Phytochemical Study of the Plant Centaurea bruguieriana (DC.) Hand.-Mazz. subsp. belangeriana (DC.) Bornm. of the Family Asteraceae
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chromatographic Technics
2.3. Spectroscopy
2.4. In Silico Study
2.5. Inhibition of Linoleic Acid Lipid Peroxidation
2.6. Inhibition of Soybean Lipoxygenase (LOX)
2.7. Statistical Analysis
3. Results
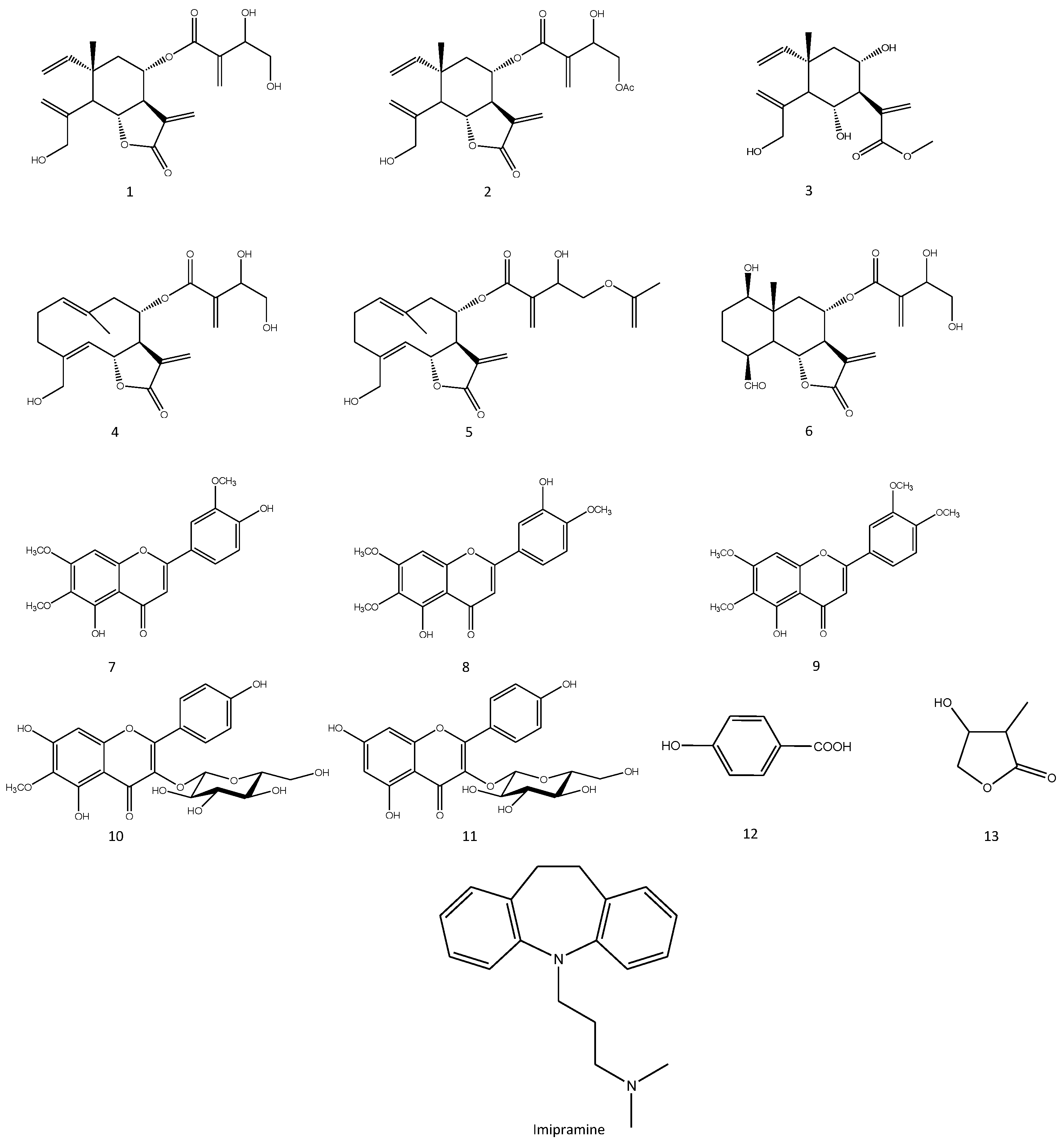
3.1. Extraction and Isolation of Compounds
3.2. Identification of the Isolated Compounds
3.3. In Silico Calculations of Physicochemical Parameters and Prediction of Biological Activities
3.4. Results of In Vitro Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, M.J.; Bedoya, L.M.; Bermejo, R. Chapter 14—Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components, 1st ed.; Rai, M.K., Kon, K.V., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 14, pp. 205–221. [Google Scholar] [CrossRef]
- Joujeh, R.; Zaid, S.; Mona, S. Pollen morphology of some selected species of the genus Centaurea L. (Asteraceae) from Syria. S. Afr. J. Bot. 2019, 125, 196–201. [Google Scholar] [CrossRef]
- Bülent Köse, Y.; Iscan, G.; Demirci, B. Antimicrobial Activity of the Essential Oils Obtained from Flowering Aerial Parts of Centaurea lycopifolia Boiss. et Kotschy and Centaurea cheirolopha (Fenzl) Wagenitz from Turkey. J. Essent. Oil-Bear. Plants 2016, 19, 762–768. [Google Scholar] [CrossRef]
