Clarification of the Cardoon (Cynara cardunculus) Blanching Wastewater by Ultrafiltration—Study of Membrane Fouling and Flux Recovery after Chemical Cleaning
Abstract
1. Introduction
2. Materials and Methods
2.1. Blanching Wastewater
2.2. Ultrafiltration System
2.2.1. Ultrafiltration Membranes
2.2.2. Membrane Evaluation
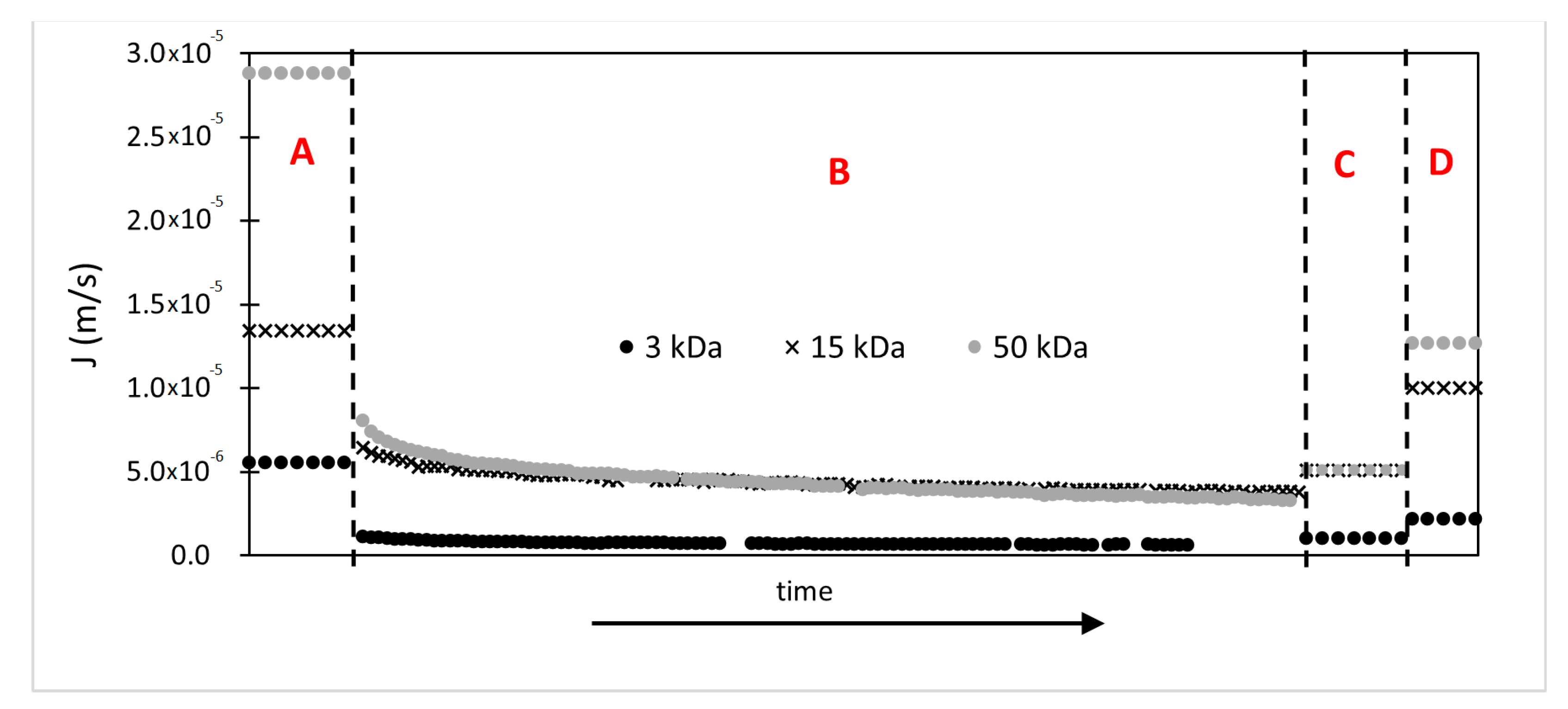
2.3. Fouling and Flux Decline Study
2.4. Analytical Determinations
2.4.1. Chemicals
2.4.2. Polyphenol Content
2.4.3. Phenolic Acids
2.4.4. Phenolic Composition
2.5. Statistical Analysis
