Basic Research on Selective Extraction of Iron from Titanium Dioxide Waste Acid to Prepare Iron Phosphate Precursors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
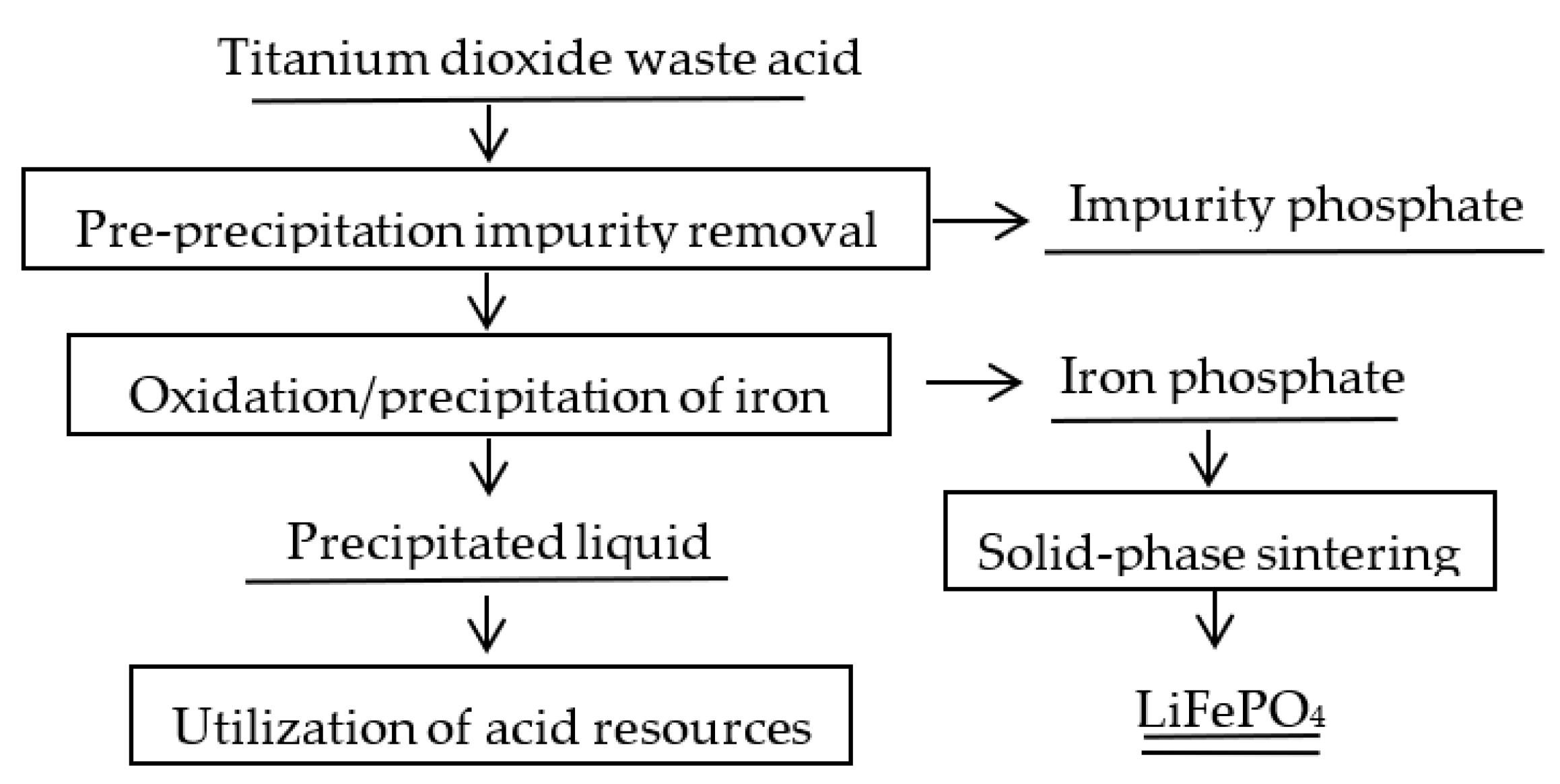
2.2. Experimental Methods
2.3. Measurement and Characterization
3. Results and Discussion
3.1. Impurity Removal Experiment in the Reduced State
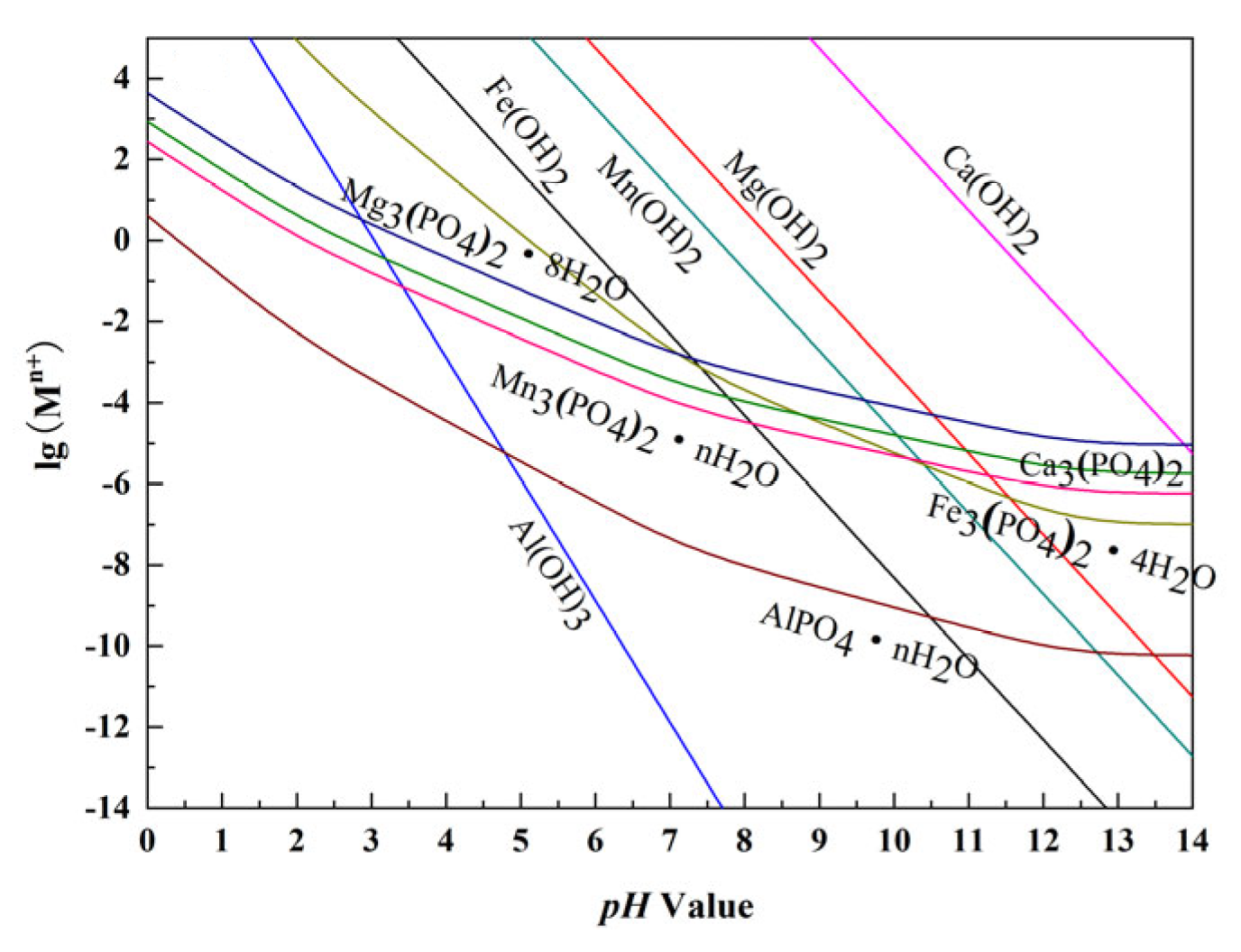
3.1.1. Thermodynamic Analysis of Impurity Removal via Pre-Precipitation
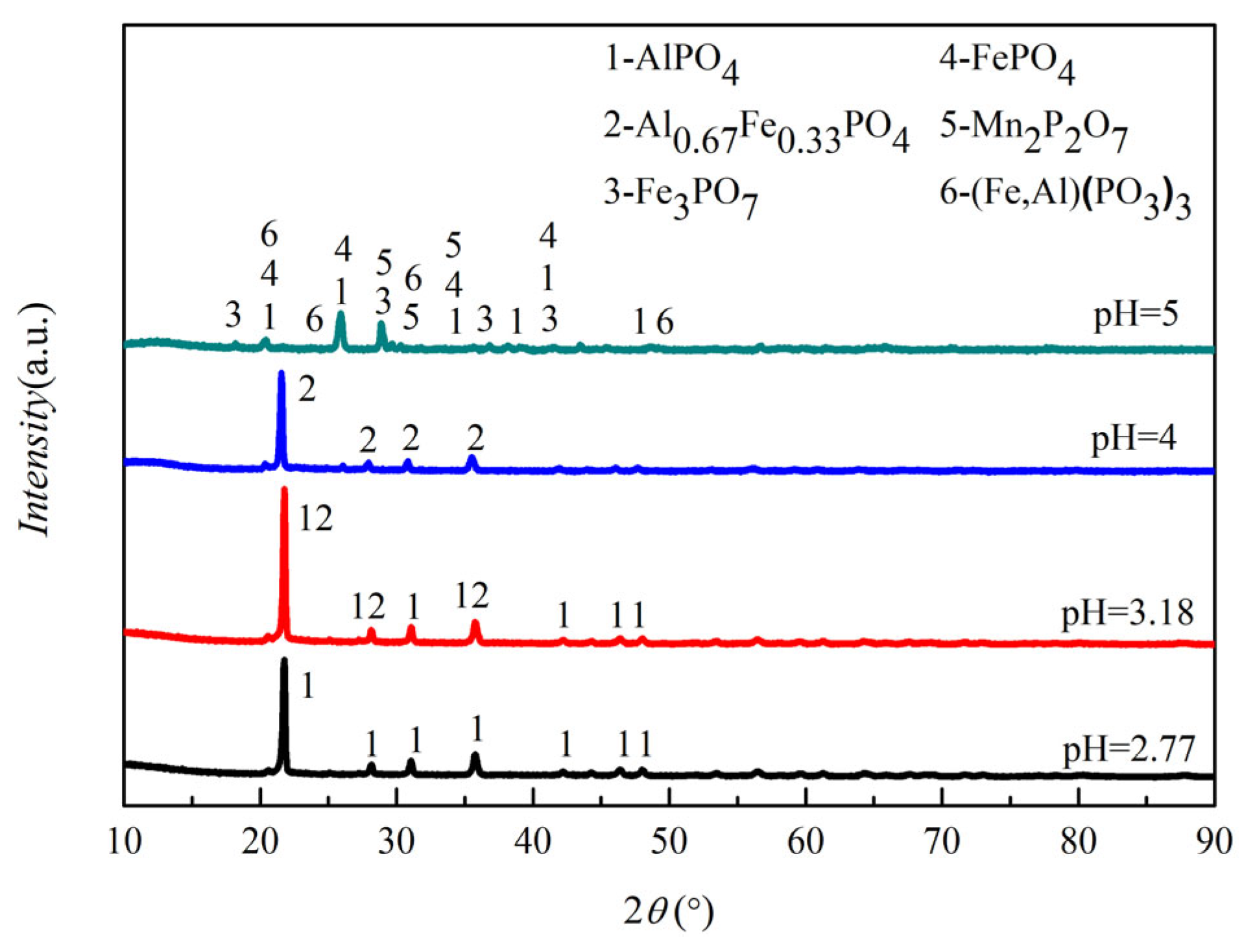
3.1.2. Impurity Removal Experiment
3.2. Study on the Preparation of Iron Phosphate in Oxidized State
3.2.1. The Effect of Temperature on the Product FePO4
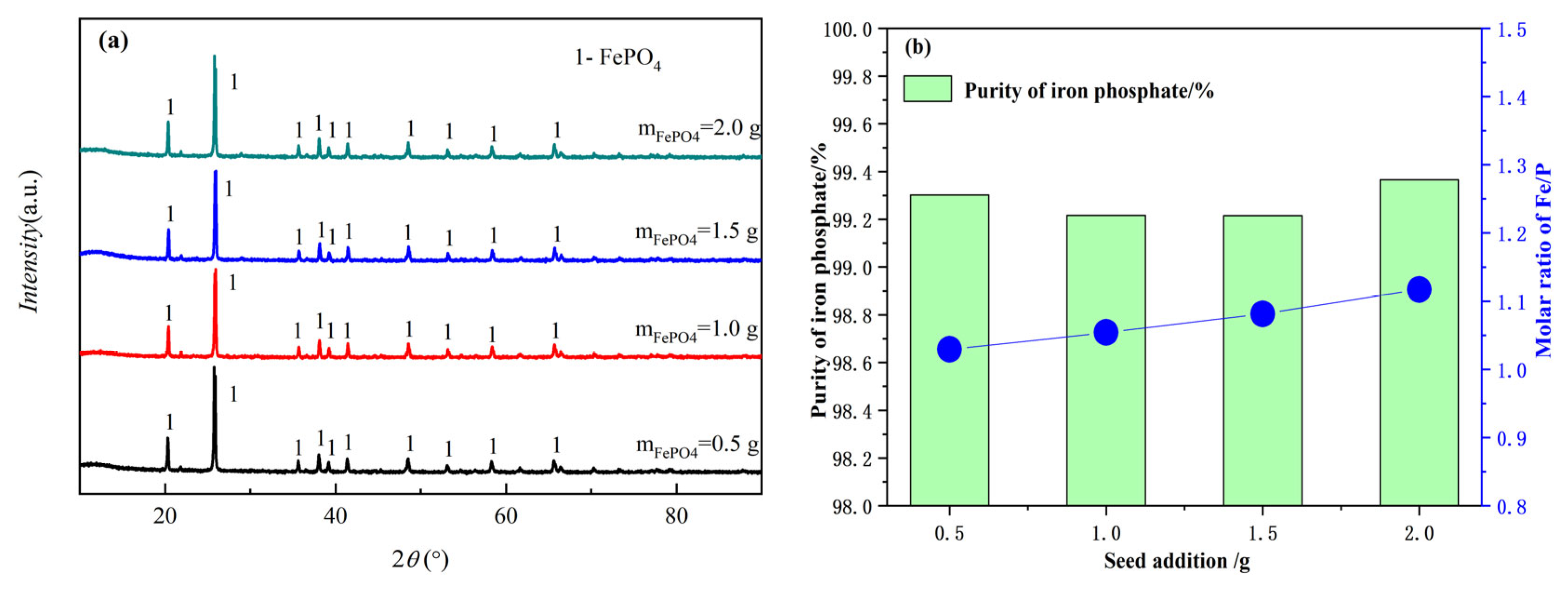
3.2.2. The Effect of Seed Crystals on the Product FePO4
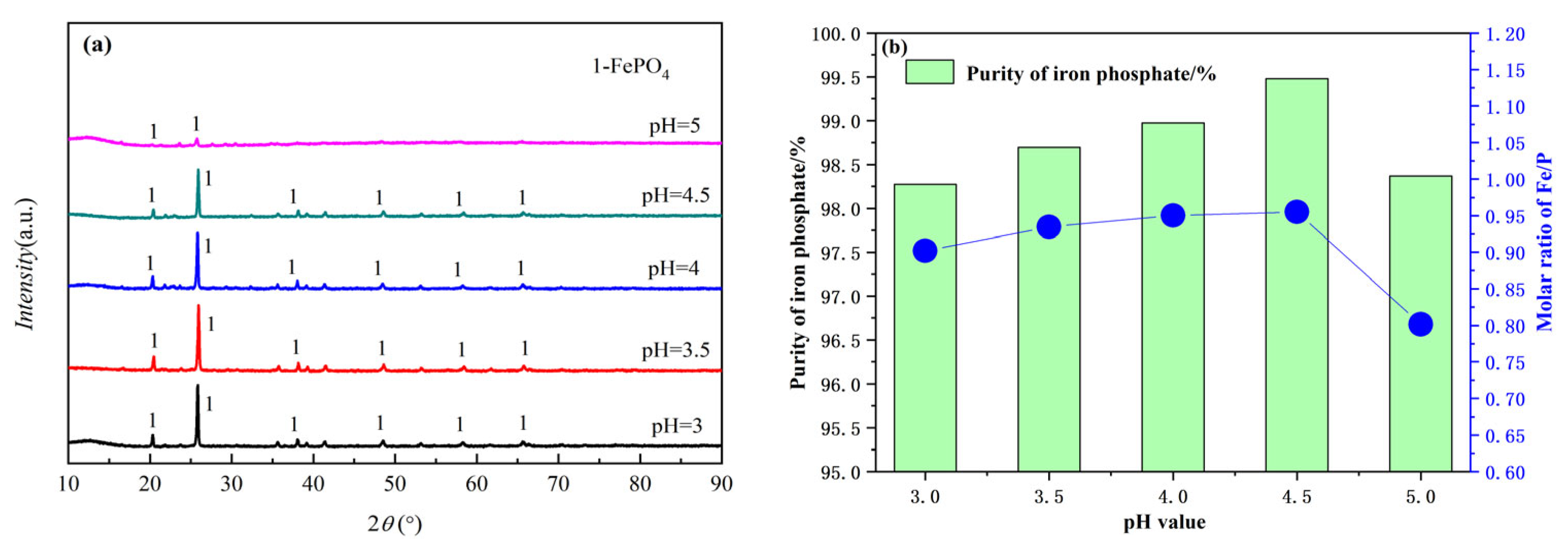
3.2.3. The Effect of pH on the Produced FePO4
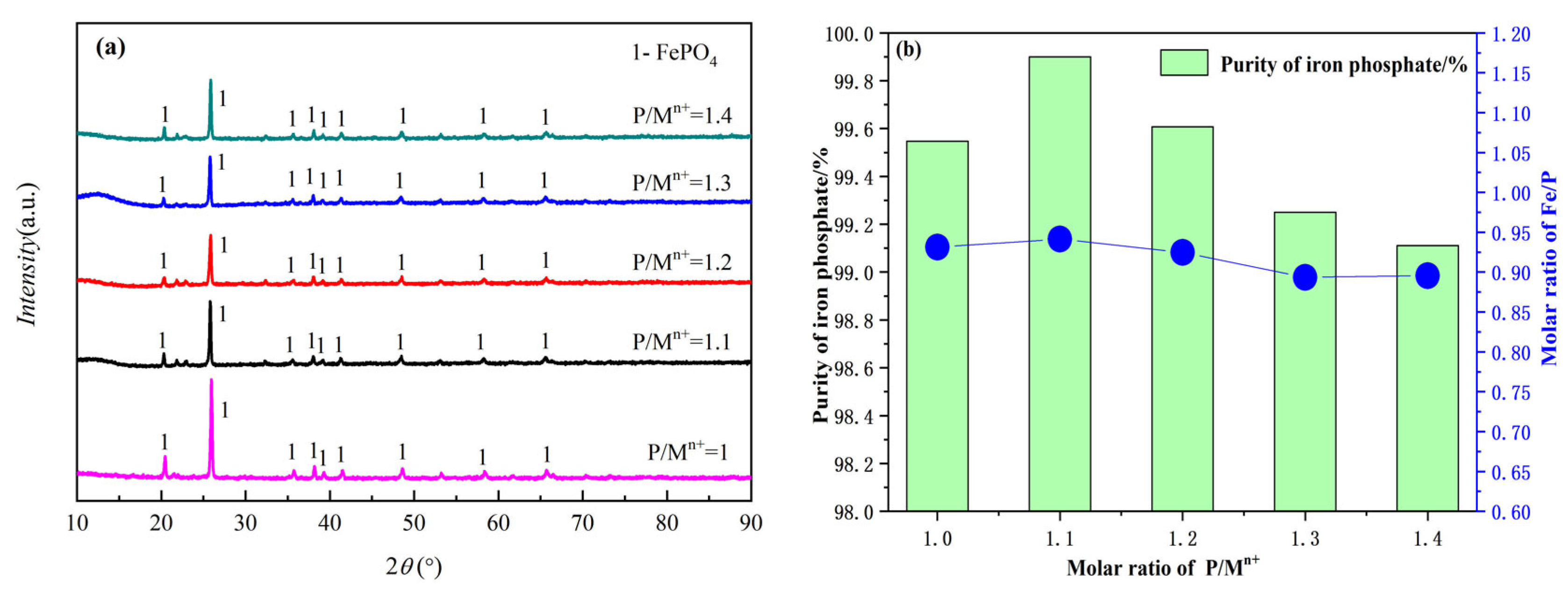
3.2.4. The Effect of P/M on the Product FePO4
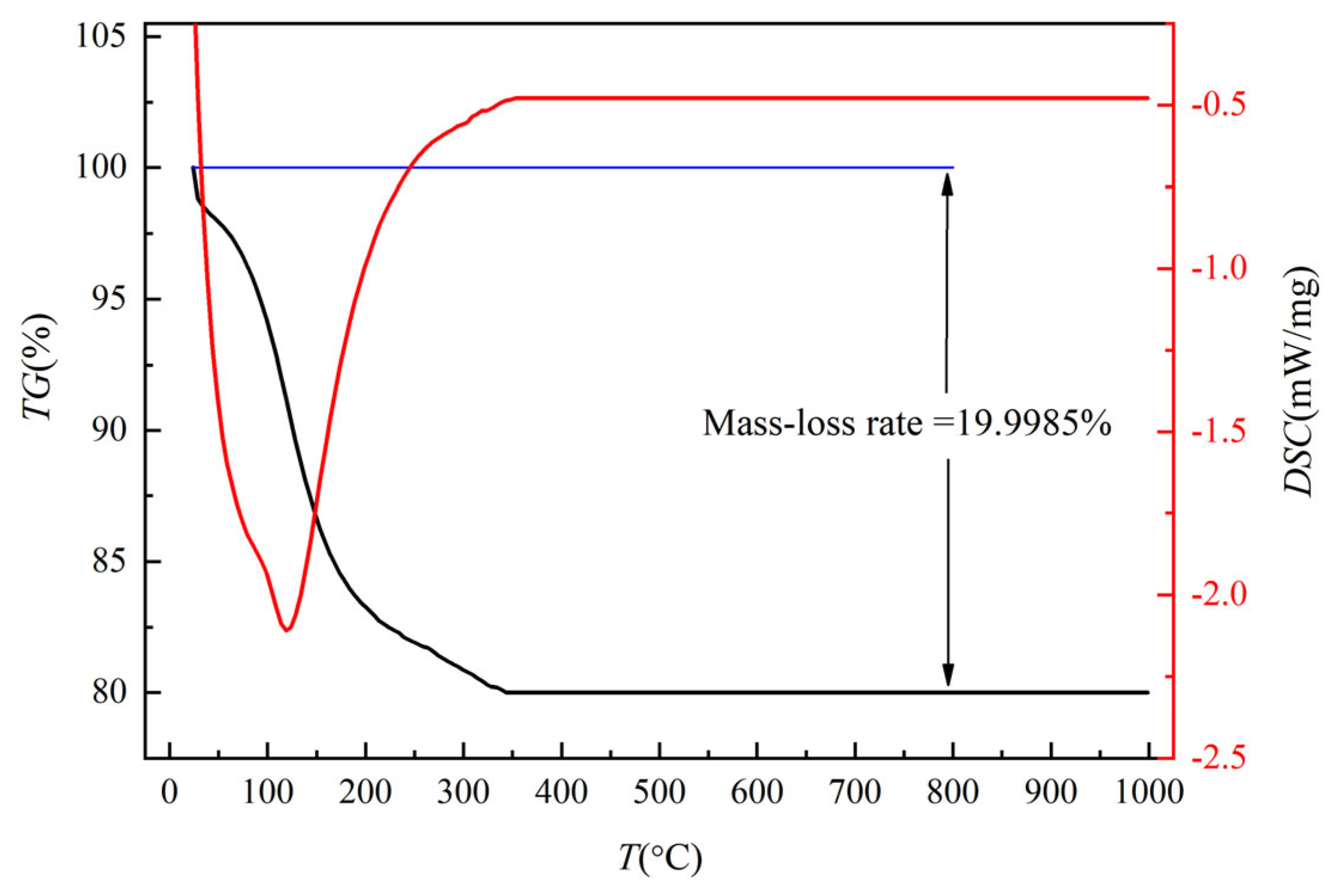
3.3. TG-DSC Analysis and Chemical Composition Analysis of Iron Phosphate
4. Conclusions
- (1)
- The effect of reaction temperature, seed crystals, pH value, and P/M on the precipitation process were investigated in detail. The experimental results show that in the reduced state, the optimal precipitation condition is a temperature of 75 °C, an initial pH value of 4.5, and an optimal P/M molar ratio of 1.1.
- (2)
