Aniline-p-Phenylenediamine Copolymer for Removal of Hexavalent Chromium from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aniline-p-Phenylenediamine Copolymer (P(ANI-PDA))
2.3. Characterizations
2.4. Cr(VI) Removal Evaluation
2.4.1. Effect of P(ANI-PDA) Dosage
2.4.2. Effect of Initial Cr(VI) Concentration
2.4.3. Effect of pH Value
2.4.4. Kinetic and Thermodynamic Study
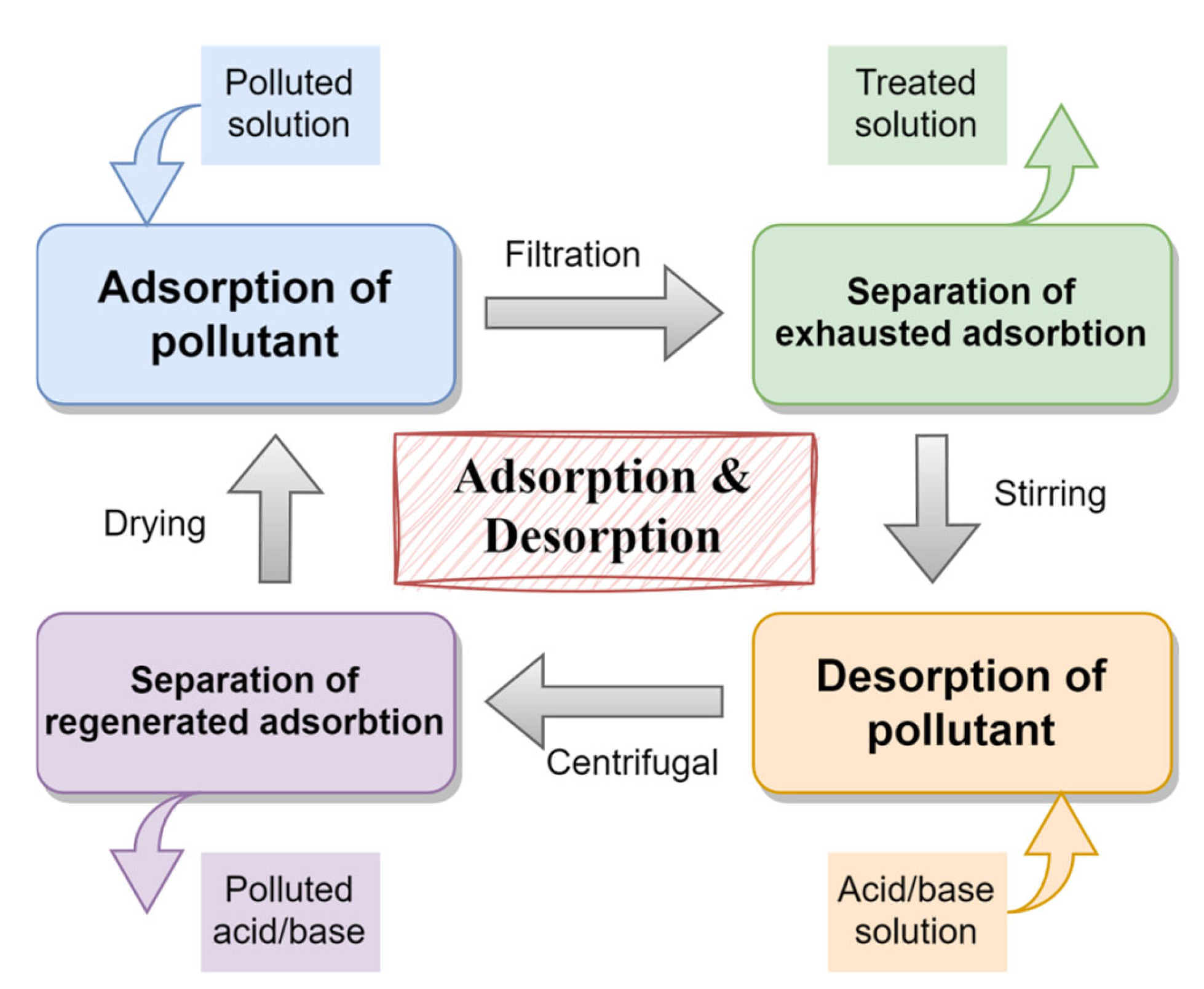
