Abstract
Chemometrics is a tool for data mining and unlocking the door for solving big data queries. Apiaceae is a family species which is commonly cultivated worldwide. Although members of this species are widely used as antioxidant, antibacterial, antifungal, and anti-inflammatory agents, their metabolites profiling remains ambiguous. Based on WHO support, chemometrics has been used in evaluating the quality and authenticity of the herbal products. The objective of this study is to profile and characterize phenolic metabolites in nine species from Egyptian cultivars and three different species of German cultivars from the Apiaceae family using multivariate analysis after LC-PDA-ESI-MS/MS and near infrared spectroscopy data are generated. Principal component analysis was successfully applied to distinguish between the nine Egyptian cultivars and the three German cultivars, and hierarchical cluster analysis also confirmed this distinctive clustering. Partial least square regression (PLS-R) models showed a relationship between phytochemicals and antioxidant activities. The metabolites responsible for the clustering pattern and variables important for projection (VIP) were identified, being twelve amongst nine Egyptian cultivar samples and thirteen amongst the Egyptian cultivar and the German cultivar comparison. The identified VIPs were also correlated with the antioxidant activity using PLS-R. In conclusion, the study showed novelty in the application of hyphenated analytical techniques and chemometrics that assist in quality control of herbal medicine.
1. Introduction
Chemometrics is the science of associating chemical information extracted from chemical systems with mathematical and statistical procedures providing comprehensive knowledge about that chemical system, facilitating decision-making [1] and linking chemical characterization and metabolite identification with different pharmacological activities of herbal drugs [2]. This approach delivers solutions for problems related to both predictive and complementary issues by being able to solve highly complicated data—matrices to justify different analytical complications. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) are unsupervised chemometric pattern recognition methods of a data matrix using different methods for the data transformation. On the other hand, partial least square regression (PLS-R) is a supervised multivariate calibration method which analyzes the spectral data into uncorrelated variables to increase the covariance of independent variables with dependent variables (i.e., pharmacological effect).
During authenticity studies, the important step in assessing drug quality is that each herbal drug contains unique characteristics that can be used to identify the plant’s species using fingerprinting techniques.
Fingerprinting is a collection of techniques that can be used for herbal drug authentication based on the extract composition of the herbal plant aiming at identifying its raw material characteristics by its chemical composition or its metabolite profiling [3,4].
To choose the best fingerprinting technique, there are several criteria aside from the plant’s nature that should be considered. These aspects can be related to the aim of the test and whether we care if the sample is intact or not while testing [5,6].
Depending on the type of analyte, analytical techniques are chosen for fingerprinting herbal drugs and investigating the chemical constituent, where chromatographic techniques such as high-performance liquid chromatography coupled with mass detection (HPLC-MS), near-infrared spectroscopy (NIR) are two of the most useful techniques [4,7,8,9].
The main challenge facing the food industry and the pharmaceutical industry is standardization of herbal preparation using validated methods [10,11]. One important aspect of herbal drug quality control is the availability of data about their authenticity. Problems associated with authenticity may include wrong identification, substitution and/or adulteration of the raw materials, or shifting to a lower-grade quality by dilution of the raw material. Therefore, the critical need for authentication analysis led to the development of new approaches such as fingerprinting techniques [9,11,12].
The Apiaceae family is considered one of the oldest families of aromatic plants and is widely cultivated in the Mediterranean region, Middle East, Southern Africa, Europe, and East Asia. It consists of 3780 species in 434 genera. Plants of this family are used in folk medicine in herbal tea preparations, as food spices, and as flavoring agents [13,14]. They are grown not only as a source of nutrition but are also used as flavoring and preservative agents in the fragrance industry and as nutraceutical agents in medicine [15]. This family is rich in vitamins, proteins, carbohydrates, flavonoids, phenolics, carotenoids, terpenoids, and fatty acids [16]. The phytochemicals that exert a range of pharmacological effects can be listed as apigenin, quercetin, luteolin, chlorogenic acid, kämpferol, and many others [17,18]. Phenolics are naturally occurring secondary metabolites which are present as simple phenols of polyphenols and are proven to enhance the well-being and life expectancy of individuals through their biological activities acting as antioxidants, antimicrobials, anti-inflammatories, anticancer, and antidiabetic agents. The herbal parts are used in different pharmaceutical preparations either as powdered dried fruit or by decoction [19].
The chemical diversity and wide array of bioactive phytochemicals of herbal extracts have attracted the attention of the scientific community. Hence, the challenge is to balance between the identification of bioactive compounds from highly complicated natural mixtures in a short time-cost effectiveness. Accordingly, the advancement in analytical applications of untargeted metabolomics to study the biologically active natural product mixtures has become more crucial. Previous studies did not tackle various collections of the Apiaceae family species nor compare Egyptian and German cultivar profiling. A Moroccan study assessed the total phenolic content of only three species of the Apiaceae family, in coriander, parsley, and celery [20]. Another study characterized the phenolic compounds using LC-ESI-MS/MS but for Lamiaceae family [21]. A study where chemometrics PCA and HCA were applied targeted essential oils in the Apiaceae and Lamiaceae families [22]. This encouraged our research group to study those twelve species in a novel approach, intending that the subsequent information from this work would be useful to promote the best use of these plants and contribute to further studies in the field.
This study aims to profile the pattern of phenolic metabolites in each species under investigation using LCMS and FT-NIR techniques and identify the common and variable importance projection (VIP) compounds responsible for clustering the studied nine Egyptian and three German cultivars via a metabolomic approach to serve as a quality control tool of different Apiaceae species and to correlate the VIP phytochemicals with the potential antioxidant activity of those species.
2. Materials and Methods
2.1. Reagents and Standards
2,5-dihydroxybenzoic acid, 3,4dihydroxybenzoic acid, −/+ catechin, apigenin, caffeic acid, catechol, chlorogenic acid, chrysin, cinnamic acid, epicatechin, ferulic acid, gallic acid, kaempherol, luteolin, m-coumaric acid, myricitrin, naringenin, o-coumaric acid, p-coumaric acid, quercetin, quercetin-3-galactoside, quercetin-3-glucosid, quercitrin, rosmarinic acid, rutin, salicylic acid, trans-Sinapic acid, umbelliferon, vanillic acid, and vanillin were supplied by Sigma-Aldrich, Germany. Other reagents were of analytical grade.
2.2. Plant Material from the Apiaceae Family
Nine species of family Apiaceae were collected from the botanical garden of the Faculty of Pharmacy, Cairo University in February 2018; which were established for research purposes by not using any plant protection chemicals during the cultivation stages. The plants were thankfully authenticated and identified by Eng. Sawsan Mohamed, plant taxonomy consultant. The aerial parts of the following plants were used: Ammi majus L. (bishop’s weed), Ammi visnaga L. (visnaga), Anethum graveolens L. (dill), Apium graveolens L. (celery), Carum carvi (caraway), Coriandrum sativum L. (coriander), Foeniculum vulgare L. (fennel), Petroselinum crispum L. (parsley) and Pimpinella anisum L. (anise). A voucher specimen of the authenticated plant (voucher no. PHBL 00230-00238) respectively was deposited at the herbarium of the Pharmaceutical Biology Department, Faculty of Pharmacy and Biotechnology, German University in Cairo, Cairo, Egypt.
Another three species of the German cultivar were obtained from the organic produce section of a local German market, the aerial parts of the spiecies of Anethum graveolens L. (dill), Coriandrum sativum L. (coriander), and Petroselinum crispum L. (parsley).
2.3. Extraction f Procedure
All samples were washed under fresh running water to eliminate dust, dirt, and contamination. Extra washing steps were done for the three German cultivars to minimize any possible remnants available. The aerial parts were air-dried (3 kg) and were macerated in 10 L 70% ethanol 7:3 (ethanol: water) overnight followed by filtration through filter paper. The solvents were evaporated at 60 °C using a rotary vacuum evaporator (Büchi Labortechnik AG, Series R 3000, Flawil, Switzerland). The yield for the Egyptian cultivar alcoholic fraction of water extract (AH) was 5% for coriander, 3.6% (w/w) for dill, 3.56% (w/w) for parsley, 7.13% (w/w) for caraway, 4.3% (w/w) for Ammi majus, 3.47% (w/w) for fennel, 6% (w/w) for anise, 2.3% (w/w) for celery, and 5% (w/w) for visnaga. As for the German cultivar, the (AH) yields for coriander, dill, and parsley were 2% (w/w), 1.6% (w/w), and 1.8% (w/w), respectively.
