Abstract
Protein purification is a crucial step for various downstream applications like drug development, antibody preparation, and structure determination. The constant pursuit is for methods that are more efficient and cost-effective. We propose a novel approach using an elastin-like polypeptide (ELP) as an aggregation core that serves as an anchor between the beads in a chromatography column. In this method, a chilled sample containing a [target protein type] fusion protein is loaded onto a pre-equilibrated IMAC (immobilized metal affinity chromatography) column with a low-salt buffer. The column is then washed with a warm buffer containing high salt to remove impurities. Here, the key step involves warming the column above the ELP’s transition temperature (Tt), which triggers its aggregation. This aggregation is expected to trap the target protein tightly between the beads. Subsequently, a harsh wash with high salt and high imidazole can be applied to remove even persistent contaminants, achieving high protein purity. Finally, the temperature is lowered, and a cold, low-salt buffer is introduced to reverse the aggregation and elute the purified target protein. This method has the potential to eliminate the need for sophisticated chromatography systems while still achieving high protein purity.
1. Introduction
Protein purification is crucial for various applications, particularly structure-based drug discovery, where highly purified proteins are essential [1,2]. Recombinant protein production has gained momentum due to advancements in molecular biology techniques like PCR, enabling mass production and the purification of proteins of interest [3,4]. These recombinant proteins find diverse applications in vaccines, therapeutics, and diagnostics [4]. Escherichia coli remains the primary choice for recombinant protein production due to its rapid growth, cost-effective culture, and high protein production capacity [5,6,7,8]. Gene introduction into E. coli via plasmids allows for controlled protein expression upon induction [4]. Following expression, protein purification methods like precipitation or chromatography are employed depending on the protein’s characteristics [2]. Affinity chromatography is the preferred method for the efficient purification of recombinant proteins [9].
Several strategies have been developed to expedite and enhance recombinant protein purification including fusion proteins incorporating tags like glutathione S-transferase (GST), maltose binding protein (MBP), or polyhistidine facilitate protein production and purification [10,11,12]. Alternatively, signal peptides can be used for periplasmic secretion [13,14], and aggregation tags can be employed to induce protein precipitation during purification [15]. The affinity purification of GST or MBP fusion proteins utilizes resins containing glutathione or maltose, while polyhistidine tags are purified using resins with immobilized metal ions. Periplasmic secretion simplifies purification due to the lower abundance of contaminating proteins compared to the cytoplasm [13]. Precipitation tag fusion proteins can be purified by manipulating factors like temperature or salt concentration [15]. The elastin-like polypeptide (ELP) employs a method called inverse temperature cycling (ITC), which necessitates centrifugation at elevated temperatures, posing limitations for low-temperature compatible centrifuges [16].
This study presents a novel method termed ‘aggregation-assisted chromatography’ for the high-purity purification of a fusion protein harboring both a precipitation tag and a polyhistidine tag. The method utilizes an immobilized metal affinity chromatography (IMAC) column with temperature and salt adjustments to achieve high purity. Notably, this technique leverages buffer temperature control to adjust column temperature, enabling high-purity manual purification without the need for expensive chromatography equipment.
2. Materials and Methods
2.1. Plasmid Construction
The starting plasmid was pVP65KR-SacB(-)I48 [17,18]. The nine proteins we tested were MBP, S-peptide, MazE, MazF, CcdB, SH3BGRL1, GLRX3, GLRX4, and I48ELP (Table 1). The corresponding gene fragments were either synthesized (S-peptide, MazE, MazF, CcdB, SH3BGRL1, GLRX3, GLRX4) or amplified from templates (MBP). All fragments were equipped with AsiSI and PmeI restriction sites at 5′- and 3′-terminus, respectively. The vector pVP65KR-MBP-I48 and each fragment were digested with AsiSI and PmeI, purified, and ligated together. The correctly inserted plasmids were screened and selected. We also produced I48 alone from I48 ELP plasmid [19].

Table 1.
Summary of proteins tested in this report. Values were predicted by ProtParam in Expasy server (https://www.expasy.org, accessed on 16 May 2024).
2.2. Protein Production
The Rosetta2(DE3)pLysS cells harboring the plasmid with the target gene were inoculated to 500 mL of LB medium from a frozen stock and grown at 37 °C. The culture was induced with IPTG to the final concentration of 0.1 mM when it reached OD600 of 0.8. The culture was further grown at 14 °C and harvested 24 h later. The harvested cells were resuspended in 20 mL of 10 mM PBS buffer (10 mM sodium phosphate pH 7.4 with 150 mM NaCl) and stored at −80 °C.