- Khammar, A.; Djeddi, S. Pharmacological and biological properties of some Centaurea species. Eur. J. Sci. Res. 2012, 84, 398–416. [Google Scholar]
- Demir, S.; Karaalp, C.; Bedir, E. Specialized metabolites from the aerial parts of Centaurea polyclada DC. Phytochemistry 2017, 143, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hodaj, E.; Tsiftsoglou, O.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Lignans and indole alkaloids from the seeds of Centaurea vlachorum Hartvig (Asteraceae), growing wild in Albania and their biological activity. Nat. Prod. Res. 2017, 31, 1195–1200. [Google Scholar] [CrossRef]
- Fattaheian-Dehkordi, S.; Hojjatifard, R.; Saeedi, M.; Khanavi, M. A Review on Antidiabetic Activity of Centaurea spp.: A New Approach for Developing Herbal Remedies. Evid. Based Complement Alternat. Med. 2021, 2021, 5587938. [Google Scholar] [CrossRef]
- Mandaville, J.P. Flora of Eastern Saudi Arabia, 1st ed.; Routledge: London, UK; New York, NY, USA, 2011; p. 313. [Google Scholar] [CrossRef]
- Atiyah, B.A.S.; Khesraji, T.O. Taxonomic Characteristics and Phytochemicals of Centaurea bruguieriana (DC.) Hand-Mazz (Asreraceae) From Western Desert of Iraq. IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 042029. [Google Scholar] [CrossRef]
- Euro+Med Plantbase. Centaurea bruguiereana (DC.) Hand.-Mazz. Available online: http://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameId=134328&PTRefFk=7000000 (accessed on 7 September 2024).