2.4. Characterization of Phenolics Using LC-PDA-ESI-MS/MS
LC-PDA-ESI-MS/MS analysis was performed on Agilent 1290 Infinity UHPLC system (Agilent Technologies, Inc., Santa Clara, CA, USA), equipped with Acquity CSH C18 1.7 µm, 2.1 × 150 mm column (Waters Corporation, Milford, CO, USA), coupled to a Q Exactive™ Hybrid Quadrupole-Orbitrap mass spectrometer operated with an ESI Source. The instruments were controlled by Xcalibur 4.4 (Thermo Fisher, Waltham, MA, USA). The mass spectra were acquired in negative and positive mode ionization and the spray capillary voltage was set to 3.5 kv and the desolvation temperature was 360 °C. Mass spectra were acquired using a scan range from 100 to 1500 mz at a resolution of 70,000. Data-dependent MS/MS spectra were generated for the five most abundant precursor ions with a resolution of 17,500 and the collision energy was set to 51 eV. The column temperature was set to 40 °C, injection volume was 5 uL, and the UV detection was performed at wavelength from 190–550 nm. The used mobile phase was comprised of water (solvent A) and acetonitrile (solvent B) and adjusted to pH 3.0 with formic acid (0.2%). The negative ionization mode was used for polyphenolic compound assessment. The phenolic compound separation was performed using binary gradient elution as follows: the initial 3% solvent B was increased to 20% for 15 min (0.01–15 min), then increased to 35% for 27 min (15–27 min), increased to 95% for 40 min and maintained at 95% for 44 min (40–44 min), decreased to 3% for 45 min, and maintained at 3% for 48 min for the rest of the elution (45–48 min). The flow rate was 0.4 mL/min. The Identification of the metabolites was done using Compound Discoverer™ and Xcalibur™ (Thermo Scientific™ Version 4.4). The identification was done with both polarity modes and the screening of the phenolic acids and relative quantification was done with extracted ion chromatograms (XIC) in the negative mode.
2.5. Identification of Phenolics Using near Infrared Spectroscopy
The NIR spectra were collected by the MPA II Multi-Purpose FT-NIR analyzer (Bruker Optik GmbH, Ettlingen, Germany) equipped with a rotating sample holder used for diffuse reflectance NIR measurements. All spectra were recorded between 12,500 and 3600 cm−1 in a resolution of 8 cm−1 at 10 kHz scanning speed using OPUS 6.0 (Bruker Optik GmbH, Ettlingen, Germany). The spectra of the twelve extracts were recorded with three repetitions averaging thirty-two scans each.
2.6. In-Vitro Assays
In vitro assays were evaluated based on the calibration curves with their corresponding standards constructed on the same day of the spectrophotometric measurements.
2.7. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
The total phenolic and flavonoid contents were assayed using the microtiter-plate adapted Folin–Ciocalteu and aluminum chloride methods colorimetric assay, respectively [23,24]. Gallic acid and quercetin were used as the standards to determine the TPC (mg GAE/µg extract) and TFC (mg QAR/µg extract), respectively. For the calibration curve establishment in Figures S1 and S2, gallic acid concentrations and quercetin concentrations ranged from 12.5 to 100 µg/mL.
2.8. DPPH Free Radical Scavenging Assay
The antioxidant activity of the twelve extracts (9 Egyptian cultivar and 3 German cultivar) was evaluated using DPPH assay according to [25,26]. All assays were carried out in three replicates. Trolox was used as the positive control as shown in Figure S3.
2.9. ABTS (2,2′-Azinobis-(3-ethylbenothiazoline-6-sulphonate) Assay
The antioxidant power of the extracts was assessed according to the literature [27]. Quercetin was used as standard (mg QAR/µg extract or fraction) and a calibration curve as shown in Figure S4 was conducted where quercetin concentrations ranged from 5 to 80 µg/mL.
2.10. FRAP (Ferric Reducing/Oxidant Power) Assay
Antioxidant activity was estimated according to the literature [28]. Serial dilutions of ferrous sulfate (FeSO4) (1.25–160 µg/mL) were used for the calibration curve as shown in Figure S5, and the extracts’ antioxidant effects were prescribed in terms of FeSO4 equivalent μg/mg extract.
2.11. CUPRAC (Cupric Ion Reducing Antioxidant Capacity) Assay
CUPRAC assay was carried out according to the literature [29] where BHA was used as a standard and serial dilutions of BHA (12.5–200 µg/mL) were used for the calibration curve as shown in Figure S6.
2.12. Statistical Analysis
2.12.1. Chemometrics Analysis
The plant sample (n = 12) was evaluated in triplicate as a minimum requisite ensuring adequate statistical power with cost effectiveness and minimizing consumption of non-benign solvents. The obtained dataset comprised concentration values of quantified polyphenols. All chemometrics analysis was done using UnscramblerX® (CAMO Analytics, Oslo, Norway).
Data clustering was done by principal component analysis (PCA) leading to plant sample grouping. Before calculating principal components, the input variables were centered and scaled. Loadings and scores were calculated using NIPALS-algorithm. Sample patterns were deduced from the simultaneous interpretation of score and loading plots of the first two principal components. Like PCA, hierarchical cluster analysis single linkage clustering is an unsupervised method used to determine the extent to which variables are related and find patterns in the data. It was used for the validation of the obtained varietal separation. The average data of the three replicates was used for each sample in calculating the score plots leading to the best explained distinctive score plots for clear comparisons.
Further discriminatory studies to assign plant samples to their corresponding metabolites included partial least squares-regression analysis (PLS-R), a supervised pattern recognition technique employing the NIPALS (nonlinear iterative partial least squares) algorithm with two latent variables to build the classification models, and cross validation.
2.12.2. Univariate Analysis
TPC, TFC, DPPH free radical scavenging assay, ABTS assay, FRAP assay, and CUPRAC assay results were analyzed by one-way analysis of variance (ANOVA), and statistical significance was assessed by multiple comparison Tukey HSD post hoc test. The results are presented as mean difference, standard error, (p < 0.05) and 95% confidence interval. IBM SPSS statistics for Windows, Version 22.0. Chicago Inc. was used in the analysis.
3. Results
3.1. Characterization of Phytochemicals
Identification of compounds was done by combining the chemical data of each metabolite, retention time, molecular weight, MS/MS fragmentation pattern, and software library, and comparing them to available standard data. A dataset of 273 variables was extracted where a total of 166 metabolites were obtained from this work and from the literature; fifty-three metabolites were tentatively identified (Table S1). Tentative identification using mzCloud, ChemSpider databases, and spectra of commercially available standards of phenolic metabolites. The detected (AH) extracts in the Egyptian and the German cultivars were matched with their molecular formula; average retention time (RT), mass spectral data, molecular weight, and fragmentation pattern are shown in Figure S1A–M, and the total ion chromatograms of each extract are illustrated in Figures S7–S18.
3.2. Measurement Results of Total Phenolic Content and Total Flavonoid Content and In Vitro Antioxidant Assasys
The TPC and TFC are depicted in Table 1 in addition to the values of several antioxidant assays. Most of the species showed high antioxidant scavenging activity of more than 85% as dill, German dill, anise, fennel, coriander, Ammi majus, celery, and visnaga. Meanwhile, ABTS assay for coriander presented 102.97 ± 0.63 quercetin equivalent concentration (μg/mg). In FRAP assay, visnaga showed 405.965 ± 15.7 ferrous sulfate equivalent concentration (μg/mg). For CUPRAC, fennel displayed 100.769 ± 6.9 BHA equivalent concentration (μg/mg).

Table 1.
Total phenolic content (TPC), total flavonoid content (TFC); antioxidant assays using DPPH, ABTS, FRAP and CUPRAC assays.
