Isolation, Purification, and Antioxidant Activity of Polyphenols from Cynanchum auriculatum Royle ex Wight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Materials and Reagents
2.3. Preparation of Ethanol Extract
2.4. Preparation of Ethyl Acetate Extract
2.5. Separation and Purification of Ethyl Acetate Extract
2.6. Compound Structure Identification
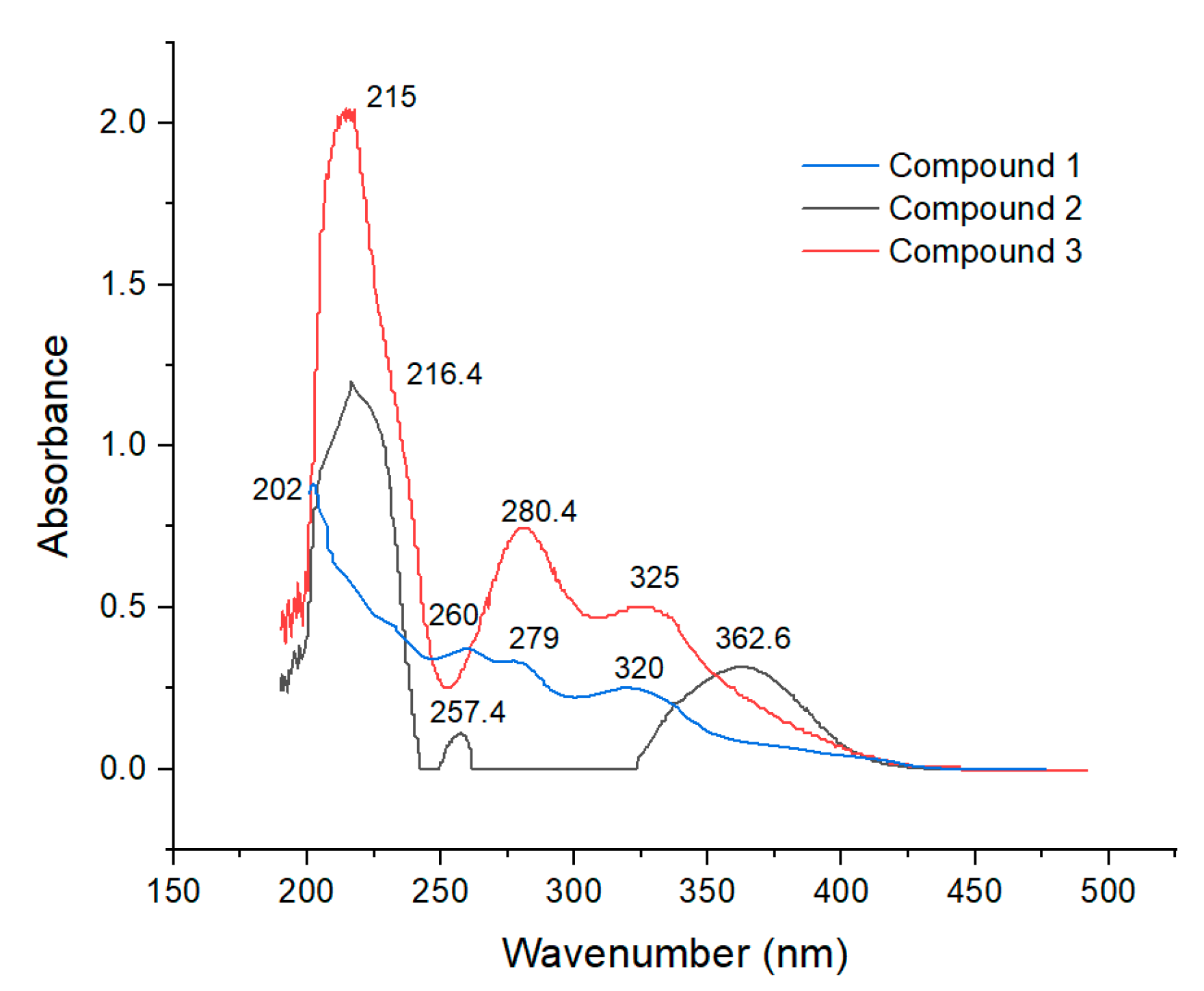
2.6.1. UV Analysis
2.6.2. Infrared Spectroscopy Analysis
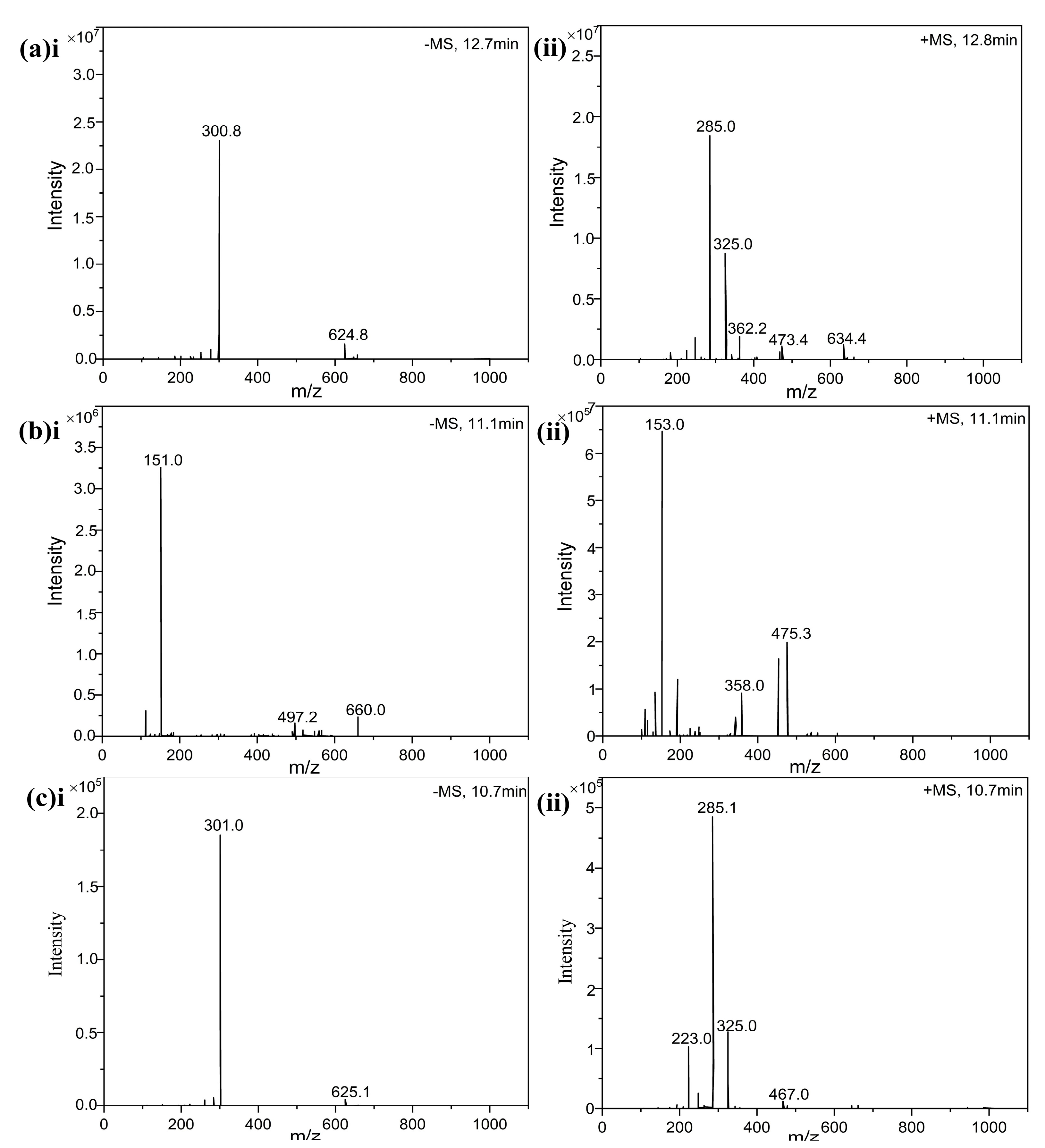
2.6.3. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
2.6.4. Nuclear Magnetic Resonance Analysis (NMR)
2.7. Antioxidant Activity Determination
2.7.1. DPPH Test
2.7.2. Hydroxyl Free Radical Test
2.7.3. ABTS Test
2.7.4. Total Reducing Power Measurement
2.8. Statistical Analysis
3. Results and Discussion
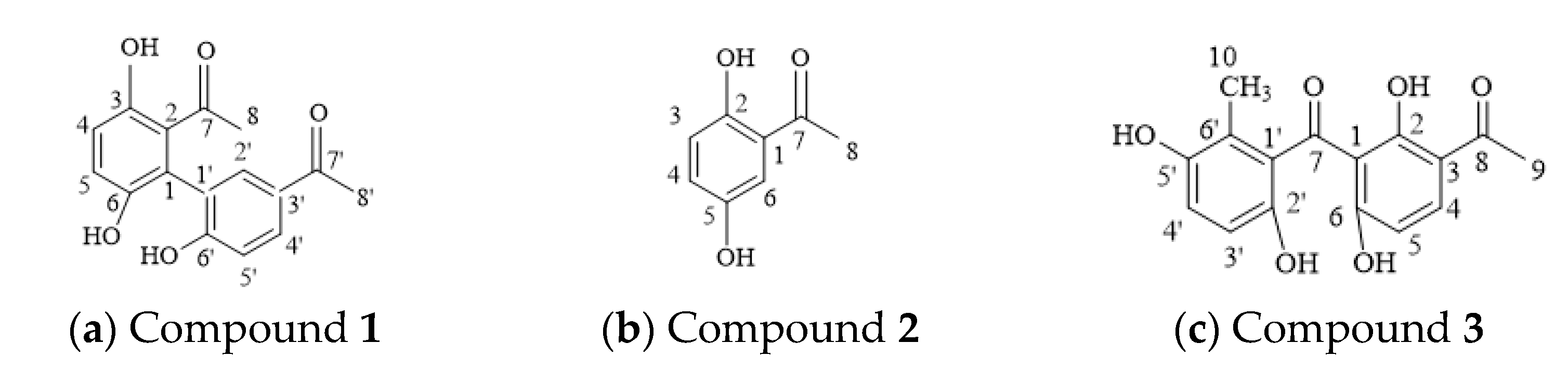
3.1. Chemical Structure Identification
3.1.1. Purified Compound 1
3.1.2. Purified Compound 2
3.1.3. Purified Compound 3
3.2. Antioxidant Activity
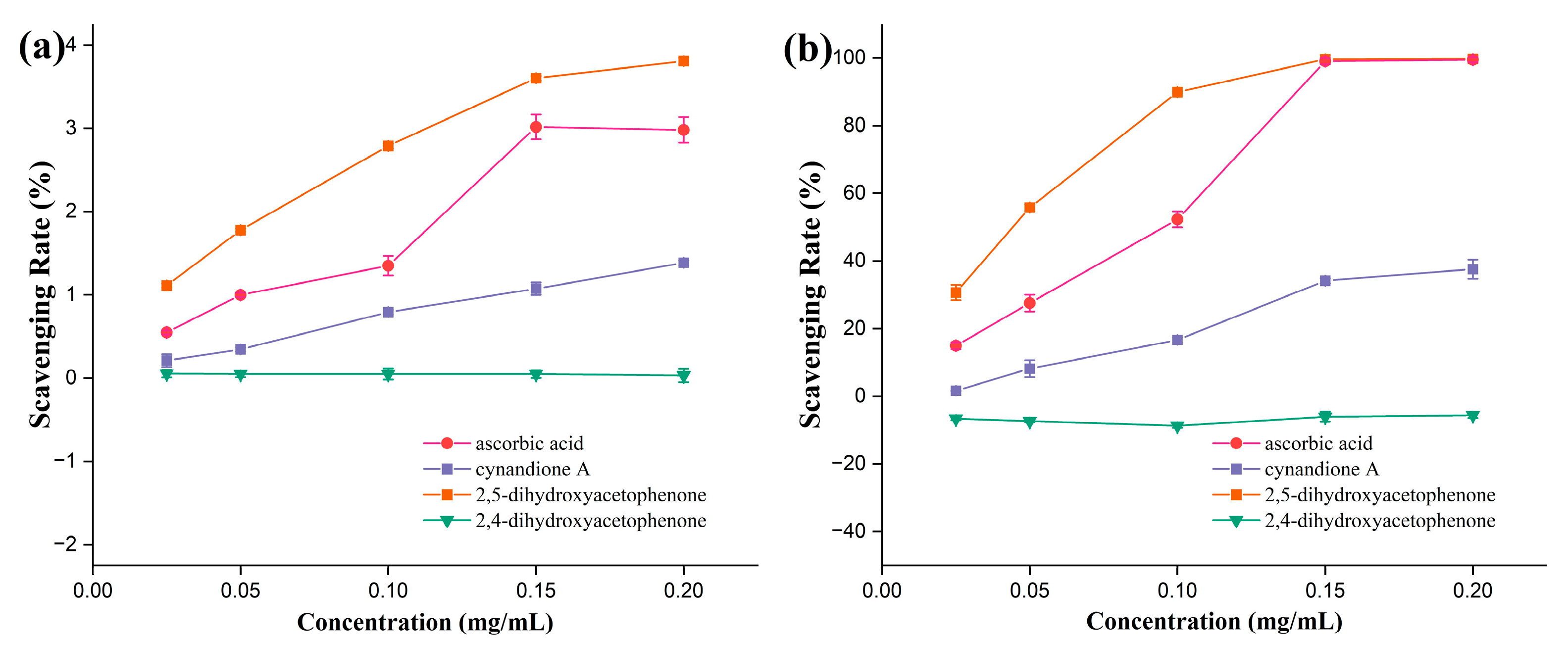
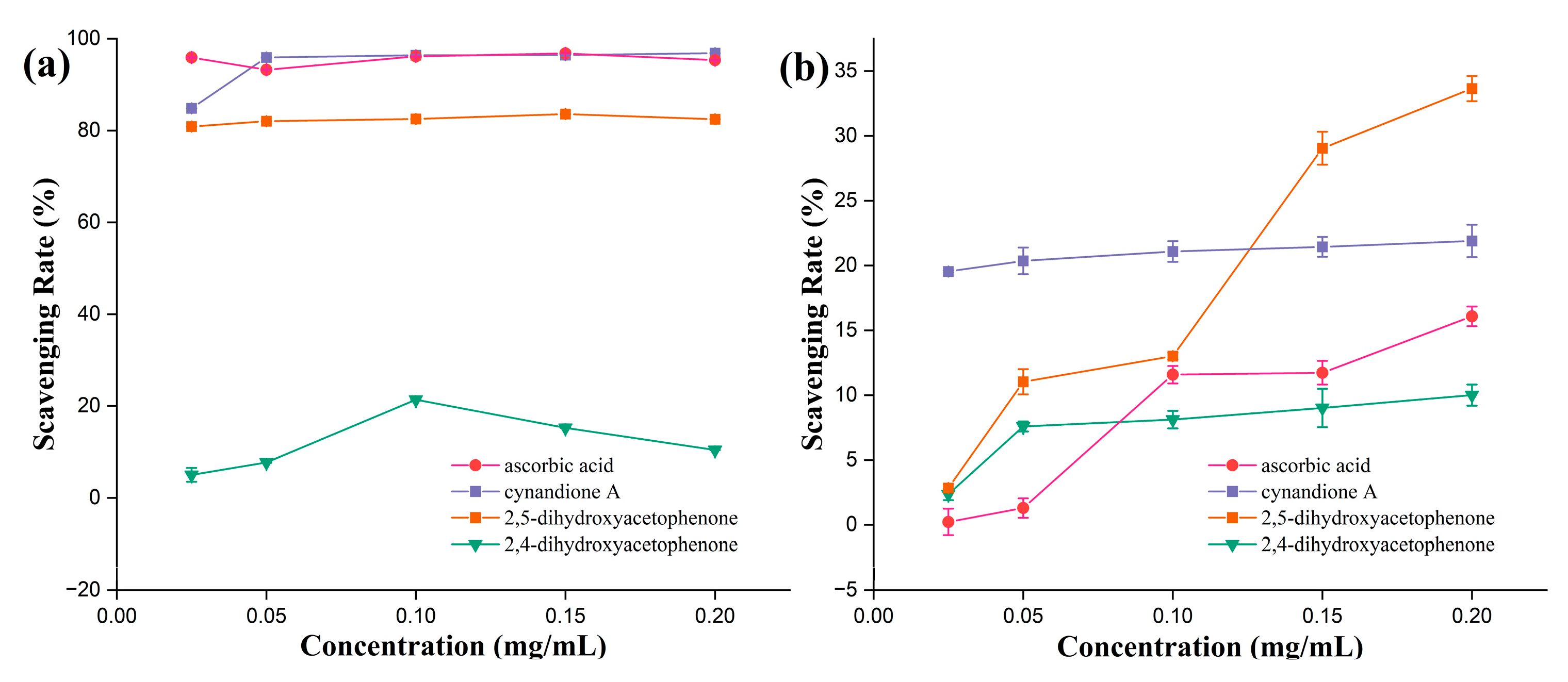
3.2.1. ABTS Radical Scavenging Activity
3.2.2. DPPH· Radical Scavenging Ability
