Abstract
Honey intake is advantageous to human health due to its antioxidant, anticancer, anti-inflammatory, and antimicrobial properties, all of which are attributed to the rich bioactive compound contents. Moreover, hepatoprotective, wound healing, and gastrointestinal protective properties have been documented. Honey’s nutritional value is significantly affected by its chemical composition, which varies depending on botanical and geographical origin. In particular, after Manuka honey, Sidr honey from the Ziziphus species is the most popular. The chemical compositions, physicochemical properties, bioactive compounds, and sensory characteristics of two Sidr honey samples from Egypt and Saudi Arabia were investigated in the current study. Moisture content, electrical conductivity (EC), pH, free acidity (FA), total acidity, lactone hydroxymethylfurfural (HMF) content, and diastase (α-amylase) activity were measured. By using high-performance liquid chromatography (HPLC), mass spectrometry (LC-MS/MS), nuclear magnetic resonance (1HNMR), and solid-phase micro-extraction (SPME) coupled with gas chromatography (GC-MS) analyses, the sugar profile, non-volatile, and volatile compounds were also identified. The physicochemical analysis revealed the following results for Sidr honey from Saudi Arabia and Egypt, respectively: a moisture content of 18.03 ± 0.05% and 19.03 ± 0.06%, EC values of 1.18 ± 0.05 and 1.16 ± 0.01 mS/cm, pH values of 4.87 ± 0.08 and 5.10 ± 0.01, FA of 37.50 ± 0.05 and 36.50 ± 0.05 meq/kg, total acidity of 41.06 ± 0.05 and 37.50 ± 0.05 meq/kg, lactone of 3.49 ± 0.005 and 1 ± 0.0 meq/kg, HMF of 20.92 ± 0.02 and 11.33 ± 0.01 mg/kg, and diastase of 59.97 ± 0.05 and 8.64 ± 0.06g/100 g. Honey from Saudi Arabia and Egypt displayed 22.51 ± 0.05 and 26.62 ± 0.16 % glucose, 40.33 ± 0.06 and 35.28 ± 0.01% fructose, 8.94 ± 0.17, and 8.87 ± 0.01% sucrose, and 8.22 ± 0.006 and 8.13 ± 0.01% maltose, respectively. According to the International Honey Commission (IHC) and GCC Standardization Organization (GSO) regulations, the levels of glucose, fructose, sucrose, and maltose were near the standard levels. Flavonoids, sugars, vitamins, and nitrogen contents were additionally measured using LC-MS/MS, whereas GC-MS was employed to identify aldehydes, ketones, phenols, acids, esters, anthraquinone, hydrocarbons, and nitrogenous compounds. The results of a study on the effect of honey’s geographic origin on its broad quality are summarized. As a result, knowing its optimal chemical and physical characteristics served as the criterion and indicator of the honey’s quality.
1. Introduction
Honey is a delicious viscous fluid food manufactured by Apis mellifera L. domestic bees. Honey is a highly appreciated and commonly consumed nutritious food that has been utilized for many centuries as a natural sweetener or flavoring for mixtures [1]. Honey belongs to the most complex natural products because it contains approximately 200 substances, the majority of which are carbohydrates, particularly reducing sugars such as fructose and glucose. Honey contains enzymes, proteins, free amino acids, minerals, vitamins, phenolic acids, flavonoids, organic acids, other organic acids, as well as other phytochemicals in small amounts [2]. Honey’s bioactive components have been connected to its health-promoting and nutritional properties. Honey seems to have antioxidant, anticancer, anti-inflammatory, antimicrobials well as antidiabetic, anti-obesity, and wound-healing properties [3,4,5,6,7,8,9,10] and have been used and proposed against viral diseases such as the COVID-19 virus [11,12]. In animal and human models, honey has effects on the cardiovascular system and has been revived as a therapy for gastrointestinal diseases, and asthma [13,14,15].
Honey’s chemical composition is claimed to be influenced by geographical and floral conditions [16], seasonal variations, as well as the manufacturing, handling, and storage processes [17]. Thus, the chemical composition, physical qualities, sensory attributes, and medicinal properties of honey from diverse botanical sources vary widely [18]. Chromatographic and spectroscopic techniques such as high-performance liquid chromatography (HPLC), ultraviolet spectroscopy (UV), near-infrared (NIR), tandem MS (MS/MS), LC-MS/MS, solid-phase micro-extraction (SPME) coupled with gas chromatography (GC-MS), mass spectrometry (MS), nuclear magnetic resonance (NMR) and chemometric analyses were used to identify the distinct characteristics of honey [19,20,21,22,23]. For instance, honey quality is determined by its moisture contents, electrical conductivity (EC), pH, free acid, insoluble matter, ash, carbohydrate level, sucrose-specific rotation, sensory, and microbiological properties [19,20,21]. The identification of honey components’ health-promoting criteria not only helps to maintain the price of honey or increase customer preference, but also helps to facilitates control/verification procedures. Accordingly, monofloral honeys have a higher market value than multifloral honeys. This is due to the distinct aroma profiles of multifloral honeys, which are the result of their unique volatile compounds composition, in addition to the rich medicinal and dietary values [24].
Sidr honey is a type of monofloral honey from the Ziziphus species, gaining popularity after Manuka honey [25]. Sidr, also known as Jujuba, grows mostly in the desert areas of Pakistan, Libya, Saud Arabia, Egypt, and Yemen [26,27,28,29]. Because of its scarcity and high price, Sidr honey is frequently adulterated [30]. As a result, determining authenticity is critical for the economy as well as consumer and producer safety. Honey’s quality is determined by its botanical source and chemical composition, which are also used to boost sales through specific labelling, such as monofloral, multifloral, and organic honeys. Researchers were assigned the task to ensure the accurate labelling of the honey type for customers, the honey industry, as well as food law enforcement organizations [31]. Researchers should also suggest techniques and metrics for assessing whether honey complies with legal requirements. In recent decades, a great deal of research has been conducted in an effort to find a suitable chemical marker reflecting the unique characteristics of honey associated with its origin. These investigations focus on the physical and chemical characteristics of honey [18,32,33,34].
The purpose of this research was to identify Sidr honey samples from Egypt and Saudi Arabia based on physicochemical qualities utilizing modern techniques, i.e., HPLC, LC-MS/MS, NMR, and SPME-GC-MS as part of our ongoing project, with particular emphasis on honeybees and bee products [35,36,37,38,39,40,41,42].
2. Materials and Methods
2.1. Honey Sampling
One kilogram of Saudi Arabian Sidr honey was obtained in 2020 from a regional honey market in Riyadh, Saudi Arabia, and Egyptian Sidr honey sample was collected directly from a beekeeper from Luxor areas of Upper Egypt. The honey samples were kept at 4 °C in glass jars in the dark until they were used for further analysis.
2.2. Standard Physicochemical Parameters and Pollen Analysis
The following parameters were determined for the Sidr honey samples: water content, EC, pH, free acidity (FA), total acidity, lactone, hydroxymethylfurfural (HMF) content, and diastase. All procedures have been carried out according to the International Honey Commission (IHC) regulations [43].
The moisture was determined using a digital refractometer PAL22S (Atago, Tokyo, Japan). In brief, the honey was dissolved in a water bath at 50 °C. The refractive index was measured at 20 °C after 6 min of equilibration [18].