- The thermodynamics results show that the content of Al3+, Mn2+, Mg2+, and Ca2+ in the reaction system can be adjusted by adjusting the pH during the pre-precipitation process. In the first step, these impurity ions should be settled as much as possible; then, Fe2+ should be oxidized to Fe3+ so as to obtain iron phosphate with higher purity in the next step of the precipitation process.
- (3)
- In the oxidized state, the following ideal conditions were determined: a temperature of 60 °C, a solution pH = 2.5, and a reaction time of 25 min. After calcination, the precipitate mainly consisted of iron phosphate, which basically meets the requirements of an iron phosphate precursor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pei, R. Production of Titanium Dioxide by Sulfuric Acid Method; Chemical Industry Press: Beijing, China, 1982; pp. 272–275. (In Chinese) [Google Scholar]
- Xu, H.; Fu, M. Research process in comprehensive utilization of spent acid and waste water in titanium dioxide production. Multipurp. Util. Miner. Resour. 2006, 4, 34–37. (In Chinese) [Google Scholar]
- Li, L. Research Progress on Comprehensive Recycle of Waste Acid in Titanium Pigment Production at Home and Abroad. Hydrometall. China 2010, 29, 150–155. (In Chinese) [Google Scholar]
- Zhao, Y.; Zhang, Y.; Xing, W.; Xu, N. Treatment of titanium white waste acid using ceramic microfiltration membrane. Chem. Eng. J. 2005, 111, 31–38. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L.; Song, Y. Titanium white sulfuric acid concentration by direct contact membrane distillation. Chem. Eng. J. 2016, 285, 101–111. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes—Static runs. J. Membr. Sci. 2001, 183, 193–200. [Google Scholar]