3. Results and Discussion
3.1. Composition of Cardoon Blanching Wastewaters
3.2. Permeate Flux Performance
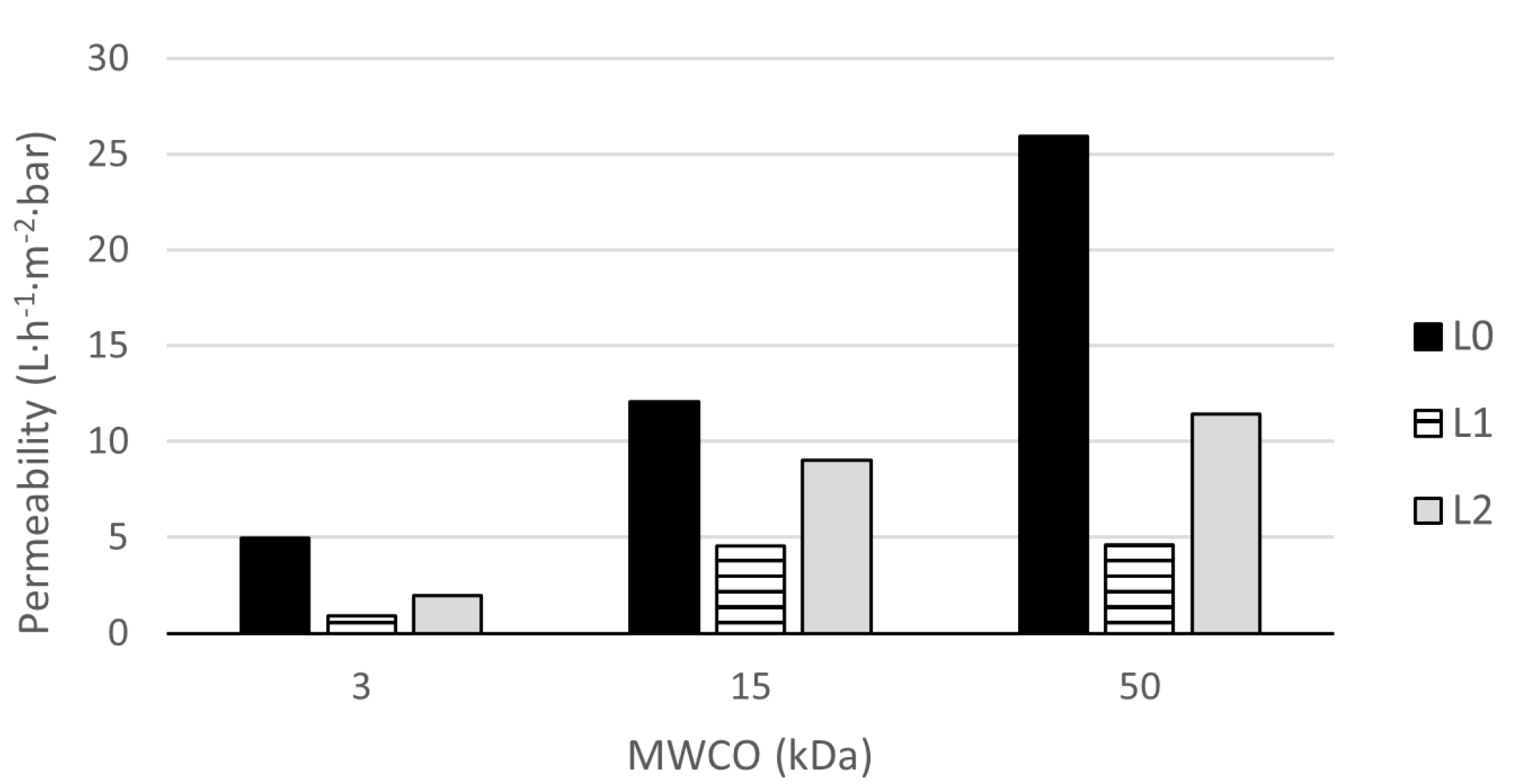
3.2.1. Membrane Characterization
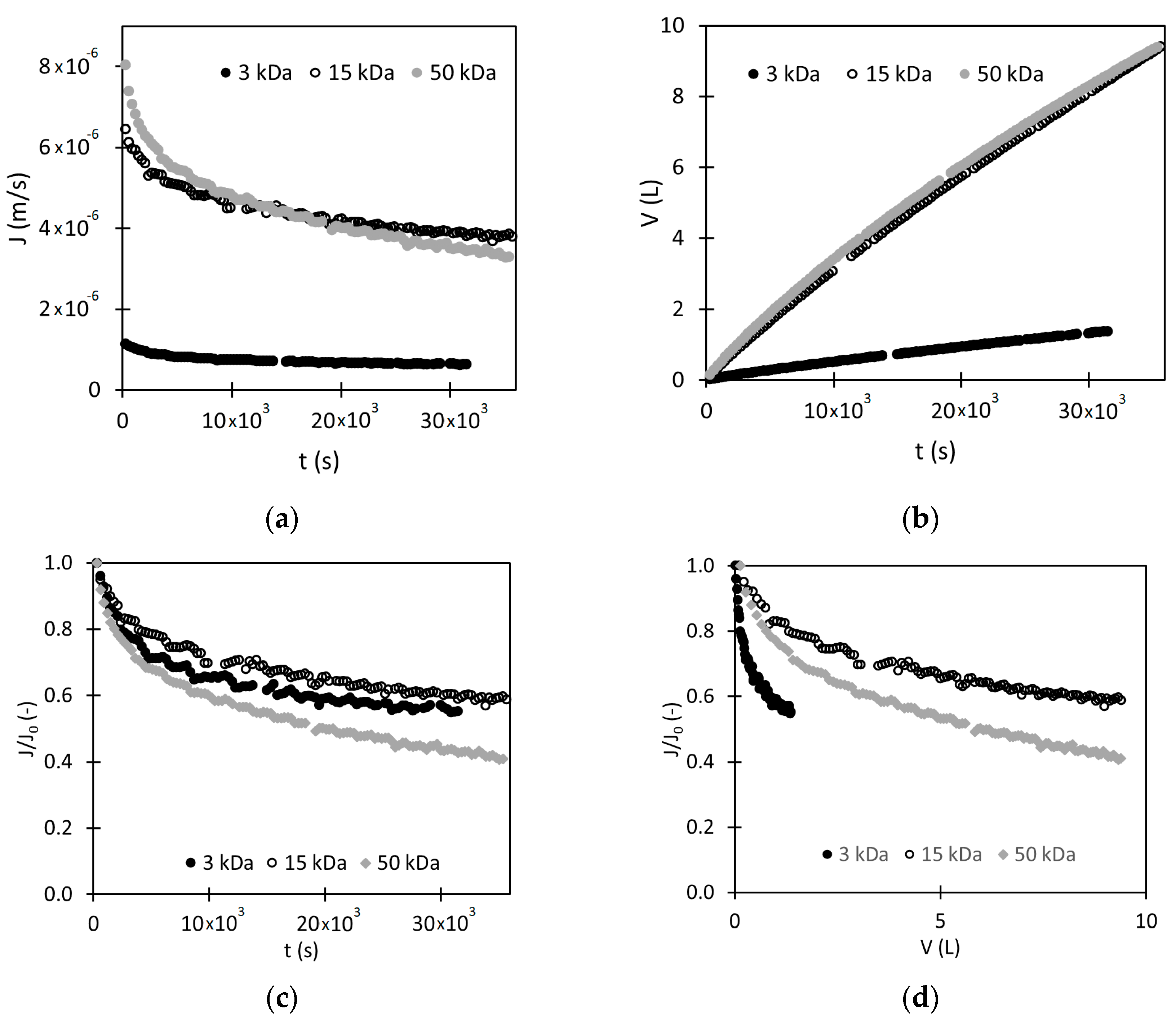
3.2.2. Evolution of the Permeate Flux
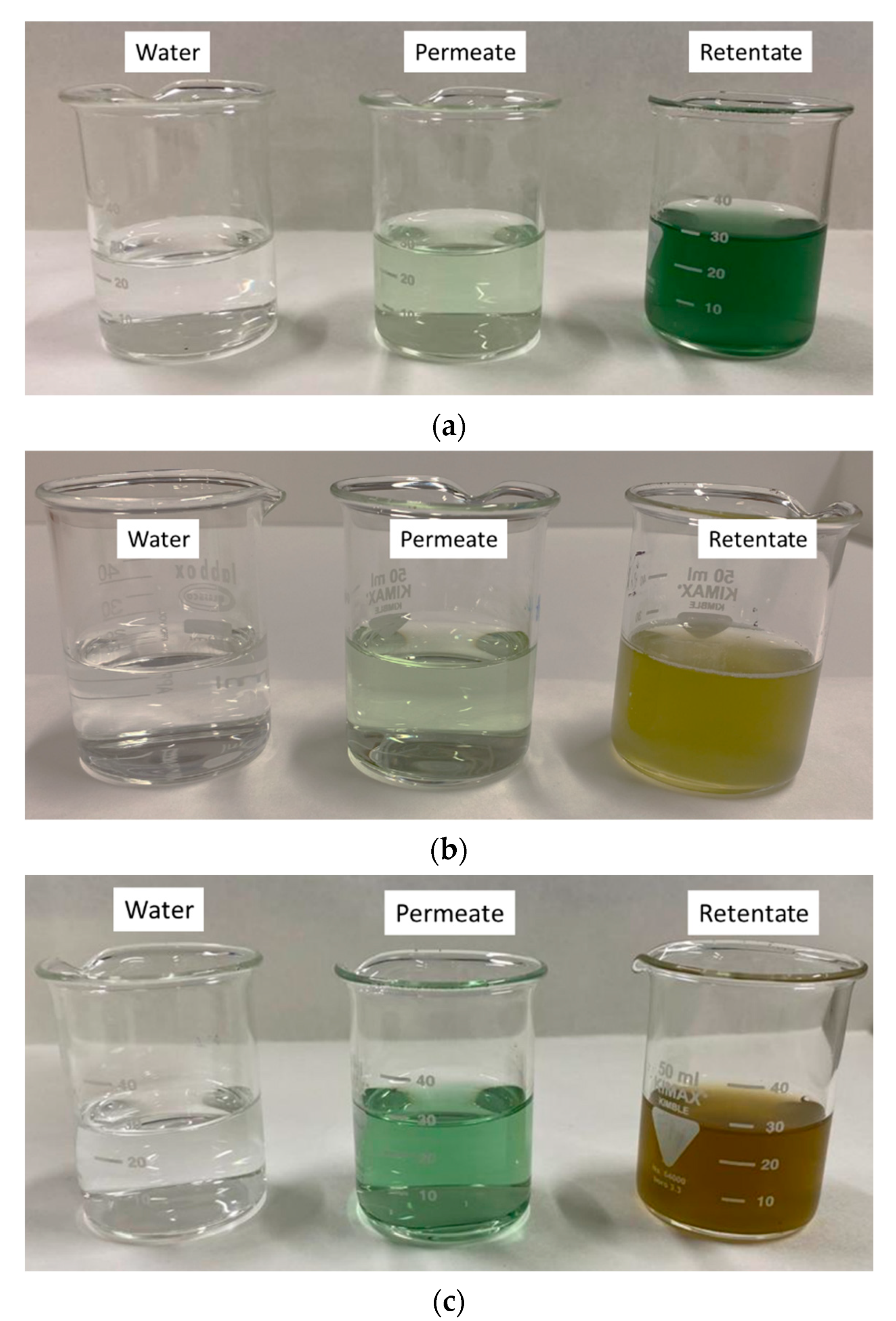
3.3. Separation of Bioactive Compounds by UF
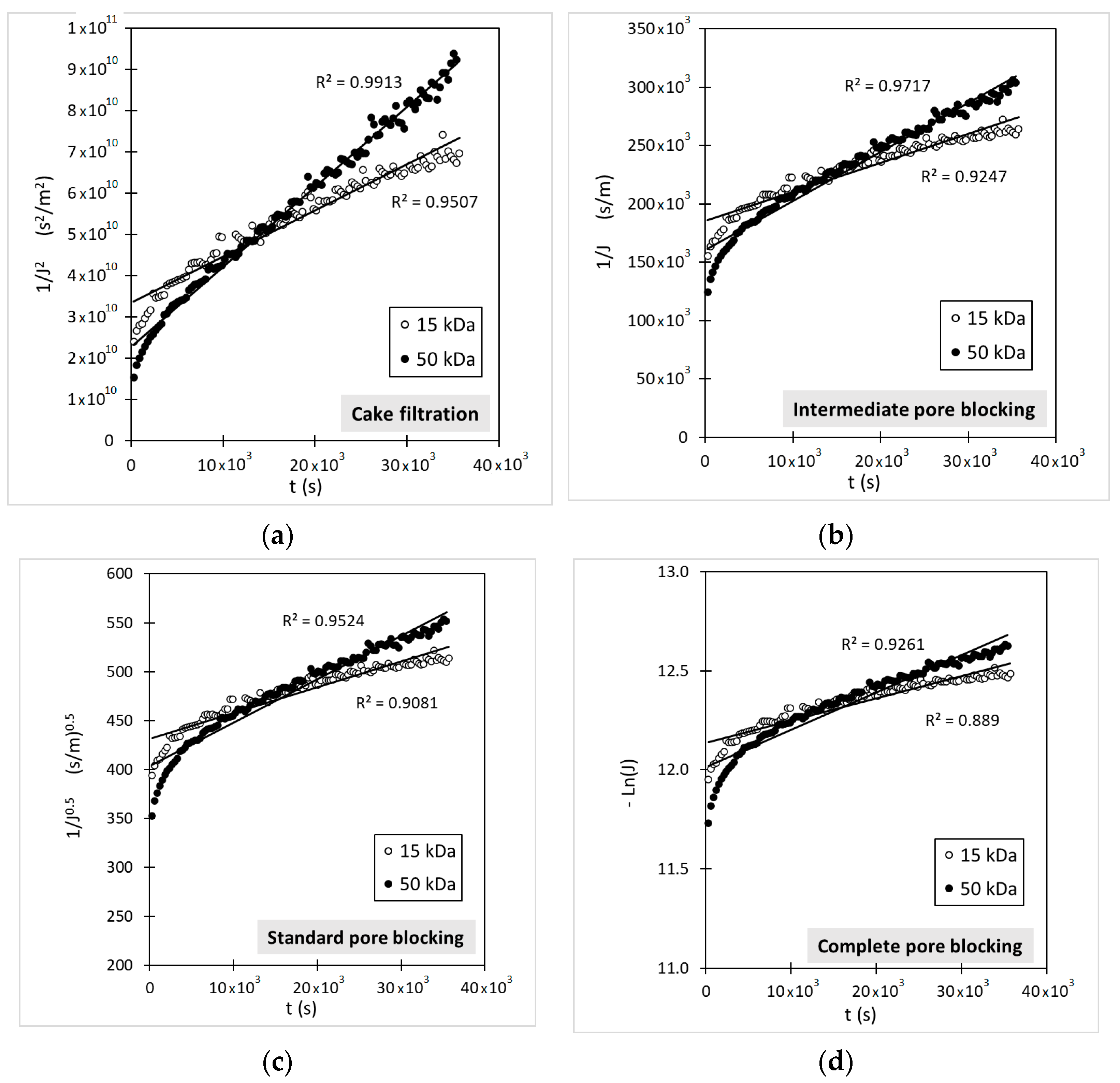
3.4. Flux Decline Mechanims
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crops Prod. 2006, 24, 222–229. [Google Scholar] [CrossRef]
