Abstract
Lichens are composite organisms that produce a wide variety of secondary metabolites; many of the compounds have a high potential as bioactive compounds. The major limitations of using bioactive compounds from lichens is their slow growth rate and the damage to environmental populations caused by massive collection. The alternative to the massive collection of lichens in the field is their culture under laboratory conditions. We chose two related lichen species of Parmeliaceae that produce similar metabolites and isolated from spores in cultures placed under axenic conditions for over 550 days. From these cultures, we sampled 35 mg of each species from different culture media at two sampling times. The samples were analyzed using high-performance liquid chromatography (HPLC) to detect and identify major compounds. We found no differences in the metabolites produced within the species in comparisons between different culture media. Our results show that the mycobiont cultures produced different secondary metabolites than those found in natural lichen thalli. Moreover, different secondary metabolites between species and different metabolites over time were observed. We conclude that mycobiont cultures are a promising alternative for determining bioactive compounds and enhancing the efficiency of growth and production. These could be a good option for eco-friendly metabolite production.
Keywords:
Parmelina carporrhizans; Parmelina quercina; mycobiont culture; phenols; polyketides; HPLC 1. Introduction
Lichens are composite symbiotic organisms, and the lichen body, which is called the thallus (plural: thalli), is formed by the mycobiont, commonly an Ascomycota fungus and one or more photoautotrophic symbionts (photobionts), which may be green algae or cyanobacteria [1]. However, the lichen thallus is more complex, and in recent decades, it has been reported that Basidiomycota yeasts belonging to the Cystobasidiomycetes class [2] and associated bacteria [3] are part of lichen symbiosis. A mycobiont is a symbiont that is responsible for naming lichens and building the complex structure of the lichen thallus, although it can only achieve this in symbiosis with a compatible photobiont [4]. With more than 30,000 species, lichens represent approximately 20% of the currently known, global fungal biodiversity species. They are widespread organisms and occupy the most extreme ecosystems on Earth, such as Antarctica or the Namib Desert. Lichens produce a wide variety of substances. Some lichen species are well known for their ethnobotanical use and have several traditional applications as wool dyes, food, and health-promoting teas in herbalism, Indian Ayurveda medicine, and traditional Chinese medicine [5,6,7,8]. Lichen-forming fungi produce a wide range of secondary metabolites with a rich potential as bioactive compound. Many of them are aromatic polyketides [9] since they are phenols with marked antioxidative properties [10]. They also have allelopathic properties against bacteria [11,12,13,14] and fungi [15,16], enabling specific pharmacological applications, such as photoprotection [17,18], cardioprotection [19], neuroprotection [20], antiviral [21], antidiabetic [22], and antitumoral applications [14,23,24] in mammals, by modulating the intracellular concentration of hydrogen peroxide [25].
The excessive collection of lichens, mainly to be used as perfume stabilizers and as dyes, has caused serious problems in the conservation of these organisms [26,27,28,29]. For example, Everniastrum nepalense is frequently picked up by Indian traders [26] and is on the IUCN red list because of the overexploitation of the species in Nepal [30]. The rare endemic lichen, Xanthoparmelia beccae, which is only known from the remote St. Helena Island where there are only four known populations and less than 1000 individuals, is listed as “vulnerable” and is also frequently collected [31]. The limiting factor in use of the secondary metabolites of lichens is often the very slow growth rates [32] of lichen thalli under natural conditions. For these reasons, isolated mycobiont growth in aposymbiotic cultures and whole thalli re-synthesis in the laboratory have been proposed as the best options for the production of secondary lichen metabolites, conserving the diversity of the natural populations of these organisms. They are also sensitive to climate change and the loss or deterioration of habitats [33,34,35,36].
Many lichen metabolites are biosynthetically related since they are produced at different stages of the same synthetical pathway. The metabolites produced by lichens are classified in three major classes based on their synthetic pathway: (i) the acetate–polymalonate pathway; (ii) mevalonic acid pathway; and (iii) shikimic acid pathway [37,38]. The secondary metabolites that can be produced by the lichen thalli in natural conditions vary between species and within species depending on environmental conditions, activating different metabolic pathways [9,39]. The major factors affecting the pattern of secondary metabolites produced by lichens are abiotic, including differences in nutrients, temperature and pH [4,40,41]. But also, among the biotic factors affecting the production pattern of secondary metabolites, the modulating effect of the photobiont [39] can be highlighted. Indeed, the phenolic pattern can vary substantially between the mycobiont growing in aposymbiotic conditions and the mycobiont associated with its photosynthetic partner (lichen symbionts).
Of special interest are the genetic and functional characterization of polyketide synthases (PKS) and the regulation of the different metabolic pathways [42]. However, there are some limitations of mycobiont cultures, including the inverse relationships between mycobiont growth and the production of secondary metabolites [4]. This can be resolved, at least in some species, by modulating culture conditions, i.e., changing the carbon source from glucose to sugar alcohols that commonly occur in lichens, such as ribitol, arabitol, and mannitol [43]. Another problem is the loss of the viability of cultures over time; somewhere between 100 and 300 days, the mycobiont culture stops the culture’s growth [4,44,45].