At 20 °C, 10 g of honey was dissolved in 50 mL of distilled water. The EC cell was used for measurement. The EC values were given in mS/cm [44].
A pH meter (Mettler Tolledo, Columbus, OH, USA) was used to determine the pH value. After diluting 25 g of honey in 75 mL of water, the pH value was measured [45].
FA was calculated using the official IHC method. In 75 mL of distilled water, 10 g of honey sample was dissolved. The solution was then titrated with 0.1 M sodium hydroxide to a pH of 8.30. The results were given in meq/kg. Moreover, HMF content was measured using the spectrophotometric technique according to IHC guidelines [43].
Diastase activity was assessed as follows: A 50 mL volumetric flask was filled with 10 g of honey samples, 5 mL of acetate buffer, and 15 mL of water. The solution was diluted to 50 mL with distilled water after adding 3 mL of sodium chloride (0.5 M). An amount of 5 mL of starch solution was added to 10 mL of honey solution after warming the cocktail to 40 °C. Every 5 min, an aliquot was collected and added to 10 mL of iodine solution. The absorbance was determined at 660 nm using a double beam UV–visible spectrophotometer directly against water blank [46].
Pollen analysis was determined via first dissolving 10 g of honey sample in 20 mL of distilled water, then have it in a water bath of 45 °C, prior to centrifugation for 15 min at 1375 g (3500 rpm), after which the supernatant was discarded. The precipitate was immersed in 10 mL of distilled water and centrifuged for another 5 min. The pollen particle deposit was dispersed on a slide. The slides were then placed on a hot plate for 10 min. After drying, a drop of glycerin gelatin was dropped into it, which was then covered with a cover slip for identification [47].
2.3. Sugar Analysis
The reducing sugars (mainly fructose and glucose), as well as the sucrose and maltose content, were all measured using HPLC [48]. Sugar was analyzed at the laboratory of the Plant Protection Research Institute in Egypt. For carbohydrate separation, an APS-2 HypersilTM column (4.6 × 150 mm, particle size 5 µ, Thermo ScientificTM) equipped with a refractive index detector (RID) was used. The injection volume was 20 µL, and the flow ratio was 1 mL/min. In the solvent system, acetonitrile (ACN)/water ratio was 80:20 (v/v). The content of each sugar was expressed as g/100 g honey. The peak quantification involved duplicate injections and the use of average peak areas. The sugar content of honey was calculated using standards such as glucose, fructose, sucrose, and maltose.
2.4. Chemical Identification of the Compounds Using LC-LTQ-MS-MS
LC-MS/MS was used to analyze honey samples. A Shimadzu LC-10 HPLC was used, along with a Grace Vydac Everest Narrowbore C-18 column (100 mm × 2.1 mm i.d., 5 µm, 300 Å). Thermo Finnigan’s LTQ Linear Ion Trap MS (San Jose, CA, USA) was used with LC-MS/MS, which has a mass range of 100–2000 m/z. An autosampler was used to inject a 2 µL sample. The following 40 min method was applied: A gradient was run for 30 min until 95% of ACN and 0.05% of FA were obtained after a 5 min isocratic run, using 5% ACN and 0.05% formic acid (FA). The column was then conditioned for 5 min with 5% ACN and 0.05% FA.
Foundation 3.1 Xcalibur 3.1.6610 was used to process and analyze the data. Additionally, MSConvert from the ProteoWizard suite (https://proteowizard.sourceforge.io/download.html; accessed on 25 December 2022) was used to convert the raw data files to mzXML format. The molecular network was created using the global natural products social molecular networking (GNPS) online workflow [42,49]. The spectra from the network were then verified against GNPS’s spectral libraries and literature data.
Cytoscape 3.5 was used to display the networks. The molecular networks were edited and analyzed using the Cytoscape program. Each node had a label that was the parent mass. The sources of the samples are indicated by colors on a pie slice that is proportional to the number of MS/MS spectra for each parent mass.
2.5. 1H-NMR Analysis
On a Jeol EX-600 spectrometer operating at 600 MHz, 1H-NMR spectra were captured [50]. Chemical shifts were referenced to the solvent peak for CD3OD at δ1H at 3.3 and 4.8 ppm.
2.6. Sampling of Volatile Compounds
A 100 mL Erlenmeyer flask (E-flask) was filled with approximately 4 g of honey. Aluminum foil was used to seal the E-opening flasks before being further tightened with a rubber band. The honey was equilibrated for 60 min at room temperature (252 °C) before the volatile compounds were collected from it. Using the SPME method, substances released from the honey were collected for 4 h (Supelco, Bellefonte, PA, USA). Immediately following the collection of volatiles, the SPME fiber was retracted, and the SPME needle was injected into a gas chromatography (GC) injector. For 5 min, the GC injector desorption was applied and SPME fiber was cleaned. Each sample was used at least three times under identical circumstances. The phytochemicals sampled from a headspace on SPME fiber were considered as volatile compounds.
2.7. GC-MS Analysis
To separate the volatile components, the samples were injected into a Hewlett Packard GC 6890 N chromatograph (Agilent Technologies Inc., California, CA, USA) fitted with a DB-5 column (30 m length, 0.25 mm internal diameter, and 0.25 μm stationary-phase film thicknesses). The temperature of the GC injector was 250 °C and remained constant isothermally throughout the analysis. The temperature of the GC oven was held isothermally at 40 °C for 2 min, then increased by 4 °C/min to 200 °C, and increased again by 10 °C/min to 280 °C, and finally held at 280 °C for 10 min phase with a constant flow of 1 mL/min. The mass spectrometer’s ion source was run at 230 °C with a solvent delay of 5 min and an electron ionization energy of 70 eV. By comparing the mass spectra and retention indices of each compound to those in the NIST-2008 MS library, all compounds were identified. A relative percentage of the total peak area is used to express the sample’s quantitative composition.
3. Results and Discussions
3.1. Physicochemical Parameters and Palynological Characteristics
Honey physicochemical characteristics are good indicators of its quality and a useful tool for the botanical identification of the honey [21]. Table 1 shows the moisture content, EC, pH, FA, total acidity, lactone, HMF, and diastase activity of the investigated samples. Moisture content is a key factor in yeast fermentation and is accordingly parameter to measure vulnerability or resistance to spoilage; moreover, as stated by the EU Directive (110/2001), it should not be more than 20% following processing and storage conditions [51,52]. In this study, the moisture content of Sidr honeys ranged from 18.03 ± 0.05 to 19.03 ± 0.06% for Saudi Arabia and Egypt, respectively [51,52]. Sidr honey from the Republic of Yemen ranged in moisture content from 13.4 to 16% [18]. Sidr honey from various origins was reported to have a moisture content that ranged from 14 to 17% [18].

Table 1.
The physicochemical parameters of the Sidr honey samples.
EC is frequently used as an alternative to ash content in routine quality control because of its close relationship to its ionic and organic acid content. Two samples of Sidr honey have high EC mS/cm of 1.18±0.05 and 1.16±0.01 for Saudi Arabia and Egypt, respectively. EC values are high compared to other honey samples from different regions of Yemen, ranging between 0.21 and 0.75 mS/cm [18]. The highest accepted value as recommended by the Council of the European Union is 0.8 mS/cm. In favor for our findings, earlier studies have suggested that the higher the EC value, the greater the mineral and acid contents [18,54,57]. EC value is determined for various samples from different regions where the EC value ranges from 0.4 to 1.18 mS/cm [18,26].