- Ierna, A.; Mauromicale, G. Cynara cardunculus L. genotypes as a crop for energy purposes in a Mediterranean environment. Biomass Bioenergy 2010, 34, 754–760. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Castanheira, I.; Fernando, A.L.; Roizzo, M.R.; Sanches Silva, A. A New Insight on Cardoon: Exploring New Uses besides Cheese Making with a View to Zero Waste. Foods 2020, 9, 564. [Google Scholar] [CrossRef]
- Ferioli, F.; A’Antuono, L.F. Phenolic compounds in local Italian types of cultivated cardoon (Cynara cardunculus L. var. altilis DC) stalks and artichoke (Cynara cardunculus L. var. scolymus L.) edible sprouts. J. Food Compos. Anal. 2022, 106, 104342. [Google Scholar]
- Mañas, P.; Castro, E.; de las Heras, J. Application of treated wastewater and digested sewage sludge to obtain biomass from Cynara cardunculus L. J. Clean. Prod. 2014, 67, 72–78. [Google Scholar] [CrossRef]
- FAOSTAT, Food and Agriculture Organization of the United Nations, Statistics Division. 2020. Available online: http://www.fao.org/faostat (accessed on 12 January 2022).
- MAPA, Ministerio de Agricultura Pesca y Alimentación (Spain). Available online: https://www.mapa.gob.es/estadistica/pags/anuario/2020/TABLAS%20PDF/CAPITULO07/pdfc07_7.5.14.4.pdf (accessed on 12 January 2022).
- Hajji Nabih, M.; El Hajam, M.; Boulika, H.; Chiki, Z.; Ben Tahar, S.; Idrissi Kandri, N.; Zerouale, A. Preparation and characterization of activated carbons from cardoon “Cynara cardunculus” waste: Application to the adsorption of synthetic organic dyes. Mater. Today Proc. 2023, 72, 3369–3379. [Google Scholar] [CrossRef]
- UN, United Nations. Available online: https://sdgs.un.org/goals (accessed on 16 January 2022).
