Abstract
The method of precipitation treatment of high-nickel waste electrolytic slag was studied, coupled with multi-step treatment to improve the recovery rate of nickel. In this study, HNO3 was used as dissolving agent, NaOH and Na2CO3 as precipitating agents, and the waste electrolytic slag was dissolved in the liquid phase and then precipitated. The results show that the electroslag with high nickel content can be completely dissolved under the optimal solid-liquid ratio of 1:10. When the pH of the leaching solution was adjusted to 7.5 by 5% NaOH solution, the iron removal rate could reach 99.3%. When the pH was adjusted to 11 with 5% Na2CO3, the nickel could be completely precipitated after standing, and the main component was basic nickel carbonate. The further recovery experiments show that high purity NiO can be obtained after the basic nickel carbonate is oxidized and calcined, and the average recovery rate of nickel is 92.4%. This study is of great practical significance to improve the treatment efficiency of the chemical precipitation method, reduce the operating cost of the chemical precipitation method, and reduce the production of secondary pollutants in the process of nickel recycling.
1. Introduction
Electronic gases are known as the “food” and “source” of semiconductor materials [1]. Electronic gas costs account for 5–6% of the total cost of semiconductor materials. Although it seems to account for a small proportion, it largely determines the performance of semiconductor devices [2]. Each order of magnitude increase in electronic gas purity will greatly promote the qualitative leap of semiconductor devices. At the same time, under the support of policies such as “Made in China 2025” and “Notice on Corporate Income Tax Policy Issues of integrated circuit Manufacturers”, China’s ultra-large scale integrated circuits, liquid crystal display devices, amorphous silicon thin film solar cells, and other industries have developed rapidly, and fluorine-containing special electronic gases are also very considerable with the rapid development of these industries.
Nitrogen trifluoride (NF3), as a class of fluorine-containing specialty gases, is the largest electronic specialty gas product in the market [3,4]. It has very excellent etching efficiency and selectivity, leaving no residue on the surface of the etched object. It is also a very good cleaning agent [5]. With the development of nanotechnology and large-scale development of electronic industry technology, China’s semiconductor industry and panel industry maintain a high prosperity. Nitrogen trifluoride is an indispensable and the largest amount of special electronic gas in the production and processing of panels and semiconductors [3]. At present, nitrogen trifluoride preparation methods are mainly divided into the chemical method and electrolysis method [2]. As the anode material for preparing nitrogen trifluoride (NF3) by the electrolytic method, Nickel (Ni) has the advantages of high electrode strength, no difficultly separating carbon tetrafluoride (CF4), and low polarization [6]. In the actual production process, in order to maintain the smooth progress of electrolysis, it is necessary to regularly clean the electrical deposits generated by the dissolution of the anode in the electrolytic cell. Nickel compounds are carcinogens and are toxic. It can lead to reduced or loss of arginase and hydroxylase activity in the body, which can cause inflammation and damage the heart muscle and liver [7]. Exposure to high nickel pollution has the potential to cause a variety of pathological effects in humans, from contact dermatitis to pulmonary fibrosis, cardiovascular and kidney diseases, and even cancer. Human exposure to nickel can come from a variety of sources (air, water, and food). Improper handling of nickel-containing compounds can also harm the environment and waste resources [8]. How to recycle waste electrolytes, especially nickel, is an urgent problem in the process of nitrogen trifluoride industrialization.
At the same time, nickel, known as the “vitamin of the steel industry”, as a national strategic resource, is widely used in various industries because of its superior performance. Nickel and its compounds and alloys are used in industrial and commercial applications. Most nickel is used in the production of stainless steel, non-ferrous alloys, and nickel-based superalloys with high corrosion and high temperature resistance. Nickel alloys are widely used in fields ranging from industrial machinery to precision electronics. Some Ni compounds and complexes are used as highly efficient catalysts in various syntheses [9]. Nickel oxide is widely used in nickel-based rechargeable batteries.
However, with the gradual depletion of nickel sulfide resources and the increase of nickel consumption, the development of more nickel resources is also an urgent concern. China’s nickel reserves of 3 million tons, accounting for about 4.05% of the world’s reserves, make it a country poor in nickel resources, with a large number of nickel resources relying on imports. The effective recovery of nickel from waste electrolytic slag can also relieve the pressure of nickel resource development and utilization to a certain extent. Therefore, the realization of harmless, reduced recycling treatment of high-nickel waste electrolytic slag not only has good economic and environmental benefits, but also meets the requirements of the current society for the sustainable development of resources.
In order to recover valuable metals from high-nickel waste electrolytic slag, a number of industrial methods based on pyrometallurgy, hydrometallurgy, or a combination of both methods have been developed [10]. In pyrometallurgy, the harmful gases released during smelting and the extremely high temperatures required are not economically compensated in the recovery process [11]. In contrast, the hydrometallurgical process allows for better control of nickel recovery, using lower temperatures. And it is theoretically more environmentally friendly due to low energy demand, low gas emissions, and low waste generation. At present, there are various methods for wet treatment of nickel-containing resources, such as chemical precipitation, solvent extraction, ion exchange, electrolysis, etc. Each has its own advantages in practical application [12].
Solvent extraction has been widely used in hydrometallurgy in recent years because of its low cost, low energy consumption, and easy automatic control [13]. There are two main process routes for nickel recovery: one is to extract nickel with appropriate extractant to separate it from other heavy metal ions. The other is to extract heavy metal impurity ions in wastewater to achieve the purpose of purifying nickel [14]. The ion exchange method is mainly used in the treatment of nickel wastewater containing chromium, nickel, alloy, etc. It has more mature experience in design, operation, and management. It is also applied to the treatment of wastewater containing copper and zinc. The ion exchange method has many advantages over other methods in processing efficiency and resource recovery. However, it has some disadvantages, such as a large one-time investment, complex system design and operation management, and large footprint. Moreover, the treatment of the regenerated eluent is difficult, and it is easy to cause “secondary pollution”. In the electrorefining process of copper anodes, the resulting outflow electrolyte has a high Ni content (up to 20 g/L). Nyirenda and Phiri [15] have shown that nickel can be recovered from electrolyte solutions either by precipitation of ammonia or by evaporative crystallization of nickel sulfate. One of the latest methods has shown that partial copper removal, crystallization, solvent extraction, and electrodeposition routes are suitable for the production of pure copper and nickel powders. In some cases, the nickel recovery efficiency is above 90% [16].
The purpose of ion exchange is to replace undesirable ions with other ions that are not harmful to the environment [17]. Ion exchange is one of the most common methods for treating wastewater containing heavy metals around the world. And the use of ion exchange resins and zeolite [18] can be used to remove nickel from wastewater [17]. In order to improve the removal efficiency of hazardous waste ions in wastewater, ion exchange technology is usually combined with other technologies. For example, Papadopoulos [18] et al., in combination with the ion exchange method and the precipitation process, achieved an Ni2+ removal efficiency of 98.3%. Dzyazko and Belyakov [19] et al. have used a combination of ion exchange and electrodialysis to remove Ni2+ from wastewater.
The precipitation method is an efficient short-process method for nickel recovery [20,21]. Nickel or other heavy metal ions are precipitated in the form of precipitation by adding precipitating agents such as hydroxide, carbonate, and sulfide. These precipitants have the characteristics of rich variety, wide source, and low price, so they are widely used in production practice [22].
This method requires that the purified substance be carried out in the liquid phase system, and different acid solvents are usually selected to oxidize the raw material into the solution. For example, in the purification and recovery of nickel in laterite nickel ore, Widi [23] et al. proposed a method of using citric acid to dissolve laterite nickel ore and using oxalate to purify and recover nickel. The method achieves a 90% nickel dissolution rate and 100% nickel precipitation recovery efficiency. Kui Liu [24] et al. used magnesium oxide for precipitation to recover nickel on the basis of sulfate solution of laterite nickel ore, which achieved a recovery efficiency of 98.1%. In addition, we can also use the precipitation method to remove impurity ions in the solution, so as to achieve the recovery of heavy metal ions. Klaehn [25] et al. showed that selective precipitation of diammonium hydrogen phosphate (DAP) in LIB leaching solution could precipitate 95–99% of Al and Fe in the form of phosphate. On the basis of retaining more than 95% of Co, Ni, and Li in the leaching solution, further treatment can recover Ni and Co in the form of double salt crystals.