2.3. Protein Purification by Aggregation-Dispersion Chromatography
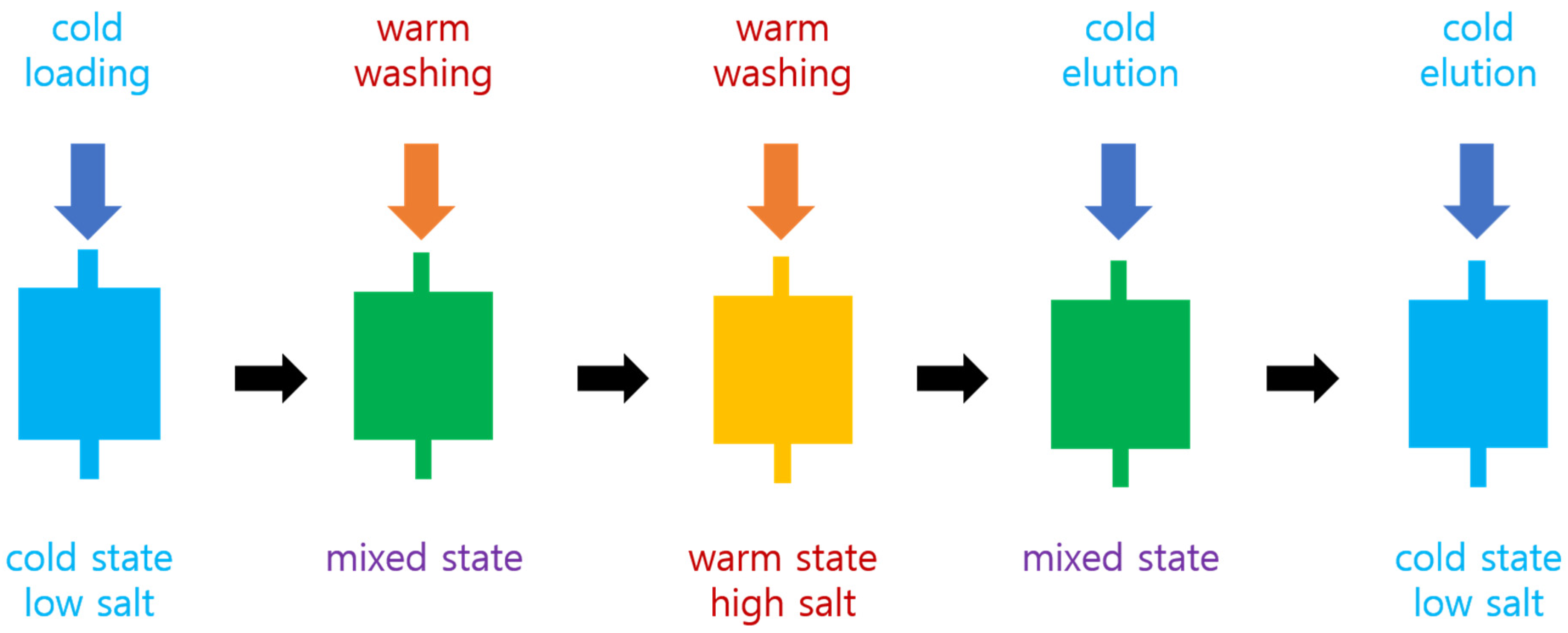
The frozen cells were thawed and disrupted by sonication. Triton X-100 was added to the final concentration of 1%. The cell lysate was centrifuged at 15,000 rpm at 4 °C for 30 min. The supernatant was retained and loaded onto the cold HiTrap Chelating HP 5 mL column (Cytiva, Marlborough, MA, USA) by using a syringe with an adaptor fitting the column inlet. This column had been placed on ice for 1 h before use. The column was washed with 15 mL of 10 mM warm (room temperature) PBS with 10 mM imidazole. This warm buffer had been placed on the lab bench at room temperature (around 22 °C) for 1 h before use. The column was successively washed with 15 and 20 mL of warm PBS with 250 and 500 mM imidazole, respectively. The column was put in ice for 20 min, and 25 mL of ice cold 10 mM PBS with 500 mM imidazole was applied to elute the bound protein. This cold buffer had been placed on ice for 1 h before use. This procedure is illustrated in Figure 1. The purity of the protein was analyzed by SDS-PAGE.
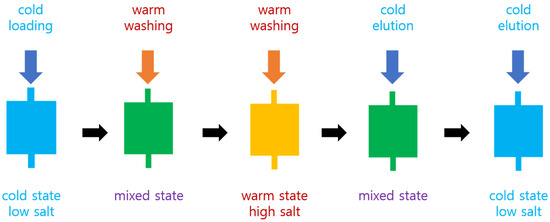
Figure 1.
Schematic diagram of aggregation-assisted chromatography. “Cold” and “warm” mean the ice-cold and room temperature, respectively.
3. Results and Discussion
3.1. Protein Production in E. coli
To facilitate the selection of clones containing the target protein during protein production, we employed a reporter gene strategy. The genes of interest were inserted into the plasmid pVP65KR-SacB-I48, replacing the SacB gene. This plasmid also contained the mCherry fluorescent protein gene under a constitutive promoter. When transformed into Rosetta2(DE3)pLysS cells, a strain optimized for protein production, this vector allowed for co-translational expression of both the target protein and mCherry. If the transformation and protein production are successful, the culture appears red/purple due to mCherry expression [17,18]. While a color change does not guarantee the successful production of the target protein itself, cultures that did not turn purple clearly indicated a failed transformation or protein production event.
