Abstract
Al-Li alloys are ideal structural materials for the aerospace industry. However, an increasing number of Al-Li alloys have reached the end of their service life and must be recycled. Unfortunately, when vacuum distillation is used to separate Al-Li alloys, metallic lithium is difficult to condense and collect. Therefore, theoretical and experimental research on lithium condensation conditions under vacuum and vacuum distillation and condensation of Al-Li alloy to prepare metallic lithium were carried out. The results show that the optimal condensation temperature range for lithium is between 523 and 560 K. More than 99.5% metallic lithium and more than 99.97% aluminum were obtained from the Al-7.87%wt Li alloy through vacuum distillation condensation. The direct yield of lithium was above 80%. This paper, therefore, provides a new and improved method for the preparation of metallic lithium.
1. Introduction
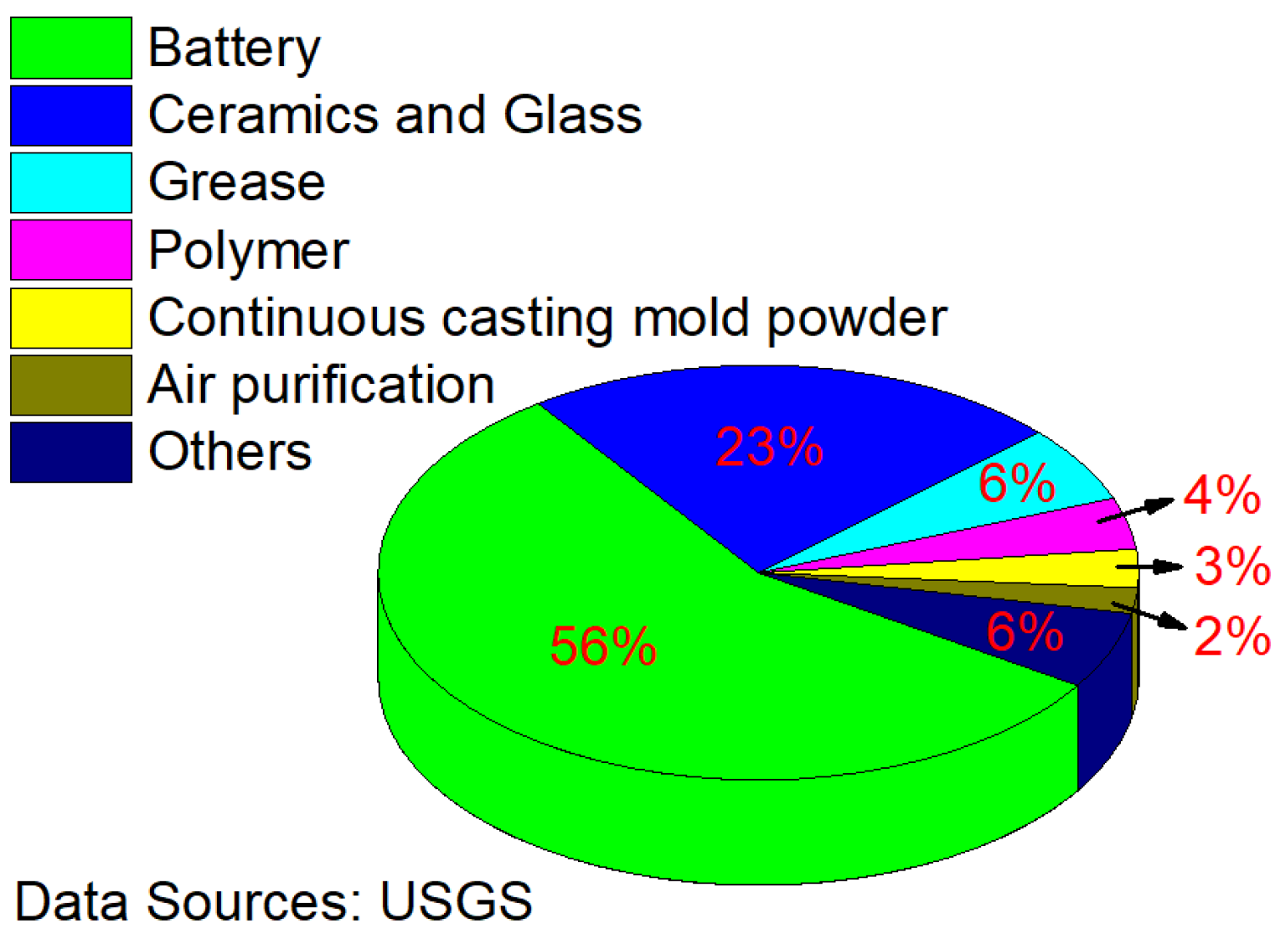
Lithium, the lightest metal, is unstable in nature. It can react with most metals and nonmetals under certain conditions [1,2,3]. However, owing to the special physical and chemical properties of lithium and its compounds, it is widely used in high-precision technical fields, such as aerospace, nuclear power generation, and high-energy batteries, as well as the creation of lightweight and high-strength alloys [4,5,6,7,8,9]. Figure 1 shows the proportion of lithium consumption worldwide in 2020 [10]. As can be seen, the battery industry is the largest source of lithium consumption and is expected to remain so for the foreseeable future.
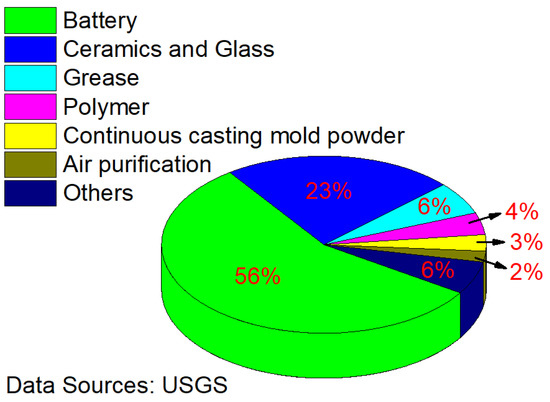
Figure 1.
Proportion of lithium consumption.
The production process of metallic lithium can be divided into two categories: molten salt electrolysis and vacuum thermal reduction [11,12]. The former is a mixture of high-purity graphite as the anode, low-carbon steel as the cathode, electrolytic melting of LiCl (45%)–KCl (55%) at a certain temperature (390–450 °C), and voltage (7–9 V). During the electrolytic process, metal cations (Li+) move towards the cathode and obtain electrons, which are reduced to lithium metal. Due to the low density, they float to the surface of molten salt and enter the special collection chamber. The anion (Cl−) moves towards the anode, loses electrons on the graphite surface, and oxidizes into chlorine gas, which volatilizes outwards and rises to the air outlet for elimination or collection. The latter involves obtaining metallic lithium by reducing Li2O or lithium-rich materials under vacuum and high-temperature conditions with metal or nonmetal as the reducing agent. Currently, 90% of the world’s lithium production comes from molten salt electrolysis, which is the only method of industrial production [13]. However, serious Cl2 pollution, high electrolyte cost, and many K and Na impurities in the product are not conducive to production needs and environmental protection, although the thermal vacuum reduction method is relatively simple and approximately 20% cheaper than molten salt electrolysis. For the secondary resource recovery of lithium, with the wide application of Al-Li alloys in aerospace and other aspects, the amount of scrap parts containing Al-Li alloy will gradually increase; in addition, in the process of processing the Al-Li alloy, due to the low yield of finished products, a large amount of processing waste is also generated, and enterprises use waste stacking storage, resulting in a huge waste of resources and high waste management costs. Therefore, the effective recovery and utilization of Al-Li alloys have become urgent problems to solve. To date, there have been limited investigations on the recovery and regeneration of Al-Li alloys. The methods employed in previous studies include electrolytic, compound, alloy, flux melting, and vacuum distillation techniques. Electrolytic method: The waste Al-Li alloy is used as the electrolytic anode, and the electrolyte is 70% LiCl, 25% KCl, and 5% LiF, which is electrolyzed in the electrolytic cell. After electrolysis, w(Li) = 0.77~1.84%, which can be reduced to 0.12~0.67% after electrolysis. Compound method: This method involves making lithium in an Al-Li alloy waste precipitate to make LiCl. Firstly, the waste is melted, and then chlorine or chlorine salt is added to the melt, so that the lithium in the Al-Li alloy waste is precipitated into LiCl. Alloy method: The so-called alloy method is to make an aluminum–lithium alloy waste through processing into another aluminum–lithium alloy process, where lithium is mixed in the liquid aluminum cathode in the LiCl melt, which becomes another aluminum–lithium alloy. Flux smelting method: The Al-Li alloy waste is mixed with a small amount of fluoride (CaF2, NaF, AlF3Or Na3AlF6); a mixture of NaCl and KCl is smelted so that metals more reactive than aluminum enter the molten salt by reacting with the fluoride salt. Vacuum method: This method is based on the difference of the saturated vapor pressure of lithium and aluminum in Al-Li alloy waste, and the separation is achieved at a certain temperature. Among these approaches, vacuum distillation has garnered significant attention from researchers due to its eco-friendly and efficient nature [14,15,16]. However, there are certain difficulties involved in obtaining elemental lithium through vacuum condensation. Therefore, we will carry out theoretical and experimental research on lithium condensation conditions under vacuum and vacuum distillation, as well as condensation of the Al-Li alloy, to prepare metallic lithium. This will provide a theoretical and experimental basis for the vacuum extraction and purification of metallic lithium.
