Abstract
Ficus natalensis subsp. leprieurii also known as the natal fig is a fruit-producing tree belonging to the family Moraceae and widely distributed in African countries and cultivated in Egypt. F. natalensis is common with a myriad of traditional and medicinal importance. Owing to the increased demand for natural products with new structural compositions, the current study aimed to separate and elucidate the structure of triterpenoid saponins from F. natalensis leaves. Our previous biological investigation of F. natalensis leaves revealed its potent antioxidant and anti-inflammatory characteristics, and its ability to alleviate Cd-induced reproductive toxicity. Phytochemical investigation of F. natalensis leaves revealed the separation and structure elucidation of seven (1–7) compounds belonging to triterpenoid saponins using NMR and MS data and identified for the first time in F. natalensis. The isolated compounds were identified as 3-O-β-D-glucopyranosyl (1 → 4) β-D-glucopyranosyl (1 → 4)–α-L-rhamnopyranosyl-quinovic acid-28-O-β-D-glucopyranosyl (1 → 4)–α-L-rhamnopyranosyl (1 → 2)–α-L-arabinopyranoside ester (1), 3-O-β-D-glucopyranosyl (1 → 4) α-L rhamnopyranosyl-quinovic acid-28-O-β-D-glucopyranosyl (1 → 4)–α-L-rhamnopyranosyl (1 → 2) α-L-arabinopyranoside (2), 3-O-β-D-glucopyranosyl–quinovic acid-28-O-β-D-glucopyranosyl ester (3), as 3-O-α-L-rhamnopyranosyl-quinovic acid-28-O-β-D-glucopyranoside ester (4), 3-O-β-D-glucopyranosyl oleanolic acid (5), 3-methoxy-oleanolic acid-28-O-α-L-rhamnopyranoside (6), and 3-O-α-L-rhamnopyranosyl-oleanolic acid-28-O-β-D-glucopyranoside ester (7). Among the identified compounds, compounds 1 and 2 were identified for the first time in nature according to Reaxys and Web of Science database.
1. Introduction
Recently, natural product specialists have made great efforts towards the identification of novel natural products with potential safety and biological activities. Plants are the main source of secondary metabolites including alkaloids, flavonoids, saponins, steroids, and terpenoids with myriad of medicinal and health value that can protect humans from several diseases [1]. Triterpenoid saponins are an important metabolite class abundant in several plant species with a myriad of biological importance [2]. Moraceae (the fig family) is a family of flowering plants comprising about 38 genera and over 1100 species and widely distributed in tropical and subtropical regions [3]. The Ficus genus including about 850 species is considered as one of the most important members of Moraceae and is generally known as fig trees or figs [4]. Several Ficus species have been reported for their traditional importance as widely used in the treatment of constipation, rheumatic disease, dyspepsia, and dysentery, besides their hypoglycemic, anthelmintic, and antihypertensive effects [4]. Ficus natalensis subsp. Leprieurii is an evergreen tree widely distributed in African countries and used traditionally alone or mixed with other medicinal plants to treat several ailments such as colds, cough, sore throat, and dysentery [5]. Several phytochemicals were reported from F. natalensis belonging to different classes including phenolics, triterpenoids, steroids, and volatiles [6,7]. Triterpenoid saponins were previously reported in the Ficus genus providing several biological importance for Ficus species [7]. Moreover, biological studies on F. natalensis leaves revealed an anti-microbial effect [8], and antibacterial activity [9]. Our previous biologically guided study on F. natalensis leaves revealed its potent antioxidant, anti-inflammatory, and effect to alleviate cadmium chloride testicular disruptions [5,10]. Moreover, three triterpenoid saponins with oleanolic acid aglycone were previously isolated from F. natalensis leaves [10]. Triterpenoid saponin with quinovic acid aglycone was previously isolated from Ficus elastica aerial root bark [11]. Hence, the main goal of this study was to separate, purify, and elucidate the structure of triterpenoid saponins from F. natalensis leaves.
2. Materials and Methods
2.1. Plant Material
Ficus natalensis subsp. leprieurii (Miq.) C.C. Berg leaves were gathered in September 2020 from the Horticultural Research Institute, Giza, Egypt. The botanical identification of the plant was supplied by Prof. Dr. Rim Hamdy, Taxonomy Department, Faculty of Science, Cairo University, Egypt. A voucher specimen was deposited at the herbarium of Pharmacognosy Department, Faculty of Pharmacy, Egyptian Russian University, Cairo, Egypt number (01 Fnal/2019).
2.2. General Experimental Procedures
NMR analysis was carried out using Bruker High-Performance Digital FT-NMR Spectrometer Advance III 400 MHZ for 1H & C 13. All samples were analyzed in DMSO-d6 and TMS was used as an internal reference, with the chemical shifts expressed in ppm and coupling constants (J) in Hertz, (The analysis was done in the NMR unit at the Faculty of Pharmacy, Cairo University, Egypt). The mass analysis was carried out on a Direct Inlet part to the mass analyzer in Thermo Scientific GCMS model ISQ at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Nasr City, Cairo. The mass spectroscopy system was used to explore the characteristic fragmentation using EI mode and the expected molecular weight at 70 ev. Glass columns packed with silica gel are used for chromatographic separation. A glass column (60 × 2.5 mm) packed with Sephadex LH-20 was used for compound purification. Silica gel 60 F254 precoated aluminum sheets (20 × 20, 0.2 mm, thickness) and (E. Merck, Darmstadt, Germany) for thin layer chromatography. Spots were visualized by ethanol/H2SO4 and vanillin/H2SO4 spray reagents. Solvent system S1 (chloroform–methanol–water (7:2.5:0.5 v/v/v)) was used as a solvent system for TLC. CHCl3 and then a CHCl3/MeOH mixture were used as solvent systems for compound elution on column chromatography.
2.3. Extraction and Fractionation
2.3.1. Extraction and Fractionation of F. natalensis Leaves
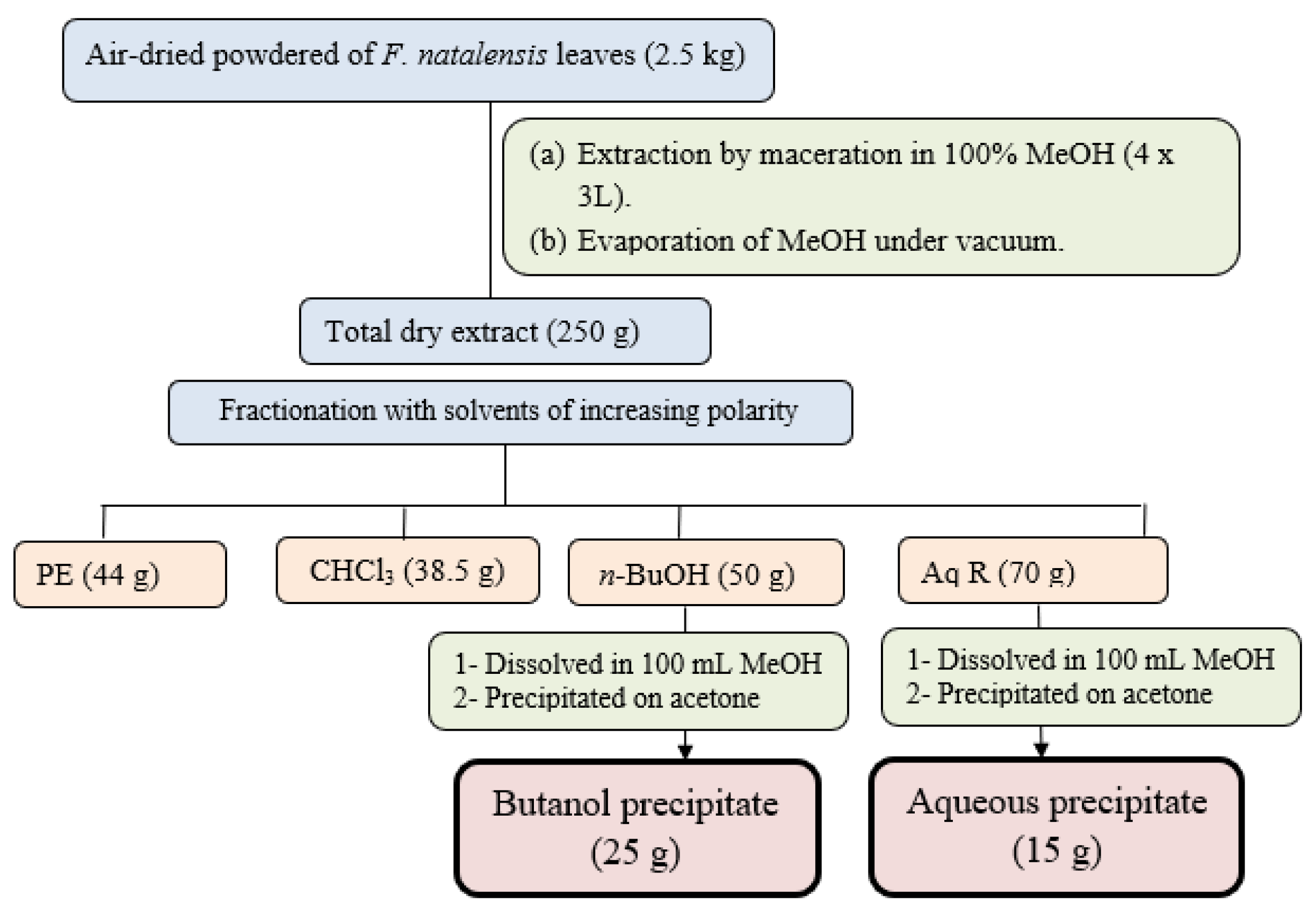
The air-dried ground powdered leaves of F. natalensis (2.5 Kg) were macerated in 100% methanol with occasional stirring at room temperature till exhaustion. The methanol extract was concentrated and dried under vacuum (45 °C) to yield a dry total extract (340 g). The dried total extract (250 g) was suspended in water (350 mL) and subjected to defatting through fractionation with petroleum ether (PE) to remove the lipophilic compounds and chlorophyll to yield petroleum ether fraction (44 g) and the defatted aqueous phase. The defatted aqueous phase was further fractionated using solvents with different polarities including chloroform and n-butanol to yield the chloroform fraction (38.5 g) and n-butanol fraction (50 g). The remaining aqueous phase was dried and labeled as the aqueous residual fraction (Aq R) (70 g). To precipitate the crude saponins, the n-butanol fraction (50 g) was suspended in 100 mL methanol and then poured onto 500 mL pure acetone with continuous stirring. The precipitate was filtrated and dried to yield (25 g) named as butanol precipitate. The aqueous residual fraction (70 g) was treated similarly to yield the second precipitate (15 g) named aqueous precipitate expected to encompass saponins and triterpenoid constituents in both precipitates (Figure 1).