Among the many elastin-like polypeptides (ELPs) tested, we chose I48 for further experiments. I48 is a polypeptide with 48 repeating units of the amino acid sequence GPGIG (glycine-proline-glycine-isoleucine-glycine). Two key factors influenced this selection. Firstly, the transition temperature (Tm) of I48 is around 22 °C, which is close to room temperature, facilitating easier handling during protein purification steps [19]. Secondly, the size of I48 is approximately 22 kDa, minimizing potential steric hindrance and improving the final yield of the target protein.
3.2. Protein Purification by Aggregation-Dispersion Chromatography
We explored a novel method for purifying target proteins fused with elastin-like polypeptides (ELPs) using immobilized metal affinity chromatography (IMAC). This method employs a prepacked IMAC column operated manually with a syringe for sample loading and buffer flow. A key feature is the stepwise modification of temperature and buffer conditions during the purification process. To minimize target protein aggregation, the samples were maintained at low temperature before loading onto the cold column. After loading, a warm buffer containing high salt and imidazole was applied to remove contaminant proteins. As shown in Figure 1, this strategy allowed for the efficient elution of target proteins under these harsh washing conditions, likely due to the stabilizing effect of the ELP tags.
It is very advantageous that a high salt and high imidazole buffer can be applied because there are several indigenous E. coli proteins known to bind to the IMAC column [20]. It is evident that the aggregate is large enough not to be eluted from the column. The SDS-PAGE results of SH3BGRL1, GLRX3, GLRX4, and I48ELP are in the Supplementary Materials. Interestingly, MBP-I48, a fusion protein containing maltose binding protein (MBP), exhibited different behavior. MBP is a well-known solubilization tag that is used to enhance the solubility of the recombinant proteins in E. coli [21]. While other target proteins eluted during the cold imidazole elution step (Figure 2), MBP-I48 eluted in the warm wash step (Figure 3). We hypothesize that the larger size of MBP compared to other fusion partners might hinder the aggregation of the ELP moiety at room temperature. This could result in the formation of smaller aggregates that could pass through the beads during the washing step. SDS-PAGE analysis (provided in the Supplementary Materials) confirmed the successful purification of other target proteins using this method.
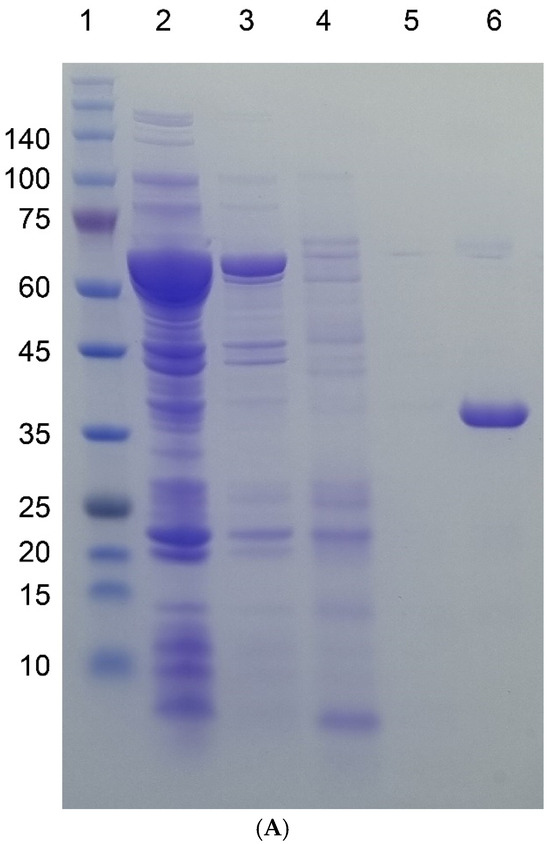
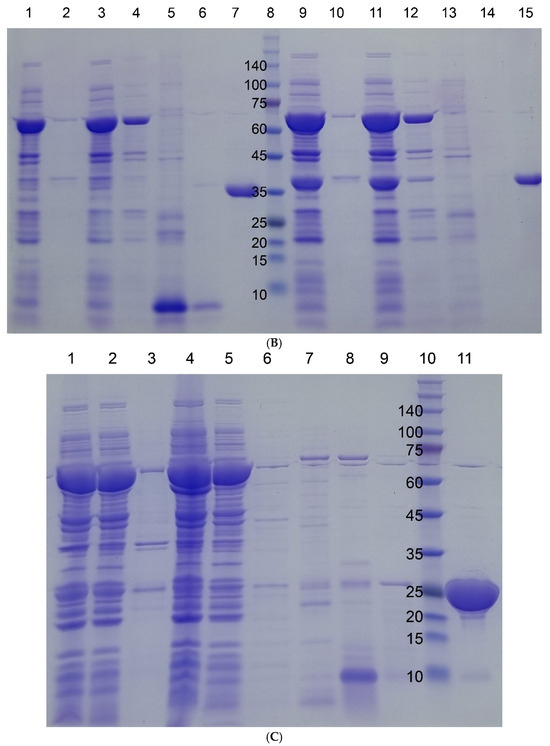
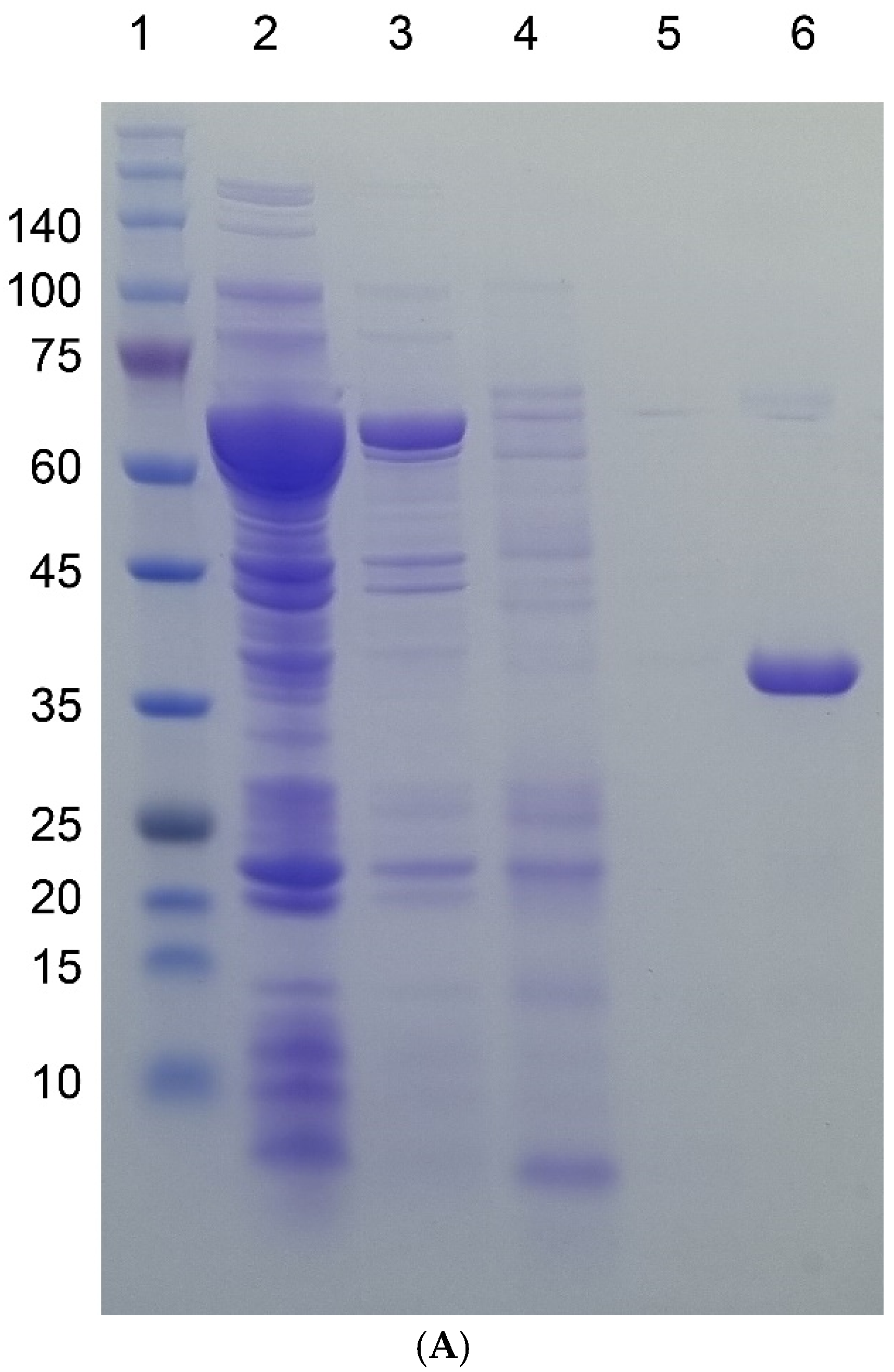
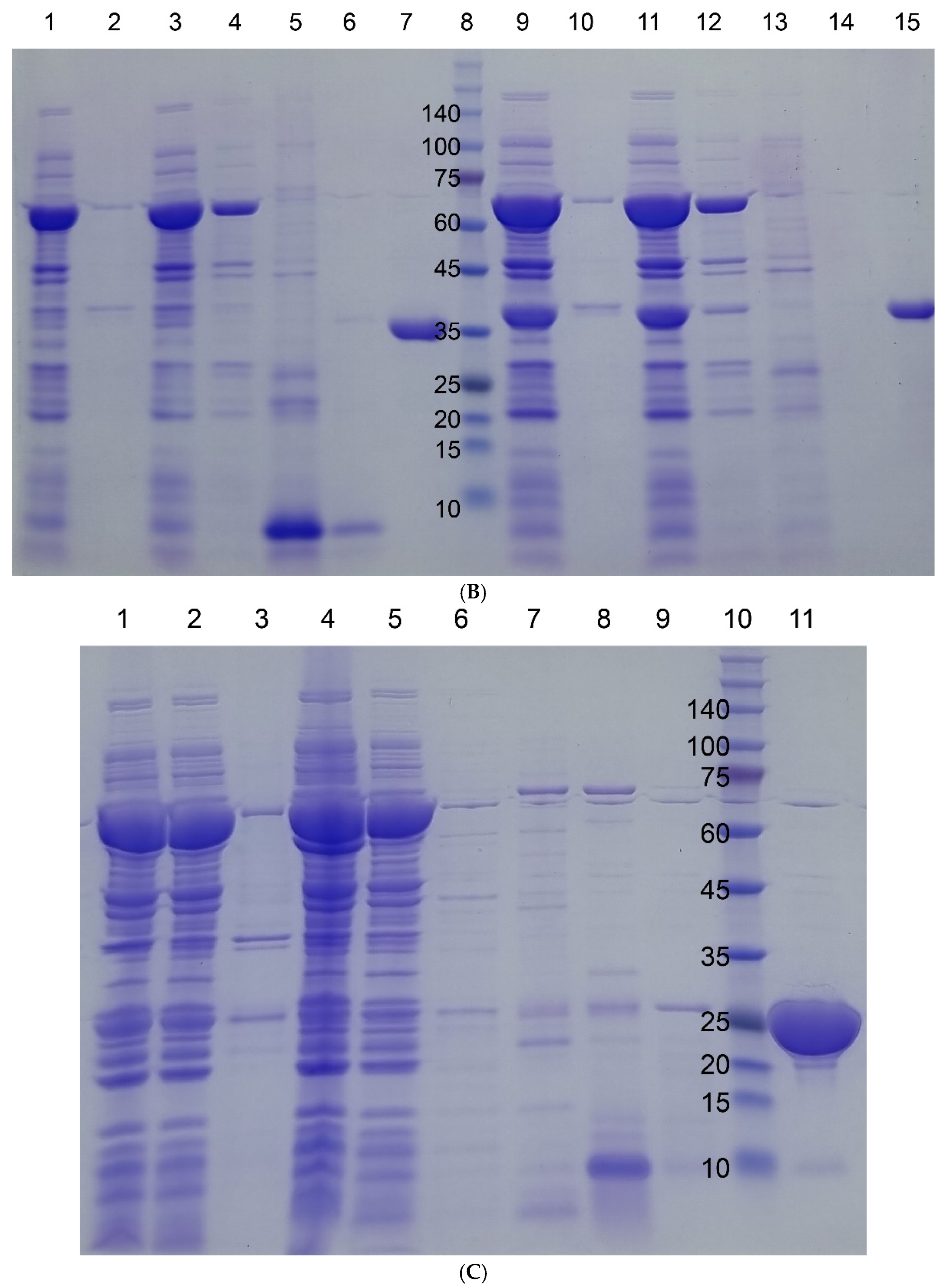
Figure 2.
