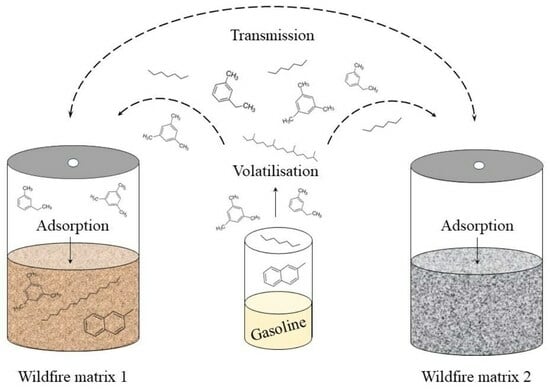
Cross-Contamination of Ignitable Liquid Residues on Wildfire Debris—Detection and Characterization in Matrices Commonly Encountered at Wildfire Scenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Analysis by GC×GC-TOFMS
2.3. Glovebox Experiment
2.4. Data Acquisition, Processing, and Analysis
3. Results and Discussion
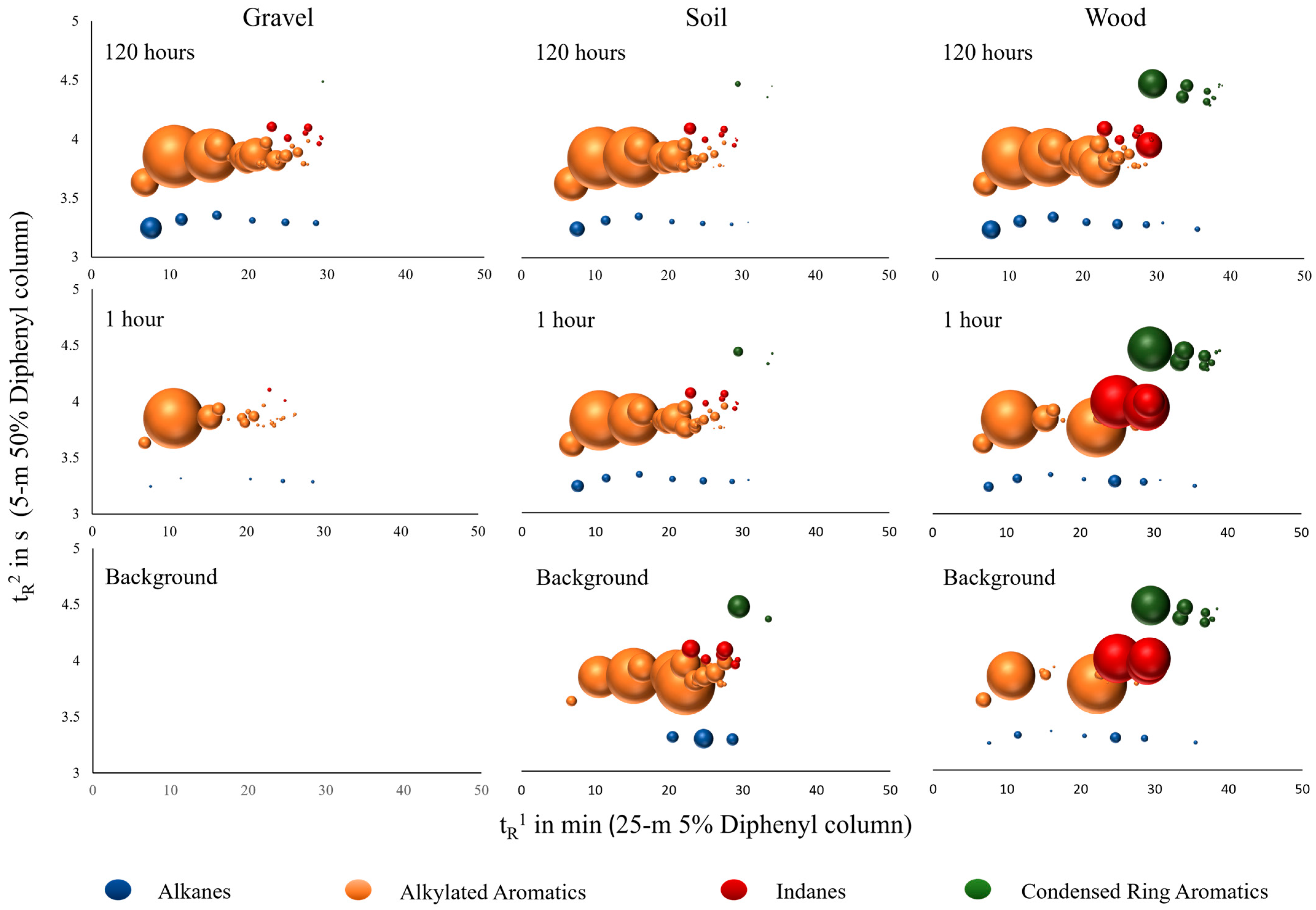
3.1. Detection of Cross-Contamination
3.1.1. Visual Comparison
3.1.2. Uptake Rates
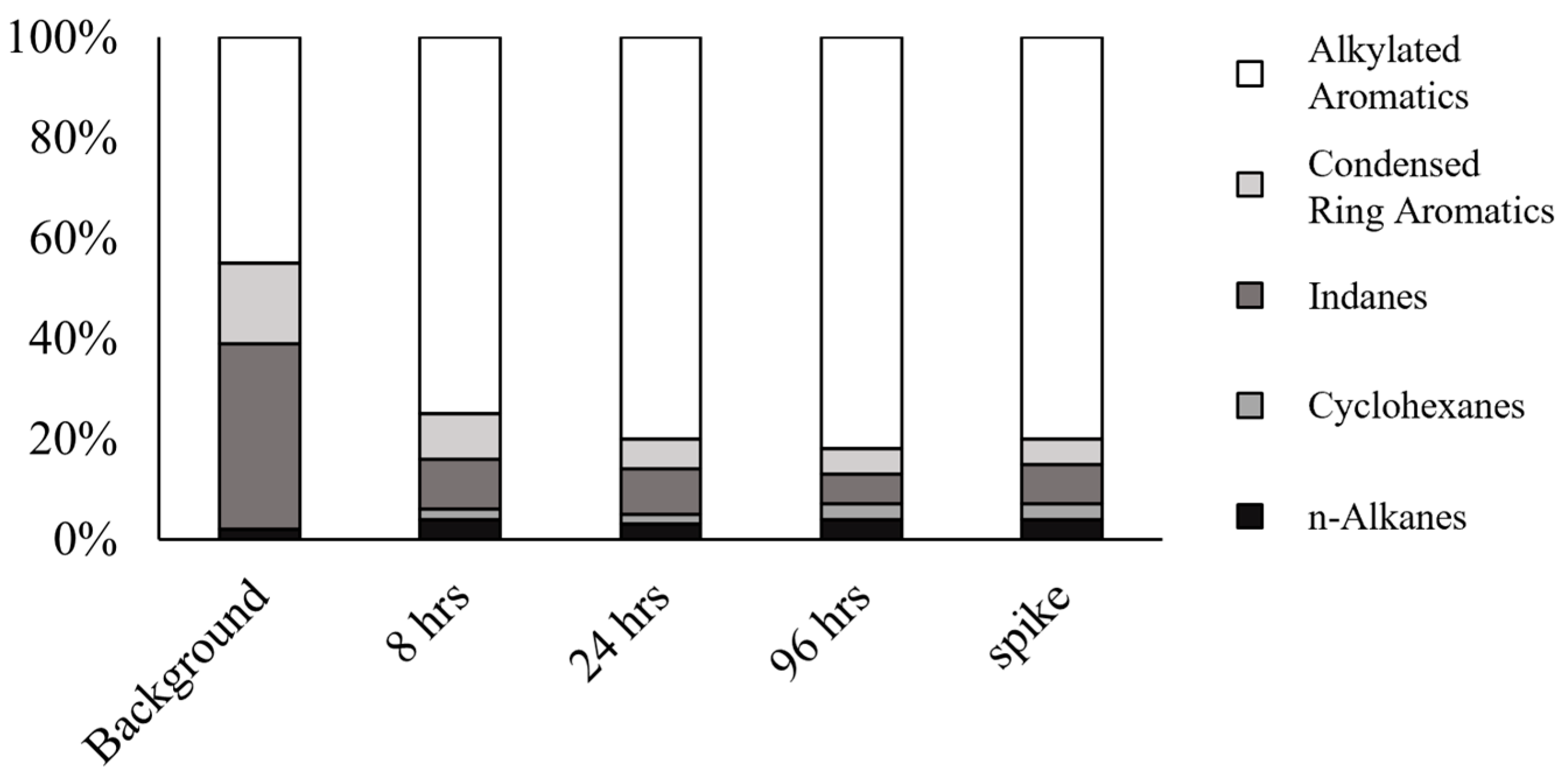
3.2. Major Compound Groups and Relative Distribution
3.3. Chemometric Analysis of Cross-Contamination
3.3.1. Principal Component Analysis and Volcano Plot Interrogation
3.3.2. Diagnostic Ratio Analysis
3.4. Practical Considerations for Sampling and Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Justice, N.I.O. Glossary for Crime Scene Investigation: Guides for Law Enforcement; U.S. Department of Justice: Washington, DC, USA, 2009. Available online: https://nij.ojp.gov/topics/articles/glossary-crime-scene-investigation-guides-law-enforcement (accessed on 10 June 2023).
- Williams, M.R.; Sigman, M. Performance Testing of Commercial Containers for Collection and Storage of Fire Debris Evidence. J. Forensic Sci. 2007, 52, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lang, T. A Study of Contamination in Fire Debris Containers. Can. Soc. Forensic Sci. J. 1999, 32, 75–83. [Google Scholar] [CrossRef]
- Borusiewicz, R.; Kowalski, R. Volatile organic compounds in polyethylene bags—A forensic perspective. Forensic Sci. Int. 2016, 266, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Grutters, M.M.P.; Dogger, J.; Hendrikse, J.N. Performance Testing of the New AMPAC Fire Debris Bag Against Three Other Commercial Fire Debris Bags. J. Forensic Sci. 2012, 57, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Belchior, F.; Andrews, S. Evaluation of Cross-contamination of Nylon Bags with Heavy-loaded Gasoline Fire Debris and with Automotive Paint Thinner. J. Forensic Sci. 2016, 61, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, D.; Yan, S.; Cassista, A.; Hrynchuk, R.; Sandercock, P. Degradation of Gasoline, Barbecue Starter Fluid, and Diesel Fuel by Microbial Action in Soil. Can. Soc. Forensic Sci. J. 2001, 34, 49–62. [Google Scholar] [CrossRef]
