Preparation of Polysilsesquioxane-Based CO2 Separation Membranes with Thermally Degradable Succinic Anhydride and Urea Units
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Membrane Preparation and Gel Characterization
2.3. Quantum Chemical Calculations
3. Results and Discussion
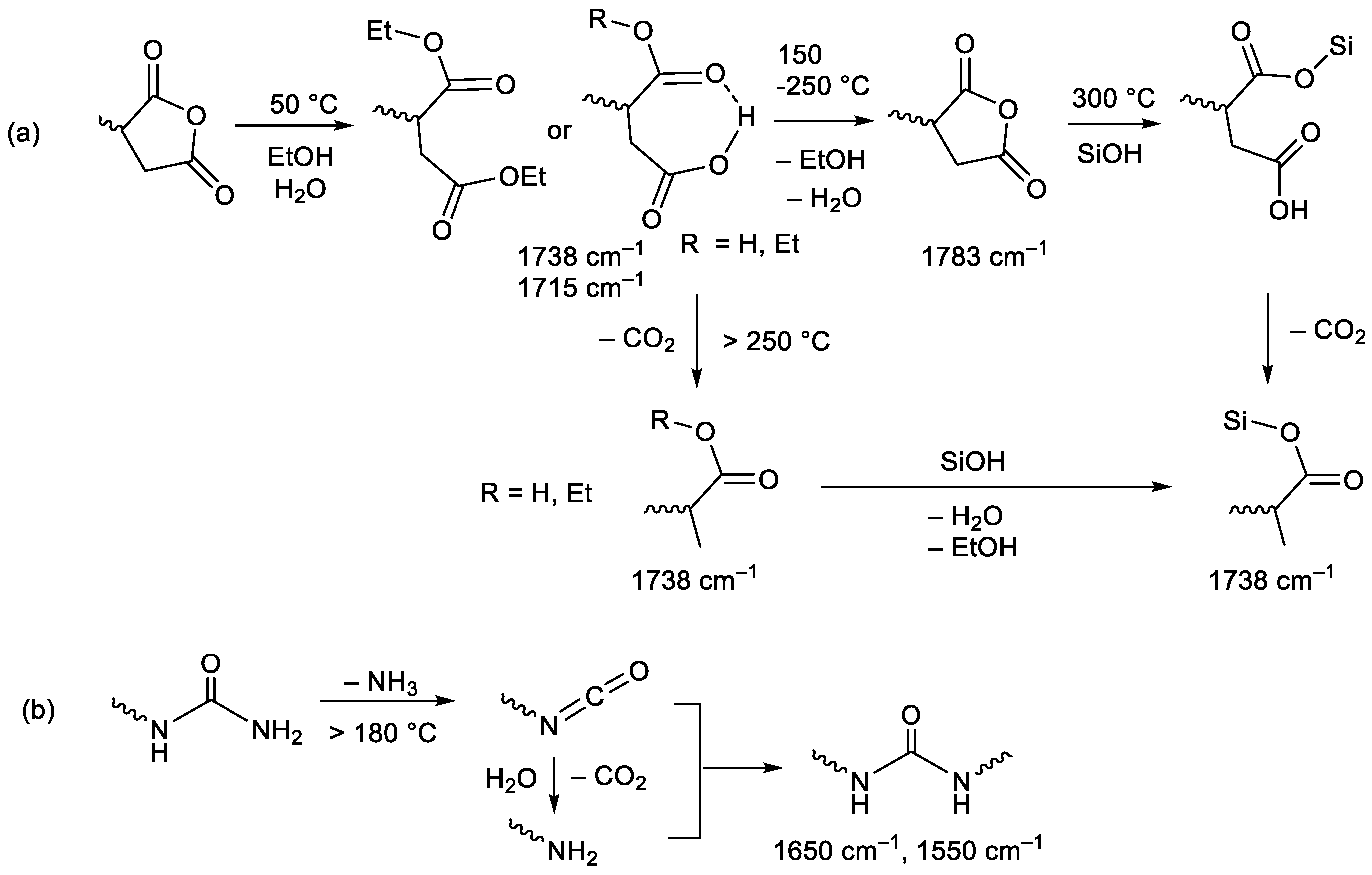
3.1. Design of CO2-Philic Groups
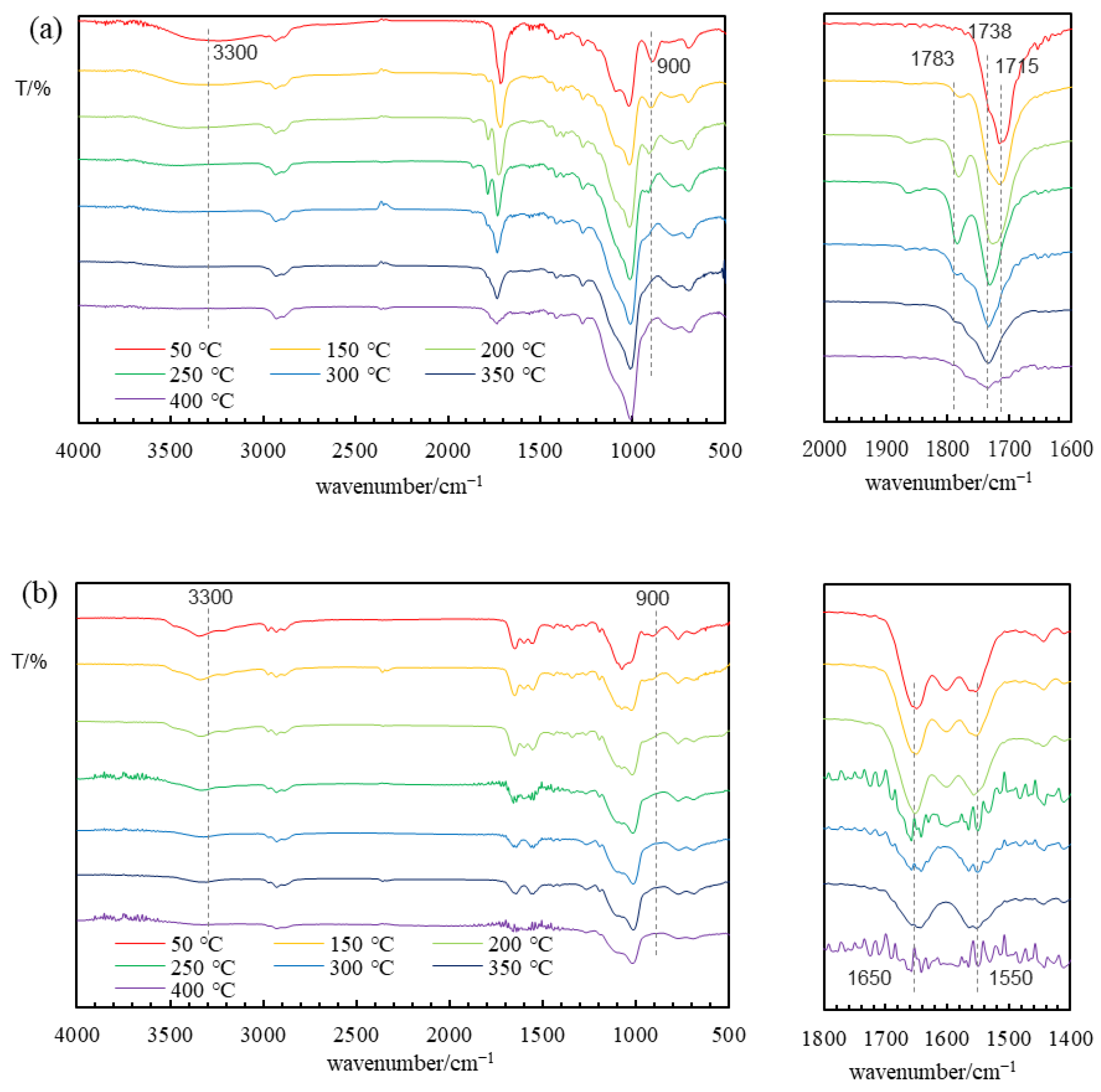
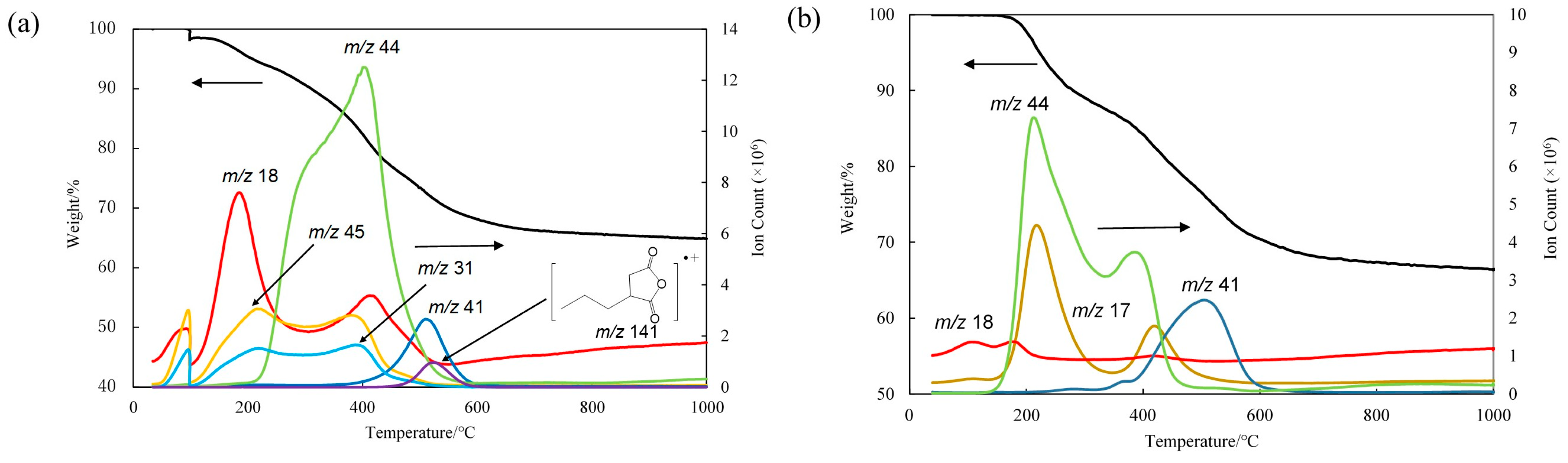
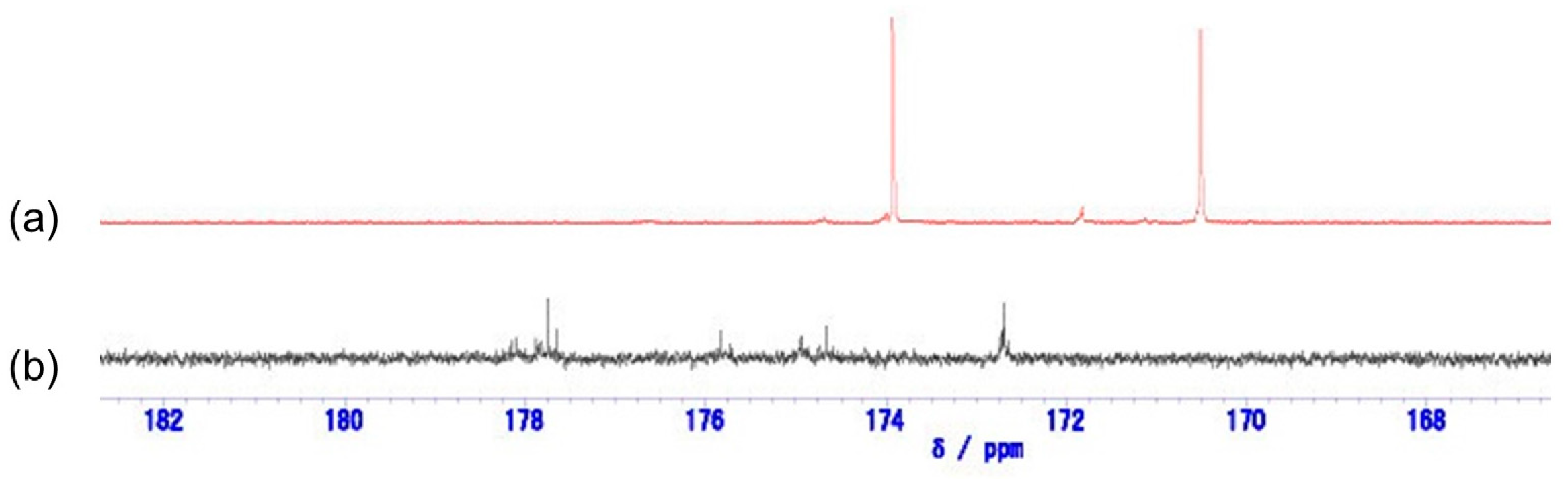
3.2. Membrane Preparation
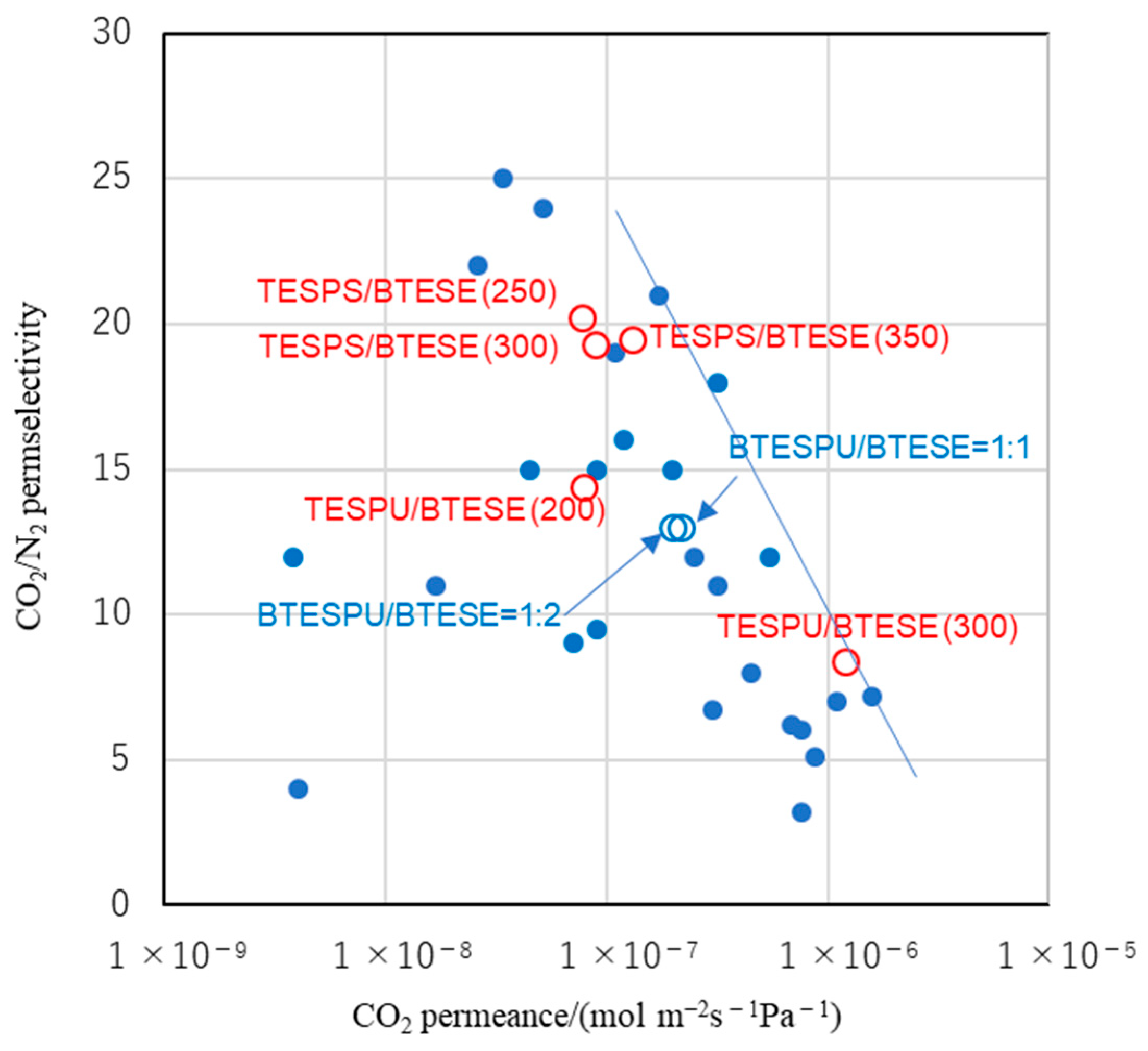
3.3. Gas Permeation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane Technologies for CO2 Separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Ma, C.; Wang, M.; Wang, Z.; Gao, M.; Wang, J. Recent Progress on Thin Film Composite Membranes for CO2 Separation. J. CO2 Util. 2020, 42, 101296. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Luo, W.; Wang, Y.; Mu, P.; Li, J. A Review on the Recent Advances in Composite Membranes for CO2 Capture Processes. Sep. Purif. Technol. 