Decoding the Volatile Profile of White Romanian Fetească Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Chemicals and Reagents
2.3. Methods
2.3.1. HS-SPME/GC-MS Analysis
2.3.2. Odor Threshold Value (OTV) and Aroma Series
2.3.3. Electronic Nose (e-Nose) Technique Sampling
2.3.4. Statistical Analysis
3. Results and Discussion
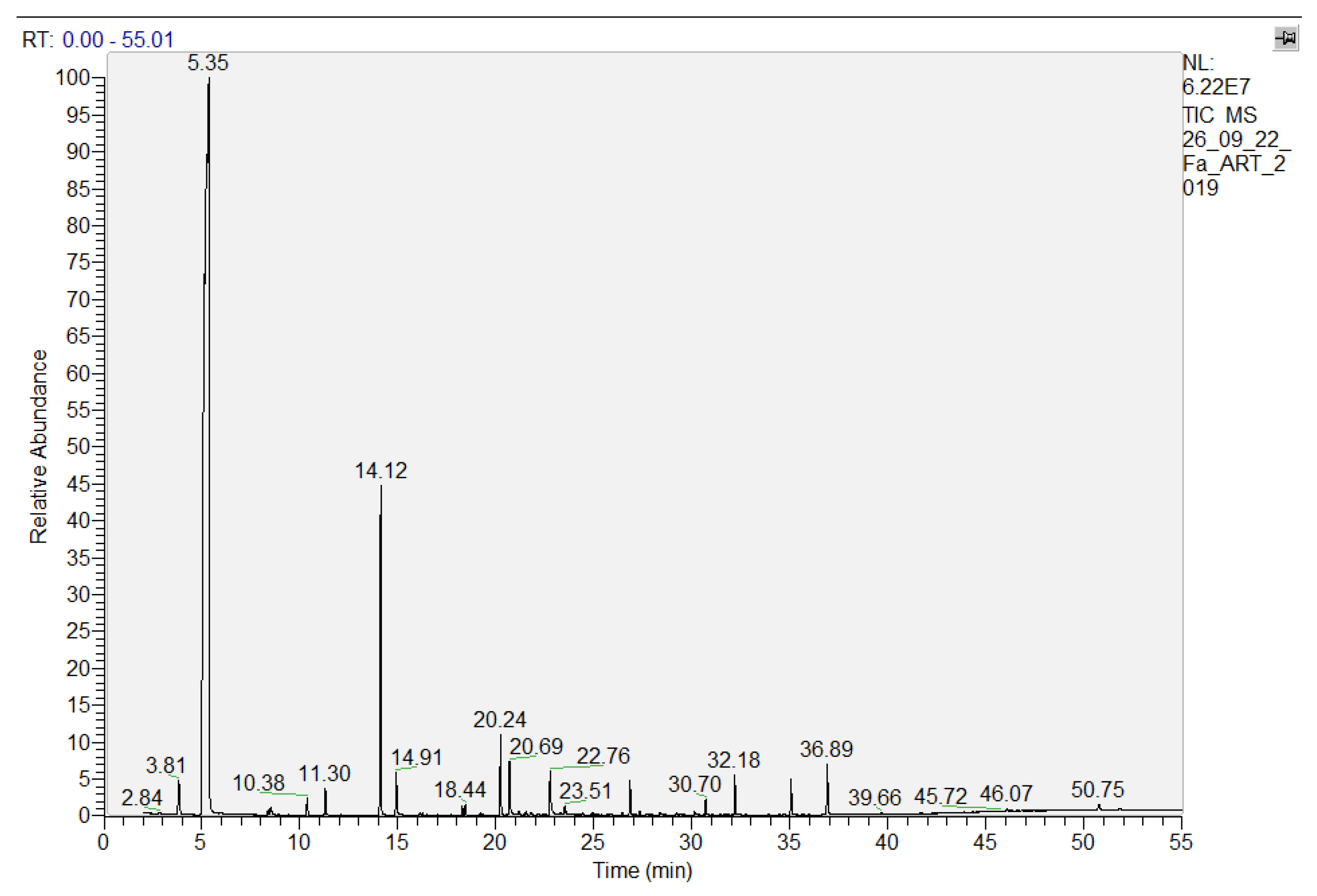
3.1. HS-SPME/GC-MS
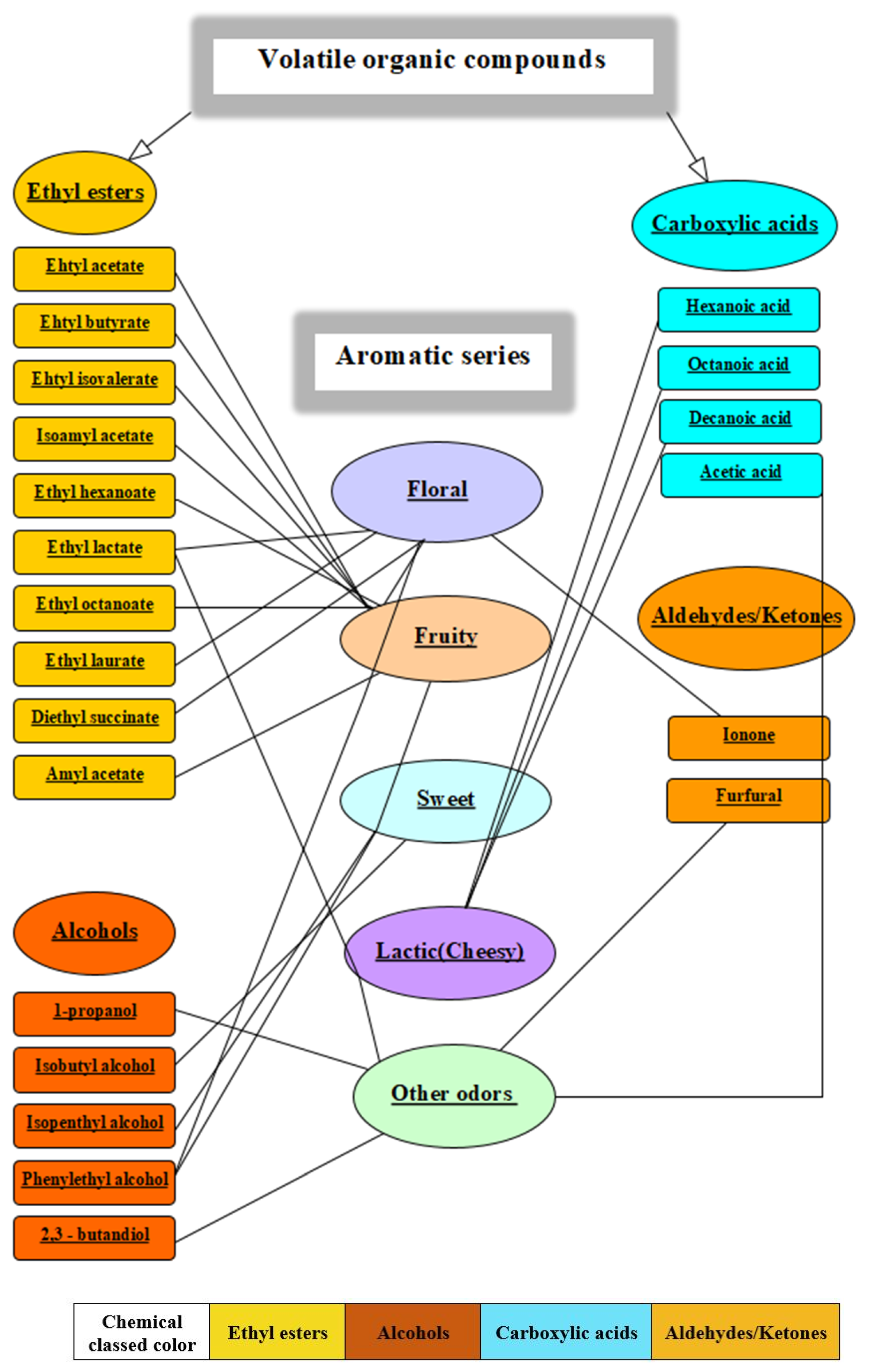
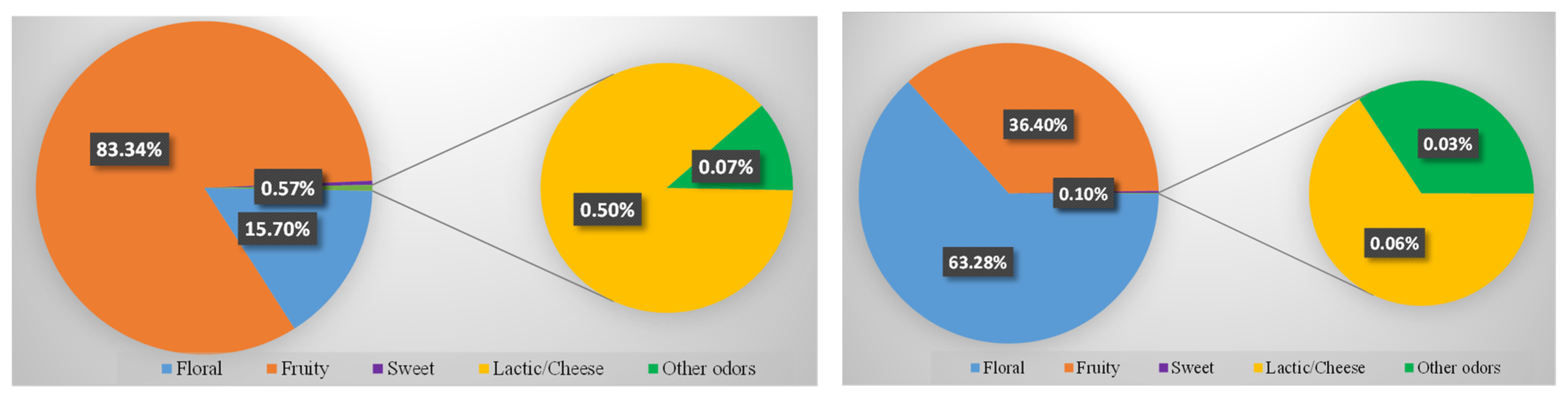
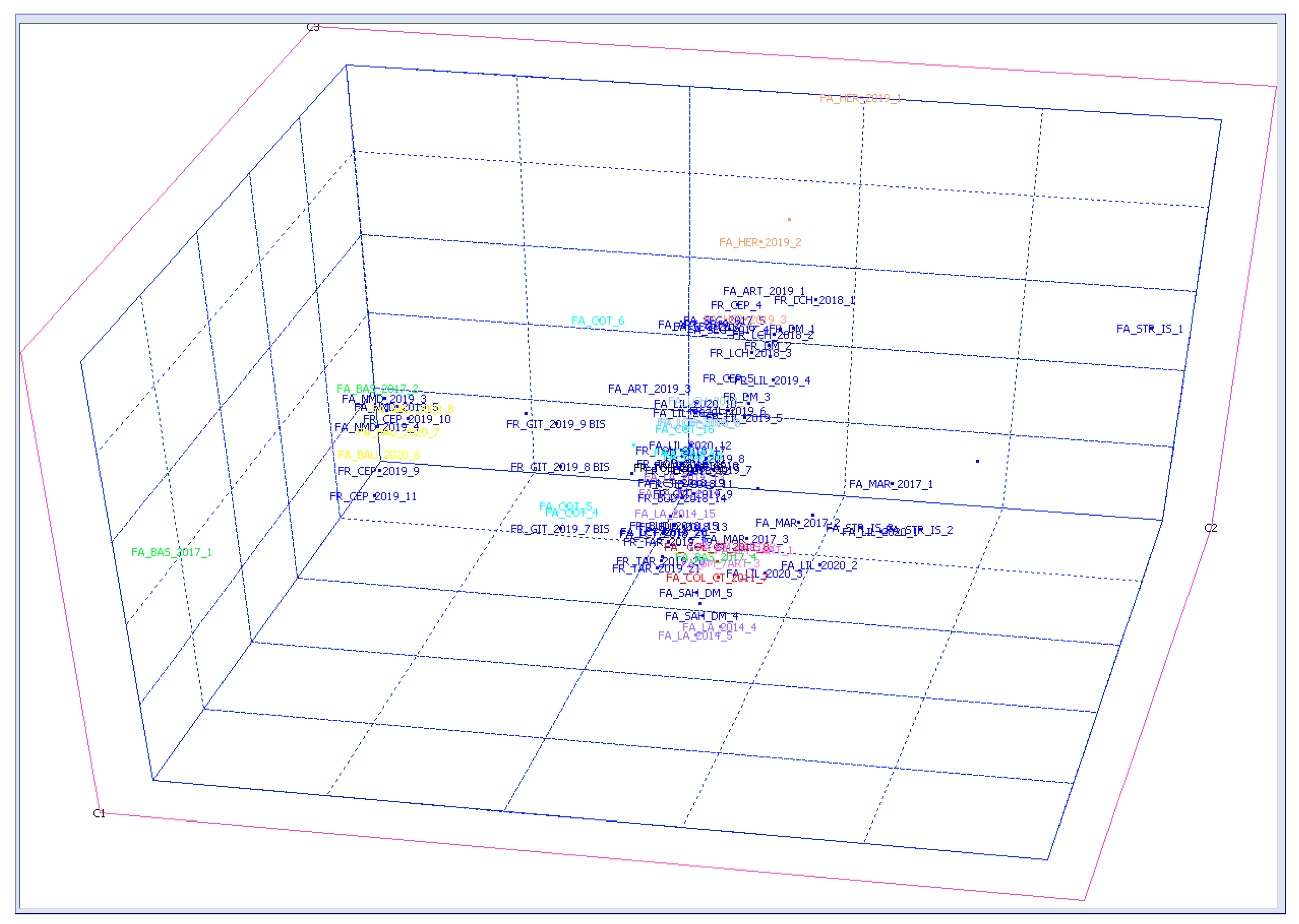
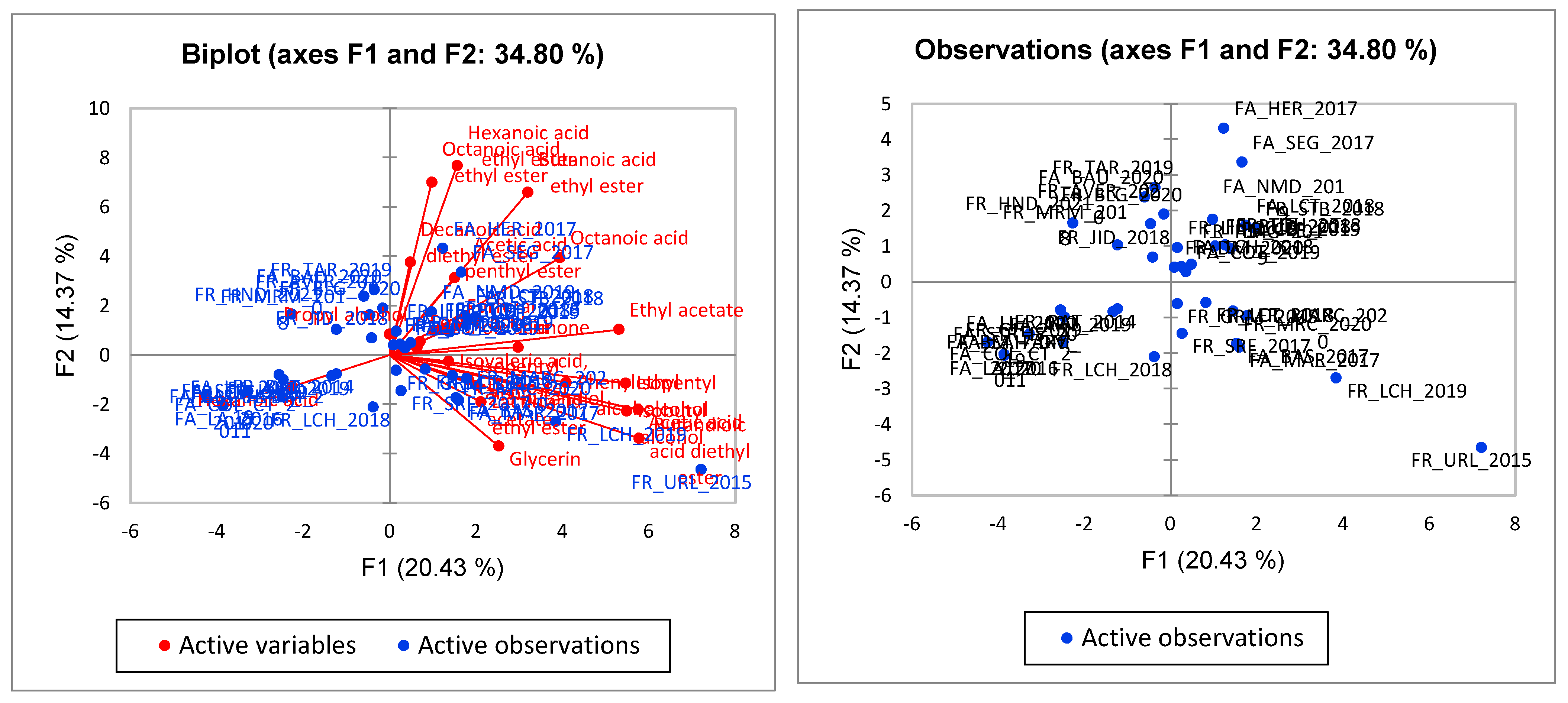
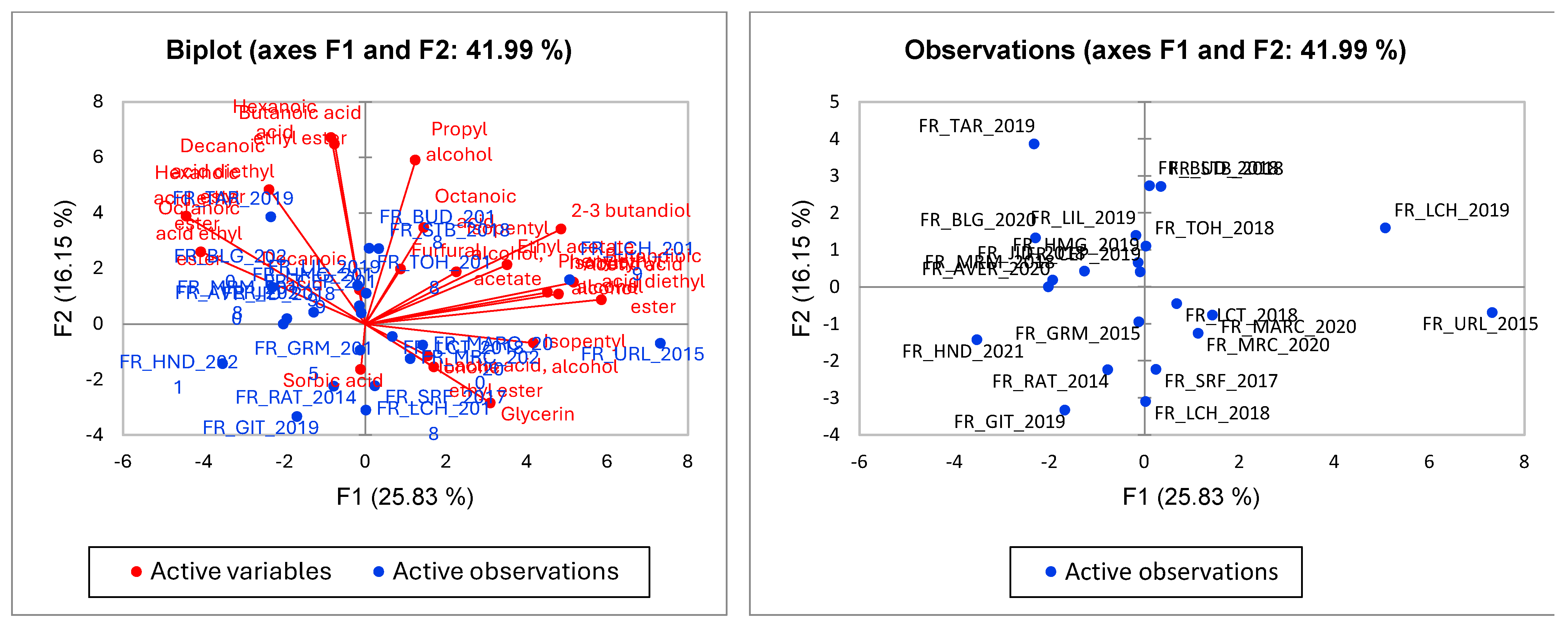
3.2. Aroma Correlation between White Wine Samples
| No. | Wine’s Volatile Compounds | Wine’s Odor Descriptors | Odor Thresholds | |
|---|---|---|---|---|
| Compound’s Chemical Classes | Volatile Compound’s Name | |||
| 1. | Ethyl esters | Ethyl acetate | Aromatic, Brandy, Grape [30] | 5 ppb to 5 ppm [31] |
| 2. | Butanoic acid ethyl ester (ethyl butanoate/ethyl butyrate) | Sour fruit, banana and strawberry flavors, floral and fruity aromas [32] | 0.1 to 18 ppb [31] | |
| 3. | Isovaleric acid, ethyl ester (ethyl isovalerate) | Apple, Fruit, Pineapple, Sour [3] | 0.01 to 0.4 ppb [31] | |
| 4. | Isopentyl alcohol, acetate (1-butanol, 3-methyl-, acetate/isoamyl acetate) | Banana, Apple, Glue, Pear [30] | 17 μg/L [3] | |