The honey’s pH values were 4.87±0.08 and 5.10±0.01 for Saudi Arabia and Egypt, respectively, which fall within the standard range established by the Codex Alimentations of 3.7 to 6 [55]. According to geographical variation, Roshan et al. reported that the pH values of various samples of Sidr honey range from 4.8 to 6.96 [18]. Hegazi et al. studied 794 samples of Sidr honey that were imported into the Saudi market from 12 different countries, and found variation of pH values of 3.6 (Egypt), 5.4 (Saudi Arabia), and 7.4 (Yemen) [26]. The pH of the Saudi Arabian Ziziphus honey on the other hand was 6.14 [58].
Honey’s acidity is caused by the relevance of organic acids, particularly citric acid, acetic acid, formic acid, oxalic acid, succinic acid, gluconic acid, pyruvic acid, tartaric acid, lactic acid, and maleic acid [59]. The majority of the acid in honey is gluconic acid. Organic acids and the acidity of honey have a very significant positive association [60]. The acidity of honey plays a role in its antimicrobial properties and stability [61]. FA levels in Sidr honey from Egypt and Saudi Arabia were 36.50 ± 0.05 and 37.50 ± 05 meq/kg, respectively, as shown in Table 1. FA exceeded the acceptable limit (50 meq/kg) in Talh honey derived from the Talh tree, Acacia gerrardii Benth, while Romanian acacia honey revealed the lowest value of FA at 8.6 meq/kg. FA is associated with the origin plant’s nature and storage conditions. FA is significantly correlated with relative humidity and EC, but not with pH, and it is known to be completely irrelevant to honey quality and maintains the freshness of honey [62,63,64]. For Egyptian and Saudi Arabian honey, the amounts of free lactone were 1 ± 0.0 and 3.49 ± 0.005 meq/kg, respectively. The significant variation in lactone acidity in honey is primarily caused by the harvest year and botanical origin of secondary nectar. The maximum permitted amount is 50.00 meq/kg [34,55]. Lactones have a sweet aroma with a faintly sour undertone. Lactones contributed significantly to the overall aroma of honey and help to explain some of its exceptional resistance to microbes [65,66].
The HMF is a relative marker of honey freshness and quality. HMF concentrations in honey samples from Egypt and Saudi Arabia were 11.33 ± 0.01 and 20.92 ± 0.02 mg/kg, respectively. The Codex Alimentarius Standard Commission has set the maximum limit for HMF in honey as 40 mg/kg. Similarly, GCC Standardization Organization (GSO) recommends 80 mg/kg for honeys originating from tropical areas. HMF value higher than 40 mg/kg is associated with the hot weather and long harvest time, implying that the sugar contents have been heated and/or processed [67,68,69], while values lower than 40 mg/kg indicate a relative freshness.
Egyptian and Saudi Arabian honey, respectively, showed a diastase activity of 8.64 ± 0.06 and 59.97 ± 0.05 g/100 g. In the current study, the samples’ diastase values fell within the acceptable range [53,55]. One of the key markers of honey freshness is diastase activity. The storage environment and heat treatment of honey affect diastase activity [70,71].
Pollen is a key ingredient in honey analysis. Pollen species are commonly used to identify the floral nectar sources that bees use for producing honey. The melissopalynological investigation of honey samples employing microscopical examination indicated the presence of the typical pollen grains. Sidr honey is distinguished by the presence of Ziziphus pollen [54,72]. In Saudi Arabia and Egypt, Ziziphus sp. (80 vs. 60%) was determined. In addition, 30% Sesamum indicum and 10% Trifolium sp. were determined for Egyptian honey, respectively [26,73]. The presence of Phoenix dactylifera, S. indicum, and Trifolium sp. distinguishes Saudi Arabian Sidr honey [73]. Relative pollen frequency is typically used to verify and label a honey sample according to the primary and minor nectar sources. The diversity of pollen types in honey reflected the broad spectrum of nectar sources present in the region where bees produce honey [73,74].
3.2. Main Sugar Profile
As shown in Table 1, sucrose, maltose, glucose, and fructose concentrations were reasonable, indicating that the honey’s total sugar content must be at least 60 g/100 g. The content of sucrose, maltose, glucose, and fructose were 8.13 ± 0.01 vs 8.22 ± 0.006, 8.87 ± 0.01 vs. 8.94 ± 0.17, 26.62 ± 0.16 vs. 22.51 ± 0.05, and 35.28 ± 0.01 vs. 40.33 ± 0.06 (g/100 g) of Egyptian and Saudi Arabian honey, respectively [75,76].
The average fructose and glucose contents of Algerian jujube (Ziziphus lotus Lam) honey were 40.8% and 30.7%, respectively [72]. Omani Sidr honey contained 17.0–27.5% and 23.9–38.9%, respectively, of glucose and fructose [69]. The fructose to glucose ratio in Egyptian and Saudi Arabian honey was 1.32 and 1.79, respectively, whereas the optimal range is known to be between 0.9 and 1.35 [75,76]. Fructose-to-glucose ratios were less than 1.0, resulting in a faster honey crystallization, as the fructose/glucose ratio and humidity are indicators of honey’s tendency to crystallize [48,77,78]. The crystal with the highest van der Waals and electrostatic interactions formed when the fructose/glucose ratio was 1.1 [79]. Maltose and sucrose were regarded as minor sugars in samples of honey. Honey’s sucrose and maltose contents may reveal information about adulteration and the honey’s botanical source [80,81]. The upper limits for sucrose and maltose were 10% and 2–16%, respectively, according to GSO [26,53]. For Sidr honey from Oman, the range of sucrose content was 0.1–17.5% [69]. Sidr honey from Saudi Arabia had maltose content values ranging from less than 1% to 3% [82,83].
3.3. Metabolites Profile Using LC-MS/MS Analysis
Utilizing GNPS networking and MS-MS data in positive ionization mode, the metabolomic mass profiles of honey were determined (Table 2 and Figure 1 and Figure 2). The elution gradient of ACN and acidified water proved effective in 40 min. MS/MS experiments in positive ionization mode, as well as comparisons with data from the literature and databases such as the Natural Products Dictionary, PubMed, and GNPS [19], aided in the identification of 10 chemical constituents, as reported in Table 2, where the corresponding retention time, molecular formula, molecular ion [M+H]+, and MS/MS fragment ions were also enlisted. The compounds are classified into flavonoids (4), carbohydrates (3), vitamins (2), and prenol lipids (1). Compounds no. 1, 2, 4,5, 6, 8, and 9 have already been reported in the literature [68,84,85,86,87,88]. Compound 1 showed ion [M+H]+ at m/z 290.27 corresponding to the molecular formula C15H14O6, was identified as catechin, and was found in both Sidr honey from Egypt and Saudi Arabia. Catechin is a flavonoid found in many natural sources, including honey, and possesses a variety of biological properties such as antioxidant, anticancer, and anti-inflammatory properties [89,90,91]. Eriodictyol is another flavonoid with m/z of 288.25 Da, MF C15H12O6, and a fragment ion at m/z 270.9870 [M-18], 253.0110 [M-36], 162.9830 [M-124], 144.9750 [M-143], and 116.9910 [M-172] due to the loss of OH−1, H2O22−, C6H4O32−, C6H7O42−, and C7H8O5, respectively [88].