- Zhang, T.; Lv, G.; Liu, Y.; Zhang, G. A Comprehensive Utilization Method of Titanium White Waste Acid. CN Patent 104178632 B, 22 June 2016. [Google Scholar]
- Zhang, T.; Zhang, W.; Lv, G.; Dou, Z.; Liu, Y. A Method of Treating Titanium White Waste Acid by Steel Slag. CN Patent 201810288264.X, 10 July 2018. [Google Scholar]
- Zhang, T.; Lv, G.; Dou, Z.; Liu, Y.; Zhang, W. A Method of Treating Titanium White Waste Acid with Steel Slag and Extracting Valuable Components. CN Patent 201810296696.5, 7 September 2018. [Google Scholar]
- Zhang, W.; Zhang, T.; Lv, G.; Zhou, W. Extraction Separation of Sc(III) and Fe(III) from a Strongly Acidic and Highly Concentrated Ferric Solution by D2EHPA/TBP. JOM 2018, 70, 2837–2845. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Dreisinger, D.; Lv, C.; Lv, G.; Zhang, W. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J. Hazard. Mater. 2019, 369, 632–641. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Zhang, Y.; Lv, G.; Liu, Y.; Liu, Z. Pressure leaching of converter vanadium slag with waste titanium dioxide. Rare Met. 2016, 35, 576–580. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Wang, Z. A novel process for producing synthetic rutile and LiFePO4 cathode material from ilmenite. J. Alloys Compd. 