3.3. Chemometric Analysis
3.3.1. Principal Component Analysis
Egyptian Cultivar
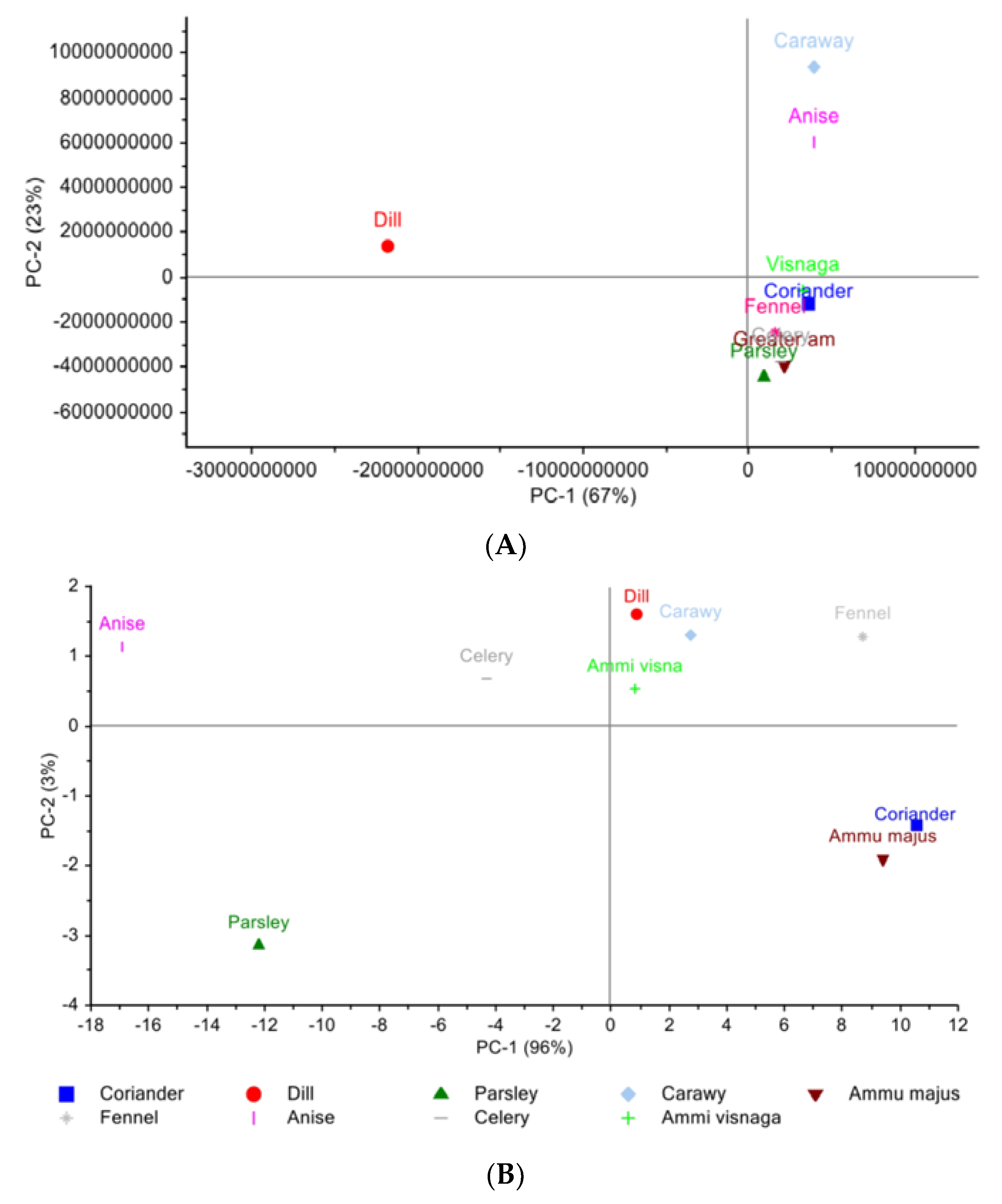
The scores plot of the PCA generated on the nine Egyptian cultivar plants’ chromatograms of LC-PDA-ESI-MS/MS are arranged (Figure 1A) where PC1 and PC2 combined represent 90% variance of the data. Along with the abscissa representing PC1, which explains 67% of the data variance, six out of the nine plants are positioned in the lower quadrant. Coriander and Ammi majus are clustered together and celery and visnaga are clustered together; fennel and parsley are found separately from each other yet considered in the same plane with the others. Dill is found on the upper left quadrant from the six clustered plants yet is found on the opposite side along PC2 which explains 23% of the variance. The metabolites that are responsible for the PCA clustering might be identified using the variable importance in projection (VIP) values that represent the most discriminatory attributes. The identified VIPs are: apigenin, caffeoyl quinic acid, chlorogenic acid, coumaroylquinic acid, ferulic acid glucoside, feruloyl quinic acid, hydroxybenzoic acid, hydroxy benzoyl hexose, isorhamnetin, isorhamnetin-rhamnoside, methoxy vinyl phenol, naringenin glucoside, and vanillin.
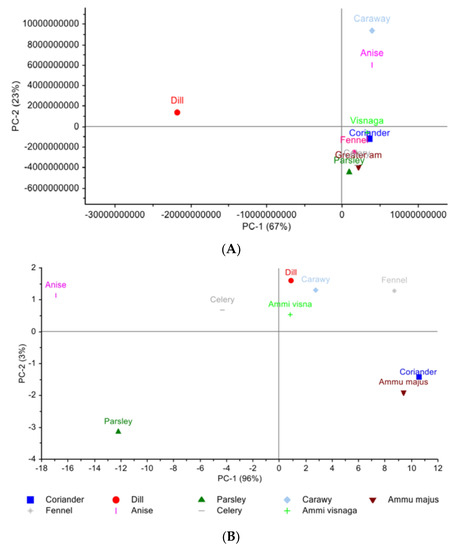
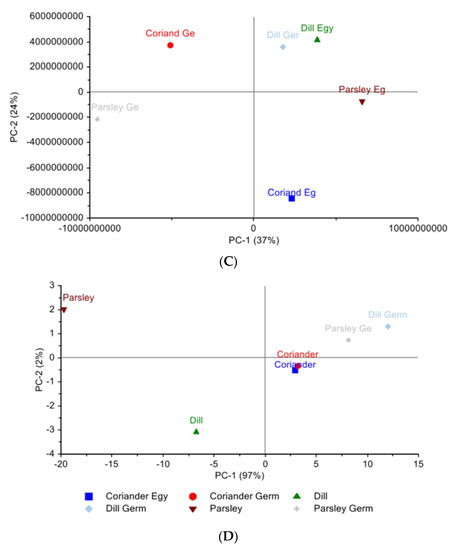
Figure 1.
Principal component analysis scores plot. (A) Egyptian cultivar samples for LC-PDA-ESI-MS/MS data (B) Egyptian cultivar samples for NIR spectra data (C) Egyptian cultivar and German cultivar for LC-PDA-ESI-MS/MS data (D) Egyptian and German cultivar for NIR spectra data.
Looking at the score plot (Figure 1B) of the NIR spectra, PC1 and PC2 together represent 99% of the data. Along PC1, we can see the cluster of coriander and Ammi majus together on the negative side while parsley is found on the opposite positive side. Along with PC2, caraway, dill and visnaga are clustered together leaving fennel behind yet in the same quadrant. On the opposite quadrant celery and anise are found yet no clustering appears.
Egyptian and German Cultivar
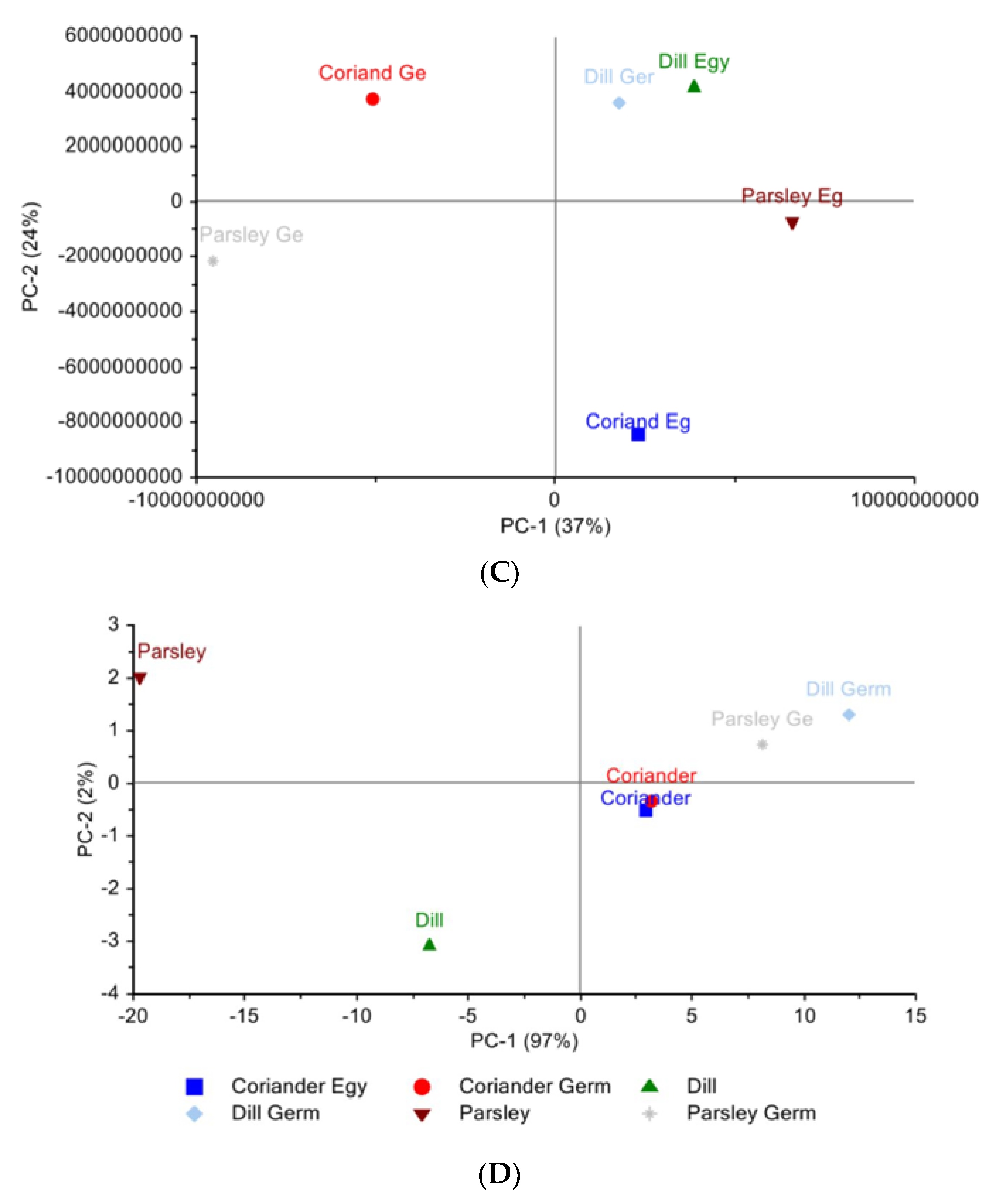
The PCA scores plot for the plants of Egyptian cultivar versus the plants of the German cultivar using LC-PDA-ESI-MS/MS data can be seen in (Figure 1C). The six plants’ metabolites are distributed among the four quadrants, where along PC1 we can see that the plants of Egyptian cultivar coriander and parsley are at a positive value, while the German parsley is located on the opposite side. With respect to PC1, both parsleys have the largest distance, indicating different compositions. Meanwhile, along PC2, it was found that Egyptian dill and German dill are clustered together, having the German coriander on the opposite side. The identified attributes with respect to VIP are: 1,3,7-trimethyluric acid, 4-methylumbelliferyl glucuronide, benzoic acid derivative, afzelechin, apigenin, caffeoyl quinic acid, caffeoyl spermidine derivative, coumaric acid hexoside, caffeic acid, ferulic acid glucoside, quinic acid, and vanillic acid glucoside.