For the present study, we chose two sexually reproducing species belonging to the small lichen genus, Parmelina (Hale), representative of the Parmeliaceae family, which is characterized by containing lecanoric acid, atranorin and chloratranorin: Parmelina carporrhizans (Taylor) Hale and Parmelina quercina (Willd.) [46]. These two foliose and morphochemically similar species have differentiated ecological distributions and different reproductive behavior. The sexual reproduction of P. quercina is based on the production of higher and smaller sizes spores with lower germination rates compared with P. carporrhizans [47]. It has been suggested that the different reproductive strategies of these species are related to their distribution. Parmelina quercina is mainly distributed in the Mediterranean climate zones of Southern Europe and Morocco, while Parmelina carporrhizans grows in more oceanic and humid areas, mainly in the Mediterranean Basin, but also in the Macaronesian region; it is even occasionally found in Great Britain.
Using two related species with different reproductive modes and distributions, we aimed to evaluate the aposymbiotic culture of lichen mycobionts as an alternative source for the production of secondary metabolites instead of natural populations of lichens growing in the field. We also aimed to compare the major compounds produced by mycobiont cultures at different time intervals between and within species.
2. Materials and Methods
2.1. Lichen Sampling
Three healthy and mature fresh thalli of Parmelina quercina and P. carporrhizans were collected in the summer and autumn of 2012 and placed in paper bags to dry lichen samples and transported to the laboratory. At the end of the experiments, representative samples were deposited in MAF herbarium (MAF-Lich 19,117, MAF-Lich 19,191, and MAF-Lich 19,192) at Farmacy Faculty of the Complutense University of Madrid.
The samples of Parmelina quercina were collected on bark of Quercus ilex (L.) in Monte de Las Pinedas, La Carlota (Spain), on 20th of July of 2012 (37°42′25.2″ N, 4°55′47.9″ W) at 182 m and Santa Cruz del Valle, Avila, on October 11th of 2012 (40°14′59.4″ N, 5°0′33.8″ W) at 689 m. Both localities were representative of Mediterranean forests, woodlands, and scrub biomes. Fresh thalli of P. carporrhizans were collected in cultivated chestnuts on bark of Castanea sativa (Mill.) in Cuevas del Valle (Spain) on October 11th of 2012 (40°18′28.4″ N, 5°00′39.0″ W) at 1007 m.
2.2. Mycobiont Isolation and Aposymbiotic Culture
We isolated the mycobionts of P. carporrhizans and P. quercina from lichen thalli collected less than two weeks prior to the isolation attempt. The aposymbiotic cultures started from ascospores following the inverted Petri dish methods of Ahmadjian [1]. Ten apothecia, five with diameters between 3 and5 mm and five apothecia with diameters greater than 5 mm, were mechanically cleaned and cut from each lichen thallus. A total of 30 apothecia from each species were washed following the protocols established by Molina and Crespo [48] and attached to the inner side of inverted Petri dish lids with petroleum jelly. The plates for spore germination contained Bold’s basal medium (BBM, ref. [49]), and the ascospores discharged upwards onto this inorganic medium, which is suitable for spore germination. The Petri dishes were covered with aluminum foil to ensure the total absence of photobionts, kept at 18–20 °C in the dark, and mycobiont cultures were periodically examined using a Nikon SMZ800 stereomicroscope and an Olympus CX40 microscope. The spores were ejected in groups of eight ascospores, and germination tubes were observed at four days after spore discharge [47]. Twenty-five to thirty days after spore discharge, a total of 90 agar pieces containing plurisporic mycelia of P. carporrhizans and a total of 35 agar pieces containing plurisporic mycelia of P. quercina were transferred to new plates containing three different types of nutrient-rich culture media: 0.2% glucose malt–yeast extract (0.2G-MY) prepared according to Molina et al. [44], cornmeal agar (CMA) prepared following the manufacturer’s instructions (Difco Laboratories, Detroit, MI, USA), and Lilly Barnett medium (LBM, [50]). Petri dishes containing the mycobiont cultures were incubated at constant 20 °C in the dark for 550 days. The mycobiont cultures were checked and pictures were taken periodically under a stereomicroscope.
We cultured both species until we obtained 35 mg of pure mycobiont culture since, with this amount of biomass, we were able to use samples for analysis. Two samplings were realized for each species, at 160 (early) and 550 days (late) for P. carporrhizans and at 250 (early) and 550 days (late) for P. quercina. We also took a sample of the agar surrounding the lichen mycobionts, which was darkened by the droplets secreted by the aposymbiotic cultures of both species, at 250 days to be analyzed. The mycobiont biomass or agar pieces of 5 mm2 were extracted in 0.5 mL of acetone for 60 min and the extract was freeze-dried until analyses.