| 5. | Hexanoic acid ethyl ester (ethyl hexanoate) | Apple Peel, Brandy, Fruit Gum, Overripe Fruit, Pineapple [30] | 0.3 to 5 ppb [31] | |
| 6. | Lactic acid, ethyl ester (ethyl lactate) | Cheese, Floral, Fruit, Pungent, Rubber [30] | 0.15 ppm [33] | |
| 7. | Octanoic acid ethyl ester (ethyl octanoate) | Apricot, Brandy, Fat, Floral, Pineapple [30] | 0.002 ppm [33] | |
| 8. | Decanoic acid diethyl ester (ethyl laurate) | Floral, Fruity [31] | 50 ppm [31] | |
| 9. | Butandioic acid diethyl ester (diethyl succinate) | Cotton, Fabric, Floral, Fruit, Wine [30] | 10 ppm [31] | |
| 10. | Acetic acid, penthyl ester (amyl acetate) | Apple, Banana, Glue, Pear [30] | 0.0052 ppm [34] | |
| 11. | Alcohols | Propyl alcohol (1-propanol) | Alcohol, ripe fruit flavors [32] | 5.7 to 40 ppm [31] |
| 12. | Isobutyl alcohol (2-methyl-1-propanol) | Sweet, Musty [31] | 360 ppb to 3.3 ppm [31] | |
| 13. | Isopentyl alcohol (isoamyl alcohol/3-methyl-1-butanol) | Mellow, Astringent, whisky-characteristic [32] | 250 ppb to 4.1 ppm [31] | |
| 14. | Phenylethyl alcohol (2-phenylethanol) | Fruit, Honey, Lilac, Rose, Wine [30] | 0.015 ppb to 3.5 ppm [31] | |
| 15. | 2–3 butandiol | Butter, creamy [27,35] | 120 ppm | |