Table 2.
The parent masses and fragments of the identified metabolites from the raw mass spectrum of honey samples from Egypt and Saudi Arabia.
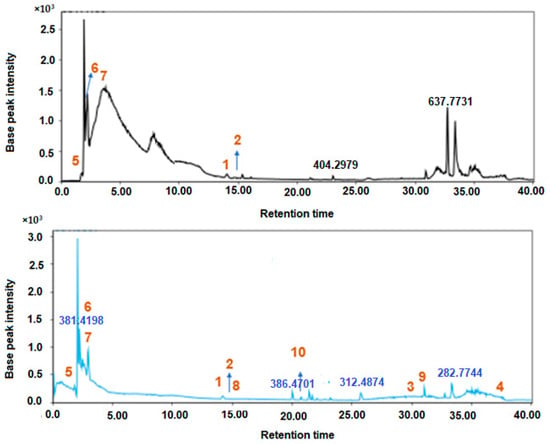
Figure 1.
LC-MS/MS chromatography of identified compounds from Sidr honey from Egypt (A) and Saudi Arabia (B). 1: catechin, 2: 5,6-dihydroxy-7,3’,4’-trimethoxyflavone, 3: isovitexin, 4: eriodictyol, 5: sucrose, 6: maltotetraose, 7: maltose, 8: biotin, 9: vitamin E, 10: (2S,3S,4S,5R,6R)-6-[[(3S,4S,6aR,6bS,8aR,9R,12aS,14bR)-9-hydroxy-4-(hydroxymethyl)-4,6a,6b,8a,11,11,14b-heptamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl]oxy]-5-[(2S,3R,4S,5R,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-3,4-dihydroxyoxane-2-carboxylic acid.
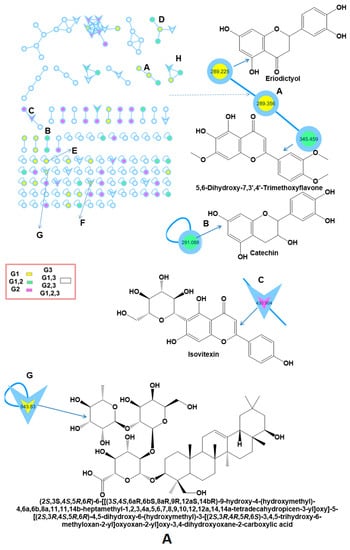
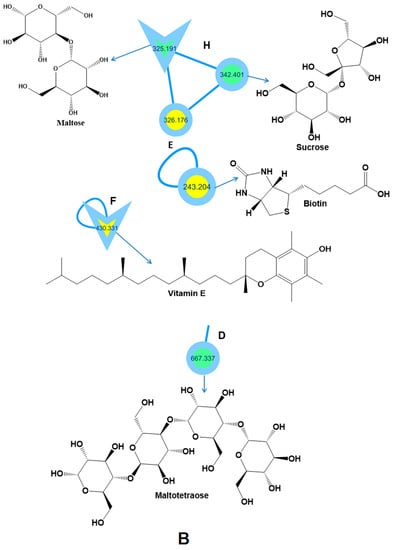
Figure 2.
Molecular networking was created for honey samples using MS/MS data in positive ionization mode. The parent mass is assigned to all nodes. The network is represented as a pie chart, with yellow (G1), green (G1,2), pink (G2), and white colors (G3, G1,2, G1,3, C1,2,3) representing the distribution of precursor ion intensity in honey from Saudi Arabia (G1), Egypt, shared compounds between honey samples, and blank solvent, respectively. The parent ions identified in the GNPS molecular network are represented by the triangle nodes. A: Flavonoids and prenol lipids have been detected. B: Sugars and vitamins were determined.
Carbohydrates constitute roughly 95% of the dry weight of honey. Sucrose and maltose (disaccharides), melezitos (trisaccharides), and maltotetraose (tetrasaccharides) were determined using LC-MS/MS analysis [68,92]. Finally, Vitamin E and maltose were identified utilizing the literature data and the GNPS library [68,88].
3.4. NMR Analysis
As shown in Figure 3, the 1H-NMR profiles of honey samples are divided into three regions based on the chemical shift, namely the aliphatic region (δ 0–3 ppm), the carbohydrate region (δ 3–6 ppm), and the aromatic region (δ 6-10 ppm). At δ 2.50 (s), the presence of CH2 adjusted to the carboxylic group could indicate the presence of succinic acid in the aliphatic region. As expected, the most intense and dominant signals in 1H-NMR spectra of honey are in the sugar regions (δ 3.0–5.5 ppm), and typically glucose and fructose are the most intense and dominant signals. The presence of anomeric proton at δ 4.59 ppm (d) and δ 5.22 ppm (d), respectively, supported the occurrence of β- glucose and α-glucose. The signals at δ 4.20 (m) and 3.65 ppm (m) indicate the presence of fructose in both samples, whereas the peak at δ 5.45 ppm (d) indicates sucrose. The two honey samples showed signals in the region δ 8.40 (s) of formic acid. In Egyptian honey, the signal at δ 6.85 (d) indicated the presence of HMF [94].
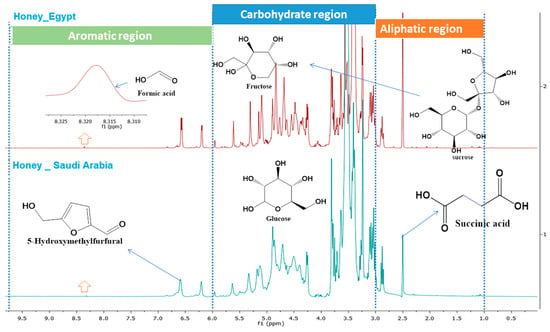
Figure 3.
Selected characteristic signals of the 1H-NMR as seen in the Egyptian and Saudi Arabian honey samples.
3.5. GC-MS Analysis
After performing a concise pretreatment, SPME was used to analyze the volatile and semi-volatile compounds found in samples of honey. A combination of GC-MS and SPME offers more information, including retention time (Rt), mass fragments for the volatile compounds, and more precise qualitative and quantitative results. The analysis of volatile compounds in foods, including fruits, vegetables, tea, wine, and honey, has been extensively conducted using this technique [47]. In the current study, 37 volatile compounds were identified and grouped into aldehydes (64.67 vs. 32.8%), ketones (16.54 vs. 32.04%), phenols (10.31 vs. 4.45%), acids and esters (7.46 vs. 23.01%), anthraquinone (0 vs. 6.38%), hydrocarbon (0.59 vs. 0%), and nitrogenous compounds (0.37 vs. 1.06%) for honey from Saudi Arabia and Egypt, respectively (Table 3).

Table 3.
Identified volatile metabolites of honey samples using SPME coupled with GC-MS analysis.