3.2.3. Hydroxyl Radical Scavenging Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, W.H.; Zhang, Z.Z.; Ban, Y.F.; Rahman, K.; Ye, B.Z.; Sun, X.L.; Tan, H.Y.; Zheng, X.H.; Liu, H.Y.; Xu, L.C.; et al. Cynanchum bungei Decne and its two related species for “Baishouwu”: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2019, 243, 112110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F. Industrial development status and countermeasures of Binhai Baishou Aconite. S. China Agric. 2019, 13, 120–121. [Google Scholar]
- Chai, Z.; Huang, W.Y.; Zhao, X.; Wu, H.; Zeng, X.X.; Li, C.Y. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of polysaccharide from Cynanchum auriculatum Royle ex Wight. Int. J. Biol. Macromol. 2018, 119, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.D.; Zhang, M.; He, M.T.; Gu, M.F.; Lin, M.; Zhang, G. Selection of solvent for extraction of antioxidant components from Cynanchum auriculatum, Cynanchum bungei, and Cynanchum wilfordii roots. Food Sci. Nutr. 2019, 7, 1337–1343. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Chen, C.L.; Li, B.C. Influence of steroidal glycosides from Cynanchum auriculatum on antioxidant indicators in H2O2-damaged PC12 cells. Pak. J. Pharm. Sci. 2022, 35, 1379–1384. [Google Scholar]