In this study, a green method was proposed to recover nickel from high-nickel waste electrolytic slag by the precipitation method, transform it into high-purity NiO products, and reduce the secondary pollutants in the recovery process. By adjusting pH, the step-by-step precipitation is realized and the treatment efficiency of chemical precipitation is improved. Compared with the conventional precipitation method, the amount of precipitating agent is less, which reduces the operating cost of the chemical precipitation method. At the same time, the production of “secondary pollutants” during the recycling process is also reduced. This is of great practical significance for nickel recovery.
2. Materials and Methods
2.1. Materials
The raw material used in this experiment is from the waste electrolytic slag produced by the electrolysis process of NF3. The electrolytic slag was analyzed by X-ray fluorescence spectrum. The results are shown in Table 1. The raw material belongs to high-nickel waste electrolytic slag. It also contains some iron and sodium elements.

Table 1.
Elemental composition of high-nickel waste electrolytic slag (%).
In the leaching experiment, nitric acid (HNO3) and Sulfuric acid (H2SO4) were used as the leaching agent from Sichuan Xilong Chemical Co., Ltd., Chengdu 611130, China. The precipitating agents used in the precipitation experiments, including sodium hydroxide (NaOH, 99%) and sodium carbonate (Na2CO3, 99%), were provided by Sinopharm Chemical Reagent Co., Ltd.
2.2. Experimental Method
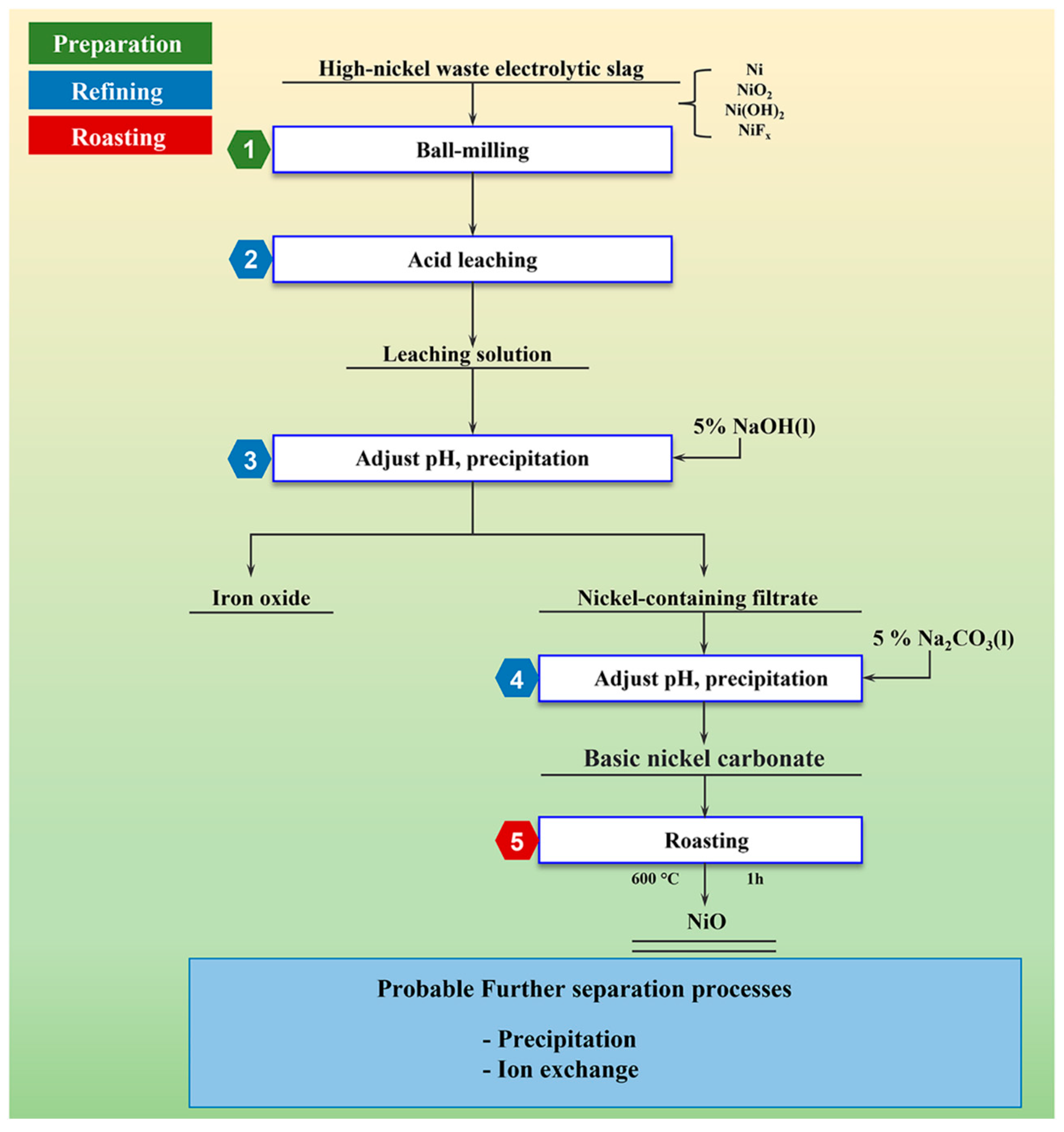
The process flow chart involved in this study is shown in Figure 1.
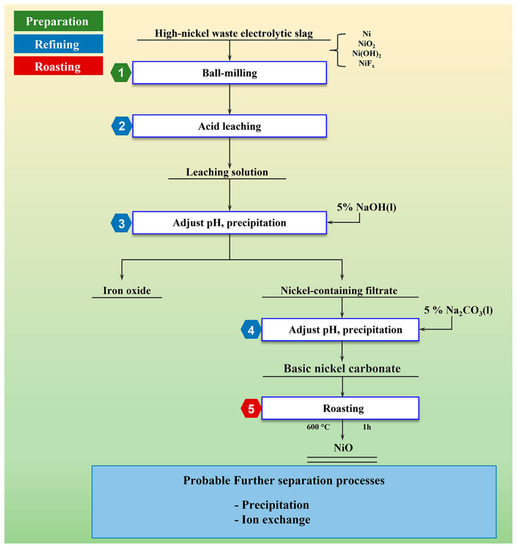
Figure 1.
Process flow chart of recovering nickel from high-nickel waste electrolytic slag by chemical precipitation.
Firstly, high-nickel electroslag was crushed and ground to obtain raw materials. The raw material was acid-soaked with acid solution to obtain the leaching solution. The pH of the leaching solution with 5% NaOH solution was adjusted until no reddish-brown precipitate was produced. The light blue-green nickel filtrate was obtained by extraction and filtration. The pH of the resulting nickel-containing filtrate was adjusted with 5% Na2CO3 solution until no light green precipitate was produced. The filtrate containing nickel was obtained by extraction and filtration. When no light green precipitate was produced, basic nickel carbonate was filtered and separated. The resulting basic nickel carbonate was dried and baked in a Muffle oven. The final product was NiO.
2.3. Analysis and Characterization Methods
The chemical composition of the high-nickel waste electrolytic slag was analyzed by X-ray fluorescence spectrometry (XRF, Axios PANalytical) and an inductively coupled plasma emission spectrometer (ICP-OES, Optima 8300, PerkinElmer, Inc., Wellesley, MA, USA). The main crystalline phases in the leaching process, precipitation process, roasting process products were analyzed by X-ray diffraction (XRD, D/Max 2200, Rigaku Corporation, Tokyo, Japan). The surface microstructure of the high-nickel waste electrolytic slag and energy dispersive spectroscopy (EDS) element content were observed by high-resolution field emission scanning electron microscopy (SEM; TESCAN MIRA4).
