Abstract
Hawthorn is a type of natural food with significant medicinal and nutritional properties; it has been listed in the “Both Food and Drug” list by the Chinese Ministry of Health Item List since 1997. However, hawthorn varieties have complex origins, and there are significant differences in the content, type, and medicinal efficacy of the chemically active ingredients in different varieties of hawthorn. This leads to the phenomenon of mixed varieties and substandard products being passed off as high-quality. In this work, by using headspace gas chromatography–ion mobility spectrometry (HS-GC-IMS), we identified and analyzed volatile organic compounds (VOCs) in four varieties of hawthorn, establishing their characteristic fingerprints. As a result, a total of 153 peaks were detected, and 139 VOCs were also identified. As shown by the fingerprint profiles, the different hawthorn samples contained different VOCs. Meanwhile, by using principal component analysis (PCA), Euclidean distance, and partial least-squares discriminant analysis (PLS-DA), the relationship between the VOCs found in the different varieties of hawthorn was revealed. This study developed a simple, fast, accurate, and sensitive method for identifying, tracking, and evaluating hawthorn varieties.
1. Introduction
Hawthorn is the dried ripe form of Crataegus pinnatifida Bge. var major N.E. Br., or Crataegus pinnatifida Bge. [1], and it is known as a “nutritious fruit” due to its richness in bioactive substances [2]. Hawthorn is not only a food product but also a medicinal plant that has many active ingredients and health benefits. In 1997, the Chinese Ministry of Health included it on its “Both Food and Drug” list, which encompasses drugs, dietary supplements, and foods. Hawthorn mainly contains flavonoids, organic acids, proanthocyanidins, triterpenes, pectin, vitamins, minerals, and other chemical components [3,4]. It has anti-atherosclerosis effects, can lower blood lipids and lower blood pressure, has antioxidant effects, improves liver damage, and has other pharmacological effects [5,6,7]. Due to its unique flavor and health benefits, hawthorn has become widely used in health foods, snacks, additives, and teas [8,9,10,11,12], such as hawthorn preserves, hawthorn pectin, hawthorn seed oil, hawthorn functional drinks, hawthorn plum, etc.
The variety of sources for hawthorns are varied and complex. In addition to Shanlihong (Crataegus pinnatifida Bge. Var. major N. E. Br.) and Shanzha (Crataegus pinnatifida Bge.), recorded in the 2020 edition of the “Chinese Pharmacopoeia”, the most commonly used edible hawthorn varieties in the domestic market include “wild hawthorn” (Crataegus cuneata Sieb. et Zicc), Malus doumeri (Bois.) Chev. Shanlihong, and Shanzha, which are mostly grown in the north; therefore, they are commonly called “Northern Hawthorn”, whereas wild hawthorn varieties mostly grow in the south; therefore, they are commonly known as “Nanshanzha”. Malus doumeri (Bois.) Chev mostly grows in Guangdong, Guangxi, and other places, and it is commonly known as “Guangshanzha” [13]. These four varieties differ greatly in terms of source varieties, chemical composition, and pharmacological effects due to a series of factors, e.g., their origin, harvest period, variety, growth environment, soil, fertilizer, harvest, processing, and storage. Nanshanzha promotes qi, disperses blood stasis, astringes, and stops diarrhea; Guangshanzha regulates qi, strengthens the spleen, and digests stagnant food. The content and types of organic acid components in Northern Hawthorn, Nanshanzha, and Guangshanzha are very different. Using Nanshanzha and Guangshanzha instead of Northern Hawthorn will have a greater impact on research results and efficacy. The quality of the different varieties of Hawthorn is irregular, and so the market prices vary. This has led to common phenomena such as mixing production areas and passing off substandard products, resulting in greatly reduced efficacy and possibly even drug use and food safety accidents. However, in the 2020 edition, the Chinese Pharmacopoeia only uses acid–base titrations and indicators to evaluate the quality of hawthorn.
This method is not suitable for the qualitative and quantitative analysis of organic acid components, and it cannot reflect the overall quality of the medicinal materials and thus cannot be used for a reasonable evaluation; this makes it impossible to carry out a reasonable quality evaluation and control. Some researchers have also used empirical identification, microscopic identification, high-performance liquid chromatography (HPLC), molecular identification (such as DNA barcoding technology), and other techniques to identify hawthorn [14,15]. Although these methods provide more choices for identification, these approaches have their limitations. For example, an empirical identification is subjective, microscopic identification is not specific, HPLC identification is time-consuming and cumbersome, and molecular identification is relatively complex. Therefore, it is important to find a fast, easy, and green way to differentiate different varieties of hawthorn.