- Wang, X.J.; Li, Z.L.; Lv, X.H.; Zuo, Q.Y.; Zhao, Y.M.; Ding, Y.F.; Pu, S.B.; Qian, S.H.; Peng, Y.R. Antitumor evaluation and multiple analysis on different extracted fractions of the root of Cynanchum auriculatum Royle ex Wight. J. Sep. Sci. 2017, 40, 3054–3063. [Google Scholar] [CrossRef]
- Peng, Y.R.; Li, Y.B.; Liu, X.D.; Zhang, J.F.; Duan, J.A. Antitumor activity of C21 steroidal glycosides from Cynanchum auriculatum Royle ex Wight. Phytomedicine 2008, 15, 1016–1020. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.W.; Choi, W.J.; Sohn, U.D.; Kim, W. The Effect of Polyphenols Isolated from Cynanchi wilfordii Radix with Anti-inflammatory, Antioxidant, and Anti-bacterial Activity. Korean J. Physiol. Pharmacol. 2015, 19, 151–158. [Google Scholar] [CrossRef]
- Jang, M.; Lim, T.G.; Lee, E.; Hong, H.D.; Rhee, Y.K.; Lee, Y.J.; Lee, J.H.; Cho, C.W. Immunostimulatory Activities of a High Molecular Weight Fraction of Cynanchum auriculatum Royle ex Wight Root Obtained by Ultrafiltration. Pharmacogn. Mag. 2020, 16, 493–498. [Google Scholar]
- Wang, Y.L.; Han, J.; Yue, Y.; Wu, Y.Z.; Zhang, W.Q.; Xia, W.; Wu, M.Q. Purification, structure identification and immune activity of a neutral polysaccharide from Cynanchum auriculatum. Int. J. Biol. Macromol. 2023, 237, 124142. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, C.S.; Hu, Y.L.; Cheng, H.Y.; Liu, T.; Liu, Y.; Xu, T.Q.; Shu, Q.; Zhou, G.X. Two new pregnane glycosides from the root of Cynanchum auriculatum. J. Asian Nat. Prod. Res. 2023, 25, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.M.; Lv, B.X.; Zhao, J.H.; He, W.B.; Li, Y.Q.; Xiao, Y.L. Study on pharmacokinetic of Baishouwu benzophenone in rats after intragastric administration the extract of Cynanchum bungei Decne. J. Taishan Med. Coll. 2020, 41, 349–352. [Google Scholar]
- Jiang, H.W.; Lin, J.; Wang, G.M.; Zhang, J.J.; Gu, S.S.; Cao, L.; Chen, Y.; Wang, L.; Jiao, H.; Zhu, W.L.; et al. Acetophenone derivatives from the root bark of Cynanchum wilfordii as potential neuroprotective agents. Phytochem. Lett. 2018, 24, 179–183. [Google Scholar] [CrossRef]
- Kong, L.Y. Natural Medicinal Chemistry, 1st ed.; The Medicine Science and Technology Press of China: Beijing, China, 2015; pp. 266–268. [Google Scholar]
- Wang, S.M.; Fu, Y.; Liu, D.; Wang, Y.F.; Li, L.G.; Huo, C.H.; Li, Y.; Liu, J.; Zhang, M.L.; Shi, Q.W. Historical story on natural medicinal chemistry: Review on the application of the UV, IR, MS and NMR spectra in the structure elucidation of natural products. CHM 2016, 47, 2779–2796. [Google Scholar]
