.; Mahaffey, K.W. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coronary. Artery. Dis. 2012, 23, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Wu, M.M.; Fang, H.; Su, Y.; Zhang, L.L.; Zhou, H. Puerarin Prevents Acute Liver Injury via Inhibiting Inflammatory Responses and ZEB2 Expression. Front. Pharmacol. 2021, 12, 727916. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.W.; Yang, H.K.; Jun, M.S.; Lee, B.C. Puerarin Attenuates Obesity-Induced Inflammation and Dyslipidemia by Regulating Macrophages and TNF-Alpha in Obese Mice. Biomedicines. 2022, 10, 175. [Google Scholar] [CrossRef]
- Kim, S.H.; Reaven, G. Obesity and Insulin Resistance: An Ongoing Saga. Diabetes 2010, 59, 2105–2106. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Brit. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef]
- Gao, D.; Cho, C.W.; Kim, J.H.; Kim, C.T.; Jeong, W.S.; Wang, Y.; Li, X.W.; Kang, J.S. Extraction and Concentration of Waste Pueraria lobata Stems with Antioxidants and Anti-Melanogenesis Activity as a Novel Skin Whitening Agent for Natural Cosmetic Prototypes. Int. J. Mol. Sci. 2022, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Bacanli, M.; Basaran, A.A.; Basaran, N. The antioxidant, cytotoxic, and antigenotoxic effects of galangin, puerarin, and ursolic acid in mammalian cells. Drug Chem. Toxicol. 2017, 40, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.L.; Zhao, Y.P.; Shu, B. The radical scavenging activities of Radix puerariae isoflavonoids:: A chemiluminescence study. Food Chem. 2004, 86, 525–529. [Google Scholar] [CrossRef]
- Li, X.F.; Yuan, T.T.; Xu, H.X.; Xin, X.; Zhao, G.L.; Wu, H.; Xiao, X.L. Whole-Cell Catalytic Synthesis of Puerarin Monoesters and Analysis of Their Antioxidant Activities. J. Agr. Food Chem. 2019, 67, 299–307. [Google Scholar] [CrossRef]
- Ye, H.; Yuan, S.; Cong, X.D. Biotransformation of puerarin into 3′-hydroxypuerarin by Trichoderma harzianum NJ01. Enzym. Microb. Technol. 2007, 40, 594–597. [Google Scholar] [CrossRef]
- Hao, C.Y.; Wang, W.T.; Shao, M.X.; Zhao, Z.Y.; Tang, L.D. Effects of Puerarin on Experimental Model of Retinal Vein Occlusion in Rats. Chin. Herb. Med. 2014, 6, 110–114. [Google Scholar] [CrossRef]
- Guan, L.N.; Li, C.; Zhang, Y.; Gong, J.Y.; Wang, G.Y.; Tian, P.; Shen, N. Puerarin ameliorates retinal ganglion cell damage induced by retinal ischemia/reperfusion through inhibiting the activation of TLR4/NLRP3 inflammasome. Life Sci. 2020, 256, 117935. [Google Scholar] [CrossRef]
- Song, Q.T.; Zhao, Y.; Li, Q.; Han, X.; Duan, J.G. Puerarin protects against iron overload-induced retinal injury through regulation of iron-handling proteins. Biomed. Pharmacother. 2020, 122, 109690. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cao, L.M.; Liu, Y.S.; Wang, X.N.; Zhang, S.M.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.M.; Gu, Y.Q.; et al. Longitudinal Associations Between Hand Grip Strength and Non-Alcoholic Fatty Liver Disease in Adults: A Prospective Cohort Study. Front. Med. 2021, 8, 752999. [Google Scholar] [CrossRef]