2010, 506, 271–278. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Wang, Z. Preparation of synthetic rutile and metal-doped LiFePO4 from ilmenite. Powder Technol. 2010, 199, 293–297. [Google Scholar] [CrossRef]
- Fan, Q.; Lei, L.; Xu, X. Direct growth of FePO4/graphene and LiFePO4/graphene hybrids for high rate Li-ion batteries. J. Power Sources 2014, 257, 65–69. [Google Scholar] [CrossRef]
- Prosini, P.; Lisi, M.; Scaccia, S. Synthesis and characterization of amorphous hydrated FePO4 and its electrode performance inlithium batteries. J. Electrochem. Soc. 2002, 149, A297–A301. [Google Scholar] [CrossRef]
- Liu, H. Synthesis of nanorods FePO4 via a facile route. J. Nanopart. Res. 2010, 12, 2003–2006. [Google Scholar] [CrossRef]
- Kahoul, A.; Hammouche, A. Electrochemical performances of FePO4-positive active mass prepared through a new sol-gel method. Ionics 2010, 16, 105–109. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Xie, K. Synthesis and characterization of multi–wall carbon nanotubes supported-hydrated iron phosphate cathode material for lithium–ion cells by a novel homogeneous precipitation method. Ionics 2012, 18, 721–729. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ma, X.; Zhang, G. Recovery of nitric and acetic acids from etching waste solution using a synergistic system of N235 and TRPO in cyclohexane. Hydrometallurgy 2019, 185, 23–29. [Google Scholar] [CrossRef]