2023, 307, 122752. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Rodrigue, D.; Kliaguine, S. In-situ Cross Interface Linking of PIM-1 polymer and UiO-66-NH2 for Outstanding Gas Separation and Physical Aging Control. J. Membr. Sci. 2018, 548, 429–438. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent Organic Frameworks (COFs) Functionalized Mixed Matrix Membrane for Effective CO2/N2 Separation. J. Membr. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Woo, R.K.; Lee, A.S.; Park, S.-H.; Baek, K.-Y.; Lee, K.B.; Lee, S.-H.; Lee, J.-H.; Hwang, S.S.; Lee, J.S. Free-standing, Polysilsesquioxane-based Inorganic/organic Hybrid Membranes for Gas Separations. J. Membr. Sci. 2015, 475, 384–394. [Google Scholar]
- Park, S.; Lee, A.S.; Do, Y.S.; Hwang, S.S.; Lee, Y.M.; Lee, J.-H.; Lee, J.S. Rational Molecular Design of PEOlated Ladder-structured Polysilsesquioxane Membranes for High Performance CO2 Removal. Chem. Commun. 2015, 51, 15308–15311. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Role of Amine Type in CO2 Separation Performance within Amine Functionalized Silica/Organosilica Membranes: A Review. Appl. Sci. 2018, 8, 1032. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Fabrication and CO2 Permeation Properties of Amine-silica Membranes Using a Variety of Amine Types. J. Membr. Sci. 2017, 541, 447–456. [Google Scholar] [CrossRef]
- Karimi, S.; Korelskiy, D.; Mortazavi, Y.; Khodadadi, A.A.; Sardar, K.; Esmaeili, M.; Antzutkin, O.N.; Shah, F.U.; Hedlund, J. High Flux Acetate Functionalized Silica Membranes Based on In-situ Co-condensation for CO2/N2 Separation. J. Membr. Sci. 2016, 520, 574–582. [Google Scholar] [CrossRef]
- Xomeriakis, G.; Tsai, C.-Y.; Brnker, C.J. Microporous Sol–gel Derived Aminosilicate Membrane for Enhanced Carbon Dioxide Separation. Sep. Purif. Technol. 2005, 42, 249–257. [Google Scholar] [CrossRef]