- Borusiewicz, R. Substrate interferences in identifying flammable liquids in food, environmental and biological samples: Case studies. Sci. Justice 2015, 55, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kates, L.N.; Richards, P.I.; Sandau, C.D. The application of comprehensive two-dimensional gas chromatography to the analysis of wildfire debris for ignitable liquid residue. Forensic Sci. Int. 2020, 310, 110256. [Google Scholar] [CrossRef]
- Baerncopf, J.; Hutches, K. Evaluation of long term preservation of ignitable liquids adsorbed onto charcoal strips: 0 to 2 years. Forensic Chem. 2020, 18, 100234. [Google Scholar] [CrossRef]
- Sinkov, N.; Sandercock, P.; Harynuk, J. Chemometric classification of casework arson samples based on gasoline content. Forensic Sci. Int. 2014, 235, 24–31. [Google Scholar] [CrossRef]
- Sigman, M.; Williams, M. Chemometric applications in fire debris analysis. WIRE’s Forensic Sci. 2020, 2, e1368. [Google Scholar] [CrossRef]
- Boegelsack, N.; Hayes, K.; Sandau, C.; Withey, J.M.; McMartin, D.W.; O’Sullivan, G. Method development for optimizing analysis of ignitable liquid residues using flow-modulated comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2021, 1656, 462495. [Google Scholar] [CrossRef] [PubMed]
- Barberis, E.; Amede, E.; Khoso, S.; Castello, L.; Sainaghi, P.P.; Bellan, M.; Balbo, P.E.; Patti, G.; Brustia, D.; Giordano, M.; et al. Metabolomics Diagnosis of COVID-19 from Exhaled Breath Condensate. Metabolites 2021, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Franchina, F.; Purcaro, G.; Burklund, A.; Beccaria, M.; Hill, J. Evaluation of different adsorbent materials for the untargeted and targeted bacterial VOC analysis. Anal. Chim. Acta 2019, 1066, 146–153. [Google Scholar] [CrossRef]