- Males, I.; Maric, T.; Vladimir-Knezevic, S.; Dragovic-Uzelac, V.; Dobrincic, A.; Skroza, D.; Males, Z.; Jerkovic, I. Determination of Phenolic Content and DPPH Radical Scavenging Activity of Functional Fruit Juices Fortified with Thymus serpyllum L. and Salvia officinalis L. Extracts. Croat. Chem. Acta 2023, 96, 51–57. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Wang, W.D. Optimization of ultrasonic-assisted extraction and in vitro, antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yu, J.L.; Xu, Q.; Zhu, J.Y.; Zhang, H.; Xia, G.Q.; Zang, H. Evaluation of total phenolic, flavonoid, carbohydrate contents and antioxidant activities of various solvent extracts from Angelica amurensis root. Nat. Prod. Res. 2021, 35, 4084–4088. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Qin, B.; Liao, S.; Liu, S.Y.; Pan, C.L.; He, Z.W.; Song, J.L. In vitro antioxidant capability of white tea ethanol extracts. Sci. Technol. Food Ind. 2018, 39, 39–43. [Google Scholar]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Wang, X.; Li, J.; Yang, P.; Yang, B. Separation and Preparation of acetophenones from Radix Aconitum Rhizome by silica gel column chromatography combined with high speed countercurrent chromatography. Chin. Mod. Tradit. Chin. Med. 2016, 18, 956–959, 973. [Google Scholar]