- Paradis, G.G.; Kreiter, R.; van Tuel, M.M.; Nijmeijer, A.; Vente, J.F. Amino-Functionalized Microporous Hybrid Silica Membranes. J. Mater. Chem. 2012, 22, 7258–7264. [Google Scholar] [CrossRef]
- Ohshita, J.; Okonogi, T.; Kajimura, K.; Horata, K.; Adachi, Y.; Kanezashi, M.; Tsuru, T. Preparation of Amine- and Ammonium-containing Polysilsesquioxane Membranes for CO2 Separation. Polym. J. 2022, 54, 875–882. [Google Scholar] [CrossRef]
- Kajimura, K.; Horata, K.; Adachi, Y.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of Urea- and Isocyanurate-containing Polysilsesquioxane Membranes for CO2 Separation. J. Sol-Gel Sci. Technol. 2023, 106, 149–157. [Google Scholar] [CrossRef]
- Karimi, S.; Mortazavi, Y.; Khodadadi, A.A.; Holmgren, A.; Korelskiy, D.; Hedlund, J. Functionalization of Silica Membranes for CO2 Separation. Sep. Purif. Technol. 2020, 235, 116207. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based Technologies for Bbiogas Separations. Chem. Rev. 2010, 39, 750–768. [Google Scholar]