- Khanavi, M.; Ahmadi, R.; Rajabi, A.; Arfaee, S.J.; Hassanzadeh, G.; Khademi, R.; Hadjiakhoondi, A.; Beyer, C.; Sharifzadeh, M. Pharmacological and Histological Effects of Centaurea bruguierana ssp. belangerana on Indomethacin-Induced Peptic Ulcer in Rats. J. Nat. Med. 2012, 66, 343–349. [Google Scholar] [CrossRef]
- Mehrnia, M.; Akaberi, M.; Amiri, M.S.; Nadaf, M.; Emami, S.A. Ethnopharmacological Studies of Medicinal Plants in Central Zagros, Lorestan Province, Iran. J. Ethnopharmacol. 2021, 280, 114080. [Google Scholar] [CrossRef]
- Sadat-Hosseini, M.; Farajpour, M.; Boroomand, N.; Solaimani-Sardou, F. Ethnopharmacological Studies of Indigenous Medicinal Plants in the South of Kerman, Iran. J. Ethnopharmacol. 2017, 199, 194–204. [Google Scholar] [CrossRef]
- Lazari, D.; Tsioumela, C.; Pegklidou, K.; Karioti, A.; Demopoulos, V.; Skaltsa, H.; Arfan, M. Inhibitory Activity of Extracts and Bioactive Constituents of Centaurea phyllocephala Boiss. (Asteraceae) on Aldose Reductase in Vitro. Planta Med. 2008, 74, PA144. [Google Scholar] [CrossRef]
- Noman, O.M.; Herqash, R.N.; Shahat, A.A.; Ahamad, S.R.; Mechchate, H.; Almoqbil, A.N.; Alqahtani, A.S. A Phytochemical Analysis, Microbial Evaluation and Molecular Interaction of Major Compounds of Centaurea bruguieriana Using HPLC-Spectrophotometric Analysis and Molecular Docking. Appl. Sci. 2022, 12, 3227. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Niknejad, A.; Aynehchi, Y. Chemical Constituents of Centaurea bruguieriana. Planta Med. 1982, 44, 185–186. [Google Scholar] [CrossRef]
- Mirzahosseini, G.; Manayi, A.; Khanavi, M.; Safavi, M.; Salari, A.; Madjid Ansari, A.; San’ati, H.; Vazirian, M. Bio-guided isolation of Centaurea bruguierana subsp. belangeriana cytotoxic components. Nat. Prod. Res. 2019, 33, 1687–1690. [Google Scholar] [CrossRef] [PubMed]
- Neu, R. Chelate von Diarylborsäuren mit aliphatischen Oxyalkylaminen als Reagenzien für den Nachweis von Oxyphenyl-benzo-γ-pyronen. Die Naturwissenschaften 1957, 44, 181–183. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics Free Web Services. Available online: https://www.molinspiration.com, Slovensky Grob, Slovakia (accessed on 13 May 2021).
- Shityakov, S.; Salvador, E.; Förster, C. In silico, in vitro, and in vivo methods to analyse drug permeation across the blood–brain barrier: A critical review. OA Anaesth. 2013, 1, 13. [Google Scholar] [CrossRef][Green Version]
- Hodaj-Celiku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat. Prod. Commun. 2017, 12, 785–790. [Google Scholar] [CrossRef]
- Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Multitarget molecular hybrids of cinnamic acids. Molecules 2014, 19, 20197–20226. [Google Scholar] [CrossRef]
- Skaltsa, H.; Lazari, D.; Georgiadou, E.; Kakavas, S.; Constantinidis, T. Sesquiterpene Lactones from Centaurea Species: C. thessala subsp. drakiensis and C. attica subsp. attica. Planta Med. 1999, 65, 393. [Google Scholar] [CrossRef]
