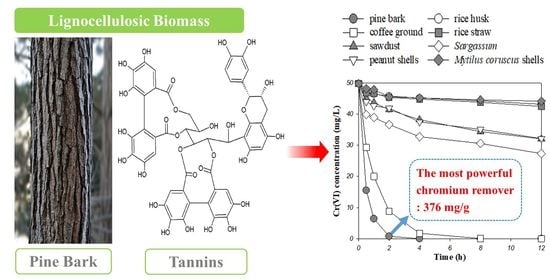
Superior Removal of Toxic Cr(VI) from Wastewaters by Natural Pine Bark
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biomass
2.2. Batch Biosorption Studies
2.3. Chromium Analysis
2.4. FTIR Analysis
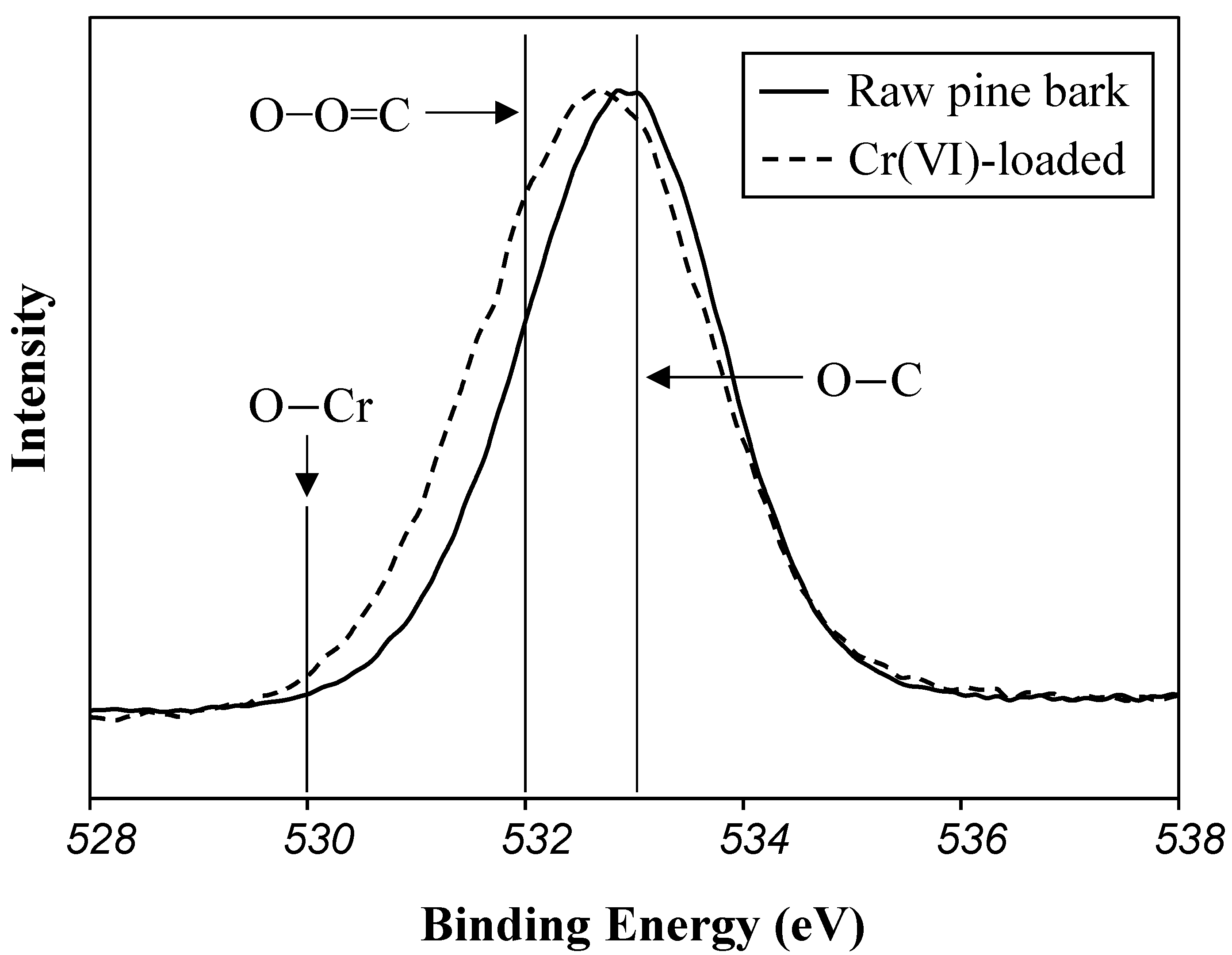
2.5. XPS Analysis
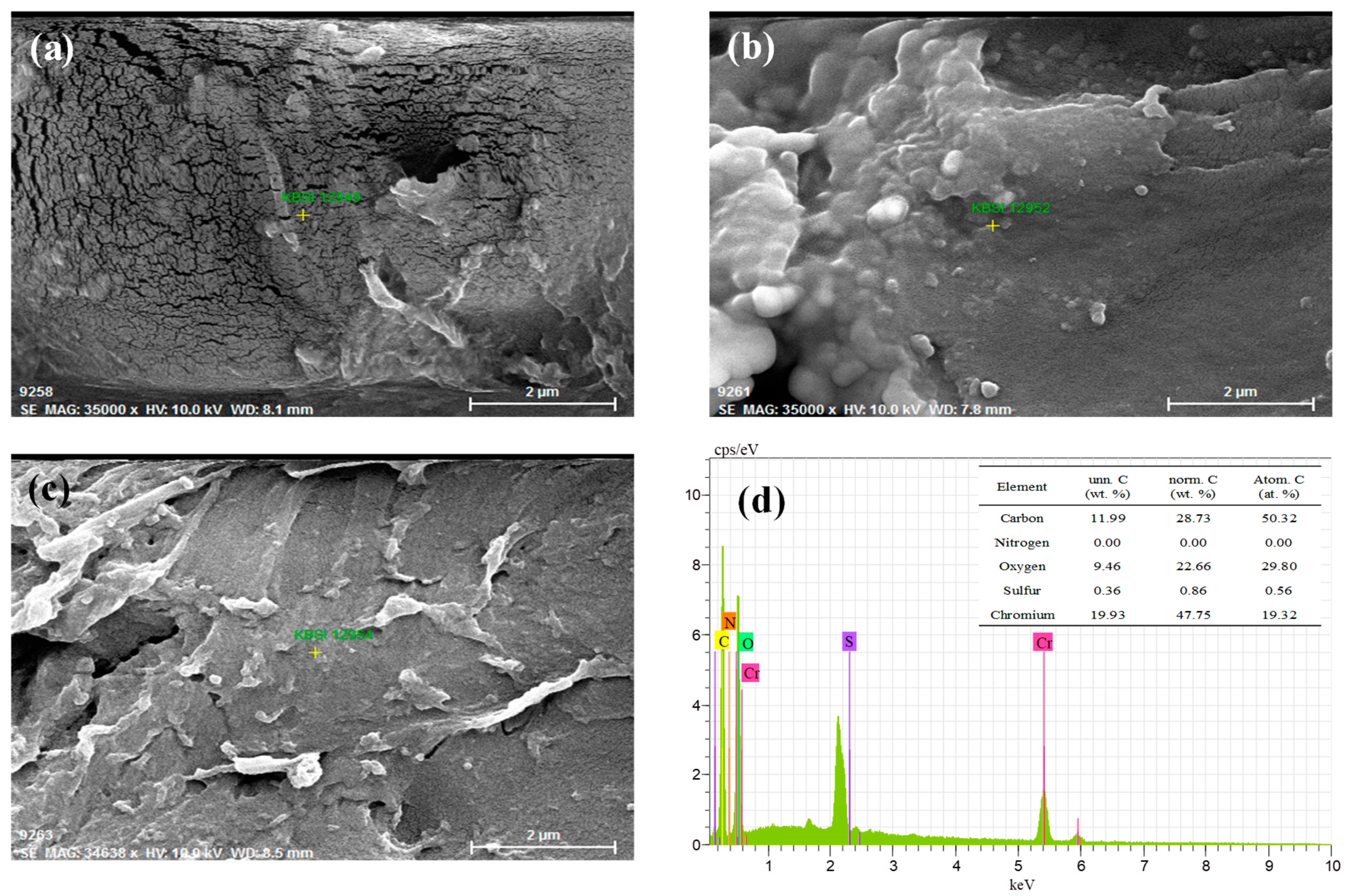
2.6. SEM-EDX Analysis
3. Results and Discussion
3.1. Characterization of Pine Bark
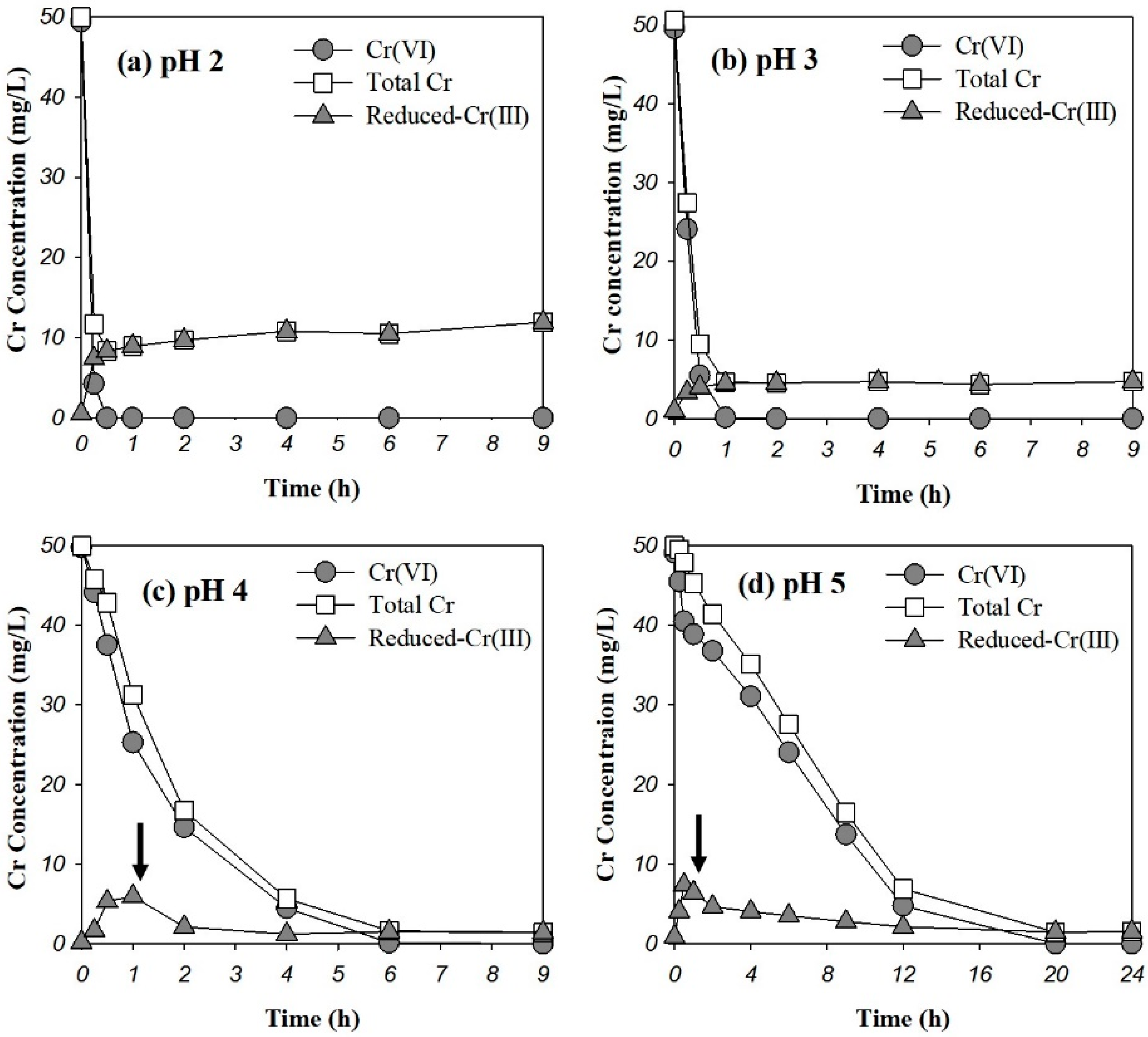
3.2. Removal Behaviors of Cr(VI) by Pine Bark
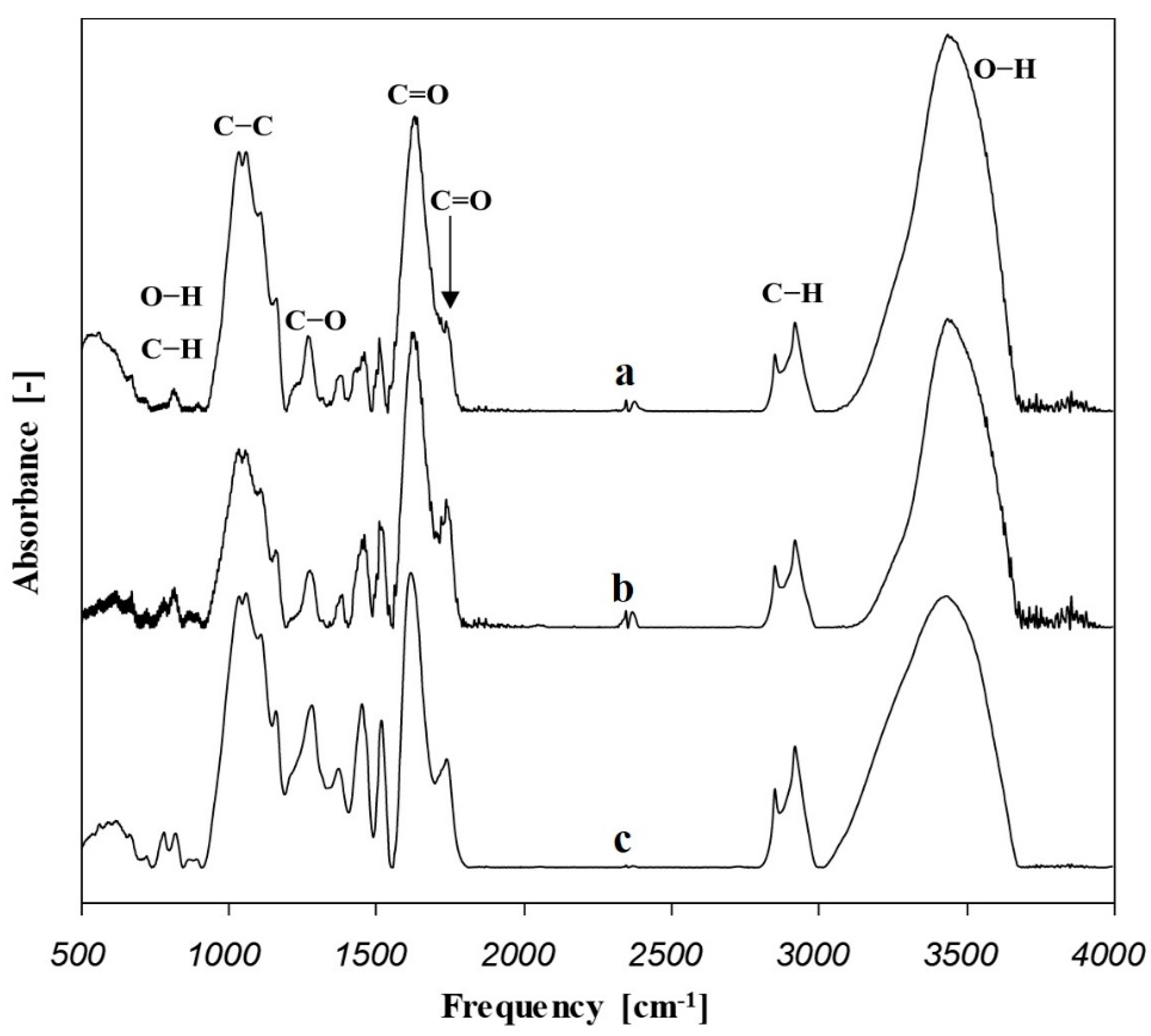
3.3. FTIR Spectroscopic Study
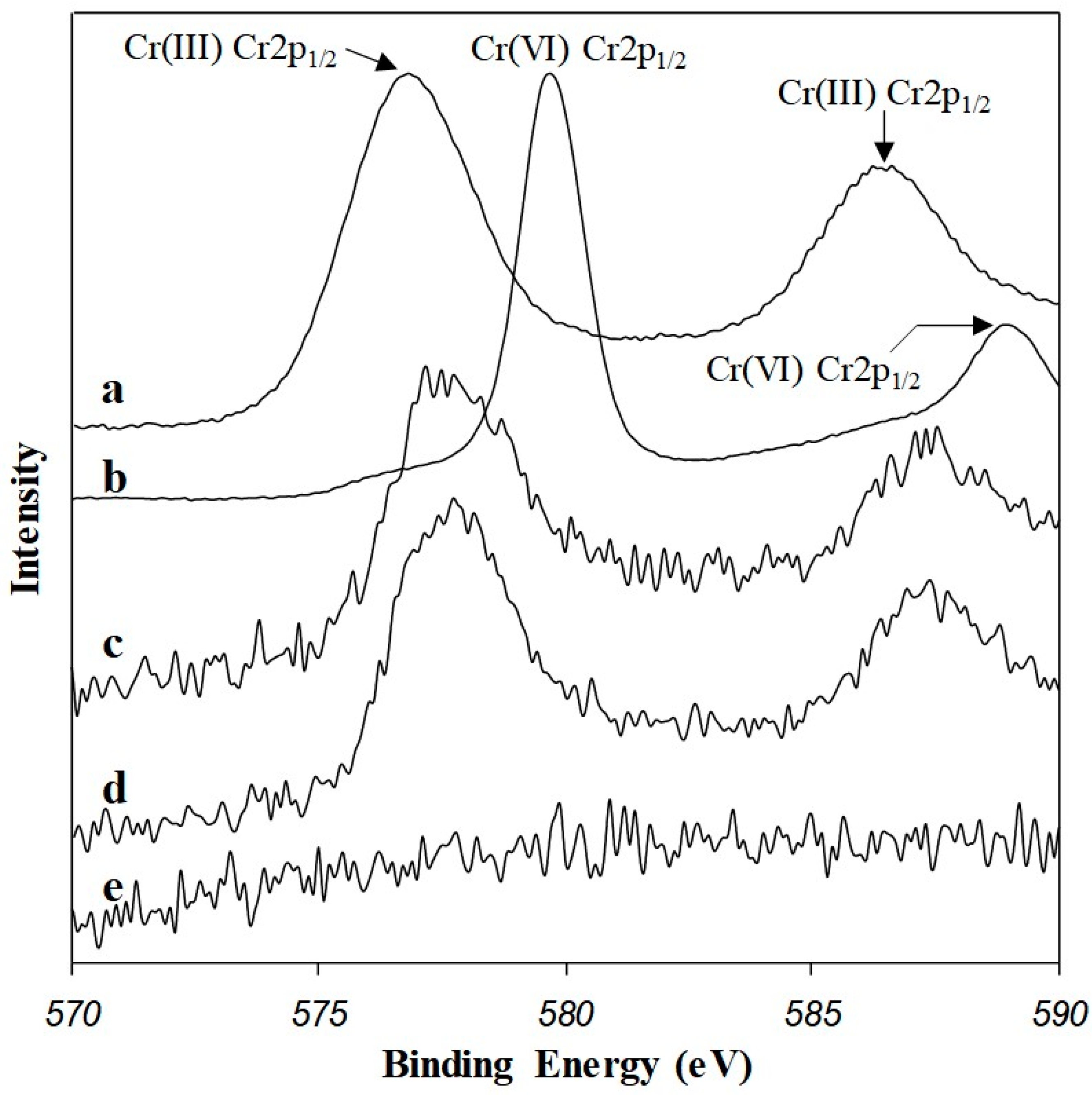
3.4. XPS Study
3.5. SEM-EDX Study
3.6. Evaluation of Cr(VI)-Reducing Power of Pine Bark
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.X.; Dou, J.F.; Xu, H.B. Removal of Cr(VI) ions by sewage sludge compost biomass from aqueous solutions: Reduction to Cr(III) and biosorption. Appl. Surf. Sci. 2017, 425, 728–735. [Google Scholar] [CrossRef]
- Liang, X.; Fan, X.; Li, R.; Li, S.; Shen, S.; Hu, D. Efficient removal of Cr(VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure. Bioresour. Technol. 2018, 250, 178–184. [Google Scholar] [CrossRef]