- Parsons, B.; Marney, L.; Siegler, W.; Hoggard, J.; Wright, B.; Synovec, R. Tile-Based Fisher Ratio Analysis of Comprehensive Two-Dimensional Gas Chromatography Time-of-Flight Mass Spectrometry (GC×GC–TOFMS) Data Using a Null Distribution Approach. Anal. Chem. 2015, 87, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- ASTM E1618-19. Standard Test Method for Ignitable Liquid Residues in Extracts from Fire Debris Samples by Gas Chromatography-Mass Spectrometry. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Boegelsack, N.; Sandau, C.; McMartin, D.; Withey, J.; O’Sullivan, G. Development of retention time indices for comprehensive multidimensional gas chromatography and application to ignitable liquid residue mapping in wildfire investigations. J. Chromatogr. A 2021, 1635, 461717. [Google Scholar] [CrossRef] [PubMed]
- Pandohee, J.; Hughes, J.G.; Pearson, J.R.; Jones, O.A.H. Chemical fingerprinting of petrochemicals for arson investigations using two-dimensional gas chromatography-flame ionisation detection and multivariate analysis. Sci. Justice 2020, 60, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.G.; Petraco, N. Interpretation of Ignitable Liquid Residues in Fire Debris Analysis: Effects of Competitive Adsorption, Development of an Expert System and Assessment of the False Positive/Incorrect Assignment Rate; U.S. Department of Justice: Washington, DC, USA, 2014; not published.
- Jin, J.; Chi, J.; Xue, T.; Xu, J.; Liu, L.; Li, Y.; Deng, L.; Zhang, J. Influence of thermal environment in fire on the identification of gasoline combustion residues. Forensic Sci. Int. 2020, 315, 110430. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, E.; Dolan, J.A.; Newman, R. Fire Debris Analysis; Academic Press: Burlington, MA, USA, 2008. [Google Scholar]
- ASTM E1412-19. Standard Practice for Separation of Ignitable Liquid Residues from Fire Debris Samples by Passive Headspace Concentration With Activated Charcoal. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM E1386-15. Standard Practice for Separation of Ignitable Liquid Residues from Fire Debris Samples by Solvent Extraction. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM E1388-17. Standard Practice for Static Headspace Sampling of Vapors from Fire Debris. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM E1413-19. Standard Practice for Separation of Ignitable Liquid Residues from Fire Debris Samples by Dynamic Headspace Concentration. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM E2154-15a. Standard Practice for Separation and Concentration of Ignitable Liquid Residues from Fire Debris Samples by Passive Headspace Concentration with Solid Phase Microextraction (SPME). ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Almirall, J.; Arkes, H.; Lentini, J.; Mowrer, F.; Pawliszyn, J. Forensic Science Assessments: A Quality and Gap Analysis—Fire Investigation. AAAS: Washington, DC, USA, 2017. [Google Scholar]


| Group | Target Compounds |
|---|---|
| n-Alkanes | heptane, octane, nonane, decane, undecane, dodecane, tridecane, tetradecane, pentadecane, hexadecane, heptadecane, pristane, octadecane, phytane, nonadecane, eicosane, heneicosane, docosane, tricosane, tetracosane, pentacosane, hexacosane, heptacosane, octacosane |
| Cyclohexanes | methylcyclohexane, ethylcyclohexane, propylcyclohexane, butylcyclohexane, pentylcyclohexane, hexylcyclohexane, heptylcyclohexane, octylcyclohexane, nonylcyclohexane, decylcyclohexane |
| Alkylated aromatics | benzene, toluene, ethylbenzene, p-&m-xylene, o-xylene, isopropylbenzene, propylbenzene, 3-ethyltoluene, 4-ethyltoluene, 1,3,5-trimethylbenzene, 2-ethyltoluene, 1,2,4-trimethylbenzene, isobutylbenzene, sec-butylbenzene, 3-, 4-, & 2-isopropyltoluene, 1,2,3-trimethylbenzene, 1,3-diethylbenzene, 3-&4-propyltoluene, 1,4-diethylbenzene, 1-ethyl-3,5-dimethylbenzene, n-butylbenzene, 1,2-diethylbenzene, 2-propyltoluene, 1-ethyl-2,5-dimethylbenzene, 1-ethyl-3,4-dimethylbenzene, 1-ethyl-2,4-dimethylbenzene, 1-ethyl-2,6-dimethylbenzene, 1-ethyl-2,3-dimethylbenzene, isopentylbenzene, 1,2,4,5-tetramethylbenzene, 1,2,3,5-tetramethylbenzene, 1-tert-butyl-2-methylbenzene, 1,2,3,4-tetramethylbenzene, pentylbenzene, 1,3,5-triethylbenzene, 1,2,4-triethylbenzene, hexylbenzene |
| Indanes | indane, 1-methylindane, 5-methylindane, 2-methylindane, 4,7-dimethylindane dimethylindane isomers |
| Condensed ring aromatics | naphthalene, 2-methylnaphthalene, 1-methylnaphthalene, 2-ethylnaphthalene, 1-ethylnaphthalene, 2,6- & 1,3- & 1,7-dimethylnaphthalene, 1,6-dimethylnaphthalene, 1,4-dimethylnaphthalene, 1,2-dimethylnaphthalene, 1,8-dimethylnaphthalene, 2,3-dimethylnaphthalene, 2,3,5-trimethylnaphthalene, trimethylnaphthalene isomers, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benz(a)anthracene |