Both honey samples yielded benzeneacetaldehyde, nonanal, and isovaleric acid. The major constituents were: benzeneacetaldehyde (6.19 vs. 32.06%), 2-furaldehyde, 5-methyl- (22.91 vs. 0%), furfural 5-methyl- (0 vs. 21.21%), 5-formylfurfural (13.28 vs. 0%), isovaleric acid (1.50 vs. 10.48%), and ethylmethylacetic acid (0 vs. 11.84%) for honey from Saudi Arabia and Egypt, respectively. Benzeneacetaldehyde was identified previously in chestnut, heather, honeydew, orange blossom, citrus, eucalyptus, and thyme honeys [32,95], as well as from Sidr honey [96]. Isovaleric acid was also identified in the marmeleiro and in buckwheat honey [65,97]. Both furfural, 5-methyl-, and 2-furaldehyde were extracted from honey [98,99]. Heat processing during SPME fractionation of honey volatiles could result in the presence of furan derivatives (furfural 5-methyl- and 5-formylfurfural). Carbohydrates are also responsible for the formation of furan derivatives such as HMF. Pentoses and hexoses in honey degrade in a slow enolization and a fast elimination of three molecules of water to form unfavorable compounds such as furans when they are heated or kept for a long time [100,101]. The two main recognized furans are furfural, which is derived from pentoses, and 5-HMF, which is derived from hexoses, such as glucose and fructose. These furans are used as indicators of food heat treatment [102].
4. Conclusions
Sidr honey has become increasingly popular due to its diversity in chemical composition. The physical and chemical properties of Sidr honey are determined using a combination of chromatographic and spectroscopic techniques such as high-performance liquid chromatography (HPLC), mass spectrometry (LC-MS/MS), nuclear magnetic resonance (1H-NMR), and solid-phase micro-extraction (SPME) coupled with gas chromatography (GC-MS). Based on the International Honey Commission (IHC) and GCC Standardization Organization (GSO) regulations, the values of moisture, electrical conductivity (EC), pH, free acidity (FA), total acidity, lactone, hydroxymethylfurfural (HMF), diastase, and sugars (glucose, fructose, sucrose, and maltose) are perfectly detected within the recommended levels [43]. The honey samples contained a significant number of phytoconstituents that were identified. Ten compounds from various classes of compounds, mainly flavonoids, were recognized by LC-MS/MS analysis. GC-MS analysis revealed 37 volatile compounds, the most abundant of which was benzeneacetaldehyde in Egyptian honey and furfural, 5-methyl- in Saudi Arabian honey. Overall, our research has aided in the assessment of the Sidr honey quality criteria of two geographical locations, namely Egypt and Saudi Arabia. The work offers basic knowledge about the ingredients for distinguishing the honeys and may serve as a helpful basis for future research. Further studies using more specimens of Sidr honey from different locations may clearly show how climate and region affect the quality of the honey.
Author Contributions
Conceptualization, H.R.E.-S.; methodology, H.R.E.-S., A.A.A.E.-W., N.Y., E.H.R. and S.A.M.K.; validation, H.R.E.-S.; writing—original draft preparation, A.A.A.E.-W., M.F.A. and S.A.M.K.; writing—review and editing, H.R.E.-S., E.H.R., K.W., A.S., C.Z., Y.A.N., S.G.M., Z.G., N.Y. and S.A.M.K.; supervision, H.R.E.-S. and S.A.M.K.; project administration, H.R.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors acknowledge the generous support from the Researchers Supporting project number (RSP 2023R122), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ioniță-Mîndrican, C.B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.E.; Mireșan, H.; et al. Honey and other beekeeping products intake among the Romanian population and their therapeutic use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Zammit Young, G.-W.; Blundell, R. A review on the phytochemical composition and health applications of honey. Heliyon 2023, 9, e12507. [Google Scholar] [CrossRef]
- Edet, U.O.; Mbim, E.N.; Ezeani, E.; Henshaw, O.U.; Ibor, O.R.; Bassey, I.U.; Asanga, E.E.; Antai, E.E.; Nwaokorie, F.O.; Edet, B.O.; et al. Antimicrobial analysis of honey against Staphylococcus aureus isolates from wound, ADMET properties of its bioactive compounds and in-silico evaluation against dihydropteroate synthase. BMC Complement Med. Ther. 2023, 23, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Ugusman, A.; Shahrin, S.A.S.; Azizan, N.H.; Pillai, S.B.; Krishnan, K.; Salamt, N.; Aminuddin, A.; Hamid, A.A.; Kumar, J.; Mokhtar, M.H. Role of honey in obesity management: A systematic review. Front. Nutr. 2022, 9, 924097–924108. [Google Scholar] [CrossRef] [PubMed]
- Frydman, G.H.; Olaleye, D.; Annamalai, D.; Layne, K.; Yang, I.; Kaafarani, H.M.A.; Fox, J.G. Manuka honey microneedles for enhanced wound healing and the prevention and/or treatment of Methicillin-resistant Staphylococcus aureus (MRSA) surgical site infection. Sci. Rep. 2020, 10, 13229–13239. [Google Scholar] [CrossRef]
- Sun, L.P.; Shi, F.F.; Zhang, W.W.; Zhang, Z.H.; Wang, K. Antioxidant and anti-inflammatory activities of safflower (Carthamus tinctorius L.) honey extract. Foods 2020, 9, 1039. [Google Scholar] [CrossRef]
- Zulkifli, M.F.; Radzi, M.N.F.M.; Saludes, J.P.; Dalisay, D.S.; Ismail, W.I.W. Potential of natural honey in controlling obesity and its related complications. J. Evid. Based Integr. Med. 2022, 27, 2515690X221103304. [Google Scholar] [CrossRef]
- Koodathil, J.; Venkatachalam, G.; Bhaskaran, K. In vitro and in vivo antidiabetic activity of bitter honey in streptozotocin-nicotinamide-induced diabetic Wistar rats. J. Med. Life 2023, 16, 91–100. [Google Scholar] [CrossRef]
- Patouna, A.; Vardakas, P.; Skaperda, Z.; Spandidos, D.A.; Kouretas, D. Evaluation of the antioxidant potency of Greek honey from the Taygetos and Pindos mountains using a combination of cellular and molecular methods. Mol. Med. Rep. 2023, 27, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Kovacova, V.; Mondockova, V.; Zemanova, N.; Babikova, M.; Biro, R.; Ciernikova, S.; Omelka, R. A promising therapeutic supplement for the prevention and management of osteoporosis and breast cancer. Nutrients 2023, 12, 567. [Google Scholar] [CrossRef]
- Ashraf, S.; Ashraf, S.; Ashraf, M.; Imran, M.A.; Kalsoom, L.; Siddiqui, U.N.; Farooq, I.; Akmal, R.; Akram, M.K.; Ashraf, S.; et al. Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multicenter placebo-controlled randomized clinical trial. Phyther. Res. 2023, 37, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.; Hatmal, M.M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and immunomodulatory effects of phytochemicals from honey against COVID-19: Potential mechanisms of action and future directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, G.S.; Mahendiran, B.; Gopalakrishnan, S.; Muthusamy, S.; Malarkodi Elangovan, S. Honey based treatment strategies for infected wounds and burns: A systematic review of recent pre-clinical research. Wound Med. 2020, 30, 100188–100199. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Schulz, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Current status of the gastrointestinal digestion effects on honey: A comprehensive review. Food Chem. 2021, 357, 129807–129820. [Google Scholar] [CrossRef]