2.4.5. Regeneration and Recyclability
3. Results and Discussion
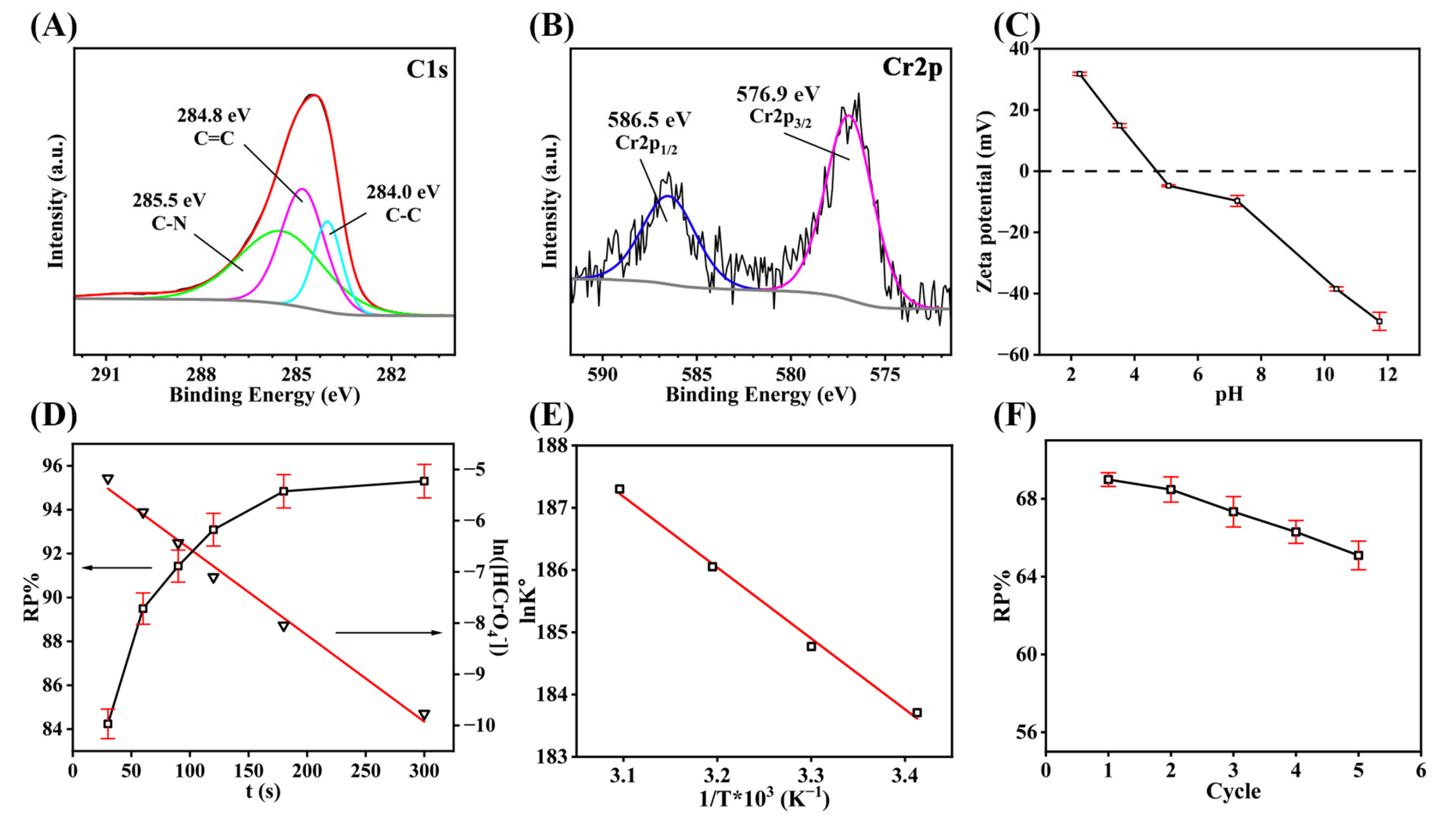
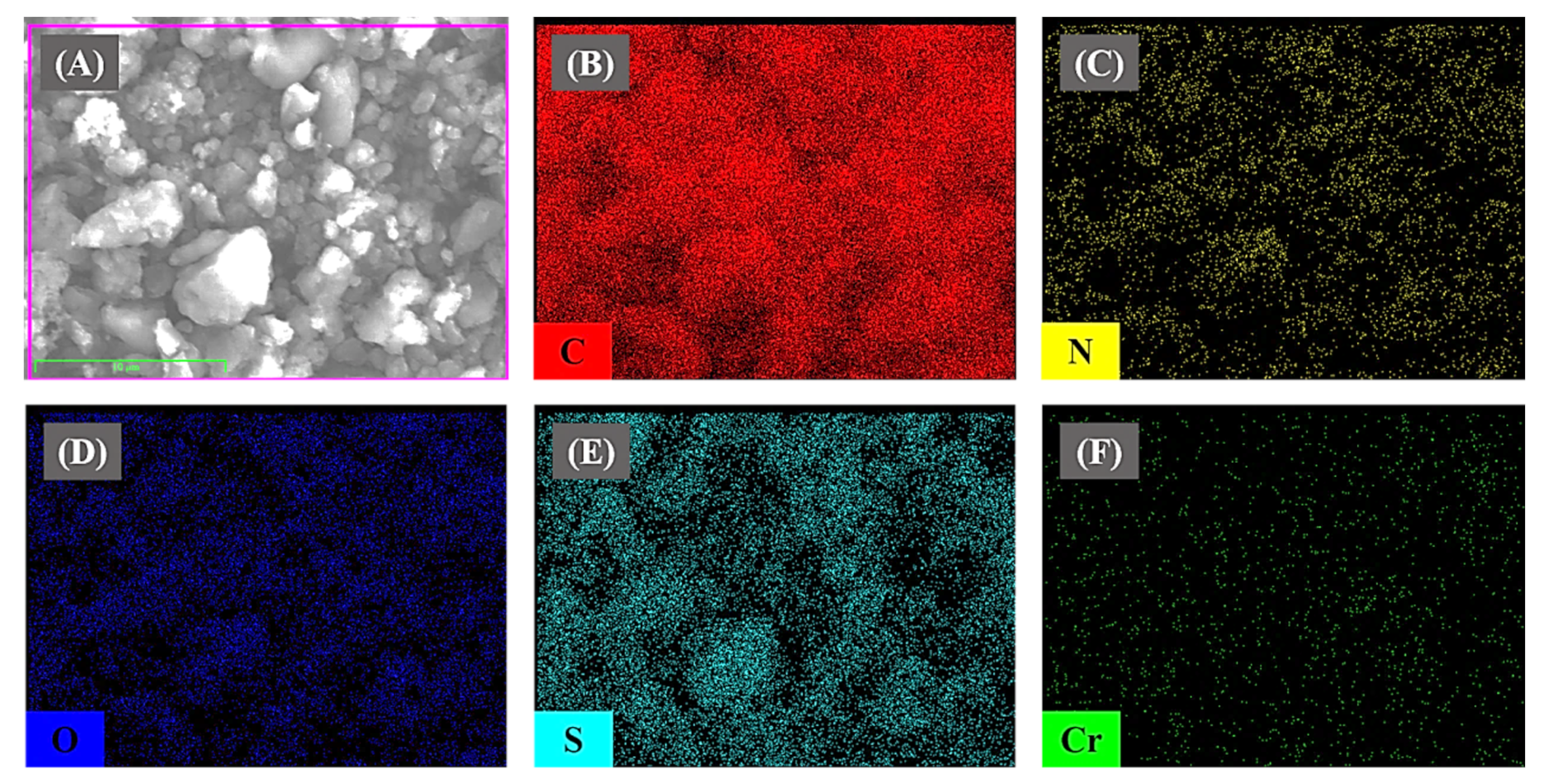
3.1. Characterization
3.2. Cr(VI) Removal Evaluation
3.3. Removal Mechanism
3.4. Removal Kinetics and Thermodynamics
3.5. Recycle and Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Uddin, M.; Xie, J.; Tao, H.; Zeidler-Erdely, P.; Kondo, K.; Yang, C. Chronic hexavalent chromium exposure upregulates the RNA methyltransferase METTL3 expression to promote cell transformation, cancer stem cell-like property, and tumorigenesis. Toxicol. Sci. 2022, 187, 51–61. [Google Scholar] [CrossRef]
- Chromium in Drinking-Water. Available online: https://iris.who.int/bitstream/handle/10665/338062/WHO-HEP-ECH-WSH-2020.3-eng.pdf (accessed on 23 October 2024).
- Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138. [Google Scholar] [CrossRef]
- Yang, Y.; Han, T.; Wang, J. Ultrafast and highly efficient Cd(II) and Pb(II) removal by magnetic adsorbents derived from gypsum and corncob: Performances and mechanisms. Ecotoxicol. Environ. Saf. 2024, 275, 116265. [Google Scholar] [CrossRef]
- Ediriweera, A.N.; Karunarathna, S.C.; Yapa, P.N.; Schaefer, D.A.; Ranasinghe, A.K.; Suwannarach, N.; Xu, J. Ectomycorrhizal mushrooms as a natural bio-indicator for assessment of heavy metal pollution. Agronomy 2022, 12, 1041. [Google Scholar] [CrossRef]
- Chaurasia, P.K.; Nagraj; Sharma, N.; Kumari, S.; Yadav, M.; Singh, S.; Mani, A.; Yadava, S.; Bharati, S.L. Fungal assisted bio-treatment of environmental pollutants with comprehensive emphasis on noxious heavy metals: Recent updates. Biotechnol. Bioeng. 2023, 120, 57–81. [Google Scholar] [CrossRef]
- Nazari, B.; Abdolalian, S.; Taghavijeloudar, M. An environmentally friendly approach for industrial wastewater treatment and bio-adsorption of heavy metals using pistacia soft shell (PSS) through flocculation-adsorption process. Environ. Res. 2023, 235, 116595. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Wang, G.F.; Zhao, L.; Cheng, H.L. Preparation and permeation recognition mechanism of Cr(VI) ion-imprinted composite membranes. e-Polymers 2022, 22, 938–948. [Google Scholar] [CrossRef]
- Moradi, G.; Heydari, R.; Zinadini, S.; Rahimi, M.; Gholami, F. High-performance nanofiltration membranes consisting of the new functionalized mesoporous for enhanced antifouling attributes and simultaneous removal of salts, dyes and heavy metals. Environ. Technol. Innov. 2021, 24, 101929. [Google Scholar] [CrossRef]
- Cheng, X.X.; Lai, C.X.; Li, J.Y.; Zhou, W.W.; Zhu, X.W.; Wang, Z.H.; Ding, J.W.; Zhang, X.Y.; Wu, D.J.; Liang, H.; et al. Toward enhancing desalination and heavy metal removal of TFC nanofiltration membranes: A cost-effective interface temperature-regulated interfacial polymerization. ACS Appl. Mater. Inter. 2021, 13, 57998–58010. [Google Scholar] [CrossRef] [PubMed]
- Aazza, M.; Ahlafi, H.; Moussout, H.; Maghat, H. Adsorption of metha-nitrophenol onto alumina and HDTMA modified alumina: Kinetic, isotherm and mechanism investigations. J. Mol. Liq. 2018, 268, 587–597. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2021, 283, 124671. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.-H.A.; Bilal, S.; Rahman, G. Basic blue dye adsorption from water using polyaniline/magnetite (Fe3O4) composites: Kinetic and thermodynamic aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef]
- Ma, T.; Liu, M.; Offiong, N.-A.O.; Duan, J.; Liu, Y.; Ren, H.; Zhou, R. Highly-efficient peroxydisulfate activation by polyaniline-polypyrrole copolymers derived pyrolytic carbon for 2,4-dichlorophenol removal in water: Coupling mechanism of singlet oxygen and electron transfer. J. Hazard. Mater. 2023, 445, 130580. [Google Scholar] [CrossRef]
- Eskandari, E.; Kosari, M.; Davood Abadi Farahani, M.H.; Khiavi, N.D.; Saeedikhani, M.; Katal, R.; Zarinejad, M. A review on polyaniline-based materials applications in heavy metals removal and catalytic processes. Sep. Purif. Technol. 2020, 231, 115901. [Google Scholar] [CrossRef]
- Rani, M.; Ramachandran, R.; Kabilan, S. A facile synthesis and characterization of semiconducting p-phenylenediamine-aniline copolymer. Synth. Met. 2010, 160, 678–684. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Su, R.; Yang, L.; Xiao, F.; Chen, L.; He, P.; Yang, D.; Zeng, Y.; Zhou, Y.; et al. PANI/GO and Sm co-modified Ti/PbO2 dimensionally stable anode for highly efficient amoxicillin degradation: Performance assessment, impact parameters and degradation mechanism. J. Environ. Manag. 2024, 364, 121435. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Y.; Xiao, J.; Fan, H.; Chen, C. Preparation and characterization of magnetic nanomaterial and its application for removal of polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2019, 371, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Shahrabi, M.J.A.; Sedighi, M. Comparative study of linearized and non-linearized modified Langmuir isotherm models on adsorption of asphaltene onto mineral surfaces. Surf. Eng. Appl. Elect. 2012, 48, 234–243. [Google Scholar] [CrossRef]