- Yin, R.V.; Phung, O.J. Effect of chromium supplementation on glycated hemoglobin and fasting plasma glucose in patients with diabetes mellitus. Nutr. J. 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, J. Occurrences, uses, and properties of chromium. Regul. Toxicol. Pharmacol. 1997, 26, S3–S7. [Google Scholar] [CrossRef]
- Escudero, C.; Fiol, N.; Poch, J.; Villaescusa, I. Modeling of kinetics of Cr(VI) sorption onto grape stalk waste in a stirred batch reactor. J. Hazard. Mater. 2009, 170, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, S.; Hong, H.J.; Choi, Y.E.; Yang, J.W. Biosorption of chromium (Cr(III)/Cr(VI)) on the residual microalga Nannochloris oculata after lipid extraction for biodiesel production. Bioresour. Technol. 2011, 102, 11155–11160. [Google Scholar] [CrossRef]
- Netzahuatl-Muñoz, A.R.; Aranda-García, E.; Cristiani-Urbina, M.D.C.; Barragán-Huerta, B.E.; Villegas-Garrido, T.L.; Cristiani-Urbina, E. Removal of hexavalent and total chromium from aqueous solutions by Schinus molle Bark. Fresenius Environ. Bull. 2010, 19, 2911–2918. [Google Scholar]