In addition to identifying food, volatile organic compounds (VOCs) also play an important role in determining a food’s nutritional and sensory properties. At present, analyses of VOCs in food comprise two parts: sensory analysis and instrumental analysis. The sensory analysis of results is a kind of subjective sensory perception that analyzes the results. An instrumental analysis is a molecular analysis that is objective. However, the traditional odor and taste identification methods, which are highly subjective and empirical, can no longer match modern development. Moreover, most flavor substances and volatile odor substances are in the ppb range in food. Therefore, the detection of differentiation needs to be more sensitive and reliable. In the past, more and more researchers have used instrumental analysis techniques to detect VOCs in food, including gas chromatography–mass spectrometry (GC-MS), chromatography–olfactometry–mass spectrometry (GC-O-MS), headspace gas chromatography–ion mobility spectrometry (HS-GC-IMS), and electronic noses (E-noses) [16]. These methods can complement sensory evaluations by providing more objective information about the substance being tested at the molecular level. There are a number of gas chromatography and mass spectrometry methods available, but GC-MS has significant limitations in distinguishing isomeric molecules, especially cyclic isomers, due to its complex sample processing methods and high mass spectrometry resolution [17]. Aroma and flavor analysis can also be performed using GC-O-MS, but it shares the same disadvantages as GC-MS [18]. An E-nose is a new type of aroma detection technology that provides rapid detection, but it also suffers from low accuracy, sensor drift, and poor repeatability, as well as a high sensitivity to the surrounding environment [19].
A recent development in HS-GC-IMS technology incorporates the advantages of both GC and IMS, combining high separation capabilities with high resolution and high sensitivity. It is equipped with a static headspace sampling device to detect trace organic components emitted from liquid or solid samples. As an emerging technology, this method is simple, fast, non-destructive, there is no need for sample pre-processing, and it has good reproducibility. It is used in the identification of foods, medicinal varieties [20,21], and food aroma analysis [22]. The impact of different drying methods and temperatures on food [23,24], component analyses of food during different harvest periods and storage periods [25,26], and other aspects have played an important role in this method. There have been very few studies conducted on HS-GC-IMS for the identification of hawthorn, and this difference is often one of the key factors contributing to the differences in quality between traditional Chinese medicines. The aim of this study was to identify and analyze the VOCs of different varieties of hawthorn using HS-GC-IMS, principal component analysis (PCA), Euclidean distance, and partial least-squares discriminant analysis (PLS-DA) methods. The characteristic VOCs in the different varieties of hawthorn were displayed in a visual form, providing technical support for the rapid analysis of VOCs and the identification of different varieties of hawthorn. Simultaneously, this study enriched the study of flavor compounds of hawthorn.
2. Materials and Methods
2.1. Medicinal Materials
Four kinds of hawthorn powder were purchased from the National Institute for Food and Drug Control, Beijing, China: Shanlihong (the dried ripe fruit of Crataegus pinnatifida Bge. Var. major, No. 121138-201206, named SZ-01), Shanzha (the dried ripe fruit of Crataegus pinnatifida Bge., No. 121626-201803, named SZ-02), Guangshanzha (the dried ripe fruit of Cantonese Crataegus, No. 120943-201903, named GSZ), and Nanshanzha (the dried ripe fruit of South Crataegus, No. 121055-201704, named NSZ).
2.2. Sample Preparation
For each kind of hawthorn powder, 1 g was accurately weighed and placed into a 20 mL headspace bottle and incubated at 80 °C for 20 min, and then the samples were injected. Each sample was measured in three parallel groups.
2.3. Headspace Sampling Conditions
The temperature of incubation was 80 °C. The incubation time was 20 min. The injection volume was 500 μL. The splitless injection method was used. The incubation speed was 500 rotations per min, and the injection needle temperature was 85 °C.