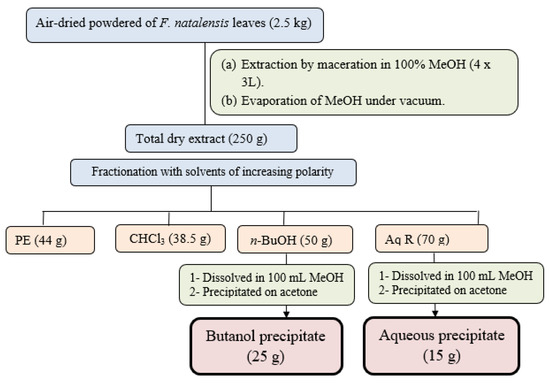
Figure 1.
Extraction and fractionation scheme of F. natalensis leaves.
2.3.2. Purification of the Butanol Precipitate Fraction
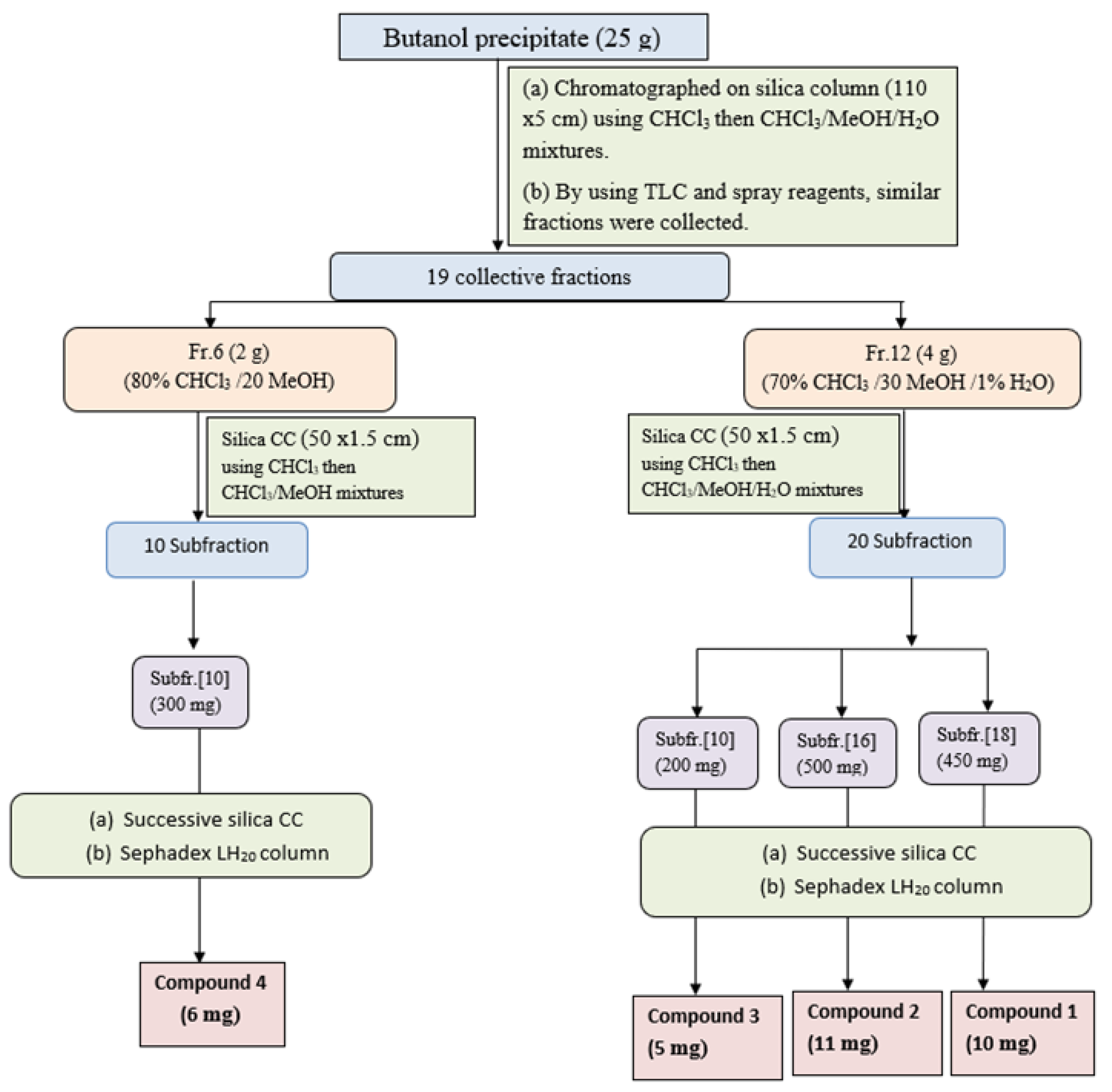
The butanol precipitate (25 g) was purified on a glass column packed with silica gel and elution with gradient CHCl3/MeOH mixtures. Similar fractions were collected according to their similarity pattern on TLC silica plates using solvent S1 and ethanol/H2SO4 and vanillin/H2SO4 spray reagents to give 19 major collective fractions (Figure 2). Fraction-6 (2 g) eluted from 80% CHCl3 and 20% MeOH, was subjected to further purification using a silica gel column and CHCl3/MeOH mixture as an eluent to give 10 main subfractions. Subfraction-5 (200 mg) was subjected to further purification using silica gel columns followed by Sephadex LH20 column using MeOH as an eluent to compound 4 (6 mg). Fraction-12 (4 g) eluted with 70% CHCl3/30% MeOH (from the main column) was subjected to further purification on a silica gel column and eluted with CHCl3/MeOH/H2O mixtures with increasing polarity, to yield 20 main subfractions. Subfraction-6 (350 mg), subfraction-10 (200 mg), and subfraction-18 (450 mg) were subjected separately to further purification using silica gel columns using CHCl3/MeOH as an eluent to yield compound 3 (5 mg), compound 2 (11 mg), and compound 1 (10 mg), respectively.
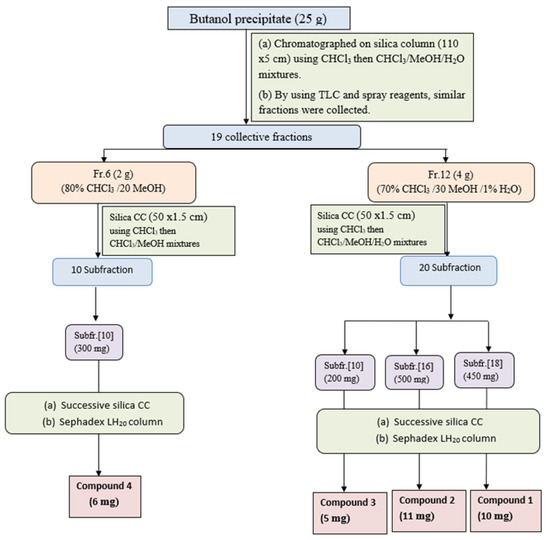
Figure 2.
Isolation and purification scheme of compounds isolated from n-butanol precipitate.