- Chen, F.; Wang, X.; Liu, W. Selective extraction of nitric and acetic acids from etching waste acid using N235 and MIBK mixtures. Sep. Purif. Technol. 2016, 169, 50–58. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Guo, H. Synthesis of FePO4 by electrolyzing ferrophosphorus. Phosphate Compd. Fertil. 2014, 29, 13–15. (In Chinese) [Google Scholar]
- Yang, J.; Lv, N.; Su, C. Research progress on the recycling of phosphorus resource from converter steelmaking slag. Appl. Chem. Ind. 2019, 48, 1440–1446. (In Chinese) [Google Scholar]
- Li, H.; Xing, S.; Liu, Y. Recovery of Lithium, Iron, and Phosphorus from Spent LiFePO4 Batteries Using Stoichiometric Sulfuric Acid Leaching System. Acs Sustain. Chem. Eng. 2017, 5, 8017–8024. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Cai, L. Preparation of Doped Iron Phosphate by Selective Precipitation of Iron from Titanium Dioxide Waste Acid. Metals 2020, 10, 789. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.; Yang, B. A versatile methed for preparing FePO4 as a promising electrode material rechargeable lithiumbatteries. J. Lanzhou Univ. (Nat. Sci.) 2007, 43, 144–146. [Google Scholar]
- Guo, X.; Ding, W.; Chen, H. Preparation and characterization of nanosized Fe-P-O catalyst. J. Fuel Chem. Technol. 2000, 28, 385–387. [Google Scholar]
- Son, D.; Kim, E.; Kim, T. Nanoparticle iron-phosphate anode material for Li-ion battery. Appl. Phys. Lett. 2004, 85, 5875–5877. [Google Scholar] [CrossRef]