- Tuzun, B.S.; Hajdu, Z.; Orban-Gyapai, O.; Zomborszki, Z.P.; Jedlinszki, N.; Forgo, P.; Kıvcak, B.; Hohmann, J. Isolation of Chemical Constituents of Centaurea virgata Lam. and Xanthine Oxidase Inhibitory Activity of the Plant Extract and Compounds. Med. Chem. 2017, 13, 498–502. [Google Scholar] [CrossRef]
- Bruno, M.; Fazio, C.; Passananti, S.; Paternostro, M.P.; Díaz, J.G.; Herz, W. Sesquiterpene lactones from Centaurea sphaerocephala ssp. sphaerocephala. Phytochemistry 1994, 35, 1371–1372. [Google Scholar] [CrossRef]
- Csapi, B.; Hajdú, Z.; Zupkó, I.; Berényi, Á.; Forgo, P.; Szabó, P.; Hohmann, J. Bioactivity-guided isolation of antiproliferative compounds from Centaurea arenaria. Phytother. Res. 2010, 24, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Ćirić, A.; Karioti, A.; Koukoulitsa, C.; Soković, M.; Skaltsa, H. Sesquiterpene Lactones from Centaurea zuccariniana and Their Antimicrobial Activity. Chem. Biodivers. 2012, 9, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Morales, V.; Alvarez, M.; Rodriguez-Garcia, I. Biomimetic cyclization of cnicin to malacitanolide, a cytotoxic eudesmanolide from Centaurea malacitana. J. Nat. Prod. 1997, 60, 1034–1035. [Google Scholar] [CrossRef][Green Version]
- Kabouche, A.; Kabouche, Z.; Touzani, R.; Bruneau, C. Flavonoids from Centaurea sulphurea. Chem. Nat. Compd. 2011, 46, 966–967. [Google Scholar] [CrossRef]
- Yam, M.F.; Lim, V.; Salman, I.M.; Ameer, O.Z.; Ang, L.F.; Rosidah, N.; Abdulkarim, M.F.; Abdullah, G.Z.; Basir, R.; Sadikun, A.; et al. HPLC and Anti-Inflammatory Studies of the Flavonoid Rich Chloroform Extract Fraction of Orthosiphon Stamineus Leaves. Molecules 2010, 15, 4452–4466. [Google Scholar] [CrossRef]
- Hou, Y.Z.; Chen, K.K.; Deng, X.L.; Fu, Z.L.; Chen, D.F.; Wang, Q. Anti-complementary constituents of Anchusa italica. Nat. Prod. Res. 2017, 31, 2572–2574. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Rasool, N.; Abbasi, M.A.; Rashid, M.A.; Kousar, F.; Zubair, M.; Ejaz, A.; Choudhary, M.I.; Tareen, R.B. Antioxidant flavonoids from Pulicaria undulata. Pol. J. Chem. 2006, 80, 745–751. [Google Scholar] [CrossRef]
- Tsiftsoglou, O.S.; Lazari, D.M.; Stefanakis, M.K.; Kokkalou, E.L. Flavonoids and phenolic acids from the aerial parts of Alyssum alyssoides L. (Brassicaceae). Biochem. Syst. Ecol. 2019, 83, 51–53. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Tsiftsoglou, O.S.; Mašković, P.Z.; Lazari, D.; Katerinopoulos, H.E. Chemical Constituents and Anticancer Activities of the Extracts from Phlomis × commixta Rech. f. (P. cretica × P. lanata). Int. J. Mol. Sci. 2024, 25, 816. [Google Scholar] [CrossRef]
- Xin, X.L.; Aisa, H.A.; Wang, H.Q. Flavonoids and phenolic compounds from seeds of the Chinese plant Nigella glandulifera. Chem. Nat. Compd. 2008, 44, 368–369. [Google Scholar] [CrossRef]