- Pandino, G.; Bonomo, A.; Scavo, A.; Mauromicale, G.; Lombardo, S. Caffeoylquinic acids and flavones profile in Cynara cardunculus L. seedlings under controlled conditions as affected by light and water-supply treatments. Sci. Hortic. 2022, 302, 111180. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alvesa, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Gerschenson, L.N.; Fissore, E.N.; Rojas, A.M.; Bernhardt, D.C.; Santo Domingo, C. Artichoke. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press-Elsevier: London, UK, 2020; Chapter 4; pp. 55–69. [Google Scholar]
- Garcia-Castello, E.M.; Mayor, L.; Calvo-Ramirez, A.; Ruiz-Melero, R.; Rodriguez-Lopez, A.D. Response Surface Optimization of Inulin and Polyphenol Extraction from Artichoke (Cynara scolymus (L.)) Solid Wastes. Appl. Sci. 2022, 12, 7957. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Cassano, A.; Rastogi, N.K.; Basile, A. Membrane technologies for water treatment and reuse in the food and beverage industries. In Advances in Membrane Technologies for Water Treatment; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Woodhead Publishing-Elsevier: Cambridge, UK, 2015; Chapter 18; pp. 551–580. [Google Scholar]
- Galanakis, C.M.; Castro-Muñoz, R.; Cassano, A.; Conidi, C. Recovery of high-added-value compounds from food waste by membrane technology. In Membrane Technologies for Biorefining; Figoli, A., Cassano, A., Basile, A., Eds.; Woodhead Publishing-Elsevier: Cambridge, UK, 2016; Chapter 8; pp. 189–215. [Google Scholar]
- Battacharjee, C.; Saxena, V.K.; Dutta, S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 136–153. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2017, 17, 149–164. [Google Scholar] [CrossRef]
- Saf, C.; Gondet, L.; Villain-Gambier, M.; Belaqziz, M.; Trebouet, D.; Ouazzani, N. Investigation of the agroecological applications of olive mill wastewater fractions from the ultrafiltration-nanofiltration process. J. Environ. Manag. 2023, 333, 117467. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.C.; Santos Amaral Moravia, M.C.; Fonseca Couto, C. Combined process of ultrafiltration and nanofiltration for vinasse treatment with and without pre-coagulation. J. Water Process. Eng. 2020, 36, 101326. [Google Scholar] [CrossRef]
- Sánchez-Arévalo, C.M.; Croes, T.; Van der Bruggen, B.; Vincent-Vela, M.C.; Álvarez-Blanco, S. Feasibility of several commercial membranes to recover valuable phenolic compounds from extracts of wet olive pomace through organic-solvent nanofiltration. Sep. Purif. Technol. 2023, 305, 122396. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Garcia-Castello, E. Valorization of artichoke wastewaters by integrated membrane process. Water Res. 2014, 48, 363–374. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Mayor, L.; Chorques, S.; Argüelles, A.; Vidal-Brotóns, D.; Gras, M.L. Reverse osmosis concentration of press liquid from orange juice solid wastes: Flux decline mechanisms. J. Food Eng. 2011, 106, 199–205. [Google Scholar] [CrossRef]
- Cui, Z.F.; Jiang, Y.; Field, R.W. Fundamentals of Pressure-Driven Membrane Separation Processes. In Membrane Technology; Cui, Z.F., Muralidhara, H.S., Eds.; Butterworth-Heinemann-Elsevier: Oxford, UK, 2010; Chapter 1; pp. 1–18. [Google Scholar]
- Hermia, J. Constant pressure blocking filtration laws-application to power law non Newtonian fluids. Inst. Chem. Eng. Trans. 1982, 60, 183–187. [Google Scholar]
