Effect of Positively Charged Lipids (DOTAP) on the Insertion of Carbon Nanotubes into Liposomes and the Separation Performance of Thin-Film Nanocomposite Membranes
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Liposomes and CNT Liposomes
2.2. Fabrication of TFC and TFN Membranes
2.3. Membrane Separation Performance
2.4. Antifouling Performance
3. Results and Discussion
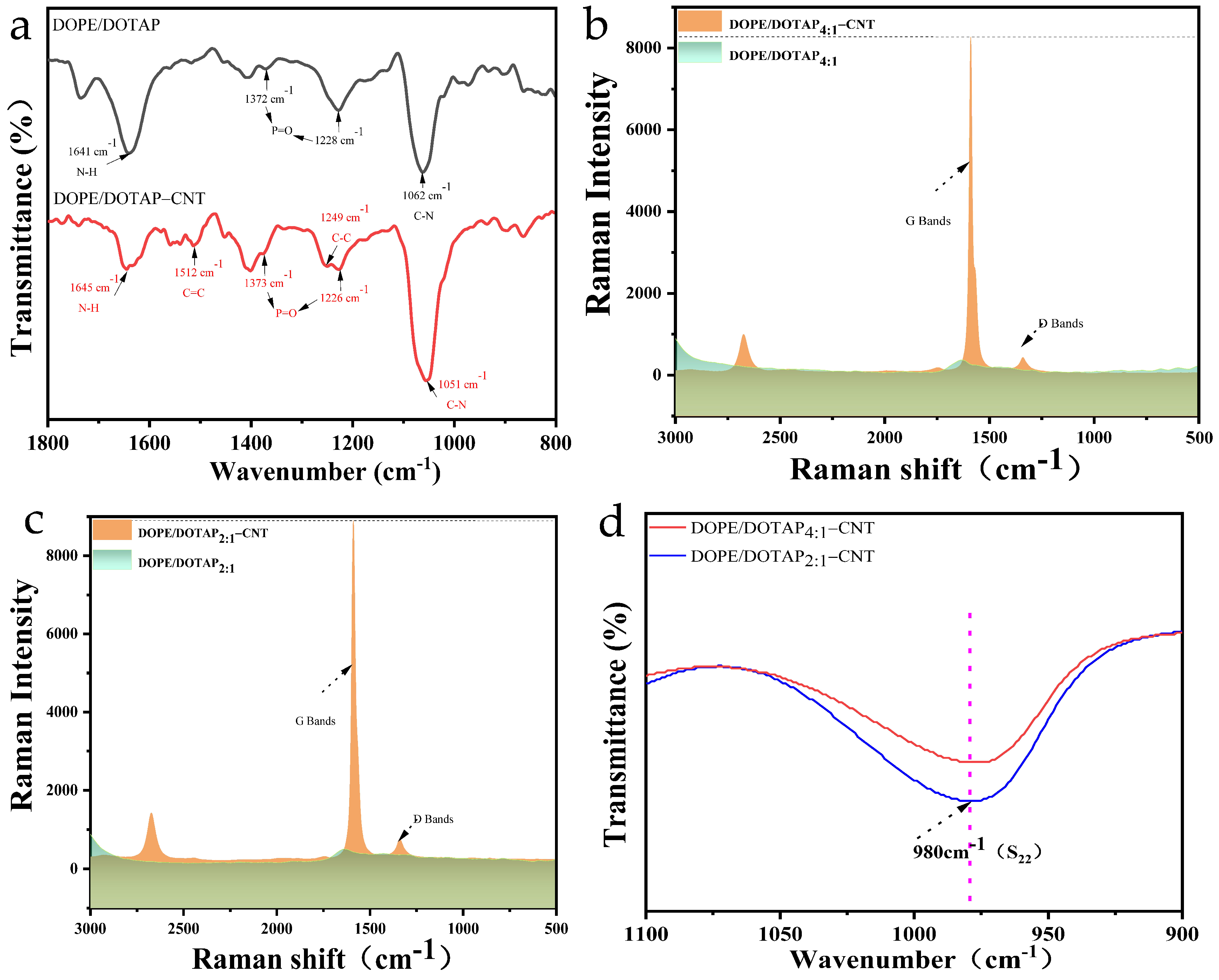
3.1. Characterization of Liposomes
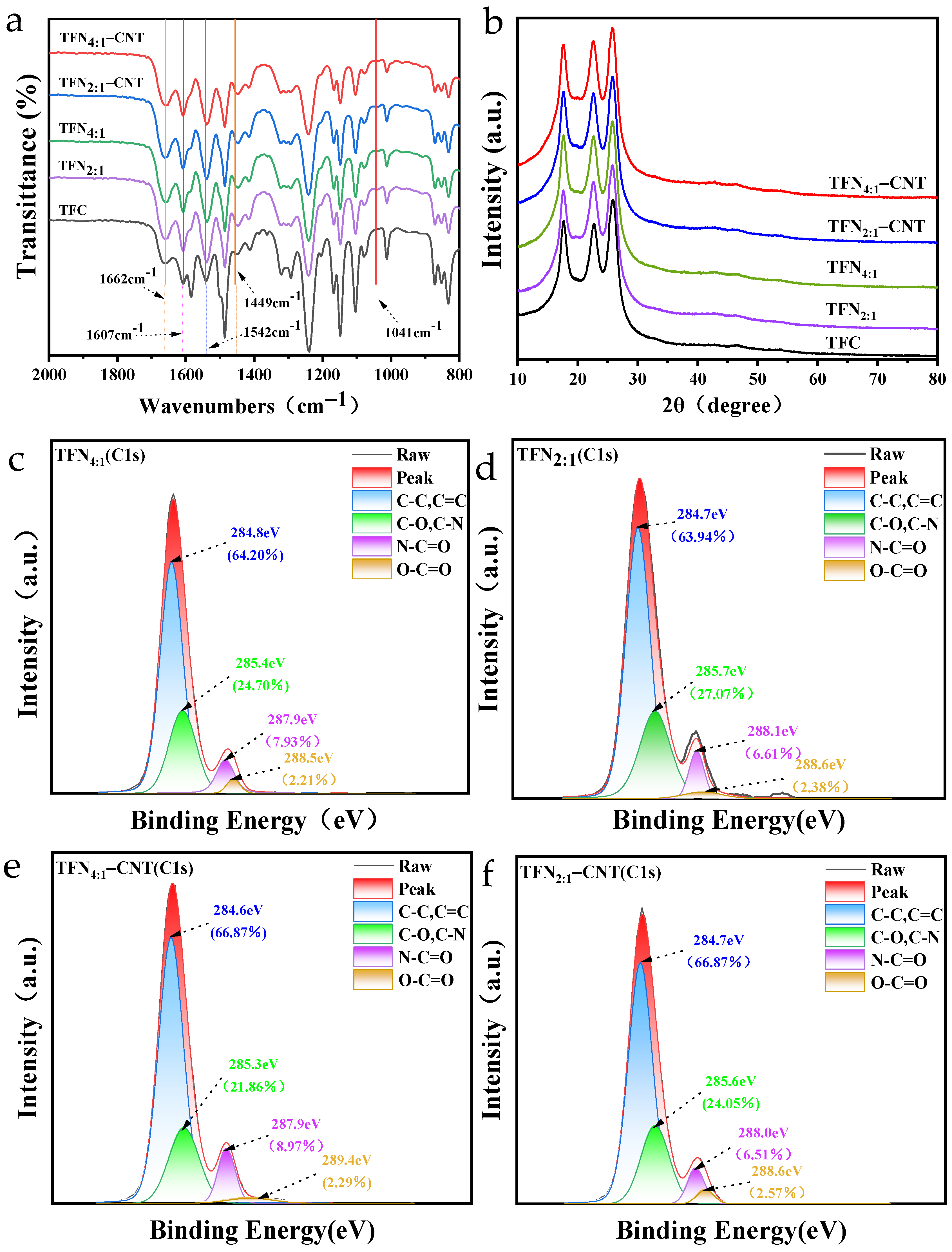
3.2. Characterization of TFC and TFN Membranes
Surface Functional Groups of TFC and TFN Membranes
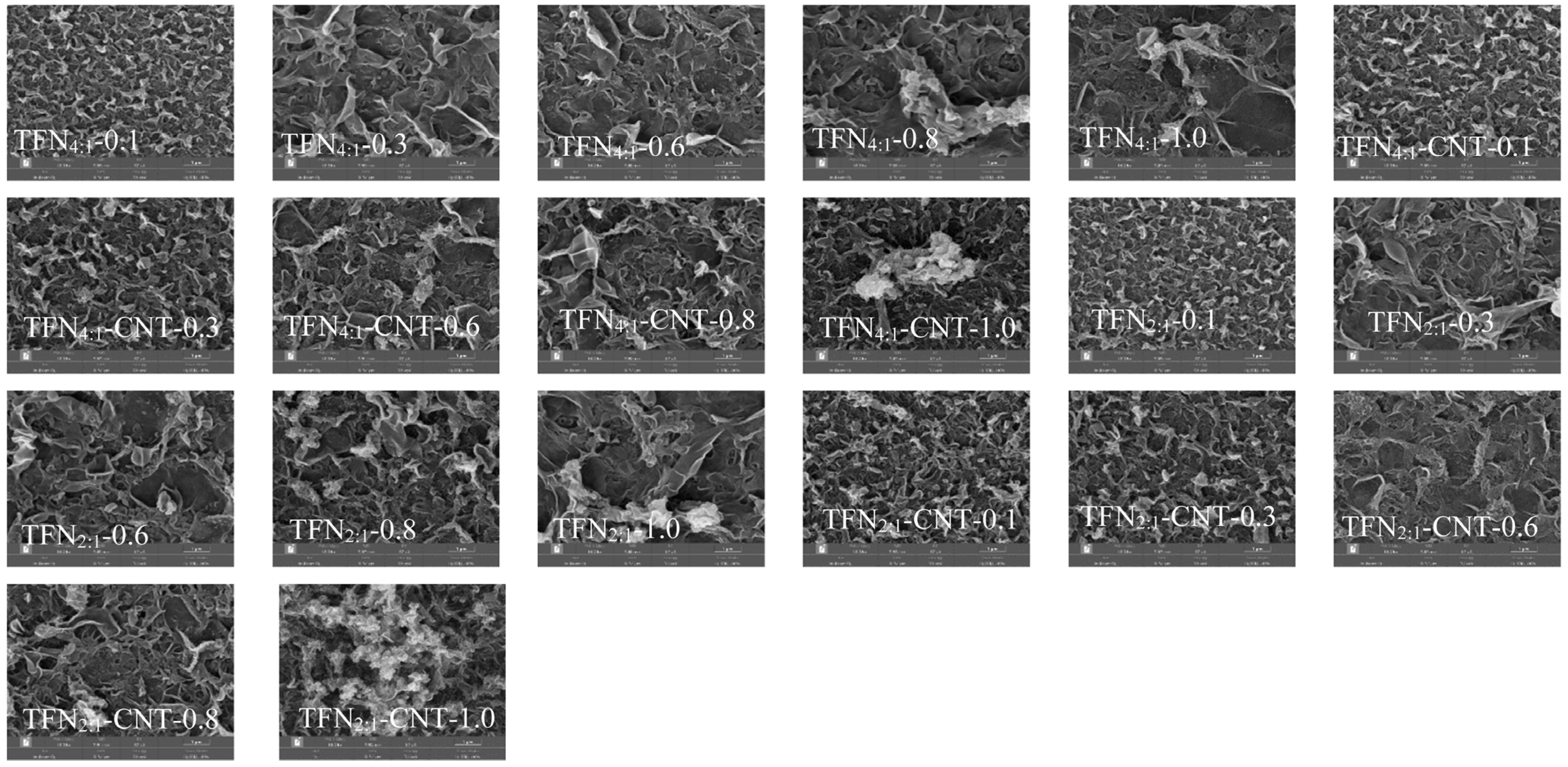
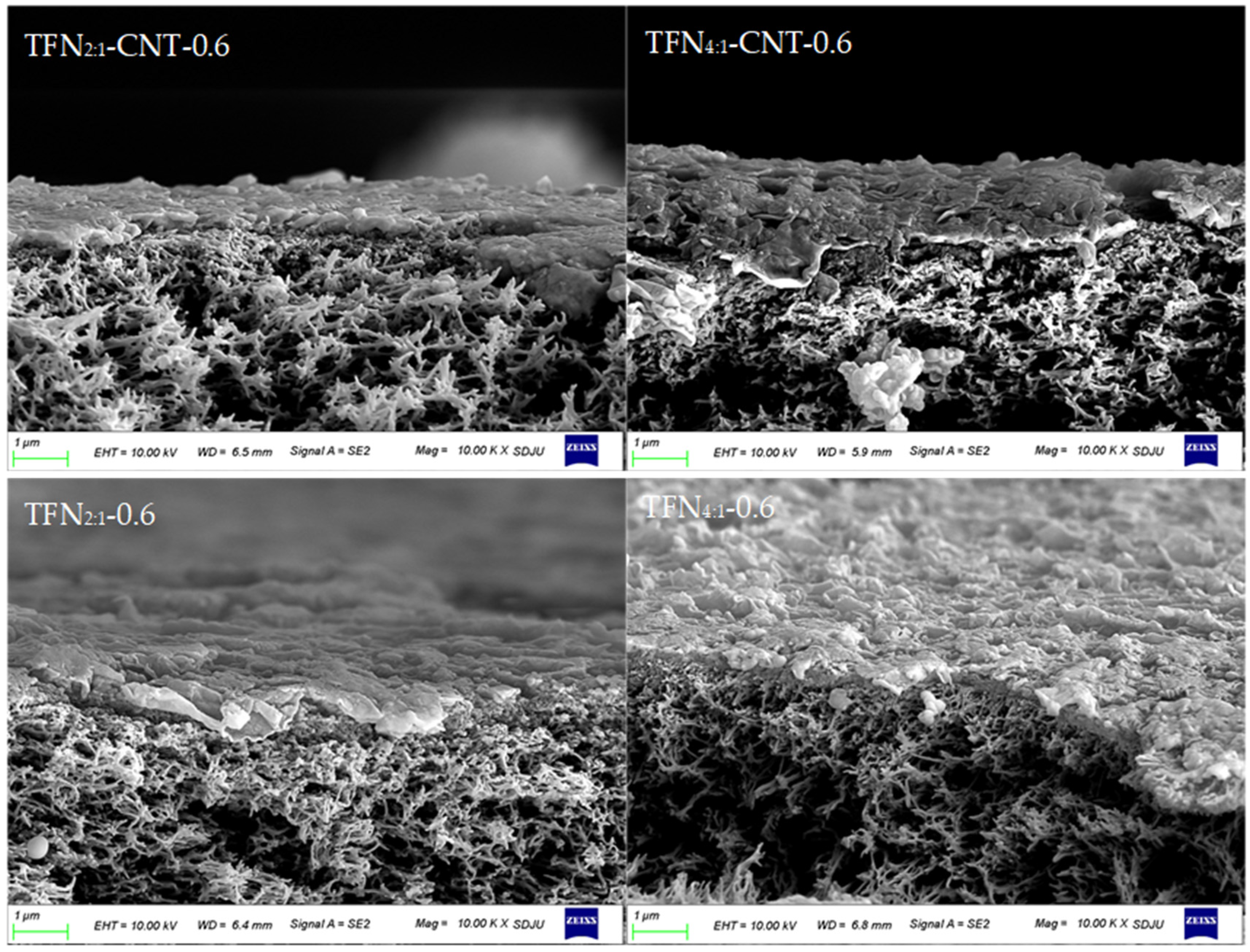
3.3. Surface Morphology and Roughness of TFC and TFN Membranes
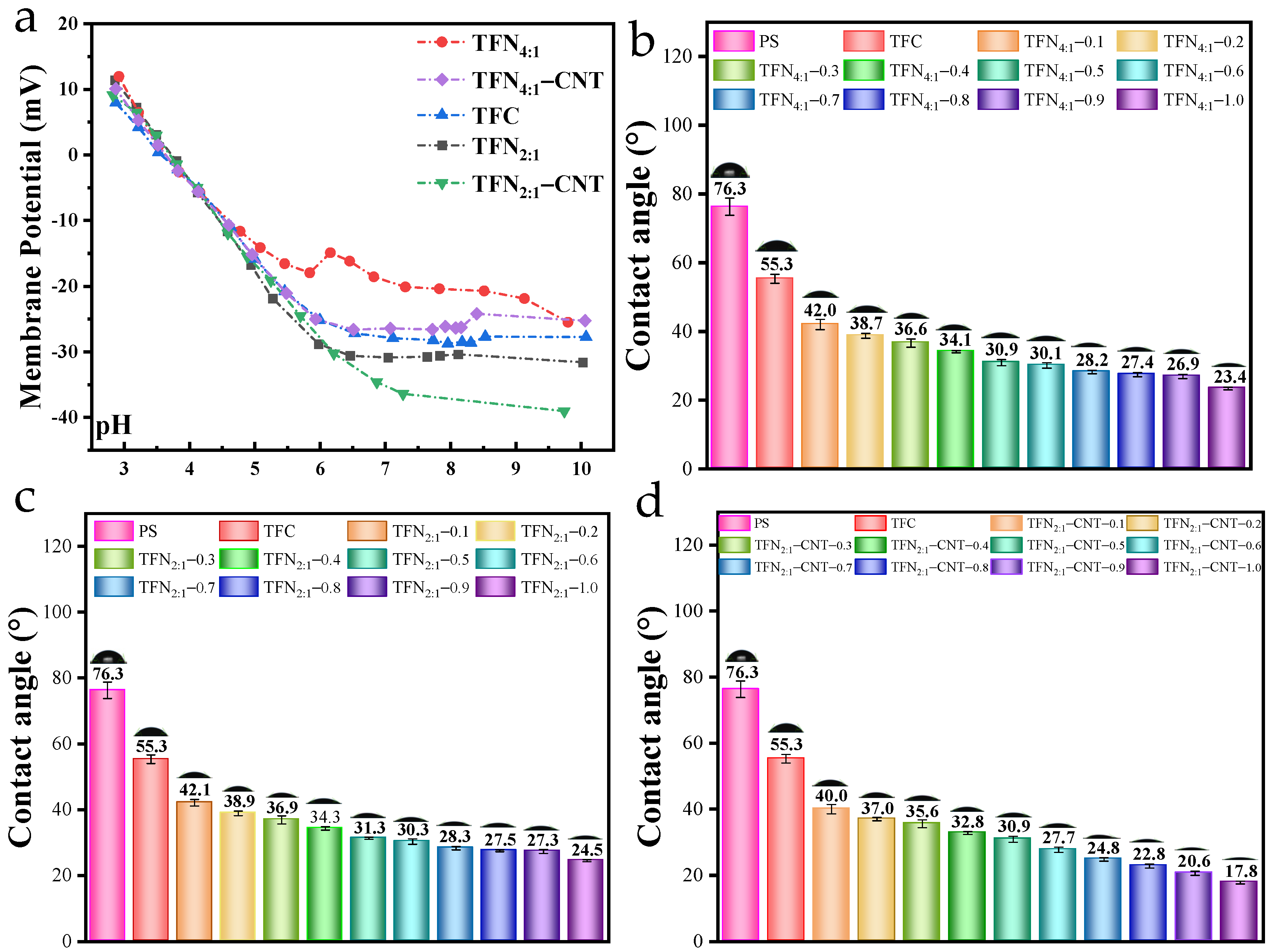
3.4. Membrane Potential and Surface Hydrophilicity of TFC and TFN Membranes
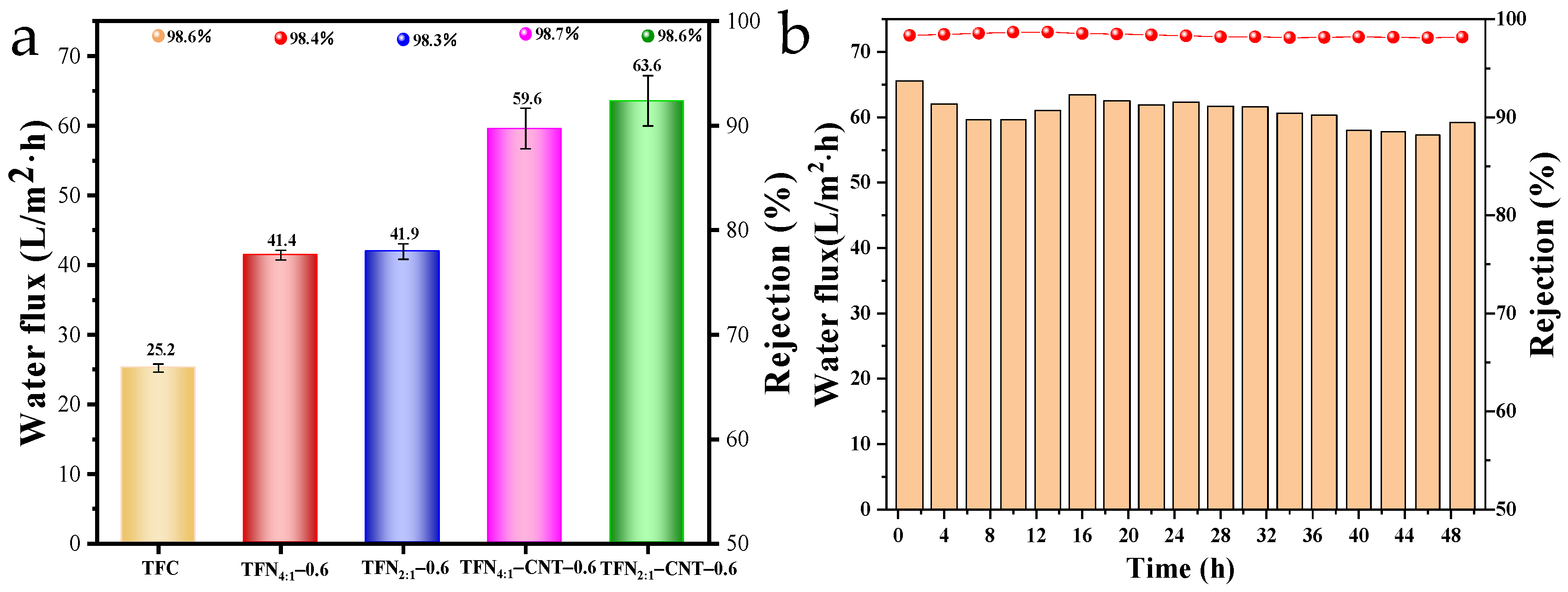
3.5. Separation Performance of TFC and TFN Membranes
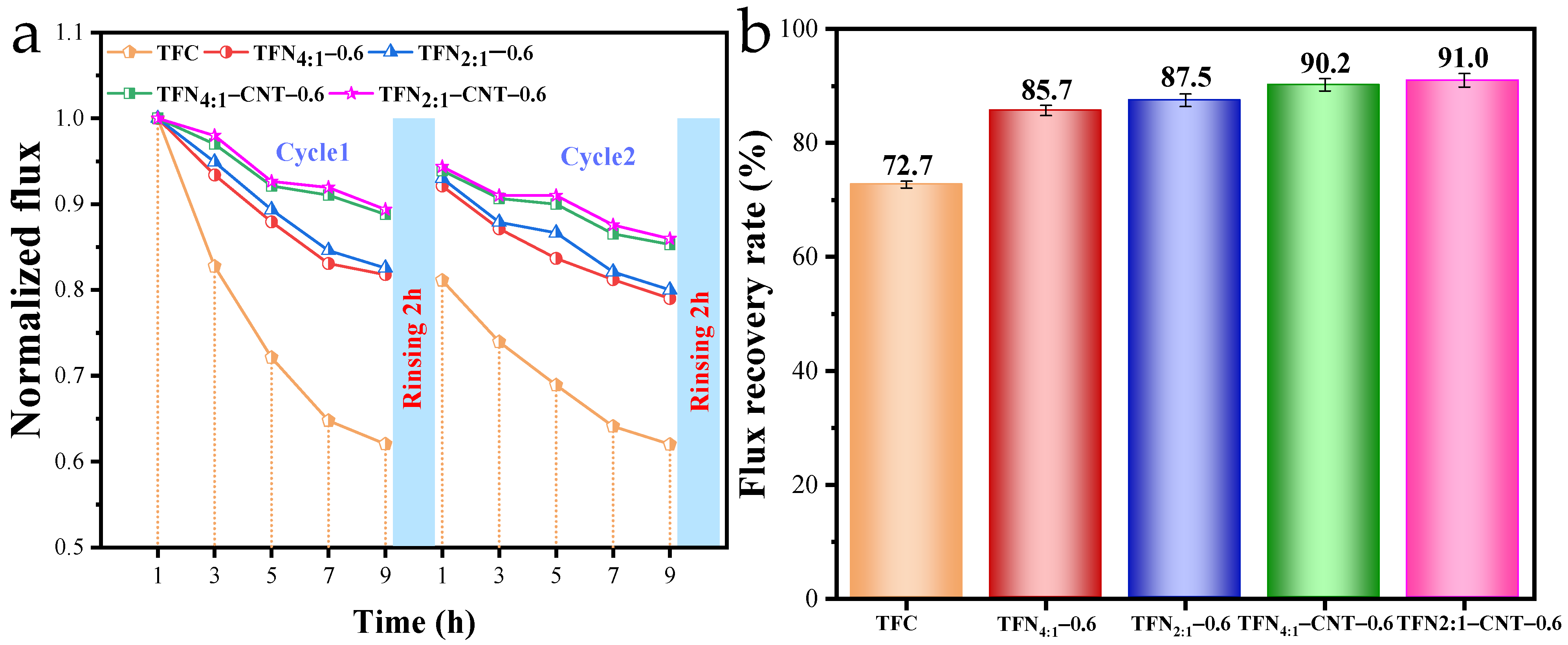
3.6. Antifouling Performance of TFC and TFN Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thakur, A.K.; Sathyamurthy, R.; Velraj, R.; Saidur, R.; Lynch, I.; Venkatesh, R.; Kumar, P.G.; Kim, S.C.; Sillanpa, M. A novel solar absorber using activated carbon nanoparticles synthesized from bio-waste for the performance improvement of solar desalination unit. Desalination 2022, 527, 115564. [Google Scholar] [CrossRef]
- Li, R.H.; Li, Y.; Wu, H.; Yan, W.T.; Yu, C.Y.; Liu, L.F.; Gao, C.J. Structure regulation for synergistically improving the permeation properties of the reverse osmosis membrane based on an amphiphilic hyperbranched polymer. J. Membr. Sci. 2020, 608, 118143. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Pan, G.Y.; Yan, H.; Yao, X.R.; Shi, H.W.; Tang, Y.J.; Wei, X.R.; Liu, Y.Q. Surface modification of polyamide reverse osmosis membrane with organic-inorganic hybrid material for antifouling. Appl. Surf. Sci. 2018, 433, 139–148. [Google Scholar] [CrossRef]