2. Theoretical Analysis and Experimental Methods
2.1. Boiling Point and Saturated Vapor Pressure of Pure Metals
Pressure is an important factor for determining the initial metal volatilization temperature. Under different pressures, pure metals in the system have different boiling points. The corresponding boiling point can be calculated using the pressure. The boiling point of a pure metal can also be used to reflect the magnitude of the interaction force between metal particles [17]. During the vacuum distillation of alloys, it is assumed that each metal is an independent pure substance without an interaction force. Under a certain system pressure, different metals display different phenomena after absorbing the same amount of heat, such as a difference in boiling point.
Saturated vapor pressure is an important indicator involved in judging the initial volatilization temperature of metals. Commonly used equations for calculating saturated vapor pressure are the Clausius–Clapeyron equation, the Clapeyron equation, and the Antoine equation [18,19,20]. We used the Clausius–Clapeyron equation to calculate the saturated vapor pressures of lithium and aluminum at different temperatures. The expression is shown in Equation (1), where T represents temperature, and A, B, C, and D are evaporation constants. The A, B, C, and D of lithium and aluminum are −8415, −1, 0, 13.465, and −16,380, −1, 0, and 14.445.
2.2. Separation Factor and Gas–Liquid Equilibrium Composition Diagram of Binary Alloy
The basis of using vacuum distillation to separate alloys involves distilling the gas phase out, resulting in different remaining liquid phase components. Dai and Yang [21] proposed the “separation factor (β)” to determine whether the components can be separated during vacuum distillation. This is related to the saturated vapor pressure and the activity coefficient of the components. β can be expressed as:
where γi and γj are the activity coefficients of components i and j, respectively. and are the saturated vapor pressures of i and j.
There are three cases of the value of βi. First, βi = 1, where the content ratio of the two components in the liquid phase and the gas phase is equal. Vacuum distillation is useless at this time. Secondly, βi > 1, wherein the content of component i in the gas phase is higher than that of the liquid phase and also enriched in the gas phase. Component j is concentrated in the liquid phase. In this situation, components i and j can be separated using vacuum distillation; the greater the βi, the better the separation effect. Finally, βi < 1, which is contrary to βi > 1, wherein i is enriched in the liquid phase and j is enriched in the gas phase. However, they can also be separated via vacuum distillation. The smaller βi is, the better the separation.
The pure metal boiling point, saturated vapor pressure, and separation coefficient can only qualitatively predict the possibility of vacuum distillation in separating alloys. Dai and Yang [21] proposed a gas–liquid equilibrium composition diagram of the alloy to quantitatively estimate the degree of alloy separation during vacuum distillation. For the binary alloy system i-j, the content wi,g of component i in the gas phase can be expressed using the following formula:
2.3. Lithium Vapor Condensation Rate
For the metal condensation process, as temperature decreases, the vapor pressure of the metal gradually reduces until it reaches equilibrium with system pressure. This results in a decrease in metal vapor in the gas phase and an increase in metal in the liquid phase. Therefore, the rate of metal condensation can be calculated by measuring changes in metal vapor density within the gas phase. Generally speaking, we express this as
where is the saturated vapor pressure of i; is the relative molar mass of i.
According to the vapor density formula, the vapor density of lithium metal at volatilization temperatures of 973 K, 1073 K, 1273 K, and 1373 K is calculated, as shown in Table 1.

Table 1.
Vapor density of lithium vapor at different temperatures.