2.4. Chromatographic Conditions
In this study, we used a FlavourSpec® gas-phase ion mobility spectrometer (G.A.S, Dortmund, Germany), a CTC-PAL 3 static headspace automatic sampling device (CTC Analytics AG, Switzerland), and a 20 mL headspace bottle (Shandong Haineng Scientific Instrument Co., Ltd., Shandong, China); an MXT-WAX capillary chromatography column (15 m × 0.53 mm × 1 μm, Restek Company of the United States, Bellefonte, PA, USA) was also used. The temperature was 60 °C. The carrier gas was high-purity N2 (purity ≥99.999%). The initial flow rate was 2.00 mL/min, which was maintained for 2 min. Then, it was linearly increased to 10.00 mL/min and then 100.00 mL/min within 10 min, and this was maintained for 40 min. The running time for the chromatography was 60 min, and the injection port temperature was 80 °C.
2.5. IMS Conditions
A tritium source (3H) was used; the flow rate was 75 mL/min; the power of the electric field was 500 V/cm; the voltage of the drift gas was 99.999%; the length of the migration tube was 53 mm; the electric field strength was 500 V/cm; the temperature of the migration tube was 45 °C; and the drift gas was high-purity nitrogen (purity = 99.999%).
2.6. Data Analysis
In order to characterize the VOCs in the samples, the GAS software (G.A.S, Dortmund, Germany, version 2.0.0), the built-in NIST retention index database, and the IMS drift time database were used. To compare VOCs between the samples, VOCal data processing software plug-ins, such as Reporter (Version 11.x), Gallery Plot (Version 1.1.0.2), and Dynamic PCA (version 0.0.3), were used to generate three-dimensional (3D) spectrums, two-dimensional (2D) spectrums, difference spectrums, fingerprints, and PCA maps, respectively. The PLS-DA VIPs were calculated using the SIMCA software (version 14.0) (Umea, Sweden).
3. Results and Discussion
3.1. GC-IMS Analysis of VOCs in Different Samples
We compared the spectral differences between the samples and the characteristic VOCs of the Shanlihong, Shanzha, Guangshanzha, and Nanshanzha samples using the VOCal software’s Reporter plug-in. The X axis represents the ion migration time, the Y axis represents the retention time of the gas chromatograph, and the Z axis represents the peak intensity used for quantification. A comparison of the VOC contents of the Shanlihong, Shanzha, Guangshanzha, and Nanshanzha varieties is shown in Figure 1. In Figure 2, a top view is used for comparison due to the inconvenience of observation. After normalizing the data, the red vertical line at 1.0 represents the reactive ion peak (RIP peak). The gas chromatography retention time is represented by the ordinate. The dots on either side of the RIP represent the VOCs. The color represents the concentration of the substance; white represents a lower concentration, and red represents a higher concentration. In general, the darker the color, the greater the concentration. The samples from the Shanlihong, Shanzha, Guangshanzha, and Nanshanzha varieties differed in terms of their VOCs, as shown in Figure 2.
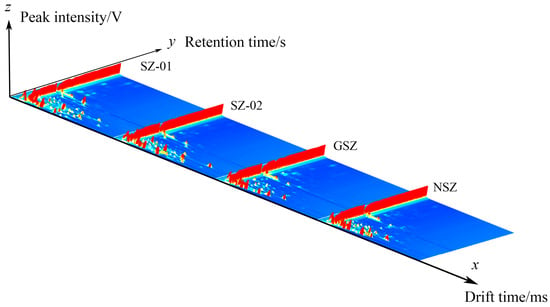
Figure 1.
Three-dimensional spectra of hawthorn VOCs.
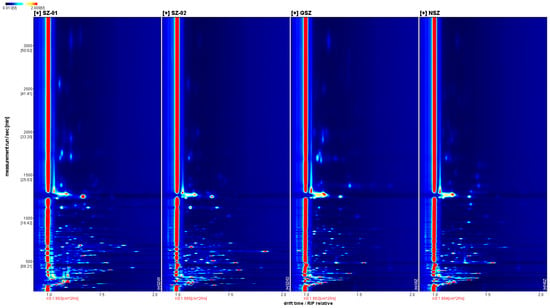
Figure 2.
Two-dimensional spectra of hawthorn VOCs.
Using the spectrum of the Shanlihong (SZ-01) variety as a reference, we can visually compare the differences in the VOCs. From the spectra of the Shanzha, Guangshanzha, and Nanshanzha samples, we were able to determine their VOCs. The differences in organic matter are shown in Figure 3. In the figure, when the measured components of Shanlihong, Shanzha, Guangshanzha, and Nanshanzha were consistent, they are shown in white after deduction. Red and blue indicate that the concentrations of the measured components in the Shanzha, Guangshanzha, and Nanshanzha varieties were higher and lower than in the Shanlihong variety, respectively. With the difference comparison chart, it is easier to identify the differences between the four samples.