- Zhao, D.L.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review. Water Res. 2020, 173, 115557. [Google Scholar] [CrossRef]
- Yu, C.J.; Cen, X.X.; Ao, D.; Qiao, Z.H.; Zhong, C.L. Preparation of thin-film composite membranes with ultrahigh MOFs loading through polymer-template MOFs induction secondary interfacial polymerization. Appl. Surf. Sci. 2023, 614, 156186. [Google Scholar] [CrossRef]
- Jeong, B.H.; Hoek, E.M.V.; Yan, Y.S.; Subramani, A.; Huang, X.F.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Yang, D.C.; Castellano, R.J.; Silvy, R.P.; Lageshetty, S.K.; Praino, R.F.; Fornasiero, F.; Shan, J.W. Fast Water Transport through Subnanometer Diameter Vertically Aligned Carbon Nanotube Membranes. Nano Lett. 2023, 23, 4956–4964. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.F.; Chen, H.Y.; Surapathi, A.; Taylor, M.G.; Shao, X.H.; Marand, E.; Johnson, J.K. Zwitterion Functionalized Carbon Nanotube/Polyamide Nanocomposite Membranes for Water Desalination. Acs Nano 2013, 7, 5308–5319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.F.; Li, M.; Yu, K.; Hu, J.H.; Zhang, X.; Wang, L.J. Sulfonated multiwall carbon nanotubes assisted thin-film nanocomposite membrane with enhanced water flux and anti-fouling property. J. Membr. Sci. 2017, 524, 344–353. [Google Scholar] [CrossRef]
- Khalid, A.; Al-Juhani, A.A.; Al-Hamouz, O.C.; Laoui, T.; Khan, Z.; Atieh, M.A. Preparation and properties of nanocomposite polysulfone/multi-walled carbon nanotubes membranes for desalination. Desalination 2015, 367, 134–144. [Google Scholar] [CrossRef]