3. Results and Discussion
3.1. The Characterization of Raw Material
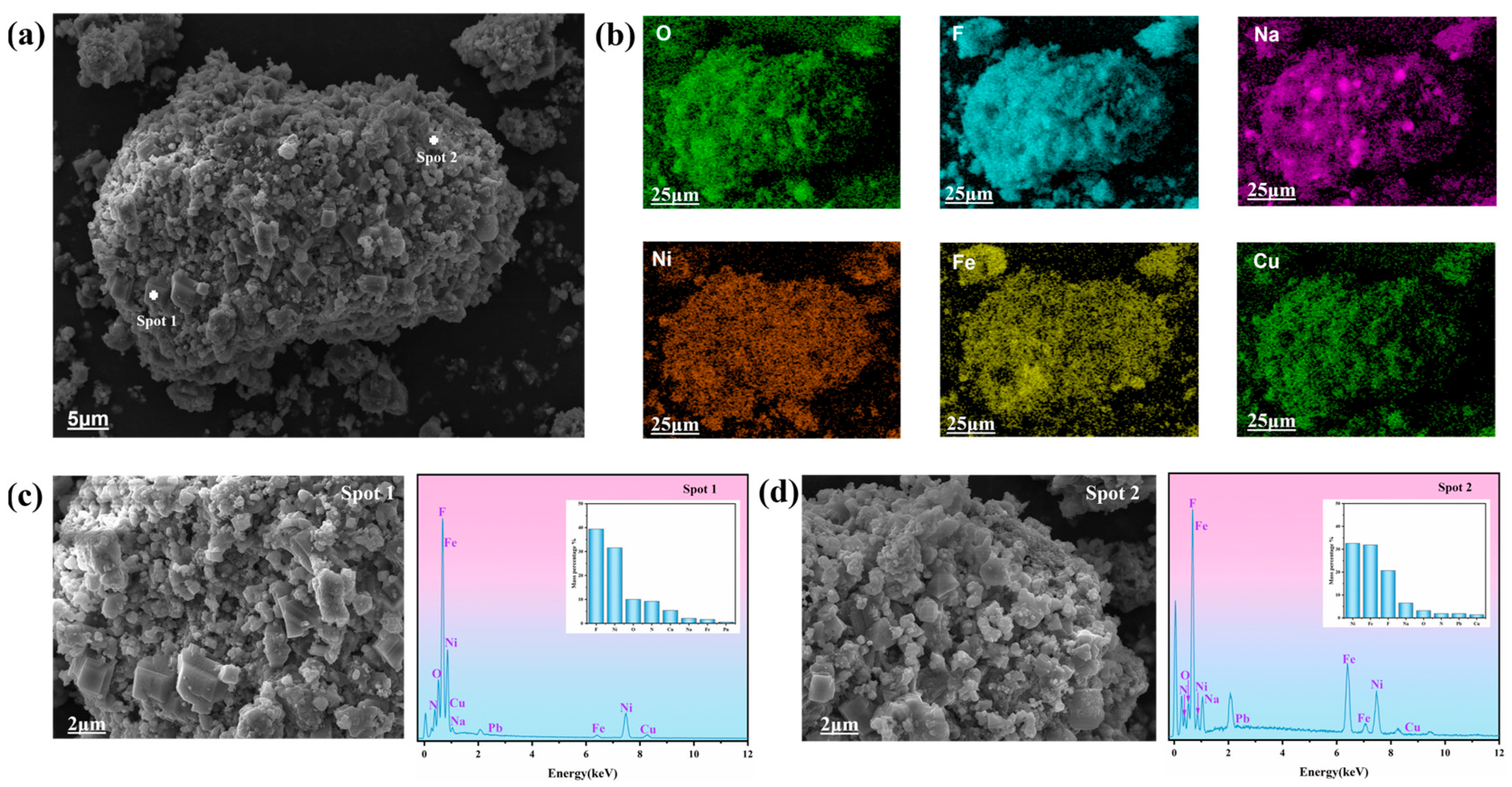
The microstructure of high-nickel electrolytic slag was observed by SEM, and the distribution of elements on its surface was analyzed by EDS. The results are shown in Figure 2. The results show that the surface of the high-nickel waste electrolytic slag presents an unsmooth and unevenly distributed lump agglomeration structure. In Figure 2b, it can be seen that F, Ni, Fe, Na, and Cu elements are uniformly distributed on its surface, which is consistent with the XRF results (Table 1). Figure 2c shows a large agglomeration structure on the surface of high-nickel waste electrolytic slag. The corresponding EDS results show that the relative contents of main elements distributed on the surface are 39.42% F, 31.57% Ni, 10.03% O, and 9.22% N. Figure 2d shows the dense distribution of agglomeration structures on the surface of high-nickel waste electrolytic slag. The corresponding EDS results show that the relative contents of main elements distributed on the surface are 32.59% Ni, 31.89% Fe, 20.69% F, and 6.52% Na. The results of XRF analysis are consistent with those of high-nickel waste electrolytic slag.
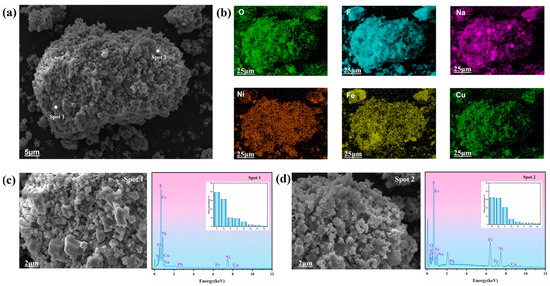
Figure 2.
SEM images (a,b) and EDS results (c,d) of high-nickel waste electrolytic slag.
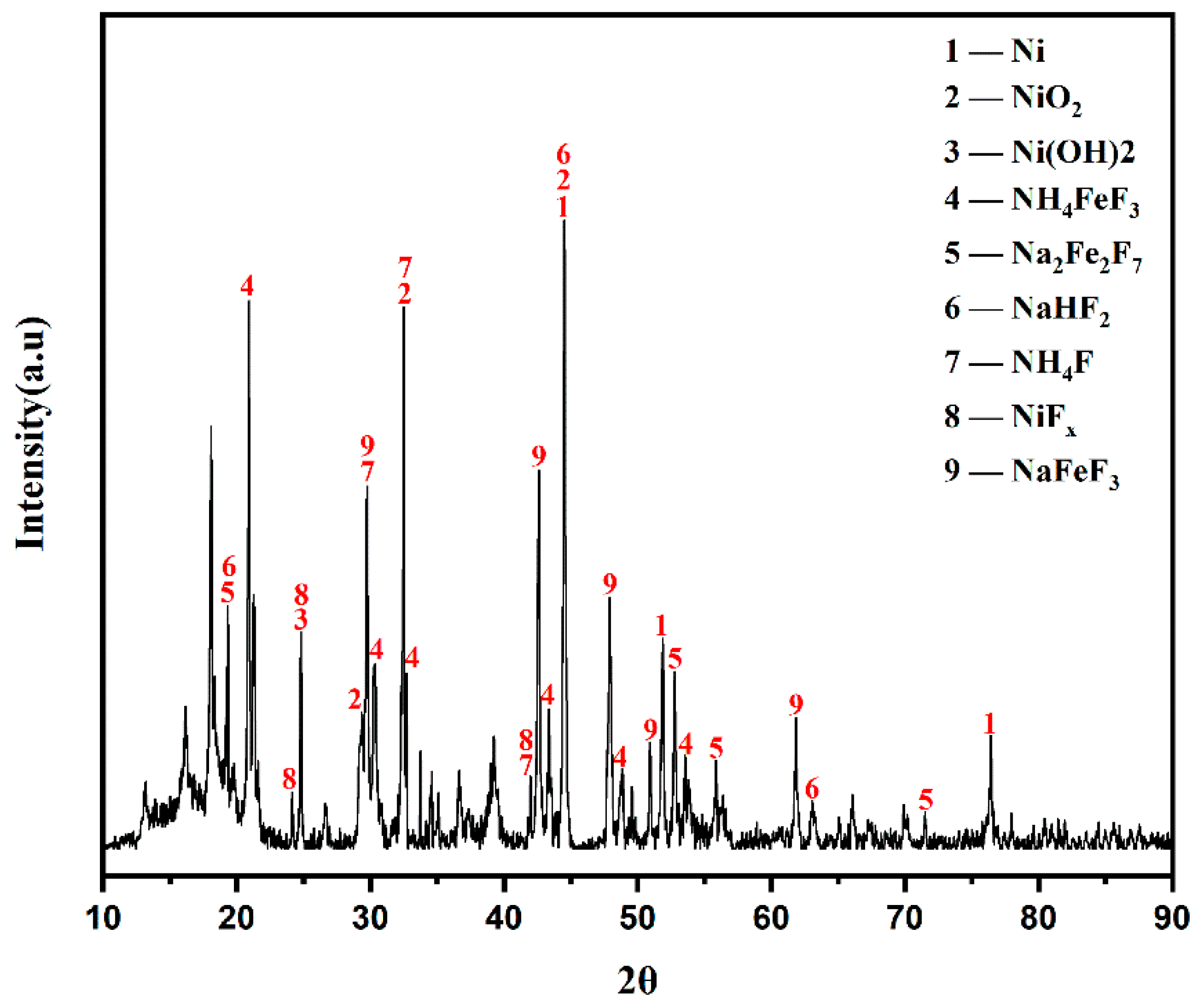
The XRD analysis result of high-nickel waste electrolytic slag is shown in Figure 3. Combined with its main element composition (Table 1), Ni in high-nickel waste electrolytic slag exists in four forms: Ni, NiO2, Ni(OH)2, and NiFx. Iron exists in the form of NH4FeF3. Sodium comes in the form of Na2Fe2F7, NaFeF3, and NaHF2. The presence of iron has an impact on the purity of nickel, so iron removal is required before nickel can be recovered.