As can be seen from Table 1, the lithium vapor density decreases with a decrease in temperature. Therefore, during the condensation process, when the lithium vapor tends to diffuse from high temperature to the region with low temperature, the lithium vapor reaches the susaturated state, which promotes the condensation of lithium vapor. When the vapor density reaches the vapor density at this temperature, the equilibrium is reached and the condensation process ends. Therefore, the process of metal condensation is accompanied by a reduction in the density of lithium vapor, and the rate of change of lithium vapor can be used to define the condensation rate of vapor.
The condensation rate can be expressed as:
where represents the metal vapor density at the beginning of i; represents the metal vapor density of i at a certain temperature.
2.4. Nucleation and Condensation of Lithium Vapor
Condensation nucleation of metal atoms is a process in which gas atoms with thermal motion in the system collide with each other or molecules are rearranged. The number of collisions between atoms is generally considered to be an important factor affecting the process. The number of atomic collisions (Z) represents the number of collisions between atoms in the system within unit time. It is a function of temperature T and pressure P and can be expressed as:
where d is the diameter of a lithium metal atom (2.90 × 10−8 m), P is the pressure, k is the Boltzmann constant, T is temperature, is the average molecular velocity, M is the relative atomic mass of lithium (6.941 × 10−3 mol·kg−1), and R is the gas constant (8.314 J/(mol·K)).
2.5. Experimental Details
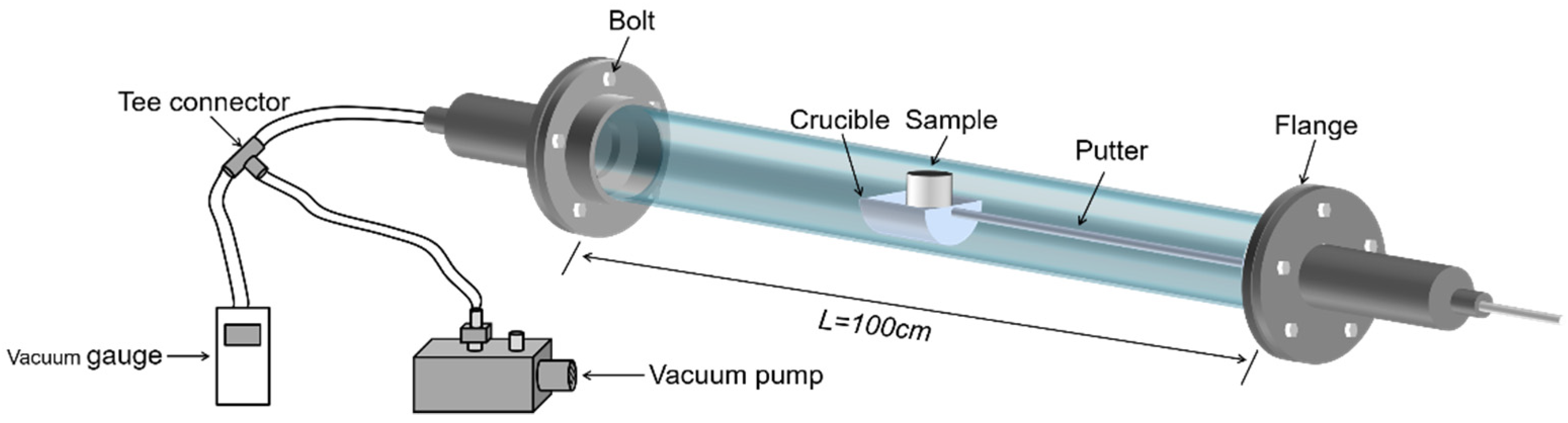
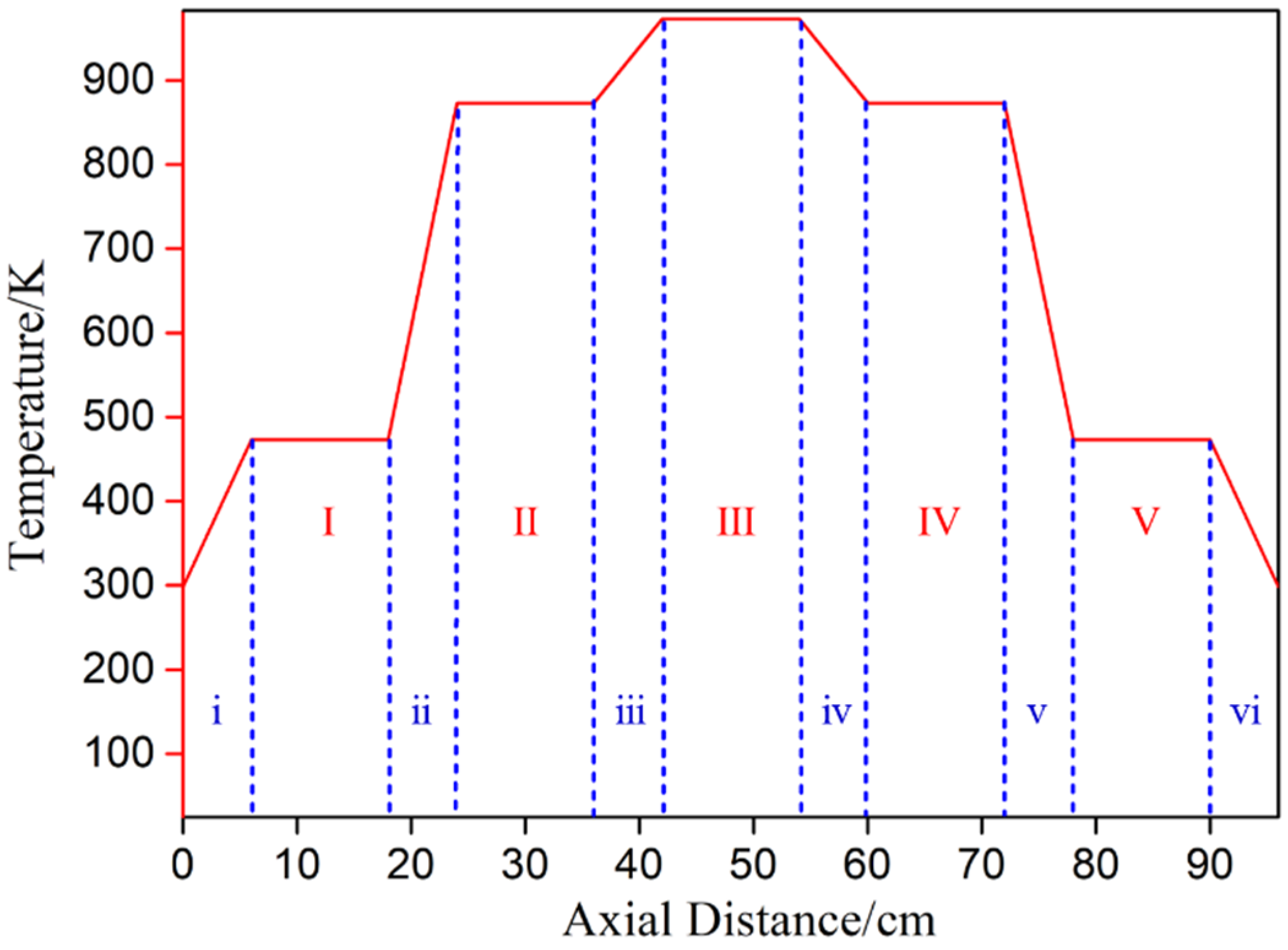
This experimental study focuses on the effect of heating temperature and temperature gradient on lithium metal vapor and the condensation law of Al-Li alloy metal vapor. The raw material, produced by Shanghai Binlian Industrial Co., Ltd. (Shanghai, China), used in the lithium vapor condensation experiment consisted of 99.9% lithium particles; its chemical composition (mass percentage) is shown in Table 2. The Al-Li alloy, obtained by reducing LiAlO2 with coking coal under vacuum conditions and condensing with aluminum liquid, was used as the raw material for this experiment. The composition of the Al-Li alloy was 7.87 % of lithium in aluminum. The experimental equipment was a vacuum grading condensing furnace with a controllable multi-temperature zone, as shown in Figure 2. The reactor consisted of three units: a heater, a catheter, and a condenser. The furnace had five heating zones and six insulation zones from left to right. The length of the heating zone was 12 cm, and there was a 6 cm heat insulation zone between each adjacent heating zone. Figure 3 shows the furnace temperature distribution, where the insulation was fixed and the heating zone temperature could be controlled and changed as needed. The sample was placed in the crucible and pushed into a quartz tube lined with a layer of graphite paper. A vacuum pump was used to evacuate the air in the system. The sample was placed in a crucible and inserted into a quartz tube. The heating rate was maintained at 8–10 K/min. After the experiment, the system was cooled to room temperature. The condensation products were then removed from the condenser for analysis.

Table 2.
Chemical composition of lithium metal (mass fraction, %).
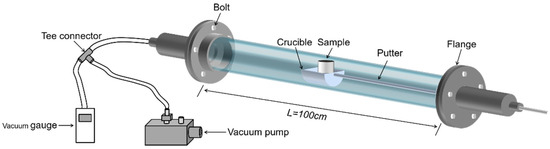
Figure 2.
Vacuum grading condensing furnace of controllable multi-temperature zone.
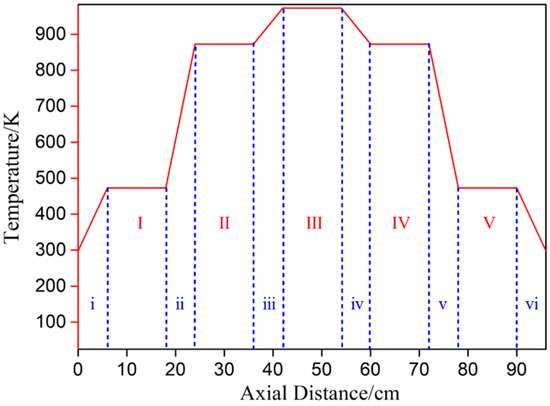
Figure 3.
Schematic diagram of furnace temperature distribution (the heating zone: I–V; the heat insulation zone: i–vi).
3. Results and Discussion
3.1. Theoretical Results
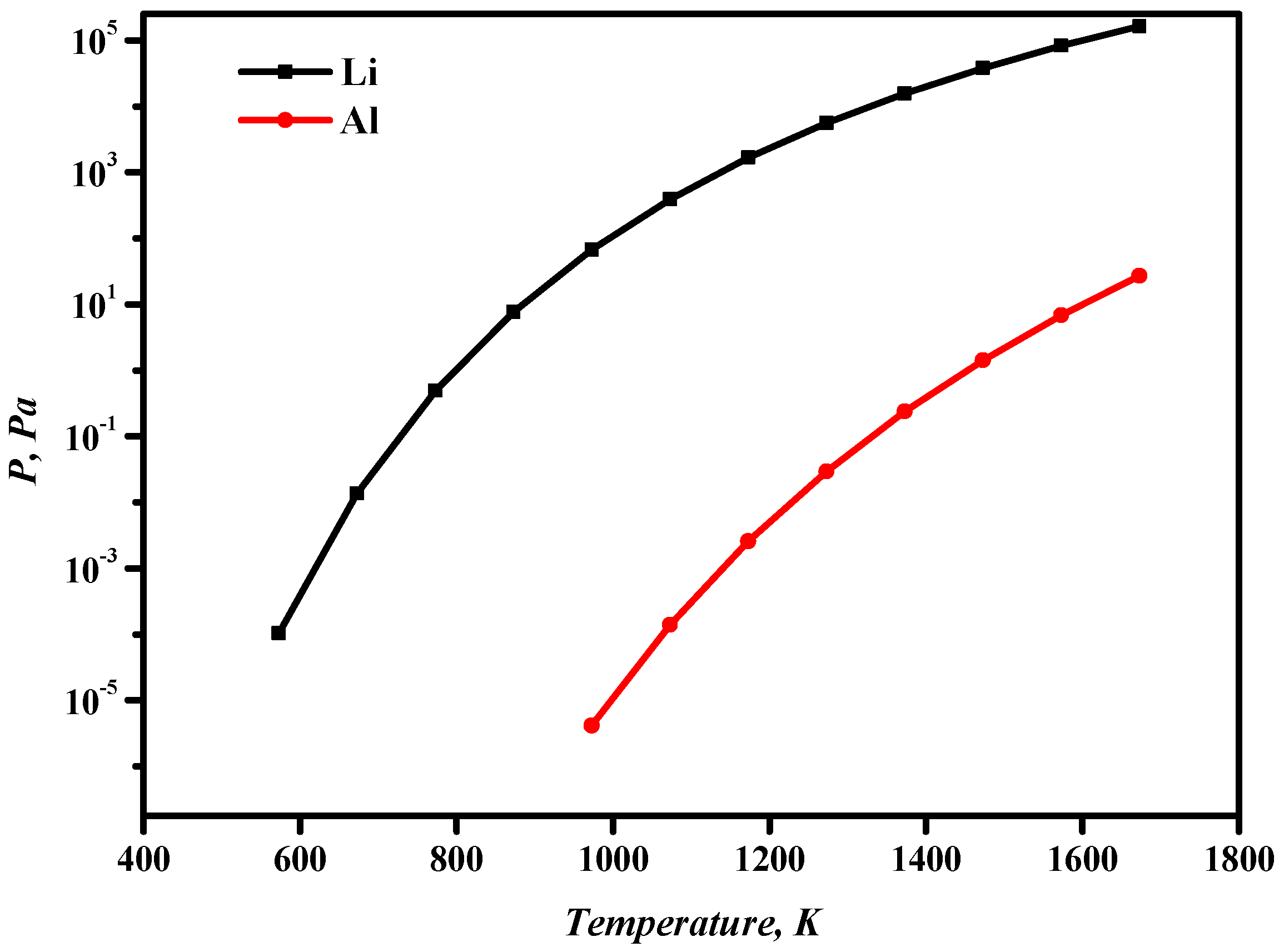
Table 3 shows the boiling points of lithium and aluminum at different temperatures. When the pressure is 13.3 Pa, the boiling point of lithium (811 K) is significantly smaller than that of aluminum (1421 K). When the system pressure is approximately 10 Pa at a certain temperature, lithium can volatilize into the gas phase, while aluminum remains in the liquid phase. The saturated vapor pressures of aluminum and lithium are calculated from Equation (1). As can be seen from Figure 4, the saturated vapor pressures of both aluminum and lithium increase as the temperature increases. The volatilization sequence of Al-Li alloy components is Li > Al. Therefore, it is theoretically possible to separate Al-Li alloys through vacuum distillation.