- Chan, W.F.; Marand, E.; Martin, S.M. Novel zwitterion functionalized carbon nanotube nanocomposite membranes for improved RO performance and surface anti-biofouling resistance. J. Membr. Sci. 2016, 509, 125–137. [Google Scholar] [CrossRef]
- Takeuchi, K.; Takizawa, Y.; Kitazawa, H.; Fujii, M.; Hosaka, K.; Ortiz-Medina, J.; Morelos-Gomez, A.; Cruz-Silva, R.; Fujishige, M.; Akuzawa, N.; et al. Salt rejection behavior of carbon nanotube-polyamide nanocomposite reverse osmosis membranes in several salt solutions. Desalination 2018, 443, 165–171. [Google Scholar] [CrossRef]
- Wang, Q.K.; Sun, J.Q.; Xue, W.J.; Zhao, G.L.; Ding, W.D.; Zhang, K.F.; Wang, S.; Li, Y.W. Effect of carbon nanotube nanochannel on the separation performance of thin-film nanocomposite (TFN) membranes. Desalination 2023, 546, 116216. [Google Scholar] [CrossRef]
- Wang, M.Q.; Wang, Z.N.; Wang, X.D.; Wang, S.Z.; Ding, W.D.; Gao, C.J. Layer-by-Layer Assembly of Aquaporin Z-Incorporated Biomimetic Membranes for Water Purification. Environ. Sci. Technol. 2015, 49, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.P.; Dharuman, V. Supported binary liposome vesicle-gold nanoparticle for enhanced label free DNA and protein sensing. Biosens. Bioelectron. 2017, 95, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Nayak, M.K.; Dash, D.; Agrawal, G.P.; Vyas, S.P. Development and characterization of site specific target sensitive liposomes for the delivery of thrombolytic agents. Int. J. Pharmaceut. 2011, 403, 254–261. [Google Scholar] [CrossRef]
- Martinez-Rubi, Y.; Gonzalez-Dominguez, J.M.; Ansón-Casaos, A.; Kingston, C.T.; Daroszewska, M.; Barnes, M.; Hubert, P.; Cattin, C.; Martinez, M.T.; Simard, B. Tailored SWCNT functionalization optimized for compatibility with epoxy matrices. Nanotechnology 2012, 23, 285701. [Google Scholar] [CrossRef] [PubMed]