2.3. DNA Extraction and Molecular Identification
Prior to DNA extraction, we extracted secondary metabolites using acetone, and then we crushed mycobiont tissue samples with pestles in liquid nitrogen and extracted genomic DNA using the DNeasy Plant Kit (QIAGEN, Redwood City, CA, USA) according to the manufacturer’s instructions. To confirm the identity of the mycobiont cultures, we amplified the internal transcribed spacer (ITS) region of the nuclear rDNA from the aposymbiotically grown cultured mycelia, using the fungal-specific forward primer ITS1F and the universal reverse primer ITS4 [51,52]. The amplifications were run using the following parameters: an initial denaturation at 94 °C for 5 min followed by 30 cycles of denaturation at 94 °C for 1 min, annealing of primers at 54 °C for 1 min and extension of primers at 72 °C for 1.5 min, and a final extension at 72 °C for 5 min. The ITS sequences were compared with the NCBI nucleotide database to confirm the identity of the mycelia cultured using Blast and deposited in GenBank (accession numbers KM357892 and KM357893). These mycobiont cultures of P. carporrhizans were used to sequence the genome using Illumina HiSeq 2000 [53].
2.4. High-Performance Liquid Chromatography
Lichen compounds were identified by HPLC using a protocol based on the study by Feige et al. [54], also described in Benatti et al. [55]. HPLC analyses were performed on an Agilent 1260 quaternary system with an incorporated degasser using a Poroshell 120 EC-C18 column (2.7 µm, 3.0 × 50 mm, Agilent, Palo Alto, CA, USA) for the separation of substances at 30 °C. Two solvents were used at a flow rate of 1.4 mL/min. Solvent A contains aqua bidest, 30% methanol, and 0.0658% trifluoroacetic acid; solvent B is 100% methanol. Thallus samples were soaked in 200 µL of methanol for one hour. Freeze-dried extracts of mycobiont cultures and culture media were resuspended in 200 µL of methanol. Next, 150 µL of these methanol extracts were filtered by centrifuging them for 1 min at 800 rpm through a Pall Acropep Advance filter plate. The filtrate was diluted tenfold with methanol, and 10 µL was automatically injected after equilibrating the HPLC system for two minutes to solvent A. The run continued isocratically for 0.18 min, solvent B was increased to 58% within 5 min, up to 100% within the next 5 min, and then for 0.82 min isocratically in 100% B. The run ended with solvent A being increased back up to 100% within 0.5 min. The column was flushed again for two minutes with 100% solvent before a new run was started. A diode array detector (DAD) detected compounds at 210, 254, 280, and 310 nm. The spectra (λ = 190–650 nm, 2 nm steps) and retention times of detected peaks were compared against a library of authentic metabolites processed under identical conditions using the OpenLAB CDS ChemStation software of Agilent at Imke Schmitt laboratory, located at Senckemberg Biodiversity and Climate Research Centre of Frankfurt am Main (Frankfurt am Main, Germany).
3. Results
3.1. Mycobiont Growth and Qualitative Observations
We successfully isolated and grew pure mycobiont cultures from ascospores of both species. The rate of success in Parmelina carporrhizans cultures was 100%, and biomass was obtained in all attempts (n = 90). Only 11.6% of the attempts resulted in growth (n = 35), and more time was needed to reach a high enough level of biomass to analyze the content of secondary metabolites (35 mg), whereas in P. quercina, we had less success. In P. carporrhizans, the secondary metabolites of the early culture were extracted after 160 days, instead of the 250 days that P. quercina needed to reach 35 mg, allowing us to analyze the secondary metabolites produced in the early stage of the mycobiont in the sample biomass.
At 80 days of growth, the cultures of Parmelina mycobiont produced striking brownish droplets in both species (Figure 1A,C). After 160 days, in most cases, the culture medium around the mycobiont culture was stained brown (Figure 1B); in other cases, the dark brown substances crystalized in the contact zone of agar where mycobionts were observed (Figure 1D). After 240 days, the culture media were more intensely stained (Figure 1E) by an unknown mycobiont metabolite.
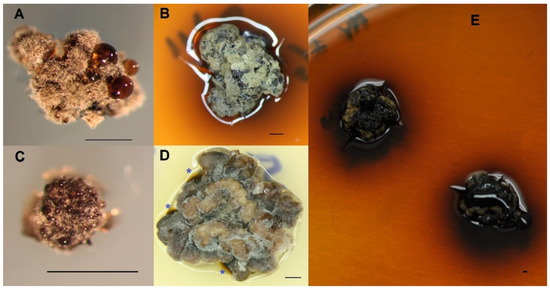
Figure 1.
Plurisporic mycobiont culture in axenic conditions. (A) P. carporrhizans at 80 days (0.2G-MY) showing drops of a phenolic nature. (B) P. carporrhizans at 160 days showing 0.2G-MY agar stained media. (C) Parmelina quercina at 80 days showing drops of a phenolic nature growing on LBM. (D) Parmelina quercina at 160 days on CMA, showing crystalized brown substance, marked with *. (E) P. carporrhizans at 340 days of culture showing intense staining of the 0.2G-MY agar culture media. Scale bar: 1 mm.