- Idrus, R.B.H.; Sainik, N.Q.A.V.; Nordin, A.; Bin Saim, A.; Sulaiman, N. Cardioprotective effects of honey and its constituent: An evidence-based review of laboratory studies and clinical trials. Int. J. Environ. Res. Public Health 2020, 17, 3613. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey phenolic compound profiling and authenticity assessment using HRMS targeted and untargeted metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef]
- Mohammed, M.E.A. Factors affecting the physicochemical properties and chemical composition of bee’s honey. Food Rev. Int. 2022, 38, 1330–1341. [Google Scholar] [CrossRef]
- Roshan, A.R.A.; Gad, H.A.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Khanbash, M.S.; Al-Azizi, M.M. Characterization and discrimination of the floral origin of Sidr honey by physicochemical data combined with multivariate analysis. Food Anal. Methods 2017, 10, 137–146. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef]
- Labsvards, K.D.; Rudovica, V.; Kluga, R.; Rusko, J.; Busa, L.; Bertins, M.; Eglite, I.; Naumenko, J.; Salajeva, M.; Viksna, A. Determination of floral origin markers of latvian honey by using IRMS, UHPLC-HRMS, and 1H-NMR. Foods 2022, 11, 42. [Google Scholar] [CrossRef]
- Sichilongo, K.; Padiso, T.; Turner, Q. AMDIS-Metab R data manipulation for the geographical and floral differentiation of selected honeys from Zambia and Botswana based on volatile chemical compositions using SPME–GC–MS. Eur. Food Res. Technol. 2020, 246, 1679–1690. [Google Scholar] [CrossRef]
- Nunes, A.; Azevedo, G.Z.; Dos Santos, B.R.; Borges, C.V.; Lima, G.P.P.; Crocoli, L.C.; Moura, S.; Maraschin, M. Characterization of Brazilian floral honey produced in the states of Santa Catarina and São Paulo through ultraviolet–visible (UV–vis), near-infrared (NIR), and nuclear magnetic resonance (NMR) spectroscopy. Food Res. Int. 2022, 162, 111913. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Rodríguez-Flores, M.S.; Míguez, M.; Seijo, M.C. Multivariate statistical approach for the discrimination of honey Samples from Galicia (NW Spain) using physicochemical and pollen parameters. Foods 2023, 12, 1493. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral honeys as a potential source of natural antioxidants, minerals and medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Bouali, N.; Hamadou, W.S.; Badraoui, R.; Lajimi, R.H.; Hamdi, A.; Alreshidi, M.; Adnan, M.; Soua, Z.; Siddiqui, A.J.; Noumi, E.; et al. Phytochemical composition, antioxidant, and anticancer activities of Sidr honey: In vitro and in silico computational investigation. Life 2023, 13, 35. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Al Guthami, F.M.; Ramadan, M.F.A.; Al Gethami, A.F.M.; Craig, A.M.; Serrano, S. Characterization of Sidr (Ziziphus spp.) honey from different geographical origins. Appl. Sci. 2022, 12, 9295. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Ibrahim, E.H.; Kilany, M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr. 2020, 8, 445–455. [Google Scholar] [CrossRef]
- Al-Yahya, M.; Mothana, R.; Al-Said, M.; Al-Dosari, M.; Al-Musayeib, N.; Al-Sohaibani, M.; Parvez, M.K.; Rafatullah, S. Attenuation of CCl4 induced oxidative stress and hepatonephrotoxicity by Saudi Sidr honey in rats. Evid. Based Complement. Altern. Med. 2013, 2013, 569037. [Google Scholar] [CrossRef]
- El-sofany, A.; Naggar, Y.A.; Naiem, E.; Giesy, J.P.; Seif, A.; Naggar, Y.A.; Naiem, E.; Giesy, J.P. Authentication of the botanical and geographic origin of Egyptian honey using pollen analysis methods. J. Apic. Res. 2020, 59, 946–955. [Google Scholar] [CrossRef]
- Ali, H.; Rafique, K.; Ullah, R.; Iftikhar, M.S. Classification of Sidr honey and detection of sugar adulteration using right angle fluorescence spectroscopy and chemometrics. Eur. Food Res. Technol. 2022, 248, 1823–1829. [Google Scholar] [CrossRef]
- Mădaş, M.N.; Mărghitaş, L.A.; Severus, D.; Bobiş, O.; Abbas, O.; Danthine, S.; Francis, F.; Haubruge, E.; Nguyen, B.K. Labeling regulations and quality control of honey origin: A review labeling regulations and quality control of honey origin: A Food. Food Rev. Int. 2019, 36, 215–240. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Maia, M.; Karabagias, V.K.; Gatzias, I.; Badeka, A.V. Quality and origin characterisation of Portuguese, Greek, Oceanian, and Asian honey, based on poly-parametric analysis hand in hand with dimension reduction and classification techniques. Eur. Food Res. Technol. 2020, 246, 987–1006. [Google Scholar] [CrossRef]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical characteristics and bioactive compounds of different types of honey and their biological and therapeutic properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef]
- Addi, A.; Bareke, T. Botanical origin and characterization of monofloral honeys in Southwestern forest of Ethiopia. Food Sci. Nutr. 2021, 9, 4998–5005. [Google Scholar] [CrossRef] [PubMed]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- Yosri, N.; El-Wahed, A.A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against Sars-COV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; El-Wahed, A.; Du, M.; El-Seedi, H.; Büttner, S. Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: Limitations and possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef]
- El-Wahed, A.A.A.; Farag, M.A.; Eraqi, W.A.; Mersal, G.A.M.; Zhao, C.; Khalifa, S.A.M.; El-Seedi, H.R. Unravelling the beehive air volatiles profile as analysed via solid-phase microextraction (SPME) and chemometrics. J. King Saud Univ. Sci. 2021, 33, 101449–101456. [Google Scholar] [CrossRef]
- El-Aarag, B.; Magdy, M.; AlAjmi, M.F.; Khalifa, S.A.M.; El-Seedi, H.R. Melittin exerts beneficial effects on paraquat-induced lung injuries in mice by modifying oxidative stress and apoptosis. Molecules 2019, 24, 1498. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Abd El-Wahed, A.A.; Shehata, M.G.; El-Seedi, H.R.; Masry, S.H.D.; Khalifa, S.A.M.; Mahfouz, H.M.; El-Sohaimy, S.A. Chemical profiling and nutritional evaluation of bee pollen, bee bread, and royal jelly and their role in functional fermented dairy products. Molecules 2023, 28, 227. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Guthami, F.M.A.; Ramadan, M.F.A.; Gethami, A.F.M.A.; Craig, A.M.; El-seedi, H.R.; Rodr, I.; Serrano, S. The bioactive value of Tamarix gallica honey from different geographical origins. Inescts 2023, 14, 319. [Google Scholar] [CrossRef]