- Tokgöz, S.R.; Kara, A.; Peksoz, A. Synthesis and characterization of poly(EGDMA--VPCA)/SWCNT composite films by surface polymerization method. Mat. Sci. Semicon. Proc. 2020, 116, 105144. [Google Scholar] [CrossRef]
- Song, X.J.; Wang, L.; Tang, C.Y.; Wang, Z.N.; Gao, C.J. Fabrication of carbon nanotubes incorporated double-skinned thin film nanocomposite membranes for enhanced separation performance and antifouling capability in forward osmosis process. Desalination 2015, 369, 1–9. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Chen, X.; Belliveau, A.; Allen, F.I.; Noy, A. High-Yield Synthesis and Optical Properties of Carbon Nanotube Porins. J. Phys. Chem. C 2017, 121, 3117–3125. [Google Scholar] [CrossRef]
- Sanborn, J.R.; Chen, X.; Yao, Y.C.; Hammons, J.A.; Tunuguntla, R.H.; Zhang, Y.L.; Newcomb, C.C.; Soltis, J.A.; De Yoreo, J.J.; Van Buuren, A.; et al. Carbon Nanotube Porins in Amphiphilic Block Copolymers as Fully Synthetic Mimics of Biological Membranes. Adv. Mater. 2018, 30, 1803355. [Google Scholar] [CrossRef]
- Genova, J.; Chamati, H.; Petrov, M. Study of SOPC with embedded pristine and amide-functionalized single wall carbon nanotubes by DSC and FTIR spectroscopy. Colloid Surf. A 2020, 603, 125261. [Google Scholar] [CrossRef]
- Porter, C.J.; Werber, J.R.; Zhong, M.J.; Wilson, C.J.; Elimelech, M. Pathways and Challenges for Biomimetic Desalination Membranes with Sub-Nanometer Channels. Acs Nano 2020, 14, 10894–10916. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, Y.; Hu, Z.Q.; Deng, B.L. Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Membr. Sci. 2013, 441, 73–82. [Google Scholar] [CrossRef]