Figure 1D presents the PCA score plot of the six plants NIR spectra; PC1 and PC2 together represent 99% of the data. Along PC1, the cluster of coriander of both Egyptian cultivar and of German cultivars clustered together can be seen. On the contrary, dill and especially parsley, of the Egyptian cultivars are positioned far away from their corresponding German cultivar. Therefore, the NIR supports the finding of LC-PDA-ESI-MS/MS data with respect to parsley.
3.3.2. Hierarchical Cluster Analysis
Egyptian Cultivar
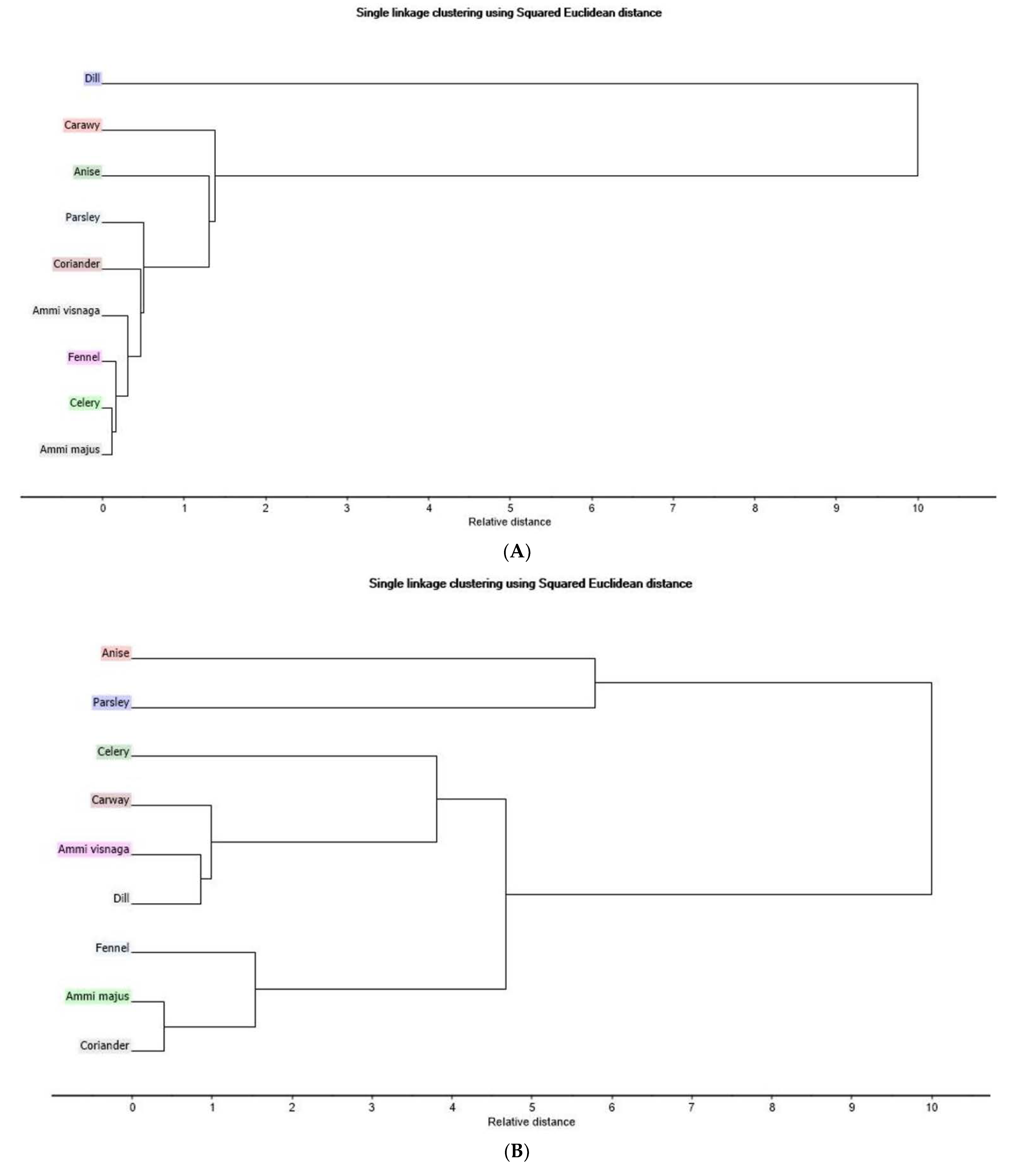
The second unsupervised pattern recognition method using HCA supports the results obtained by PCA as shown in the dendrogram (Figure 2A). Dill (blue) is found too far away from Ammi majus (grey) and the rest of the seven plants. The results obtained by HCA dendrogram (Figure 2B) of NIR spectra recorded for nine plants of Egyptian cultivar reveals the same exploratory results of how coriander and Ammi majus (light grey) are close to each other where fennel is somehow close to them. The contrary is seen with anise, which is the farthest plant sample. In addition, the distance between dill, visnaga, and caraway (pink) is small, which explains their closeness in the PCA scores plot.
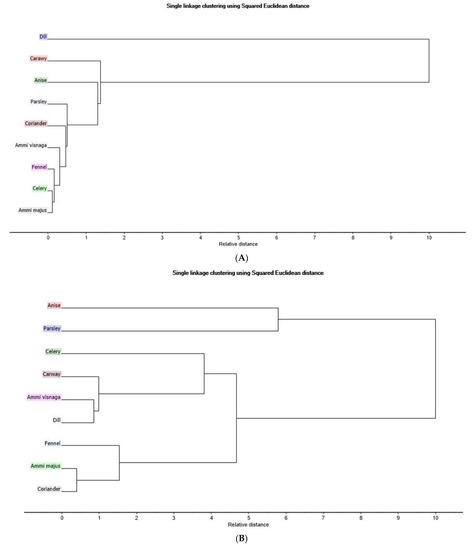
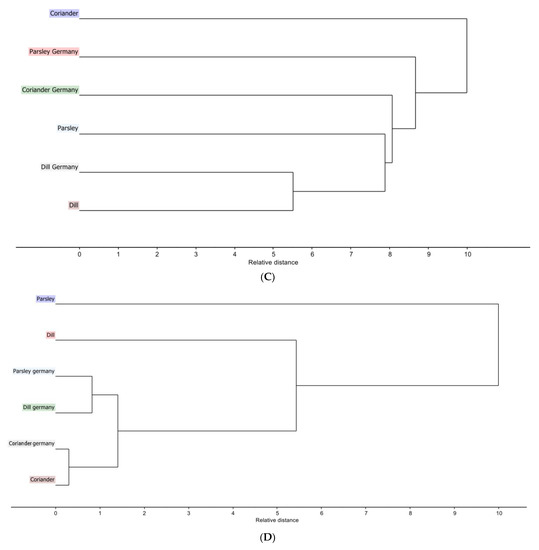
Figure 2.