| 16. | Carboxylic acids | Hexanoic acid (Caproic acid) | Cheese, Oil, Pungent, Sour [30] | 93 ppb to 10 ppm [31] |
| 17. | Octanoic acid (Caprylic acid) | Cheese, Fat, Grass, Oil [30] | 10 ppm [31] | |
| 18. | Decanoic acid (Capric acid) | Dust, Fat, Grass [3] | 1.6 ppm | |
| 19. | Acetic acid | Sour and pungent vinegar odor [32] | 15 ppm [31] | |
| 20. | Aldehydes/ Ketones | Ionone | Violet, Wood, Floral [30,33] | 0.9 ppb |
| 21. | Furfural | Almond, Baked Potatoes, Bread, Burnt, Spice [3] | 65 ppm | |
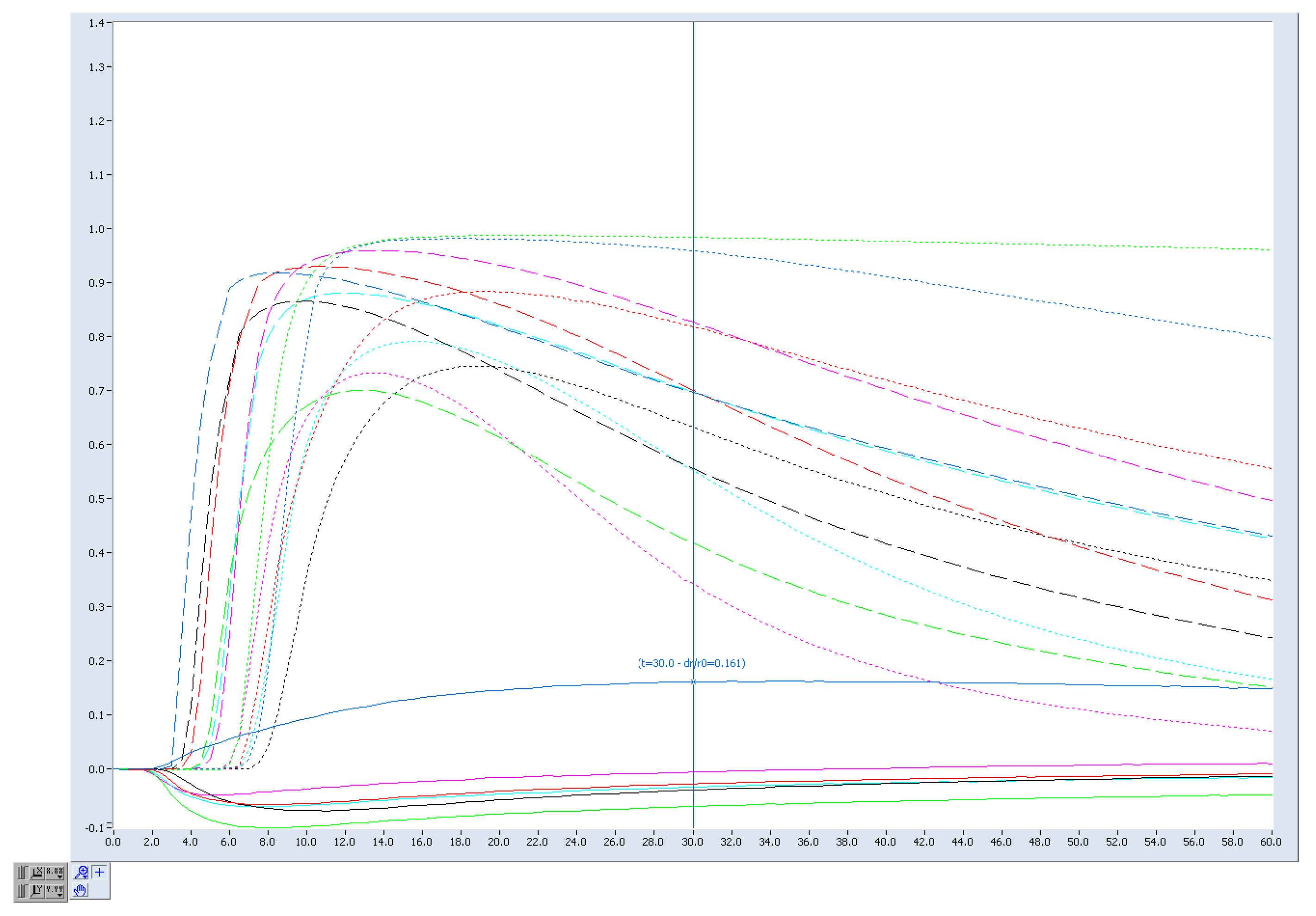
3.3. Electronic Nose (e-Nose) Results
3.4. Statistical Analysis of Wine Samples Based on HS SPME/GC-MS Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khakimov, B.; Bakhytkyzy, I.; Fauhl-Hassek, C.; Engelsen, S.B. Non-Volatile Molecular Composition and Discrimination of Single Grape White of Chardonnay, Riesling, Sauvignon Blanc and Silvaner Using Untargeted GC–MS Analysis. Food Chem. 2022, 369, 130878. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A. Supplementary Material Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: The Case Study of Portuguese Wines by Chemical Families, Odor Threshold, Content, and Odor Active Value (OAV). Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Luzzini, G.; Slaghenaufi, D.; Ugliano, M. Volatile Compounds in Monovarietal Wines of Two Amarone Della Valpolicella Terroirs: Chemical and Sensory Impact of Grape Variety and Origin, Yeast Strain and Spontaneous Fermentation. Foods 2021, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Xu, K.; Wang, J.; Meng, F.; Wang, B. Based on HS-SPME-GC-MS Combined with GC-O-MS to Analyze the Changes of Aroma Compounds in the Aging Process of Citri Reticulatae Pericarpium. Food Biosci. 2023, 54, 102798. [Google Scholar] [CrossRef]
- Gu, W.; Wei, Y.; Fu, X.; Gu, R.; Chen, J.; Jian, J.; Huang, L.; Yuan, C.; Guan, W.; Hao, X. HS-SPME/GC×GC-TOFMS-Based Flavoromics and Antimicrobial Properties of the Aroma Components of Zanthoxylum Motuoense. Foods 2023, 12, 2225. [Google Scholar] [CrossRef]
- Rossi, L.; Foschi, M.; Biancolillo, A.; Maggi, M.A.; D’Archivio, A.A. Optimization of HS-SPME-GC/MS Analysis of Wine Volatiles Supported by Chemometrics for the Aroma Profiling of Trebbiano d’Abruzzo and Pecorino White Wines Produced in Abruzzo (Italy). Molecules 2023, 28, 1534. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, M.; Carlin, S.; Lotti, C.; Vrhovsek, U.; Mattivi, F. Development of a Fully Automated Method HS-SPME-GC-MS/MS for the Determination of Odor-Active Carbonyls in Wines: A “Green” Approach to Improve Robustness and Productivity in the Oenological Analytical Chemistry. J. Agric. Food Chem. 2024, 72, 1995–2007. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Badeka, A.V. Volatilome of White Wines as an Indicator of Authenticity and Adulteration Control Using Statistical Analysis. Aust. J. Grape Wine Res. 2021, 27, 269–279. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Li, Y.; Liu, S.; Tu, Q.; Yuan, C. Characterization of Wine Volatile Compounds from Different Regions and Varieties by HS-SPME/GC-MS Coupled with Chemometrics. Curr. Res. Food Sci. 2023, 6, 100418. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; Valle-Herrero, H.d.; Bueno-Herrera, M.; López-de-la-Cuesta, P.; Pérez-Magariño, S. Volatile and Non-Volatile Characterization of White and Rosé Wines from Different Spanish Protected Designations of Origin. Beverages 2021, 7, 49. [Google Scholar] [CrossRef]
- Fuentes, S.; Viejo, C.G. Novel Use of E-Noses for Digital Agriculture, Food, and Beverage Applications. In Nanotechnology-Based E-Noses: Fundamentals and Emerging Applications; Gupta, R.K., Nguyen, T.A., Bilal, M., Ahmadi, M., Eds.; Woodhead Publishing: Shaston, UK, 2023; pp. 415–432. [Google Scholar]
- Celdrán, A.C.; Oates, M.J.; Molina Cabrera, C.; Pangua, C.; Tardaguila, J.; Ruiz-Canales, A. Low-Cost Electronic Nose for Wine Variety Identification through Machine Learning Algorithms. Agronomy 2022, 12, 2627. [Google Scholar] [CrossRef]
- Erwanto, D.; Wahyudi, D.; Fatkhur Rizal, R. Sistem Electronic Nose Untuk Deteksi Aroma Pada Fasilitas Kamar Mandi Berbasis IoT. J. Zetroem 2023, 5, 43–50. [Google Scholar] [CrossRef]
- Meléndez, F.; Arroyo, P.; Gómez-Suárez, J.; Palomeque-Mangut, S.; Suárez, J.I.; Lozano, J. Portable Electronic Nose Based on Digital and Analog Chemical Sensors for 2,4,6-Trichloroanisole Discrimination. Sensors 2022, 22, 3453. [Google Scholar] [CrossRef]
- Pati, S.; Tufariello, M.; Crupi, P.; Coletta, A.; Grieco, F.; Losito, I. Quantification of Volatile Compounds in Wines by HS-SPME-GC/MS: Critical Issues and Use of Multivariate Statistics in Method Optimization. Processes 2021, 9, 662. [Google Scholar] [CrossRef]
- Jin, X.; Wu, S.; Yu, W.J.; Xu, X.; Huang, M.; Tang, Y.; Yang, Z. Wine Authentication Using Integration Assay of MIR, NIR, E-Tongue, HS-SPME-GC-MS, and Multivariate Analyses: A Case Study for a Typical Cabernet Sauvignon Wine. J. AOAC Int. 2019, 102, 1174–1180. [Google Scholar] [CrossRef]
- Harta A3 Podgorii Spate Romana 2023. Available online: https://www.crameromania.ro/upload/HartaA3podgoriispate%20romana%202023.png (accessed on 26 January 2024).