- Bolton, G.; LaCasse, D.; Kuriyel, R. Combined models of membrane fouling: Development and application to microfiltration and ultrafiltration of biological fluids. J. Membr. Sci. 2006, 277, 75–84. [Google Scholar] [CrossRef]
- Charfi, A.; Ben Amar, N.; Harmand, J. Analysis of fouling mechanisms in anaerobic membrane bioreactors. Water Res. 2012, 46, 2637–2650. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barredo-Damas, S.; Iborra-Clar, A.; Pascual-Garrido, J.; Iborra-Clar, M.I. Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions. Sustainability 2021, 13, 13682. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Montesano, V.; Negro, D.; Sonnante, G.; Laghetti, G.; Urbano, M. Polyphenolic Compound Variation in Globe Artichoke Cultivars as Affected by Fertilization and Biostimulants Application. Plants 2022, 11, 2067. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, A.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crops Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L) by HPLC-DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastro-intestinal digestion. J. Funct. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- Lutz, M.; Henriquez, C.; Escobar, M. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.), raw and cooked. J. Food Compos. Anal. 2011, 24, 49–54. [Google Scholar] [CrossRef]
- Negro, D.; Montesano, V.; Grieco, S.; Crupi, P.; Sarli, G.; De Lisi, A.; Sonnante, G. Polyphenols compounds in artichoke plant tissues and varieties. J. Food Sci. 2012, 77, C244–C251. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Cabezas, M.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Use of ultrafiltration ceramic membranes as a first step treatment for olive oil washing wastewater. Food Bioprod. Process. 2022, 135, 60–73. [Google Scholar] [CrossRef]
- Corbatón-Báguena, M.-J.; Álvarez-blanco, S.; Vincent-Vela, M.C. Cleaning of ultrafiltration membranes fouled with BSA by means of saline solutions. Sep. Purif. Technol. 2014, 125, 1–10. [Google Scholar] [CrossRef]
- Ruby Figueroa, R.A.; Cassano, A.; Drioli, E. Ultrafiltration of orange press liquor: Optimization for permeate flux and fouling index by response surface methodology. Sep. Purif. Technol. 2011, 80, 1–10. [Google Scholar] [CrossRef]
- Forestier, A.; Belguesmia, Y.; Krier, F.; Drider, D.; Dhulster, P.; Firdaous, L. Recovery of nisin from culture supernatants of Lactococcus lactis by ultrafiltration: Flux properties and separation efficiency. Food Bioprod. Process. 2022, 136, 196–210. [Google Scholar] [CrossRef]
- Esteves Costa, C.A.; Rodrigues Pinto, P.C.; Rodrigues, A.E. Lignin fractionation from E. Globulus kraft liquor by ultrafiltration in a three stage membrane sequence. Sep. Purif. Technol. 2018, 192, 140–151. [Google Scholar] [CrossRef]



| Hermia’s Mechanisms | ||||
| n | Flux Decline Mechanism | Equation | Fitted Parameters | |
| 0.0 | Cake Filtration (CF) | (s·m−2) | (5) | |
| 1.0 | Intermediate Pore Blocking (IB) | (m−1) | (6) | |
| 1.5 | Standard Pore Blocking (SB) | (m−1) | (7) | |
| 2.0 | Complete Pore Blocking (CB) | (s−1) | (8) | |
| Combined Hermia’s Mechanisms | ||||
| Flux Decline Mechanism | Equation | Fitted Parameters | ||
| Cake Filtration and Complete Pore Blocking (CF-CB) | Kcf (s·m−2); Kcb (s−1) | (9) | ||
| Cake Filtration and Intermediate Pore Blocking (CF-IB) | Kcf (s·m−2); Kib (m−1) | (10) | ||
| Cake Filtration and Standard Pore Blocking (CF-SB) | Kcf (s·m−2); Ksb (m−1) | (11) | ||
| Complete Pore Blocking and Standard Pore Blocking (CB-SB) | Kcb (s−1); Ksb (m−1) | (12) | ||