2.3.3. Isolation and Purification of the Aqueous Precipitate Fraction
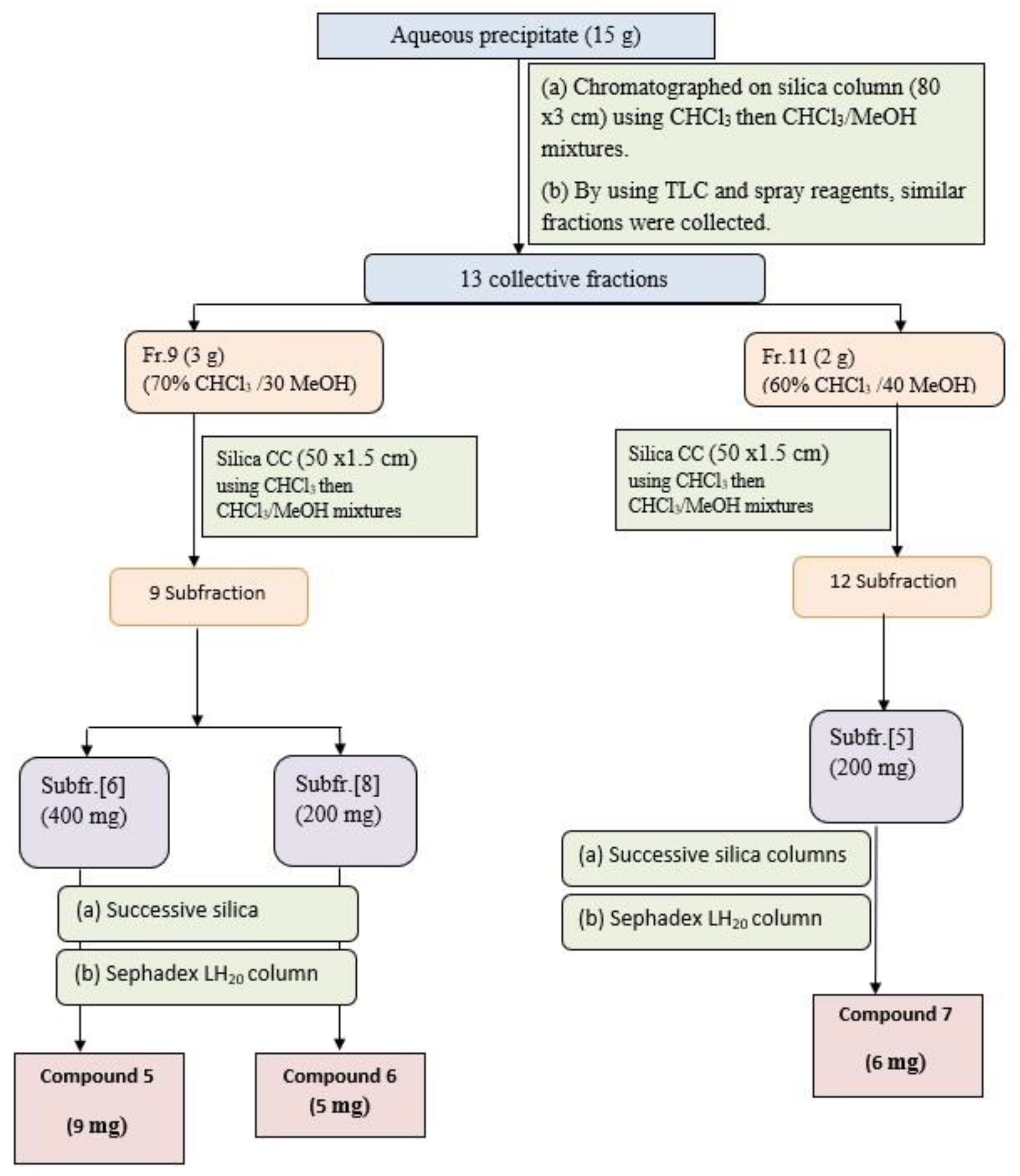
The aqueous precipitate (15 g) was purified on a silica gel column and elution using CHCl3/MeOH mixtures with increasing polarity, to afford 51 individual fractions each (250 mL). Similar fractions were collected using TLC silica plates using the solvent S1 and ethanol/H2SO4 and Vanillin/H2SO4 as spray reagents to give 13 major collective fractions, (Figure 3). Fraction-9 (3 g) eluted with 70% CHCl3 and 30%MeOH, was subjected to further purification on a silica gel subcolumn and eluted with CHCl3/MeOH mixture to yield 9 main subfractions. Subfractions-6 (400 mg) and subfraction-8 (200 mg) were subjected separately to further purification using silica gel columns followed by Sephadex LH-20 column using MeOH as an eluent to give compound 5 (9 mg) and compound 6 (5 mg), respectively. Fraction-11 (2 g) eluted with 60% CHCl3 and 40% MeOH, was further purified on a silica subcolumn and eluted with CHCl3/MeOH mixture to yield 12 main subfractions. Subfraction-5 (200 mg) was subjected to more purification on the silica column followed by Sephadex LH-20 column to yield compound 7 (6 mg).
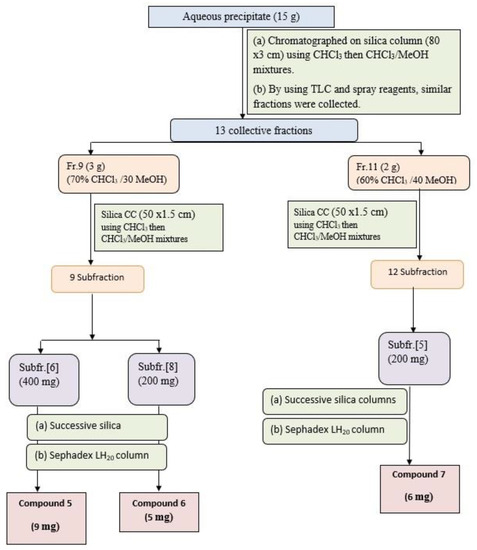
Figure 3.
Isolation and purification scheme of compounds isolated from aqueous precipitate.
2.3.4. Acid Hydrolysis for Sugars
Acid hydrolysis for all compounds containing sugars was performed using 1 M HCL on water bath for 2 h at 80 °C, then removing solvent under reduced pressure. Sugars were extracted from the aqueous phase (10 mL) and washed with methylene chloride (15 mL). The combined methylene chloride extract was washed with water to yield the aglycone moiety after evaporation [2,12].
3. Results and Discussion
Phytochemical investigation of F. natalensis leaves led to the isolation and identification of 7 compounds belonging to triterpenoid saponins and the detailed identification of the pure compounds as mentioned below.
3.1. Compound 1
Compound 1 was obtained as a creamy white amorphous powder (10 mg). Chromatographic properties: Rf = 0.33 (S1) on silica gel TLC plate and appeared as purple color with ethanol/H2SO4 and vanillin/H2SO4 spray reagents. 1HNMR (400MHz, DMSO-d6) and 13CNMR (100 MHz, DMSO-d6) spectroscopic data are summarized in Table S1. The 1HNMR spectrum (Figures S1 and S2) of compound 1 revealed the presence of six overlapped methyl signals that appeared at δH 0.84, 0.90, 0.86, 0.87, 0.99, and 1.01 ppm assigned to methyl groups H-23, H-24, H-25, H-26, H-29, and H-30, respectively. Two proton resonances were detected corresponding to oxygenated methine (originally secondary alcoholic groups) at δH 3.80 (m) assigned for H-3 and olefinic proton at δH 5.31 (brt) assigned for H-12, together with the absence of a dd signal in the range of δH 2.2–3.0, suggestive that compound 1 belongs to the ursane series [13,14]. The 13CNMR spectrum (Figure S3) showed signals for two carboxyl carbons assigned for C-27 and C-28 at δC 179.90 and δC 174.36, respectively. Moreover, an ethylenic carbon C-12 was detected at δC 130.25, a quaternary carbon C-13 at δC 136.97 and C-19 at δC 38.82, as well as C-20 at δC 39.02. 1HNMR and 13CNMR revealed the characteristic signals corresponding to an ursane-type triterpene skeleton for the aglycone having an olefinic bond at C-12 and two carboxyl groups at C-27 and C-28, which falls in accordance with the structure of quinovic acid (3β-ol-urs-12-en-27,28-dioic acid) [14].