- Torrens, J.; Riu-Aumatell, M.; López-Tamames, E.; Buxaderas, S. Volatile Compounds of Red and White Wines by Headspace-Solid-Phase Microextraction Using Different Fibers. J. Chromatogr. Sci. 2004, 42, 310–316. [Google Scholar] [CrossRef]
- Dumitriu, G.D.; Sánchez-Suárez, F.; Peinado, R.A.; Cotea, V.V.; de Lerma, N.L.; Gabur, I.; Simioniuc, V. Metabolomics of Red Wines Aged Traditionally, with Chips or Staves. Foods 2024, 13, 196. [Google Scholar] [CrossRef]
- Urcan, D.E.; Lung, M.L.; Giacosa, S.; Torchio, F.; Ferrandino, A.; Vincenzi, S.; Rió Segade, S.; Pop, N.; Rolle, L. Phenolic Substances, Flavor Compounds, and Textural Properties of Three Native Romanian Wine Grape Varieties. Int. J. Food Prop. 2016, 19, 76–98. [Google Scholar] [CrossRef]
- Colibaba, C.; Cotea, V.; Liliana, R.; Marius, N.; Vararu, F.; Luchian, C. Studies of the Influence of Some Conditioning Treatments on Some Volatile Compounds in Fetească Albă Wines. Sci. Pap. Ser. Hortic. 2014, 55, 181–186. [Google Scholar]
- Cojocaru, G.A.; Antoce, A.O. Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile. Biosensors 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma Characteristics of Volatile Compounds Brought by Variations in Microbes in Winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef] [PubMed]
- Petretto, G.; Urgeghe, P.P.; Cabizza, R.; Del Caro, A. Evaluation of Volatile and Chemical Profile of Sherry-like White Wine Vernaccia Di Oristano from Sardinia by Comprehensive Targeted and Untargeted Approach. Eur. Food Res. Technol. 2023, 249, 1887–1897. [Google Scholar] [CrossRef]
- Cordente, A.G.; Nandorfy, D.E.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic Higher Alcohols in Wine: Implication on Aroma and Palate Attributes during Chardonnay Aging. Molecules 2021, 26, 4979. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.-S.; Li, H. Active Volatiles of Cabernet Sauvignon Wine from Changli County. Health 2009, 1, 176–182. [Google Scholar] [CrossRef]
- Avram, V.; Floare, C.G.; Hosu, A.; Cimpoiu, C.; Măruţoiu, C.; Moldovan, Z. Characterization of Romanian Wines by Gas Chromatography–Mass Spectrometry. Anal. Lett. 2015, 48, 1099–1116. [Google Scholar] [CrossRef]
- The Role of Acetic Acid in Wine. Available online: https://www.calwineries.com/learn/wine-chemistry/wine-acids/acetic-acid (accessed on 1 February 2024).
- Flavor Ingredient Library. Available online: https://www.femaflavor.org/flavor-library (accessed on 15 January 2024).
- Chemical Book. Available online: https://www.chemicalbook.com/ (accessed on 15 January 2024).
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [PubMed]
- Amyl Acetate. Available online: https://pubchem.ncbi.nlm.nih.gov/#query=amyl%20acetate (accessed on 15 January 2024).
- Hao, N.; Liu, J.; Wang, F.; Cao, J.; Chen, Q.; He, L.; Guan, X.; Liu, S.; Shi, K. Malolactic Fermentation Performance of Indigenous Oenococcus Oeni Strains from Shaanxi Wine Region (China) and Their Mutants on Pinot Noir and Chardonnay Wines. Lwt 2023, 185, 115170. [Google Scholar] [CrossRef]
- Plawiak, P.; Maziarz, W. Comparison of Artificial Intelligence Methods on the Example of Tea Classification Based on Signals from E-Nose Sensors. Adv. Signal Process. 2013, 1, 19–32. [Google Scholar] [CrossRef]
- Pławiak, P.; Rzecki, K. Approximation of Phenol Concentration Using Computational Intelligence Methods Based on Signals from the Metal-Oxide Sensor Array. IEEE Sens. J. 2015, 15, 1770–1783. [Google Scholar] [CrossRef]





| Group | Growing Area | Samples Details | Sample Codification | Year of Production | pH * | Alc.% ** |
|---|---|---|---|---|---|---|
| Fetească albă | Muntenia | Dealu Mare (PH) | FA_LA_2014 | 2014 | 3.29 | 12.5 |
| D.O.C—C.M.D Ștefănești | FA_MAR _2017 | 2017 | 3.31 | 12.5 | ||
| Urlați (PH) | FA_BAS_2017 | 2017 | 3.51 | 13.0 | ||
| Dealu Mare (PH) | FA_NMD_2019 | 2019 | 3.39 | 10.0 | ||
| Dealu Mare (PH) | FA_ART_2019 | 2019 | 3.54 | 13.5 | ||
| Dealu Mare (PH) | FA_SAH_DM_2020 | 2020 | 3.73 | 14.3 | ||
| Oltenia | D.O.C—C.M.D Banu | FA_BM_7ART_2017 | 2017 | 3.35 | 13 | |
| Mărăcine, Craiova | FA_SEG_2017 | 2017 | 3.54 | 12 | ||
| D.O.C—C.M.D, Dolj Drăgășani, Vâlcea | FA_BAU_2020 | 2020 | 3.27 | 13 | ||
| Moldova | Cotnari (IS) | FA_COL_CT_2011 | 2011 | 3.54 | 13.5 | |