- Guo, X.; Ding, W.; Wang, X. Synthesis of a novel mesoporous iron phosphate. Chem. Commun. 2001, 8, 709–710. [Google Scholar] [CrossRef]
- Zaghiba, K.; Julien, C. Structure and electrochemistry of FePO4·2H2O hydrate. Power Sources 2005, 142, 279–284. [Google Scholar] [CrossRef]
- Song, Y.; Zavalij, P.; Suzuki, M. New Iron(III) Phosohate: Crystal structure and electrochemical and magnetic properties. Inorg. Chem. 2002, 41, 5778–5786. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Xiao, C.; Xiao, L.; Gao, C. Thermodynamic study on removal of magnesium from lithium chloride solutions using phosphate precipitation method. Sep. Purif. Technol. 2015, 156, 582–587. [Google Scholar] [CrossRef]
- He, L.; Xu, W.; Song, Y.; Liu, X.; Zhao, Z. Selective removal of magnesium from a lithium-concentrated anolyte by magnesium ammonium phosphate precipitation. Sep. Purif. Technol. 2017, 187, 214–220. [Google Scholar] [CrossRef]
- Xiao, C.; Zeng, L.; Wei, J.; Xiao, L.; Zhang, G. Thermodynamic analysis for the separation of tungsten and aluminium in alkaline medium using solvent extraction. Hydrometallurgy 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Lv, G. Thermodynamic study on the V(V)-P(V)-H2O system in acidic leaching solution of vanadium-bearing converter slag. Sep. Purif. Technol. 2019, 218, 164–172. [Google Scholar] [CrossRef]

| Element | Fe | V | Mg | Mn | Ca | Al |
|---|---|---|---|---|---|---|
| Concentration/g·L−1 | 37.64 | 0.41 | 5.00 | 2.15 | 0.23 | 1.52 |