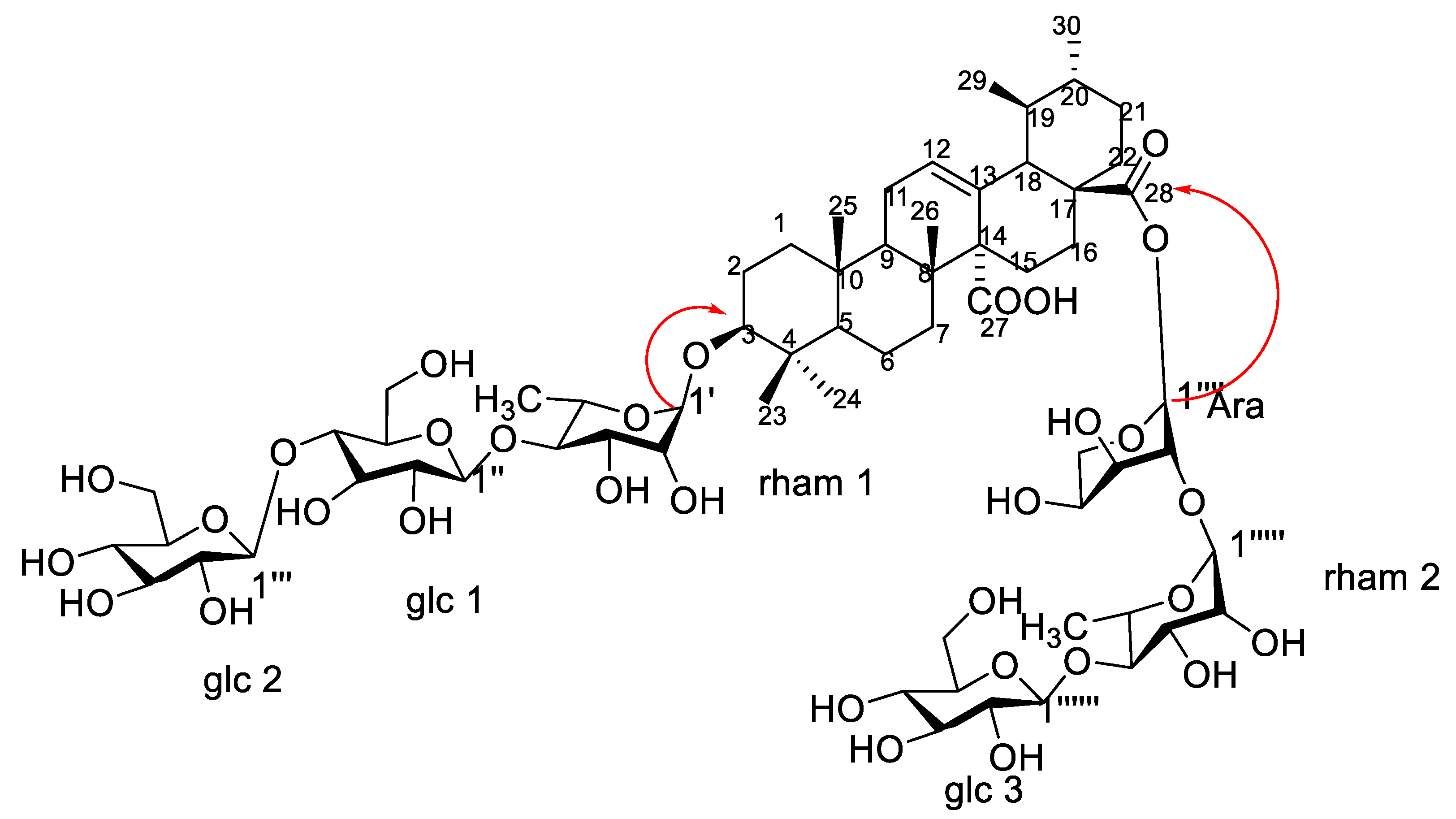
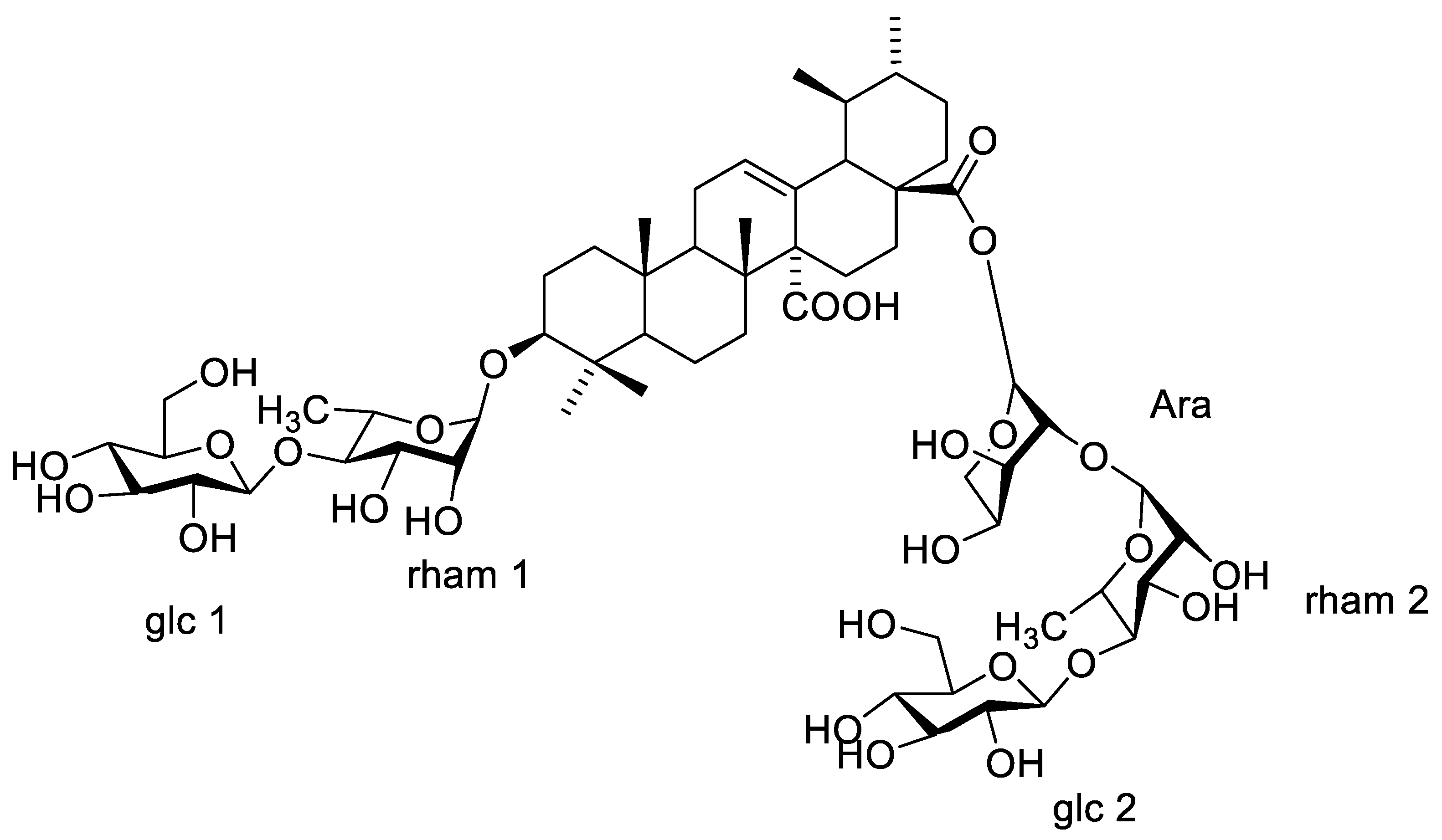
1H-NMR spectrum showed six anomeric proton signals at δH 5.61 (d, J = 1.5), 4.36 (d, J = 7.8), 4.64 (d, J = 7.8), 4.55 (d, J = 3.0), 5.15 (d, J = 1.5), and 4.78 (d, J = 7.8) assigned for H-1′, H-1″, H-1′′′, H-1′′′′, H-1′′′′′, and H-1′′′′′′, respectively, two methyl signals were observed at δH 0.99 and 1.01 corresponding to C-6 of two rhamnose moieties. 13CNMR spectrum exhibited six anomeric signals at δC 98.5, 100.25, 102.41, 92.65, 97.32, and 104.60 corresponding to C-1′, C-1″, C-1′′′, C-1′′′′, C-1′′′′′, and C-1′′′′′′, respectively, indicating the presence of six saccharide moieties. The downfield shift of C-3 at δC 82.32 and the upfield shift due to esterification of C-28 at δC 174.36 showed that the sugar chains are located at these positions. The acid hydrolysis using 1 N HCl and the comparison of the hydrolysate with authentic sugars using PC co-chromatography led to the identification of sugars as rhamnose, glucose and arabinose. All 1D NMR signals assigned in NMR spectra were finally confirmed using homo- and hetero-nuclear long-range coupling 2D HSQC, and HMBC spectra. A direct correlation was observed in HSQC and HMBC spectra of compound 1, different J2 and J3 sets of correlations between hydrogens and carbons were assigned. HSQC (Figure S4) revealed the major correlation of olefinic proton H-12 (5.31) with the corresponding carbon at δC 130.25 and of H-3 at δH 3.80 with the corresponding carbon C-3 at δC 82.32 confirmed the down field shift of C-3, which confirmed the substitution of C-3 position. The absence of any downfield shift of any of the carbons of two glucose units (glc 2 and glc 3) suggested that they are terminal sugars. The small coupling constant of the anomeric protons of rhamnose moieties (J = 1.5) led to configuration assignment as α sugar, while the large coupling of the glucose units’ anomeric protons (J = 7.8) led to the identification of their configuration as β sugars [14]. HMBC spectrum (Figures S5 and S6) revealed that the attachment of one of the sugar chains is at C-3 from the correlation of H-1′ (rham 1) at δH 5.61 with C-3 at δC 82.32. The downfield shift of C-4 of the rhamnose moiety together with the downfield shift of C-4 of the glucose unit (glc 1) led to the assumption of the sequence of the first saccharide chain to be D-glucopyranosyl (1 → 4)-D-glucopyranosyl (1 → 4)-L-rhamnopyranosyl. The attachment of the second sugar chain at C-28 was recognized from the upfield shift of the carbon of the carboxyl group at δC 174.36 (as compared to non-esterified quinovic acid), indicating its esterification by the sugar chain. The chemical shift of C-1 arabinose assigned it as the first sugar attached to the carboxylic group. Moreover, the downfield shift of its C-2 at δC 75.27 suggested that it is the point of attachment of the second rhamnose moiety (rahm. 2). The downfield shift of C-4 of the second rhamnose allowed for the identification of the point of attachment of the terminal glucose (glc 3), with the second saccharide chain proposed to be D-glucopyranosyl (1 → 4)-L-rhamnopyranosyl (1 → 2)arabinopyranosyl. The EI-MS had a molecular ion peak [M]+ at m/z 1365.51 (calcd. 1365.60), corresponding to the molecular formula C63H96O32 (calcd. C63H97O32). Consequently, from the aforementioned NMR data and comparing with previously reported literature of structurally related compounds [13,14], compound 1 was assigned as 3-O-β-D-glucopyranosyl (1 → 4) β-D-glucopyranosyl (1 → 4)–α-L-rhamnopyranosyl-quinovic acid-28-O-β-D-glucopyranosyl (1 → 4)–α-L-rhamnopyranosyl (1 → 2)–α-L arabinopyranoside ester (Figure 4) which is reported for the first time from F. natalensis leaves. By searching for the compound novelty in nature on Reaxys and Web of Science databases, compound 1 was identified for the first time from nature.