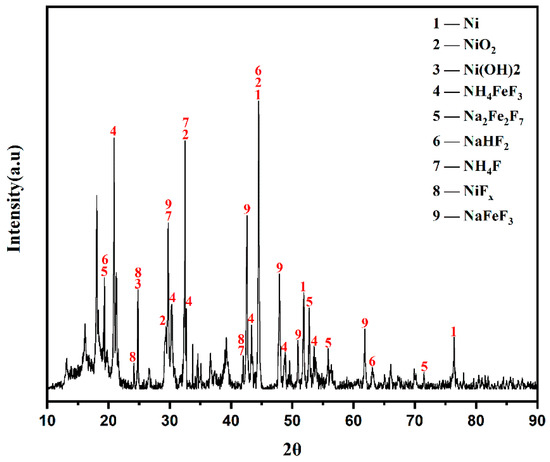
Figure 3.
XRD patterns of high-nickel waste electrolytic slag.
3.2. Solvent Selection
It is necessary to select a suitable solvent to completely dissolve the high-nickel waste electrolytic slag into the liquid phase, and purify nickel and its compounds in the liquid phase system. Since Fe2+ may be present in the sample, its presence will affect the purity of subsequent nickel. Therefore, a strong oxidizing acid is selected to oxidize it to Fe3+ to remove metal ion impurities. In order to make the experiment fully carried out, the dissolving time was up to 5 h. The dissolution effect is shown in Table 2.

Table 2.
Comparison of results of solvent dissolved samples at room temperature.
Figure 4 shows the change of solution before and after the start of the experiment, which is consistent with the description in Table 2. The above results show that only when nitric acid is used as the acid solvent, nickel slag can be completely dissolved into the liquid phase.

Figure 4.
Dissolution of high-nickel slag in solution at room temperature: at the beginning of dissolution experiment (a); at the end of the dissolution experiment (b).
In further experiments, we found that when the volume fraction of 24% nitric acid was used for the experiment, the amount of nitric acid was reduced by half, and the experimental purpose of dissolving nickel slag could be achieved. Therefore, in the follow-up experiment, we adopted a solid-liquid ratio of 1:10 when using nitric acid to dissolve nickel slag.
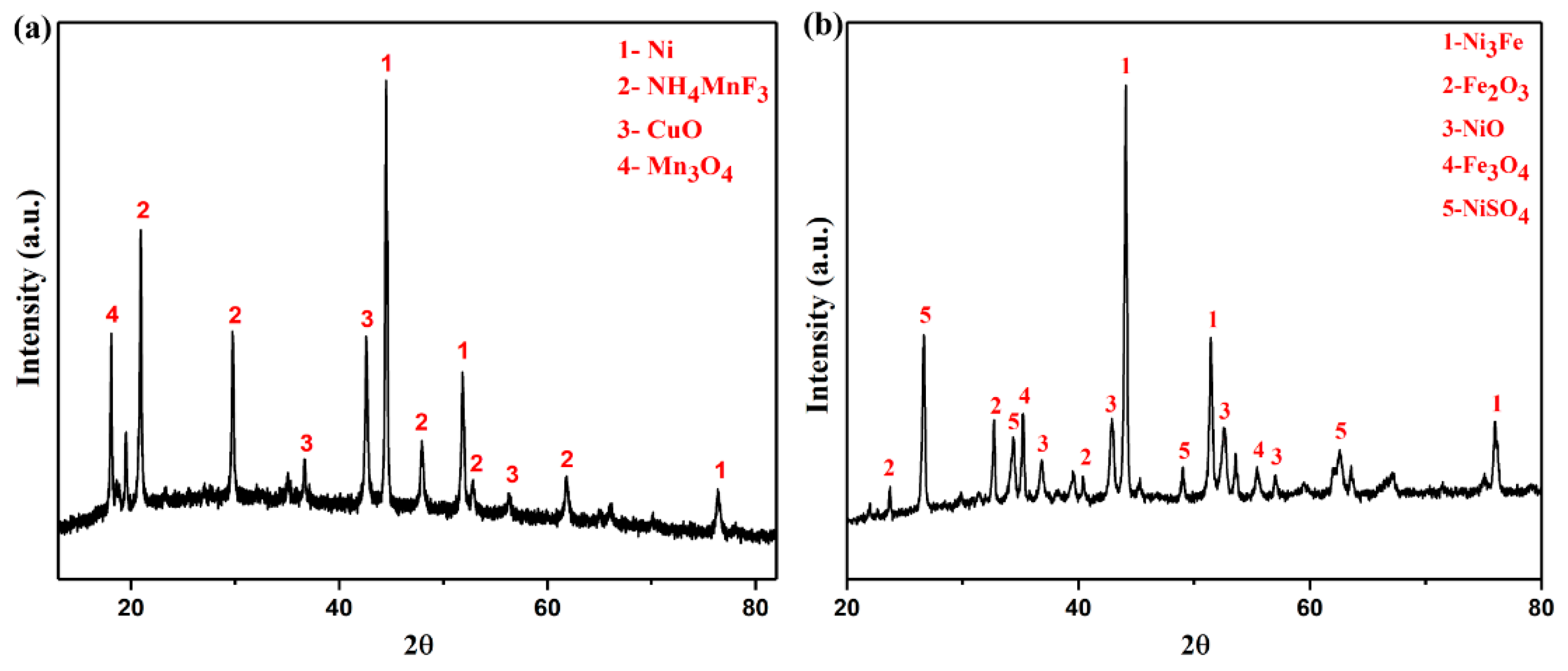
According to the above results, XRD was used to detect the tailings which were not completely dissolved by using the other two solvents. The results are shown in Figure 5.
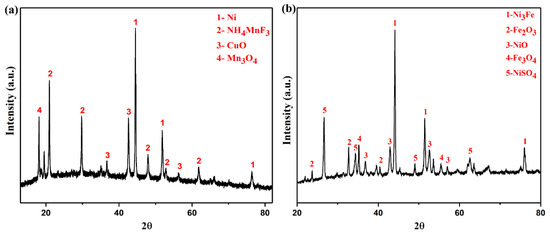
Figure 5.
XRD patterns of water leaching residue (a), sulfuric acid leaching residue (b).
Figure 5a shows that in the water leaching system, the leaching residue contains Ni, NH4MnF3, CuO, and Mn3O4 phases. Nickel oxides, iron salts, sodium salts, and some ammonium fluoride salts enter the liquid phase. Figure 5b shows that in the sulfuric acid leaching system, the leaching residue contains phases of Ni3Fe, Fe2O3, NiO, Fe3O4, and NiSO4. This is due to Ni(OH)2 and fluoride ammonium salt into the liquid phase, and the hydrolysis of fluoride ammonium salt makes Fe into insoluble iron oxide remaining in the leaching residue. Sulfuric acid cannot dissolve Ni completely. The above analysis is consistent with the results shown in Table 2, that is neither water nor sulfuric acid as solvents can completely dissolve nickel, which is not conducive to nickel recovery. The mass fraction of 20~28% nitric acid can completely dissolve the sample. Moreover, the amount of acid required is small, the dissolution efficiency is fast, and the reaction is stable and controllable. Therefore, the choice of mass fraction of 24% nitric acid solution as the solvent is the most suitable.
3.3. Principle Analysis of Precipitation
The chemical reactants that may be involved in this experiment are as follows.
The purification process is usually carried out in the liquid phase system, and the high-nickel waste electrolytic slag can be dissolved by acidic solvent. At the same time, because the raw material contains Fe2+ (Figure 3), strong oxidizing acid can be used to oxidize it to Fe3+, and then remove it in the following steps to reduce its impact on the purity of nickel. In 3.2, we completed the selection of solvent. The results show that nitric acid with volume fraction of 20~28% can achieve the desired effect. Equations (1)–(7) show the chemical principles that may be involved in the precipitation experimental phase.