| Substance | State | Solubility Product Constant |
|---|---|---|
| Fe3(PO4)2·H2O | solid | Ksp = 9.94 × 10−29 |
| Mn3(PO4)2·nH2O | solid | Ksp = 6.13 × 10−32 |
| AlPO4·1.5H2O | solid | Ksp = 3.5 × 10−21 |
| Mg3(PO4)2·8H2O | solid | Ksp = 6.31 × 10−26 |
| Fe3(PO4)2 | solid | Ksp = 1.3 × 10−22 |
| Ca3(PO4)2 | solid | Ksp = 2 × 10−29 |
| Fe(OH)3 | solid | Ksp = 2.79 × 10−39 |
| Mn(OH)2 | solid | Ksp = 1.9 × 10−13 |
| Al(OH)3 | solid | Ksp = 1.3 × 10−33 |
| Mg(OH)2 | solid | Ksp = 5.61 × 10−12 |
| Ca(OH)2 | solid | Ksp = 5.5 × 10−6 |
| Equilibrium Reactions | Equilibrium Constants (lgK) | Mathematical Relationships |
|---|---|---|
| Fe3+ + OH− = FeOH2+ | 11.87 | [FeOH2+]=1011.87[Fe3+][OH−] |
| Fe3+ + 2OH− = Fe(OH)2+ | 21.17 | [Fe(OH)2+] = 1021.17[Fe3+][OH−]2 |
| Fe3++ 3OH− = Fe(OH)3 | 29.67 | [Fe(OH)3] = 1029.67[Fe3+][OH−]3 |
| Fe3+ + SO42− = Fe(SO4)+ | 2.03 | [Fe(SO4)+] = 102.03[Fe3+][SO42−] |
| Fe3+ + 2SO42− = Fe(SO4)2− | 2.98 | [Fe(SO4)2−] = 102.98[Fe3+][SO42−]2 |
| Fe(OH)3 = Fe3+ +3OH− | −38.55 | [Fe2+][OH−]3 = 10−38.55 |
| FePO4 = Fe3+ + PO43− | −23 | [Fe2+][PO43−] = 10−23 |
| Metal Ion | Mn2+ | Mg2+ | Ca2+ | Fe2+ | Al3+ |
|---|---|---|---|---|---|
| lg[Mn+] | 0.33 | 0.70 | −0.64 | 1.58 | 0.18 |
| Initial precipitation pH of orthophosphate | 3.07 | 1.45 | 3.4 | 4.1 | <0 |
| Initial precipitation pH of hydroxide | 7.31 | 8.04 | 11.7 | 5.05 | 3.0 |
| Component/wt.% | Fe | P | Fe/P Ratio | H2O | Ca | Mg | Na/K | Cu/ Zn/Ni | Sulfate | D50, μm |