- Bolster, C.H.; Hornberger, G.M. On the use of linearized langmuir equations. Soil Sci. Soc. Am. J. 2008, 72, 1848. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Bhadra, J.; Alkareem, A.; Al-Thani, N. A review of advances in the preparation and application of polyaniline based thermoset blends and composites. J. Polym. Res. 2020, 27, 122. [Google Scholar] [CrossRef]
- Samadi, A.; Xie, M.; Li, J.; Shon, H.; Zheng, C.; Zhao, S. Polyaniline-based adsorbents for aqueous pollutants removal: A review. Chem. Eng. J. 2021, 418, 129425. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Du, L.; Gao, P.; Liang, N.; Wu, S.; Minami, T.; Zang, L.; Yu, C.; Xu, X. Preparation of polyaniline/emulsion microsphere composite for efficient adsorption of organic dyes. Polymers 2020, 12, 167. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Bhatti, H.N.; Khan, A.; Iqbal, M.; Kausar, A. Polypyrole, polyaniline and sodium alginate biocomposites and adsorption-desorption efficiency for imidacloprid insecticide. Int. J. Biol. Macromol. 2020, 147, 217–232. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Hossein Baghdadi, A.; Kartom Kamarudin, S.; Abdul Rashid, S.; Jakmunee, J.; Shaari, N. Recent progress in polyaniline and its composites; synthesis, properties, and applications. Eur. Polym. J. 2024, 210, 112948. [Google Scholar] [CrossRef]
- Samadi, A.; Kong, L.; Guo, W.; Sillanpää, M.; Boztepe, I.; Song, C.; Zeng, Q.; Zhao, S. Standardized methodology for performance evaluation in using polyaniline-based adsorbents to remove aqueous contaminants. J. Environ. Chem. Eng. 2024, 12, 112650. [Google Scholar] [CrossRef]
- Ma, X.L.; Fei, G.T.; Xu, S.H. Synthesis of polyaniline coating on the modified fiber ball and application for Cr(VI) removal. Nanoscale Res. Lett. 2021, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Atassi, Y. Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes. Water Sci. Eng. 2021, 14, 129–138. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manage. 2014, 133, 309–314. [Google Scholar] [CrossRef]
- Li, S.; You, T.; Guo, Y.; Yao, S.; Zang, S.; Xiao, M.; Zhang, Z.; Shen, Y. High dispersions of nano zero valent iron supported on biochar by one-step carbothermal synthesis and its application in chromate removal. RSC Adv. 2019, 9, 12428–12435. [Google Scholar] [CrossRef]
- Shi, S.; Yang, J.; Liang, S.; Li, M.; Gan, Q.; Xiao, K.; Hu, J. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci. Total Environ. 2018, 628–629, 499–508. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Rafiaee, S.; Samani, M.R.; Toghraie, D. Removal of hexavalent chromium from aqueous media using pomegranate peels modified by polymeric coatings: Effects of various composite synthesis parameters. Synth. Met. 2020, 265, 116416. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, Q.; Chen, J.; Ma, B.; Chen, S. Carbothermal preparation of porous carbon-encapsulated iron composite for the removal of trace hexavalent chromium. Chem. Eng. J. 2014, 253, 24–33. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, J.; Tan, X.; Lou, H.; Gu, H. Aniline-p-Phenylenediamine Copolymer for Removal of Hexavalent Chromium from Wastewater. Separations 2024, 11, 327. https://doi.org/10.3390/separations11110327
Li Y, Chen J, Tan X, Lou H, Gu H. Aniline-p-Phenylenediamine Copolymer for Removal of Hexavalent Chromium from Wastewater. Separations. 2024; 11(11):327. https://doi.org/10.3390/separations11110327
Chicago/Turabian StyleLi, Yifeng, Jingyue Chen, Xiwei Tan, Han Lou, and Hongbo Gu. 2024. "Aniline-p-Phenylenediamine Copolymer for Removal of Hexavalent Chromium from Wastewater" Separations 11, no. 11: 327. https://doi.org/10.3390/separations11110327
APA StyleLi, Y., Chen, J., Tan, X., Lou, H., & Gu, H. (2024). Aniline-p-Phenylenediamine Copolymer for Removal of Hexavalent Chromium from Wastewater. Separations, 11(11), 327. https://doi.org/10.3390/separations11110327