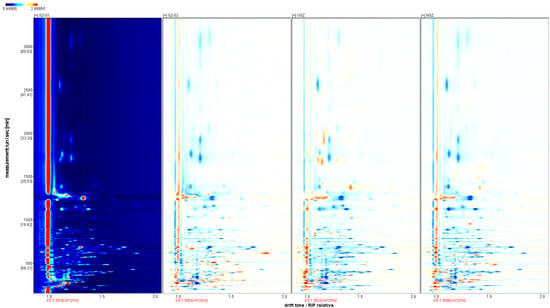
Figure 3.
A comparison chart of the differences between varieties of hawthorn.
3.2. Comparison of the Fingerprints of VOCs
To compare the specific VOCs from the hawthorn samples, the Gallery Plot plug-in of VOCal was used to select the peaks for fingerprint comparison (Figure 4). Each row in the figure represents a sample; the rightmost side is the name of the sample, each column is a compound, and the bottom is the qualitative result of the samples. Some substances are followed by M and D, which indicate monomers and dimers of the same substance, respectively. The color indicates the concentration of the compounds, with darker colors indicating a higher concentration of the compounds, and black colors indicating a very low or undetectable concentration.

Figure 4.
Fingerprints of VOCs of different varieties of hawthorn.
A comparison of the VOCs of the different hawthorn samples is shown in Figure 4. The contents of carvone, 6-methyl-5-hepten-2-one, 5-methyl-2(3H)-furanone, 2-methyltetrahydrofuran-3-one, 2-octanone, cyclopentanone, 1-penten-3-one, 3-methyl-2-pentanone, 2-pentanone, neryl acetate, diethyl succinate, methyl octanoate, ethyl enanthate, phytyl acetate, methyl caproate, isopentyl acetate, methyl 3-methylbutyrate, methyl acetate, isobutyl isobutyrate, ethyl isobutyrate, 2-furanmethanol, α-terpineol, 4-terpineol, linalool, linalool oxide, tetrahydrolinalool, 1-octen-3-ol, 1-Penten-3-ol, 3-furanmethanol, 2-methyl-3-furanthiol, 5-methyl-2-furfural, furfural, Z-4-heptenal, E-2-hexenal, E-2-pentenal, E-2-butenal, acetal, butyraldehyde, 2-acetylfuran, 2-ethyl-3-methylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 2,6-dimethylpyridine, γ-terpinene, 1,8-cineole, 1,4-cineole, and 46 other substances were high in SZ-01.
The contents of 1-octanol, Z-2-penten-1-ol, 2-butanol, 1-propanethiol, 2-cyclohexen-1-one, 4-methyl-3-pentene-2-one, acetone, isoamyl propionate, isobutyl acetate, methyl isobutyrate, hexyl acetate, β-pinene, Z-2-pentenal, 3-methyl-2-butene aldehyde, nonanal, octanal, 2-methylbutyraldehyde, 2-methylpropionaldehyde, methylthiopropionaldehyde, diethyl disulfide, diallyl sulfide, dimethyl sulfide, 2,5-dimethylfuran, o-xylene, and 24 other substances were high in SZ-02.
The contents of geranyl acetate, γ-butyrolactone, acetophenone, 1-hydroxy-2-propanone, acetoin, butyric acid, 2-methyl propionic acid, propionic acid, acetic acid, E-2-octenal, E-2-heptenal, 2,4-hexadienal, 2-propenal, heptanal, valeraldehyde, propionaldehyde, benzaldehyde, ethanol, 2-ethyl-1-hexanol, 3-ethylpyridine, styrene, myrcene, and 22 other substances were high in GSZ.
The contents of E-2-nonenal, hexanal, 3-methyl butyraldehyde, salicylaldehyde, citronellal, α-thujone, 2-heptanone, 2-butanone, 1-hexanol, 1-pentanol, 3-methyl-1-butanol, 1-butanol alcohol, 2-methyl-1-propanol, 1-propanol, 2-methyl-2-propanol, 2-pentylfuran, ethyl acetate, linalyl acetate, 3-carene, (E, E), α-farnesene, p-xylene, thiophene, pyridine, and 23 other substances were high in NSZ.