Table 3.
Boiling point of Li and Al at different pressures (K).
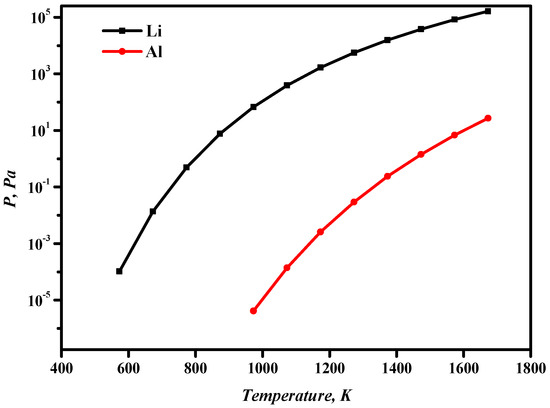
Figure 4.
Relationship between aluminum- and lithium-saturated vapor pressure and temperature.
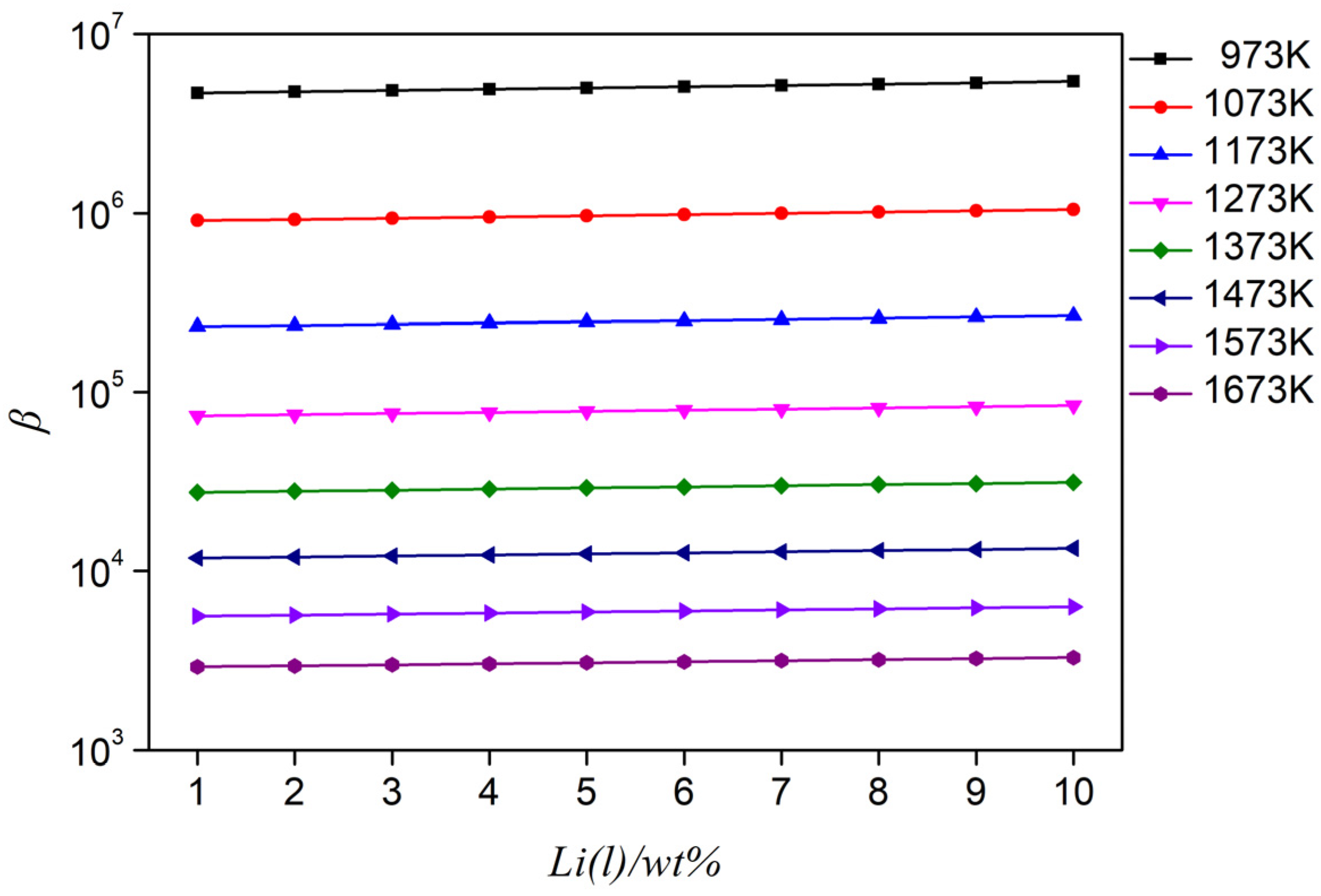
Based on the Wilson equation, which predicts the Al-Li alloy activity coefficient at different temperatures, in combination with the saturated vapor pressure and Equation (2), the separation coefficient of the Al-Li alloy in a range of 973–1673 K was calculated, as shown in Figure 5. In the calculation of the separation coefficient, the influence of temperature on the activity coefficient is considered; thus, the accuracy of the calculated separation coefficient is guaranteed. It can be seen from Figure 5 that the separation coefficient is β >> 1 when the lithium content is 1–10%. This indicates that in the Al-Li alloy system, vacuum distillation can be used to separate aluminum and lithium under certain conditions.
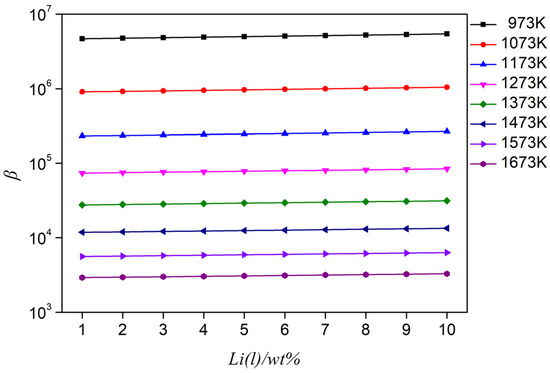
Figure 5.
Separation coefficient of Al-(1–10 wt%) Li alloy at different temperatures.
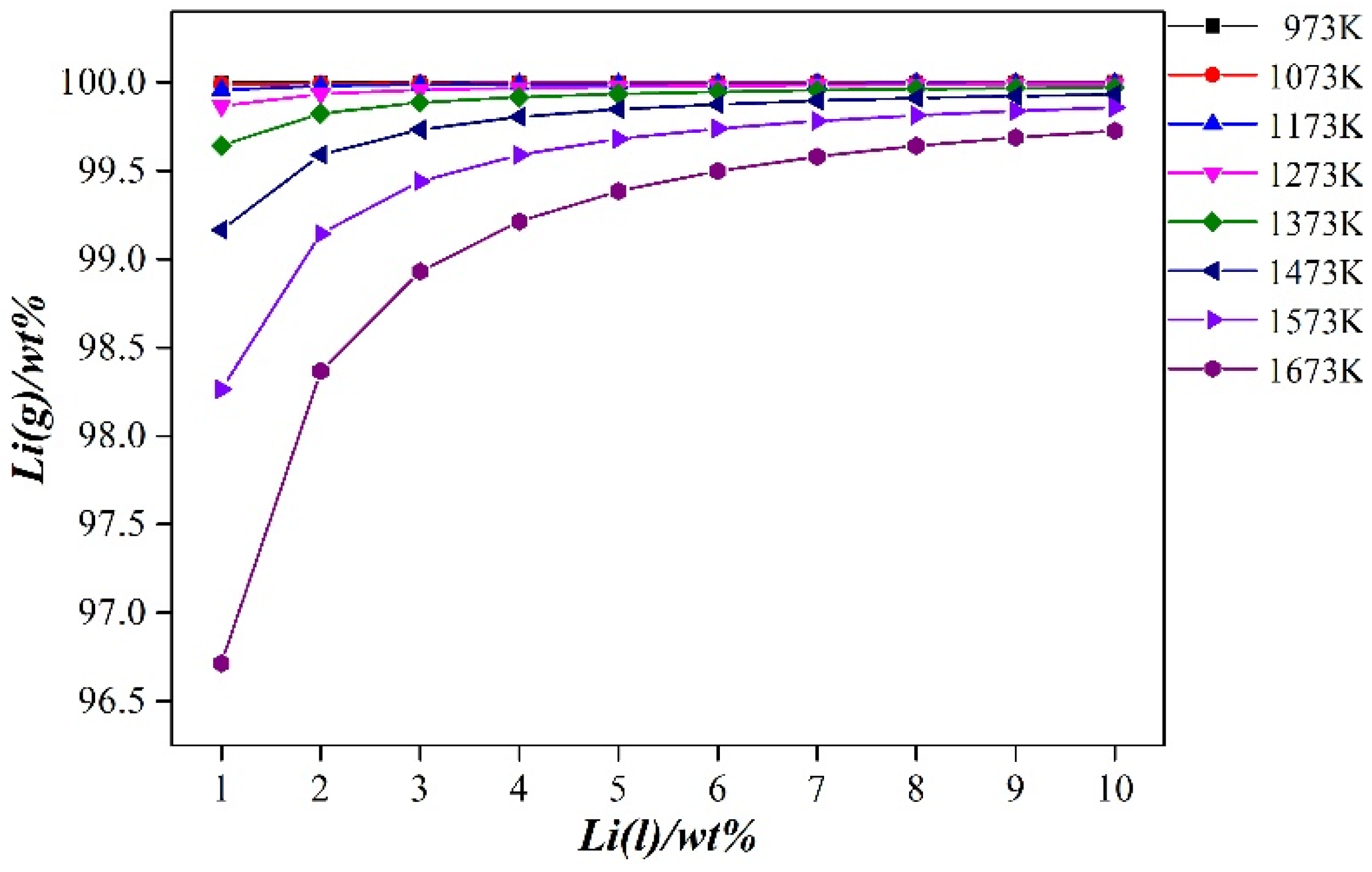
From Figure 6 (calculated using Equation (3)), it can be seen that during the vacuum distillation process of the Al-Li alloy, when the distillation temperature is constant, the lithium volatilizing into the gas phase increases as the lithium content in the binary alloy increases. When the lithium content in the binary alloy is fixed, the lithium content volatilized into the gas phase decreases as the distillation temperature increases. The lithium content in the binary alloy varied from 1 to 10%, the distillation temperature was in a range of 973–1673 K, and the lithium content volatilized into the gas phase was between 96.713 and 99.998%. Therefore, the method of vacuum distillation can effectively separate aluminum and lithium in the Al-Li alloy. In the gas phase, metallic lithium with a purity of more than 99.99% can be obtained.