SDS-PAGE gel images of four target proteins fused to I48 along the purification steps. Molecular sizes are denoted next to the size marker bands. (A) mazF-I48 (34.9k). Lane 1, Size marker; lane 2, flow-through fraction upon loading on to HisTrap column; lanes 3, 4, and 5, elution fractions with 10, 250, and 500 mM imidazole at room temperature; lane 6, elution fraction with 500 mM imidazole at ice cold temperature. (B) mazE-I48 (32.2k), lanes 1 to 7, and ccdB-I48 (34.5k), lanes 8 to 15. Lanes 2 and 3, soluble and insoluble fractions of cell lysate; lane 3, flow-through fraction upon loading on to HisTrap column; lanes 4, 5, and 6, elution fractions with 10, 250, and 500 mM imidazole at room temperature; lane 7, elution fraction with 500 mM imidazole at ice cold temperature; lane 8, size marker; lanes 9 and 10, soluble and insoluble fractions of cell lysate; lane 11, flow-through fraction upon loading on to HisTrap column; lanes 12, 13, and 14, elution fractions with 10, 250, and 500 mM imidazole at room temperature; lane 15, elution fraction with 500 mM imidazole at ice cold temperature. (C) S-I48 (25k). Lane 1, whole cell lysate; lanes 2 and 3, soluble and insoluble fractions of cell lysate; lanes 4 and 5, flow-through fractions upon loading on to HisTrap column; lanes 6, 7, 8, and 9, elution fractions with 10, 50, 250, and 500 mM imidazole at room temperature; lane 10, size marker; lane 11, elution fraction with 500 mM imidazole at ice cold temperature.