3.2. Identification of secondary metabolites by High-Performance Liquid Chromatography
We identified lecanoric acid as a major compound and atranorin and chloratranorin as minor substances in the thalli of P. carporrhizans and P. quercina by HPLC. (Table 1). In addition, we identified evernic acid in P. carporrhizans at the trace level. In the early mycobiont culture of P. carporrhizans, confluentic acid was identified as a major compound, whereas in the early mycobiont culture of P. quercina, we detected an unknown substance with a retention time (RT) of 6.97 min and identified pulvinic acid as a minor substance. This unknown substance was secreted by both species, P. carporrhizans and P. quercina, in the culture medium at 240 days. Norlobaridone was identified in late mycobiont cultures of P. carporrhizans as a major substance, while in late mycobiont cultures of P. quercina, different unknown substances with retention times of 5.79 and 6.97 min (Table 1) were detected as major substances. Notably, we did not find differences in the lichen substances produced in different culture media. The UV spectra of the unknown substances reported in Table 1 are provided in Figure 2.

Table 1.
Lichen substances and retention times (RTs) of Parmelina carporrhizans and Parmelina quercina found in natural thalli and mycobiont cultures at different time intervals. Early time interval is 160 days for P. carporrhizans and 250 days for P. quercina. Late time intervals are 550 days for both species. Secretion was analyzed from agar pieces at 250 days in both species. The quantity of lichen substances is categorized according to if they were present as major or minor components of the extract, or if they were only present at trace levels as Ma, mi, or tra.
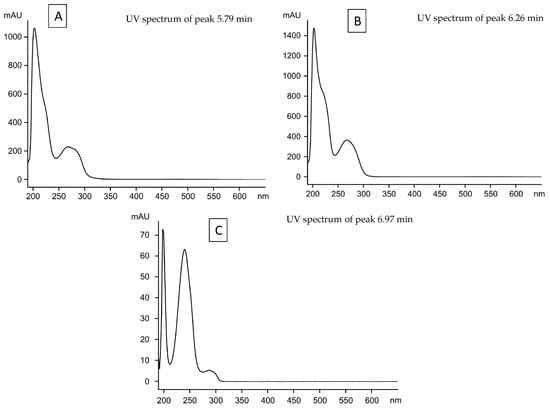
Figure 2.
UV spectra of the unknown substances detected as major substances. (A) Unknown substance with retention time of 5.79 (B); unknown substance with retention time of 6.26; and (C) unknown substance with retention time of 6.97.
4. Discussion
Although the thalli of Parmelina carporrhizans and Parmelina quercina produce similar secondary metabolites in the field under different ecological conditions, we found that their mycobionts in aposymbiotic cultures produce different secondary metabolites under the same conditions. We found in natural thalli almost the same secondary metabolites as those described above. In addition to lecanoric acid, atranorin, and chloratranorin produced by thalli of both species [46], we found evernic acid in P. carporrhizans at a trace level. Notably, the presence of evernic acid in the natural thallus of P. carporrhizans thallus is not reported in the literature. Contrarily, we did not find gyrophoric acid in P. quercina, which was reported at a trace level only once by Clerc and Truong [56].
Comparing the secondary metabolites produced by the Parmelina lichen thalli and the aposymbiotic cultures of Parmelina, we found that completely different metabolites are produced by the isolated mycobiont. In mycobiont cultures, we found confluentic acid, norlobaridone as major substances produced in the early and late stages of P. carporrhizans and one unknown substance secreted by mycobionts of P. carporrhizans, whereas pulvinic acid and unknown substances were detected in P. quercina. In P. carporrhizans, the metabolites that we found in thalli (lecanoric acid, atranorin acid and chloratranorin) and the metabolites found in the mycobiont in aposymbiotic culture (confluentic acid in early cultures and norlobaridone in late cultures) are produced at different stages in the same pathway. Contrarily, the metabolites found in P. quercina mycobiont cultures (pulvinic acid), with respect of the metabolites found in natural thalli (lecanoric acid, atranorin acid and chloratranorin), implied a change of the metabolic pathway [57] from the acetate–mevalonate pathway to the shikimic acid pathway via the expressions of different polyketide synthases [25]. Similar results for the variation between secondary metabolites produced by thalli and mycobiont cultures were reported [4,39,44], arguing that the change of biosynthetic pathway also changed metabolite production [57] via the expression of different polyketide synthases [4]. This is attributed to the absence of carbon sources provided by the photobionts [39] or suboptimal environmental conditions that caused stress [4].