- Amr, A.; Abd El-Wahed, A.; El-Seedi, H.R.; Khalifa, S.A.M.; Augustyniak, M.; El-Samad, L.M.; Abdel Karim, A.E.; El Wakil, A. UPLC-MS/MS analysis of naturally derived Apis mellifera products and their promising effects against cadmium-induced adverse effects in female rats. Nutrients 2023, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Harmonised methods of the international IHC. Int. Honey Comm. 2009, 5, 1–62. [Google Scholar]
- Laaroussi, H.; Bouddine, T.; Bakour, M.; Ousaaid, D. Physicochemical properties, mineral content, antioxidant activities, and microbiological quality of Bupleurum spinosum Gouan honey from the Middle Atlas in Morocco. J. Food Qual. 2020, 2020, 7609454. [Google Scholar] [CrossRef]
- Pascual-Maté, A.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T. Methods of analysis of honey Ana. J. Apic. Res. 2018, 57, 38–74. [Google Scholar] [CrossRef]
- Daka, S.; Dessalegn, E.; Hassen, Y.; Zula, A.T. Physicochemical properties and antioxidant activities of thermally treated poly-floral honey from Chire woreda, Sidamo regional State, Ethiopia. Cogent Food Agric. 2023, 9, 2172989–2173005. [Google Scholar] [CrossRef]
- Tedesco, R.; Scalabrin, E.; Malagnini, V.; Strojnik, L.; Ogrinc, N.; Capodaglio, G. Characterization of botanical origin of Italian honey by carbohydrate composition and volatile organic compounds (VOCs). Foods 2022, 11, 2441. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Alshumrani, E.S.; Saeed, M.S.B.; Rawas, G.M.; Alharthi, N.T.; Baeshen, M.N.; Helmi, N.M.; Alam, M.Z.; Suhail, M. Analysis of sugar composition and pesticides using HPLC and GC–MS techniques in honey samples collected from Saudi Arabian markets. Saudi J. Biol. Sci. 2020, 27, 3720–3726. [Google Scholar] [CrossRef]
- El-Din, M.I.G.; Fahmy, N.M.; Wu, F.; Salem, M.M.; Khattab, O.M.; El-Seedi, H.R.; Korinek, M.; Hwang, T.-L.; Osman, A.K.; El-Shazly, M. Comparative LC–LTQ–MS–MS analysis of the leaf extracts of Lantana camara and Lantana montevidensis growing in Egypt with insights into their anti-Inflammatory, and cytotoxic activities. Plants 2022, 11, 1699. [Google Scholar] [CrossRef]
- Noiset, P.; Cabirol, N.; Rojas-Oropeza, M.; Warrit, N.; Nkoba, K.; Vereecken, N.J. Honey compositional convergence and the parallel domestication of social bees. Sci. Rep. 2022, 12, 18280–18285. [Google Scholar] [CrossRef]
- Muhati, G.L.; Warui, M.W. Physicochemical properties and floral sources of honey produced in Marsabit Forest Reserve, Northern Kenya. J. Food Qual. 2022, 2022, 3841184–3841193. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Ropciuc, S.; Oroian, M. Advanced characterization of monofloral honeys from Romania. Agric. 2022, 12, 526. [Google Scholar] [CrossRef]
- Bicudo de Almeida-Muradian, L.; Monika Barth, O.; Dietemann, V.; Eyer, M.; Freitas, A.D.S.D.; Martel, A.C.; Marcazzan, G.L.; Marchese, C.M.; Mucignat-Caretta, C.; Pascual-Maté, A.; et al. Standard methods for Apis mellifera honey research. J. Apic. Res. 2020, 59, 1–62. [Google Scholar] [CrossRef]
- Aljohar, H.I.; Maher, H.M.; Albaqami, J.; Al-Mehaizie, M.; Orfali, R.; Orfali, R.; Alrubia, S. Physical and chemical screening of honey samples available in the Saudi market: An important aspect in the authentication process and quality assessment. Saudi Pharm. J. 2018, 26, 932–942. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Draft Revised Standard for Honey (At Step 10 of the Codex Procedure); Codex Alimentarius Commission; FAO: Rome, Italy, 2001; Volume 1, pp. 19–26. [Google Scholar]
- Karabagias, I.K. Seeking of reliable markers related to Greek nectar honey geographical and botanical origin identification based on sugar profile by HPLC-RI and electro-chemical parameters using multivariate statistics. Eur. Food Res. Technol. 2019, 245, 805–816. [Google Scholar] [CrossRef]
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem. 2007, 101, 695–703. [Google Scholar] [CrossRef]
- Al-waili, N.; Ghamdi, A.; Ansari, J.; Al-attal, Y.; Al-mubarak, A.; Salom, K. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi. Arch. Med. Res. 2013, 44, 307–3016. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H.; Samples, H. Determination of organic acids in honey by liquid chromatography with tandem mass spectrometry. Food Anal. Methods 2020, 13, 2249–2257. [Google Scholar] [CrossRef]
- Ropciuc, S.; Dranca, F.; Oroian, M.; Pauliuc, D.; Ciurs, P. Physicochemical parameters prediction and authentication of different monofloral honeys based on FTIR spectra. J. Food Compos. Anal. 2021, 102, 104021–104032. [Google Scholar]
- Mieles, J.Y.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An advanced antimicrobial and wound healing biomaterial for tissue engineering applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Albu, A.; Radu-Rusu, C.G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality assessment of raw honey issued from Eastern Romania. Agricultre 2021, 11, 247. [Google Scholar] [CrossRef]
- Raweh, H.S.A.; Ahmed, A.Y.B.H.; Iqbal, J.; Alqarni, A.S. Monitoring and evaluation of free acidity levels in Talh honey originated from Talh tree Acacia gerrardii Benth. J. King Saud Univ. Sci. 2022, 34, 101678–101687. [Google Scholar] [CrossRef]
- Benth; Raweh, H.S.A.; Badjah-hadj-ahmed, A.Y.; Iqbal, J. Physicochemical characteristics, especially free acidity in Talh honey. Molecules 2022, 27, 5959–5978. [Google Scholar]
- Moreira, R.F.A.; Trugo, L.C.; Pietroluongo, M.; De Maria, C.A.B. Flavor composition of cashew (Anacardium occidentale) and marmeleiro (Croton species) honeys. J. Agric. Food Chem. 2002, 50, 7616–7621. [Google Scholar] [CrossRef] [PubMed]
- Fröschle, M.; Horn, H.; Spring, O. Characterization of Jatropha curcas honeys originating from the southern highlands of Madagascar. LWT Food Sci. Technol. 2018, 93, 525–533. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, C.; Li, C.; Huang, Z.Y.; Miao, X. Pathway of 5-hydroxymethyl-2-furaldehyde formation in honey. J. Food Sci. Technol. 2019, 56, 2417–2425. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Belushi, S.; Al-Amri, A.; Al-Hadhrami, A.; Al-Rusheidi, M.; Al-Alawi, A. Quality evaluation of Omani honey. Food Chem. 2018, 262, 162–167. [Google Scholar] [CrossRef]
- Bako, T.; Mamai, E.A.; Bature, B.J. Determination of quality parameters of honey from Taraba State—Nigeria. Chem. Biomol. Eng. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Dżugan, M.; Miłek, M.; Kielar, P.; Stępień, K.; Sidor, E.; Bocian, A. SDS-PAGE Protein and HPTLC polyphenols profiling as a promising tool for authentication of goldenrod honey. Foods 2022, 11, 2390. [Google Scholar] [CrossRef]