- Park, D.; Yun, Y.S.; Park, J.M. Reduction of hexavalent chromium with the brown seaweed Ecklonki biomass. Environ. Sci. Technol. 2004, 38, 4860–4864. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Jo, J.H.; Park, J.M. Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger. Water Res. 2005, 39, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. Use of dead fungal biomass for the detoxification of hexavalent chromium: Screening and kinetics. Process Biochem. 2005, 40, 2559–2565. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Lautner, S.; Saake, B. Removal of hexavalent chromium by different modified spruce bark adsorbents. J. Wood Chem. Technol. 2014, 34, 273–290. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Braghiroli, F.L.; Amaral-Labat, G.; Boss, A.F.N.; Lacoste, C.; Pizzi, A. Tannin gels and their carbon derivatives: A review. Biomolecules 2019, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Lopez-Nuñez, P.V.; Aranda-García, E.; Cristiani-Urbina, M.C.; Morales-Barrera, L.; Cristiani-Urbina, E. Removal of hexavalent and total chromium from aqueous solutions by plum (P. domestica L.) tree bark. Environ. Eng. Manag. J. 2014, 13, 1927–1938. [Google Scholar]
- Han, X.; Wong, Y.S.; Wong, M.H.; Tam, N.F.Y. Biosorption and bioreduction of Cr(VI) by a microalgal isolate, Chlorella miniata. J. Hazard. Mater. 2007, 146, 65–72. [Google Scholar] [CrossRef]