- Fajardo-Diaz, J.L.; Takeuchi, K.; Morelos-Gomez, A.; Cruz-Silva, R.; Yamanaka, A.; Tejima, S.; Izu, K.; Saito, S.; Ito, I.; Maeda, J.; et al. Enhancing boron rejection in low-pressure reverse osmosis systems using a cellulose fiber-carbon nanotube nanocomposite polyamide membrane: A study on chemical structure and surface morphology. J. Membr. Sci. 2023, 679, 121691. [Google Scholar] [CrossRef]
- Ding, W.D.; Cai, J.; Yu, Z.Y.; Wang, Q.H.; Xu, Z.N.; Wang, Z.N.; Gao, C.J. Fabrication of an aquaporin-based forward osmosis membrane through covalent bonding of a lipid bilayer to a microporous support. J. Mater. Chem. A 2015, 3, 20118–20126. [Google Scholar] [CrossRef]
- Tayefeh, A.; Poursalehi, R.; Wiesner, M.; Mousavi, S.A. XPS study of size effects of Fe3O4 nanoparticles on crosslinking degree of magnetic TFN membrane. Polym. Test. 2019, 73, 232–241. [Google Scholar] [CrossRef]
- Shan, M.; Kang, H.; Xu, Z.; Li, N.; Jing, M.; Hu, Y.; Teng, K.; Qian, X.; Shi, J.; Liu, L. Decreased cross-linking in interfacial polymerization and heteromorphic support between nanoparticles: Towards high-water and low-solute flux of hybrid forward osmosis membrane. J. Colloid Interf. Sci. 2019, 548, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Goh, K.; Tan, C.H.; Wang, R. Liposomes-assisted fabrication of high performance thin film composite nanofiltration membrane. J. Membr. Sci. 2021, 620, 118833. [Google Scholar] [CrossRef]
- Liu, J.W. Interfacing Zwitterionic Liposomes with Inorganic Nanomaterials: Surface Forces, Membrane Integrity, and Applications. Langmuir 2016, 32, 4393–4404. [Google Scholar] [CrossRef]
- Baroña, G.N.B.; Lim, J.; Choi, M.; Jung, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef]
- Asempour, F.; Akbari, S.; Bai, D.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Improvement of stability and performance of functionalized halloysite nano tubes-based thin film nanocomposite membranes. J. Membr. Sci. 2018, 563, 470–480. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Li, K.; Lee, B.; Kim, Y. High performance reverse osmosis membrane with carbon nanotube support layer. J. Membr. Sci. 2019, 592, 117358. [Google Scholar] [CrossRef]