3.3. Identifying VOCs in Different Samples
Our qualitative analysis was conducted using the NIST and IMS databases built into the VOCal software (Version 0.4.03, GAS Deutschland, Dortmund, Germany), as well as the Reporter, Gallery Plot, and Dynamic PCA plugins. Ultimately, we detected 153 peaks and identified 139 VOCs (Table 1 and Table 2) from all the samples, including 31 aldehydes, 29 alcohols, 20 ketones, 20 esters, 16 terpenes, 5 acids, 4 furans, 3 pyridines, 3 pyrazines, 1 thiophene, and 7 other compounds.

Table 1.
Results of the VOC analysis of different varieties of hawthorn.

Table 2.
The average area of VOCs in different varieties of hawthorn.
3.4. PCA of the Four Hawthorn Samples
The PCA method is a multivariate statistical analysis method that reduces the dimensions of a dataset by linearly combining variables and retaining the variability of the original data [27], in which the relationship between samples is more clearly and intuitively compared [28]. An analysis of PCA plots shows that the greater the similarity between samples, the greater the clustering degree, while the greater the difference between samples, the greater the distance [29]. As shown in Figure 5, the PCA of the VOCs in the four varieties of hawthorn was performed. Each color represents a different hawthorn sample, and the distance between each point indicates how similar they were.
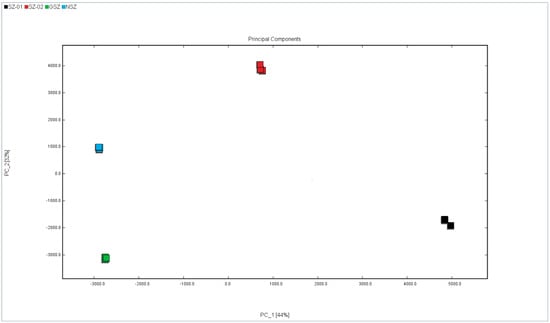
Figure 5.
PCA analysis of different hawthorn varieties.
We can obviously see that the differentiation of the four samples and the distance between the Nanshanzha and Guangshanzha varieties was relatively close, which was because there were similarities in the VOC parts of the two samples, which was basically consistent with the signal peak information of the samples in the fingerprint spectrum. These results indicate that PCA combined with HS-GC-IMS could clearly distinguish differences in the VOCs among the four varieties of hawthorn.
3.5. Fingerprint Similarity Analysis Using Euclidean Distance
The distance coefficient is used to determine similarity in terms of the Euclidean distance, as is carried out in a PCA. This means that the difference is small if the distance between the samples is close, and this means that the difference is obvious if the distance is far [30]. Based on the Euclidean distance analysis, we created a fingerprint map with more intuitive characteristics than the PCA analysis. Based on the Euclidean distances between the four hawthorn varieties, Figure 6 depicts the fingerprint similarity. Thus, the Shanlihong and Nanshanzha varieties were close to each other, as were the Nanshanzha and Guangshanzha varieties, but the difference in VOCs between the Shanlihong and Nanshanzha varieties was of most significance. The results were in good agreement with the PCA analysis results. This may be because the Shanlihong and Shanzha varieties (the two are commonly known as “Northern Hawthorn”) mostly grow in north China, while the Nanshanzha and Guangshanzha varieties mostly grow in south China, such as in Guangdong and Guangxi. The north and south of China have very different climates, soils, altitudes, and so on, which causes the VOC levels to differ between varieties of hawthorn.
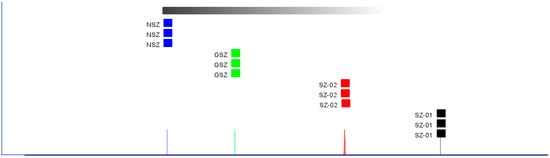
Figure 6.
Fingerprint similarity based on the Euclidean distance of different varieties of hawthorn.