- Tsuru, T.; Nakasuji, T.; Oka, M.; Kanezashi, M.; Yoshioka, T. Preparation of Hydrophobic Nanoporous Methylated SiO2 Membranes and Application to Nanofiltration of Hexane Solutions. J. Membr. Sci. 2011, 384, 149–156. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Reverse Osmosis Performance of Organosilica Membranes and Comparison with the Pervaporation and Gas Permeation Properties. AIChE J. 2013, 59, 1298–1307. [Google Scholar] [CrossRef]
- Raveendran, P.; Wallen, S. Cooperative C-H···O Hydrogen Bonding in CO2-Lewis Base Complexes: Implications for Solvation in Supercritical CO2. J. Am. Chem. Soc. 2001, 124, 12590–12599. [Google Scholar] [CrossRef]
- McNeil, I.C.; Mohammad, M.H. A Comparison of the Thermal Degradation Behavior of Ethylene-ethyl Acrylate Copolymer, Low Density polyethylene and Poly(ethyl acrylate). Polym. Degrad. Stab. 1995, 48, 175–187. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, T.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal Decomposition (Pyrolysis) of Urea in an Open Reaction Vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Honorien, J.; Fournet, R.; Gaude, P.-A.; Sirjean, B. Theoretical Study of the Thermal Decomposition of Urea Derivatives. J. Phys. Chem. A 2022, 126, 6264–6277. [Google Scholar] [CrossRef] [PubMed]
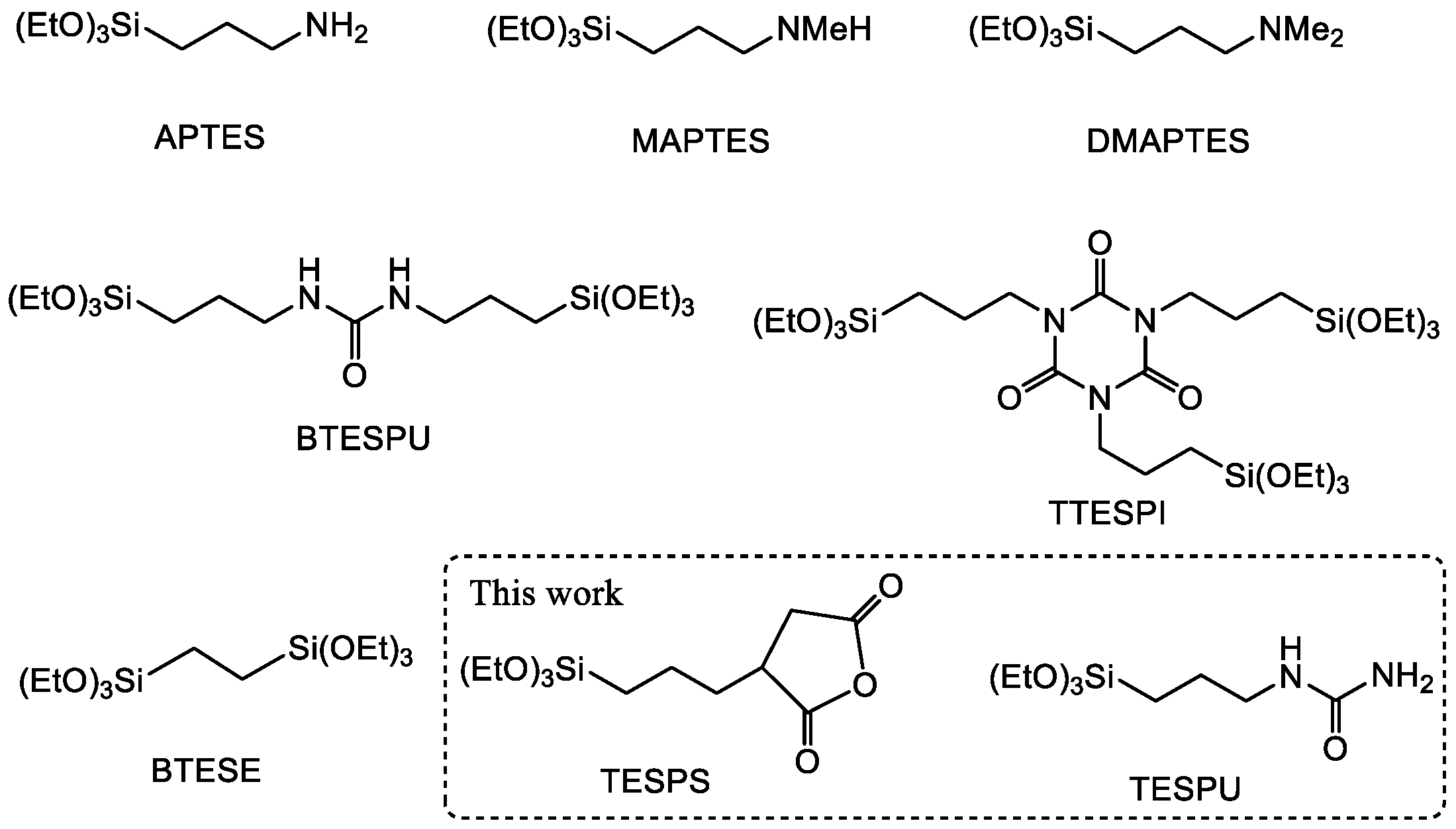
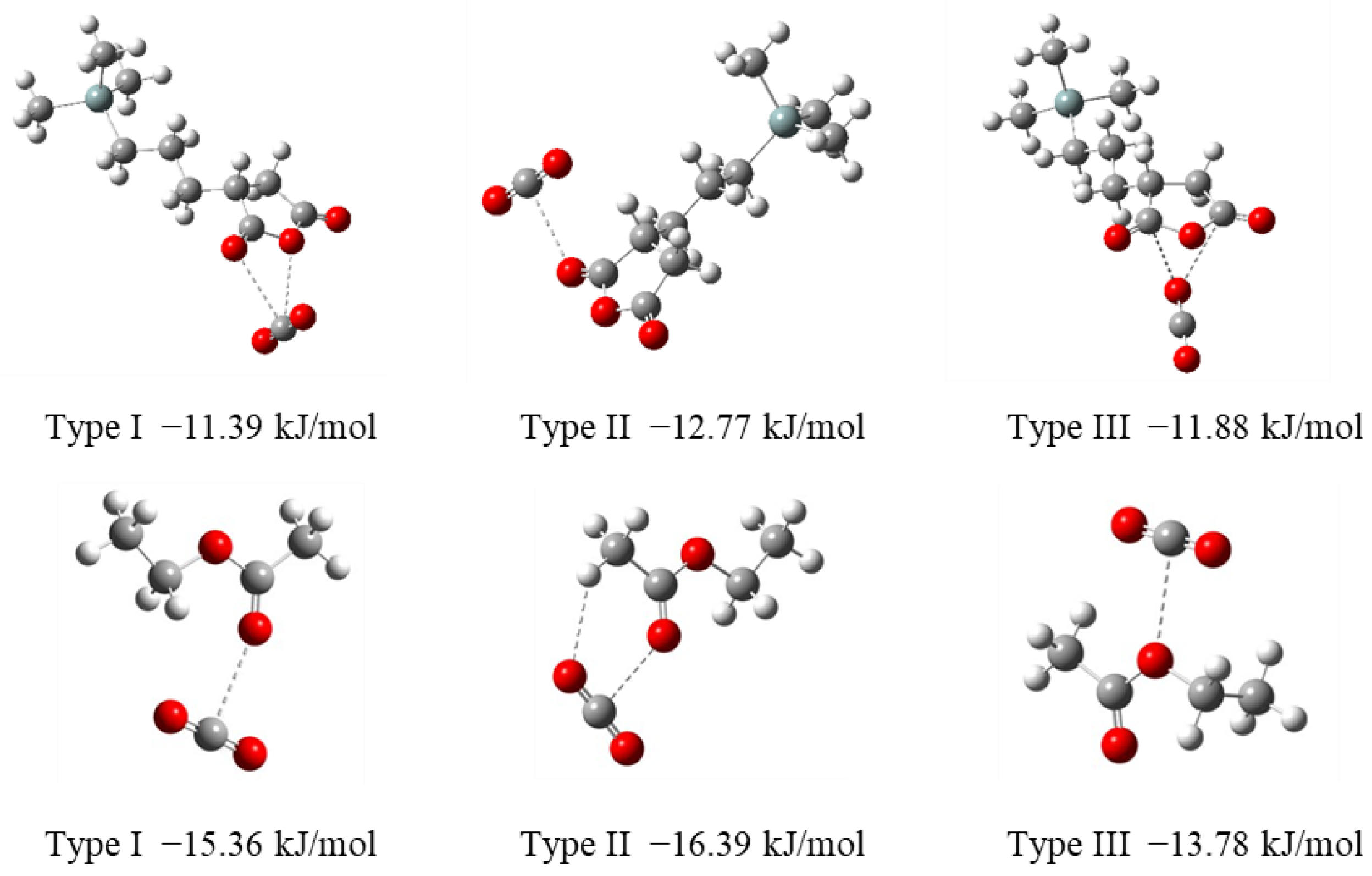
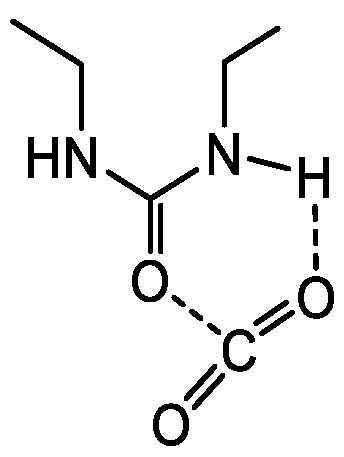
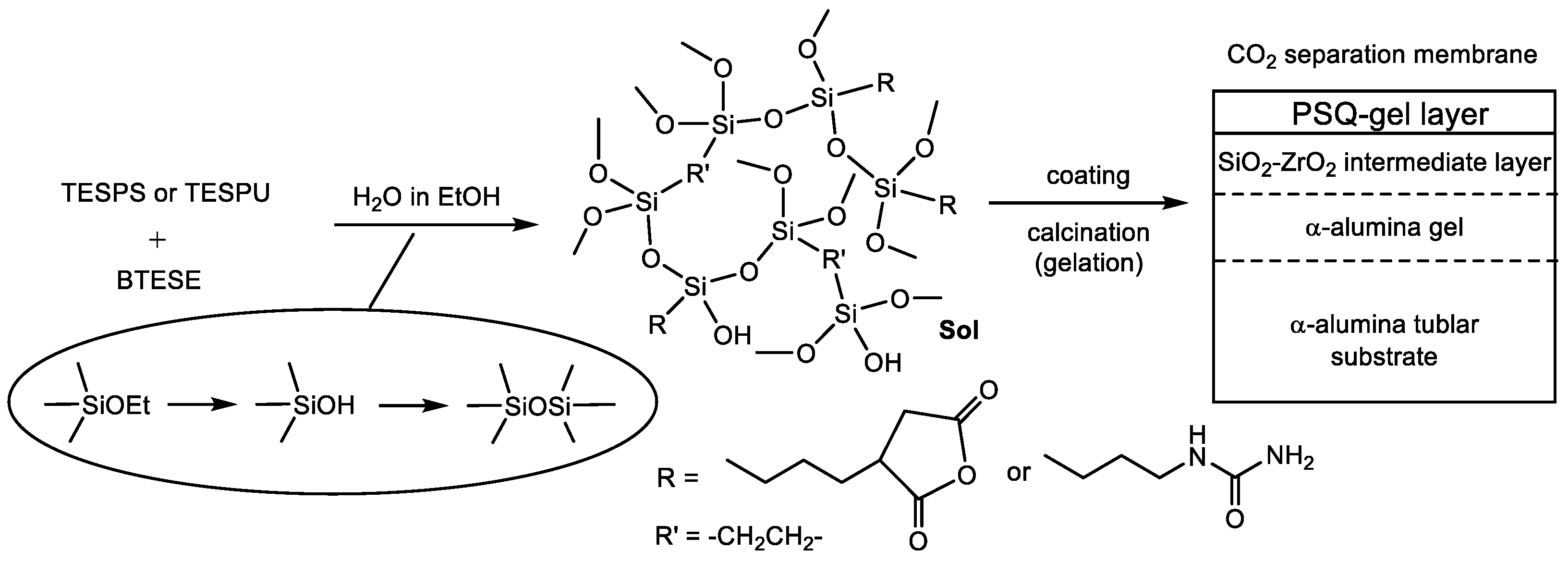
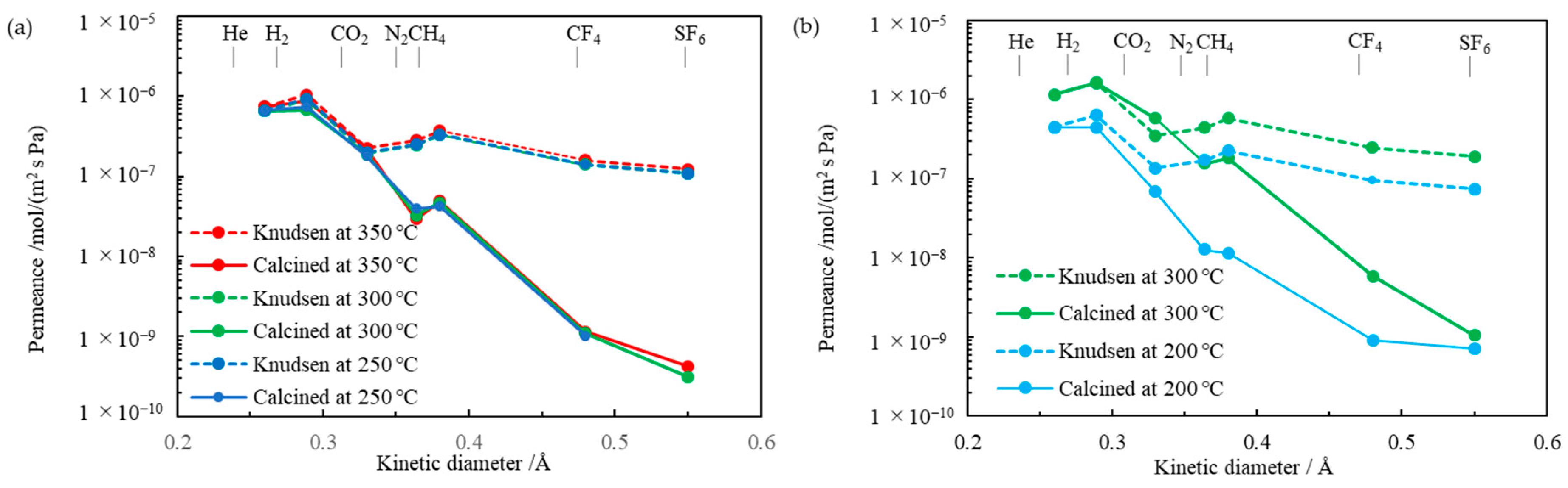
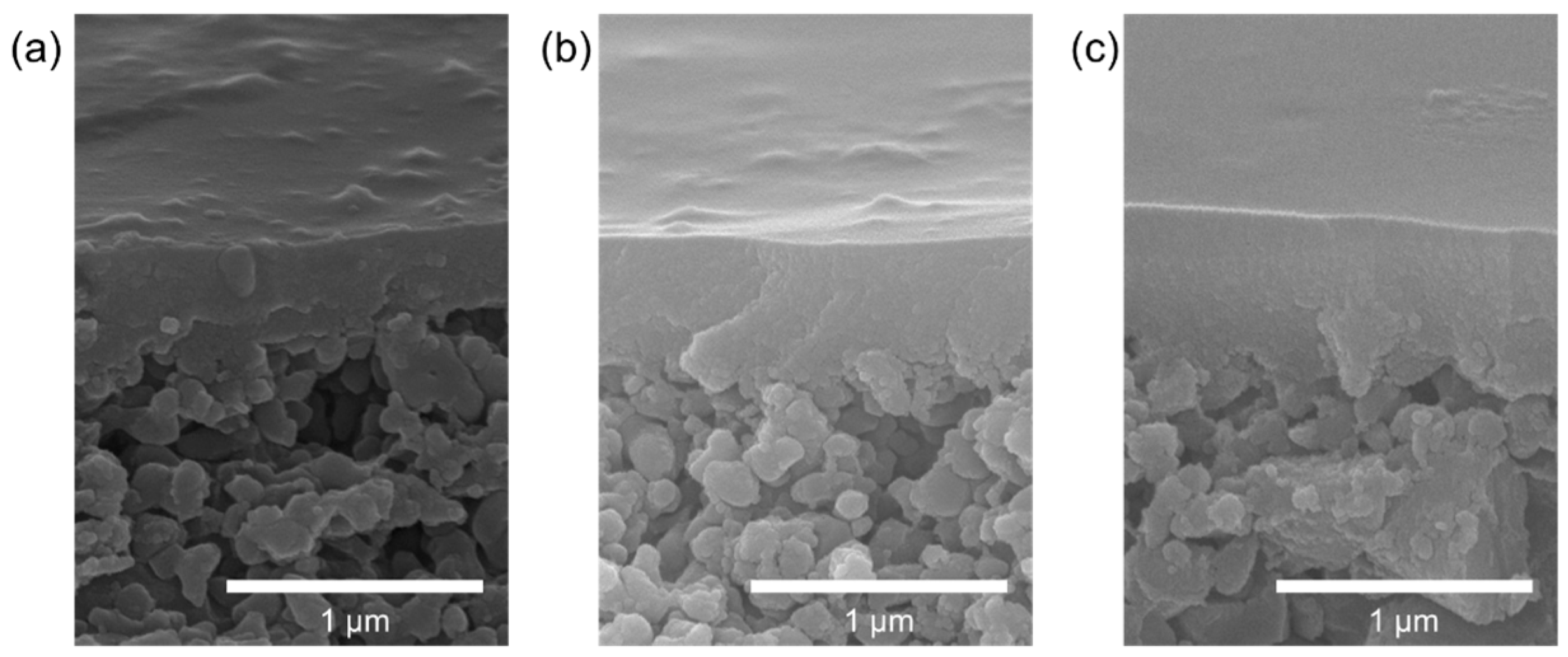
| Run | Precursor /g (/mmol) | BTESE /g (/mmol) | Ethanol /g | Water /g (/mmol) | Reaction Time/h |
|---|---|---|---|---|---|
| 1 | TESPS 0.30 (0.99) | 0.30 (0.85) | 7.43 | 3.30 (183) | 48 |
| 2 | TESPU 0.31 (1.2) | 0.30 (0.85) | 7.11 | 4.28 (238) | 96 |
| Precursor | Calcination Temp/°C | CO2 Permeance /mol m−2·s−1·Pa−1 1 | CO2/N2 1 | Eact/kJmol−1 2 | |