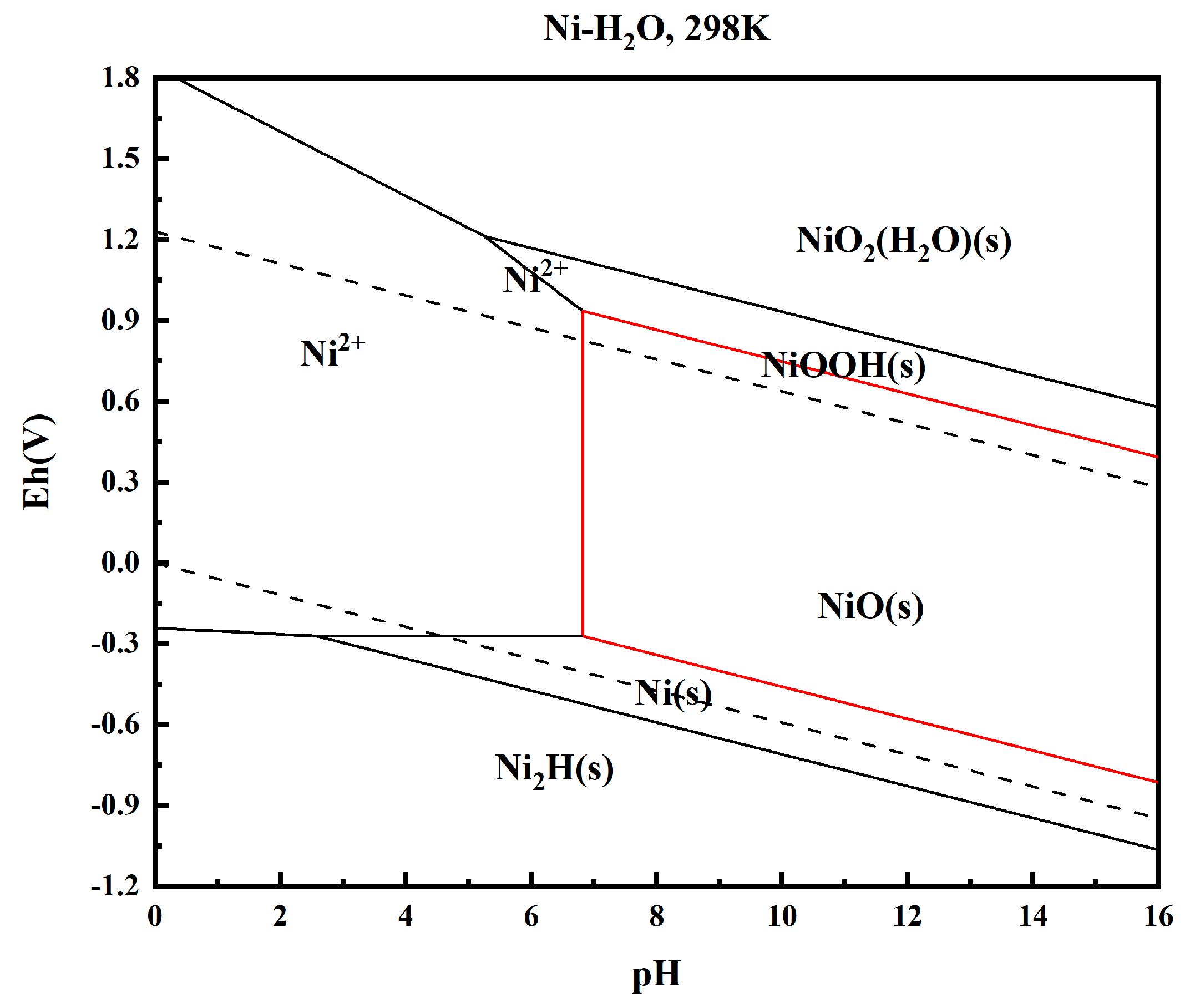
The high-nickel solution treated with acid was analyzed at room temperature. The potential-pH diagram corresponding to Ni is drawn using the electrochemical principle, and the result is shown in Figure 6.
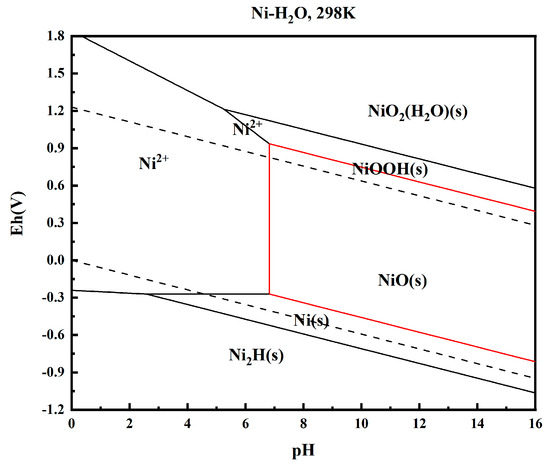
Figure 6.
e—pH diagram of Ni-H2O system.
The results show that theoretically, Ni precipitates from the solution in solid form (NiO, NiOOH) in the range of pH = 6.8~16, which is the part surrounded by the red line in Figure 6. Precipitation begins when pH = 6.8, and is completely precipitated when pH = 16. Therefore, by using the precipitation rule of Ni2+ in this range, nickel can be precipitated by adjusting pH to achieve the purification of nickel in high-nickel solution, which is feasible in this experiment.
3.4. Selection of Precipitating Agent
The leaching solution mainly contains Ni2+ and Fe3+, which can form precipitation with most of the precipitating agents. Based on the theoretical analysis of the precipitation principle mentioned in Section 3.3, when NaOH solution is used for pH regulation, Ni2+ will begin to precipitate when the pH is adjusted to about 6–7. At the same time, in the previous step we need to use OH− to remove Fe3+. Therefore, the experiment at this stage is mainly divided into three parts: the first part uses OH− to regulate pH. The second part uses the introduced OH− to remove excess Fe3+. The third part uses CO32− to purify Ni2+ on the basis of removing Fe3+. The experimental results are shown in Table 3.

Table 3.
The results of Ni2+, Fe3+ precipitation experiment at room temperature.
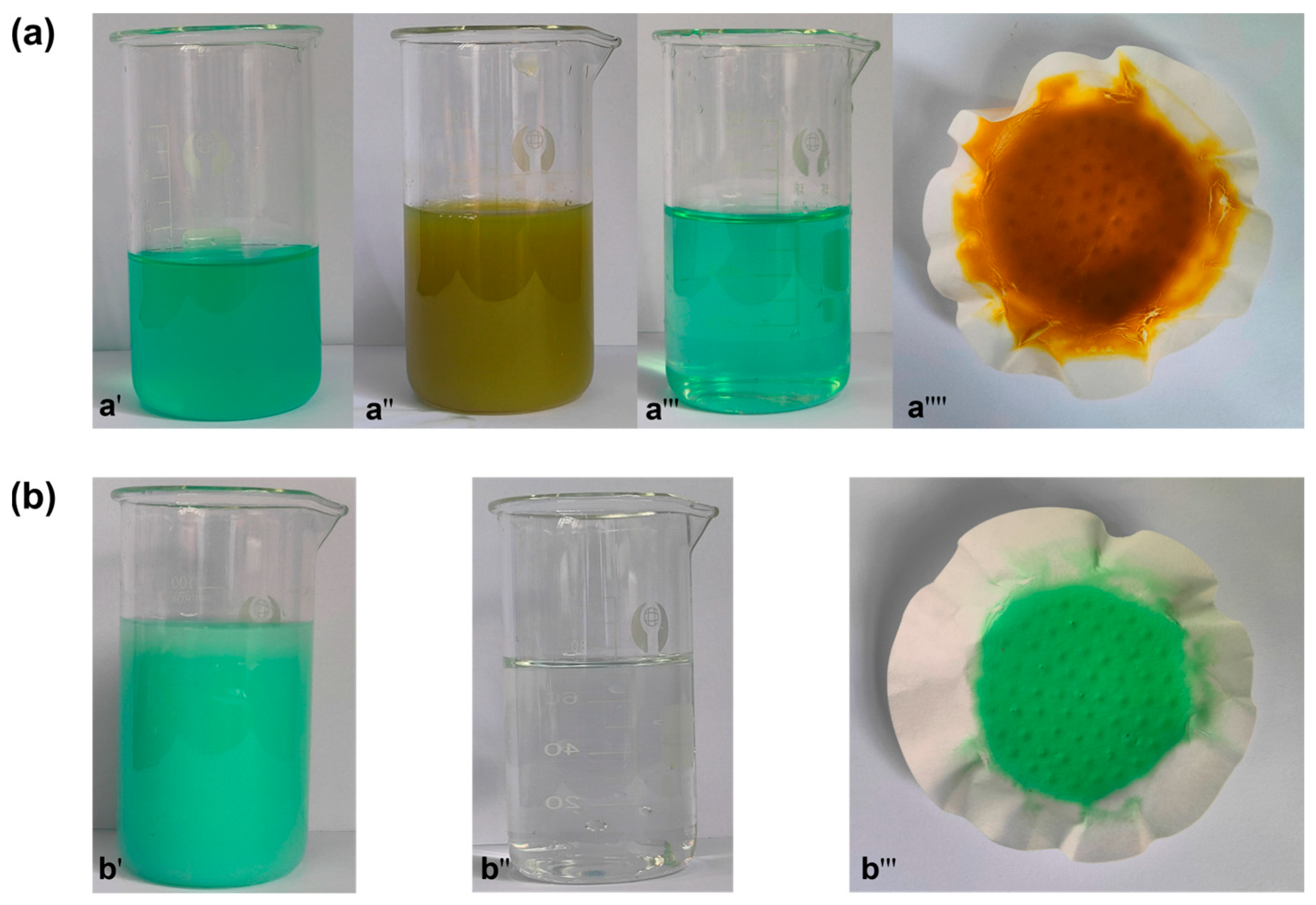
When precipitated, the color of the solution will also change. The color change phenomenon of the solution and the precipitation phenomenon at each step are shown in Figure 7. It turns out that this is almost the same as the phenomenon mentioned in Table 4. Figure 7a shows the change of solution and the color of precipitate during the experiment with NaOH solution. Figure 7b shows the color change of Na2CO3 solution and the color of obtained precipitate after the solution is added at a pH greater than 7.