| Compound | Compound Group | Henry’s Law Constant (KH) a | Octanol/Water Coefficient (log KOW) b | Langmuir Isotherm Adsorption Rates | ||
|---|---|---|---|---|---|---|
| Gravel (r²) | Soil (r²) | Wood (r²) | ||||
| Heptane | n-alkanes | 5.40 × 10−6 | 4.66 | 128.6 (0.88) | 13.3 (0.69) | 23.8 (0.89) |
| Dodecane | n-alkanes | 1.10 × 10−6 | 6.1 | 10.5 (0.63) | 23.3 (0.69) | 6.4 (0.29) |
| Indane | indanes | 1.20 × 10−2 | 3.18 | 3 × 10−5 (0.82) | 3 × 10−6 (0.60) | 1 × 10−5 (0.97) |
| 4,7-Dimethylindane | indanes | n/a | 3.5 | n/a | 3 × 10−6 (0.60) | 3 × 10−7 (0.25) |
| 3-Ethyltoluene | alkylated aromatics | 1.30 × 10−3 | 3.98 | 5 × 10−6 (0.97) | 7 × 10−7 (0.74) | 3 × 10−6 (0.94) |
| 4-Ethyltoluene | alkylated aromatics | 1.40 × 10−3 | 3.63 | 7 × 10−6 (0.56) | 1 × 10−6 (0.67) | 1 × 10−5 (0.97) |
| 1,3,5-Trimethylbenzene | alkylated aromatics | 1.40 × 10−3 | 3.42 | 4 × 10−6 (0.87) | 1 × 10−6 (0.70) | 3 × 10−6 (0.84) |
| 2-Ethyltoluene | alkylated aromatics | 1.80 × 10−3 | 3.53 | 1 × 10−5 (0.93) | 2 × 10−6 (0.70) | 1 × 10−5 (0.92) |
| 1,2,4-Trimethylbenzene | alkylated aromatics | 2.10 × 10−3 | 3.63 | 3 × 10−6 (0.93) | 4 × 10−7 (0.66) | 2 × 10−6 (0.96) |
| Naphthalene | condensed ring aromatics | 3.20 × 10−2 | 3.3 | n/a | −1 × 10−6 (0.26) | −7 × 10−8 (0.51) |
| 2-Methylnaphthalene | condensed ring aromatics | 2.80 × 10−2 | 3.86 | n/a | −6 × 10−7 (0.00) | −3 × 10−7 (0.37) |
| 1-Methylnaphthalene | condensed ring aromatics | 2.60 × 10−2 | 3.87 | n/a | −1 × 10−5 (0.11) | −3 × 10−7 (0.44) |
| tR1 (min) | tR2 (s) | m/z | Library Match | Target Affiliation |
|---|---|---|---|---|
| 14.3 | 2.9 | 55 | 2,6-dimethyloctane | none |
| 15.1 | 2.9 | 85 | 3-Octene, 2,2-dimethyl- | none |
| 15.1 | 3.7 | 84 | Cyclopropane, 1,1,2-trimethyl-3-(2-methylpropyl)- | none |
| 19.4 | 3.7 | 91 | 3-&4-ethyltoluene | Alkylated Aromatics |
| 21.0 | 3.7 | 116 | 1,2,4-trimethylbenzene | Alkylated Aromatics |
| 23.4 | 3.7 | 119 | 1-Ethyl-3,5-Dimethylbenzene | Alkylated Aromatics |