- Sun, X.; Guo, L.; Kang, Y. A study on the chemical kinetics of Polygonum multiflorum concoction. Propr. Chin. Med. 1988, 7, 20. [Google Scholar]


| 1 (CD3OD) | 2 (CD3OD) | 3 (CD3OD) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | δC | δH | Multiplicity; J (Hz) | δC | δH | Multiplicity; J (Hz) | δC | δH | Multiplicity; J (Hz) |
| 1 | 127.7 | 126.3 | 111.3 | ||||||
| 2 | 120.3 | 152.7 | 162.9 | ||||||
| 3 | 152.2 | 115.8 | 6.78 | 1H; d; 8.9 | 112.6 | ||||
| 4 | 118.2 | 6.79 | 1H; d; 8.8 | 121.8 | 7.00 | 1H; dd; 8.9; 3.0 | 133.1 | 7.83 | 1H; d; 8.9 |
| 5 | 121.7 | 6.93 | 1H; d; 8.9 | 151.2 | 108.0 | 6.82 | 1H; d; 8.8 | ||
| 6 | 149.0 | 7.22 | 1H; d; 3.0 | 162.4 | |||||
| 7 | 207.4 | 200.1 | 204.2 | ||||||
| 8 | 30.8 | 2.17 | 3H; s | 26.3 | 2.58 | 3H; s | 203.2 | ||
| 9 | 29.9 | 2.58 | 3H; s | ||||||
| 10 | 25.6 | ||||||||
| 1’ | 114.4 | 119.2 | |||||||
| 2’ | 163.7 | 148.1 | |||||||
| 3’ | 113.1 | 118.1 | 6.54 | 1H; d; 8.8 | |||||
| 4’ | 133.9 | 7.78 | 1H; d; 8.9 | 122.6 | 6.97 | 1H; d; 8.7 | |||
| 5’ | 108.8 | 6.49 | 1H; d; 8.9 | 153.1 | |||||
| 6’ | 163.7 | 123.9 | 2.22 | 3H; s | |||||
| 7’ | 204.6 | ||||||||