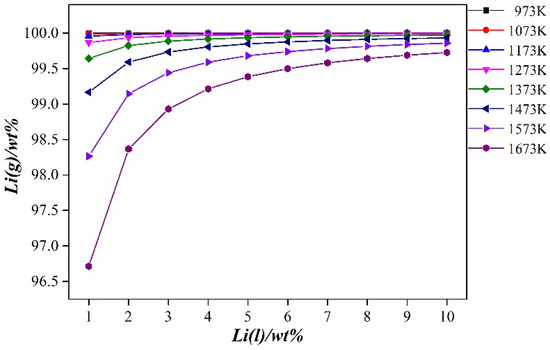
Figure 6.
Gas–liquid equilibrium composition diagram of Al-(1–10 wt. %) Li alloy at different temperatures.
When the distillation temperature is 973 K, 1073 K, 1173 K, and 1273 K, respectively, and the temperature in the condensation region is 473 K, 573 K, 673 K, 773 K, and 873 K, respectively, the condensation rate of lithium vapor is calculated using Equation (5), as shown in Table 4.

Table 4.
Lithium condensation rate at different temperatures.
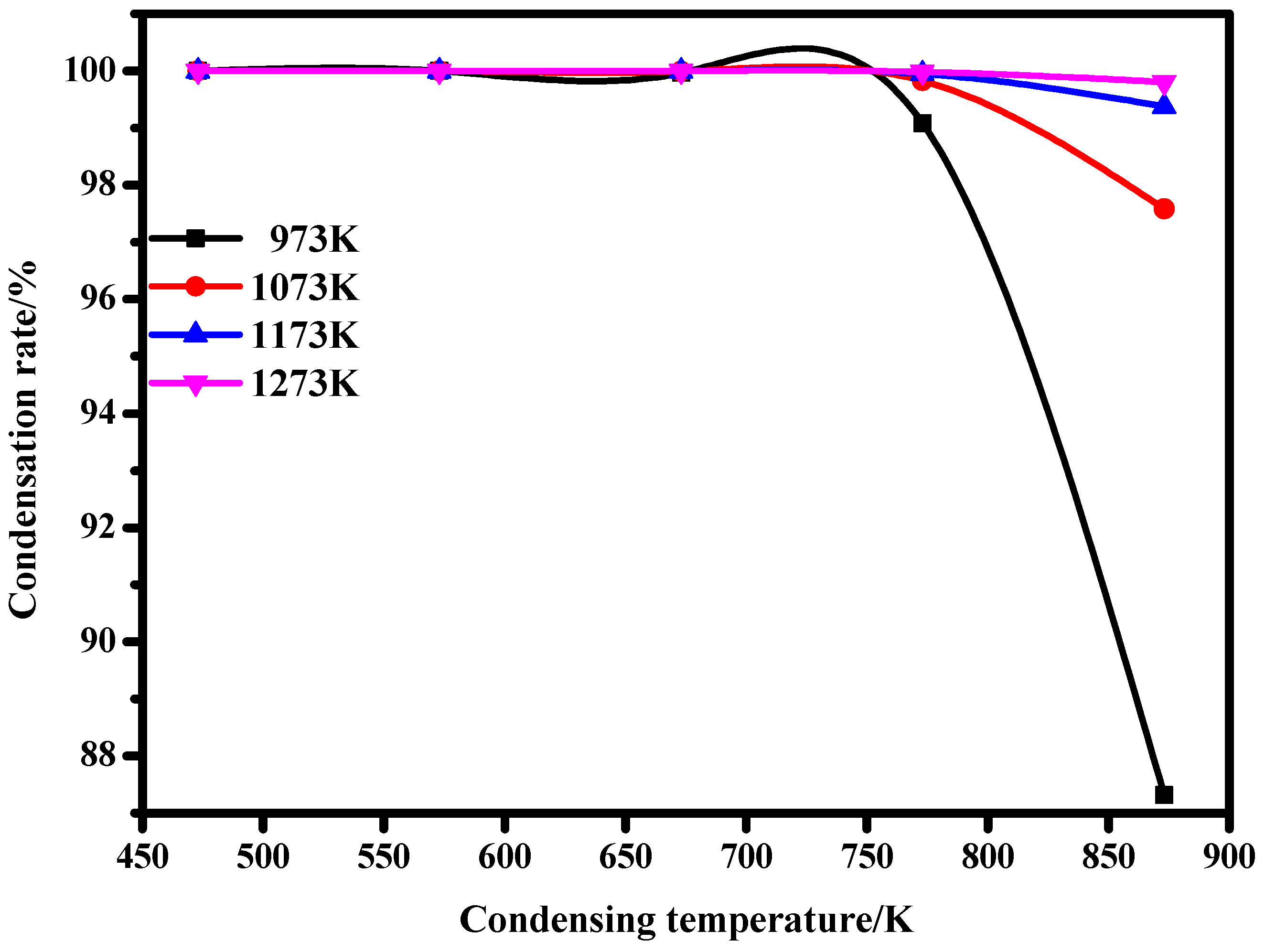
As can be seen from Figure 7, when lithium metal is condensed from 973 K, 1073 K, 1173 K, and 1273 K to 473 K, 573 K, 673 K, 773 K, and 873 K, respectively, the condensation rate of lithium vapor increases gradually. When the condensation temperature is 673 K, the condensation rate of lithium vapor almost reaches 100%. This indicates that the condensation efficiency of lithium metal vapor is high when the condensation temperature is lower than 673 K, which is conducive to the condensation of lithium metal vapor.
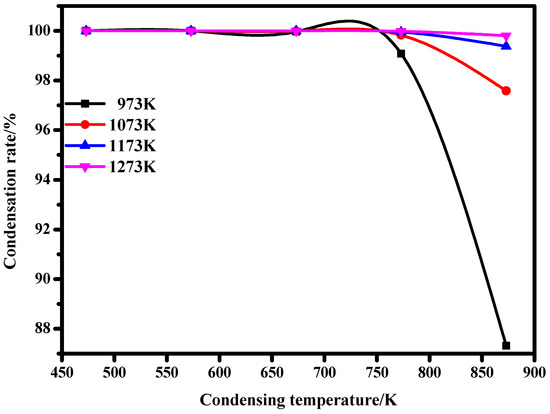
Figure 7.
Relationship between condensation rate and temperatures.