- Netzahuatl-Muñoz, A.R.; Morales-Barrera, L.; Cristiani-Urbina, M.d.C.; Cristiani-Urbina, E. Hexavalent chromium reduction and chromium biosorption by Prunus serotina bark. Fresenius Environ. Bull. 2012, 21, 1793–1801. [Google Scholar]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids: Potential therapeutic agents by their antioxidant capacity. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 265–288. [Google Scholar]
- Shen, Y.S.; Wang, S.L.; Huang, S.T.; Tzou, Y.M.; Huang, J.H. Biosorption of Cr(VI) by coconut coir: Spectroscopic investigation on the reaction mechanism of Cr(VI) with lignocellulosic material. J. Hazard. Mater. 2010, 179, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Pereira, H.; Olivella, M.A.; Villaescusa, I. Heavy metals removal in aqueous environments using bark as a biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. Chemical state quantification of iron and chromium oxides using XPS: The effect of the background subtraction method. Surf. Sci. 2005, 578, 108–123. [Google Scholar] [CrossRef]
- Choudhary, B.; Paul, D.; Singh, A.; Gupta, T. Removal of hexavalent chromium upon interaction with biochar under acidic conditions: Mechanistic insights and application. Environ. Sci. Pollut. Res. 2017, 24, 16786–16797. [Google Scholar]
- Xu, X.; Huang, H.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr (VI) during its sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Tang, P.L.; Lee, C.K.; Low, K.S.; Zainal, Z. Sorption of Cr(VI) and Cu(II) in aqueous solution by ethylenediamine modified RCE hull. Environ. Technol. 2003, 24, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M. Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: Kinetic and equilibrium studies. Bioresour. Technol. 2004, 91, 317–321. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J. Hazard. Mater. 2005, 124, 192–199. [Google Scholar] [CrossRef]
- Bansal, M.; Singh, D.; Garg, V.K. A comparative study for the removal of hexavalent chromium from aqueous solution by agriculture wastes' carbons. J. Hazard. Mater. 2009, 171, 83–92. [Google Scholar] [CrossRef]
- Aoyama, M.; Sugiyama, T.; Doi, S.; Cho, N.S.; Kim, H.E. Removal of hexavalent chromium from dilute aqueous solution by coniferous leaves. Holzforschung 1999, 53, 365–368. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer'eb, M. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Chand, R.; Narimura, K.; Kawakita, H.; Ohto, K.; Watari, T.; Inoue, K. Grape waste as a biosorbent for removing Cr(VI) from aqueous solution. J. Hazard. Mater. 2009, 163, 245–250. [Google Scholar] [CrossRef]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; El-Turki, A.; Hallam, K.R.; Allen, G.C. Banana peel: A green and economical sorbent for the selective removal of Cr(VI) from industrial wastewater. Colloids Surf. B 2009, 70, 232–237. [Google Scholar] [CrossRef]

| Parameters | Values |
|---|---|
| Moisture (%) | 5.651 |
| Ash (%) | 21.25 |
| Volatile organic matter (%) | 73.10 |
| Carbon (%) | 55.57 |
| Hydrogen (%) | 5.549 |
| Nitrogen (%) | 0.080 |
| Sulfur (%) | 0.077 |
| Oxygen (%) | 37.19 |
| Zeta potential (mV) | −27 |
| Point of zero charge (pHpzc) | 3.79 |
| Biomass | Proposed Mechanism of the Cr(VI) Removal | Cr(VI) Removal Capacity (mg/g) | pH | Ref. |
|---|---|---|---|---|
| Ethylenediamine-modified rice hull | Adsorption a | 23.4 | 2.0 | [25] |
| Hazelnut shell | Adsorption a | 170 | 1.0 | [26] |
| Sawdust activated carbon | Adsorption a | 65.8 | 2.0 | [27] |
| Sugar beet pulp | Adsorption a | 17.2 | 2.0 | [28] |