3.6. PLS-DA Analysis of the Four Hawthorn Samples
PLS-DA is a discriminant-analysis-supervised model that identifies hidden feature variables that damage model robustness and highlight the differences between groups [31]. Similarities and differences between samples are directly reflected in the PLS-DA score plot. In the score plot, a farther distance between two locations indicates a greater difference between two samples. The PLS-DA score plots of the four hawthorn samples were used to explore the differences in the VOC levels between the Shanlihong, Shanzha, Guangshanzha, and Nanshanzha varieties. The results are shown in Figure 7. It is evident that the samples of the different varieties of hawthorn were clearly distinguished based on the results of the principal component analysis. The SIMCA software (version 14.0) revealed that R2X = 0.993 and Q2 = 0.999, indicating that the model had a relatively accurate generalization and prediction ability and could both distinguish between the different varieties and identify their differences. Figure 8 shows the validation of the model carried out by the PLS-DA analysis after the permutation test (n = 200 times), with R2 = 0.0309 and Q2 = −0.605. It is evident from the slopes of the two regression lines that the model has a good prediction ability and does not show overfitting.
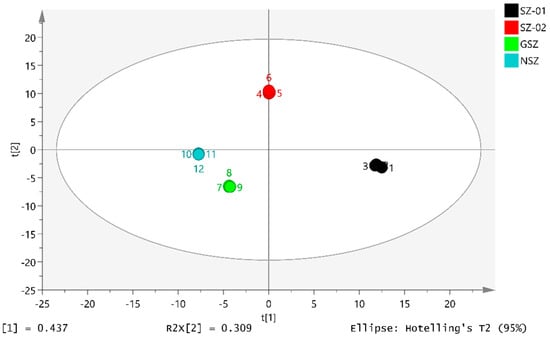
Figure 7.
PLS-DA score chart.
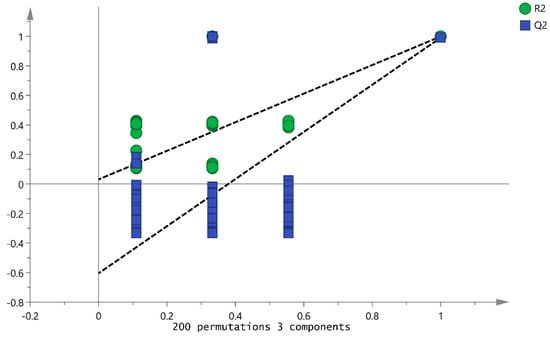
Figure 8.
PLS-DA fitting curve.
It can be seen from the PLS-DA score chart that the supervised analysis method could better distinguish the four varieties of hawthorn and could obtain the variable weight value (variable important in projection: VIP). The results are shown in Figure 9. The VIP value is usually used to reflect the importance of PLS-DA model variables. The higher the column height and contribution value to the model in the figure, the more significant the difference [32]. The results show that there were 70 types of VOC with VIP >1 (marked in red boxes). Among them, the VOCs with the highest VIP values were 2-heptanone-M, alpha-thujone-M, (E)-2-pentenal-M, citronellal, 2-heptanone-D, and 2-cyclohexen-1-one. The abovementioned VOCs can be used as important indicators for the classification and identification of hawthorn, and they can provide a reference for the rapid identification of the four different types of hawthorn.
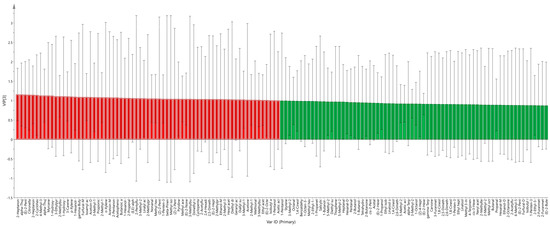
Figure 9.
VIP diagram of the PLS-DA model.
4. Conclusions
This study used HS-GC-IMS for detecting and analyzing VOCs in hawthorn varieties. A total of 153 peaks were detected, and 139 VOCs were identified, including aldehydes, esters, alcohols, ketones, terpenes, acids, furans, pyridines, pyrazines, and thiophenes. By using the fingerprints, PCA, Euclidean distance, and PLS-DA, we were able to quickly and effectively distinguish the four hawthorns. According to the results, the four hawthorns could be distinguished accurately and objectively without using appearance features. This study used HS-GC-IMS to analyze the VOCs in four varieties of hawthorn, which is a simple, rapid, and accurate method. In addition, this approach requires fewer sample preparation steps and less analysis time than other analytical methods. As a result, the detection and comparison methods applied in this study provide a valuable reference tool for identifying hawthorns from food VOCs. Simultaneously, this research enriched the study of flavor compounds in hawthorn.