- Mavridis, E.; Bermperoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D. 5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules. Molecules 2020, 25, 3173. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Chakrabarti, M.; Costanzi, S. Prediction of passive blood–brain partitioning: Straightforward and effective classification models based on in silico derived physicochemical descriptors. J. Mol. Graph. Modell. 2010, 28, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.B.; Geethavani, M.; Ramakrishna, C. Synthesis Characterization and Molinspiration Analysis, Anti-bacterial activity of Novel 2,4,6-tri Substituted Pyrimidines. J. Young Pharm. 2022, 14, 174–178. [Google Scholar] [CrossRef]
- Bruno, M.; Bancheva, S.; Rosselli, S.; Maggio, A. Sesquiterpenoids in subtribe Centaureinae (Cass.) Dumort (tribe Cardueae, Asteraceae): Distribution, C-13 NMR spectral data and biological properties. Phytochemistry 2013, 93, 19–93. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.; Di Gioia, F.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.; Barros, L.; et al. Wild and cultivated Centaurea raphanina subsp. mixta: A valuable source of bioactive compounds. Antioxidants 2020, 9, 314. [Google Scholar] [CrossRef]
- Radan, M.; Carev, I.; Tešević, V.; Politeo, O.; Čulić, V.Č. Qualitative HPLC-DAD/ESITOF- MS Analysis, Cytotoxic, and Apoptotic Effects of Croatian Endemic Centaurea ragusina L. Aqueous Extracts. Chem. Biodivers. 2017, 14, e1700099. [Google Scholar] [CrossRef]
- Grafakou, M.E.; Djeddi, S.; Tarek, H.; Skaltsa, H. Secondary metabolites from the aerial parts of Centaurea papposa (Coss.) Greuter. Biochem. Syst. Ecol. 2018, 76, 15–22. [Google Scholar] [CrossRef]
- Aliouche, L.; Mosset, P.; León, F.; Brouard, I.; Benayache, S.; Sarri, D.; Benayache, F. Characterization of Chemical Compounds and Antioxidant Activity of Centaurea solstitialis sp. schouwii (DC.) Q. et S. (Asteraceae). Curr. Bioact. Compd. 2020, 16, 618–626. [Google Scholar] [CrossRef]
- Zengin, G.; Bulut, G.; Mollica, A.; Picot-Allain, N.C.M.; Mahomoodally, M.F. In vitro and in silico evaluation of Centaurea saligna (K.Koch) Wagenitz—An endemic folk medicinal plant. Comput. Biol. Chem. 2018, 73, 120–126. [Google Scholar] [CrossRef]
- Suganya, M.; Hemamalini, M.; Jose Kavitha, S.; Rajakannan, V. In silico Studies of Molecular Property and Bioactivity of Organic Crystalline Compounds using Molinspiration. IRJET 2020, 07, 519–523. [Google Scholar]
- Fatullayev, H.; Paşayeva, L.; Celik, I.; İnce, U.; Tugay, O. Phytochemical Composition, In Vitro Antimicrobial, Antioxidant, and Enzyme Inhibition Activities, and In Silico Molecular Docking and Dynamics Simulations of Centaurea lycaonica: A Computational and Experimental Approach. ACS Omega 2023, 8, 22854–22865. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Hamedi, A.; Pasdaran, A.; Heidari, R.; Azam, M.S.; Tabassum, S.; Mehmood, R.; Peng, J. Anti-inflammatory potential of some eudesmanolide and guaianolide sesquiterpenes. Inflammopharmacology 2024, 32, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Lazanaki, M.; Tsikalas, G.; Tsiftsoglou, O.S.; Katerinopoulos, H.; Hadjipavlou-Litina, D.; Lazari, D. Secondary Metabolites and Their Biological Evaluation from the Aerial Parts of Staehelina uniflosculosa Sibth. & Sm. (Asteraceae). Int. J. Mol. Sci. 2024, 25, 10586. [Google Scholar] [CrossRef]