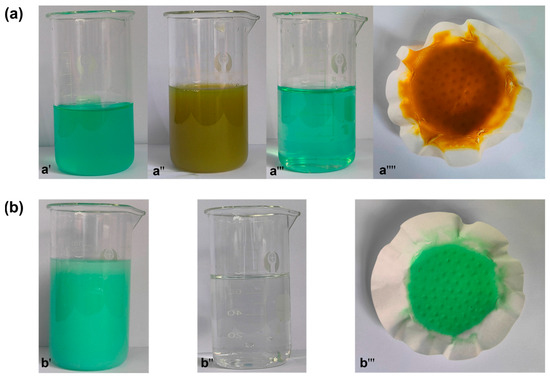
Figure 7.
The color change of solution and residue at temperature. Adding NaOH soluion (a): pH = 2–3 (a′), pH = 5 (a′′), pH = 7 (a′′′), the residue (a′′′′); adding Na2CO3 solution (b): pH = 9.8 (b′), pH= 10.5 (b′′), the residue (b′′′).

Table 4.
Experimental results of nickel recovery at room temperature.
The results show that NaOH can be used as an iron precipitator. When the pH range is adjusted to 4–5, iron precipitates in the form of brownish yellow. However, when the pH range is adjusted to 7–9, the free NH4+ in the leaching solution is easily complexed with Ni2+ to form colloid under alkaline conditions. The colloid will dissolve in ammonia water, cannot achieve the purpose of nickel iron separation, and is not conducive to nickel recovery. By comparing the precipitation effect, Na2CO3 is more suitable as a nickel precipitator than NaOH. Especially in the presence of a large amount of free NH4+ in the solution, Na2CO3 has a weaker ability to regulate pH. Therefore, the advantages of NaOH and Na2CO3 should be comprehensively considered in the selection of precipitating agents.
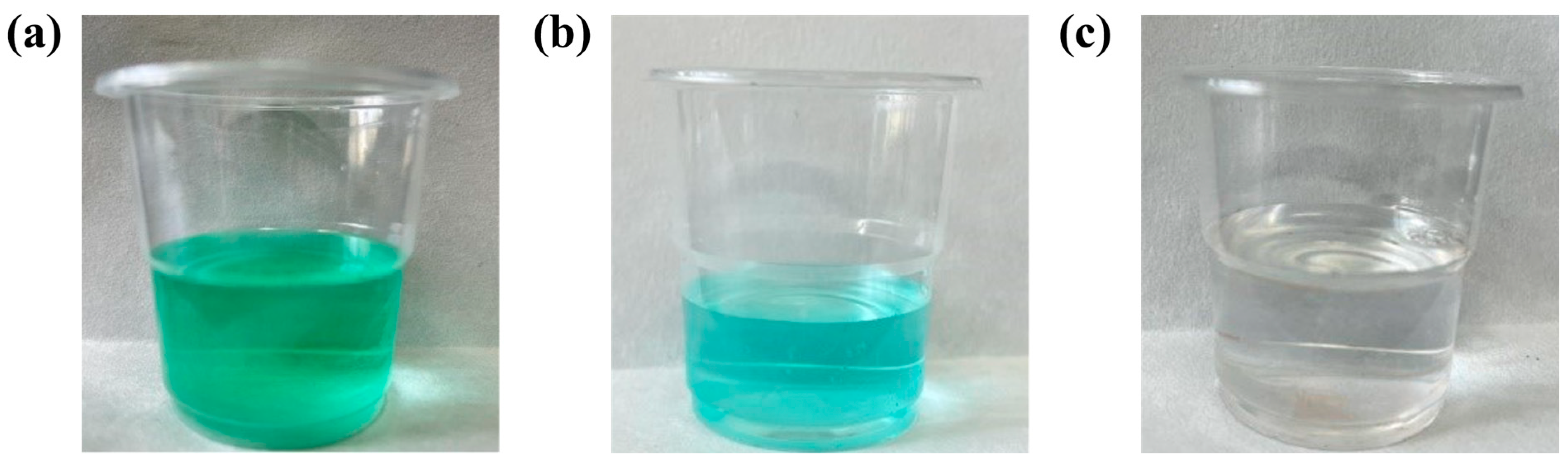
Firstly, a 5% NaOH solution is used to adjust the pH of the solution to 7. After the iron oxide is completely filtered, the solution pH is adjusted to 11 using 5% Na2CO3 solution. When there is no light-green precipitation in the solution, the basic nickel carbonate can be obtained by filtering and drying the filter cake. The color change of the solution during the precipitation process is shown in Figure 8, which changes from grass green to clear eventually.
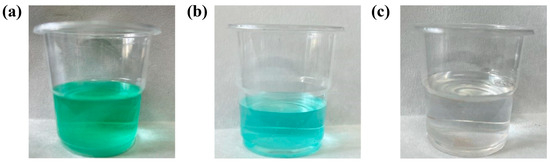
Figure 8.
Color change of leaching solution during precipitation at room temperature: the solution after HNO3 completely dissolves the waste electrolytic slag, pH = 2 (a); after pH adjustment, the solution that has removed Fe3+ after filtration, pH = 7 (b); adding Na2CO3, the solution after filtration, pH = 11 (c).