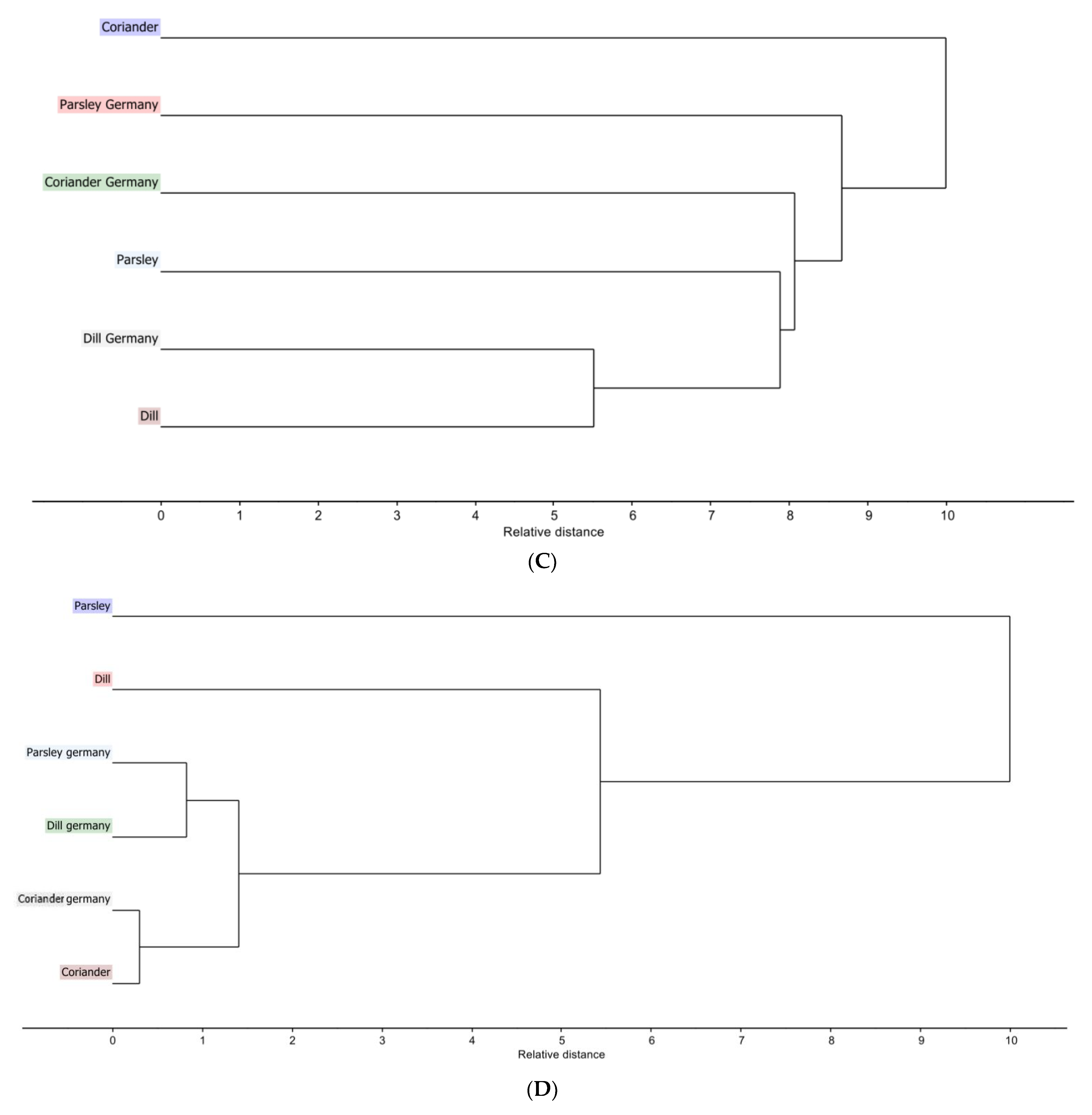
Hierarchical clustering analysis dendrogram based on single linkage clustering (A) Egyptian cultivar samples for LC-PDA-ESI-MS/MS data (B) Egyptian cultivar samples for NIR spectra data (C) Egyptian cultivar and German cultivar for LC-PDA-ESI-MS/MS data (D) Egyptian and German cultivar for NIR spectra data.
Egyptian and German Cultivars
HCA dendrogram (Figure 2C) with single linkage clustering using squared elucidation distance shows that the closest distance is between dill of Egyptian cultivar (pink) and dill of German cultivar (light grey), while the largest distance is between dill of Egyptian cultivar (pink) and coriander of Egyptian cultivar (blue). This supports the PCA analysis results.
The dendrogram (Figure 2D) gives another view of the PCA score plot of NIR data for coriander of the Egyptian and German cultivars as close to each other. Somehow, dill (green) and parsley (light blue) of German cultivars are related to each other and distant from their corresponding Egyptian cultivar.
3.3.3. Partial Least Square Regression
Egyptian Cultivar
Partial least square regression (PLS-R) analysis was conducted to establish a correlation between the nine Egyptian cultivars and their total phenolic content, total flavonoid content, and antioxidant activities. PLS-R was constructed by the data matrix X containing peak area of the LC/MS and the response vector Y containing the total phenolic content, total flavonoid content, and antioxidant activity data. In addition, PLS-R was performed on the VIP peak area of LC/MS represented as X and the response vector Y containing the total phenolic content, total flavonoid content, and antioxidant activity. Unfortunately, the results were not representative enough to the data due to small sample size which led to the determination coefficient R2 calibration and validation below 0.8, and they are not closely related. Applying transformation to the data matrix X values, log and ln transformations, no improvement to the R2 was observed (Table S2).
The NIR spectra of nine Egyptian cultivars were evaluated in the same way as LC/MS data and data matrix X containing NIR spectra and the response vector Y containing the total phenolic content, total flavonoid content, and antioxidant activity data. The PLS calibration R2 and validation R2 were of negligible correlation for the non-transformed and the transformed data types (Table S3).
Egyptian Cultivar versus German Cultivar
PLS-R analysis was conducted to establish a correlation between the three Egyptian cultivars, coriander, dill, and parsley vs. the three German cultivars, coriander, dill, and parsley and their total phenolic content, total flavonoid content, and antioxidant activities. PLS-R was constructed by the same procedure done with the Egyptian cultivar. The non-transformed data showed no correlation while applying transformation of the data matrix X values, log and ln transformations showed a calibration R2 0.960 and validation R2 for 0.889 with FRAP only (Table S4). The same procedure was applied for the VIP data of the 3 German cultivars and three corresponding Egyptian cultivars non-transformed data resulted in calibration R2 0.977 and validation R2 0.7819 for FRAP assay. As for transformed data, the log and ln transformed peak areas for ABTS assays showed calibration R2 for one, validation R2 for 0.895 (Table S4) while the inverse transformation showed no good predictive model.
For the three Egyptian cultivars and three German cultivars the NIR spectra of six extracts were evaluated in the same way. The PLS calibration R2 and validation R2 had negligible correlation for the non-transformed and the transformed data types (Table S5).
3.4. Evaluation of In-Vitro Assays of Extracts Using One-Way ANOVA
Applying one-way ANOVA showed significant difference between TPC, TFC, and the three antioxidant assays FRAP, ABTS, and CUPRAC (Table S6). These finds lead to the exploration of the significant extracts that lead to these effects, so we conducted post hoc Tukey HSD (Table S7). Interestingly, the results of the post hoc showed that all the extracts with (p > 0.05) are the extracts that have similar antioxidant activities.
4. Discussion
Going through the plants under investigation, PCA score plot variations in the clustering patterns (Figure 1A) were found, revealing that the similarity between anise and caraway was due to the presence of seven common metabolites: chlorogenic acid, caffeoylquinic acid, dicaffeoylquinic acid, neochlorogenic acid, quinic acid, phenylalanine, and tryptophan. In addition, the presence of Coumaroylquinic acid, isoorientnm (luteolin hexoside), isovitexin, luteolin glucuronide, miqueliuanin (quercetin-3-galacturonide) only in anise and not in caraway might be affecting the location of the two plants on the PCA plot. This coincides with the fact that anise and caraway have phenolic acids, chlorogenic acid isomers, caffeoylquinic acid, kämpferol, and flavonoid luteolin as previously reported [30].
In the lower right quadrant along PC1, there are two clusters that can be explained separately and collectively. The clustering of coriander, fennel, and visnaga at the same position is due to sharing of three common metabolites, quinic acid, tryptophan, and phenylalanine, which were also found in the second clustering of parsley, Ammi majus, and celery. In addition, caffeoyl quinic acid and feruloylquinic acid were found to be common between fennel, celery, and visnaga. Coriander had the caffeic acid metabolite which was also reported, among with quercetin and chlorogenic acid, to be present in visnaga and coriander [30,31].
Dill was found to be the only plant having isorhamnetin glucuronide which explains its position away from the other eight plants. This type of flavanol glucuronide was also found in dill in other reported references [32]. Among other available phenolic compounds such as chlorogenic acid, neochlorogenic acid, caffeic acid, caffeoyl quinic acid, quinic acid, quercetin glucuronide, phenylalanine, and tryptophan, this characterizes dill as having antioxidant activity used in the management of diabetes and hypertriglyceridemia [30,33,34].
Taking a closer look at the loadings of the metabolites which were identified by LC-PDA-ESI-MS/MS chemical profiling, the first two PCs explained 90% of the total data variance (PC1 67% and PC2 23%). The results revealed that there are thirteen variables important to projection (VIP) metabolites which explains the above variation and clustering of the PCA. The VIPs present are apigenin, caffeoyl quinic acid, chlorogenic acid, Coumaroylquinic acid, ferulic acid glucoside, feruloyl quinic acid, hydroxybenzoic acid, hydroxy benzoyl hexose, isorhamnetin, isorhamnetin-rhamnoside, methoxy vinyl phenol, naringenin glucoside, and vanillin.
The PCA of the three Egyptian cultivars and the three German cultivars (Figure 2B) was PC1 37%, PC2 24%, and PC3 17%; the position of dill from both cultivars is supported by the presence of common metabolites: caffeic acid, caffeoyl quinic acid, isorhamnetin glucuronide, neochlorogenic acid, quinic acid, quercetin glucuronide, phenylalanine, and tryptophan. While looking at the metabolites of the coriander from the Egyptian cultivar and coriander from the German cultivar, we found that there are some common metabolites and some distinctive metabolites found in each on their own. The common metabolites are isopropylmalic acid, quinic acid, phenylalanine, and tryptophan.