- Zengin, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Nedialkov, P.; Mocan, A.; Ćirić, A.; Glamočlija, J.; Soković, M.; Aktumsek, A.; Mahomoodally, M.F. Identification of phenolic components via LC–MS analysis and biological activities of two Centaurea species: C. drabifolia subsp. drabifolia and C. lycopifolia. J. Pharm. Biomed. Anal. 2018, 149, 436–441. [Google Scholar] [CrossRef]
- Zengin, G.; Llorent-Martínez, E.J.; Sinan, K.I.; Yildiztugay, E.; Picot-Allain, C.; Mahomoodally, M.F. Chemical profiling of Centaurea bornmuelleri Hausskn. aerial parts by HPLC MS/ MS and their pharmaceutical effects: From nature to novel perspectives. J. Pharm. Biomed. Anal. 2019, 174, 406–413. [Google Scholar] [CrossRef]
- Şen, A. Antioxidant and Anti-inflammatory Activity of Five Centaurea Species. Eur. J. Biol. 2023, 82, 311–316. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Screening for in vitro antioxidant properties and fatty acid profiles of five Centaurea L. species from Turkey flora. Food Chem. Toxicol. 2011, 49, 2914–2920. [Google Scholar] [CrossRef]
- Csupor, D.; Widowitz, U.; Blazsó, G.; Laczkó-Zöld, E.; Tatsimo, J.S.N.; Balogh, Á.; Boros, K.; Dankó, B.; Bauer, R.; Hohmann, J. Anti-inflammatory Activities of Eleven Centaurea Species Occurring in the Carpathian Basin. Phytother. Res. 2013, 27, 540–544. [Google Scholar] [CrossRef]
- Grafakou, M.E.; Barda, C.; Heilmann, J.; Skaltsa, H. In vitro cytotoxic and anti-inflammatory activities of sesquiterpene lactones from Centaurea papposa (Coss.) Greuter. Nat. Prod. Res. 2021, 36, 3211–3215. [Google Scholar] [CrossRef]
- Baykan-Erel, S.; Karaalp, C.; Bedir, E.; Kaehlig, H.; Glasl, S.; Khan, S.; Krenn, L. Secondary metabolites of Centaurea calolepis and evaluation of cnicin for anti-inflammatory, antioxidant, and cytotoxic activities. Pharm. Biol. 2011, 49, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, G.; Fua, J.; Lu, L.; Cheesman, M.J.; Cock, I.E. A Review of the Traditional Uses, Medicinal Properties and Phytochemistry of Centaurea benedicta L. Pharmacogn. J. 2021, 13, 798–812. [Google Scholar] [CrossRef]
- Neganova, M.E.; Afanas’eva, S.V.; Klochkov, S.G.; Shevtsova, E.F. Mechanisms of Antioxidant Effect of Natural Sesquiterpene Lactone and Alkaloid Derivatives. Bull. Exp. Biol. Med. 2012, 152, 660–663. [Google Scholar] [CrossRef] [PubMed]
| Compound | miLogP | TPSA | Logbb | natoms | MW | nON | nOHON | Violations | nrotb | Volume |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.44 | 113.29 | −0.90 | 27 | 378.42 | 7 | 3 | 0 | 8 | 349.47 |
| 2 | 1.15 | 119.37 | −0.85 | 30 | 420.46 | 8 | 2 | 0 | 10 | 385.99 |
| 3 | 1.55 | 86.99 | −0.47 | 21 | 296.36 | 5 | 3 | 0 | 6 | 287.55 |
| 4 | 0.53 | 113.29 | −0.89 | 27 | 378.42 | 7 | 3 | 0 | 6 | 349.12 |
| 5 | 1.24 | 119.37 | −0.84 | 30 | 420.46 | 8 | 2 | 0 | 8 | 385.63 |
| 6 | −0.21 | 130.37 | −1.17 | 28 | 394.42 | 8 | 3 | 0 | 6 | 352.61 |
| 7 | 2.60 | 98.37 | −0.42 | 25 | 344.32 | 7 | 2 | 0 | 4 | 292.67 |