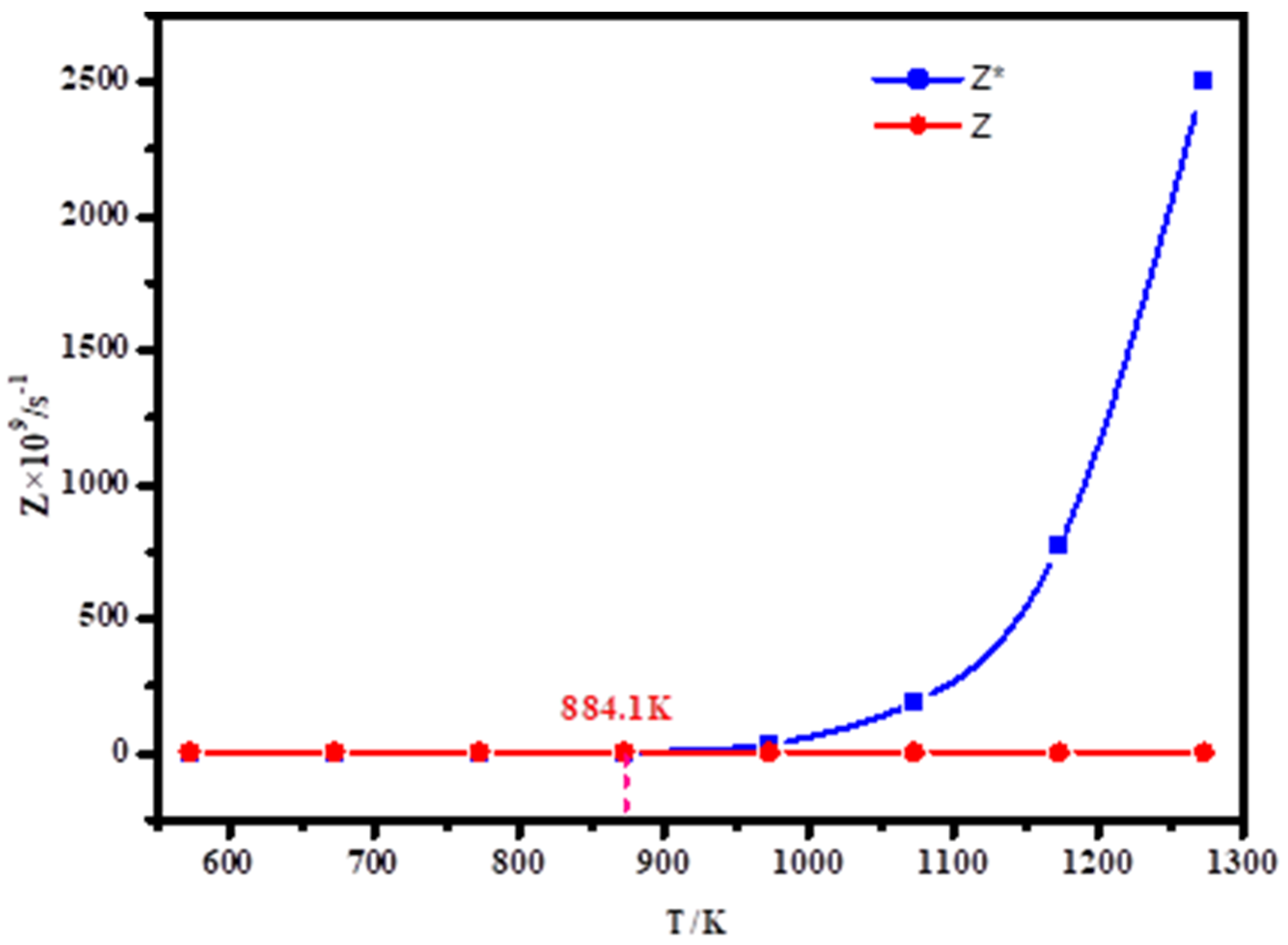
According to the formula, the relationship between atomic collision times and temperature of lithium metal vapor at saturation vapor pressure and system pressure of 10 Pa is obtained, as shown in Figure 8.
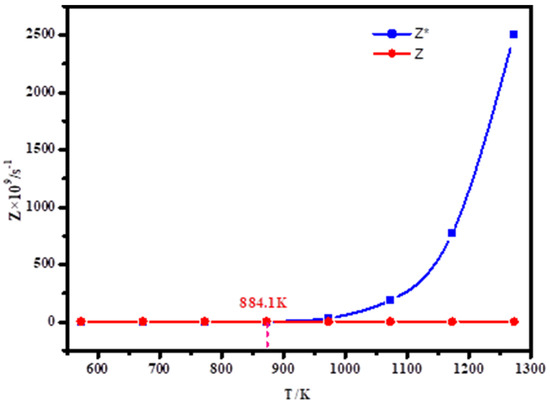
Figure 8.
Relation between number of collisions per unit time and temperature of lithium under different pressures.
Figure 8 shows the relation between the number of collisions per unit time and temperature of lithium in a range of 573 K–1273 K. Z* represents the relationship between the number of collisions of lithium atoms and temperature when the pressure is saturated vapor pressure, that is, p = p*, Z represents the relationship between the number of atomic collisions and temperature when the pressure in the system is p = 10 Pa. As can be seen from the figure, Z* generally decreases when the temperature is reduced from 1273 K to 573 K, but when the temperature is gradually reduced from 1273 K to 884.1 K, Z* drops sharply. When the temperature is lower than 884.1 K, Z* slowly decreases with the temperature. For Z, Z gradually increases as the temperature decreases. If Z* > Z, that is, when the temperature is higher than 884.1 K, the evaporation of Li atoms at this time increases, which is not conducive to Li agglomeration. If Z* < Z is, that is, when the temperature is less than 884.1 K, at this time, when the system pressure is 10 Pa, the number of collisions of Li atoms is greater than the number of collisions at saturated vapor pressure, which is conducive to Li nucleation and vapor condensation. Therefore, in the condensation of lithium vapor, when the temperature of controlling the condensation zone is lower than 884.1 K, it is conducive to the condensation of lithium metal vapor.
To sum up, the temperature of the condensation zone is selected to be lower than 884.1 K for research, and the optimal condensation temperature range of lithium vapor under 10 Pa is obtained by controlling different temperatures of the condensation zone for experiments.
3.2. Condensation Law of Pure Li
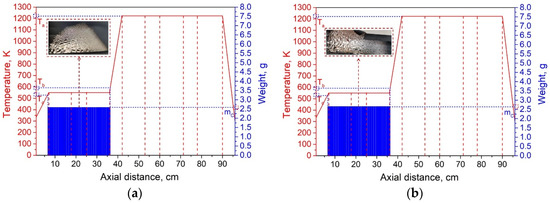
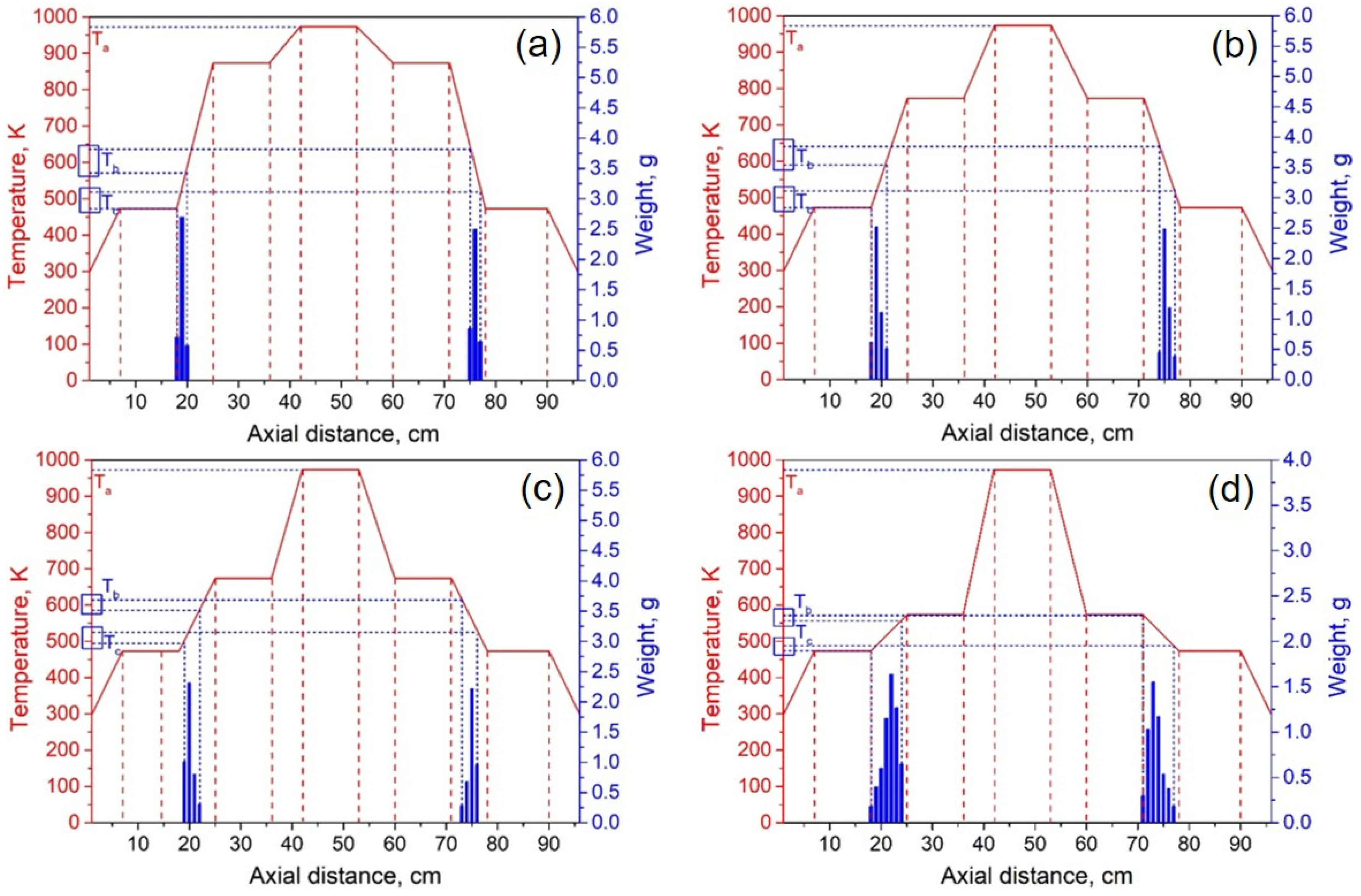
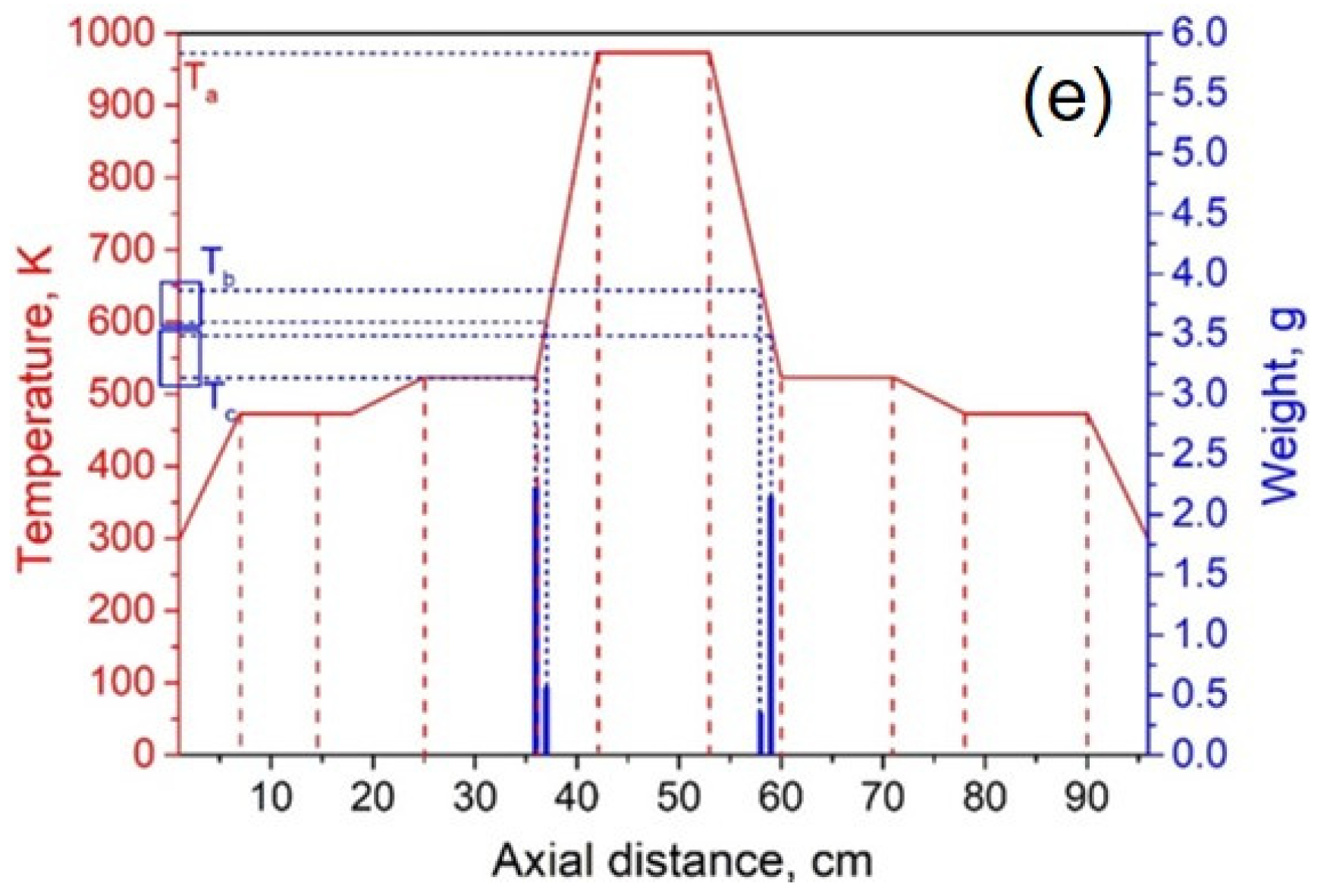