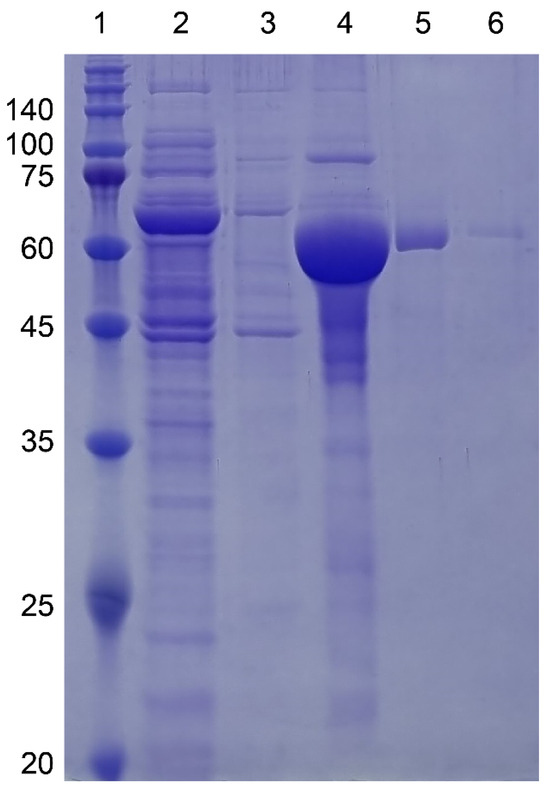
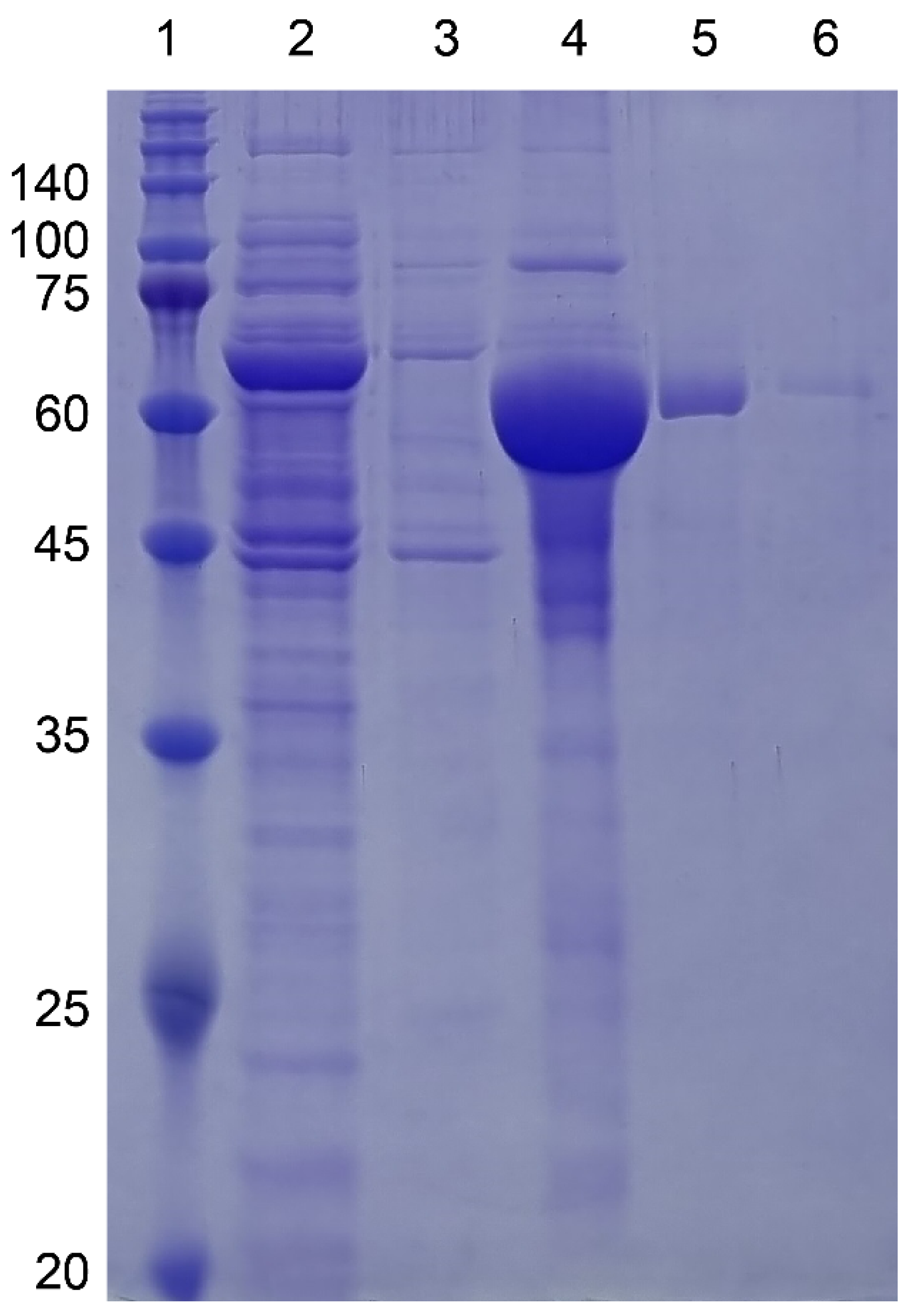
Figure 3.
SDS-PAGE gel images of MBP-I48 (63.4kDa) along the purification steps. All buffers contained 10 mM sodium phosphate pH 7.4 with 150 mM NaCl. Lane 1, Size marker; lane 2, flow-through fraction upon loading on to HisTrap column; lanes 3, 4, and 5, elution fractions with 10, 250, and 500 mM imidazole at room temperature; lane 6, elution fraction with 500 mM imidazole at ice cold temperature. Molecular sizes are denoted next to the size marker bands.