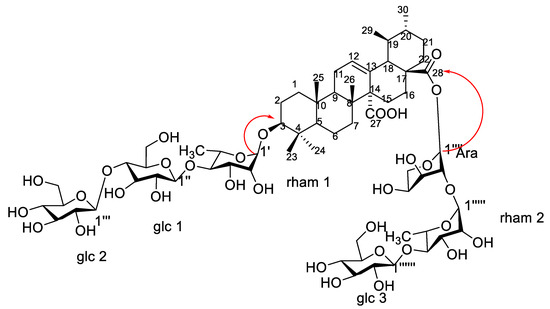
Figure 4.
Structure and major correlations of compound 1.
3.2. Compound 2
Compound 2 was isolated as a yellowish-white amorphous powder (11 mg). Chromatographic properties: Rf = 0.35 (S1) on the silica gel TLC plate gave a pink color with ethanol/H2SO4 and vanillin/H2SO4 spray reagents. 1HNMR (400 MHz, DMSO-d6) and 13CNMR (125 MHz, DMSO-d6): Spectroscopic data are summarized in Table S1. 1H-NMR spectrum (Figure S7) of compound 2 revealed the presence of six angular methyl overlapped signals that appeared at δH 0.84, 0.86, 0.99, 0.87, 0.86, and 1.01 ppm assigned for methyl groups H-23, H-24, H-25, H-26, H-29, and H-30, respectively. Another two proton resonances were noticed at δH 3.18 (m) and δH 5.32 (brt) assigned to H-3 (oxygenated methine) and H-12 (olefinic proton) characteristic for the triterpenoid skeleton.
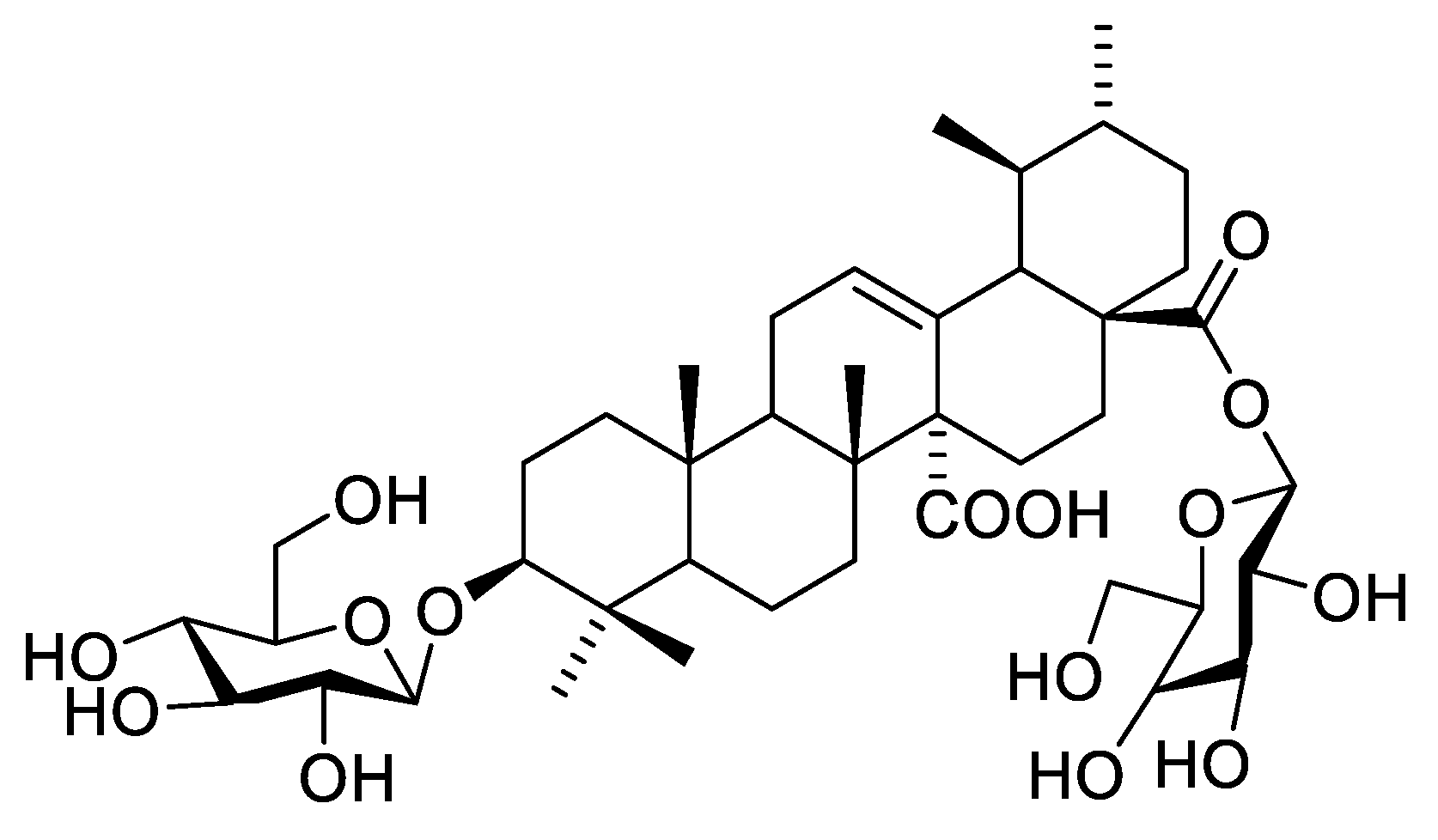
13CNMR spectrum (Figures S8 and S9) showed two signals at δC 179.73 and δC 177.82, characteristic of carboxyl carbons C-27 and C-28, respectively. Moreover, an ethylenic carbon C-12 was detected at δC 130.15 as well as a quaternary carbon C-13 at δC 138.36. The comparison of 1HNMR and 13CNMR spectral data of 2 with that of compound 1 proved their similarity as ursane-type triterpene aglycones, with an olefinic bond at C-12 and two carboxyl groups at C-27 and C-28, which is identified as quinovic acid (3β-ol-urs-12-en-27,28-dioic acid). 1HNMR spectrum showed five anomeric proton signals at δH 4.84 brs, 4.26 (d, J = 8), 5.15 (d, J = 3.0), 4.69 brs, and 4.90 (d, J = 7.8) assigned for H-1′, H-1″, and H-1′′′, and H-1′′′′ and H-1′′′′′, respectively. Moreover, 13CNMR spectra exhibited five anomeric signals at δC 98.51, 104.63, 92.66, 97.35, and 102.44, corresponding to C-1′, C-1″, C-1′′′, C-1′′′′, and C-1′′′′′, respectively, indicating the presence of five sugar moieties attached to the aglycone. 13CNMR data of C-3 at δC 82.35 and of C-28 at δC 177.82 confirmed sugar attachments at these positions. Acid hydrolysis using 1 N HCl and the comparison of the hydrolysate with authentic sugars using PC co-chromatography led to the identification of sugars to be rhamnose, glucose, and arabinose. The spectral data of the sugar moieties of compound 2 showed a large similarity with compound 1, though without one of the glucose units. This led us to the assumption that the sequence of the first saccharide chain is D-glucopyranosyl (1 → 4)-L-rhamnopyranosyl which is located at C-3, and the second saccharide chain is D-glucopyranosyl (1 → 4)-L-rhamnopyranosyl (1 → 2)-L arabinopyranosyl esterifying C-28. The EI-MS with molecular ion peak [M]+ at m/z 1234.41 (calcd. 1234.61) corresponds to the molecular formula to be C59H94O27 (calcd. C59H94O27). Consequently, from the aforementioned 1D NMR data and comparison with previously reported literature of related compounds [13,14], compound 2 is proposed to be 3-O-β-D-glucopyranosyl (1 → 4)–α-L rhamnopyranosyl-quinovic acid-28-O-β-D-glucopyranosyl (1 → 4)–α-L rhamnopyranosyl–(1 → 2) α-L arabinopyranoside (Figure 5) which is isolated for the first time from F. natalensis leaves. By searching for the compound novelty in nature on Reaxys and Web of Science databases, compound 1 was identified for the first time from nature.
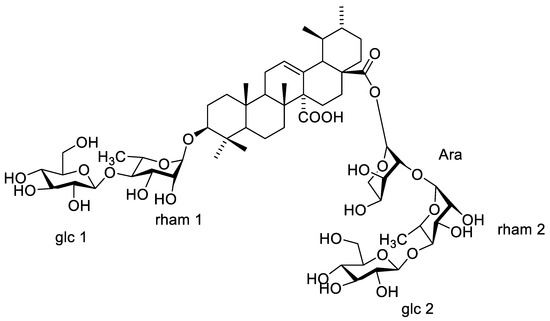
Figure 5.
Structure of compound 2.