The experimental system pressure was 10 Pa. According to the theoretical results, the temperature of heating zone III was set to 973 K, and the temperatures of I and V were 473 K. By adjusting the temperature of heating zones II and IV, we investigated the effect of condensation temperature gradient on lithium condensation location at temperatures of 873 K, 773 K, 673 K, 573 K, and 523 K. Figure 9 shows the experimental results of the condensation position of lithium vapor in the quartz tube at temperatures of 873, 773, 673, 573, and 523 K in heating zones II and IV. The abscissa indicates the position of the condensing furnace from left to right, the solid red line indicates the temperature of each location in the furnace, and the blue column indicates the mass of lithium condensate at that location. Ta represents the temperature of the heating zone, Tb represents the temperature at which lithium vapor starts to condense, and Tc represents the minimum condensation temperature of lithium vapor.
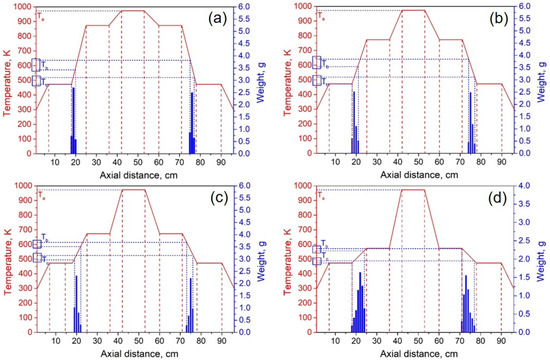
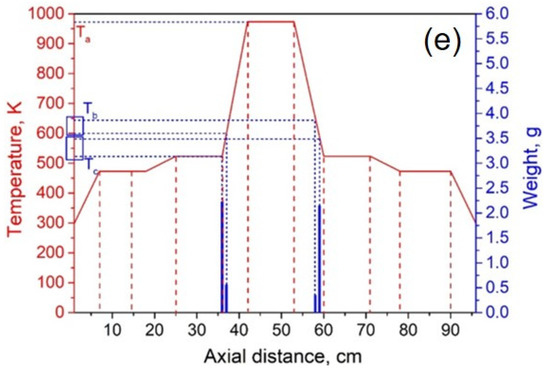
Figure 9.
Lithium vapor condensation experiment results at different temperatures of II and IV (a—873 K; b—773 K; c—673 K; d—573 K; e—523 K).
It can be seen from Figure 9a–e that as the temperature of the heating zone II gradually decreased from 873 to 523 K, the temperature gradients of III and II increased in sequence, while the gradients of I and II gradually decreased. As the temperature decreased, the position of the lithium vapor condensation zone tended to move to the right, that is, in the direction of III, and the range of condensation gradually increased. However, the location of condensation was near the heat insulation layer. When the temperature was lowered to 523 K, the location of the condensing zone changed greatly and moved to III. Since heating zone III was used as the volatilization area of the metal, the positions and temperature settings of the condensation zones on both sides were completely symmetrical. Therefore, the condensation area and temperature of the lithium vapor on both sides of III are theoretically the same. However, the experimental results found that the location of lithium vapor condensation at the vacuum pump end was farther from III than at the other side, and the condensation temperature was slightly lower than on the other side. This shows that the suction power of the vacuum pump has a certain effect on the condensation of the metal vapor. Combining the condensation temperature range of lithium vapor, we found that the optimal condensation temperature range under vacuum was 523–560 K.