The metabolites exclusive to the Egyptian cultivar are astragalin, caffeic acid, chlorogenic acid, neochlorogenic acid, feruloyl quinic acid, kämpferol-3-O-rutinoside, quercetin glucuronide, and rutin. Adding to the potent antioxidant, anti-inflammatory, and cardioprotective activities that most phenolics share, rutin was reported to have neuroprotective effects by increasing the production of glutathione which stimulates the expression of antioxidant enzymes. In addition, it acts directly as a free radical scavenger and inhibits the xanthin oxidase enzyme which helps in the production of reactive oxygen species (ROS) [35]. Astragalin is known to have antifibrotic, antiulcer, and antidiabetic effects [36]. Caffeic acid and neochlorogenic acid are chlorogenic acid isomers, produced from caffeic acid and quinic acid that differ in the number of caffeoyl quinic acids and binding site position. Chlorogenic acid has shown in vivo and in vitro effects acting in multimodal ways as a food additive and nutraceutical in the treatment and prevention of metabolic syndrome [37,38]. In addition, coriander (Coriandrum sativum L.) extracts showed antiaging in vivo effects, having protective lipid peroxidation activity [39].
The metabolites that are only available In the German cultivars were found to be apiin, malonyl apiin, and coumaroyl hexoside. Having parsley of both cultivars in opposite positions is due to the presence of chrysoeriol and its glucuronide derivatives, apigenin and one of its glucosides, and apigetrin only present in parsley from the Egyptian cultivar.
The loadings of the three PCs, which account for 78% of variation revealed a list of VIP metabolites that have the highest influence on the distinction between both cultivars. They are listed as follows: 1,3,7-trimethyluric acid, 4-methylumbelliferyl glucuronide, benzoic acid derivative, afzelechin, apigenin, caffeoyl quinic acid, caffeoyl spermidine derivative, coumaric acid hexoside, caffeic acid, ferulic acid glucoside, quinic acid, and vanillic acid glucoside.
The relative distance in the HCA dendrogram (Figure 2A) confirms that the dill of the Egyptian cultivar and the coriander of the Egyptian cultivar are the farthest points from each other, which was also shown by the PCA.
The PCA of the original data of the nine Egyptian cultivars shows a variation of 99% of both PC1 and PC2. Along PC1 which represents 96% of the data variation, the only major clustering appears between coriander and Ammi majus, while parsley is on the opposite side of that clustering. Along PC2, which represents 3%, dill, caraway, fennel, and visnaga are present in the same quadrant, while celery and anise are present in the same quadrant. This can be confirmed by the HCA dendrogram (Figure 2D) where the closest distance is between coriander and Ammi majus and the two plants that are shown to be present at the farthest points between the nine plants are anise and parsley.
The PCA and the HCA reveal how close the coriander plant is in both cultivars. PCA shows a variation of 99% where PC1 is 97% and PC2 is 2%. Moving along PC1 shows that dill is present in the left quadrant whereas coriander plants are in the right quadrant. As for PC2, the dill of the German cultivar and the parsley of the German cultivar are close to each other, positioned oppositely to the parsley of Egyptian origin.
The NIR scatter plot (Figure S19) of the mean of the nine Egyptian cultivar spectra for all original NIR data and the NIR scatter plot for the three Egyptian cultivars versus the three German cultivars (Figure S20) showed a pattern in the composition of the plants, stating that the overall plants of the same family show similar pattern when using the same NIR spectrophotometer yet difference in intensities and absorption band. There was no spectral enhancement after applying the first and second derivatization preprocessing. The results of PCA, HCA, and NIR spectra are complementary to each other, describing the position of the extract along with the phenolics availability in each extract.
By applying the PLS model, the PLS-R model for the Egyptian cultivars LC-PDA-ESI-MS/MS, and NIR data revealed that all the nine extracts did not exhibit a good correlation model with TPC, TFC, ABTS, FRAP, and CUPRAC antioxidant assays. On the contrary, comparing the Egyptian cultivars to the German cultivars the log- transformed LC-PDA-ESI-MS/MS led to calibration R2 of 0.960 and validation R2 of 0.889 with FRAP assay. The VIP of the phenolic compounds exhibited antioxidant activity with FRAP assay as the calibration R2 0.977 and validation R2 of 0.7819 and the log- transformed VIP, calibration R2 1 and validation R2 of 0. 895. This highlights that the six extracts are potent antioxidants with reducing power to reduce ferric (Fe3+) to ferrous (Fe2+) in vitro. FRAP assay is the only assay that measures the antioxidant capacity directly compared to other antioxidant assays [40]. It is considered a reliable assessment tool for total antioxidants in plants that are consumed by humans [41].
The antioxidant activity applied to the tested samples was done through different antioxidant assays, (TPC, TFC, DPPH, ABTS, FRAP, and CUPRAC) to assess different mechanisms of antioxidant effects. The number of phenolics and flavonoids present in the extracts, the free radical scavenging ability, reducing antioxidant property, and metal chelating ability of the extracts reflect the capability of the sampled to overcome oxidative stresses which in sequence lead to a wide range of pharmacological effects such as neuroprotection, anti-obesity, and anti-aging [30].
The evaluated polyphenol level in Table 1 revealed that the highest value of total polyphenols by Ammi majus 160.73 ± 4.42 gallic acid equivalent conc. (μg/mg) and the lowest level was found in coriander 50.33 ± 16.03 gallic acid equivalent conc. (μg/mg). Our results are somehow like those found by Tang et al. (Tang et al. 2015) which displayed the highest measured total phenolic content in parsley of 42.31 ± 0.50 mg GAE/g DW where parsley had a phenolic content of 53.67 gallic acid equivalent conc. (μg/mg). Nevertheless, El-Sayed et al. [42] reported that parsley exhibited higher polyphenols levels (121.95 ± 2.15 mg GAE/g extract). Alternatively, Msaada et al. [43] (recorded lower levels of phenolic content in Egyptian coriander (0.94 ± 0.05 mg GAE/g DW), Tunisian coriander (1.00 ± 0.06 mg GAE/g DW), and Syrian coriander (1.09 ± 0.02 mg GAE/g DW). Looking at celery polyphenol content of 75.763 ± 8.10 gallic acid equivalent conc. (μg/mg) was found to be lower in content than another study by Uddin et al. [44]. Comparing the three plants, coriander, dill, and parsley grown in Egypt, versus those grown in Germany, it was found that the coriander and parsley had higher phenolic content of 67.806 ± 3.31 and 62.15 ± 1.08 gallic acid equivalent conc. (μg/mg) in the German cultivar, respectively, compared to the Egyptian cultivar coriander and parsley as follows: 50.33 ± 16.03 and 52.57 ± 3.46 gallic acid equivalent conc. (μg/mg). Dill showed the opposite results, where dill of the German cultivar had lower phenolic content of 46.04 ± 1.45 gallic acid equivalent conc. (μg/mg) while the Egyptian cultivar was 67.36 ± 6.36 dill. These variations in total polyphenols level may be due to genetic variations, conditions of cultivation, extraction time, or the extracting solvent [45].
The total flavonoid content of the methanolic extract of the nine plants (Table 1) was calculated from the linear regression equation of the standard curve of quercetin. The highest was in Ammi majus concentration 32.90 ± 0.12 quercetin equivalent conc. (μg/mg) and the lowest concentration was in dill of 11.70 ± 0.02 quercetin equivalent conc. (μg/mg). Comparing the three German cultivar plants together, we concluded that parsley had the highest flavonoid content of 20.03 ± 0.01 quercetin equivalent conc. (μg/mg), while dill had a concentration of 13.41 ± 0.01 quercetin equivalent conc. (μg/mg); looking at the German cultivar plants, coriander had the highest flavonoid content of the twelve plants.
With the DPPH free radical scavenging assay, for the Egyptian cultivar herbs (Table 1), dill and celery had similar percentage of antioxidant activity of 90.22% which was the highest percentage of free radical scavenging, and the lower percentage was recorded for parsley for 69.25%. Within the German cultivar herbs, dill had the highest antioxidant activity at 90.47% and the lowest antioxidant activity in coriander at 55.01%. Surprisingly, comparing the twelve herbs together, dill recorded the highest free radical scavenging percentage in both German and Egyptian cultivar plants.
The case of ABTS assay, the experimental data for the Egyptian cultivar plants (Table 1) revealed that the crude methanolic extract was found to be effective in scavenging the ABTS+ radical, and the highest activity was shown in coriander with a concentration 102.97 ± 0.63 quercetin equivalent conc. (μg/mg), while the lowest concentration of 33.86 ± 0.36 quercetin equivalent conc. (μg/mg). In the German cultivar plants, dill noted the highest quercetin equivalent conc. (μg/mg) the concentration of 70.56 ± 0.38. Comparing German and Egyptian cultivar plants, parsley showed the highest concentration than Egyptian dill for 68.04 ± 1.04 quercetin equivalent conc. (μg/mg), while both German coriander and dill were of lower concentration than the Egyptian coriander and dill.