|---|---|---|---|---|---|
| CO2 | N2 | ||||
| TESPS-BTESE (1:1) | 250 | 7.7 × 10−8 | 20.2 | 7.3 | 17.2 |
| 300 | 8.9 × 10−8 | 19.3 | 6.4 | 14.7 | |
| 350 | 1.3 × 10−7 | 19.5 | 4.5 | 10.3 | |
| TESPU-BTESE (1:1) | 200 | 7.9 × 10−8 | 14.4 | −1.3 | 7.1 |
| 300 | 1.2 × 10−6 | 8.4 | −6.4 | 0.5 | |
| TESPS | 250 | 4.8 × 10−9 | 9.4 | 27.5 | 31.0 |
| 300 | 6.4 × 10−9 | 2.2 | 24.1 | 21.2 | |
| 350 | 1.2 × 10−7 | 11.5 | 11.5 | 17.7 | |
| BTESPU-BTESE (1:1) 3 | 300 | 2.2 × 10−7 | 13 | 0.3 | 9.1 |
| BTESPU-BTESE (1:2) 3 | 300 | 2.0 × 10−7 | 13 | 0.35 | 10.1 |
| TTESPI 3 | 300 | 3.2 × 10−7 | 18 | 3.3 | 14.4 |
| TTESPI-BTESE (1:1) 3 | 300 | 5.5 × 10−7 | 12 | −2.5 | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horata, K.; Yoshio, T.; Miyazaki, R.; Adachi, Y.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of Polysilsesquioxane-Based CO2 Separation Membranes with Thermally Degradable Succinic Anhydride and Urea Units. Separations 2024, 11, 110. https://doi.org/10.3390/separations11040110
Horata K, Yoshio T, Miyazaki R, Adachi Y, Kanezashi M, Tsuru T, Ohshita J. Preparation of Polysilsesquioxane-Based CO2 Separation Membranes with Thermally Degradable Succinic Anhydride and Urea Units. Separations. 2024; 11(4):110. https://doi.org/10.3390/separations11040110
Chicago/Turabian StyleHorata, Katsuhiro, Tsubasa Yoshio, Ryuto Miyazaki, Yohei Adachi, Masakoto Kanezashi, Toshinori Tsuru, and Joji Ohshita. 2024. "Preparation of Polysilsesquioxane-Based CO2 Separation Membranes with Thermally Degradable Succinic Anhydride and Urea Units" Separations 11, no. 4: 110. https://doi.org/10.3390/separations11040110
APA StyleHorata, K., Yoshio, T., Miyazaki, R., Adachi, Y., Kanezashi, M., Tsuru, T., & Ohshita, J. (2024). Preparation of Polysilsesquioxane-Based CO2 Separation Membranes with Thermally Degradable Succinic Anhydride and Urea Units. Separations, 11(4), 110. https://doi.org/10.3390/separations11040110