| 8 | 2.60 | 98.37 | −0.42 | 25 | 344.32 | 7 | 2 | 0 | 4 | 292.67 |
| 9 | 2.91 | 87.38 | −0.26 | 26 | 358.35 | 7 | 1 | 0 | 5 | 310.20 |
| 10 | 0.14 | 199.51 | −1.81 | 34 | 478.41 | 12 | 7 | 2 | 5 | 389.73 |
| 11 | 0.12 | 190.28 | −1.72 | 32 | 448.38 | 11 | 7 | 2 | 4 | 364.19 |
| 12 | 1.37 | 57.53 | −0.20 | 10 | 138.12 | 3 | 2 | 0 | 1 | 119.06 |
| 13 | −1.01 | 46.53 | −0.46 | 8 | 116.12 | 3 | 1 | 0 | 0 | 104.80 |
| Imipramine | 4.16 | 6.48 | 0.74 | 21 | 280.42 | 2 | 0 | 0 | 4 | 287.31 |
| GPCR Ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor | |
|---|---|---|---|---|---|---|
| 1 | 0.14 | −0.07 | −0.18 | 0.90 | 0.13 | 0.61 |
| 2 | 0.07 | −0.08 | −0.21 | 0.76 | 0.11 | 0.54 |
| 3 | −0.02 | −0.07 | −0.43 | 0.75 | 0.12 | 0.43 |
| 4 | 0.33 | 0.12 | −0.13 | 0.98 | 0.06 | 0.76 |
| 5 | 0.25 | 0.10 | −0.17 | 0.83 | 0.04 | 0.68 |
| 6 | 0.12 | −0.04 | −0.29 | 0.75 | 0.18 | 0.61 |
| 7 | −0.09 | −0.23 | 0.20 | 0.13 | −0.29 | 0.14 |
| 8 | −0.09 | −0.23 | 0.20 | 0.13 | −0.29 | 0.14 |
| 9 | −0.10 | −0.23 | 0.18 | 0.12 | −0.27 | 0.12 |
| 10 | 0.01 | −0.14 | 0.09 | 0.07 | −0.16 | 0.35 |
| 11 | 0.06 | −0.05 | 0.10 | 0.20 | −0.05 | 0.41 |
| 12 | −0.98 | −0.39 | −1.21 | −0.62 | −1.19 | −0.41 |
| 13 | −2.52 | −2.44 | −3.42 | −2.91 | −2.47 | −2.24 |
| Adenosine | 1.10 | |||||
| Capsazepine | −0.03 | |||||
| K-252α | 1.27 | |||||
| Corticosterone | 1.02 | |||||
| Z-VAD-(OMe)-FMK | 1.03 | |||||
| Ebselen | 0.65 | |||||
| NDGA | 0.13 |
| % Lipid Peroxidation Inhibition | % Lipoxygenase Inhibition | |
|---|---|---|
| CEP-Crude extract | 22.1 a ± 0.23 | n.a. |
| CEP-Organic phase Β | 6.1 b ± 0.29 | 18.4 c ± 0.37 |
| 1 | n.a. | 74.4 a ± 0.65 |
| 2 | n.a. | 73.3 b ± 0.31 |
| 4 | 2.7 c ± 0.06 | n.a. |
| 6 | n.a. | n.a. |
| NDGA | n.m. | 93.0 |
| TROLOX | 95.0 | n.m. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, K.M.; Tsiftsoglou, O.; Hadjipavlou-Litina, D.; Arfan, M.; Lazari, D. Phytochemical Study of the Plant Centaurea bruguieriana (DC.) Hand.-Mazz. subsp. belangeriana (DC.) Bornm. of the Family Asteraceae. Separations 2024, 11, 319. https://doi.org/10.3390/separations11110319
Dimitriadis KM, Tsiftsoglou O, Hadjipavlou-Litina D, Arfan M, Lazari D. Phytochemical Study of the Plant Centaurea bruguieriana (DC.) Hand.-Mazz. subsp. belangeriana (DC.) Bornm. of the Family Asteraceae. Separations. 2024; 11(11):319. https://doi.org/10.3390/separations11110319
Chicago/Turabian StyleDimitriadis, Kyriakos Michail, Olga Tsiftsoglou, Dimitra Hadjipavlou-Litina, Mohammad Arfan, and Diamanto Lazari. 2024. "Phytochemical Study of the Plant Centaurea bruguieriana (DC.) Hand.-Mazz. subsp. belangeriana (DC.) Bornm. of the Family Asteraceae" Separations 11, no. 11: 319. https://doi.org/10.3390/separations11110319
APA StyleDimitriadis, K. M., Tsiftsoglou, O., Hadjipavlou-Litina, D., Arfan, M., & Lazari, D. (2024). Phytochemical Study of the Plant Centaurea bruguieriana (DC.) Hand.-Mazz. subsp. belangeriana (DC.) Bornm. of the Family Asteraceae. Separations, 11(11), 319. https://doi.org/10.3390/separations11110319