The capacity of the HisTrap column used was 40 mg of protein per mL of resin. Based on the manufacturer’s specifications, the average bead size was 34 μm (radius of 17 μm). Calculations assuming spherical beads suggest that a single bead can bind up to 1.2 × 1010 protein molecules (e.g., a 40 kDa protein). At maximum binding capacity, the proteins would essentially occupy the entire surface area of the bead. In this scenario, we propose that the ELP moieties from different fusion proteins are in close proximity, potentially allowing them to form interlocked aggregates at room temperature and high salt concentrations. These aggregates, formed either from the same bead or from neighboring beads, could become physically trapped within the column matrix, hindering elution during the high imidazole and salt wash step. Furthermore, smaller proteins might form even stronger interlocking networks due to their ability to pack more densely on the bead surface.
Typical parameters of the tested proteins were compared, and no parameters unique to MBP were seen (Table 1). What is unique about MBP is that it is larger than the other proteins tested, and the size may hinder the aggregation of I48 by non-specific interaction. The net result would have been earlier elution from the column during the washing phase. However, as in our previous paper, MBP-I48 was shown to form aggregates above its transition temperature. We speculate that MBP-I48 forms an aggregate whose size is small enough to move through the beads. That is, the size of the aggregate does seem to be affected by that of the fusion partner.
The reason why we chose IMAC for this aggregation-dispersion method was quite fortuitous. In another research project, we produced and purified ELP tagged proteins to study protein–protein interaction. We used a low pressure liquid chromatography system, and purified the proteins without the ELP tag using the conventional imidazole gradient elution. However, when we tried to purify the desired protein with the ELP tag, it did not elute from the column, no matter how much we increased the NaCl or imidazole concentrations. This was when we realized the potential of the ELP tag to form aggregates at room temperature. By simply lowering the temperature of the column by applying the cold elution buffer, thus dispersing the large aggregates into monomers or smaller oligomers, we could obtain what we wished.
3.3. Comparison with Other Aggregation Tags
Researchers have developed various aggregation tags for column-free protein purification methods [15]. Two representative methods are the inclusion body strategy and ITC using ELP constructs [22,23]. The inclusion body strategy, while applicable to proteins expressed in mammalian cells, presents a significant challenge in the refolding step. This refolding process often requires specific and carefully controlled conditions such as precisely regulated pH, temperature, and the presence of specific molecules to assist protein folding. Unfortunately, these optimal conditions can be difficult to predict and vary greatly between different proteins. As a result, the inclusion body strategy can be time-consuming and have a low success rate, especially for complex proteins. On the other hand, the ITC employing ELP constructs suffers from inefficiencies in both yield and processing time. This drawback stems from the necessity of repeated cycles of rinsing and centrifugation. These cycles are crucial for achieving the desired level of protein purity. However, each rinse-centrifugation step results in some protein loss, ultimately reducing the overall yield. Additionally, the repetitive nature of this process significantly extends the purification time. This becomes a major bottleneck, especially when dealing with large-scale protein production. The simplicity of our method by using stepwise washing/elution offers an advantage of performing a manual operation of column chromatography, yielding highly pure proteins in a short amount of time without the need for sophisticated, expensive, and automated machines. Even though IMAC is an affinity chromatography, there are proteins that bind to the Ni-agarose/sepharose resins through non-specific interactions, and these interactions are quite strong, so the contaminants are not separated well from the desired one, even with the automated gradient elution. This especially becomes a problem when the desired protein elutes at low imidazole concentrations. As for large proteins like MBP, we speculate that if a longer ELP is used, our method can be applied better, because the size of the ELP can overcome that of MBP or larger proteins. However, since a longer ELP has a lower transition temperature, it may aggregate at too low a temperature, and the dispersion may become difficult, even with the ice-cold buffer. To counter this lowering of the transition temperature, the X residue in the VPGXG pentapeptide repeat of ELP should be chosen to have a lower aggregation tendency: such an ELP will maintain its transition temperature slightly lower than the room temperature like I48. In this way, the cold and warm buffers can be within the easily controllable range, and the desired protein can be in the native conformation throughout the purification procedure.
4. Conclusions
We developed a new chromatography technique that employed the physical aggregation of ELP and showed promising results through our target proteins. Our technique offers a simple and rapid purification of a desired protein by manually operating an IMAC column through the use of cold and warm buffers, and a harsh washing condition such as 500 mM imidazole. By carefully selecting the X residue in the pentapeptide repeat of ELP and the repetition number, this method can be applied to the purification of a large variety of proteins.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations11120335/s1, Figure S1: SH3BGRL1-I48; Figure S2: GLRX3-I48; Figure S3: GLRX4-I48; Figure S4: I48 ELP; Figure S5: His-malFP2; Figure S6: His-SH3; Figure S7: His-S-protein; Figure S8: Plasmid map of pVP65KR-SacB-I48.