3.3. Compound 3
Compound 3 was isolated as a yellowish-white amorphous powder (5 mg). Chromatographic properties: Rf = 0.38 (S1) on silica gel TLC plate gave a rose-pink color upon spraying with ethanol/H2SO4 and vanillin/H2SO4 spray reagents. 1HNMR spectrum (400 MHz, DMSO-d6) and 13CNMR (125 MHz, DMSO-d6): Spectroscopic data are summarized in Table S2. 1H-NMR and 13CNMR revealed the characteristic peaks of quinovic acid (3β-ol-urs-12-en-27,28-dioic acid). From 1H-NMR, it was possible to identify the six methyl signals at δH 0.84, 0.88, 0.88, 0.87, 0.86, 0.90 ppm that were assigned for methyl groups H-23, H-24, H-25, H-26, H-29, and H-30, respectively. Oxygenated methine of H-3 and H-12 olefinic proton resonances were detected at δH 3.52 and at δH 5.32 (brt). 13CNMR spectrum showed two signals at δC 178.86 and δC 175.11 characteristic for carboxyl carbons C-27 and C-28, respectively. Moreover, an ethylenic carbon C-12 was detected at δC 130.11 as well as a quaternary carbon C-13 at δC 138.58. Moreover, 1HNMR spectrum showed two anomeric proton signals at δH 5.18 (d, J = 7.8) and 4.81 (d, J = 7.8) assigned for H-1′ and H-1″, respectively, indicating the presence of two β sugar moieties. 13CNMR spectrum also exhibited two anomeric signals at δC 107.71 and 104.47 corresponding to C-1′ and C-1″, respectively, proving the presence of two sugar moieties. The rest of the sugar carbon shifts led to the identification of the sugar units to be two glucose units (Table S1). The data of C-3 and C-28 at δC 82.99 and δC 175.11, respectively, verified the location of the glucose moieties. The EI-MS with molecular ion peak [M]+ at m/z 810.98 (calcd. 810.44), corresponding to the molecular formula to be C42H66O15 (calcd. C42H66O15). All the aforementioned 1D NMR data are in accordance with that published for glycoside B isolated from Anthocephalus cadamba bark [15,16]. Consequently, compound 3 was assigned as 3-O-β-D-glucopyranosyl-quinovic acid-28-O-β-D-glucopyranosyl ester (Figure 6) which is isolated for the first time from F. natalensis leaves.
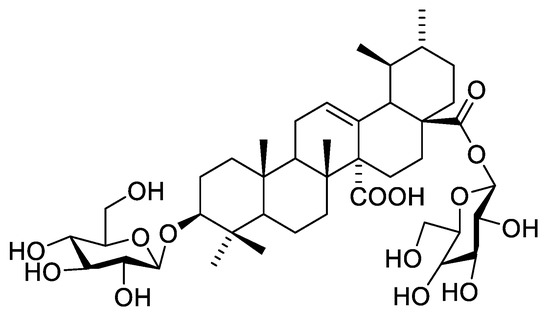
Figure 6.
Structure of compound 3.
3.4. Compound 4
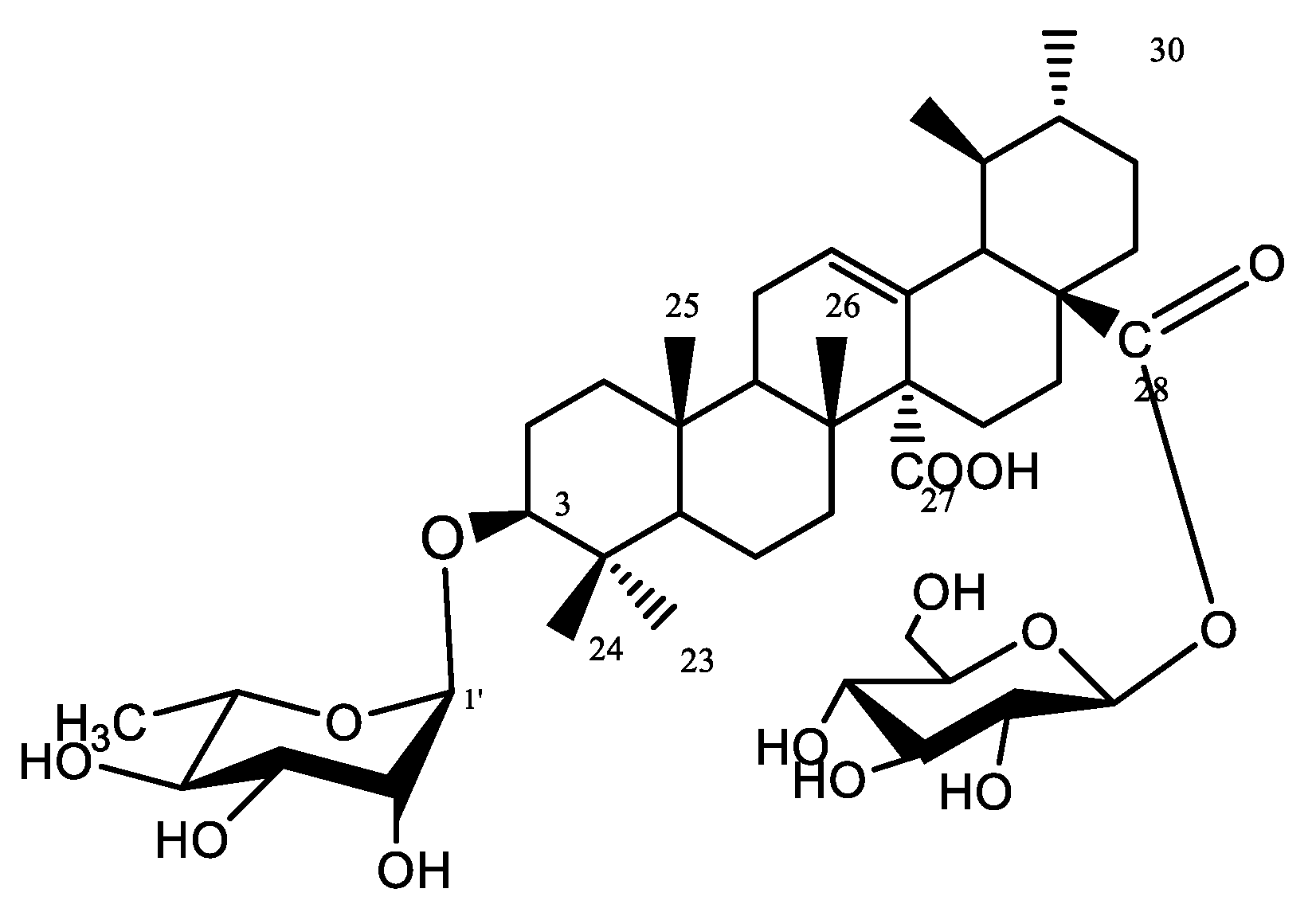
Compound 4 was isolated as a yellowish-white amorphous powder (6 mg). Chromatographic properties: Rf = 0.37 (S2) on silica gel TLC plate yielded a rose-pink color upon spraying with ethanol/H2SO4 and vanillin/H2SO4 spray reagents. 1HNMR spectrum (400 MHz, DMSO-d6) and 13CNMR (125 MHz, DMSO-d6); Spectroscopic data are summarized in Table S2. 1HNMR and 13CNMR data showed characteristic NMR signals of another quinovic acid (3β-ol-urs-12-en-27,28-dioic acid). 1HNMR chemical shifts of compound 4 revealed the presence of six methyl signals at δH 0.84, 0.95, 0.90, 0.87, 0.97, 1.04 ppm assigned for methyl groups H-23, H-24, H-25, H-26, H-29, and H-30, respectively. H-3 oxygenated methine appeared at δH 3.52, while olefinic H-12 was assigned at δH 5.33 (brt). 13CNMR spectrum showed two signals at δC 181.82 and δC 175.32, characteristic of carboxyl carbons C-27 and C-28, respectively. Moreover, carbon C-12 was detected at δC 129.39 as well as a quaternary carbon C-13 at δC 134.27 proving the presence of a double bond between C-12 and C-13. 1HNMR spectrum showed two anomeric proton signals at δH 5.41 (d, J = 1.5, 1H′) and 4.90 (d, 1H″) together with a methyl doublet at δH 1.01 (6′-H). Additionally, the 13CNMR spectrum exhibited two anomeric signals at δC 97.05 and 103.54, corresponding to C-1′ and C-1″, indicating the presence of one rhamnose moiety and one glucose moiety. Other sugar carbons were assigned at the range of δC 60–80. The attachment of rhamnose at C-3 was confirmed from the downfield shift of C-3 at δC 90.57 and the value of its anomeric proton while the location of glucose at C-28 was assigned by the upfield shift of C-28 at δC 175.32. The structure was confirmed by EI-MS which showed a molecular ion peak [M]+ at m/z 794.82 and prominent fragments at m/z 632.28 [M-162]+, corresponding to the removal of one hexose unit, and at m/z 484.8, corresponding to [M-glc-rham-2H]+, consistent with the molecular formula to be C42H66O14. All the previous data are in accordance with that published for gongganoside B isolated from Bhesa paniculata bark [16,17]. Therefore, compound 4 was confirmed as 3-O-α-L-rhamnopyranosyl-quinovic acid 28-O-β-D-glucopyranoside ester (Figure 7), which was previously reported from Ficus elastica aerial root bark [11] and identified for the first time from F. natalensis leaves.
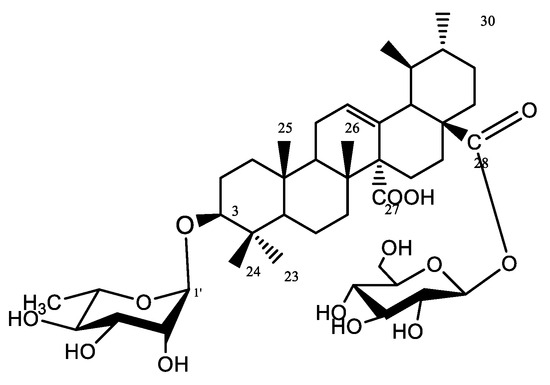
Figure 7.