- Sun, C.B.; Zhang, J.; Hou, J.; Hui, M.L.; Qi, H.L.; Lei, T.; Zhang, X.S.; Zhao, L.X.; Du, H.W. Induction of autophagy via the PI3K/Akt/mTOR signaling pathway by Pueraria flavonoids improves non-alcoholic fatty liver disease in obese mice. Biomed. Pharmacother. 2023, 157, 114005. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, Z.; Su, D.; Yang, M.; Tao, S.; Li, J. Comparison between the efficacies of curcumin and puerarin in C57BL/6 mice with steatohepatitis induced by a methionine- and choline-deficient diet. Exp. Ther. Med. 2014, 7, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Zhang, N.H.; Aldhahrani, A.; Soliman, M.M.; Zhang, L.B.; Zhou, F. Puerarin ameliorates nonalcoholic fatty liver in rats by regulating hepatic lipid accumulation, oxidative stress, and inflammation. Front. Immunol. 2022, 13, 956688. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.Q.; Pan, L.; Huang, Z.C.; Liang, Y.Z.; Liao, Y.H.; Xia, N. Development and Validation of a Prediction Model for Survival in Diabetic Patients With Acute Kidney Injury. Front. Endocrinol. 2021, 12, 737996. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liang, T.; Duan, X.Q.; Xu, L.Y.; Zhang, K.F.; Li, R. Anti-diabetic effects of puerarin, isolated from Pueraria lobata (Willd.), on streptozotocin-diabetogenic mice through promoting insulin expression and ameliorating metabolic function. Food Chem. Toxicol. 2013, 60, 341–347. [Google Scholar] [CrossRef] [PubMed]
- She, S.Y.; Liu, W.J.; Li, T.; Hong, Y.K. Effects of puerarin in STZ-induced diabetic rats by oxidative stress and the TGF-β1/Smad2 pathway. Food Funct. 2014, 5, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.; Prasain, J.K.; Peng, N.; Dai, Y.Y.; Wyss, J.M. Acute and Chronic Kudzu Improves Plasma Glucose Tolerance in Non-Diabetic CD-1 Mice. J. Endocrinol. Diabetes Mellit. 2014, 2, 70–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, W.C.; Hayakawa, S.; Yamamoto, T.; Su, H.C.; Liu, I.M.; Cheng, J.T. Mediation of β-endorphin by the isoflavone puerarin to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2004, 70, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Mukhlynina, E.A.; Moroz, G.A.; Gette, I.F.; Danilova, I.G.; Kovaleva, E.G. Anti-diabetic effect of isoflavone rich kudzu root extract in experimentally induced diabetic rats. J. Funct. Foods 2020, 68, 103922. [Google Scholar] [CrossRef]
- Szulc, M.; Kujawski, R.; Baraniak, J.; Kania-Dobrowolska, M.; Kaminska, E.; Gryszczynska, A.; Czora-Poczwardowska, K.; Winiarska, H.; Mikolajczak, P.L. Differential Influence of Pueraria lobata Root Extract and Its Main Isoflavones on Ghrelin Levels in Alcohol-Treated Rats. Pharmaceuticals 2022, 15, 25. [Google Scholar] [CrossRef]
- Gloppen, K.M.; Roesler, J.S.; Farley, D.M. Assessing the Costs of Excessive Alcohol Consumption in Minnesota. Am. J. Prev. Med. 2022, 63, 505–512. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Y.L.; Zhu, W.L.; Shi, J.; Liu, Y.; Ling, W.; Kosten, T.R. Traditional Medicine in the Treatment of Drug Addiction. Am. J. Drug Alcohol Abus. 2009, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.C.; Guthrie, S.; Xie, C.Y.; Mai, K.; Lee, D.Y.; Lumeng, L.; Li, T.K. Isoflavonoid compounds extracted from Pueraria lobata suppress alcohol preference in a pharmacogenetic rat model of alcoholism. Alcohol. Clin. Exp. Res. 1996, 20, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Penetar, D.M.; Toto, L.H.; Farmer, S.L.; Lee, D.Y.W.; Ma, Z.Z.; Liu, Y.Z.; Lukas, S.E. The isoflavone puerarin reduces alcohol intake in heavy drinkers: A pilot study. Drug Alcohol. Depen. 2012, 126, 251–256. [Google Scholar] [CrossRef]
- Penetar, D.M.; Toto, L.H.; Lee, D.Y.W.; Lukas, S.E. A single dose of kudzu extract reduces alcohol consumption in a binge drinking paradigm. Drug Alcohol. Depen. 2015, 153, 194–200. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).