However, there were still some shortcomings in this study, such as the limited sample size of the test, the fact that established model may not be the optimal model, the fact that the ion migration spectrum database is not perfect, and the fact that the database of various commercial instruments is not universal, resulting in the identification of some ions. Next, we will carry out a study with a larger sample size and establish a comprehensive and detailed database of GC-IMS, which can also be combined with mass spectrometry to further improve the qualitative ability.
Author Contributions
Conceptualization, L.Z. (Lijun Zhu); methodology, L.Z. (Lijun Zhu) and F.O.; software, Y.M. and L.Z. (Lingfeng Zhu); validation, Y.X. and B.W.; formal analysis, L.Z. (Lijun Zhu) and F.O.; investigation, Y.X.; resources, B.W.; data curation, C.L.; writing—original draft preparation, L.Z. (Lijun Zhu); writing—review and editing, C.L.; visualization, Y.M. and Q.Z.; supervision, Q.Z.; project administration, Y.M. and C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yunnan Key Laboratory of Screening and Research on Anti-pathogenic Plant Resources from Western Yunnan (No. APR202302), the Hunan Agricultural Science and Technology Innovation Fund Project (No. 2023CX30), the Hunan Provincial Natural Science Foundation of China (No. 2021JJ40406), the Undergraduate Research and Innovation Fund Project of Hunan University of Chinese Medicine (No. 2023), and the domestic First-Class Construction Discipline of Chinese Medicine in Hunan University of Chinese Medicine (No. 2024).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Lou, X.; Yuan, B.; Wang, L.; Xu, H.; Hanna, M.; Yuan, L. Evaluation of Physicochemical Characteristics, Nutritional Composition and Antioxidant Capacity of Chinese Organic Hawthorn Berry (Crataegus pinnatifida). Int. J. Food Sci. Technol. 2020, 55, 1679–1688. [Google Scholar] [CrossRef]
- Duan, L.; Xiong, H.; Du, Y.; Wang, Z.; Li, Y.; Zhao, S.; Chen, J.; Si, D.; Pan, H. High-throughput LC-MS method for the rapid characterisation and comparative analysis of multiple ingredients of four hawthorn leaf extracts. Phytochem. Anal. 2022, 33, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, P.; Sun, J.; Zhao, C.; Song, Y.; Wu, J. Research progress on chemical constituents and pharmacological action of hawthorn. J. Northwest Pharm. 2021, 3, 521–523. [Google Scholar]
- Wang, S.Z.; Wu, M.; Chen, K.J.; Liu, Y.; Sun, J.; Sun, Z.; Ma, H.; Liu, L.T. Hawthorn Extract Alleviates Atherosclerosis through Regulating Inflammation and Apoptosis Related Factors: An Experimental Study. Chin. J. Integr. Med. 2019, 25, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gao, S.; Zhu, T.; Sun, G.; Zhang, P.; Huang, Y.; Qu, S.; Du, X.; Mou, D. Hawthorn fruit acid consumption attenuates hyperlipidemia-associated oxidative damage in rats. Front. Nutr. 2022, 9, 936229. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jang, E.; Lee, J.H. Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases. Nutrients 2022, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, S.; Huang, X.; Zhang, X.; Cui, Y.; Zhang, Z.; Ma, Y.; Zhang, X.; Yu, Q.; Yang, S. Biological Properties and Potential Application of Hawthorn and Its Major Functional Components: A Review. J. Funct. Foods 2022, 90, 104988. [Google Scholar] [CrossRef]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An Updated Overview on Its Beneficial Properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Liu, J.; Chitrakar, B.; Wang, B.; Wang, Y. Hawthorn pectin: Extraction, function and utilization. Curr. Res. Food Sci. 2021, 4, 429–435. [Google Scholar] [CrossRef]
- Kwek, E.; Yan, C.; Ding, H.; Hao, W.; He, Z.; Liu, J.; Ma, K.Y.; Zhu, H.; Chen, Z.Y. Effects of hawthorn seed oil on plasma cholesterol and gut microbiota. Nutr. Metab. (Lond.) 2022, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Research Progress on Character Identification and Organic Acid Compositions of Shanzha (Crataegus pinnatifida), Nanshanzha (South Crataegus) and Guangshanzha (Cantonese Crataegus). J. Liaoning Univ. Chin. Med. 2023, 25, 132–137. [Google Scholar] [CrossRef]