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment products | 28.24 | 17.52 | 0.89 | 19.38 | 0 | 0.024 | / | / | 0.0065 | 16.81 |
| Technical indicators (standards) | 29.0~30.0 | 16.2~17.2 | 0.97~1.02 | 19.0~21.0 | <0.005 | <0.005 | <0.01 | <0.005 | <0.01 | 2~6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Chen, Y.; Liang, X.; Li, Y.; Zhang, W.; Cai, Z.; Zhang, T. Basic Research on Selective Extraction of Iron from Titanium Dioxide Waste Acid to Prepare Iron Phosphate Precursors. Separations 2023, 10, 400. https://doi.org/10.3390/separations10070400
Cao X, Chen Y, Liang X, Li Y, Zhang W, Cai Z, Zhang T. Basic Research on Selective Extraction of Iron from Titanium Dioxide Waste Acid to Prepare Iron Phosphate Precursors. Separations. 2023; 10(7):400. https://doi.org/10.3390/separations10070400
Chicago/Turabian StyleCao, Xuejiao, Yang Chen, Xinxing Liang, Yibing Li, Weiguang Zhang, Zhenlei Cai, and Ting’an Zhang. 2023. "Basic Research on Selective Extraction of Iron from Titanium Dioxide Waste Acid to Prepare Iron Phosphate Precursors" Separations 10, no. 7: 400. https://doi.org/10.3390/separations10070400
APA StyleCao, X., Chen, Y., Liang, X., Li, Y., Zhang, W., Cai, Z., & Zhang, T. (2023). Basic Research on Selective Extraction of Iron from Titanium Dioxide Waste Acid to Prepare Iron Phosphate Precursors. Separations, 10(7), 400. https://doi.org/10.3390/separations10070400