3.3. Vacuum Distillation Condensation of Al-Li Alloy
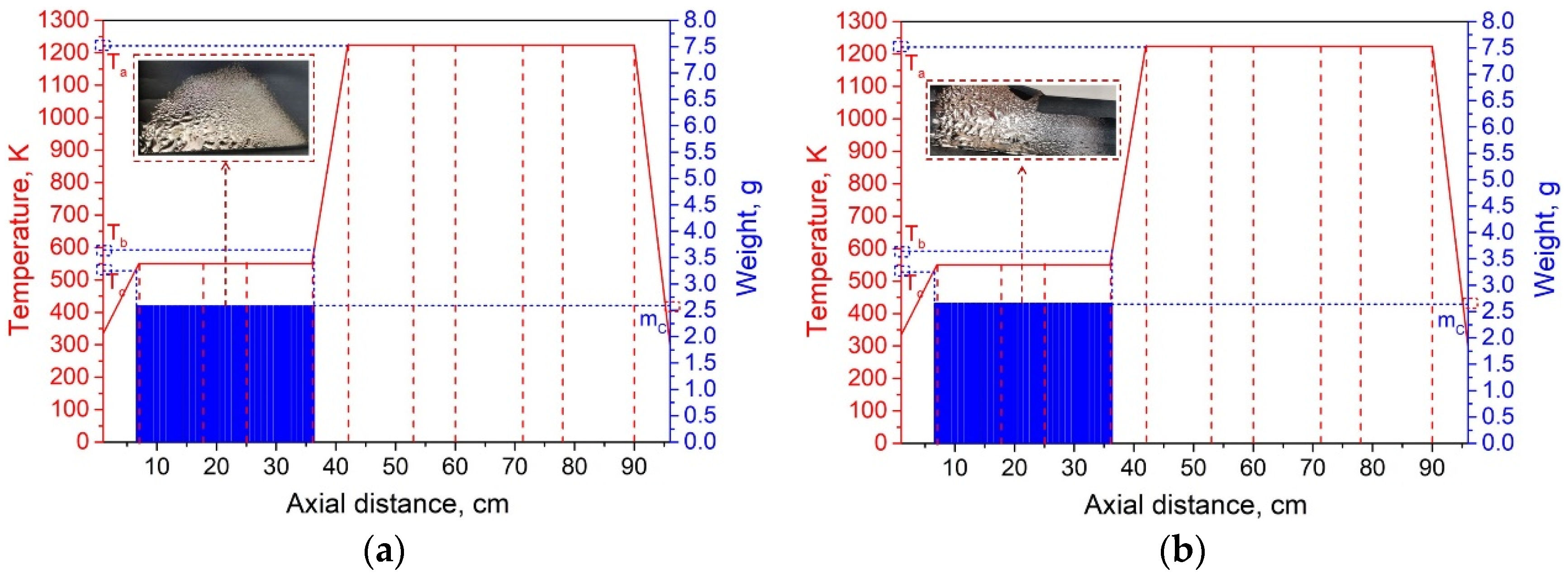
According to the results of the condensation experiment on lithium vapor, in order to condense the metal vapor volatilized under vacuum in the Al-Li alloy at a specific temperature, the temperature zones of the condensation furnace were set asymmetrically. Drawing on prior research findings [22], the system pressure was set to 10 Pa, and the experiment time was 60 min. The temperature zone of heating zones I and II was 550 K (the optimal condensation temperature of lithium vapor at 10 Pa), and the temperature of heating zones III, IV, and V was 1223 K. The Al-Li alloy was placed in heating zone III close to the condensation zone, so that the vaporized lithium vapor condensed along the condensation zone. Two sets of comparative experiments were conducted, and the results are listed in Table 5.

Table 5.
Experimental results of Al-7.87 wt% Li vacuum distillation condensation.
The distribution of the condensate of the two groups of Al-7.87 wt% Li alloy condensation experiments is shown in Figure 10. From these results, in combination with those of Table 3, we can see that the Al-Li alloy is well separated. The purity of aluminum in the residue exceeded 99.97%. The purity of lithium in the condensate exceeded 99.5%, which meets the requirements of China’s national standards for industrial lithium. The metal lithium in the condensate presents as “droplets”. The condensation temperature range is consistent with the results for elemental lithium. The direct yield of lithium was above 80%. This method, therefore, shows good prospects for industrial application.
4. Conclusions
According to the analysis of the boiling point, saturated vapor pressure of pure metals, separation factor, gas–liquid equilibrium composition diagram of the binary alloy, lithium vapor condensation rate, and nucleation and condensation of lithium vapor, the method of vacuum distillation condensation can be used to efficiently separate Al-Li alloys. The vacuum distillation condensation experiments involving lithium show that the condensation range of lithium is between 523 and 560 K at a pressure of 10 Pa. The purity of the Al-7.87 wt% Li alloy after vacuum distillation and condensation of aluminum and lithium exceed 99.97 and 99.5%, respectively.
These experimental results confirm that the method of vacuum distillation condensation can be used to efficiently separate Al-Li alloys. However, there is scope for developing this method further in regard to the separation method of azeotropic alloys and multicomponent alloys through the control of temperature gradient and extending the condensing system.
Author Contributions
Data curation, Z.P. and H.Z.; formal analysis, L.S. and H.Z.; funding acquisition, L.S., P.N. and T.Q.; investigation, P.N. and X.S.; methodology, L.S. and Z.P.; project administration, H.Z.; software, Z.P. and X.S.; supervision, P.N.; writing—original draft, L.S. and Z.P.; writing—review and editing, L.S., P.N., X.S., K.L. and T.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Yunnan Fundamental Research Projects (No. 202201AU070194), National Science Foundation of China (No. 52204362) and Analysis and Testing Foundation of Kunming University of Science and Technology (No. 2022T20210200).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, M.L.; Yan, Y.D. Lithium and Lithium Compounds; Science Press: Beijing, China, 2015; pp. 10–22. [Google Scholar]
- Qiao, Z.Y. Handbook of Rare Metal; Metallurgical Industry Press: Beijing, China, 1992; pp. 11–18. [Google Scholar]
- Atkins, P.W. Physical Chemistry; Oxford University Press: Oxford, UK, 2014; pp. 25–58. [Google Scholar]
- Sverdlin, A.; Drits, A.M.; Krimova, T.V. Aluminum-lithium alloys for the aerospace. Adv. Mater. Process. 1998, 153, 49–52. [Google Scholar]
- Babel, H.; Gibson, J.; Tarkanian, M.; Parrish, C.; Prietto, M.; Ordonez-Chu, A.; Haberl, H.; Kabisch, J.; Clark, R.; Ogrenet, J.; et al. 2099 aluminum-lithium with key-locked inserts for aerospace applications. J. Mater. Eng. Perform. 2007, 16, 584–591. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, S.C.; Duan, J.H.; Sun, J.H.; Zhong, P.Y.; Zhang, Y.J. Review of nuclear power development in China: Environment analysis, historical stages, development status, problems and countermeasures. Renew Sust Energ Rev. 2016, 59, 1369–1383. [Google Scholar] [CrossRef]
- Xie, Q.; Li, W.; Manthiram, A. A Mg-doped high-nickel layered oxide cathode enabling safer, high-energy-density Li-ion batteries. Chem. Mater. 2019, 31, 938–946. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. ChemInform Abstract: Optimized LiFePO4 for Lithium Battery Cathodes. ChemInform 2010, 32. [Google Scholar] [CrossRef]
- Arnold, A.; Sauermann, R.; Friedrich, B. Recycling of Li-containing aluminum alloys. Light Met. Age 2011, 69, 32–37. [Google Scholar]
- Collins, B.D.; Jibson, R.W. Assessment of Existing and Potential Landslide Hazards Resulting from the April 25, 2015 Gorkha, Nepal Earthquake Sequence; US Geological Survey: Menlo Park, CA, USA, 2015. [CrossRef]
- Ostroushko, Y.I. Lithium, Its Chemistry and Technology; US Atomic Energy Commission, Division of Technical Information: Washington, DC, USA, 1962.
- Smeets, A.A.J.; Fray, D.J. Extraction of lithium by vacuum thermal reduction with aluminum and silicon. Trans. Inst. Min. Metall. Sect. C-Miner. Process. Extr. Metall. 1991, 100, 42–55. [Google Scholar]
- Chen, W.L. Research on Vacuum Refining of Lithium and Preliminary Study on Vacuum Carbothermal Reduction of Lithium Oxide; Kunming University of Science and Technology: Kunming, China, 2000. [Google Scholar]
- Lee, H.J.; Cagin, T.; Goddard, I.W.A.; Johnson, W.L. Molecular dynamics simulations of glass formation and crystallization in binary liquid metals. J. Metastable Nanocryst. Mater. 2003, 15–16, 181–186. [Google Scholar] [CrossRef]
- Kluge, M.D.; Ray, J.R.; Rahman, A. Amorphous-silicon formation by rapid quenching: A molecular-dynamics study. Phys. Rev. B 1987, 36, 4234–4237. [Google Scholar] [CrossRef] [PubMed]
- Holender, J.M. Molecular-dynamics studies of the thermal properties of the solid and liquid fcc metals Ag, Au, Cu, and Ni using many-body interactions. Phys. Rev. B 1990, 41, 8054–8061. [Google Scholar] [CrossRef] [PubMed]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book; Elsevier Press: Amsterdam, The Netherland, 2003. [Google Scholar]
- Herrmann, H.; Bucksch, H. Dictionary Geotechnical Engineering/Wörterbuch GeoTechnik: English-German/Englisch-Deutsch; Springer: Berlin/Heidelberg, Germany, 2014; p. 229. [Google Scholar]
- Yang, C.C.; Li, J.C.; Jiang, Q. Effect of pressure on melting temperature of silicon determined by Clapeyron equation. Chem. Phys. Lett. 2003, 372, 156–159. [Google Scholar] [CrossRef]
- Surhone, L.M.; Tennoe, M.T.; Henssonow, S.F. Antoine Equation; Betascript Publishing: Beau Bassin, Mauritius, 2011. [Google Scholar]
- Dai, Y.N.; Yang, B. Vacuum Metallurgy of Non-Ferrous Metals; Metallurgical Industry Press: Beijing, China, 2009; pp. 508–514. [Google Scholar]
- Shi, L.; Jia, L.; Ning, P.; Sun, X.; Wang, C.; Ma, Y.X.; Wang, F.; Qu, T.; Li, K. Vacuum distillation and ab initio molecular dynamic simulation of Al–Li alloys. Vacuum 2023, 210, 111877. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).