- Roy, K.; Mukherjee, A.; Maddela, N.R.; Chakraborty, S.; Shen, B.; Li, M.; Du, D.; Peng, Y.; Lu, F.; García Cruzatty, L.C. Outlook on the bottleneck of carbon nanotube in desalination and membrane-based water treatment—A review. J. Environ.Chem. Eng. 2020, 8, 103572. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G.-Z.; Qiu, S.; Cheng, L.-H.; Chen, H.-L. Preparation of high-flux thin film nanocomposite reverse osmosis membranes by incorporating functionalized multi-walled carbon nanotubes. Desalin. Water Treat. 2011, 34, 19–24. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Wan Azelee, I.; Goh, P.S.; Lau, W.J.; Ismail, A.F. Facile acid treatment of multiwalled carbon nanotube-titania nanotube thin film nanocomposite membrane for reverse osmosis desalination. J. Clean. Prod. 2018, 181, 517–526. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, H.J.; Kim, S.-H.; Lee, J.-C.; Yoon, J. Evaluation of carbon nanotube-polyamide thin-film nanocomposite reverse osmosis membrane: Surface properties, performance characteristics and fouling behavior. J. Ind. Eng. Chem. 2017, 56, 327–334. [Google Scholar] [CrossRef]
- Inukai, S.; Cruz-Silva, R.; Ortiz-Medina, J.; Morelos-Gomez, A.; Takeuchi, K.; Hayashi, T.; Tanioka, A.; Araki, T.; Tejima, S.; Noguchi, T.; et al. High-performance multi-functional reverse osmosis membranes obtained by carbon nanotube·polyamide nanocomposite. Sci. Rep. 2015, 5, 13562. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhsh, J.; Delnavaz, M.; Vatanpour, V. Investigation of raw and oxidized multiwalled carbon nanotubes in fabrication of reverse osmosis polyamide membranes for improvement in desalination and antifouling properties. Desalination 2017, 410, 1–9. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2021, 499, 114857. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Wang, W.; Wang, Y.; Gao, B.; Wang, Z. Enhanced antifouling and antimicrobial thin film nanocomposite membranes with incorporation of Palygorskite/titanium dioxide hybrid material. J. Colloid Interf. Sci. 2019, 537, 1–10. [Google Scholar] [CrossRef]
| Liposome Type | Average Size (nm) | PDI | Zeta Potential (mV) | k (s−1) | Pf (µm/s) |
|---|---|---|---|---|---|
| DOPE/DOTAP4:1 | 73.66 | 0.124 | 10.60 | 21.48 | 50.52 |
| DOPE/DOTAP4:1-CNT | 93.16 | 0.228 | −7.13 | 206.17 | 616.21 |
| DOPE/DOTAP2:1 | 77.18 | 0.131 | 25.03 | 23.47 | 57.84 |
| DOPE/DOTAP2:1-CNT | 100.70 | 0.237 | −2.45 | 221.73 | 712.91 |
| Membrane | Surface Elemental Composition | |||||
|---|---|---|---|---|---|---|
| C (%) | N (%) | O (%) | P (%) | RO/N | D (%) | |
| TFN4:1 | 76.35 | 10.63 | 12.59 | 0.43 | 1.18 | 74.7 |
| TFN4:1-CNT | 74.22 | 11.73 | 13.66 | 0.38 | 1.16 | 77.2 |
| TFN2:1 | 71.84 | 11.82 | 15.95 | 0.39 | 1.35 | 55.4 |
| TFN2:1-CNT | 74.81 | 10.74 | 16.3 | 0.15 | 1.33 | 57.3 |
| Membrane | Roughness Parameters | ||
|---|---|---|---|
| Ra (nm) | Rq (nm) | Rmax (nm) | |
| TFC | 87.4 | 111 | 812 |
| TFN4:1-0.6 | 143 | 177 | 1129 |
| TFN2:1-0.6 | 145 | 187 | 1307 |
| TFN4:1-CNT-0.6 | 151 | 191 | 1457 |
| TFN2:1-CNT-0.6 | 158 | 211 | 1724 |
| CNT Nanofiller | Water Flux (L/m2·h) | Salt Rejection | References |
|---|---|---|---|
| CNT-COOH/PA | 26→71 | NaCl: 95%→82% | [38] |
| CNT-Zwitterion/PA | 11→48 | NaCl: 97%→98% | [9] |
| MWCNT-COOH/PA | 14→28 | NaCl: 95%→90% | [39] |
| MWCNT/PA | 27→71 | NaCl: 97%→90% | [12] |
| CNT-Zwitterionic/PA | 14→34 | NaCl: 98%→98% | [40] |