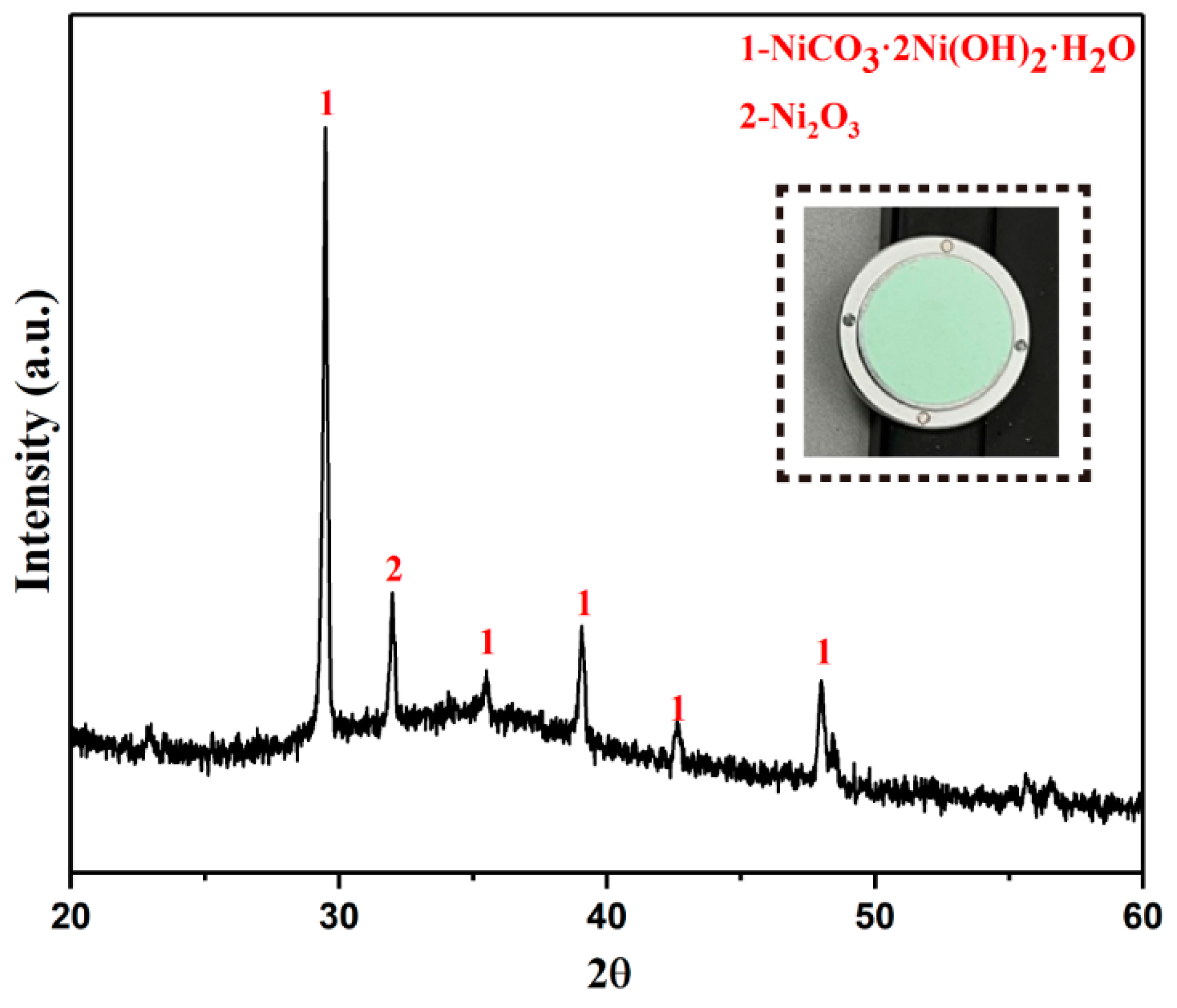
The final product XRD is shown in Figure 9. The results show that the final product is light green solid. Its main phase is nickel carbonate. It also contains a small amount of Ni2O3. The phase of Ni2O3 appears because a small part of the basic nickel carbonate reacts with solvent nitric acid during the precipitation process.
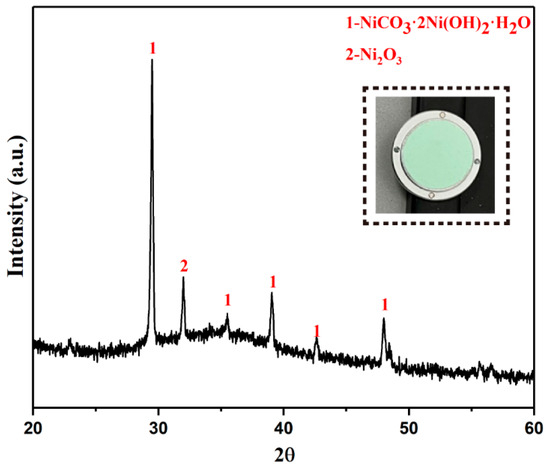
Figure 9.
XRD pattern of basic nickel carbonate.
3.5. The Preparation of NiO
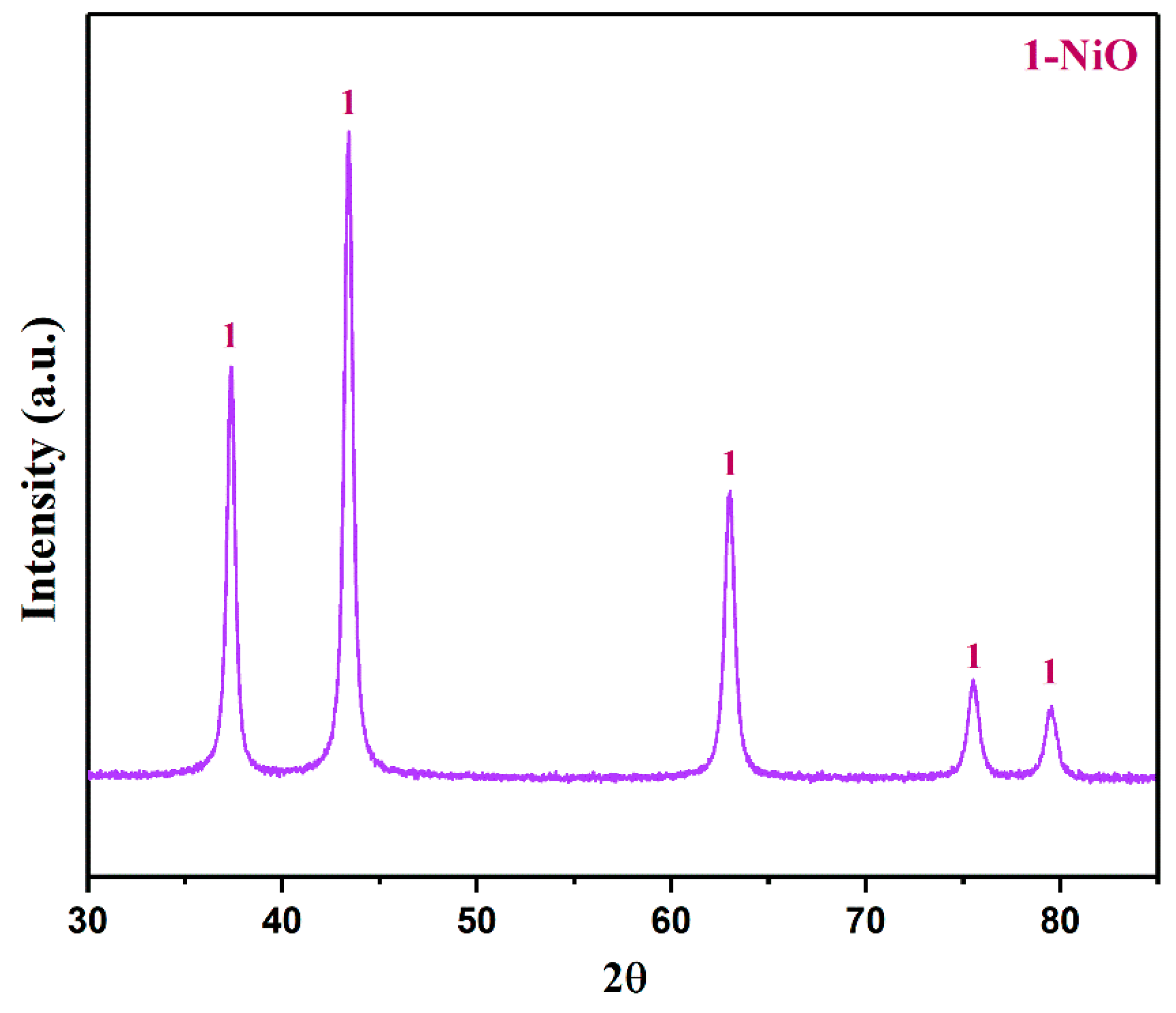
The basic nickel carbonate was roasted in a Muffle furnace at 600 °C for 1 h. The XRD pattern of the roasted product is shown in Figure 10. The results showed that the main phase of the roasted product was NiO. This is because after the basic nickel carbonate is calcined in an oxygen atmosphere, the excess CO32− and water molecules are removed.
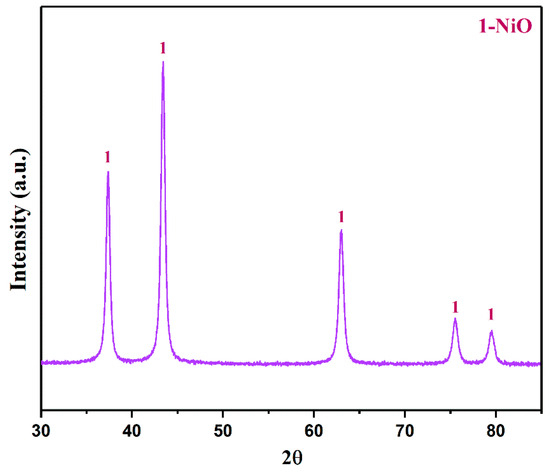
Figure 10.
The XRD pattern of the roasted product (roasted temperature: 600 °C).