| 8’ | 26.3 | 2.56 | 3H; s | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiru, M.M.; Sun, Y.-E.; Zhao, L.; Bunhok, T.; Roth, C.M.; Sovath, S.; Pharith, H.; Wang, W.; Li, C. Isolation, Purification, and Antioxidant Activity of Polyphenols from Cynanchum auriculatum Royle ex Wight. Separations 2024, 11, 316. https://doi.org/10.3390/separations11110316
Nasiru MM, Sun Y-E, Zhao L, Bunhok T, Roth CM, Sovath S, Pharith H, Wang W, Li C. Isolation, Purification, and Antioxidant Activity of Polyphenols from Cynanchum auriculatum Royle ex Wight. Separations. 2024; 11(11):316. https://doi.org/10.3390/separations11110316
Chicago/Turabian StyleNasiru, Mustapha Muhammad, Yue-E Sun, Lingyun Zhao, Taing Bunhok, Chuon Mony Roth, Sun Sovath, Hay Pharith, Weidong Wang, and Chunyang Li. 2024. "Isolation, Purification, and Antioxidant Activity of Polyphenols from Cynanchum auriculatum Royle ex Wight" Separations 11, no. 11: 316. https://doi.org/10.3390/separations11110316
APA StyleNasiru, M. M., Sun, Y.-E., Zhao, L., Bunhok, T., Roth, C. M., Sovath, S., Pharith, H., Wang, W., & Li, C. (2024). Isolation, Purification, and Antioxidant Activity of Polyphenols from Cynanchum auriculatum Royle ex Wight. Separations, 11(11), 316. https://doi.org/10.3390/separations11110316