- Fei, C.; Dai, H.; Wu, X.; Li, L.; Lu, T.; Li, W.; Cai, B.; Yin, W.; Yin, F. Quality evaluation of raw and processed Crataegi Fructus by color measurement and fingerprint analysis. J. Sep. Sci. 2018, 2, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhong, L.; Huang, R.; Yue, J.; Li, L.; Nie, L.; Wu, A.; Huang, S.; Yang, C.; Cao, G.; et al. Identification and determination of different processed products and their extracts of Crataegi Fructus by infrared spectroscopy combined with two-dimensional correlation analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 310, 123922. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.; Lin, R.; Li, X.; Ding, H.; Han, L.; Yang, W.; Song, X.; Li, W.; Qu, H. Application and development trends of gas chromatography-ion mobility spectrometry for traditional Chinese medicine, clinical, food and environmental analysis. Microchem. J. 2021, 168, 106527. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; Zheng, X.; Chen, Z.H.; Zhang, Y.Y.; Du, M.; Dong, X.P.; Qin, L.; Zhu, B.W. Fresh and grilled eel volatile fingerprinting by e-Nose, GC-O, GC-MS and GC×GC-QTOF combined with purge and trap and solvent-assisted flavor evaporation. Food Res. Int. 2019, 115, 32–43. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, D.; Zhou, Y.; Duan, H.; He, Y.; Jiang, Y.; Yan, W. Characteristic Volatile Organic Compound Analysis of Different Cistanches Based on HS-GC-IMS. Molecules 2022, 27, 6789. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, S.; Yang, L.; He, Y.; Guo, X.; Ma, Y.; Li, S.; Huang, D. Explorative study for the rapid detection of Fritillaria using gas chromatography-ion mobility spectrometry. Front. Nutr. 2024, 11, 1361668. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; He, Y.; Ji, X.J.; Tang, H.; Dong, Y.M.; Yan, W.J. HS-GC-IMS detection of volatile organic compounds in Acacia honey powders under vacuum belt drying at different temperatures. Food Sci. Nutr. 2021, 9, 4085–4093. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Jin, W.; Sun, S.; Hu, K.; Li, J. Effects of Drying Methods on the Physicochemical Aspects and Volatile Compounds of Lyophyllum decastes. Foods 2022, 11, 3249. [Google Scholar] [CrossRef]
- Duan, H.; Zhou, S.; Guo, J.; Yan, W. HS-GC-IMS Analysis of Volatile Organic Compounds in Different Varieties and Harvesting Times of Rhizoma gastrodiae (Tian Ma) in Yunnan Province. Molecules 2023, 28, 6705. [Google Scholar] [CrossRef]
- Zhang, Y.; Tong, X.; Chen, B.; Wu, S.; Wang, X.; Zheng, Q.; Jiang, F.; Qiao, Y. Novel application of HS-GC-IMS for characteristic fingerprints and flavor compound variations in citrus reticulatae pericarpium during storage with different Aspergillus niger fermentation. Food Chem. X 2023, 18, 100653. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of Fatty Acid, Amino Acid and Volatile Compound Compositions and Bioactive Components of Seven Coffee (Coffea robusta) Cultivars Grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef] [PubMed]
- Christmann, J.; Rohn, S.; Weller, P. gc-ims-tools—A new Python package for chemometric analysis of GC–IMS data. Food Chem. 2022, 394, 133476. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Duan, H.; Zhou, S.; Guo, J.H.; Yan, W.J. Detection and analysis of volatile flavor compounds in different varieties and origins of goji berries using HS-GC-IMS. LWT 2023, 187, 115322. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Feng, D.; Zhou, Y.X.; Zhao, J.; Zhao, J.Y.; Guo, Y.; Yan, W.J. HS-GC-IMS detection of volatile organic compounds in cistanche powders under different treatment methods. LWT—Food Sci. Technol. 2022, 165, 113730. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; Martín-Torres, S.; Ortega-Gavilán, F.; Camacho, J. PLS-DA vs sparse PLS-DA in food traceability. A case study: Authentication of avocado samples. Talanta 2021, 224, 121904. [Google Scholar] [CrossRef]
- Li, H.; Mao, Y.; Liu, Y.; Li, D.; Wang, M.; Ren, X.; Dou, Z. Comparative investigation of raw and processed products of Gardeniae Fructus and Gardenia jasminoides var. radicans using HPLC coupled with chemometric methods. Biomed. Chromatogr. 2021, 35, e5051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).