Structure of compound 4.
3.5. Compound 5
Compound 5 was obtained as a white amorphous powder (9 mg). Chromatographic properties: Rf = 0.46 (S1) on silica gel TLC plate. It gave a violet color with ethanol/H2SO4 and vanillin/H2SO4 spray reagents. 1HNMR spectrum (400 MHz, DMSO-d6) and 13CNMR (100 MHz, DMSO-d6): Spectroscopic data are summarized in Table S3. The 13CNMR spectrum showed seven methyl carbon signals at δC 29.31, 17.62, 14.95, 18.03, 26.21, 33.84, and 23.49 assigned to C-23, C-24, C-25, C-26, C-27, C-29, and C-30, respectively. An ethylenic carbon was observed at δC 123.71 ppm assigned for C-12, together with a quaternary carbon signal C-13 at δC 139.30 ppm and one carboxylic carbon at δC 183.99 ppm corresponding to C-28. The signals of C-19 and C-20 were observed at δC 46.31 and 31.73, respectively, proving the location of C-29 and C-30 methyls at C-20.
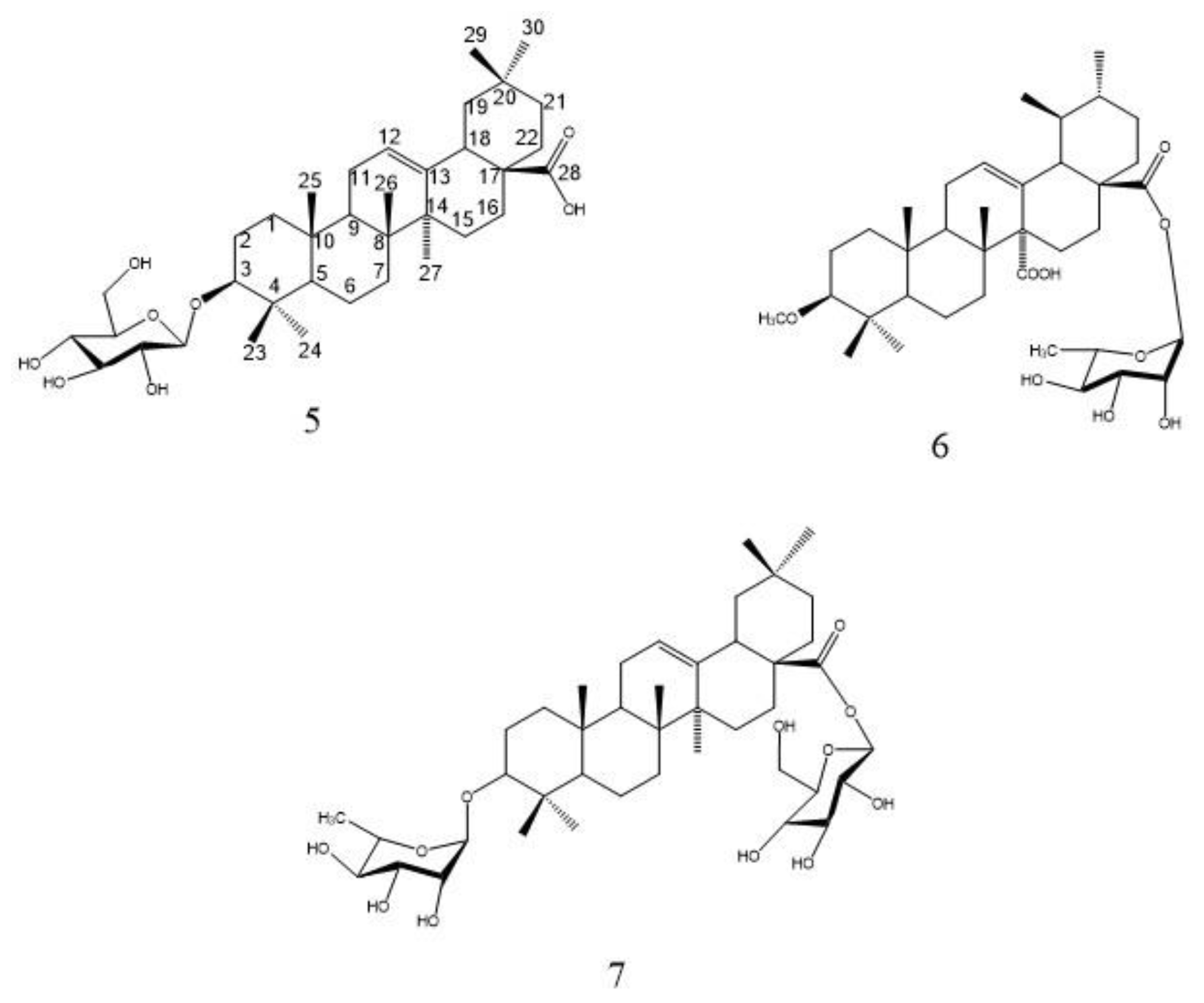
The 1HNMR spectrum showed the presence of 7 overlapped methyl signals at δH 0.84, 0.88, 0.88, 0.87, 1.34, 0.90, and 0.86 ppm corresponding to protons of seven angular methyls H-23, H-24, H-25, H-26, H-27, H-29, and H-30. An oxygenated methine proton of a terpene moiety appeared at δH 3.20 ppm attributed to H-3, and a trisubstituted olefinic proton appeared as a broad triplet at δH 5.32 ppm. 1HNMR and 13CNMR spectra led to the identification of 8 to be an oleanane-type triterpene skeleton, with an olefinic bond at C-12 and one carboxyl group at C-28, known as oleanolic acid [18]. Additionally, the 1HNMR spectrum showed one anomeric proton signal at δH 5.18 (d, J = 7.8), indicating the presence of one β sugar unit. An anomeric carbon was assigned at δC 101.53 in 13CNMR, the data of the sugar carbons led to the identification of the sugar unit as glucose [2]. The attachment of this glucose moiety at C-3 was confirmed by the downfield shift of C-3 carbon at δC 84.40. The EI-MS with molecular ion peak [M]+ at m/z 618.42 (calcd. 618.41), corresponds to the molecular formula to be C36H58O8 (calcd. C36H58O8). Consequently, based on 1D NMR data and by comparing with reported literature of related compounds [19,20], compound 8 was assigned as 3-O-β-D-glucopyranosyl oleanolic acid (Figure 8), which was isolated from Albizia gummifera bark and the whole plant of Patrinia saniculaefolia [19,20], and is reported for the first time from F. natalensis leaves.
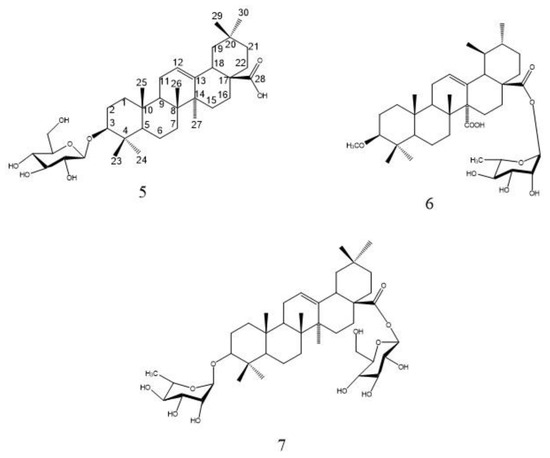
Figure 8.
Structure of compounds 5, 6, and 7.
3.6. Compound 6
Compound 6 was isolated as a yellowish-white amorphous powder (5 mg). Chromatographic properties: Rf = 0.43 (S1) on silica gel TLC plate. It gave a pink color with ethanol/H2SO4 and vanillin/H2SO4 spray reagents. 1HNMR spectrum (400 MHz, DMSO-d6) and 13CNMR (100 MHz, DMSO-d6): Spectroscopic data are summarized in Table S3. Spectral data of compound 6 were superimposed with those of compound 5 which prove the presence of the same oleanane-type triterpene skeleton, with an olefinic bond at C-12 and one carboxyl group at C-28 (oleanolic acid) [13,21]. The point of difference between 6 and 5 lies in the presence of one anomeric proton signal at δH 4.87 (d, J = 1.5) at 1HNMR spectrum assigned for H-1′ together with methyl signal at δC 18.35 (C-6′) indicating the presence of one α-L-rhamnose moiety. Additionally, the 1HNMR spectrum revealed the presence of a singlet signal at δH 3.51 integrated for three protons of the methoxy group. 13CNMR showed one anomeric signal at δC 98.34 corresponding to C-1′ and methoxy carbon at δC 51.67. The upfield shift of C-3 at δC79.49 as compared to the non-substituted one led to the identification of the site of attachment of the methoxy group, while the upfield shift of C-28 at δC 179.9 proved the location of the rhamnose moiety. The EI-MS showed a molecular ion peak [M]+ at m/z 616.48, consistent with the molecular formula to be C37H60O7. From the discussed 1D NMR, EI-MS data and by comparing with previously reported literature of structurally related compounds [13,21], compound 6 was assigned as 3-methoxy-oleanolic acid-28-O-α-L-rhamnopyranoside which is isolated for the first time from F. natalensis leaves.