The secondary metabolites that we found in the mycobiont of Parmelina species in aposymbiotic cultures are reported to be bioactive molecules. Norlobaridone and confluentic acid, both produced by P. carporrhizans mycobionts are antioxidants and have antimicrobial activity [14,58]. Additionally, the mycobiont of P. quercina produces pulvinic acid, which has marked antioxidant properties, and showed photoprotection against UV rays [59]. We can speculate that if these metabolites are also synthesized by mycobionts under natural conditions, they may have an adaptive advantage related to their habitat. The photoprotection offered by pulvinic acid could be useful in the exposed bark of the Mediterranean forests of evergreen Quercus species, which P. quercina inhabits. Furthermore, the antimicrobial activity of confluentic acid could be suitable for P. carporrhizans, which adapts to shade and humid places, where fast growing organisms such as bacteria can proliferate. The metabolites produced by the mycobionts of P. carporrhizans and the mycobionts of P. quercina are different, but interestingly, the secreted substance present in the agar was the same in both species, and this was also the major metabolite found in late cultures of P. quercina. Following the same reasoning applied to metabolites found in the mycobiont cultures, the substance secreted to agar by both mycobionts, if it is produced by mycobionts in natural conditions, should be adaptative for both species in their respective habitats. We failed to identify this metabolite, which, based on our observations of the droplets, looks like a phenol; however, we know with certainty that they have a retention time of 6.97 min (Table 1). Further studies are needed to identify the substance secreted by Parmelina mycobionts, and if it is confirmed that this substance has bioactive properties, using it would be advantageous since it does not need to be extracted. Although the change in metabolites produced by mycobionts as a result of growth in different culture media has previously been reported [21,60], we observed no differences in the secondary metabolites produced by Parmelina mycobionts in aposymbiotic growth between the tested culture media (0.2G-MY, CMA, and Lilly Barnett medium). This result is unexpected. A possible explanation could be that the culture media are not sufficiently different in terms of pH, carbon source, nucleotides and other ingredients that could lead to a change in the secondary metabolism of lichen mycobionts.
We found that different secondary metabolites were produced at different times. Confluentic acid was produced as a major substance by the early mycobiont cultures of P. carporrizans, while norlobaridone acid was the major substance produced by the late mycobiont cultures. Similarly, early mycobiont cultures of P. quercina produced pulvinic acid as minor secondary metabolites and a substance with 6.97 min of retention time as a major substance, while the late mycobiont culture produced substances with 5.79 and 6.26 min retention [21] times, both as major substances. As far as we know, this is the first study on the change in secondary metabolites of mycobiont cultures over time. The mycobiont cultures often did not survive for the long time period of 550 days (this study), showing a loss of viability between 100 and 300 days [4,44,45]. Moreover, only few studies analyzed the variation of metabolites in “late cultures”. For example, Cordeiro et al. [60] compared early stages of 2 months with late cultures of 6 months for aposymbiotically grown mycobionts of Ramalina species. In this study, we did not find any depsides at the early stage but did find sekikaic acid after 6 months. On the other hand, Fazio et al. [21] studied changes in the profiles of metabolites in mycobionts of the Parmeliaceae lichen (Parmotrema reticulatum) for up to 10 months, combining the growth on different culture media with stress to desiccation (which simulates senescence). However, they did not find differences in metabolites between 5 and 10 months.
Our findings suggest that mycobiont cultures could be an alternative source of bioactive secondary metabolites of potential interest instead of natural populations of lichens. Furthermore, the study of the changes in secondary metabolites over time enriches the catalogue of substances produced by mycobionts. Our study also shows that it is possible to maintain cultures for long periods of time (up to 550 days), and current research is solving problems such as pH variation over time in cultures by improving growth [43].
5. Conclusions
We conclude that the aposymbiotic cultures of lichen mycobionts have a high potential as a source of bioactive compounds since they increase the catalogue of substances produced by the lichen-forming fungus, most importantly, without harming natural lichen populations or the environment. Our study shows a longevity superior to that commonly achieved by mycobiont cultures and points to the importance of time (“early” vs. “late” stages) in characterizing the metabolites produced by mycobionts. We not only found additional secondary metabolites in aposymbiotically grown mycobionts but also different secondary metabolites over time. Future studies in mycobiont cultures are needed to better characterize the secondary metabolites produced by more species, under different conditions and at different times. Additional research is needed for the modulation of the cultures to improve the growth rates.
Author Contributions
M.C.M., D.A. and A.C. (Ana Crespo) conceived and designed the experiments; A.C. (Anjuli Calchera) and I.S. performed the experiments; M.C.M., D.A. and P.K.D. analyzed the data; D.A. and M.C.M. contributed reagents/materials/analysis tools; D.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministerio de Economia y Competitividad (CGL2013-42498-P), Ministerio de Ciancia e Innovación (PID2019-105312GB-I00), the research funding program Landes-Offensive zur Entwicklung Wissenschaftlich-Oekonomischer Exzellenz (LOEWE) of Hesse’s Ministry of Higher Education, Research, and the Arts through the Senckenberg Biodiversity and Climate Research Centre (BiK-F).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data which are not declared within text are available in a publicly accessible repository and can be requested from the corresponding author.