| Maize cob | Adsorption a | 13.8 | 1.5 | [29] |
| Sugarcane baggase | Adsorption a | 13.4 | 2.0 | [30] |
| Coniferous leaves | Adsorption a | 6.3 | 3.0 | [29] |
| Pine needle | Adsorption a | 21.5 | 2.0 | [30] |
| Grape waste | Adsorption-coupled reduction b | 99.3 | 4.0 | [31] |
| Banana peel | Adsorption-coupled reduction b | 96.2 | 2.0 | [32] |
| Pine bark | Adsorption-coupled reduction b | 376.3 | 2.0 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Kim, N.; Park, D. Superior Removal of Toxic Cr(VI) from Wastewaters by Natural Pine Bark. Separations 2023, 10, 430. https://doi.org/10.3390/separations10080430
Yang H, Kim N, Park D. Superior Removal of Toxic Cr(VI) from Wastewaters by Natural Pine Bark. Separations. 2023; 10(8):430. https://doi.org/10.3390/separations10080430
Chicago/Turabian StyleYang, Hanui, Namgyu Kim, and Donghee Park. 2023. "Superior Removal of Toxic Cr(VI) from Wastewaters by Natural Pine Bark" Separations 10, no. 8: 430. https://doi.org/10.3390/separations10080430
APA StyleYang, H., Kim, N., & Park, D. (2023). Superior Removal of Toxic Cr(VI) from Wastewaters by Natural Pine Bark. Separations, 10(8), 430. https://doi.org/10.3390/separations10080430