- Zerrouk, S.; Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Characterization of Ziziphus lotus (jujube) honey produced in Algeria Salim. J. Apic. Res. 2018, 57, 166–174. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Ioana, C.; Cornea-cipcigan, M.; Suharoschi, R.; Erler, S. Honey botanical origin and honey-specific protein pattern: Characterization of some European honeys. LWT Food Sci. Technol. 154 2022, 154, 112883–112892. [Google Scholar]
- Del Campo, G.; Zuriarrain, J.; Zuriarrain, A.; Berregi, I. Quantitative determination of carboxylic acids, amino acids, carbohydrates, ethanol and hydroxymethylfurfural in honey by 1H NMR. Food Chem. 2016, 196, 1031–1039. [Google Scholar] [CrossRef]
- Taylor, M.A.; Robertson, A.W.; Biggs, P.J.; Richards, K.K.; Jones, D.F.; Parkar, S.G. The effect of carbohydrate sources: Sucrose, invert sugar and components of mānuka honey, on core bacteria in the digestive tract of adult honey bees (Apis mellifera). PLoS ONE 2019, 14, e0225845. [Google Scholar] [CrossRef]
- Scripca, L.A.; Amariei, S. Research on honey crystalization. Rev. Chim. 2018, 69, 2953–2957. [Google Scholar] [CrossRef]
- Baloš, M.M.Ž.; Popov, N.S.; Radulović, J.Z.P.; Stojanov, I.M.; Jakšić, S.M. Sugar profile of different floral origin honeys from Serbia. J. Apic. Res. 2020, 59, 398–405. [Google Scholar] [CrossRef]
- Naik, R.R.; Gandhi, N.S.; Thakur, M.; Nanda, V. Analysis of crystallization phenomenon in Indian honey using molecular dynamics simulations and artificial neural network. Food Chem. 2019, 300, 125182. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Sugar profiling of honeys for authentication and detection of adulterants using high-performance thin layer chromatography. Molecules 2020, 25, 5289. [Google Scholar] [CrossRef]
- Tosun, M.; Keles, F. Investigation methods for detecting honey samples adulterated with sucrose syrup. J. Food Compos. Anal. 2021, 101, 103941–103945. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Khan, K.A.; Ahmed, Z.; Ansari, M.J. Quality evaluation of Saudi honey harvested from the Asir province by using high-performance liquid chromatography (HPLC). Saudi J. Biol. Sci. 2020, 27, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.K.A.; Al-Kahtani, S.; Taha, R. Comparison of the physicochemical characteristics of sidr (Ziziphus spp.) honey produced by Apis florea F. and Apis mellifera L. J. Apic. Res. 2021, 60, 470–477. [Google Scholar] [CrossRef]
- Neggad, A.; Benkaci-Ali, F.; Laurent, S.; Ayata, G. A new method of extracting polyphenols from honey using a biosorbent compared to the commercial resin amberlite XAD2. J. Sep. Sci. 2021, 44, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Yu, X.; Jiang, Y.; Zhang, J.; Liu, B. Characterization and identification of the metabolites of Menthae Haplocalycis Herba water extracts in rat plasma, urine, and feces by ultra-high performance liquid chromatography with linear ion trap-Orbitrap mass spectrometry. J. Sep. Sci. 2020, 43, 1051–1062. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abd El-Hady, F.K. Influence of honey on the suppression of human low density lipoprotein (LDL) peroxidation (in vitro). Evid. Based Complement. Altern. Med. 2009, 6, 113–121. [Google Scholar] [CrossRef]
- Guerrini, A.; Bruni, R.; Maietti, S.; Poli, F.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Scalvenzi, L.; Sacchetti, G. Ecuadorian stingless bee (Meliponinae) honey: A chemical and functional profile of an ancient health product. Food Chem. 2009, 114, 1413–1420. [Google Scholar] [CrossRef]
- Cadoná, F.C.; Dantas, R.F.; de Mello, G.H.; Silva, F.P., Jr. Natural products targeting into cancer hallmarks: An update on caffeine, theobromine, and (+)-catechin. Crit. Rev. Food Sci. Nutr. 2022, 62, 7222–7241. [Google Scholar] [CrossRef]
- Li, T.; Li, F.; Liu, X.; Liu, J.; Li, D. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4–MyD88-mediated NF-κB and MAPK signaling pathways. Phyther. Res. 2019, 33, 756–767. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Soto-Valdez, H.; Peralta, E.; Ayala-Zavala, J.F.; Auras, R.; Gámez-Meza, N. Antioxidant activity and diffusion of catechin and epicatechin from antioxidant active films made of poly(l-lactic acid). J. Agric. Food Chem. 2012, 60, 6515–6523. [Google Scholar] [CrossRef]
- Tedesco, R.; Barbaro, E.; Zangrando, R.; Rizzoli, A.; Malagnini, V.; Gambaro, A.; Fontana, P.; Capodaglio, G. Carbohydrate determination in honey samples by ion chromatography–mass spectrometry (HPAEC-MS). Anal. Bioanal. Chem. 2020, 412, 5217–5227. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Boffo, E.F.; Tavares, L.A.; Tobias, A.C.T.; Ferreira, M.M.C.; Ferreira, A.G. Identification of components of Brazilian honey by 1H NMR and classification of its botanical origin by chemometric methods. LWT 2012, 49, 55–63. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Badeka, A.V. The Honey volatile code: A collective study and extended version. Foods 2019, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Odeh, I.; Abu-Lafi, S.; Al-Najjar, I. Determination of unifloral honey volatiles from Centaurea iberica and Zizyphus spinachristi by solid-phase microextraction and gas chromatography-mass spectrometry. Acta Chromatogr. 2014, 26, 485–493. [Google Scholar] [CrossRef]
- Panseri, S.; Manzo, A.; Chiesa, L.M.; Giorgi, A. Melissopalynological and volatile compounds analysis of buckwheat honey from different geographical origins and their role in botanical determination. J. Chem. 2013, 2013, 904202. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Zaldivar-Cruz, J.M.; Kuri, V.; Fernández-López, J.; Carbonell-Barrachina, Á.A.; Pérez-Álvarez, J.Á. Aroma profile and physico-chemical properties of artisanal honey from Tabasco, Mexico. Int. J. Food Sci. Technol. 2010, 45, 1111–1118. [Google Scholar] [CrossRef]
- Salis, S.; Spano, N.; Ciulu, M.; Floris, I.; Pilo, M.I.; Sanna, G. Electrochemical determination of the ‘furanic index’ in honey. Molecules 2021, 26, 4115. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Kezić, J.; Gugić, M. Headspace, volatile and semi-volatile organic compounds diversity and radical scavenging activity of ultrasonic solvent extracts from amorpha fruticosa honey samples. Molecules 2009, 14, 2717. [Google Scholar] [CrossRef]
- Owczarek-Fendor, A.; De Meulenaer, B.; Scholl, G.; Adams, A.; Van Lancker, F.; Eppe, G.; De Pauw, E.; Scippo, M.L.; De Kimpe, N. Furan formation in starch-based model systems containing carbohydrates in combination with proteins, ascorbic acid and lipids. Food Chem. 2012, 133, 816–821. [Google Scholar] [CrossRef]
- Kuś, P.M.; Czabaj, S.; Jerković, I. Comparison of volatile profiles of meads and related unifloral honeys: Traceability markers. Molecules 2022, 27, 4558. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).