Acknowledgments
DA was the recipient of a short stay grant from the Spanish Ministry of Economia y Competitividad. The herbarium MAF–Lich is sincerely thanked for providing herbarium numbers and samples for this study. MCM would like to dedicate this article to Professor Carlos Vicente, lichenologist and expert in phenolic compounds, who passed away a year and a half ago.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmadjian, V. The Lichen Symbiosis; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete Yeasts in the Cortex of Ascomycete Macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef]
- Meeßen, J.; Ott, S. Recognition Mechanisms during the Pre-Contact State of Lichens: I. Mycobiont-Photobiont Interactions of the Mycobiont of Fulgensia bracteata. Symbiosis 2013, 59, 121–130. [Google Scholar] [CrossRef]
- Timsina, B.A.; Sorensen, J.L.; Weihrauch, D.; Piercey-Normore, M.D. Effect of Aposymbiotic Conditions on Colony Growth and Secondary Metabolite Production in the Lichen-Forming Fungus Ramalina dilacerata. Fungal Biol. 2013, 117, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Casselman, K.D. Lichen Dyes: The New Source Book, 2nd ed.; Dover Publications: Mineola, NY, USA, 2003. [Google Scholar]
- Wang, L.-S.; Narui, T.; Harada, H.; Culberson, C.F.; Culberson, W.L. Ethnic Uses of Lichens in Yunnan, China. Bryologist 2001, 104, 345–349. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Hulme, A.N.; McNab, H.; Quye, A. The Natural Constituents of Historical Textile Dyes. Chem. Soc. Rev. 2004, 33, 329–336. [Google Scholar] [CrossRef]
- Devkota, S.; Chaudhary, R.P.; Werth, S.; Scheidegger, C. Indigenous Knowledge and Use of Lichens by the Lichenophilic Communities of the Nepal Himalaya. J. Ethnobiol. Ethnomed. 2017, 13, 15. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Metabolic Diversity of Lichen-Forming Ascomycetous Fungi: Culturing, Polyketide and Shikimatemetabolite Production, and PKS Genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef]
- White, P.A.S.; Oliveira, R.C.M.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.S.; Gelain, D.P.; Moreira, J.C.F.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J.; et al. Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Darokar, M.P.; Saikia, D.; Pal, A.; Fatima, A.; Khanuja, S.P.S. Antimycobacterial Activity of Lichens. Pharm. Biol. 2007, 45, 200–204. [Google Scholar] [CrossRef]
- Cheng, B.; Cao, S.; Vasquez, V.; Annamalai, T.; Tamayo-Castillo, G.; Clardy, J.; Tse-Dinh, Y.-C. Identification of Anziaic Acid, a Lichen Depside from Hypotrachyna sp., as a New Topoisomerase Poison Inhibitor. PLoS ONE 2013, 8, e60770. [Google Scholar] [CrossRef]
- Kosanić, M.; Manojlović, N.; Janković, S.; Stanojković, T.; Ranković, B. Evernia prunastri and Pseudoevernia furfuraceae Lichens and Their Major Metabolites as Antioxidant, Antimicrobial and Anticancer Agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Honda, N.; Freitas, D.; Micheletti, A.; Carvalho, N.; Spielmann, A.; Canêz, L. Parmotrema screminiae (Parmeliaceae), a Novel Lichen Species from Brazil with Potent Antimicrobial Activity. Orbital Electron. J. Chem. 2016, 8, 334–340. [Google Scholar] [CrossRef]
- Jeon, H.-S.; Lokos, L.; Han, K.S.; Ryu, J.-A.; Kim, J.-A.; Koh, Y.-J.; Hur, J.-S. Isolation of Lichen-Forming Fungi from Hungarian Lichens and Their Antifungal Activity Against Fungal Pathogens of Hot Pepper Anthracnose. Plant Pathol. J. 2009, 25, 38–46. [Google Scholar] [CrossRef]
- Kowalski, M.; Hausner, G.; Piercey-Normore, M.D. Bioactivity of Secondary Metabolites and Thallus Extracts from Lichen Fungi. Mycoscience 2011, 52, 413–418. [Google Scholar] [CrossRef]
- Rancan, F.; Rosan, S.; Boehm, K.; Fernández, E.; Hidalgo, M.E.; Quihot, W.; Rubio, C.; Boehm, F.; Piazena, H.; Oltmanns, U. Protection against UVB Irradiation by Natural Filters Extracted from Lichens. J. Photochem. Photobiol. B 2002, 68, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.-H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-Protectant Metabolites from Lichens and Their Symbiotic Partners. Nat. Prod. Rep. 2013, 30, 1490–1508. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Mahadik, N.; Morey, M. Antioxidative and Cardiovascular-Protective Activities of Metabolite Usnic Acid and Psoromic Acid Produced by Lichen Species Usnea complanata under Submerged Fermentation. Pharm. Biol. 2012, 50, 968–979. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. In Vitro Neuroprotective Potential of Lichen Metabolite Fumarprotocetraric Acid via Intracellular Redox Modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.T.; Bertoni, M.D.; Adler, M.T.; Ruiz, L.B.; Rosso, M.L.; Muggia, L.; Hager, A.; Stocker-Wörgötter, E.; Maier, M.S. Culture Studies on the Mycobiont Isolated from Parmotrema reticulatum (Taylor) Choisy: Metabolite Production under Different Conditions. Mycol. Progress. 2009, 8, 359–365. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Karunaratne, V. Potential of Lichen Compounds as Antidiabetic Agents with Antioxidative Properties: A Review. Oxid. Med. Cell. Longev. 2017, 2017, 2079697. [Google Scholar] [CrossRef]
- Rezanka, T.; Dembitsky, V.M. The Colleflaccinosides, Two Chiral Bianthraquinone Glycosides with Antitumor Activity from the Lichen Collema flaccidum Collected in Israel and Russia. Nat. Prod. Res. 2006, 20, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-S.; Kim, T.K.; Kim, J.E.; Hong, J.-M.; Nguyen, T.T.T.; Han, S.J.; Youn, U.J.; Yim, J.H.; Kim, I.-C. Anticancer Activity of Ramalin, a Secondary Metabolite from the Antarctic Lichen Ramalina terebrata, against Colorectal Cancer Cells. Molecules 2017, 22, 1361. [Google Scholar] [CrossRef] [PubMed]
- Carlos, I.Z.; Quilles, M.B.; Carli, C.B.A.; Maia, D.C.G.; Benzatti, F.P.; Lopes, T.I.B.; Gianini, A.S.; Brum, R.L.; Vilegas, W.; dos Santos, L.C.; et al. Lichen Metabolites Modulate Hydrogen Peroxide and Nitric Oxide in Mouse Macrophages. Z. Nat. C. J. Biosci. 2009, 64, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Upreti, D.K.; Divakar, P.K.; Nayaka, S. Commercial and Ethnic Use of Lichens in India. Econ. Bot. 2005, 59, 269–273. [Google Scholar] [CrossRef]
- Joulain, D.; Tabacchi, R. Lichen Extracts as Raw Materials in Perfumery. Part 1: Oakmoss. Flavour Fragr. J. 2009, 24, 49–61. [Google Scholar] [CrossRef]
- Shukla, P.; Upreti, D.K. Lichen Dyes: Current Scenario and Future Prospects. In Recent Advances in Lichenology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 209–230. [Google Scholar] [CrossRef]
- Shaheen, S.; Iqbal, Z.; Hussain, M. First Report of Dye Yielding Potential and Compounds of Lichens; a Cultural Heritage of Himalayan Communities, Pakistan. Pak. J. Bot. 2019, 51, 341–360. [Google Scholar] [CrossRef]
- Devkota, S.; Weerakoon, G. Everniastrum nepalense. In The IUCN Red List of Threatened Species 2017; IUCN: Gland, Switzerland, 2017. [Google Scholar] [CrossRef]
- Aptroot, A.; Perez-Ortega, S. Xanthoparmelia beccae. In IUCN Red List Assessment; IUCN: Gland, Switzerland, 2018. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary Metabolite Profiling of Species of the Genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2017, 23, 54. [Google Scholar] [CrossRef]
- Horák, J.; Materna, J.; Halda, J.P.; Mladenović, S.; Bogusch, P.; Pech, P. Biodiversity in Remnants of Natural Mountain Forests under Conservation-Oriented Management. Sci. Rep. 2019, 9, 89. [Google Scholar] [CrossRef]
- Pykälä, J. Habitat Loss and Deterioration Explain the Disappearance of Populations of Threatened Vascular Plants, Bryophytes and Lichens in a Hemiboreal Landscape. Glob. Ecol. Conserv. 2019, 18, e00610. [Google Scholar] [CrossRef]
- Díaz, E.M.; Zamora, J.C.; Ruibal, C.; Divakar, P.K.; González-Benítez, N.; Le Devehat, F.; Chollet, M.; Ferron, S.; Sauvager, A.; Boustie, J.; et al. Axenic Culture and Biosynthesis of Secondary Compounds in Lichen Symbiotic Fungi, the Parmeliaceae. Symbiosis 2020, 82, 79–93. [Google Scholar] [CrossRef]
- Zakeri, Z.; Junne, S.; Jäger, F.; Dostert, M.; Otte, V.; Neubauer, P. Lichen Cell Factories: Methods for the Isolation of Photobiont and Mycobiont Partners for Defined Pure and Co-Cultivation. Microb. Cell Factories 2022, 21, 80. [Google Scholar] [CrossRef]
- Culberson, C.F.; Culberson, W.L. Chemosyndromic Variation in Lichens. Syst. Bot. 1976, 1, 325–339. [Google Scholar] [CrossRef]
- Elix, J.A. Biochemistry and Secondary Metabolites. In Lichen Biology; Nash, T., III, Ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 154–180. [Google Scholar]
- Brunauer, G.; Hager, A.; Grube, M.; Türk, R.; Stocker-Wörgötter, E. Alterations in Secondary Metabolism of Aposymbiotically Grown Mycobionts of Xanthoria elegans and Cultured Resynthesis Stages. Plant. Physiol. Biochem. 2007, 45, 146–151. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Elix, J.A.; Grube, M. Secondary Chemistry of Lichen-Forming Fungi: Chemosyndromic Variation and DNA-Analyses of Cultures and Chemotypes in the Ramalina farinacea Complex. bryo 2004, 107, 152–162. [Google Scholar] [CrossRef]