| MWCNT-COOH/PA | 20→28 | NaCl: 97%→97% | [41] |
| MWCNT-TNT/PA | 7→17 | NaCl: 98%→96% | [42] |
| CNT/PA | 36→42 | NaCl: 99%→97% | [43] |
| DOPE/DOTAP2:1-CNT | 25.2→63.6 | NaCl: 98.6%→98.6% | This work |
| Membrane | Rt (×1013 m−1) | Rm (×1013 m−1) | Rr (×1012 m−1) | Rir (×1012 m−1) |
|---|---|---|---|---|
| TFC | 3.52 | 2.56 | 6.07 | 9.60 |
| TFN4:1-0.6 | 1.46 | 1.25 | 1.24 | 2.09 |
| TFN2:1-0.6 | 1.39 | 1.22 | 1.30 | 1.74 |
| TFN4:1-CNT-0.6 | 1.12 | 1.01 | 6.46 | 1.10 |
| TFN2:1-CNT-0.6 | 1.19 | 1.08 | 6.94 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Sun, J.; Zhang, K.; Wang, S.; Ding, W.; Li, Z. Effect of Positively Charged Lipids (DOTAP) on the Insertion of Carbon Nanotubes into Liposomes and the Separation Performance of Thin-Film Nanocomposite Membranes. Separations 2024, 11, 75. https://doi.org/10.3390/separations11030075
Zhao J, Sun J, Zhang K, Wang S, Ding W, Li Z. Effect of Positively Charged Lipids (DOTAP) on the Insertion of Carbon Nanotubes into Liposomes and the Separation Performance of Thin-Film Nanocomposite Membranes. Separations. 2024; 11(3):75. https://doi.org/10.3390/separations11030075
Chicago/Turabian StyleZhao, Jianjun, Junqing Sun, Kefeng Zhang, Shan Wang, Wande Ding, and Zhengping Li. 2024. "Effect of Positively Charged Lipids (DOTAP) on the Insertion of Carbon Nanotubes into Liposomes and the Separation Performance of Thin-Film Nanocomposite Membranes" Separations 11, no. 3: 75. https://doi.org/10.3390/separations11030075
APA StyleZhao, J., Sun, J., Zhang, K., Wang, S., Ding, W., & Li, Z. (2024). Effect of Positively Charged Lipids (DOTAP) on the Insertion of Carbon Nanotubes into Liposomes and the Separation Performance of Thin-Film Nanocomposite Membranes. Separations, 11(3), 75. https://doi.org/10.3390/separations11030075