According to the process route of this study (Figure 1), nickel in high-nickel waste electrolytic slag was recovered to obtain NiO. The content of Ni in the product was analyzed by ICP to determine the recovery efficiency of the process and verify the repeatability of the experiment. The experimental results are shown in Table 4.
With the progress of NiO production technology, in order to gradually develop the production technology and quality control of NiO in the direction of refinement, the preparation of NiO in this experiment conforms to the national standard.
4. Conclusions
In this study, the chemical precipitation method was used to recover and treat the nitrogen trifluoride waste electrolytic slag prepared by electrolysis, and the following conclusions were obtained:
- (1)
- The chemical precipitation method was used to select the appropriate solvent and precipitator, and optimize the experimental conditions. The nickel in high-nickel waste electrolytic slag was recovered. The problem of industrial production of nitrogen trifluoride by the electrolytic method was solved, realizing the recycling of resources;
- (2)
- An optimized recovery process was selected in the experiment. The waste electrolytic slag with high nickel content was treated by acid leaching, fractional precipitation, and oxidation roasting, and finally, the product NiO was obtained. The average mass fraction of nickel is 76.6%. The average recovery efficiency of nickel reached 92.4%;
- (3)
- The process not only realizes the recovery of nickel resources, but also has good economic benefits, and provides experimental support for realizing industrialization.
Author Contributions
Conceptualization, Y.R. (Yongzhuan Ren) and J.Y.; Funding acquisition, Y.W.; Investigation, J.Y. and Y.R. (Yongzhuan Ren); Methodology, Y.R. (Yongzhuan Ren), J.Y.; Resources, Y.W.; Supervision, Y.W.; Validation, J.Y. and Y.R. (Yuxuan Ran); Writing—original draft, Y.R. (Yongzhuan Ren), Y.R. (Yuxuan Ran) and J.Y.; Writing—review and editing, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Open Project of Yunnan Precious Metals Laboratory Co., Ltd. (No. YPML-2023050273).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Li, X.; Yang, C.; Du, S.; Wu, Y.; Huang, B.; Tan, A.; Liang, Z.; Xiao, J. Dynamic Adsorption Separation of C-C4F8/C3F8 for Effective Purification of Perfluoropropane Electronic Gas. Chem. Eng. Sci. 2023, 273, 118656. [Google Scholar] [CrossRef]
- Clark, J.R. Chemistry of Electronic Gases; ACS Publications: Washington, DC, USA, 2006. [Google Scholar]
- Miyazaki, T.; Mori, I.; Umezaki, T.; Yonezawa, S. NF3 Synthesis Using ClF3 as a Mediator. J. Fluor. Chem. 2019, 219, 55–61. [Google Scholar] [CrossRef]
- Tasaka, A. Electrochemical Synthesis and Application of NF3. J. Fluor. Chem. 2007, 128, 296–310. [Google Scholar] [CrossRef]
- Panda, R.; Jha, M.K.; Pathak, D.D.; Gupta, R. Recovery of Ag, Cu, Ni and Fe from the Nitrate Leach Liquor of Waste ICs. Miner. Eng. 2020, 158, 106584. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, K.M.; Ko, M.S.; Kim, Y.S. A Study of the NF3 Plasma Etching Reaction with Cobalt Oxide Films Grown on an Inorganic Compounds. Nucl. Eng. Technol. 2022, 54, 4449–4459. [Google Scholar] [CrossRef]
- Meng, L.; Qu, J.; Guo, Q.; Xie, K.; Zhang, P.; Han, L.; Zhang, G.; Qi, T. Recovery of Ni, Co, Mn, and Mg from Nickel Laterite Ores Using Alkaline Oxidation and Hydrochloric Acid Leaching. Sep. Purif. Technol. 2015, 143, 80–87. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Y.; Jiao, S.Q.; Chou, K.C.; Zhang, G.H. Recovery of Ni Matte from Ni-Bearing Electroplating Sludge. J. Environ. Manag. 2023, 326, 116744. [Google Scholar] [CrossRef]
- Perosa, A.; Tundo, P. Selective Hydrogenolysis of Glycerol with Raney Nickel. Ind. Eng. Chem. Res. 2005, 44, 8535–8537. [Google Scholar] [CrossRef]
- Tian, B.; Cui, Y.; Zhao, J.; Liu, M.; Shang, H.; Gao, W.; Wen, J.; Ma, J. Stepwise Recovery of Ni, Cu, Zn, and Cr: A Green Route to Resourceful Disposal of Electroplating Sludge. J. Environ. Chem. Eng. 2023, 11, 109767. [Google Scholar] [CrossRef]
- Guo, X.Y.; Shi, W.T.; Li, D.; Tian, Q.H. Leaching Behavior of Metals from Limonitic Laterite Ore by High Pressure Acid Leaching. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2011, 21, 191–195. [Google Scholar] [CrossRef]
- Johnson, J.A.; Cashmore, B.C.; Hockridge, R.J. Optimisation of Nickel Extraction from Laterite Ores by High Pressure Acid Leaching with Addition of Sodium Sulphate. Miner. Eng. 2005, 18, 1297–1303. [Google Scholar] [CrossRef]
- Benali, K.; Benhida, R.; Khaless, K. A Novel and Complete Process for Iodine Extraction and Recovery from Industrial Wet-Process Phosphoric Acid Based on Chemical Oxidation and Solvent Extraction. Process Saf. Environ. Prot. 2023, 176, 332–345. [Google Scholar] [CrossRef]
- Virolainen, S.; Fallah Fini, M.; Laitinen, A.; Sainio, T. Solvent Extraction Fractionation of Li-Ion Battery Leachate Containing Li, Ni, and Co. Sep. Purif. Technol. 2017, 179, 274–282. [Google Scholar] [CrossRef]
- Coman, V.; Robotin, B.; Ilea, P. Nickel Recovery/Removal from Industrial Wastes: A Review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Argun, M.E. Use of Clinoptilolite for the Removal of Nickel Ions from Water: Kinetics and Thermodynamics. J. Hazard. Mater. 2008, 150, 587–595. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective Removal of the Heavy Metal Ions from Waters and Industrial Wastewaters by Ion-Exchange Method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.; Fatta, D.; Parperis, K.; Mentzis, A.; Haralambous, K.J.; Loizidou, M. Nickel Uptake from a Wastewater Stream Produced in a Metal Finishing Industry by Combination of Ion-Exchange and Precipitation Methods. Sep. Purif. Technol. 2004, 39, 181–188. [Google Scholar] [CrossRef]
- Dzyazko, Y.S.; Belyakov, V.N. Purification of a Diluted Nickel Solution Containing Nickel by a Process Combining Ion Exchange and Electrodialysis. Desalination 2004, 162, 179–189. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, Z.; Yang, J.; Cao, L.; Zhang, W. Recovery of Mo and Ni from Spent Acrylonitrile Catalysts Using an Oxidation Leaching-Chemical Precipitation Technique. Hydrometallurgy 2016, 164, 64–70. [Google Scholar] [CrossRef]
- BATTI, N.R.; MANDRE, N.R. Nickel and Aluminium Recovery from Spent Reforming Catalyst through Selective Leaching, Crystallization and Precipitation. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2022, 32, 345–353. [Google Scholar] [CrossRef]
- Yang, X.; Peng, X.; Kong, L.; Hu, X. Removal of Ni(II) from Strongly Acidic Wastewater by Chelating Precipitation and Recovery of NiO from the Precipitates. J. Environ. Sci. 2021, 104, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Astuti, W.; Nurjaman, F.; Rofiek Mufakhir, F.; Sumardi, S.; Avista, D.; Cleary Wanta, K.; Tri Bayu Murti Petrus, H. A Novel Method: Nickel and Cobalt Extraction from Citric Acid Leaching Solution of Nickel Laterite Ores Using Oxalate Precipitation. Miner. Eng. 2023, 191, 107982. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Long, H.; Cheng, Y.; Wu, Y.; Cai, Y.; Jiang, J. Recovery of Cobalt and Nickel from Magnesium-Rich Sulfate Leach Liquor with Magnesium Oxide Precipitation Method. Miner. Eng. 2021, 169, 106961. [Google Scholar] [CrossRef]
- Klaehn, J.R.; Shi, M.; Diaz, L.A.; Molina, D.E.; Reich, S.M.; Palasyuk, O.; Repukaiti, R.; Lister, T.E. Removal of Impurity Metals as Phosphates from Lithium-Ion Battery Leachates. Hydrometallurgy 2023, 217, 106041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).