| Intermediate Pore Blocking and Standard Pore Blocking (IB-SB) | Kib (m−1); Ksb (m−1) | (13) | ||
| Rejection Index (%) | |||
|---|---|---|---|
| Total Phenolic Index | Phenolic Acids | Total Polyphenols (HPLC) | |
| 3 kDa | 65.4 | 55.1 | 68.2 |
| 15 kDa | 42.8 | 40.9 | 42.7 |
| 50 kDa | 23.6 | 18.3 | 19.9 |
| Model | Membrane | Fitted Parameters | r2fitting | SSE | Error (%) | r2corr |
|---|---|---|---|---|---|---|
| CF | 15 kDa | Kcf = 1.12 × 106 s/m2 | 0.9507 | 3.43 × 10−12 | 2.02 | 0.9494 |
| 50 kDa | Kcf = 2 × 106 s/m2 | 0.9913 | 3.34 × 10−12 | 2.51 | 0.9911 | |
| IB | 15 kDa | Kib = 2.49 m−1 | 0.9247 | 4.16 × 10−12 | 2.48 | 0.9226 |
| 50 kDa | Kib = 4.22 m−1 | 0.9717 | 8.67 × 10−12 | 2.65 | 0.9714 | |
| SB | 15 kDa | Ksb = 2.6 × 10−3 m−1 | 0.9081 | 4.64 × 10−12 | 2.80 | 0.9054 |
| 50 kDa | Ksb = 4.4 × 10−3 m−1 | 0.9524 | 1.05 × 10−11 | 3.21 | 0.9519 | |
| CB | 15 kDa | Kcb = 1.12 × 10−5 s−1 | 0.8890 | 4.97 × 10−12 | 3.01 | 0.8857 |
| 50 kDa | Kcb = 2.0 × 10−5 s−1 | 0.9261 | 1.22 × 10−11 | 3.82 | 0.9252 |
| Model | Membrane | Fitted Parameters | SSE | Error (%) |
|---|---|---|---|---|
| CF-CB | 15 kDa | Kcf = 1.00 × 109 s·m−2 Kcb = 6.09 × 10−6 s−1 | 9.09 × 10−5 | 40.11 |
| 50 kDa | Kcf = 1.00 × 109 s·m−2 Kcb = 1.11 × 10−15 s−1 | 6.69 × 10−5 | 30.33 | |
| CF-SB | 15 kDa | Kcf = 4.71 × 10−3 s·m−2 Ksb = 5.34 × 108 m−1 | 9.52 × 10−5 | 41.04 |
| 50 kDa | Kcf = 4.37 × 10−3 s·m−2 Ksb = 5.34 × 108 m−1 | 7.42 × 10−5 | 32.62 | |
| CF-IB | 15 kDa | Kcf = 4.50 × 10−2 s·m−2 Kib = 7.88 × 102 m−1 | 3.40 × 10−4 | 77.24 |
| 50 kDa | Kcf = 4.45 × 10−2 s·m−2 Kib = 7.98 × 102 m−1 | 3.27 × 10−4 | 67.91 | |
| CB-SB | 15 kDa | Kcb = 9.86 × 10−6 s−1 Ksb = 3.41 × 102 m−1 | 5.92 × 10−4 | 102.20 |
| 50 kDa | Kcb = 1.11 × 10−15 s−1 Ksb = 3.34 × 102 m−1 | 5.94 × 10−4 | 91.98 | |
| IB-SB | 15 kDa | Kib = 7.88 × 102 m−1 Ksb = 1.00 × 10−12 m−1 | 3.40 × 10−4 | 77.24 |
| 50 kDa | Kib = 7.98 × 102 m−1 Ksb = 1.00 × 10−12 m−1 | 3.27 × 10−4 | 67.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Castello, E.M.; Moratalla, M.; Reig, M.; Iborra-Clar, M.I.; Iborra-Clar, A.; Rodriguez-Lopez, A.D. Clarification of the Cardoon (Cynara cardunculus) Blanching Wastewater by Ultrafiltration—Study of Membrane Fouling and Flux Recovery after Chemical Cleaning. Separations 2023, 10, 418. https://doi.org/10.3390/separations10070418
Garcia-Castello EM, Moratalla M, Reig M, Iborra-Clar MI, Iborra-Clar A, Rodriguez-Lopez AD. Clarification of the Cardoon (Cynara cardunculus) Blanching Wastewater by Ultrafiltration—Study of Membrane Fouling and Flux Recovery after Chemical Cleaning. Separations. 2023; 10(7):418. https://doi.org/10.3390/separations10070418
Chicago/Turabian StyleGarcia-Castello, Esperanza Maria, Monica Moratalla, Milagro Reig, Maria Isabel Iborra-Clar, Alicia Iborra-Clar, and Antonio Diego Rodriguez-Lopez. 2023. "Clarification of the Cardoon (Cynara cardunculus) Blanching Wastewater by Ultrafiltration—Study of Membrane Fouling and Flux Recovery after Chemical Cleaning" Separations 10, no. 7: 418. https://doi.org/10.3390/separations10070418
APA StyleGarcia-Castello, E. M., Moratalla, M., Reig, M., Iborra-Clar, M. I., Iborra-Clar, A., & Rodriguez-Lopez, A. D. (2023). Clarification of the Cardoon (Cynara cardunculus) Blanching Wastewater by Ultrafiltration—Study of Membrane Fouling and Flux Recovery after Chemical Cleaning. Separations, 10(7), 418. https://doi.org/10.3390/separations10070418