The method of FRAP to assess the reducing power of the different methanolic extracts of Egyptian cultivar (Table 1), visnaga showed the highest ferrous sulfate equivalent conc (μg/mg) of 405.96 ± 15.7 and the lowest concentration was recorded in celery 30.37 ± 59.8 ferrous sulfate equivalent conc (μg/mg). Exploring the German cultivar plants, parsley has the highest ferrous sulfate equivalent conc (μg/mg) of 82.64 ± 34.4. Of the twelve plants (Figure), visnaga remained in the highest rank above them all.
The reducing power of the crude methanolic extract of the twelve plants was evaluated using ferric to ferrous reducing activity using CUPRAC as determined spectrophotometrically from the formation of Perl’s Prussian blue color complex. In our study, the reducing power of the methanolic extract was compared to BHA. Taking a closer look at the Egyptian cultivar plants (Table 1), fennel showed the highest BHA equivalent conc. (μg/mg) of 100.76 ± 6.9 while parsley showed 22.96 ± 2.3, lowest BHA equivalent conc. (μg/mg). In addition, in the German cultivar plants, it was observed that parsley has the highest BHA equivalent conc. (μg/mg) of 28.09 ± 8.5 and coriander has the lowest BHA equivalent conc. (μg/mg) 6.25 ± 0.5. While comparing the German cultivar plants with the Egyptian cultivar plants, the parsley of the German cultivar was highest, and the Egyptian coriander and dill were of higher concentration than their match in the alternative German cultivar plants.
For the ANOVA findings, the correlation between the assays showed significant values (p < 0.05) which means that all the in vitro assays with the total phenolic content and total flavonoid content show no relation to each other. While going further in the analysis and applying post hoc Tukey HSD, the TPC content showed to be similar for corianders of both cultivars, having a sig > 0.05 with dill of both cultivars and parsley of both cultivars, caraway, fennel, anise, celery, and visnaga. In addition, Ammi majus showed a statistically insignificant value with caraway.
The TFC gave related results with the coriander of both cultivars and dill of both cultivars and parsley of the Egyptian cultivar. Parsley of the German cultivar showed similar TFC with coriander of German cultivar, fennel, anise, celery, and visnaga. Caraway with Ammi majus, fennel, celery and visnaga. Fennel shows a related TFC with parsley Germany, caraway, anise, celery, and visnaga. Anise with coriander of both cultivars, dill, parsley of German cultivars, fennel, celery, and visnaga. Celery with parsley of German cultivar, caraway, fennel, anise, and visnaga. Visnaga with coriander and parsley of German cultivar, caraway, fennel anise, and celery. Ammi majus showed similar TPC and TFC with caraway.
Moving to the in vitro assays, FRAP also showed a sig. > 0.05 with coriander of the Egyptian cultivar and coriander of the German cultivar, dill of both cultivars, parsley of both cultivars, caraway, Ammi majus, and fennel. On the other hand, the coriander of the German cultivar had similar ferrous inhibition of free radicals’ activity with the coriander of the German cultivar, dill of both cultivars, parsley of both cultivars, and celery. Dill of Egyptian cultivar showed the same pattern with all plants except with caraway, anise, and visnaga, while dill of the German cultivar compared with coriander of the German cultivar, dill of the Egyptian cultivar, parsley of both cultivars, and celery. Looking at the parsley of both cultivars, they showed similar FRAP activity to the coriander of both cultivars, dill of both cultivars, parsley of the Egyptian cultivars, and celery.
The CUPRAC assay post hoc results find that coriander of both cultivars has similar activity with dill of both cultivars, Ammi majus, and celery. Dill of the Egyptian cultivar, coriander of both cultivars, dill of the German cultivar, parsley of both cultivars, Ammi majus, celery and visnaga showed similar cupric ion-reducing antioxidant capacity. As for the dill of the German cultivar, it showed a similarity with the coriander of the Egyptian cultivar, dill, and parsley of both cultivars, Ammi majus, celery, and visnaga. Parsley of Egyptian cultivar was compared with coriander of the German cultivar, dill of both cultivars, parsley of German cultivar, Ammi majus and celery, and parsley of German cultivar with coriander and dill of both cultivars, parsley of Egyptian cultivar, Ammi majus, celery, and Ammi visnaga.
The in vitro assay ABTS showed different results for the coriander; from the Egyptian cultivar, it was only like anise, while coriander of the German cultivar was like parsley of both cultivars, caraway, Ammi majus, and visnaga. Dill of both cultivars showed a relative ability to scavenge free radicals with dill and parsley of both cultivars, caraway, fennel, anise, and visnaga. Meanwhile, parsley of both cultivars also had similar activity with coriander of both cultivars, dill of both cultivars, caraway, fennel, and visnaga.
5. Conclusions
Secondary metabolite identification techniques are becoming modernized to keep up with the speed of new instrumentation innovation and the vast amount of data output. Thus, the use of multivariate analysis combined with fingerprinting techniques gives a broad image to help in decision-making.
After intense investigation, considerable variations were detected between different samples. As expected, spectroscopic techniques revealed different outcomes regarding the profiling of phenolic metabolites. The unsupervised pattern recognition techniques PCA and HCA helped in visualization of the complex dataset and underlying metabolites responsible for clustering were observed. The PCA score plot of the nine Egyptian cultivars LC-PDA-ESI-MS/MS presented 90% of data variance, and 12 VIPs were identified, whereas the PCA of the three Egyptian cultivars versus the three German cultivars displayed 61% of data variance with 13 VIP metabolites. As for the FT-NIR PCA score plots of both types of analysis, nine Egyptian cultivar and three Egyptian cultivars versus the three German cultivars showed 99% of variation. Due to presence of high phenolic content, total phenolic content, total flavonoid content, and in vitro antioxidants assays were assessed on all the investigational samples of Egyptian and German cultivars, showing a wide variety of antioxidant activity. Ammi majus had the highest TPC and TFC, fennel and anise showed almost the same CUPRAC effect, celery with the highest ABTS result; dill of both cultivars showed same % scavenging activity, and parsley of both cultivars also presented same % scavenging ability and caraway, Ammi majus, fennel, anise, celery, and visnaga exhibited similar pattern of % scavenging effect. The German cultivar parsley has the highest FRAP and CUPRAC. Further analysis was done, using one-way ANOVA post hoc to confirm the in vitro assays results showing a potent antioxidant that can be used in herbal medicine. The correlation between the VIPs and phytochemicals within the tested extracts facilitated the predication of chemical structures in correlation with antioxidant activities without isolation; this strategy is highly effective regarding the cost and time to identify bioactive compounds or chemical markers for complicated herbal extracts. Novelty is demonstrated in profiling phenolic secondary metabolites in the Apiaceae family and using chemometric tools to investigate the big data produced from hyphenated techniques. This will be a leading step in the field of analytical data interpretation and application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10060347/s1. Figure S1: Gallic acid standard calibration curve for the determination of TPC; Figure S2: Quercetin standard calibration curve for the determination of TFC; Figure S3: Trolox standard calibration curve for determination of DPPH; Figure S4: Quercetin standard calibration curve for determination of ABTS; Figure S5: Ferrous sulfate standard calibration curve for the determination of FRAP; Figure S6: BHA standard calibration curve for determination of CUPRAC; Figure S7: LC-PDA chromatogram of metabolites detected in coriander (Coriandrum sativum L.) negative mode; Figure S8: LC-PDA chromatogram of metabolites detected in dill (Annethum graveolens L.) negative mode; Figure S9: LC-PDA chromatogram of metabolites detected in parsley (Petroselinum sativum L.) negative mode; Figure S10: LC-PDA chromatogram of metabolites detected in caraway (Carum carvi L.) negative mode; Figure S11: LC-PDA chromatogram of metabolites detected in greater ammi (Ammi majus L.) negative mode; Figure S12: LC-PDA chromatogram of metabolites detected in fennel (Foeniculum vulgare L.) negative mode; Figure S13: LC-PDA chromatogram of metabolites detected in anise (Pimpinella anisum L.) negative mode; Figure S14: LC-PDA chromatogram of metabolites detected in celery (Apium graveolens L.) negative mode; Figure S15: LC-PDA chromatogram of metabolites detected in visnaga (Ammi visnaga L.) negative mode; Figure S16: LC-PDA chromatogram of metabolites detected in coriander (Coriandrum sativum L.) negative mode; Figure S17: LC-PDA chromatogram of metabolites detected in dill (Annethum graveolens L.) negative mode; Figure S18: LC-PDA chromatogram of metabolites detected in parsley (Petroselinum sativum L.) negative mode; Figure S19: FT-NIR absorbance average spectrum of Egyptian cultivar samples; Figure S20: FT-NIR absorbance average spectrum of Egyptian versus German cultivar samples; Table S1: Peaks assignment using LC-PDA-ESI-MS/MS of metabolites detected in detected in alcoholic fraction of water extract of Egyptian and German extracts; Table S2: The partial least squares regression model parameters used for prediction of Egyptian cultivar LC-PDA-ESI-MS/MS data; Table S3: The partial least squares regression model parameters used for prediction of Egyptian cultivar NIR data; Table S4: The partial least squares regression model parameters used for prediction of Egyptian cultivar and German cultivar LC-PDA-ESI-MS/MS data; Table S5: The partial least squares regression model parameters used for prediction of Egyptian cultivar and German cultivar NIR data; Table S6: Antioxidant assays one way ANOVA results; Table S7: Antioxidant assays one way ANOVA post hoc Tukey results.