3.7. Compound 7
Compound 7 was isolated as a white amorphous powder (6 mg). Chromatographic properties: Rf = 0.41 (S2) on a silica gel TLC plate. It gave a violet color with ethanol/H2SO4 and vanillin/H2SO4 spray reagents. 1HNMR spectrum (400 MHz, DMSO-d6) and 13CNMR (100 MHz, DMSO-d6), spectroscopic data are summarized in Table S3. Comparison of the spectral data of 7 with those of compounds 5 and 6 led to the identification of its aglycone as oleanane-type triterpene, with an olefinic bond at C-12 and one carboxyl group at C-28 (oleanolic acid) [12,18]. The points of difference were the presence of two anomeric proton signals at δH 5.19 with a small coupling constant together with a doublet signal of CH3-6′ at δC 1.02 indicating the presence of rhamnose moiety and at δH 4.69 indicating the presence of β sugar in 1H NMR. Moreover, two anomeric carbons at δC 104.47 and 102.43 were identified in the 13C NMR spectrum. The EI-MS with molecular ion peak [M]+ at m/z 764.41 (calcd. 764.99), corresponding to the molecular formula to be C42H68O12 (calcd. C42H68O12). A comparison of the sugar carbon values with the reported literature confirmed the presence of one β-D-glucose unit and one α-L-rhamnose unit [13,21]. The attachment of the sugar units at C-3 and C-28 was confirmed by their chemical shift at δC 82.21 and 175.0, respectively. Consequently, compound 7 was identified as 3-O-α-L-rhamnopyranosyl-oleanolic acid-28-O-β-D-glucopyranoside ester which is isolated for the first time from F. natalensis leaves.
4. Conclusions
The detailed chromatographic separation of seven triterpenoid saponins was introduced herein. The isolated compounds were identified according to their spectroscopic analysis including 1D 1HNMR, 13CNMR, 2D HSQC, and HMBC along with MS to confirm the deduced structure. Seven compounds (1–7) were identified for the first time from F. natalensis belonging to bidesmoside triterpenoid saponins with quinovic acid and an oleanolic acid aglycone. Compounds 1 and 2 were reported for the first time from nature according to Reaxys and Web of Science database. Further screening of the biological activities of the isolated compounds is recommended in future work. In addition, the application of large-scale metabolomic approaches including UPLC-MS/MS is recommended for metabolites profiling of F. natalensis leaves to profile more saponins present at lower levels and compare it with fruits as well as plants from other sources to provide conclusive evidence on saponins profile in F. natalensis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10090478/s1, Table S1.1H- and 13C-NMR spectroscopic data of compounds (1–2) in DMSO-d6; Table S2: 1H -and 13C-NMR spectroscopic data of compounds (3–4) in DMSO-d6; Table S3: 1H -and 13C-NMR spectroscopic data of compounds (5–7) in DMSO-d6. Figure S1:1HNMR spectrum of compound 1; Figure S2: Magnified 1HNMR spectrum of compound 1; Figure S3:13CNMR spectrum of compound 1; Figure S4: HSQC spectrum of compound 1; Figure S5: HMBC spectrum of compound 1; Figure S6: Magnified HMBC spectrum of compound 1; Figure S7:1HNMR spectrum of compound 2; Figure S8: 13CNMR spectrum of compound 2; Figure S9: Magnified 13CNMR spectrum of compound 2.
Author Contributions
Conceptualization, M.H.B. and A.T.; methodology, M.H.B. and S.E.A.E.; formal analysis, S.E.A.E., S.A. and W.H.E.-T.; investigation, M.H.B., A.T. and W.H.E.-T.; data curation, M.H.B.; writing—original draft preparation, S.E.A.E. and M.H.B.; writing—review and editing, M.H.B., A.T. and S.A.; supervision, A.T. and M.H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are included in the manuscript.
Acknowledgments
The authors acknowledge Rim Hamdy, Taxonomy Department, Faculty of Science, Cairo University, Egypt, for efforts in botanical identification of the plant. The authors acknowledge Mohamed Ali Farag, in Pharmacognosy, Cairo University, for reviewing the English Writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baky, M.H.; Kamal, A.M.; Haggag, E.G.; Elgindi, M.R. Flavonoids from Manilkara hexandra and antimicrobial and antioxidant activities. Biochem. Syst. Ecol. 2022, 100, 104375. [Google Scholar] [CrossRef]
- Eskander, J.Y.; Haggag, E.G.; El-Gindi, M.R.; Mohamedy, M.M. A novel saponin from Manilkara hexandra seeds and anti-inflammatory activity. Med. Chem. Res. 2014, 23, 717–724. [Google Scholar] [CrossRef]
- Somashekhar, M.; Nayeem, N.; Sonnad, B. A review on family Moraceae (Mulberry) with a focus on Artocarpus species. World J. Pharm. Pharm. Sci. 2013, 2, 2614–2621. [Google Scholar]
- Olaokun, O.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC Complement. Altern. Med. 2013, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Elish, S.E.; Sanad, F.A.; Baky, M.H.; Yasin, N.A.; Temraz, A.; El-Tantawy, W.H. Ficus natalensis extract alleviates Cadmium chloride-induced testicular disruptions in albino rats. J. Trace Elem. Med. Biol. 2022, 70, 126924. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jian, L.; Chen, G.; Song, X.; Han, C.; Wang, J. Chemical constituents and in vitro anticancer cytotoxic activities of Polyalthia plagioneura. Chem. Nat. Compd. 2014, 49, 1172–1174. [Google Scholar] [CrossRef]
- Awolola, G.V. Phytochemical Analyses and Biological Activities of Four South African Ficus Species (Moraceae). Ph.D. Thesis, University of Kwazulu-Natal, Westville, South Africa, 2015. [Google Scholar]
- Tkachenko, H.; Buyun, L.; Terech-Majewska, E.; Osadowski, Z. In vitro antimicrobial activity of ethanolic extracts obtained from Ficus spp. leaves against the fish pathogen Aeromonas hydrophila. Arch. Pol. Fish. 2016, 24, 219–230. [Google Scholar] [CrossRef]
- Tkachenko, H.; Buyun, L.; Osadowski, Z.; Honcharenko, V.; Prokopiv, A. Studies on antibacterial activity of Ficus natalensis Hochst. subsp. leprieurii (Miq.) CC Berg (Moraceae) leaf extract. Med. Herbs Past New Technol. 2016, 28, 256. [Google Scholar]
- Elish, S.E.; Baky, M.H.; Temraz, A. Phytochemical Profile and Antioxidant Capacity of Ficus natalensis Subsp. leprieurii (miq) Cultivated in Egypt: In-vitro Study. Azhar Int. J. Pharm. Med. Sci. 2023, 3, 70–76. [Google Scholar]
- Mbosso, E.J.T.; Nguedia, J.C.A.; Meyer, F.; Lenta, B.N.; Ngouela, S.; Lallemand, B.; Mathieu, V.; Van Antwerpen, P.; Njunda, A.L.; Adiogo, D. Ceramide, cerebroside and triterpenoid saponin from the bark of aerial roots of Ficus elastica (Moraceae). Phytochemistry 2012, 83, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Elgindi, M.R.; Shawky, E.M.; Ibrahim, H.A. Phytochemical investigation of Ludwigia adscendens subsp. diffusa aerial parts in context of its biological activity. BMC Chem. 2022, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- El-Wakil, E.A. Phytochemical and molluscicidal investigations of Fagonia arabica. Z. Nat. C 2007, 62, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Serag, A.; Baky, M.H.; Döll, S.; Farag, M.A.J.R.a. UHPLC-MS metabolome-based classification of umbelliferous fruit taxa: A prospect for phyto-equivalency of its different accessions and in response to roasting. RSC Adv. 2020, 10, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Bach, N.X.; Thu, V.K.; Trang, D.T.; Van Kiem, P. Quinovic acid glycosides from Mussaenda pilosissima Valeton. Vietnam. J. Chem. 2019, 57, 64–69. [Google Scholar] [CrossRef]
- Sahu, N.P.; Koike, K.; Jia, Z.; Banerjee, S.; Mandal, N.B.; Nikaido, T. Triterpene glycosides from the bark of Anthocephalus cadamba. J. Chem. Res. 2000, 2000, 22–23. [Google Scholar] [CrossRef]
- Lamidi, M.; Ollivier, E.; Faure, R.; Debrauwer, L.; Nze-Ekekang, L.; Balansard, G. Quinovic acid glycosides from Nauclea diderrichii. Phytochemistry 1995, 38, 209–212. [Google Scholar] [CrossRef]
- Ohashi, K.; Kojima, H.; Tanikawa, T.; Okumura, Y.; Kawazoe, K.; Tatara, N.; Shibuya, H.; Kitagawa, I. Indonesian medicinal plants. IX. Chemical structures of gongganosides A, B, and C, three new quinovic acid glycosides from the bark of Bhesa paniculata (Celastraceae). Chem. Pharm. Bull. 1994, 42, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- An, R.-B.; Na, M.-K.; Min, B.-S.; Lee, H.-K.; Bae, K.-H. Anti-complement activity of triterpenoids from the whole plant of Patrinia saniculaefolia. Nat. Prod. Sci. 2008, 14, 249–253. [Google Scholar]
- Rukunga, G.; Waterman, P. A new oleanane glycoside from the stem bark of Albizia gummifera. Fitoterapia 2001, 72, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Hichri, F.; Jannet, H.B.; Cheriaa, J.; Jegham, S.; Mighri, Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. Comptes Rendus Chim. 2003, 6, 473–483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).