| 24.2 | 3.7 | 105 | 1-Ethyl-2,5-dimethylbenzene | Alkylated Aromatics |
| 24.7 | 3.7 | 119 | 1-Ethyl-2,4-dimethylbenzene | Alkylated Aromatics |
| Ratio | 1 | 2 | 3 | |
|---|---|---|---|---|
| Matrix | Heptane: 1-Ethyl-2,4-dimethylbenzene | Toluene: 1-Ethyl-2,4-dimethyl-benzene | 4-Isopropyltoluene: 2-Methylnaphthalene | |
| Gravel | Background (n = 3) | n/a | n/a | n/a |
| Contaminated (n = 33) | 0.1–7.4 | 15.7–555.1 | n/a | |
| Spiked Background (n = 3) | 0.5–0.6 | 4.6–5.7 | 0.1–0.2 | |
| Soil | Background (n = 3) | 0 | 4.2–5.1 | 57.6–104.9 |
| Contaminated (n = 33) | 0.3–3.0 | 20.5–71.2 | 20.8–293.6 | |
| Spiked Background (n = 3) | 0.6–0.8 | 4.4–4.9 | 0.6–1.0 | |
| Wood | Background (n = 3) | n/a | n/a | 14.1–15.4 |
| Contaminated (n = 34) | 1.1–6.3 | 17.9–192.8 | 8.1–14.9 | |
| Spiked Background (n = 3) | 0.6–0.7 | 4.3–4.8 | 1.5–1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boegelsack, N.; Walker, J.; Sandau, C.D.; Withey, J.M.; McMartin, D.W.; O'Sullivan, G. Cross-Contamination of Ignitable Liquid Residues on Wildfire Debris—Detection and Characterization in Matrices Commonly Encountered at Wildfire Scenes. Separations 2023, 10, 491. https://doi.org/10.3390/separations10090491
Boegelsack N, Walker J, Sandau CD, Withey JM, McMartin DW, O'Sullivan G. Cross-Contamination of Ignitable Liquid Residues on Wildfire Debris—Detection and Characterization in Matrices Commonly Encountered at Wildfire Scenes. Separations. 2023; 10(9):491. https://doi.org/10.3390/separations10090491
Chicago/Turabian StyleBoegelsack, Nadin, James Walker, Court D. Sandau, Jonathan M. Withey, Dena W. McMartin, and Gwen O'Sullivan. 2023. "Cross-Contamination of Ignitable Liquid Residues on Wildfire Debris—Detection and Characterization in Matrices Commonly Encountered at Wildfire Scenes" Separations 10, no. 9: 491. https://doi.org/10.3390/separations10090491
APA StyleBoegelsack, N., Walker, J., Sandau, C. D., Withey, J. M., McMartin, D. W., & O'Sullivan, G. (2023). Cross-Contamination of Ignitable Liquid Residues on Wildfire Debris—Detection and Characterization in Matrices Commonly Encountered at Wildfire Scenes. Separations, 10(9), 491. https://doi.org/10.3390/separations10090491