Author Contributions
Conceptualization, R.S.H. and H.H.; methodology, R.S.H., H.H. and N.H.A.; software, N.H.A.; data curation, I.K. and N.H.A.; writing—original draft preparation, N.H.A.; writing—review and editing, R.S.H., H.H., B.H. and N.H.A.; supervision, R.S.H., H.H. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article and the supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vandeginste, B. i-Chemometrics. J. Chemom. 2015, 29, 435–441. [Google Scholar] [CrossRef]
- Martens, H. Quantitative Big Data: Where chemometrics can contribute. J. Chemom. 2015, 29, 563–581. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Ruiz-Samblás, C.; Valverde-Som, L.; Pérez-Castaño, E.; González-Casado, A. Chromatographic fingerprinting: An innovative approach for food “identitation” and food authenticatione—A tutorial. Anal. Chim. Acta 2016, 909, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Yongyu, Z.; Shujun, S.; Jianye, D.; Wenyu, W.; Huijuan, C.; Jianbing, W.; Xiaojun, G. Quality Control Method for Herbal Medicine—Chemical Fingerprint Analysis. In Quality Control of Herbal Medicines and Related Areas; Shoyama, Y., Ed.; IntechOpen Limited: London, UK, 2011; pp. 171–194. [Google Scholar] [CrossRef]
- Kharbach, M.; Marmouzi, I.; El Jemli, M.; Bouklouze, A.; Vander Heyden, Y. Recent advances in untargeted and targeted approaches applied in herbal-extracts and essential-oils fingerprinting—A review. J. Pharm. Biomed. Anal. 2020, 177, 112849. [Google Scholar] [CrossRef]
- Schulz, H. Analytical Techniques for medicinal and aromatic plant. Stewart Postharvest Rev. 2007, 4, 1–12. [Google Scholar] [CrossRef]
- Bhusnure, O.G.; Suryawanshi, S.; Vijayendra Swamy, S.M.; Gholve, S.B.; Girm, P.S.; Birajdar, M.J. Standardization and Quality Evaluation of Herbal Drugs. Drug Deliv. Ther. 2019, 9, 1058–1063. Available online: https://jddtonline.info/index.php/jddt/article/view/2941 (accessed on 19 April 2023).
- De Lima, M.D.; Barbosa, R. Methods of Authentication of Food Grown in Organic and Conventional Systems Using Chemometrics and Data Mining Algorithms: A Review. Food Anal. Methods 2019, 12, 887–901. [Google Scholar] [CrossRef]
- Rohman, A.; Putri, A.R. The chemometrics techniques in combination with instrumental analytical methods applied in Halal authentication analysis. Indones. J. Chem. 2019, 19, 262–272. [Google Scholar] [CrossRef]
- Rohman, A.; Nugroho, A.; Lukitaningsih, E.; Sudjadi. Application of Vibrational Spectroscopy in Combination with Chemometrics Techniques for Authentication of Herbal Medicine. Appl. Spectrosc. Rev. 2014, 49, 603–613. [Google Scholar] [CrossRef]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2016, 53, 1071–1083. [Google Scholar] [CrossRef]
- Dhiman, A.; Sharma, K.; Sharma, A.; Sindhu, P. A review on the status of quality control and standardization of herbal drugs in India. Drug Dev. Ther. 2016, 7, 107–112. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Methanolic extract of Pimpinella anisum L. prevents dementia by reducing oxidative stress in neuronal pathways of hypermnesic mice. Pak. J. Zool. 2020, 52, 1779–1786. [Google Scholar] [CrossRef]
- Thiviya, P.; Gunawardena, N.; Gamage, A.; Madhujith, T.; Merah, O. Apiaceae Family as a Valuable Source of Biocidal Components and their Potential Uses in Agriculture. Horticulturae 2022, 8, 614. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Villera-Castrejón, J.; Gutiérrez-Uribe, J. Bioactive Molecules in Food, 1st ed.; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Li, M.Y.; Feng, K.; Hou, X.L.; Jiang, Q.; Xu, Z.S.; Wang, G.L.; Liu, J.X.; Wang, F.; Xiong, A.S. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic. Res. 2020, 7, 9. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. Industrial Crops & Products The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Arias-Alpizar, K.; Nuñez, O.; Saurina, J. Hydrophilic interaction liquid chromatography to characterize nutraceuticals and food supplements based on flavanols and related compounds. Separations 2021, 8, 17. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Hmidani, A.; Bammou, M.; Bourkhis, B.; Sellam, K.; Alem, C. Assessment of total polyphenols, flavonoids and anti-inflammatory potential of three Apiaceae species grown in the Southeast of Morocco. Sci. Afr. 2020, 9, e00507. [Google Scholar] [CrossRef]
- Onder, A.; Izgi, M.N.; Cinar, A.S.; Zengin, G.; Yilmaz, M.A. The characterization of phenolic compounds via LC-ESI-MS/MS, antioxidant, enzyme inhibitory activities of Salvia absconditiflora, Salvia sclarea, and Salvia palaestina: A comparative analysis. S. Afr. J. Bot. 2022, 150, 313–322. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Socaciu, M.I.; Socaci, S.A.; Muresan, V.; Fogarasi, M.; Rotar, A.M. Chemometric comparison and classification of some essential oils extracted from plants belonging to Apiaceae and Lamiaceae families based on their chemical composition and biological activities. Molecules 2018, 23, 2261. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic content, Flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Fredotović, Ž.; Puizina, J.; Nazlić, M.; Maravić, A.; Ljubenkov, I.; Soldo, B.; Vuko, E.; Bajić, D. Phytochemical characterization and screening of antioxidant, antimicrobial and antiproliferative properties of allium × cornutum clementi and two varieties of allium cepa l. Peel extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Gülçin, I.; Beyza, S.; Sarikaya, Ö.; Bursal, E. In vitro antioxidant activity of silymarin. J. Enzyme Inhib. Med. Chem. 2009, 24, 395–405. [Google Scholar] [CrossRef]
- Shahinuzzaman, M.; Yaakob, Z.; Anuar, F.H.; Akhtar, P.; Kadir, N.H.A.; Hasan, A.K.M.; Sobayel, K.; Nour, M.; Sindi, H.; Amin, N.; et al. In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Sci. Rep. 2020, 10, 10852. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Armstrong, D. (Ed.) Advanced Protocols in Oxidative Stress I, 1st ed.; Humana: Totowa, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Clinically tested medicinal plants View project Medical Education View project Phenolics and flavonoids contents of medicinal plants, as natural ingredients for many therapeutic purposes—A review. IOSR J. Pharm. 2020, 10, 42–81. [Google Scholar]
- Slimestad, R.; Fossen, T.; Brede, C. Flavonoids and other phenolics in herbs commonly used in Norwegian commercial kitchens. Food Chem. 2020, 309, 125678. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Calín-Sánchez, Á.; Nowicka, P.; Martínez-Tomé, J.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017, 226, 179–186. [Google Scholar] [CrossRef]
- Christova-Bagdassarian, V.L.; Bagdassarian, K.S.; Atanassova, M.S. Phenolic Profile, Antioxidant and Antibacterial Activities from the Apiaceae family (Dry Seeds). Mintage J. Pharm. Med. Sci. 2013, 2, 26–31. [Google Scholar]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Chen, Y. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 1943911. [Google Scholar] [CrossRef]
- Cisneros-zevallos, L.; Jacobo-vel, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Martins, F.; Mancini, J. In vivo antioxidant effect of aqueous and etheric coriander (Coriandrum sativum L.) extracts. Eur. J. Lipid Sci. Technol. 2003, 105, 483–487. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Payne, A.C.; Mazzer, A.; Clarkson, G.J.J.; Taylor, G. Antioxidant assays—Consistent findings from FRAP and ORAC reveal a negative impact of organic cultivation on antioxidant potential in spinach but not watercress or rocket leaves. Food Sci. Nutr. 2013, 1, 439–444. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Metwally, N.H.; Ibrahim, I.A.; Abdel-Hady, H.; Abdel-Wahab, B.S.A. Antioxidant Activity, Total Phenolic and Flavonoid Contents of Petroselinum crispum Mill. J. Appl. Life Sci. Int. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Msaada, K.; Ben Jemia, M.; Salem, N.; Bachrouch, O.; Sriti, J.; Tammar, S.; Bettaieb, I.; Jabri, I.; Kefi, S.; Limam, F.; et al. Antioxidant activity of methanolic extracts from three coriander (Coriandrum sativum L.) fruit varieties. Arab. J. Chem. 2017, 10, S3176–S3183. [Google Scholar] [CrossRef]
- Din, Z.U.; Shad, A.A.; Bakht, J.; Ullah, I.; Jan, S. In vitro antimicrobial, antioxidant activity and phytochemical screening of Apium graveolens. Pak. J. Pharm. Sci. 2015, 28, 1699–1704. [Google Scholar]
- Asensio, E.; Vitales, D.; Peralba, L.; Viruel, J. Phenolic Compounds Content and Genetic Diversity at Population Level across the Natural Distribution. Plants 2020, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).