Author Contributions
Conceptualization, Y.K.C. and H.B.S.; Methodology, H.B.S.; Validation, Y.K.C.; Investigation, Y.K.C. and H.B.S.; Resources, Y.K.C.; Writing—original draft, Y.K.C.; Writing—review and editing, Y.K.C.; Visualization, Y.K.C.; Supervision, Y.K.C.; Project administration, Y.K.C.; Funding acquisition, Y.K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1F1A1046500).
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Labrou, N.E. (Ed.) Protein Purification: An Overview. In Protein Downstream Processing: Design, Development and Application of High and Low-Resolution Methods; Humana Press: Totowa, NJ, USA, 2014; pp. 3–10. [Google Scholar] [CrossRef]
- Batool, M.; Ahmad, B.; Choi, S. A Structure-Based Drug Discovery Paradigm. Int. J. Mol. Sci. 2019, 20, 2783. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, P.T. Overview of the Purification of Recombinant Proteins. Curr. Protoc. Protein Sci. 2015, 80, 6.1.1-6.1.35. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 1999, 10, 411–421. [Google Scholar] [CrossRef]
- Rudge, S.R.; Ladisch, M.R. Industrial Challenges of Recombinant Proteins. In Current Applications of Pharmaceutical Biotechnology; Silva, A.C., Moreira, J.N., Lobo, J.M.S., Almeida, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Rosli, W.R.W.; Tahir, A.R.M.; Razak, F.S.A.; Kee, P.E.; Ng, H.S.; Chew, Y.-L.; Lee, S.-K.; Ramasamy, M.; Tan, C.S.; et al. Evolving Paradigms of Recombinant Protein Production in Pharmaceutical Industry: A Rigorous Review. Sci 2024, 6, 9. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Nong, F.-T.; Wang, Y.-Z.; Yan, C.-X.; Gu, Y.; Song, P.; Sun, X.-M. Strategies for efficient production of recombinant proteins in Escherichia coli: Alleviating the host burden and enhancing protein activity. Microb. Cell Factories 2022, 21, 191. [Google Scholar] [CrossRef]
- Pina, A.S.; Lowe, C.R.; Roque, A.C.A. Challenges and opportunities in the purification of recombinant tagged proteins. Biotechnol. Adv. 2014, 32, 366–381. [Google Scholar] [CrossRef]
- Lebendiker, M.; Danieli, T. Purification of proteins fused to maltose-binding protein. Methods Mol. Biol. 2011, 681, 281–293. [Google Scholar] [CrossRef]
- Harper, S.; Speicher, D.W. Purification of proteins fused to glutathione S-transferase. Methods Mol. Biol. 2011, 681, 259–280. [Google Scholar] [CrossRef]
- Loughran, S.T.; Walls, D. Purification of poly-histidine-tagged proteins. Methods Mol. Biol. 2011, 681, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Karyolaimos, A.; de Gier, J.-W. Strategies to Enhance Periplasmic Recombinant Protein Production Yields in Escherichia coli. Front. Bioeng. Biotechnol. 2021, 9, 797334. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, Q.; Xing, L.; Zhou, B.; Wang, X. Aggregating tags for column-free protein purification. Biotechnol. J. 2015, 10, 1877–1886. [Google Scholar] [CrossRef]
- Hassouneh, W.; Christensen, T.; Chilkoti, A. Elastin-Like Polypeptides as a Purification Tag for Recombinant Proteins. Curr. Protoc. Protein Sci. 2010, 61, 6.11.1-6.11.16. [Google Scholar] [CrossRef]
- Chae, Y.K.; Um, Y.; Kim, H. A simple and sensitive detection of the binding ligands by using the receptor aggregation and NMR spectroscopy: A test case of the maltose binding protein. J. Biomol. NMR 2021, 75, 371–381. [Google Scholar] [CrossRef]
- Chae, Y.K.; Kim, H. Development of an Autoinducible Plasmid for Recombinant Protein Production. Protein Pept. Lett. 2021, 28, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Aluri, S.; Lin, Y.-A.; Shah, M.; Edman, M.; Dhandhukia, J.; Cui, H.; MacKay, J.A. Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo. J. Control. Release 2013, 171, 330–338. [Google Scholar] [CrossRef]
- Bartlow, P.; Uechi, G.T.; Cardamone, J.J.; Sultana, T.; Fruchtl, M.; Beitle, R.R.; Ataai, M.M. Identification of native Escherichia coli BL21 (DE3) proteins that bind to immobilized metal affinity chromatography under high imidazole conditions and use of 2D-DIGE to evaluate contamination pools with respect to recombinant protein expression level. Protein Expr. Purif. 2011, 78, 216–224. [Google Scholar] [CrossRef]
- Sun, P.; Tropea, J.E.; Waugh, D.S. Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner. Methods Mol. Biol. 2011, 705, 259–274. [Google Scholar] [CrossRef]
- Fan, Y.; Miozzi, J.M.; Stimple, S.D.; Han, T.-C.; Wood, D.W. Column-Free Purification Methods for Recombinant Proteins Using Self-Cleaving Aggregating Tags. Polymers 2018, 10, 468. [Google Scholar] [CrossRef]
- Singh, A.; Upadhyay, V.; Upadhyay, A.K.; Singh, S.M.; Panda, A.K. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb. Cell Factories 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).