- Hager, A.; Stocker-Wörgötter, E. Secondary Chemistry and DNA-Analyses of the Australian Lichen Heterodea muelleri (Hampe) Nyl. and Culture of the Symbionts. Symbiosis 2005, 39, 13–19. [Google Scholar]
- Bertrand, R.L.; Sorensen, J.L. Lost in Translation: Challenges with Heterologous Expression of Lichen Polyketide Synthases. ChemistrySelect 2019, 4, 6473–6483. [Google Scholar] [CrossRef]
- Pichler, G.; Candotto Carniel, F.; Muggia, L.; Holzinger, A.; Tretiach, M.; Kranner, I. Enhanced Culturing Techniques for the Mycobiont Isolated from the Lichen Xanthoria parietina. Mycol. Progress. 2021, 20, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.C.; Divakar, P.K.; Zhang, N.; González, N.; Struwe, L. Non-Developing Ascospores in Apothecia of Asexually Reproducing Lichen-Forming Fungi. Int. Microbiol. 2013, 16, 145–155. [Google Scholar] [CrossRef]
- Molina, M.; Divakar, P.; González-Benítez, N. Success in the Isolation and Axenic Culture of Anaptychia ciliaris (Physciaceae, Lecanoromycetes) Mycobiont. Mycoscience 2015, 56, 351–358. [Google Scholar] [CrossRef]
- Hale, M.E. A Monograph of the Lichen Genus Parmelia Acharius Sensu Stricto (Ascomycotina: Parmeliaceae); Smithsonian Institution Press: Washington, DC, USA, 1987; pp. 1–64. [Google Scholar]
- Alors, D.; Cendón-Flórez, Y.; Divakar, P.K.; Crespo, A.; Benítez, G.N.; Molina, M.C. Differences in the Sexual Aposymbiotic Phase of the Reproductive Cycles of Parmelina carporrhizans and P. quercina. Possible Implications for Their Reproductive Biology. Lichenologist 2019, 51, 175–186. [Google Scholar] [CrossRef]
- del Molina, M.C.; Crespo, A. Comparison of Development of Axenic Cultures of Five Species of Lichen-Forming Fungi. Mycol. Res. 2000, 104, 595–602. [Google Scholar] [CrossRef]
- Deason, T.R.; Bold, H.C. Psychological Studies: I. Exploratory Studies of Texas Soil Algae; The University of Texas Publication: Austin, TX, USA, 1960. [Google Scholar]
- Lilly, H.L.; Barnett, V.G. Physiology of the Fungi; McGraw-Hill: New York, NY, USA, 1951. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Sninsky, J.J., White, T.J., Eds.; Academic Press: Millbrae, CA, USA, 1989; Volume 38, pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Alors, D.; Dal Grande, F.; Schmitt, I.; Kraichak, E.; Lumbsch, H.T.; Crespo, A.; Divakar, P.K. Characterization of Fungus-Specific Microsatellite Markers in the Lichen-Forming Fungus Parmelina carporrhizans (Parmeliaceae). Appl. Plant. Sci. 2014, 2, 1400081. [Google Scholar] [CrossRef] [PubMed]
- Feige, G.B.; Lumbsch, T.; Huneck, S.; Elix, J. Identification of Lichen Substances by a Standardize High-Performance Liquid Chromatography Method. J. Chromatogr. A 1993, 646, 417–427. [Google Scholar] [CrossRef]
- Benatti, M.N.; Gernert, M.; Schmitt, I. Parmotrema hydrium, a New Species of Parmeliaceae in Southeastern Brazil. Acta Bot. Bras. 2013, 27, 810–814. [Google Scholar] [CrossRef]
- Clerc, P.; Truong, C. The Non-Sorediate and Non-Isidiate Parmelina Species (Lichenized Ascomycetes, Parmeliaceae) in Switzerland—Parmelina atricha (Nyl.) P. Clerc Reinstated in the European Lichen Flora. Sauteria 2008, 15, 175–194. [Google Scholar]
- Molina, M.C.; Crespo, A.; Vicente, C.; Elix, J.A. Differences in the Composition of Phenolics and Fatty Acids of Cultured Mycobiont and Thallus of Physconia distorta. Plant Physiol. Biochem. 2003, 41, 175–180. [Google Scholar] [CrossRef]
- Liu, X.-T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.A.; Monte, A. Antibacterial Compounds from Mushrooms I: A Lanostane-Type Triterpene and Prenylphenol Derivatives from Jahnoporus hirtus and Albatrellus flettii and Their Activities against Bacillus cereus and Enterococcus faecalis. Planta Med. 2010, 76, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Nadal, B.; Thetiot-Laurent, S.; Pin, S.; Renault, J.-P.; Cressier, D.; Rima, G.; Le Roux, A.; Meunier, S.; Wagner, A.; Lion, C.; et al. Synthesis and Antioxidant Properties of Pulvinic Acids Analogues. Bioorganic Med. Chem. 2010, 18, 7931–7939. [Google Scholar] [CrossRef]
- Cordeiro, L.M.C.; Iacomini, M.; Stocker-Wörgötter, E. Culture Studies and Secondary Compounds of Six Ramalina Species. Mycol. Res. 2004, 108, 489–497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).