| Dealurile Moldovei (IS) | FA_STR_IS_2019 | 2019 | 3.59 | 12.5 | ||
| D.O.C—C.M.D, Cotnari (IS) | FA_COT_2019 | 2019 | 3.22 | 12.0 | ||
| D.O.C—C.M.D, Bivolari (IS) | FA_HER_2019 | 2019 | 3.57 | 13.2 | ||
| Transylvania | D.O.C—C.M.D, Lechința (BN) | FA_LCH_2018 | 2018 | 3.37 | 12.7 | |
| Valea Ascunsă, Teaca (BN) | FA_LCT_2018 | 2018 | 3.17 | 12.5 | ||
| D.O.C—C.M.D, Lechința (BN) | FA_LIL_2020 | 2020 | 3.29 | 12.5 | ||
| Fetească regală | Transylvania | Dealurile Crișanei (AL) | FR_RAT_2014 | 2014 | 3.19 | 13.9 |
| D.O.C—C.M.D, Târnave (AL) | FR_JID_2018 | 2018 | 3.28 | 12.0 | ||
| D.O.C—C.M.D, Jelba (BN) | FR_LCH_2018 | 2018 | 3.29 | 12.4 | ||
| D.O.C—C.M.D, Valea Ascunsă, Teaca (BN) | FR_LCT_2018 | 2018 | 3.05 | 13.5 | ||
| D.O.C—C.M.D, Târnava (MS) | FR_TAR_2019 | 2019 | 3.36 | 13.0 | ||
| D.O.C—C.M.D, Lechința (BN) | FR_LIL_2019 | 2019 | 3.14 | 12.5 | ||
| Muntenia | D.O.C—C.M.D, Lechința (BN) | FR_LCH_2019 | 2019 | 3.12 | 13 | |
| D.O.C—C.M.D, Dealu Mare, Urlați (PH) | FR_URL_2015 | 2015 | 3.06 | 13.5 | ||
| D.O.C—C.M.D, Mizil (PH) D.O.C—Dealu Mare, Gura Vadului (PH) | FR_SRF_2017 FR_BUD_2018 | 2017 2018 | 3.23 3.05 | 12 13 | ||
| Gura Vadului (PH) | FR_TOH_2018 | 2018 | 3.44 | 11 | ||
| D.O.C—C.M.D, Ceptura (PH) | FR_CEP_2019 | 2019 | 3.37 | 13 | ||
| Gura Vadului (PH) | FR_DM_2020 | 2020 | 3.34 | 13 | ||
| Ștefănești (AG) | FR_MRC_2020 | 2020 | 3.22 | 12 | ||
| Dealu Mare, Urlați (PH) | FR_BLG_2020 | 2020 | 3.6 | 13 | ||
| Moldova | Bucium (IS) | FR_GRM_2015 | 2015 | 3.23 | 12 | |
| Huși (VS) | FR_AVER_2020 | 2020 | 3.01 | 12.5 | ||
| Oltenia | Dealurile Olteniei | FR_STB_2018 | 2018 | 2.92 | 13.5 | |
| Dealurile Segarcea, Dolj | FR_MRM_2018 | 2018 | 3.48 | 12.2 | ||
| Dobrogea | Valea lui Traian (CT) | FR_GIT_2019 | 2019 | 3.22 | 13.5 | |
| Banat | Babadag (TL) | FR_HMG_2019 | 2019 | 3.43 | 13.5 | |
| Miniș-Măderat (AR) | FR_MARC_2020 | 2020 | 3.47 | 13.5 | ||
| Recaș (TM) | FR_HND_2021 | 2021 | 3.30 | 11.5 |
| No.crt. | Compounds Name | RT | Molecular Formula | Molecular Weight, g/mol | Integrated Ions |
|---|---|---|---|---|---|
| 1. | Ethyl acetate | 4.02 | C4H8O2 | 88.11 | 43/61/45 |
| 2. | Butanoic acid ethyl ester | 8.5 | C6H12O2 | 116.16 | 71/43/29 |
| 3. | Propyl alcohol | 8.64 | C3H8O | 60.09 | 31/29/27 |
| 4. | Isobutyl alcohol | 10.41 | C4H10O | 74.13 | 43/41/42 |
| 5. | Acetic acid, pethyl ester | 11.39 | C7H14O2 | 130.19 | 43/70/42 |
| 6. | Isopentyl alcohol | 14.15 | C5H12O | 88.15 | 29/42/57 |
| 7. | Hexanoic acid ethyl ester | 14.95 | C8H16O2 | 144.21 | 88/29/43 |
| 8. | Lactic acid ethyl ester | 18.3 | C5H10O3 | 118.13 | 45/29/27 |
| 9. | 2-octanol | 20.25 | C8H18O | 130.23 | 45/55/41 |
| 10. | Octanoic acid ethyl ester | 20.7 | C10H20O2 | 172.27 | 88/101/57 |
| 11. | Acetic acid | 21.52 | C2H4O2 | 60.05 | 43/45/60 |
| 12. | Ionone | 23.25 | C13H20O | 192.30 | 121/93/136 |
| 13. | 2–3 butandiol | 23.52 | C4H10O2 | 90.12 | 45/43/57 |
| 14. | Decanoic acid, ethyl ester | 25.8 | C12H24O2 | 200.32 | 88/101/29 |
| 15. | Butandioic acid diethyl ester | 26.85 | C8H14O4 | 174.20 | 101/29/129 |
| 16. | Hexanoic acid | 30.7 | C6H12O2 | 116.16 | 60/73/41 |
| 17. | Phenylethyl alcohol | 32.19 | C8H10O | 122.16 | 91/92/65 |
| 18. | Octanoic acid | 35.05 | C8H16O2 | 144.21 | 60/73/43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolache, F.-A.; Duță, D.-E.; Criveanu-Stamatie, G.D.; Iordache, T.-A.; Todașcă, M.-C. Decoding the Volatile Profile of White Romanian Fetească Wines. Separations 2024, 11, 141. https://doi.org/10.3390/separations11050141
Manolache F-A, Duță D-E, Criveanu-Stamatie GD, Iordache T-A, Todașcă M-C. Decoding the Volatile Profile of White Romanian Fetească Wines. Separations. 2024; 11(5):141. https://doi.org/10.3390/separations11050141
Chicago/Turabian StyleManolache, Fulvia-Ancuța, Denisa-Eglantina Duță, Gabriela Daniela Criveanu-Stamatie, Teodora-Alexandra Iordache, and Maria-Cristina Todașcă. 2024. "Decoding the Volatile Profile of White Romanian Fetească Wines" Separations 11, no. 5: 141. https://doi.org/10.3390/separations11050141
APA StyleManolache, F.-A., Duță, D.-E., Criveanu-Stamatie, G. D., Iordache, T.-A., & Todașcă, M.-C. (2024). Decoding the Volatile Profile of White Romanian Fetească Wines. Separations, 11(5), 141. https://doi.org/10.3390/separations11050141









